Abstract
The innate immune components that modulate allergic contact hypersensitivity (CHS) responses are poorly defined. Using human skin from contact dermatitis patients and a mouse model of CHS, we find that hapten allergens disrupt the Arginase1 (Arg1) and inducible nitric oxide synthase (iNOS or Nos2) dynamic in monocytes/macrophages, which renders those cells ineffectual in suppressing skin inflammation. Mice lacking Arg1 in macrophages develop increased CHS characterized by elevated ear thickening, monocytes/macrophage-dominated dermal inflammation, and increased iNOS and IL-6 expression compared to control mice. Treatment of Arg1flox/flox; LysMCre+/− mice with a selective NOS inhibitor or knockout of iNOS significantly ameliorated CHS. Our findings suggest a critical role for Arg1 in monocytes/macrophages in suppressing CHS through dampening Nos2 expression. These results may support that increasing Arg1 may be a potential therapeutic avenue in treating allergic contact dermatitis.
Keywords: skin, monocytes/macrophages, CHS, allergy, inflammation, arginase, iNOS, human, mouse
Introduction
Allergic contact dermatitis (ACD) is one of the leading causes of occupational skin disease (1–4) The sensitization phase of ACD is facilitated by early activation of the innate immune system to prime T cells (5–9). Ag-specific effector T cells then migrate into the skin, where they persist for a long time, and then are called tissue-resident T cells (TRM). When re-challenging the skin with the same allergen (elicitation phase), early activation of innate immune cells and Ag-specific TRM mediate tissue damage causing by inflammation, edema, and skin swelling (10). Notably, the relevance of innate immune cells including macrophages (MΦ) and monocytes (mono) in CHS elicitation has been now recognized (11, 12), yet the underlying functional mechanisms remain unknown. We here identified that cutaneous mono/MΦ from ACD patients express high levels of inducible nitric oxide synthase (iNOS or Nos2) and Arginase1(Arg1). While previous studies have utilized iNOS and Arg1 simply as markers for M1 (killer phenotype classically-activated MΦ) and M2 (regulatory phenotype, alternatively activated MΦ), respectively, we here demonstrate novel functional roles for these L-arginine-metabolizing enzymes in CHS. iNOS was found critical for CHS. In vitro stimulation of MΦ with an allergen increases Nos2 and decreases Arg1 expression, while dexamethasone restores this response to baseline levels. Significantly elevated iNOS and IL-6 levels in mono/MΦ from Arg1flox/flox; LysMCre+/− was associated with increased inflammation in vivo. Increased CHS in Arg1flox/flox; LysMCre+/− was normalized to WT levels by treatment with a selective iNOS inhibitor. Together, these studies demonstrate that Arg1 in mono/MΦ resolves CHS responses by tempering iNOS-mediated inflammation.
Materials and Methods
Human tissue from ACD patients
All human tissues were collected at the Department of Dermatology, Mount Sinai University, New York, NY and written informed consent was received prior to obtaining skin biopsies from patients undergoing patch testing and showing a positive reaction to nickel (n=2), fragrance (n=1), and Balsam of Peru (n=1). Biopsies from the positive patch test area and from non-lesional skin from the same patients were obtained. In addition, skin samples from healthy donors undergoing plastic surgery (n=2) were collected at the Department of Dermatology, Duke University, Durham, NC and were used as an additional control or were used for FACS-sorting (see below).
Mice
Female C57BL6/J (2–5 months of age, Jackson laboratory, ME, USA), Arg1flox/flox, Arg1flox/flox; Tie2Cre (Jackson laboratory, ME, USA), and Arg1flox/flox; LysMCre+/− mice and iNOS−/− mice (13) were used in our studies. Mice were maintained under a specific pathogen-free condition and were sustained under regulated conditions with food and water ad libitum in the pathogenic-free facility at Duke University. All mice were in the same hair cycle while in vivo experiments were performed. Mice were single-housed during the time of the CHS studies. The protocol was approved by the Institutional of Duke University Animal Care and Use Committee under protocol A175-14-07.
Allergic and irritant contact dermatitis mouse models
For the allergic contact hypersensitivity model, mice were sensitized via topical application of 0.5% (v/v) DNFB (Sigma-Aldrich, St. Louis, MO) in 4:1 acetone/olive oil on their shaved back (50µl) and were challenged 4–5 days later with 0.25% DNFB or vehicle control (5µl on the dorsal and 5µl on the ventral side of the ear). For the irritant contact dermatitis model, 2% (v/v) croton oil (Sigma-Aldrich, St. Louis, MO) in 4:1 acetone/olive oil or vehicle control was used. Ear thickness was measured using an engineer’s micrometer (Mitutoyo, Tokyo, Japan). For depletion of macrophages, a single dose of 100µl clophosome was injected intraperitoneally (i.p.) and a single dose of 20µL clophosome (FormuMax Scientific, Inc. Palo Alto, CA) was injected intradermal (i.d.) into mice 24 hrs prior to the sensitization or the elicitation phase of CHS. Injections of the same amount of empty liposomes (FormuMax Scientific, Inc. Palo Alto, CA) into mice served as control. The efficiency of clopohosomes to deplete macrophages was confirmed by flow cytometry analysis (data not shown). In some studies, a selective iNOS inhibitor, N6-(1-iminoethyl)-L-lysine, dihydrochloride (14) (L-NIL; Cayman Chemical Company, Ann Arbor, MI) was injected ip.6 mg/kg for the first dose and then 3 mg/kg twice daily or PBS were injected into animals during the time of the experiment.
Flow cytometry and FACS
Abs and appropriate IgG controls were conjugated to FITC, AF488, PE, PerCPCy5.5, PeCy7, AmCyan, Brilliant Violet 421, Pacific Blue, efluor 450, allophycocyanin (APC), APCVIO770, APC-Cy7. Abs against CD3, CD11b, CD161, B220, and Gr-1 were purchased from Tonbo Biosciences, San Diego, CA. Ab against F4/80 was purchased from AbD Serotec, Raleigh, NC. Abs to CD45, CD163, CD64, Ly6G and CD14 were purchased from Biolegend San Diego, CA. Ab to CD1a was purchased from eBioscience, Inc. San Diego, CA. Abs to IL-6 was purchased from Miltenyi Biotec Inc.San Diego, CA. Abs to Arg1 was purchased from R&D Systems, Inc., Minneapolis, MN. Cells were acquired with DiVa 5.0 software on a digital LSRII and analyzed with FlowJo software (Tree Star).
