Abstract
Cr(VI) is a well known environmental carcinogen, but its mechanism of action and the measures required to mitigate its effects remain to be investigated. Our previous studies showed that exposure of human bronchial epithelial (BEAS-2B) cells to Cr(VI) caused malignant transformation, that these transformed cells progressed through tumorigenesis, and that luteolin, a natural compound, inhibited both of these processes. The present study investigates the underlying mechanisms by which luteolin protects cells against Cr(VI)-induced transformation and tumorigenesis. The present study shows that luteolin activates inducible Nrf2 to inhibit Cr(VI)-generated reactive oxygen species (ROS) in normal BEAS-2B cells. The decreased ROS level is likely responsible for the protective effect of luteolin against Cr(VI)-induced malignant cell transformation in normal cells. By contrast, in cells that have been transformed by Cr(VI), Nrf2 is constitutively activated, and its target proteins, heme oxygenase 1 (HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), and superoxide dismutase 1/2 (SOD1/SOD2) are all constitutively activated, and ROS levels are low. Bcl-2, an anti-apoptotic protein and target protein of Nrf2 is elevated. Cr(VI)-transformed BEAS-2B cells develop apoptosis resistance, increasing the survival of these transformed cells. Luteolin decreases interactions between Nrf2 and the antioxidant response element sites of its target anti-apoptotic and antioxidant proteins, Bcl-2, Bcl-XL, and HO-1, which results in decreased constitutive Nrf2 activation. The decreased constitutive Nrf2 activation, decrease in Nrf2 target proteins and consequent apoptosis resistance by luteolin are possible mechanisms that mediate the protective effect of luteolin in Cr(VI)-transformed cells.
Keywords: Hexavalent chromium, luteolin, Nrf2, apoptosis resistance, carcinogenesis
Introduction
It has been well established that environmental and occupational exposure to Cr(VI) causes cancer in various organs, including lungs (Agency for Toxic Substances and Disease Registry, 1982; Costa, 1997; De Flora, 2000; IARC, 1990; Langard, 1990, 1993; Machle and Gregorius, 1948; Pellerin and Booker, 2000; Simonato et al., 1991; Sorahan et al., 1998; Woodruff et al., 1990). Most studies on Cr(VI)- induced carcinogenesis focused on the mechanistic elements of transformation; few studies direct attention to mechanism-based prevention. In our recent study (Pratheeshkumar et al., 2014), we showed that luteolin inhibits Cr(VI)-induced malignant cell transformation, the first stage of Cr(VI)-induced carcinogenesis, whereby normal cells are transformed into cancer cells. Luteolin can also inhibit tumorigenesis of Cr(VI)-transformed cells, the second stage, whereby the Cr(VI)-transformed cells progress to tumors. Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a common dietary flavonoid found in fruits, vegetables, and medicinal herbs with recognized antioxidant, anti-inflammatory, and anticancer activities. In the last decade, cancer prevention using naturally occurring dietary agents has gained immense interest because of their safety, low toxicity, and general availability. Although our study (Pratheeshkumar et al., 2014) showed that luteolin inhibits Cr(VI)-induced carcinogenesis at both stages, the underlying mechanisms responsible for this protection are unclear. The present study investigates the mechanisms by which luteolin protects cells against Cr(VI)-induced malignant transformation and the progression of tumorigenesis in Cr(VI)-transformed cells.
While detailed information on the mechanism of Cr(VI)-induced carcinogenesis is still under investigation, it is generally believed that Cr(VI)-generated reactive oxygen species (ROS) are an important component (Leonard et al., 2004; Stohs et al., 2000; Wang et al., 2011; Yao et al., 2008; Ye et al., 1995). Our previous studies showed that exposure of human lung bronchial epithelial (BEAS-2B) cells to Cr(VI) generated a whole spectrum of ROS, including superoxide radical (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH) (Leonard et al., 2004; Wang et al. 2011). Inhibition of these species by SOD (against O2•−) and catalase (against H2O2, a precursor of •OH) decreased Cr(VI)-induced cell transformation, indicating that ROS are important in this process (Wang et al., 2011). Thus, inhibition of ROS is an important approach to protect cells from Cr(VI)-induced transformation. It has been reported that luteolin has an antioxidant property, through activation of inducible Nrf2, which is a key transcription factor that regulates antioxidant proteins to neutralize ROS and restore cellular redox balance (Chan et al., 2011; Kobayashin et al., 2006). Upregulation of a variety of important antioxidants occurs through the binding of Nrf2 to the antioxidant response elements (ARE) of antioxidant target proteins such as SOD, catalase, and HO-1. Numerous reports demonstrate that induction of inducible Nrf2 by a natural compound up-regulates its target antioxidant proteins, leading to a decrease in ROS and a subsequent cancer protective effect by this natural compound (Jeong et al., 2006; Kumar et al., 2014; Kou et al., 2013; Park et al., 2006). It is very possible that luteolin activates inducible Nrf2 in normal BEAS-2B cells, which increases cellular levels of antioxidants, and decreases Cr(VI)-generated ROS. The outcome is inhibition of Cr(VI)-induced malignant cell transformation, the first stage of Cr(VI)-induced carcinogenesis. In this study, we show that activation of inducible Nrf2 and decrease of ROS by luteolin are likely the underlying mechanisms that this natural compound employs as protection against Cr(VI)- induced malignant cell transformation of BEAS-2B cells reported in our recent study (Pratheeshkumar et al., 2014).
In contrast to the widely reported studies on the mechanism of Cr(VI)-induced malignant cell transformation, only limited studies are available that focus on the mechanism responsible for the oncogenic progression of Cr(VI)-transformed cells to the second stage, tumor formation. In Cr(VI)-transformed cells, ROS may function as an inhibitor of tumorigenesis in Cr(VI)-transformed cells by the induction of apoptosis. A variety of studies show that activation of inducible Nrf2 is anti-carcinogenic in the early stage of carcinogenesis (from normal cells to malignantly transformed cells) by up-regulation of its target antioxidants to reduce the levels of ROS (Hu et al., 2010; Kansanen et al., 2013; Kostova, 2010; Sporn and Liby, 2012). In contrast, constitutive expression of Nrf2 is carcinogenic through a protection to cancer cells from oxidative stress and chemotherapeutic agents in the late stages of carcinogenesis (from malignantly transformed cells or cancer cells to tumor) (Komatsu, 2011; Sporn and Liby, 2012; Tew, 2011; Wang et al., 2008 12). Constitutive expression of Nrf2 is conspicuous in several types of human cancer cell lines and tumors (Lau et al., 2013; Ohat et al., 2008; Sporn and Liby, 2012; Tong et al., 2006). Therefore, the stage of carcinogenesis determines whether Nrf2 acts as a cancer preventive or oncogenic agent (Komatsu, 2011; Sporn and Liby, 2012; Tew, 2011). Activation of inducible Nrf2, which would reduce oxidative stress or mutagenic stress, appears to be beneficial during the pre-malignant state (Komatsu, 2011; Sporn and Liby, 2012; Tew, 2011). As such, the biological timing context is important: inducible Nrf2 activation is desirable in the early stage of carcinogenesis, when the host is seeking to control pre-malignant carcinogenesis, but is undesirable in the later stage, when it can make malignant cancer cells resistant to apoptotic cell death or treatment (Sporn and Liby, 2012). Reports indicate that constitutively activated Nrf2 in cancer cells bind to ARE sites of Bcl-2 and Bcl-XL gene promoters, leading to up-regulation of Bcl-2 and Bcl-XL and development of resistance to apoptosis (Calvert et al., 2009). In our recent studies (Son et al., 2014, 2015), we showed that in arsenic- or Cd(II)-transformed cells, Nrf2 is constitutively activated, which upregulates antioxidant and anti-apoptotic proteins through the binding of Nrf2 to ARE sites of these target proteins. The outcome is decreased levels of ROS and the development of apoptosis resistance, an environment favorable for progression of transformed cells and tumor formation. In the present study, we show that in Cr(VI)-transformed cells, Nrf2 is constitutively activated and its inducible nature is lost. Constitutive Nrf2 activation in Cr(VI)-transformed cells is oncogenic through a decrease of ROS and development of apoptosis resistance. In the present study, we also show that in Cr(VI)-transformed cells, luteolin inhibits constitutive Nrf2 activation, which is likely the mechanism by which luteolin interrupts the process of tumorigenesis in Cr(VI)-transformed cells observed in our recent study (Pratheeshkumar et al., 2014).
