Abstract
Background
Limited evidence suggests bariatric surgery may not reduce opioid analgesic use, despite improvements in pain.
Objective
To determine if use of prescribed opioid analgesics changes in the short- and long-term following bariatric surgery and to identify factors associated with continued and post-surgery initiated use.
Setting
Ten US hospitals.
Methods
The Longitudinal Assessment of Bariatric Surgery-2 is an observational cohort study. Assessments were conducted pre-surgery, 6 months post-surgery and annually post-surgery for up to 7 years until January 2015. Opioid use was defined as self-reported daily, weekly or “as needed” use of a prescribed medication classified as an opioid analgesic.
Results
Of 2258 participants with baseline data, 2218 completed follow-up assessment(s) (78.7% were female, median body mass index 46; 70.6% underwent Roux-en-Y gastric bypass). Prevalence of opioid use decreased following surgery from 14.7% (95% CI, 13.3–16.2) at baseline to 12.9% (95% CI, 11.5–14.4) at month-6, but then increased to higher than baseline levels as time progressed to 20.3% (95% CI, 18.2–22.5) at year-7. Among participants without baseline opioid use (N=1892), opioid use prevalence increased from 5.8% (95% CI, 4.7–6.9) at month-6 to 14.2% (95%CI, 12.2–16.3) at year-7. Public versus private health insurance, more pain pre-surgery, undergoing subsequent surgeries, worsening/less improvement in pain, and starting or continuing non-opioid analgesics post-surgery were significantly associated with higher risk of post-surgery initiated opioid use.
Conclusion
Following bariatric surgery, prevalence of prescribed opioid analgesic use initially decreased, but then increased, surpassing baseline prevalence, suggesting the need for alternative methods of pain management in this population.
Introduction
In the last three decades, efforts to improve patient care(1;2) have led to a significant increase in prescriptions for opioid analgesics to manage acute severe and chronic pain(3;4). An epidemic of opioid abuse, addiction and overdose has been an unforeseen consequence(5). Given recent reports suggesting several surgical procedures are associated with an increased risk of chronic opioid use(6), coupled with evidence that bariatric surgery patients are overrepresented in substance use treatment facilities and that Roux-en-Y Gastric Bypass (RYGB) increases the risk of alcohol use disorder(7), there is growing concern over opioid analgesic use among bariatric surgery patients.
Severe obesity increases risk of pain via mechanical factors, chemical mediators, depression and lifestyle(8). Bariatric surgery is an effective treatment for severe obesity, resulting in long-term weight loss and improvement in many comorbidities(9;10), including musculoskeletal and nonspecific pain and headaches(11–14). However, these improvements may not be followed by a reduction in use of opioid analgesics(15;16) for a number of reasons. First, due to an increased risk of marginal ulcers, non-steroidal anti-inflammatory drugs (NSAIDs) are contraindicated following bariatric surgery(17), leaving fewer non-opioid analgesic options. Second, administering systemic opioid therapy for short-term postoperative pain may increase risk for chronic opioid use(6;18;19). Third, RYGB accelerates the rate of morphine solution absorption(20), and, in animal models, has been shown to increase motivation for taking morphine(21). Collectively, these data suggest that the risk for opioid use and dependence may increase following at least some bariatric surgical procedures.
Utilizing a large multisite cohort study with 7 year follow-up, this study evaluated short- and long-term changes in use of prescribed opioid analgesics, and identified factors associated with continuation and post-surgery initiation of such use.
Methods
PARTICIPANTS
The Longitudinal Assessment of Bariatric Surgery-2 study is an observational study of 2458 adults who underwent an initial bariatric surgical procedure as part of clinical care between March 14, 2006 and April 24, 2009 at one of 10 hospitals at 6 clinical centers in the United States(22;23). The institutional review boards at each center approved the protocol and all participants gave written informed consent to participate in the study. The study is registered at: https://www.clinicaltrials.gov/ct2/show/NCT00465829.
Baseline assessments were conducted by research staff independent of clinical care following clearance for surgery and within 30 days prior to scheduled surgery date. Follow-up assessments were conducted at six months and annually following surgery, within 6 months of the surgery anniversary date, for seven years or until January 31, 2015. Participants who completed the baseline and at least one follow-up medication assessment were included in this report (N=2218; Figure 1).