For experiments using fresh human skin obtained from plastic surgery procedures, epidermis and dermis was separated after incubation with Dispase (2.4 Units/ml, Thermo Fisher Scientific, Waltham, MA) for overnight at 4 °C degrees. Epidermis was peeled off the dermis using tweezers and epidermis was incubated for 20 minutes in Dispase for 20 minutes at 37 °C degrees. Dermal components were further manually disrupted using scissors and were processed using Dispase II and Collagenase type II 2mg/ml, Thermo Fisher Scietific, Waltham, MA) at a 1:1 ratio for 2 hours at 37 °C degrees with intermittent rigorous shaking for dermal single cell isolation.
Human skin cells were purified by FACS-sorting: Langerhans cell cells were sorted from epidermal cell suspensions (15) being CD45+CD3−CD1a+, MΦ were CD45+CD3−CD14+CD163hi, CD14+ dermal dendritic cells (dDC, that are now recognized being transcriptionally closer to MΦ, 23) were CD45+CD3−CD163−CD14+, CD1a+dDC were CD45+CD3−CD1a+. For experiments using mouse skin, whole skin was digested with 0.3% trypsin/0.1% glucose, 14.8 mM NaCl, 5.3 mM KCL (GNK) with 0.1% DNase at 4 °C overnight. MΦ from mouse skin were purified by FACS-sorting and were CD45+CD3−F4/80+CD11B+GR1−. The purity of sorted cell populations was between 91–99%.
Quantitative RT-qPCR
Total RNA was isolated from cells and tissue using TRIzol (Thermo Fisher Scientific, Waltham, MA). RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and resulting cDNA were amplified using Fast Start Universal SYBR Green Master Mix (Roche, Basel, Switzerland). PCR was performed with primers as shown in the supplementary table.
Immunofluorescence
Sections of frozen specimens (either human or mouse) were incubated overnight at 4°C with primary antibodies (anti-human/mouse iNOS (Thermo Fisher Scientific, Waltham, MA); Mouse IgG1 Isotype Control (MOPC-21), (Tonbo Biosciences, San Diego, CA); Anti-Human CD14 (61D3) and Anti-human CD3 (Tonbo Biosciences, San Diego, CA), anti-mouse F4/80 (BioRad, Raleigh, NC), mouse IgG1, rat IgG2b (Biolegend San Diego, CA), anti-human Arg1 and Goat IgG Control (Santa Cruz Biotechnology, Inc. Dallas, Texas) followed by reaction with Cy3, Cy5, or FITC-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA). Nuclei were counterstained with Hoechst 33342, washed in PBS, and mounted with Antifade Mounting Media (Thermo Fisher Scientific, Waltham, MA).
Generation of bone marrow-derived dendritic cells (BMDC) and bone marrow-derived macrophages (BMDM)
BM cells were stimulated with GM-CSF (5 ng/ml, Tonbo Biosciences, San Diego, CA) for 6 days to induce BMDC (12). On day 6, non-adherent cells were collected for gene analysis. BMDM were generated with MΦ colony-stimulating factor (10 ng/ml, Sigma-Aldrich, St. Louis, MO). For generation of M2, BMDM were treated with IL-4 (10 ng/ml, Tonbo Biosciences, San Diego, CA ) and IL-13 (10 ng/ml, Tonbo Biosciences, San Diego, CA) for additional 3 days (16, 17). DNBS (0.05%, Sigma-Aldrich, St. Louis, MO) and 1µM dexamethasone (Sigma-Aldrich, St. Louis, MO) were used to stimulate cells for 16 hrs as outlined in figure legends and in text.
Mouse microarray expression data
The microarray data GSE49358 from study (18) was retrieved and downloaded from the Gene Expression Omnibus (19). Robust Multiarray Average (RMA) normalization was applied to the data to eliminate systematic differences across the array using the affy (20) Bioconductor (21) package in the R statistical programming environment (http://www.r-project.org).
Statistical Analysis
For comparisons between multiple groups, the overall differences were analyzed by ANOVA with Bonferroni’s multiple comparison tests. For comparisons between two groups, two-tailed student’s T-tests were used.