Materials and methods
Chemicals, antibodies, and laboratory wares
Unless specified otherwise, all chemicals and laboratory wares were purchased from Sigma Chemical Company (St. Louis, MO) and Falcon Labware (Bectone-Dickinson, Franklin Lakes, NJ), respectively. Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco Company (Gibco BRL, NY).
Cell culture and treatments
The human lung bronchial epithelial cell line BEAS -2B was obtained from American Type Culture Collection (Manassas, VA). Cr(VI)-transformed cells were generated as described previously (Wang et al., 2011). Cr(VI)-transformed BEAS-2B cells and their parent non-transformed BEAS-2B cells were maintained in DMEM supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin and then processed as indicated.
Clonal assay
BEAS-2B cells were treated without or with luteolin for 48 hours. The cells were reseeded and cultured for two weeks. The cells were fixed with 2% formalin for 10 min and stained with 0.5% crystal violet for 5 min prior to quantitation of colonies by light microscopic inspection.
ROS measurement by fluorescence staining
Cells were incubated with 10 μmol/L 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate ethyl ester (DCF; Molecular Probes) or 5 μmol/L dihydroethidium (DHE; Molecular Probes). Treated cells were subsequently harvested with trypsin, washed twice with cold PBS, and analyzed by fluorescence-activated cell sorting (FACS Calibur, BD Biosciences). The fluorescence intensity of DCF was measured at an excitation wavelength of 492 nm and an emission wave length of 517 nm. The fluorescence intensity of DHE was measured at an excitation wavelength of 535 nm and an emission wavelength of 610 nm.
ROS measurement by electron spin resonance (ESR) spin trapping assay
This assay was performed using a Bruker EMX spectrometer (Bruker Instrume nts, Billerica, MA) and a flat cell assembly, as described previously (Son et al., 2010). 5,5-Dimethyl-1-pyrroline-1-oxide (DMPO) (50 μM) was used as a radical trapping agent. Spectrometer settings included magnetic field 3220 ± 50 G; microwave frequency, 9.6 GHz; incident microwave power, 10 mW; scan time, 1 min; modulation frequency, 100 kHz; and modulation amplitude, 0.2 G.
Western blot analyses
Cells lysates were prepared in ice-cold RIPA buffer (Sigma-Aldrich) with freshly added protease inhibitor cocktail. The lysate was then centrifuged at 12,000 g for 10 min at 4°C and the supernatant (total cell lysate) was collected, aliquoted and stored at −80°C. The protein concentration was determined using Coomassie Protein Assay Reagent (Thermo, Rockford, IL). Approximately 40 μg cellular proteins were separated through 6%–12% SDS-polyacrylamide gel, and then transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). Nonspecific binding was blocked with 5% fat- free dry milk in 1X Tris-buffered saline (TBS) and the membrane incubated with antibody, as indicated. Protein bands, detected with horseradish peroxidase-conjugated antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD), were visualized with enhanced chemiluminescence reagent (Perkin Elmer, Boston, MA).
Assessment of apoptosis
The extent of apoptosis was evaluated by flow cytometric analysis using FITC-conjugated Annexin V/propidium iodide (PI; BD PharMingen) staining per the manufacturer’s instructions. Both early apoptotic (Annexin V–positive, PI-negative) and late apoptotic (Annexin V–positive and PI-positive) cells were included in cell death determinations.
Chromatin immunoprecipitation (ChIP) assay
ChIP analysis was performed using a Pierce™ Agarose ChIP Kit (Thermo Scientific, Rockford, IL) as described previously (Son et al., 2014). The relative Nrf2 binding to the ARE regions of Bcl-2, Bcl-XL, and HO-1 were assessed by the MyiQ™ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with SYBR Green PCR master mix. General PCR amplification was also performed by Mastercycler® thermal cyclers (Eppendorf, Foster City, CA).
Statistical analysis
All the data are expressed as mean ± standard error (SE). One-way analysis of variance (ANOVA) using IBM SPSS Statistics 21 was used for multiple comparisons. A value of P < 0.05 was considered statistically significant.
Results
Luteolin does not exhibit any significant toxicity in BEAS-2B cells
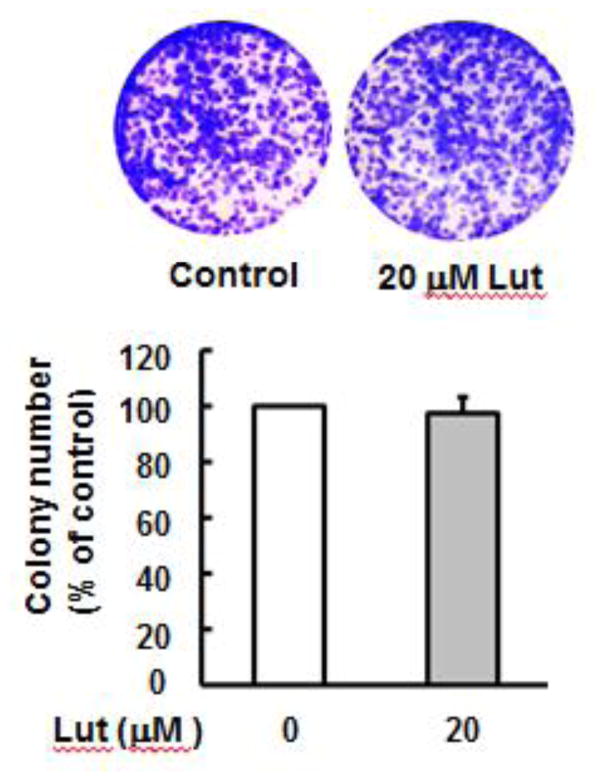
The effect of luteolin on cell viability was determined by clonogenic assay. BEAS-2B cells were treated with 20 μM luteolin for 48 hours, reseeded, cultured for an additional 2 weeks and stained with crystal violet. Luteolin at 20 μM does not cause significant cell death (Fig. 1), indicating that at the concentration used for these studies luteolin does not exhibit any significant cytotoxicity.