Figure 1.
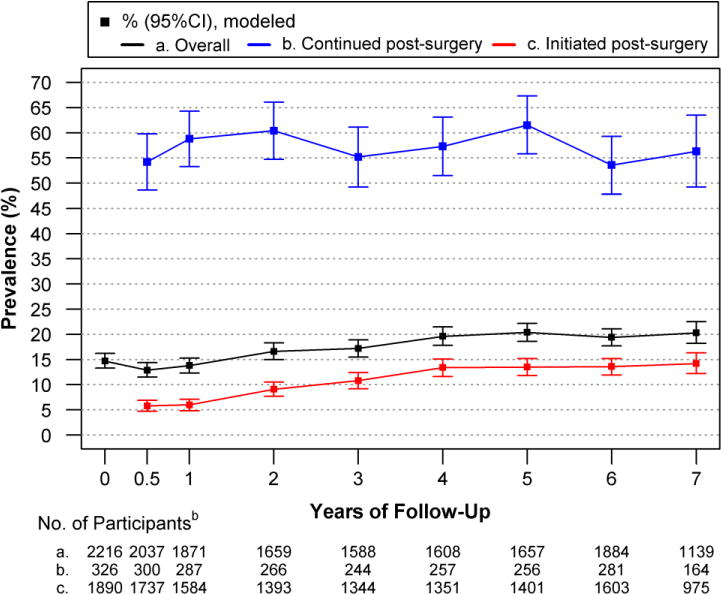
Modeled Prevalence of Prescribed Opioid Analgesic Use in Relation to Bariatric Surgery, Overall and by Pre-surgery Usea
Overall, there was a quadratic trend in the prevalence of opioid use from baseline to 7 years post-surgery (P<.01); prevalence initially dropped following surgery from 14.7% (95% CI, 13.3–16.2) at baseline to 12.9% (95% CI, 11.5–14.4) at 6 months (P=0.04), but then increased to higher than baseline levels as time progressed (20.3%, 95% CI, 18.2–22.5, at year-7; P<.001). Among those with regular opioid use at baseline, the prevalence of opioid use was 54.2% (95%CI 48.6–59.8) at 6 months post-surgery; there was not a trend over time (P=0.33). However, the prevalence of opioid use increased over follow-up (P<.001) among those with post-surgery initiation of opioid use from 5.8% (95%CI, 4.7–6.9) at 6 months post-surgery to 14.2% (95% CI, 12.2–16.3) at year -7.
aModels were adjusted for baseline factors related to missing follow-up data (i.e., site, age, smoking status). Observed and modeled data are reported in eTables 2 and 3 [Supplement], respectively.
bData are based on observations until January 31, 2015; data collection ended before 634 participants were eligible for a year-7 assessment.
COLLECTION OF DATA
Prescription analgesic medication use
Participants were asked to bring their prescription medication bottles from the past 90 days to research assessments, during which they completed the LABS-2 Medication Form (23). The form instructed participants to, “print the name (as listed on your medication bottle) of each prescription medication that you have taken in the past 90 days.” Participants were then asked if they took each medication daily (1 or more times/day), weekly (1–6 times/weekly), monthly/rarely (0–3 times/month), or as needed, or if they were no longer taking. Indication and dose were not assessed. Participants who forgot their medication bottles or could not attend an in-person assessment were able to complete the form at home and return it by mail.
Therapeutic and pharmacological classes of medications were used to determine use of opioid analgesic medications [opioid agonist alone or in combination with acetaminophen, a barbiturate, a NSAID, or a salicylate/aspirin; or opioid agonist/antagonist with acetaminophen], and non-opioid analgesic medications most commonly used for pain [acetaminophen alone or in combination with anything except an opioid agonist; aspirin or other salicylate in combination with anything except an opioid agonist; skeletal muscle relaxant; cyclooxygenase-2 selective or nonselective NSAID]. Medications typically prescribed for opioid dependency were identified by therapeutic and pharmacological class [buprenorphine hydrochloride, Subutex®, buprenorphine/naloxone, Suboxone®] or medication name [methadone hydrocholoride, Dolophine®, Methadone HCL Intensol®, and Methadose®] and considered separately. Drug names were also used to exclude some medications from the non-opioid analgesic medication category, despite a qualifying therapeutic/pharmacological class, due to their likely use for indications other than typical forms of pain (i.e., aspirin alone for prophylaxis against a cardiac event, clonidine for hypertension or attention deficit hyperactivity disorder, and phenazopyridine for a urinary tract infection).