Results
Identification of iNOS- and Arg1-expressing CD14+ dermal cells in human allergic contact dermatitis (ACD)
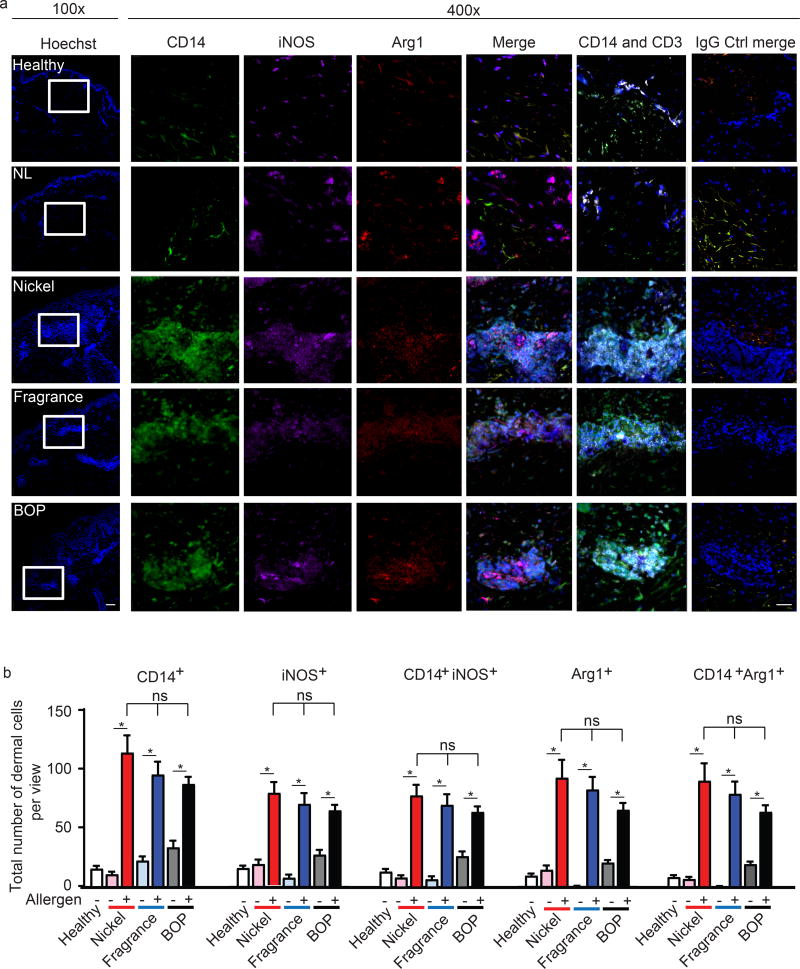
Under steady state conditions, healthy human skin expresses both Arg1 and iNOS in epidermal keratinocytes and endothelial cells (22) as well as in immune cells, including Langerhans, dermal DC and MΦ (23) (Suppl. Fig. 1a and e). Following environmental insults or allergen expsoure of the barrier tissues like the skin, CD14+ monocytes infiltrate from the blood to the skin, of which some but not all cells, become sessile and differentiate into MΦ, or undergo cell death. Notably, ACD skin lesions from positive patch-tested patients (nickel, fragrance, and Balsam of Peru; BOP) presented a significantly higher number of dermal CD14+ cells compared to healthy control or non-lsional skin (Fig 1). Within ACD lesions, iNOS and Arg1 predominantly co-localized in the dermis in CD14+ cells, typically within the recenlty described dermal T cell clusters (Fig. 1a–b). These clusters have been recently reported to play an integral role in CHS responses (11). Our results suggested to us that infiltrating monocytes and macrophages expressing iNOS and Arg1 are present in active human ACD lesions and may be invloved in skin inflammation.
Figure 1. iNOS and Arg1-expressing MΦ infiltrate ACD lesions.
(a) Representative immunohistochemistry analysis depicting CD14 (green), iNOS (purple), Arg1 (red), CD3 (white), Hoechst (blue) expression in ACD skin (Nickle, Balsam of Peru; BOP, and fragrance), non-lesional (NL), and healthy controls. Data are representative of 1–2 patient samples per tested condition with similar results, original magnification ×100(left) and x400 with scale bar 100 µm and 50 µm, respectively.
(b) Analysis depicting total numbers of dermal CD14+, iNOS+, Arg1 and co-expression of CD14 with iNOS and Arg1 in ACD lesions (Nickel, Balsam of Peru; BOP, and fragrance), non-lesional (NL), and healthy samples. Data are expressed as positive cells ± SEM from at least three seperate microscopic fields, 400x, from 1–2 patients, *p<0.05.
iNOS contributes to CHS-associated inflammation in vivo
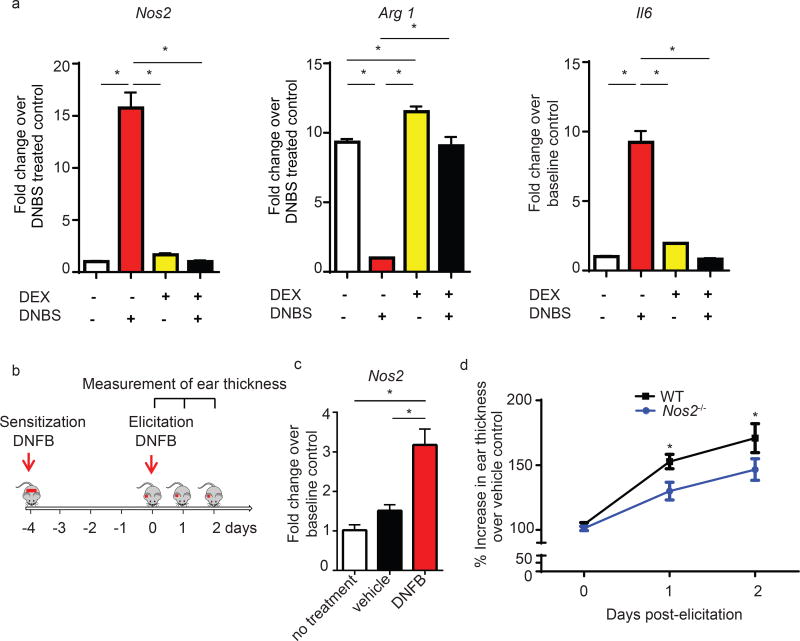
Mice with genetic depletion of mono/MΦ are resistant to CHS (11). However, it is not clear whether mono/MΦ impact immune responses in the sensitization and/or in the elicitation phase of CHS. We found that mono/MΦ depletion before the elicitation, but not before sensitization, significantly inhibited CHS (Suppl. Fig. 1b). Because hapten allergens are reported to modify macrophage immune responses (18, 24), we further investigated whether mono/MΦ exposed to hapten allergens alter their levels of Nos2 expression. We performed in vitro stimulation experiments with 4-dinitrobenzene sulfonic acid sodium salt (DNBS), a commonly used hapten allergen for in vitro studies (25–27). Following DNBS stimulation, BMDM (polarized into M2) increased not only Nos2 but also Il6, a key cytokine mediating CHS responses (Fig. 2a) (28). Interestingly, FACS-sorted skin mono/MΦ from mice challenged with DNFB, but not from un-challenged skin, demonstrated significant upregulation of Nos2 as well (Fig. 2b, c). In contrast, Arg1 expression in BMDM was significantly suppressed at 16 hrs post-DNBS treatment (Fig. 2a). These responses could be normalized in vitro by (pre-)treatment of the cells with dexamethasone, a therapeutic corticosteroid used to suppress CHS responses through targeting mono/MΦ (12). Our results demonstrate that the hapten DNBS disrupts the existing balance of Arg1 and Nos2 levels in mono/MΦ. Furthermore, these findings suggest that the therapeutic action of dexamethasone may function –at least in part- through regulation of Arg1 and iNOS in agreement with a previous report (12).
Figure 2. Hapten allergen induces Nos2.