Fig. 1. Luteolin does not exhibit any cytotoxic property.
Cell viability was measured by clonogenic assay. BEAS-2B cells were treated without or with luteolin (20 μM) for 48 hours, reseeded and cultured for an additional 2 weeks and stained with crystal violet. The data are expressed as the mean±SE (n=6). Lut; luteolin.
Scavenging of Cr(VI)-generated ROS by luteolin
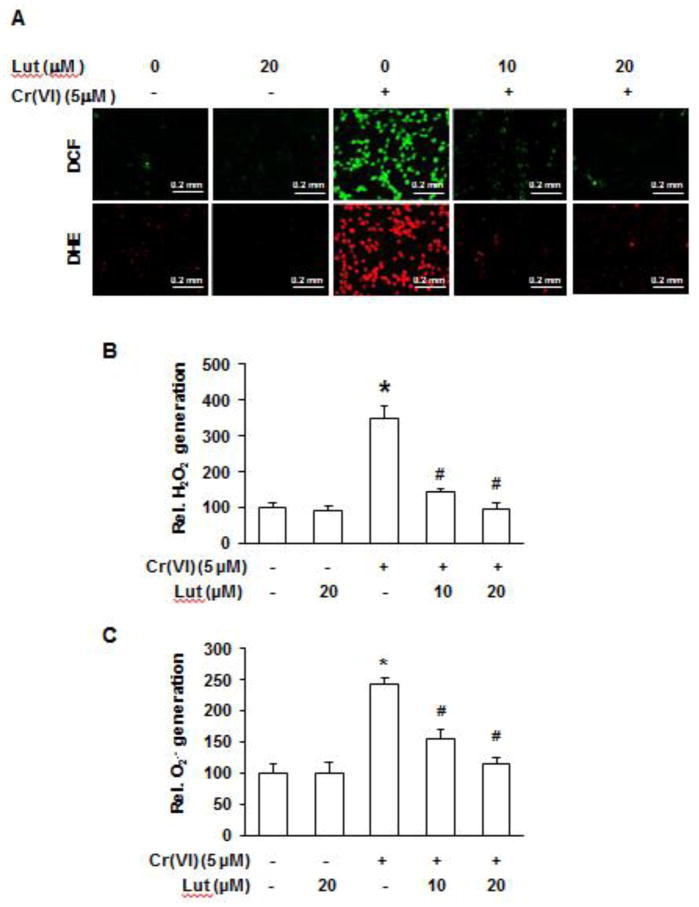
In our previous studies(Leonard et al., 2004; Wang et al. 2011), we showed that exposure of BEAS-2B cells to Cr(VI) generates ROS. In the present study, we measured the Cr(VI)-induced O2•− and H2O2 productions using fluorescent probes DCF (for H2O2 detection) and DHE (for O2•− detection), respectively. As shown in Figure 2, treatment of BEAS-2B cells with Cr(VI) generates both O2•− and H2O2, and luteolin decreases the cellular levels of these reactive oxygen species.
Fig. 2. Luteolin decreases the levels of H2O2 and O2•− generated by Cr(VI).
BEAS-2B cells were exposed to Cr(VI) (0 or 5 μM) with or without luteolin (0, 10, 20 μM) for 12 h and then were labeled with DHE (5 μM) or DCFDA (10 μM). (A) Images were taken with fluorescence microscopy and fluorescent intensity determined by flow cytometry. Micrographs represent one of six replicates from each treatment group. Graphic representation of fluorescence intensity for (B) H2O2 and (C) O2•− with *p<0.05 compared to control, #p<0.05 compared to Cr(VI) alone. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells, Lut; luteolin.
Constitutive expression of Nrf2 in Cr(VI)-transformed cells
As outlined in the Introduction, Cr(VI)-induced cell transformation is considered as the first stage of Cr(VI) carcinogenesis (from normal cells to malignant/transformed or cancer cells). The progression of Cr(VI)-transformed cells to tumor occurs subsequently. In our recent report, we showed that luteolin inhibits tumorigenesis of Cr(VI)-transformed cells (Pratheeshkumar et al., 2014). In order to understand the mechanism of this tumorigenic process and its protection by luteolin, we measured the protein level of Nrf2 in Cr(VI)-transformed cells. As shown in Figure 3, the protein level of Nrf2 is much higher in Cr(VI)-transformed BEAS-2B cells than that in the parental BEAS-2B cells, and this high Nrf2 level is constitutive. In addition, the expression level of Nrf2 was increased in the presence of 1 μM of Cr(VI) in the Cr(VI)-transformed BEAS-2B cells.
Fig. 3. Increased levels of Nrf2 in Cr(VI)-transformed cells.
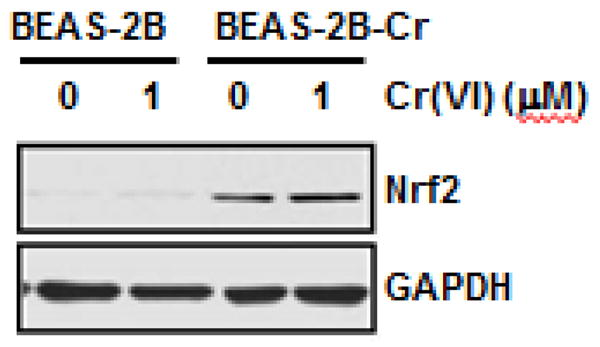
Both normal parental and Cr(VI)-transformed BEAS-2B cells were treated with and without 1 μM of Cr(VI) for 24 hours. The cells were harvested and whole protein lysates was obtained. The expression of Nrf2 was analyzed by immunoblot. Presented results are representative of three independent experiments. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells.
Increased expressions of antioxidant enzymes in Cr(VI)-transformed cells
As a transcription factor, Nrf2 regulates various antioxidant proteins positively through binding to the ARE sites of their gene promoter. To investigate the up-regulation of antioxidant proteins by Nrf2, we measured expression levels of several key antioxidant enzymes. As shown in Figure 4, HO-1, NQO1, SOD1, and SOD2 were constitutively up-regulated in Cr(VI)-transformed BEAS-2B cells compared to levels in the non-transformed parental cells.
Fig. 4. Increased levels of antioxidant proteins in Cr(VI)-transformed cells.
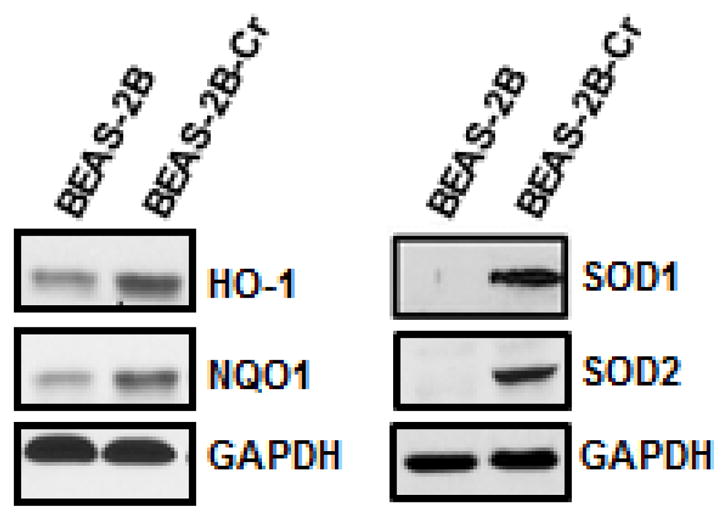
Whole cell lysates were extracted from Cr(VI)-transformed cells and passage-matched normal cells (BEAS-2B) for immunoblot analysis. Presented results are representative of three independent experiments. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells.