The primary outcome was regular (defined as daily, weekly or as needed) use of a prescribed opioid analgesic (referred to as “opioid use” throughout). Secondary outcomes were regular use of any prescribed analgesic (opioid or non-opioid), a prescribed non-opioid analgesic, and a prescribed NSAID. Daily use of opioid dependency medications was an exploratory outcome. When participants reported more than one medication within a category, the more frequent use was selected.
Covariates
Daily use of a prescribed anti-depressant medication and current use of a prescribed benzodiazepine medication were also determined by therapeutic and pharmacological classes of medications reported on the LABS-2 Medication Form. Anthropometric measurements followed standardized protocols. Sociodemographics, psychiatric hospitalizations, binge eating, loss of control eating, substance use (smoking, alcohol intake, symptoms of alcohol use disorder and illicit drug use), back, hip, knee and ankle surgeries, and subsequent bariatric procedures (i.e. revisions, reversals, new procedures) were determined with study-specific forms (23;24;25). The Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) assessed physical and mental health(26), with scores transformed to a mean of 50 and standard deviation of 10 in the general U.S. population(27); a lower score indicates worse function. The Western Ontario and McMaster Universities Osteoarthritis index (WOMAC) assessed hip and knee pain and function level during various activities(28), with scores transformed to a 0–100 scale; a higher score indicates more pain. The 12-item Interpersonal Support Evaluation List (ISEL-12) measured perceived social support. A lower score (range 0–12) indicates less support availability(29). The Beck Depression Inventory, version 1 (BDI), assessed depressive symptoms in the past week. A higher score (range 0–63) indicates greater symptomatology(30).
Analysis
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). All reported P-values are two-sided; P-values less than 0.05 were considered to be statistically significant. The Pearson chi square test for categorical and the Wilcoxon rank sum test for continuous variables were used to compare characteristics of LABS-2 participants included versus excluded from analysis (eTable 1 [Supplement]). Descriptive statistics summarize baseline characteristics of the analysis sample overall and by surgical procedure.
Longitudinal analyses were performed with mixed models with random intercept assuming the compound symmetry covariance matrix, with control for baseline age, smoking status and site, which were associated with missing follow-up data(31). Sensitivity analyses were performed to examine the robustness of results with respect to the missing at random assumption. Among those missing versus not missing the medication assessment at a particular time point, the prevalence of opioid use at other time points was similar, suggesting that missing data did not induce bias (eAppendix 1 [Supplement]). Sensitivity analyses were also performed to examine the potential impact of secular trends. Year of data collection was not significantly related to opioid use, and adjustment for year of data collection had negligible impact on parameter estimates (prevalence or relative risks).
Poisson mixed models with robust error variance were used to estimate and test for change in the prevalence of outcomes over time. In addition to testing for a quadratic or linear trend over time (baseline to year-7), pair-wise comparisons were made between baseline and 6 months and baseline and year-7, to examine short- and long-term changes, respectively, using the t statistic with P-values adjusted to control for overall type I error(32). Likewise, the prevalence of continued opioid use (i.e., post-surgery use among those with pre-surgery use) and post-surgery initiated opioid use (i.e., post-surgery use among those without pre-surgery use) was modeled, and linear and quadratic trends from 6 months to year-7 were tested. If change in the prevalence of outcomes over time differed by surgical procedure (tested for each outcome with a time*procedure interaction term), only analyses stratified by surgical procedure are reported.
Poisson mixed models with robust error variance were also used to identify factors related to continued opioid use and post-surgery initiated opioid use, respectively. Two sets of models were constructed for each outcome. The first set considered baseline factors and surgical procedure/approach only. The second set also considered post-surgery status. Independent variables were selected and either forced or considered based on the literature(3;6;7;18;19;33–34) as detailed in eAppendix 2 [supplement]. Due to the large number of variables under consideration, first a univariable model with each factor was tested. Next, factors with P<0.20 were entered into a multivariable model with the forced variables. Variables that were not forced or significant in the multivariable model were removed by using backward elimination.