(a) qPCR of Arg1, Nos2, and Il6 mRNA in M2 MΦ 16hrs post-stimulation with DNBS and pre-treatment with 1µM dexamethasone (DEX). Data are summarized as mean ± SEM, *p<0.05 and are representative of 3 independent experiments.
(b) Experimental outline for the analysis in Figures 2c and d.
(c) qPCR of Nos2 in CD11b+F4/80+Gr1 sorted cells at 24 hrs after DNFB elicitation. Data are expressed as mean ± SEM, pooled from 4–5 mice, *p<0.05.
(d) C57BL/6J and iNOS−/− mice were sensitized and elicited with DNFB as outlined in 2b. Ear swelling was calculated as the % increase in ear thickness over vehicle control ±SEM from 18–27 mice, *p<0.05
To test the functional role of iNOS in CHS, we next utilized mice with a constitutive genetic deletion in the Nos2 gene and evaluated ear swelling in response to epi-cutaneous DNFB challenge. Nos2−/− mice displayed significantly lower CHS responses than their wild-type (WT) controls (Fig. 2d), suggesting that epicutaneous antigen challenge leads to increased iNOS levels and that iNOS functionally reinforces CHS.
Arg1flox/flox; LysMCre+/− mice have increased allergic and irritant contact hypersensitivity responses
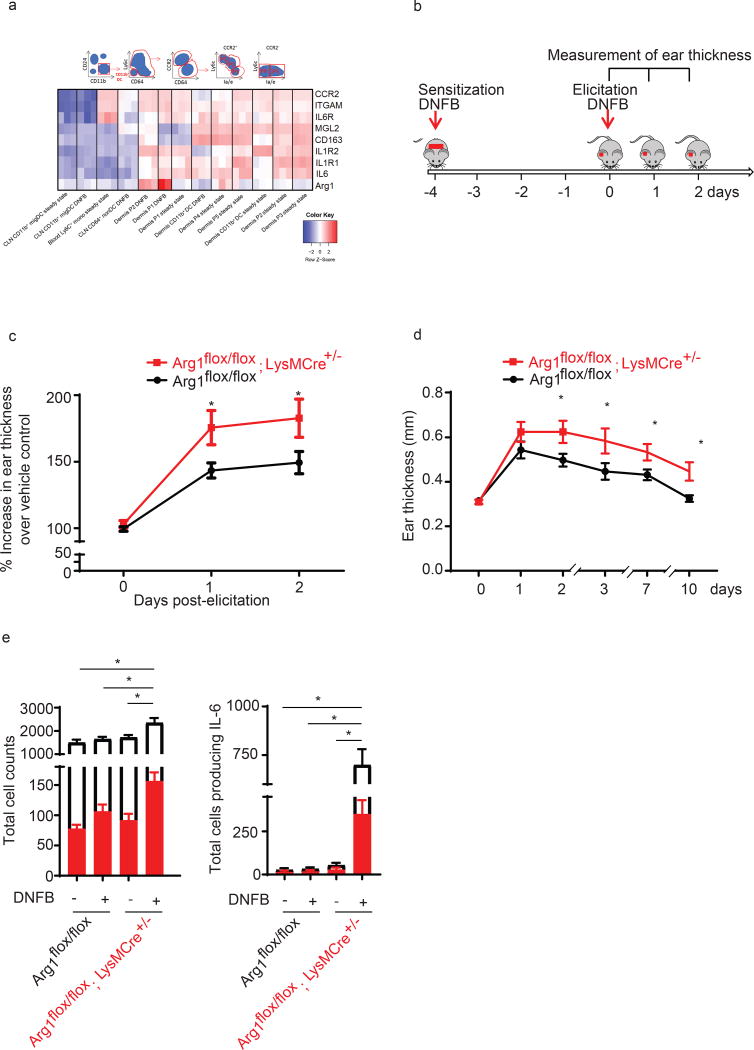
When testing Arg1 levels in FACS-sorted skin mono/MΦ (CD11b+F4/80+Gr1−) from mice challenged with DNFB in vivo, we found surprisingly that in addition to iNOS, Arg1 was upregulated at 24 hrs post-elicitation (Fig. 2b,c and Suppl. Fig. 1d). Work from Henri’s group (18) described that a large number of monocytes infiltrate DNFB-elicitetd skin and analysis of microarray GSE49358 from this study further illuminates that monocytic cells in the skin highly upregulate Arg1 expression following DNFB challenge (Fig. 3a) To better delineate the function of Arg1 in mono/MΦ, we next investigated functional consequences of Arg1 deletion using Arg1flox/flox; LysMCre+/− and Arg1flox/flox Tie2Cre+/− mice. Efficiency of LyzMCre and Tie2Cre can vary and successful depletion of Arg1 in both LysMCre and Tie2Cre strains was verified (Suppl. Fig. 2a, b). Compared to control mice, Arg1flox/flox; LysMCre+/− mice and Arg1flox/flox; Tie2Cre mice develop increased CHS and ear thickening in vivo either following a one-time or following multiple applications of the hapten DNFB to the skin site (Fig. 3c, d and Suppl. Fig. 2c). Multiple exposures to DNFB increases generation and lodging of allergen-specific memory T cells into the skin (10) (data not shown). Both, the one-time sensitization model as well as following multiple exposures to DNFB, led to an significantly increased CHS response in the Arg1flox/flox; LysMCre+/− mice.
Figure 3. Arg1 deletion in mono/MΦ promotes CHS in vivo.
(a) Heat map showing various genes which were 2- log fold up or down regulated in murine monocyte, macrophage and DC populations under steady state or following DNFB (microarray GSE49358 from Tamoutounour et al., 2013 (18)) Normalized gene expression was plotted across the samples after z-score normalization. Genes are clustered using a correlation distance with complete linkage.
(b) A timeline indicates experiment chronology.
(c) Arg1flox/flox; LysMCre+/−and Arg1flox/flox were sensitized and elicited with DNFB. Ear swelling was measured daily and is depicted as mean increase over vehicle control ± SEM from 15–22 mice, *p<0.05
(d) Arg1flox/flox; LysMCre+/−and Arg1flox/flox mice were sensitized for 5 times before re-elicitation with DNFB on the ear. Ear swelling was measured daily and is depicted as mean ± SEM from 5 mice, *p<0.05, data were analyzed by two-way ANOVA test followed by least-significant differences multi-comparison (LSD) test.