Reduced capability of ROS generation in Cr(VI)-transformed cells
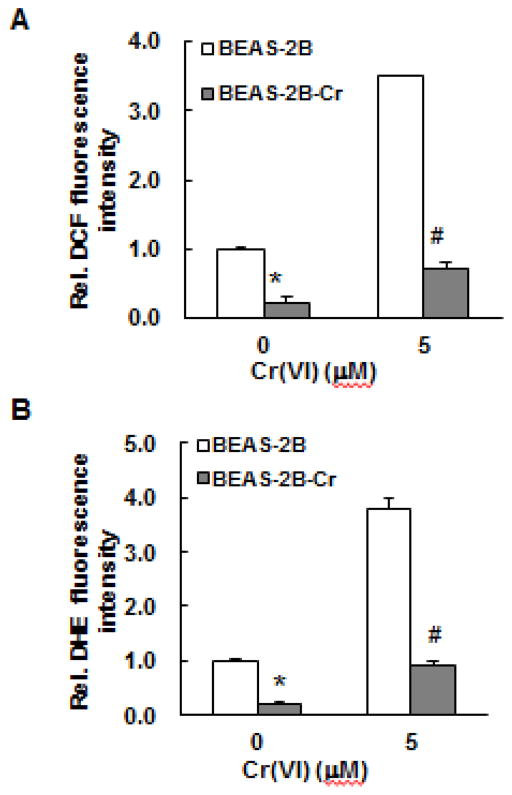
The increase in Nrf2 and its target proteins in Cr(VI)-transformed cells suggests that the decrease in ROS levels in these cells may be related to Nrf2. We used two methods to investigate this possibility: 1) ESR spin trapping and 2) fluorescence staining. Since oxygen radicals are reactive and have a very short lifetime, direct ESR measurement cannot be used for their detection. Instead, ESR spin trapping, an indirect method, is used. This technique involves the addition-type reaction of a short- lived radical with a paramagnetic compound (spin trap) to form a relatively long-lived free radical product (spin adduct), which can then be measured by ESR. The intensity of the ESR signal is used to measure the amount of short-lived radicals trapped, and the hyperfine couplings or line-shape of the spin adduct are generally characteristic for the trapped radicals and used for identification. Spin trapping is the method of choice for the detection and identification of free radicals generated in biological systems due to its specificity and sensitivity. Using this method, we measured ROS generation in Cr(VI)-transformed and non-transformed BEAS-2B cells with and without Cr(VI) exposure. The results are provided in Figure 5. As noted from this figure, BEAS-2B cells alone generated a detectable basal level of oxygen radicals and Cr(VI) exposure enhanced this level. Similar results were obtained using Cr(VI)-transformed BEAS-2B cells except that overall signal intensities are low, indicating a reduced capability of oxygen radical generation in these cells. We also measured ROS generation in Cr(VI)-transformed and non-transformed BEAS-2B cells with and without Cr(VI) exposure by flow cytometry together with DHE or DCF as fluorescent probes for O2•− and H2O2, respectively. The results show that both the DHE and DCF fluorescence intensities with and without Cr(VI) treatment were substantially lower in Cr(VI)-transformed BEAS-2B cells than those from the non-transformed cells (Fig. 6). These results indicate that Cr(VI)-transformed cells exhibited a reduced capacity for ROS generation.
Fig. 5. Reduced ROS generation in Cr(VI)-transformed cells as measured by electron spin resonance spin trapping.
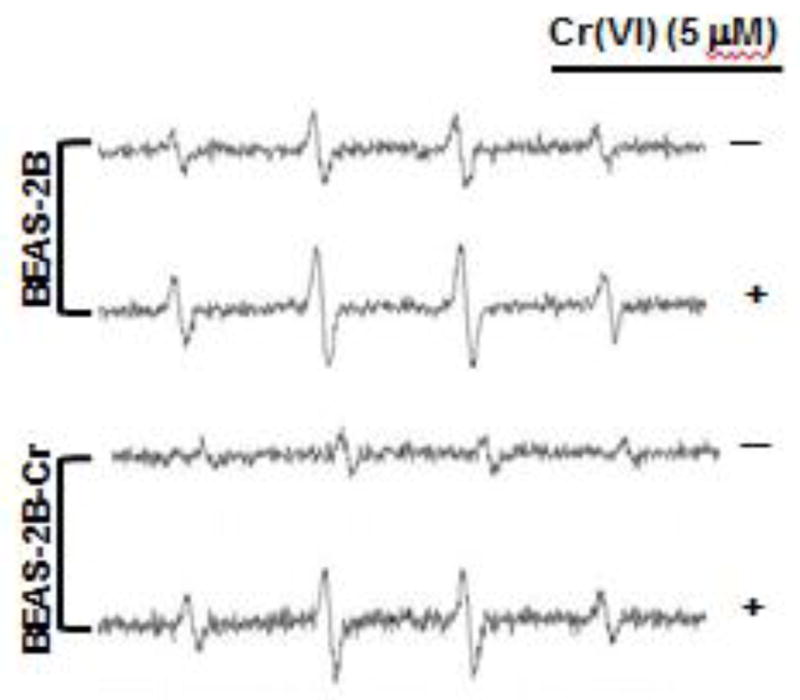
Both normal parental BEAS-2B cells and Cr(VI)-transformed BEAS-2B cells were treated with and without 5 μM Cr(VI) for 12 hours. The oxygen radical generations were measured by ESR spin trapping using 5,5-Dimethyl-1-pyrroline N-oxide (DMPO. The results are representative of three independent experiments. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells.
Fig. 6. Reduced ROS generation in Cr(VI)-transformed cells as measured by fluorescence.
Both normal parental BEAS-2B cells and Cr(VI)-transformed BEAS-2B cells were treated with and without 5 μM Cr(VI) for 12 hours and analyzed for DCF (A) and DHE (B) fluorescence intensities by flow cytometry as described in previously. Data are mean±SE (n=6). * and # indicates p<0.05 compared to normal parent BEAS-2B cells without and with 5 μM Cr(VI) treatment, respectively. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells.