Sensitivity analysis of frequency threshold
Because there is not an established frequency threshold for clinically relevant opioid analgesic use (i.e., that is associated with opioid abuse or dependence), analyses of opioid use were repeated applying a daily use threshold requirement. A frequency recorded as “weekly (1–6 times/weekly)” for fentanyl was considered “daily” because one patch provides 72 hours of pain relief. In addition to the variables already described (eAppendix 2;supplement), baseline weekly or as needed opioid use was considered as a risk factor for post-surgery initiated daily opioid use(8).
Results
Of 2258 participants with baseline medication data, 2218 (98.0%) completed the medication assessment at least once during follow-up and are included in the analysis sample. Follow-up rates are 90.2% (2037/2258), 83.2% (1874/2253), 73.9% (1661/2247), 71.0% (1590/2240), 72.0% (1609/2235), 74.6% (1659/2224), 85.4% (1885/2206), and 73.0% (1141/1563) at month 6 and year 1, 2, 3, 4, 5 and 7, respectively (eFigure 1 [Supplement]).
Baseline characteristics are presented in Table 1. The majority of participants (79%) were female. Median age was 46 (range 18–78) years; median BMI was 46 (range 34–94) kg/m2. RYGB was the most common surgical procedure (70.6%), most (88.9%) of which were performed laparoscopically. One quarter (24.9%) of participants underwent laproscopic adjustable gastric banding (LAGB). The “other procedure” group (4.3%) consisted of 51 sleeve gastrectomy, 30 banded RYGB and 17 biliopancreatic diversion with duodenal switch. In the seven years following their initial bariatric procedure, 7.9% (n=175) of participants reported a subsequent bariatric procedure.
Table 1.
Baseline Socioemographic and Clinical Characteristics of Adults prior to Bariatric Surgery.a
| Total N = 2218 |
RYGBb N = 1567 |
LAGB N = 553 |
Other Procedurec N = 98 |
|
|---|---|---|---|---|
|
|
|
|
|
|
| Sociodemographics | ||||
| Female, No. (%) | 1746/2218 (78.7) | 314/1567 (80.0) | 129/553 (76.7) | 29/98 (70.4) |
| Age, y, median (IQR) | 46 (37,55) | 45 (37,54) | 48 (38,57) | 47 (38,54) |
| Race, No. (%) | (n = 2198) | (n = 1551) | (n = 551) | (n = 96) |
| White | 1907 (86.8) | 1322 (85.2) | 497 (90.2) | 88 (91.7) |
| Black | 225 (10.2) | 176 (11.3) | 42 (7.6) | 7 (7.3) |
| Otherd | 66 (3.0) | 53 (3.4) | 12 (2.2) | 1 (1.0) |
| Hispanic/Latino ethnicity, No./total No. (%) |
108/2217 (4.9) | 78/1567 (5.0) | 25/552 (4.5) | 5/98 (5.1) |
| Marital status, No. (%) | (n= 2067) | (n= 1525) | (n= 544) | (n= 98) |
| Married or living as married |
1391 (64.2) | 955 (62.6) | 376 (69.1) | 60 (61.2) |
| Divorced, separated or widowed | 437 (20.2) | 321 (21.0) | 97 (17.8) | 19 (19.4) |
| Never married | 339 (15.6) | 249 (16.3) | 71 (13.1) | 19 (19.4) |
| Education, No. (%) | (n = 2067) | (n= 1527) | (n= 543) | (n= 97) |