(e) Left: Total number of CD45+ cells (white bar) and CD45+CD11b+CD64+ (red bar ), and CD45+CD11b+CD64+( red bar ); Right : Total number of CD45+ cells producing IL-6 (white bar) and CD45+CD11b+CD64+ producing IL-6 (red bar) in skin from Arg1flox/flox; LysMCre+/−and Arg1flox/flox mice at 12 hrs post-DNFB. Data are summarized as mean ± SEM from 3 mice, *p<0.05
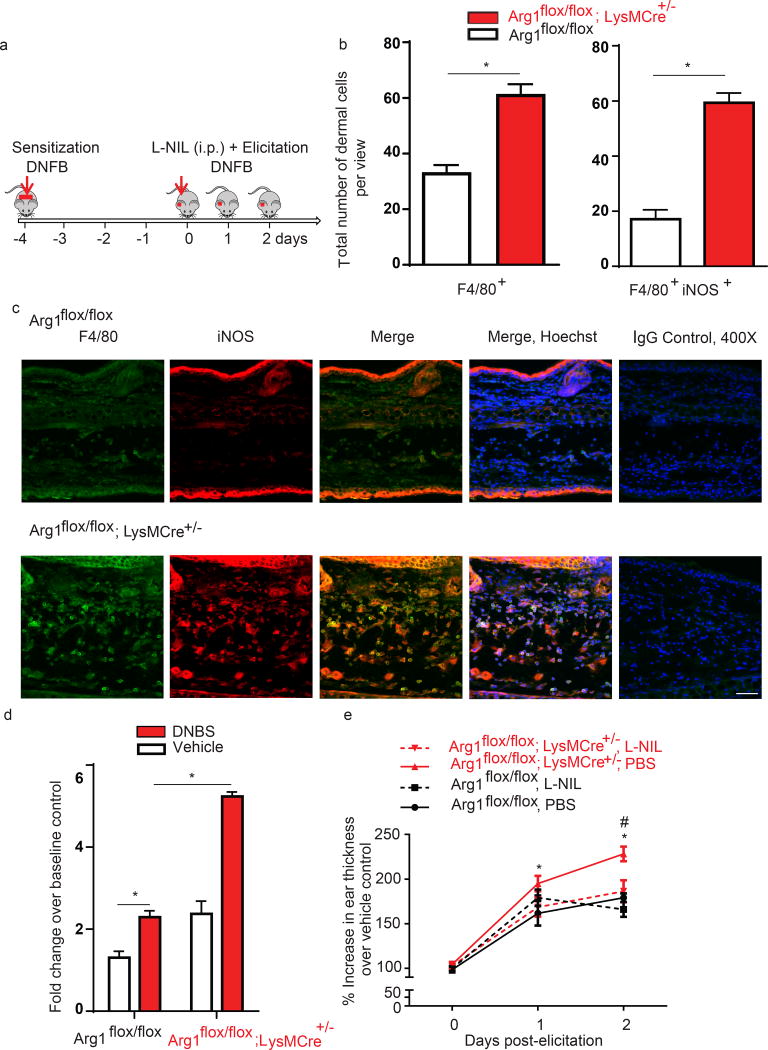
We next tested if the absence of Arg1 in Mono/MΦ would affect also IL-6 production. Notably, the total number of myeloid cells and MΦ expressing IL-6 were significantly elevated in Arg1flox/flox; LysMCre+/− mice compared to control mice at 12 hrs post-elicitation (Fig. 3e). Increased ear thickening in Arg1flox/flox; LysMCre+/− mice was associated with marked and significant increases in the total number of F4/80+ dermal MΦ co-expressing iNOS compared to Arg1flox/flox mice (Fig. 4a, b, c). In addition, BMDM from Arg1flox/flox; LysMCre+/− mice had significantly elevated Nos2 levels upon DNBS stimulation compared to BMDM from Arg1flox/flox mice (Fig. 4d). Together, these findings suggested that Arg1 in myeloid cells controls CHS, possibly via negative regulation of iNOS.
Figure 4. Arg1 deletion in mono /MΦ promotes CHS in vivo via increased iNOS.
(a) A timeline indicates experiment chronology
(b,c) Representative immunohistochemistry depicting F4/80 (green staining), iNOS (red staining) Hoechst (blue) and co-localization (merged image) in DNFB-elicited ears from Arg1flox/flox and Arg1flox/flox; LysMCre+/− mice. Data are representative of 3 independent samples per tested condition; original magnification x400 with scale bar 100µm. Analysis shows total numbers of F4/80+ iNOS+ cells per microscopic view and is expressed as mean ± SEM from at least three views of microscopic fields, *p<0.05.
(d) qPCR on Nos2 in DNBS-treated BMDM from Arg1flox/flox; LysMCre+/− mice compared to Arg1flox/flox cells at 16 hrs after treatment. Data are summarized as mean ± SEM, *p<0.05 and representative of 3 independent experiments.
(e) Arg1flox/flox; LysMCre+/− and Arg1flox/flox mice were sensitized and elicited with DNFB and treated with NOS inhibitor (L-NIL, ip. 6 mg/kg for the first dose, then 3 mg/kg twice daily), or vehicle (PBS). Ear swelling is depicted as mean ± SEM from 4–6 mice, *p≤0.05 Arg1flox/flox; LysMCre+/−, PBS versus Arg1flox/flox, PBS and #p≤0.05 Arg1flox/flox; LysMCre+/−, PBS versus Arg1flox/flox; LysMCre+/−, L-NIL
Next, we utilized an irritant contact dermatitis (ICD) mouse model to study the function of Arg1 in MΦ in a system that largely relies on innate immune cell-mediated inflammation. Using the ICD model, we find that Arg1flox/flox; LysMCre+/− mice treated with the irritant croton oil exhibited increased ear thickness compared to Arg1flox/flox mice within the first 3 hours (Suppl. Fig. 3). Together, our results from CHS and ICD in vivo studies demonstrate that Arg1 in MΦ/mono attenuates contact dermatitis in both antigen-dependent and antigen-independent contexts.