Acquired apoptosis resistance and constitutive expression of Bcl-2
Previous studies showed that Nrf2 is able to bind to the ARE in the promoter region of the Bcl-2 gene, leading to an up-regulation of Bcl-2 and decrease in apoptosis (Calvert et al, 2009; Lau et al., 2013; Mukherjee et al, 2013; Ohat et al., 2008; Sporn and Liby, 2012; Tong et al., 2006). Constitutive Nrf2 activation in Cr(VI)-transformed BEAS-2B cells promotes elevated Bcl-2 levels in this transformed cells. Figure 7A shows that Bcl-2 protein levels are much higher in Cr(VI) transformed BEAS-2B cells than in normal parental cells, both with and without Cr(VI) treatment. Similar to Nrf2, the high Bcl-2 levels in Cr(VI)-transformed cells is constitutive. Because Bcl-2 is an anti-apoptosis protein and the acquired resistance to apoptosis is a critical cellular event in tumor formation and malignant progression, we investigated whether Cr(VI)-transformed cells develop resistance to apoptosis. Consistent with Bcl-2 up-regulation, we observed a decreased level of cleaved PARP (c-PARP) in Cr(VI)-transformed cells with Cr(VI)-treatment (Fig. 7A), indicating that Cr(VI)-transformed cells have the property of apoptosis resistance. We also used FITC-conjugated Annexin V/PI staining together with flow cytometry to measure the apoptotic potential of Cr(VI)-transformed cells. In the results presented in Figure 7B confirm that Cr(VI)-transformed cells exhibit a resistance to apoptosis.
Fig. 7. Acquired apoptosis resistance in Cr(VI)-transformed BEAS-2B cells.
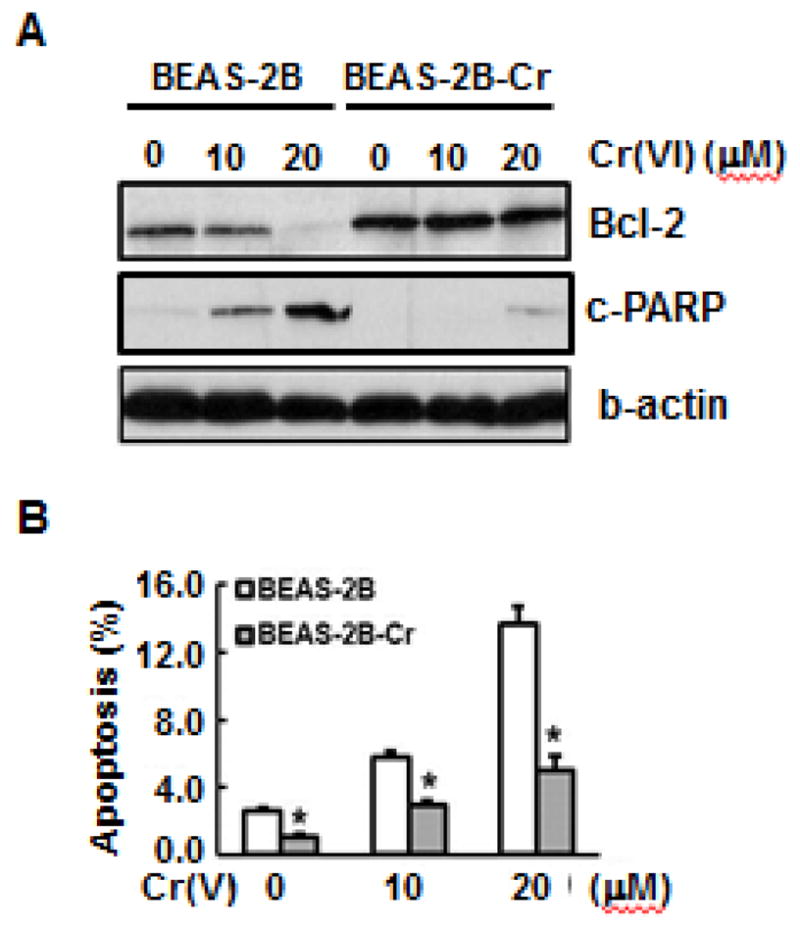
(A) Both BEAS-2B and Cr(VI)-transformed cells were treated with different concentrations of Cr(VI) for 24 hours. The whole cell lysates were collected to examine expressions of Bcl-2 and cleaved PARP (c-PARP) by immunoblot analysis. (B) Apoptosis was measured using FITC-conjugated Annexin V/PI staining by flow cytometry. Both BEAS-2B and Cr(VI)-transformed cells were seeded into 6-well culture plates (1×105cells/well). The cells were treated with different concentrations of Cr(VI). After 24 hours, 5 μL of fluorescein isothiocyanate (FITC)-labeled PI solution (10 μg/mL) was added into the cells. The percentage of apoptotic cells was measured using flow cytometry. Data are mean±SE (n=6). * indicates a p < 0.05, compared to normal BEAS-2B cells. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells.
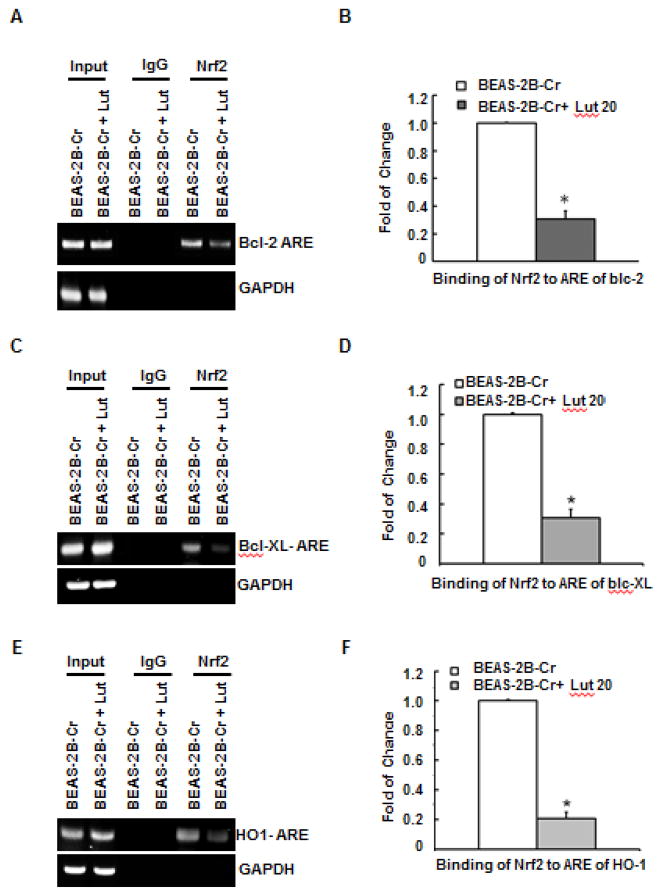
Luteolin inhibits Nrf2 binding to ARE sites in the Bcl-2, Bcl-XL, and HO-1 gene promoters in Cr(VI)-transformed cells
Previous studies showed that Nrf2 binds to ARE sites in the promoter region of Bcl-2, Bcl-XL, and HO-1 genes, leading to an up-regulation of these genes (Lau et al., 2013; Ohat et al., 2008; Sporn and Liby, 2012; Tong et al., 2006). In addition, it has been demonstrated that Nrf2- mediated up-regulation of Bcl-2 plays a significant role in increased cell survival (Calvert et al, 2009; Lau et al., 2013; Mukherjee et al, 2013; Ohat et al., 2008; Sporn and Liby, 2012; Tong et al., 2006). Toward the goal of understanding the mechanism by which luteolin resists tumorigenesis in Cr(VI)-transformed cells, we examined the possibility that this natural compound inhibits the binding between Nrf2 and individual ARE sites of anti-apoptotic and antioxidant proteins by ChIP analysis. The results (Fig. 8) show that luteolin dramatically reduced the binding of Nrf2 to the ARE sites of these genes.
Fig. 8. Luteolin inhibits binding of Nrf2 to ARE sites on the Bcl-2, Bcl-XL, and HO-1 gene promoters in Cr(VI)-transformed cells using ChIP assay.
Cr(VI)-transformed cells in the presence or absence of luteolin (20 μM) were fixed with formaldehyde and cross-linked. The chromatin was sheared and immunoprecipitated with anti-Nrf2 antibody or control IgG. Binding of Nrf2 to Bcl-2, Bcl-XL, and HO-1 promoters was analyzed by PCR using specific primers for ARE regions of their promoters (A, C, and E). ChIP and quantitative RT-PCR analysis was performed. The immunoprecipitated DNA was normalized to the input levels and plotted (B, D, and F). Data are mean ±SE (n=6). * indicates a p < 0.05, compared to levels obtained without luteolin. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells, Lut; luteolin.
Luteolin increases Nrf2 expression in normal cells and decreases constitutive Nrf2 expression in Cr(VI)-transformed cells
Results presented in Figure 2 show that luteolin reduces Cr(VI)- induced ROS generation. Previous studies showed that luteolin up-regulates Nrf2 in a variety of cellular systems (Huang et al., 2012; Zhang et al., 2013). Based on the results obtained in Figure 2 and those reported in the literature (Huang et al., 2012; Zhang et al., 2013), we hypothesized that luteolin is capable of activating Nrf2 in normal BEAS-2B cells. As shown in Figure 9A, luteolin does indeed up-regulate Nrf2 expression in BEAS-2B cells. In addition, we showed that Nrf2 is constitutively activated in Cr(VI)-transformed BEAS-2B cells (Fig. 3). It has been reported that luteolin decreases activation of Nrf2 in cancer cells (Tang, 2011). It is very likely that luteolin may inhibit the constitutive expression of Nrf2 in Cr(VI)-transformed BEAS-2B cells. This indeed the case as shown in Figure 9B, that luteolin decreased the protein level of Nrf2 in Cr(VI)-transformed BEAS-2B cells.
Fig. 9. Luteolin increases inducible Nrf2 expression in normal cells and decreases constitutive Nrf2 expression in Cr(VI)-transformed cells.
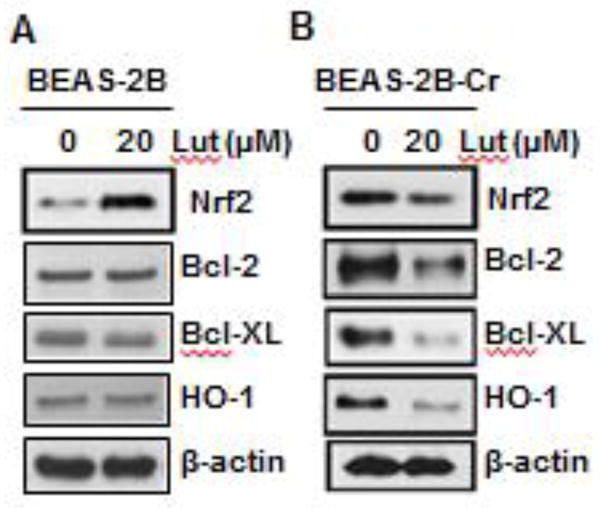
Normal parental BEAS-2B cells (A) and Cr(VI)-transformed BEAS-2B cells (B) were treated with luteolin (10 μM) for 24 hours. Cells were harvested and whole protein lysates were extracted. The expression levels of Nrf2 and its target proteins were examined by immunoblot analysis. Presented results are representative of three independent experiments. BEAS-2B-Cr; Cr(VI)-transformed BEAS-2B cells, Lut; luteolin.
Luteolin inhibits constitutive expressions of Bcl-2, Bcl-XL, and HO-1 proteins in Cr(VI)-transformed cells
Bcl-2 and Bcl-XL are well known anti-apoptotic proteins and HO-1 is an antioxidant protein. In Figure 9B, we showed that luteolin decreased constitutive activation of Nrf2 in Cr(VI)-transformed BEAS-2B cells. Our results (Fig. 8) also show that luteolin decreased the binding of Nrf2 to the ARE sites of its target antioxidant and anti-apoptotic proteins. Based on these results, it is expected that luteolin decreases the protein level of Nrf2 target proteins. As shown in Figure 9B, luteolin inhibits expressions of Bcl-2, Bcl-XL, and HO-1 in Cr(VI)-transformed cells.
Discussion
The goal of the present study is to understand the underlying mechanisms related to our recent report that luteolin inhibits Cr(VI)- induced malignant cell transformation, the first stage of Cr(VI) carcinogenesis (from normal cells to malignantly transformed cells or cancer cells), as well as the tumorigenic processes of Cr(VI)-transformed cells in the second stage (from malignantly transformed cells or cancer cells to tumor). Our previous studies showed that ROS generated by activation of NAD(P)H oxidase are important for Cr(VI)-induced malignant cell transformation (Wang et al., 2011). The results obtained from the present study indicate that inhibition of ROS is the key mechanism by which luteolin prevents Cr(VI)-induced cell transformation. The following results support this conclusion. (1) Fluorescence staining shows that luteolin inhibits Cr(VI)- induced ROS generation. This result is in an agreement with other reports that focus on the antioxidant property of this natural compound (Park et al., 2006). (2) Luteolin upregulates inducible Nrf2 in normal BEAS-2B cells. The Nrf2 pathway plays an vital role in cellular redox homeostasis and activation of this pathway is one of the main defense mechanisms against oxidative or electrophilic stress (Chan et al., 2001; Kobayashi et al., 2006). Nrf2 upregulates its target antioxidant proteins through binding of Nrf2 to ARE sites in their regulatory regions. These Nrf2 target enzymes are expressed in various isoforms and are distributed in a variety of organelles and subcellular compartments and cooperatively participate in metabolic reactions that eliminate ROS at their sites of origin. Various natural compounds exhibit their cancer-preventive properties through activation of inducible Nrf2 (Jeong et al., 2006; Kou et al., 2013). Thus, in normal BEAS-2B cells, ROS generated by Cr(VI) are oncogenic by changing normal BEAS-2B cells to malignantly transformed cells. Activation of inducible Nrf2 in non-cancer cells by luteolin, which up-regulates its target antioxidant proteins, reduces ROS, and inhibits cell transformation; these activities are cancer preventive or anti-oncogenic. The mechanism of our previously reported protection of Cr(VI)- induced cell transformed by luteolin is likely through an upregulation of Nrf2 and decrease of ROS by this natural compound.