CHS responses in Arg1flox/flox; LysMCre+/− mice can be attenuated by iNOS inhibition
Given our findings that iNOS expression is higher in MΦ/mono from Arg1flox/flox LyzMCre+/−mice, we next tested whether pharmacological inhibition of iNOS could normalize CHS responses. To examine this, we utilized the iNOS inhibitor L-N6-(1-Iminoethyl)lysine (L-NIL) and found that treatment with L-NIL during the elicitation phase significantly attenuates DNFB-induced ear thickening in Arg1flox/flox; LysMCre+/− mice at 48 hrs post-elicitation (Fig. 4d). This result strongly suggested that Arg1 in MΦ/mono controls CHS by attenuating Nos2-associated inflammation.
Discussion
Our results demonstrate that Arg1 in MΦ/mono limits allergic and irritant hypersensitivity responses in the skin. Arg1 catalyzes the production of ornithine and urea, whereas iNOS catalyzes the production of nitric oxide. Both L-arginine metabolizing enzymes are present in MΦ/mono and their abundance and activity depends on multiple micro-environmental influences. Following cutaneous tissue damage, such as an allergic hypersensitivity reaction, MΦ consume and digest damaged tissue and allergens aimed at easing inflammation. Enzyme dysfunctions within MΦ may impair their ability to perform these important roles and consequently, inflammation remains unchecked. We here made the novel observation that hapten allergens disrupt the balance of Arg1 and Nos2 in MΦ and that in the absence of Arg1, MΦ cannot successfully resolve skin inflammation resulting in elevated CHS responses. Our experiments substantiate that Arg1 deficiency in MΦ unleashes an exaggerated pro-inflammatory response, including upregulation of iNOS and IL-6.
CHS has been characterized as a classical T-cell-mediated immune response. Our findings and recent studies by the groups of Kabashima and Schutz, respectively, highlight the relevant and novel roles of MΦ as important contributors to the effector phase of CHS responses (11, 12). In human and murine skin, Arg1 is expressed in multiple cells, including keratinocytes, fibroblasts, dendritic cells, neutrophils and MΦ (22, 29–32). Following hapten exposure, MΦ/mono abundantly infiltrate both human and mouse skin, manifesting a possible involvement of MΦ/mono in allergic contact hypersensitivity responses in both, humans and mice. Furthermnore, the relevance of Arg1 and iNOS to human ACD is supported by the finding that Arg1 and iNOS are expressed by CD14+ dermal cells in human ACD lesions. CD14+ dermal cells comprise a population of transient dermal monocyte-derived MΦ with weak T cell-stimulatory activity (23). Mono/ MΦ have been shown to play critical roles in many diseases because they can regulate both innate and adaptive immune responses.
In our mouse CHS studies, we here show that MΦ are required at the elicitation, but not at the sensitization phase of CHS, which is in agreement with a recently published study (11, 12). Using two cre-driver strains for myeloid Arg1 deletion, LysMCre and Tie2Cre, we identify exaggerated CHS responses at 24 hrs post-elictation. This supports that mono/macrophage-specific Arg1 controls CHS. LysMCre targets mono/ MΦ and neutrophils, while Tie2Cre targets endothelial cells, macrophages and additional cells of the hematopoetic lineage. (33–35). Since neutrophils are not required to mediate CHS (11), our studies focused on the Arg1flox/flox; LysMCre+/− strain. However, we cannot fully exclude that Arg1 expression in neutrophils also contributes to the immune responses observed. We did observe a significant increase in the CHS ear thickening response in Arg1flox/flox; Tie2Cre mice at the 24 hrs timepoint post-elicitation but not thereafter, which is in contrast to findings we have in the Arg1flox/flox LyzMCre mice. A potential explination for this is that Tie2Cre can target endothelial cells. Arg1 is a well-studied mediator of endothelial cell inflammation (36–39) and deletion of Arg1 in endothelial cells targeted via Tie2Cre (35), may protect the vascular integrity and therefore reduce further immune cell infiltration into the injured area. Because CHS causes potent infiltration of immune cells into the hapten-exposed area, this inflammatory response may be limited by the deletion of Arg1 driven by the Tie2 promotor. Future studies could investigate the role of endothelial cells expressing Arg1 in the context of CHS.
iNOS and Arg1 have been identified as markers for M1 and M2, respectively (11, 40–42). Our study provides novel insight into the functional roles of arginase and iNOS during cutaneous CHS. We here identified a relevant role for Arg1 in limiting T cell -mediated and innate-immune –mediated skin inflammatory responses. Our findings significantly expand our knowledge gained from previous studies on Arg1 in infectious model systems, cancer, and cutaneous wound repair (30, 43) and furthermore highlight not only the intricate regulation between Arg1 and iNOS, but also the regulation of these enzymes by the microenvironment, a notion that has been made previously by Murray and others (44–46) (47–50). Notably, our work demonstrates that both Arg1 and iNOS are upregulated during allergic contact dermatitis responses in humans and mice. Whether Arg1 and iNOS are reciprocally regulated in our model system is not known. In addition, alternative mechanisms of Arg1 and iNOS regulation, such as substrate competition for L-arginine and IL-6-regulated Arg1 and iNOS expression may exist (45, 51) and are an important area of research. It will be very interesting in the future to investigate in detail the role of IL-6 in regulating Arg1 and/or iNOS in our model system in vivo given previous findings from elegant studies showing that IL-6 can promote Arg1 expression via STAT3 (51) and that IL-6 can induce M2 and a myeloid-derived suppressor cells program (52, 53). However, IL-6 can serve both immune-regulatory and immune-stimulatory roles (28, 52, 54, 55) and therefore, it will be critical to decipher its precise effects by the use of conditional IL-6 knockout mice and by investigating the timing and source of production and its signaling pathway and associated immune response downstream, respectively. In our study, we find that IL-6 as well as iNOS production was induced by haptens in vitro and was significantly elevated in Arg1flox/flox; LysMCre+/− mice compared to control mice following allergen elicitation, suggesting an additional layer of complexity and possible feed-back loop between macrophage-derived Arg1, iNOS and IL-6 signaling in the skin.