Considering various roles of ROS, the mechanism for the prevention of tumorigenesis in Cr(VI)-transformed BEAS-2B cells by luteolin can be very different from that occurring for the prevention of malignant transformation of normal cells by luteolin. Apoptosis or programmed cell death plays an important role in the regulation of tissue development, differentiation, and homeostasis (Ellisr, et al., 1991). Dysregulation of apoptosis contributes to the pathogenesis of cancer. In this regard, acquired resistance to apoptosis is a hallmark of all cancers (Hanahan and Weinberg, 2000). Under normal circumstances, apoptosis eliminates DNA-damaged or mutated cells. Acquired resistance to apoptosis is a critical cellular event during carcinogenesis. Disruption of apoptosis plays a major role in tumor formation and malignant progression (Wyllie, et al., 1999). In the present study, we have demonstrated that in Cr(VI)-transformed BEAS-2B cells, Nrf2 is constitutively overexpressed (Fig. 3). The Nrf2 target antioxidant proteins, HO-1, NQO1, SOD1, and SOD2 are also constitutively overexpressed (Fig. 4). The high levels of these antioxidant proteins decrease cellular ROS in Cr(VI)-transformed cells as shown by ESR spin trapping and fluorescence staining (Figs. 5 and 6). Our previous studies demonstrated that ROS generated by Cr(VI) exposure causes apoptosis (Chen et al, 2001; Ye et al., 1999). The low level of ROS in Cr(VI)-transformed BEAS-2B cells reduces the apoptotic potential of these cells and increases their survival (Fig. 7), providing a favorable environmental for progression of the Cr(VI)-transformed cells to tumor. The constitutive Nrf2 activation will also increase its antioxidant target Bcl-2 through the binding of Nrf2 to the ARE site of Bcl-2 gene (Fig 8). Our results show that in Cr(VI)-transformed cells, Bcl-2 is constitutively overexpressed and apoptosis resistance was observed. This constitutive Nrf2-mediated apoptosis is likely another important contributor to the progression of Cr(VI)-transformed BEAS-2B cells to tumor.
To understand the mechanism involved in the prevention of tumorigenesis in Cr(VI)-transformed cells by luteolin reported in our recent study (Pratheeshkumar et al., 2014), we note the following important results obtained in the present study using Cr(VI)-transformed BEAS-2B cells. (1) Luteolin reduces constitutive Nrf2 activation (Fig. 9). (2) This natural compound inhibits the binding between Nrf2 and the promoter regions of the target antioxidant and anti-apoptotic proteins as demonstrated by Chip analysis (Fig 8). (3) Luteolin decreases the levels of antioxidant and anti-apoptotic proteins (Fig. 9). The overall outcome is the restoration of ROS levels and apoptotic function in Cr(VI)-transformed cells by luteolin. The processes outlined above (1–3) are the likely mechanisms by which luteolin blocks the tumorigenesis in Cr(VI)-transformed BEAS-2B cells observed in our recent study (Pratheeshkumar et al., 2014).
From above discussion, it may be noted that inducible Nrf2 in normal cells is anti-oncogenic by increasing its target antioxidant proteins and reducing ROS levels. In the first stage of Cr(VI)- induced carcinogenesis, cell transformation, ROS are oncogenic. The low level of ROS reduces the Cr(VI)-induced malignant cell transformation. In the second stage of Cr(VI) carcinogenesis, the tumorigenesis of Cr(VI)-transformed cells, the inducible property of Nrf2 is lost in Cr(VI)-transformed cells. The Nrf2 is constitutively activated in these cells. This constitutive Nrf2 activation in Cr(VI)-transformed cells is oncogenic by increasing target antioxidant and anti-apoptotic proteins, leading to low ROS levels and acquisition of apoptosis resistance. In this second stage of Cr(VI)-induced carcinogenesis, both low level of ROS and constitutive Nrf2 activation are oncogenic. The above mechanistic studies show that Nrf2 signaling can be an important target for dietary prevention of Cr(VI)- induced carcinogenesis at both stages of carcinogenesis. We have carried out literature searches and screened a variety of natural compounds in an attempt to select those with two types of properties: (a) ability to activate inducible Nrf2 and decrease ROS in non-transformed cells to prevent Cr(VI)-induced malignant transformation and (b) to inactivate constitutive Nrf2 expression and increase ROS in Cr(VI)-transformed cells to decrease survival of these transformed cells. Luteolin, a natural compound, has these dual properties. The mechanisms for the activation of inducible Nrf2 by luteolin in normal cells and inactivation of constitutive Nrf2 by the same natural compound in Cr(VI)-transformed cells are under investigation in our laboratories. Our preliminary results indicate that keap1 (an inhibitor of Nrf2) signal (canonical mechanism) is important in the activation of inducible Nrf2 by luteolin in normal cells while the p62 pathway (noncanonical mechanism) activated by autophagy deficiency of Cr(VI)-transformed cells is important for the inactivation of constitutive Nr2 by the same natural compound in these transformed cells.
The importance of the results obtained in the present study are summarized below. (1) The results from this study can be used to formulate strategies for dietary prevention against Cr(VI)-induced carcinogenesis. These strategies take advantage of a cascade of molecular events that occur over time in the carcinogenic process, i.e., enabling a moderate and carefully orchestrated promotion of Nrf2 in the early stage of Cr(VI)-induced carcinogenesis and reduction of the constitutive and high level expression of this protein in the later carcinogenesis stage. (2) The results may also be used to develop a similar strategy against other carcinogenic metals, such as arsenic (Leonard et al., 2004; Lee et al., 2012; Son et al., 2015), nickel (Pan et al., 2010), and cadmium (Son et al., 2011; 2014). These carcinogenic metals share a common mechanism with Cr(VI) and also generate ROS, cause oxidative stress, and induce cancers of various organs (Leonard et al., 2004; Lee et al., 2012; Pan et al., 2010; Son et al., 2014, 2015).
Highlight.
Constitutive Nrf2 activation in Cr(VI)-transformed cells is oncogenic
Constitutive Nrf2 activation decreases ROS with development of apoptosis resistance
Luteolin inhibits constitutive Nrf2 activation in Cr(VI)-transformed cell.