MΦ mediate and regulate both, innate immune inflammation and adaptive immune responses. In our current studies, we observed that Arg1 deficiency in mono/MΦ increases skin inflammation in both T cell –mediated CHS and in the ICD model, the latter disease being solely dependent on innate immune cells.
In summary, we have demonstrated a novel and previously unrecognized role for Arg1 and iNOS in CHS immunity. High levels of iNOS are associated with increased DNFB treatment and CHS-associated inflammation and Arg1 is a negative regulator of M1-mediated CHS. Modulating iNOS and Arg1 in MΦ may therefore be a novel avenue for future therapeutic targets in ACD.
Supplementary Material
Acknowledgments
ASM is supported by NIH K08 Award (K08 AR06379), a Duke Physician-Scientist Strong Start Award and by Dermatology Foundation Research Grant; BY is supported by the National Natural Science Foundation (grant no. 81201243) and the China Postdoctoral Science Foundation (grant no. 2014M562671); RMT is supported by NIH K08 Award (K08 HL105537).
We thankfully acknowledge Mike Cook, PhD and Bin Li, PhD from the DCI-associated flow sorting facility.
Footnotes
Conflict of interest: No conflict of interest exists.
Author Contributions
JS designed and performed most experiments and analyzed and interpreted data; MS performed ICD studies, LP imaged and counted cell infiltrates of human skin samples, BY helped conduct mouse experiments; LQ, RT, and AB provided, bred and genotyped mice; EGY collected human skin samples, DC processed the microarray data, ASM designed, analyzed, and interpreted results. JS, MS, RT and ASM wrote the manuscript.
References
- 1.Chew AL, Maibach HI. Occupational issues of irritant contact dermatitis. Int Arch Occup Environ Health. 2003;76:339–346. doi: 10.1007/s00420-002-0419-0. [DOI] [PubMed] [Google Scholar]
- 2.Hanifin JM, Reed ML, Eczema P, Impact Working G. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82–91. doi: 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- 3.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476–486. doi: 10.1111/pai.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin SF. Contact dermatitis: from pathomechanisms to immunotoxicology. Exp Dermatol. 2012;21:382–389. doi: 10.1111/j.1600-0625.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Durr C, Heimesaat MM, Bereswill S, Fejer G, Vassileva R, Jakob T, Freudenberg N, Termeer CC, Johner C, Galanos C, Freudenberg MA. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205:2151–2162. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt M, Hupe M, Endres N, Raghavan B, Kavuri S, Geserick P, Goebeler M, Leverkus M. The contact allergen nickel sensitizes primary human endothelial cells and keratinocytes to TRAIL-mediated apoptosis. J Cell Mol Med. 2010;14:1760–1776. doi: 10.1111/j.1582-4934.2009.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen PJ, Galanos C, Roth J, Skerra A, Martin SF, Freudenberg MA, Goebeler M. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11:814–819. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- 9.Weber FC, Esser PR, Muller T, Ganesan J, Pellegatti P, Simon MM, Zeiser R, Idzko M, Jakob T, Martin SF. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med. 2010;207:2609–2619. doi: 10.1084/jem.20092489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, Kupper TS. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. 2015;21:647–653. doi: 10.1038/nm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natsuaki Y, Egawa G, Nakamizo S, Ono S, Hanakawa S, Okada T, Kusuba N, Otsuka A, Kitoh A, Honda T, Nakajima S, Tsuchiya S, Sugimoto Y, Ishii KJ, Tsutsui H, Yagita H, Iwakura Y, Kubo M, Ng L, Hashimoto T, Fuentes J, Guttman-Yassky E, Miyachi Y, Kabashima K. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol. 2014;15:1064–1069. doi: 10.1038/ni.2992. [DOI] [PubMed] [Google Scholar]
- 12.Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, Clausen BE, Stride B, Forster I, Habenicht AJ, Reichardt HM, Tronche F, Schmid W, Schutz G. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest. 2007;117:1381–1390. doi: 10.1172/JCI28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 14.Sugita K, Kabashima K, Yoshiki R, Ikenouchi-Sugita A, Tsutsui M, Nakamura J, Yanagihara N, Tokura Y. Inducible nitric oxide synthase downmodulates contact hypersensitivity by suppressing dendritic cell migration and survival. J Invest Dermatol. 2010;130:464–471. doi: 10.1038/jid.2009.288. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod AS, Rudolph R, Corriden R, Ye I, Garijo O, Havran WL. Skin-resident T cells sense ultraviolet radiation-induced injury and contribute to DNA repair. J Immunol. 2014;192:5695–5702. doi: 10.4049/jimmunol.1303297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marim FM, Silveira TN, Lima DS, Jr, Zamboni DS. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One. 2010;5:e15263. doi: 10.1371/journal.pone.0015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosser DM, Zhang X. Activation of murine macrophages. Curr Protoc Immunol Chapter. 2008;14 doi: 10.1002/0471142735.im1402s83. Unit 14 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 21.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, MacDonald J, Obenchain V, Oles AK, Pages H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, Morgan M. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12:115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruch-Gerharz D, Schnorr O, Suschek C, Beck KF, Pfeilschifter J, Ruzicka T, Kolb-Bachofen V. Arginase 1 overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. Am J Pathol. 2003;162:203–211. doi: 10.1016/S0002-9440(10)63811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E, Green K, Dickinson R, Wang XN, Low D, Best K, Covins S, Milne P, Pagan S, Aljefri K, Windebank M, Miranda-Saavedra D, Larbi A, Wasan PS, Duan K, Poidinger M, Bigley V, Ginhoux F, Collin M, Haniffa M. Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity. 2014;41:465–477. doi: 10.1016/j.immuni.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D, Kim YJ, Seo JN, Kim J, Lee Y, Park CS, Kim DW, Kim DS, Kwon HJ. 2,4-Dinitrofluorobenzene modifies cellular proteins and induces macrophage inflammatory protein-2 gene expression via reactive oxygen species production in RAW 264.7 cells. Immunol Invest. 2009;38:132–152. doi: 10.1080/08820130802667499. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki Y, Yokozeki H, Awad S, Igawa K, Minatohara K, Satoh T, Katayama I, Nishioka K. Glucocorticoids augment the chemically induced production and gene expression of interleukin-1alpha through NF-kappaB and AP-1 activation in murine epidermal cells. J Invest Dermatol. 2000;115:746–752. doi: 10.1046/j.1523-1747.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen MM, Lovato P, Macleod AS, Witherden DA, Skov L, Dyring-Andersen B, Dabelsteen S, Woetmann A, Odum N, Havran WL, Geisler C, Bonefeld CM. IL-1beta-Dependent Activation of Dendritic Epidermal T Cells in Contact Hypersensitivity. J Immunol. 2014;192:2975–2983. doi: 10.4049/jimmunol.1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott NM, Ng RL, Strickland DH, Bisley JL, Bazely SA, Gorman S, Norval M, Hart PH. Toward homeostasis: regulatory dendritic cells from the bone marrow of mice with inflammation of the airways and peritoneal cavity. Am J Pathol. 2012;181:535–547. doi: 10.1016/j.ajpath.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura N, Tohyama C, Satoh M, Nishimura H, Reeve VE. Defective immune response and severe skin damage following UVB irradiation in interleukin-6-deficient mice. Immunology. 1999;97:77–83. doi: 10.1046/j.1365-2567.1999.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abeyakirthi S, Mowbray M, Bredenkamp N, van Overloop L, Declercq L, Davis PJ, Matsui MS, Weller RB. Arginase is overactive in psoriatic skin. Br J Dermatol. 2010;163:193–196. doi: 10.1111/j.1365-2133.2010.09766.x. [DOI] [PubMed] [Google Scholar]
- 30.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol. 2013;133:2461–2470. doi: 10.1038/jid.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kampfer H, Pfeilschifter J, Frank S. Expression and activity of arginase isoenzymes during normal and diabetes-impaired skin repair. J Invest Dermatol. 2003;121:1544–1551. doi: 10.1046/j.1523-1747.2003.12610.x. [DOI] [PubMed] [Google Scholar]
- 32.Witte MB, Barbul A, Schick MA, Vogt N, Becker HD. Upregulation of arginase expression in wound-derived fibroblasts. J Surg Res. 2002;105:35–42. doi: 10.1006/jsre.2002.6443. [DOI] [PubMed] [Google Scholar]
- 33.Ye M, Iwasaki H, Laiosa CV, Stadtfeld M, Xie H, Heck S, Clausen B, Akashi K, Graf T. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 2003;19:689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 34.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- 35.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 36.Caldwell RB, Toque HA, Narayanan SP, Caldwell RW. Arginase: an old enzyme with new tricks. Trends Pharmacol Sci. 2015;36:395–405. doi: 10.1016/j.tips.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas R, Czikora I, Sridhar S, Zemskov EA, Oseghale A, Circo S, Cederbaum SD, Chakraborty T, Fulton DJ, Caldwell RW, Romero MJ. Arginase 1: an unexpected mediator of pulmonary capillary barrier dysfunction in models of acute lung injury. Front Immunol. 2013;4:228. doi: 10.3389/fimmu.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas R, Fulton D, Caldwell RW, Romero MJ. Arginase in the vascular endothelium: friend or foe? Front Immunol. 2014;5:589. doi: 10.3389/fimmu.2014.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toque HA, Nunes KP, Rojas M, Bhatta A, Yao L, Xu Z, Romero MJ, Webb RC, Caldwell RB, Caldwell RW. Arginase 1 mediates increased blood pressure and contributes to vascular endothelial dysfunction in deoxycorticosterone acetate-salt hypertension. Front Immunol. 2013;4:219. doi: 10.3389/fimmu.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasegawa-Moriyama M, Ohnou T, Godai K, Kurimoto T, Nakama M, Kanmura Y. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates postincisional pain by regulating macrophage polarization. Biochem Biophys Res Commun. 2012;426:76–82. doi: 10.1016/j.bbrc.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 43.Albina JE, Mills CD, Henry WL, Jr, Caldwell MD. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J Immunol. 1990;144:3877–3880. [PubMed] [Google Scholar]
- 44.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 45.Duque-Correa MA, Kuhl AA, Rodriguez PC, Zedler U, Schommer-Leitner S, Rao M, Weiner J, 3rd, Hurwitz R, Qualls JE, Kosmiadi GA, Murray PJ, Kaufmann SH, Reece ST. Macrophage arginase-1 controls bacterial growth and pathology in hypoxic tuberculosis granulomas. Proc Natl Acad Sci U S A. 2014;111:E4024–E4032. doi: 10.1073/pnas.1408839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schleicher U, Paduch K, Debus A, Obermeyer S, Konig T, Kling JC, Ribechini E, Dudziak D, Mougiakakos D, Murray PJ, Ostuni R, Korner H, Bogdan C. TNF-Mediated Restriction of Arginase 1 Expression in Myeloid Cells Triggers Type 2 NO Synthase Activity at the Site of Infection. Cell Rep. 2016;15:1062–1075. doi: 10.1016/j.celrep.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modolell M, Choi BS, Ryan RO, Hancock M, Titus RG, Abebe T, Hailu A, Muller I, Rogers ME, Bangham CR, Munder M, Kropf P. Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl Trop Dis. 2009;3:e480. doi: 10.1371/journal.pntd.0000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 51.Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal. 2010;3:ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Bruning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skabytska Y, Wolbing F, Gunther C, Koberle M, Kaesler S, Chen KM, Guenova E, Demircioglu D, Kempf WE, Volz T, Rammensee HG, Schaller M, Rocken M, Gotz F, Biedermann T. Cutaneous innate immune sensing of Toll-like receptor 2–6 ligands suppresses T cell immunity by inducing myeloid-derived suppressor cells. Immunity. 2014;41:762–775. doi: 10.1016/j.immuni.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el B, Lehr HA, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee N, You S, Shin MS, Lee WW, Kang KS, Kim SH, Kim WU, Homer RJ, Kang MJ, Montgomery RR, Dela Cruz CS, Shaw AC, Lee PJ, Chupp GL, Hwang D, Kang I. IL-6 receptor alpha defines effector memory CD8+ T cells producing Th2 cytokines and expanding in asthma. Am J Respir Crit Care Med. 2014;190:1383–1394. doi: 10.1164/rccm.201403-0601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.