Dietary strategies using luteolin may prevent Cr(VI)-induced carcinogenesis
Acknowledgments
This research was supported by National Institutes of Health (R01ES021771, R01ES020870, and R01ES025515) and by the Cytometry and Cell Sorting Core Facility of the University of Kentucky Markey Cancer Center (P30CA177558)
Footnotes
Disclosure of potential conflicts of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for chromium. U S Dep Health & Hum Serv. 1982 [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Vallyathan V, Castranova V, Shi X. Cell apoptosis induced by carcinogenic metals. Mol Cell Biochem. 2001;222:183–188. [PubMed] [Google Scholar]
- Costa M. Toxicity and Carcinogenicity of Cr(VI) in Animal Models and Humans. Crit Rev Toxicol. 1997;27:431–442. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- De Flora S. Threshold mechanisms and site specificity in chromium (VI) carcinogenesis. Carcinogenesis. 2000;21:533–541. doi: 10.1093/carcin/21.4.533. [DOI] [PubMed] [Google Scholar]
- Ellisr E, Yuan J, Horvitzh R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hu R, Saw CL, Yu R, Kong AN. Regulation of NF-E2-related factor 2 signaling for cancer chemo-prevention: antioxidant coupled with antiinflammatory. Antioxid Redox Signal. 2010;13:1679–1698. doi: 10.1089/ars.2010.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Li CK, Lin AH, Yeh YW, Yao HT, Li CC, Wang TS, Chen HW. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 2012;87:167–178. doi: 10.1007/s00204-012-0913-4. [DOI] [PubMed] [Google Scholar]
- IARC. Chromium, Nickel and Welding. IARC Monogr Eval Carcinog Risks Hum. 1990;49:1–648. [PMC free article] [PubMed] [Google Scholar]
- Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanism of activation and dysregulatiion in cancer. Redox Biol. 2014;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- Komatsu M. Potential role of p62 in tumor development. Autophagy. 2011;7:1088–1090. doi: 10.4161/auto.7.9.16474. [DOI] [PubMed] [Google Scholar]
- Kou X, Kirberger M, Yang Y, Chen Y. Natural products for cancer prevention associated with Nrf2–ARE pathway. Food Sci Human Wellness. 2013;2:22–28. [Google Scholar]
- Kumar H, Kim IS, More SV, Kim BW, Choi DK. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat prod Rep. 2014;31:109–139. doi: 10.1039/c3np70065h. [DOI] [PubMed] [Google Scholar]
- Langard S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am J Ind Med. 1990;17:189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- Langard S. Role of chemical species and exposure characteristics in cancer among persons occupationally exposed to chromium compounds. Scand J Work Environ Health. 1993;19:81–89. [PubMed] [Google Scholar]
- Lau A, Whitman SA, Jaramillo MC, Zhang DD. Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J Biochem Mol Toxicol. 2013;27:99–105. doi: 10.1002/jbt.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Son YO, Pratheeshkumar P, Shi X. Oxidative stress and metal carcinogenesis. Free Rad Biol Med. 2012;53:742–757. doi: 10.1016/j.freeradbiomed.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Machle W, Gregorius F. Cancer of the respiratory system in the United States chromate producing industry. Public Health Rep. 1948;63:1114–1127. [PubMed] [Google Scholar]
- Mukherjee S, Ghosh S, Choudhury S, Adhikary A, Manna K, Dey S, Sa G, Das T, Chattopadhyay S. Pomegranate reverses methotrexate- induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways. J Nutr Biochem. 2013;24:2040–2050. doi: 10.1016/j.jnutbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Ohta T, Iijima K, Miyamoto M, NakaharaI I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- Pan J, Chang Q, Wang X, Son Y, Zhang Z, Chen G, Luo J, Bi Y, Chen F, Shi X. Reactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cells. Chem Res Toxicol. 2010;23:568–577. doi: 10.1021/tx9003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JN, Kang KA, Zhang R, Piao MJ, Park SY, Kim JS, Kang SS, Hyun JW. Antioxidant and Cytotoxicity Effects of Luteolin. Toxicol Res. 2006;22:391–395. [Google Scholar]
- Pellerin C, Booker SM. Reflection on hexavalent chromium: health hazards of an industrial heavy weigh. Environ Health Perspect. 2000;108:A402–407. doi: 10.1289/ehp.108-a402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheeshkumar P, Son YO, Divya SP, Roy RV, Hitron JA, Wang L, Kim D, Dai J, Asha P, Zhang Z, Wang Y, Shi X. Luteolin inhibits Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicol Appl Pharmacol. 2014;281:230–241. doi: 10.1016/j.taap.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato L, Fletcher AC, Anderson AA, Anderson K, Becker N, Chang J. An historical perspective study of European stainless steel, mild steel, and shipyard welders. Br J Ind Med. 1991;48:14–154. doi: 10.1136/oem.48.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Hitron JA, Wang X, Chang Q, Pan J, Zhang Z, Liu J, Wang S, Lee JC, Shi X. Cr(VI) induces mitochondrial- mediated and caspase-dependent apoptosis through reactive oxygen species- mediated p53 activation in JB6 Cl41 cells. Toxicol Appl Pharmacol. 2010;245:226–235. doi: 10.1016/j.taap.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Pratheeshkumar P, Roy RV, Hitron JA, Wang L, Divya SP, Xu X, Luo J, Chen G, Zhang Z, Shi X. Anti-oncogenic and oncogenic properties of Nrf2 in arsenic-induced carcinogenesis. J Biol Chem. 2015;290:27090–27100. doi: 10.1074/jbc.M115.675371. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Son YO, Pratheeshkumar P, Roy RV, Hitron JA, Wang L, Zhang Z, Shi X. Nrf2/p62 Signaling in apoptosis resistance and its role in cadmium- induced carcinogenesis. J Biol Chem. 2014;289:28660–28675. doi: 10.1074/jbc.M114.595496. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Son YO, Wang X, Hitron JA, Zang Z, Chen P, Budhraja A, Ding S, Lee JC, Shi X. Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol Appl Pharmacol. 2011;255:287–296. doi: 10.1016/j.taap.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorahan T, Burges DC, Hamilton L, Harrington JM. Lung cancer mortality in nickel/chromium platers, 1946–1995. Occup Environ Med. 1998;55:236–242. doi: 10.1136/oem.55.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D, Hassoun E, Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. 2000;19:201–213. [PubMed] [Google Scholar]
- Sporn M, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nature Rev. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, Wang XJ. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Rad Biol Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Tew KD. NrF2/Keap1 as gatekeepers of redox homeostasis-do they prevent of cause cancer? Pigment Cell Melanoma Res. 2011;24:1078–1087. doi: 10.1111/j.1755-148X.2011.00913.x. [DOI] [PubMed] [Google Scholar]
- Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Son YO, Chang Q, Sun L, Hitron JA, Budhraja A, Zhang Z, Ke Z, Chen C, Luo J, Shi X. NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicol Sci. 2011;123:399–410. doi: 10.1093/toxsci/kfr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Axelrad DA, Caldwell J, Morello-Frosch R, Rosenbaum A. Public health implications of 1990 air toxics concentrations across the United States. Environ Health Perspect. 1998;106:245–251. doi: 10.1289/ehp.98106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie AH, Bellamy CO, Bubb VJ, Clarke AR, Corbet S, Curtis L, Harrison DJ, Hooper ML, Toft N, Webb S, Bird CC. Apoptosis and carcinogenesis. Br J Cancer. 1999;80:3437. [PubMed] [Google Scholar]
- Yao H, Guo L, Jiang B, Luo J, Zhang Z, Shi X. Oxidative stress and chromium carcinogenesis. J Environ Pathol Toxicol Oncol. 2008;27:77–88. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- Ye J, Wang S, Leonard SS, Sun Y, Butterworth L, Antonini J, Ding M, Rojanasakul Y, Vallyathan V, Castranova V, Shi X. Role of reactive oxygen species and p53 in chromium(VI)- induced apoptosis. J Biol Chem. 1999;274:34974–34980. doi: 10.1074/jbc.274.49.34974. [DOI] [PubMed] [Google Scholar]
- Ye J, Zhang X, Young HA, Mao Y, Shi X. Chromium(VI)- induced nuclear factor-κB activation in intact cells via free radical reactions. Carcinogenesis. 1995;16:2401–2405. doi: 10.1093/carcin/16.10.2401. [DOI] [PubMed] [Google Scholar]
- Zhang YC, Gan FF, Shelar SB, Ng KY, Chew EH. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem Toxicol. 2013;59:272–280. doi: 10.1016/j.fct.2013.05.058. [DOI] [PubMed] [Google Scholar]