| ≤ High school | 496 (22.9) | 358 (23.4) | 123 (22.7) | 15 (15.5) |
| Some college | 874 (40.3) | 656 (43.0) | 183 (33.7) | 35 (36.1) |
| ≥ College degree | 797 (36.8) | 513 (33.6) | 237 (43.6) | 47 (48.5) |
| Household income, No. (%) |
(n = 2111) | (n= 1486) | (n= 530) | (n= 95) |
| < $25,000 | 374 (17.7) | 285 (19.2) | 69 (13.0) | 20 (21.1) |
| $25,000–$49,000 | 545 (25.8) | 423 (28.5) | 104 (19.6) | 18 (18.9) |
| $50,000–$74,999 | 500 (23.7) | 356 (24.0) | 129 (24.3) | 15 (15.8) |
| $75,000–$99,999 | 344 (16.3) | 229 (15.4) | 100 (18.9) | 15 (15.8) |
| ≥ $100,000 | 348 (16.5) | 193 (13.0) | 128 (24.2) | 27 (28.4) |
| Insurance, No. (%) | (n = 2164) | (n= 1522) | (n= 544) | (n= 98) |
| Private | 1422 (65.7) | 1002 (65.8) | 355 (65.3) | 65 (66.3) |
| Public | 522 (24.1) | 356 (23.4) | 140 (25.7) | 26 (26.5) |
| Othere | 220 (10.2) | 164 (10.8) | 49 (9.0) | 7 (7.1) |
| Employment status, No. (%) |
(n= 2163) | (n= 1529) | (n= 545) | (n= 98) |
| Employed | 1495 (69.1) | 1060 (69.7) | 368 (67.5) | 67 (68.4) |
| Unemployed | 79 (3.7) | 61 (4.0) | 11 (2.0) | 7 (7.1) |
| Disabled | 314 (14.5) | 228 (15.0) | 72 (13.2) | 14 (14.3) |
| Other | 275 (12.7) | 171 (11.3) | 94 (17.3) | 10 (1.02) |
| Physical Health | ||||
| Body Mass Index,f median (IQR) |
45.8 (41.7,51.4) | 46.5 (42.3,51.8) | 43.7 (40.4,48.1) | 51.7 (44.2,59.2) |
| History of back, hip, knee or ankle surgery, No./total No. (%) |
649/2158 (30.1) | 453/1522 (29.8) | 173/538 (32.2) | 23/98 (23.5) |
| SF-36 Physical component score, median (IQR) |
36.5 (27.8,45.0) | 35.7 (27.1,44.4) | 39.3 (30.5,46.9) | 34.8 (26.5,43.0) |
| SF-36 Bodily Pain score, median (IQR) |
39.6 (31.5,48.5) | 39.6 (31.5,48.5) | 40.0 (35.4,49.4) | 39.6 (35.4,44.3) |
| WOMAC knee pain score |
4.0 (1.0,8.0) | 5.0 (1.0,8.0) | 3.0 (0.0,7.0) | 5.0 (0.0,9.0) |
| WOMAC hip pain score | 3.0 (0.0,7.0) | 3.0 (0.0,7.0) | 2.0 (0.0,6.0) | 2.0 (0.0,8.5) |
| Total N = 2218 |
RYGB N = 1567 |
LAGB N = 553 |
Other Procedured N = 98 |
|
|---|---|---|---|---|
|
|
|
|
|
|
| Mental Health History of psychiatric hospitalization, No./Total No. (%) |
214/2166 (9.9) | 158/1527 (10.3) | 39/541 (97.2) | 17/98 (17.3) |
| Binge eating, No./Total No. (%) |
336/2132 (15.8) | 218/1502 (14.5) | 98/533 (18.4) | 20/97 (20.6) |
| Loss of control eating, No./Total No. (%) |
757/2143 (35.3) | 507/1509 (33.6) | 211/540 (39.1) | 39/94 (41.5) |
| Antidepressant medication, No./total No. (%) |
849/2201 (38.6) | 605/1551 (39.0) | 201/552 (36.4) | 43/98 (43.9) |
| Benzodiazepine medication, No./total No. (%) |
213/2214 (9.6) | 152/1564 (9.7) | 49/552 (8.9) | 12/98 (12.2) |
| SF-36 Mental component score, median (IQR) |
51.7 (42.8,57.2) | 51.8 (42.8,57.4) | 51.8 (44.2,57.0) | 48.5 (37.2,55.4) |
| ISEL-12 Belonging score, median (IQR) |
14 (12,16) | 14 (12,16) | 14 (12,16) | 14 (11,16) |
| Beck Depression Inventory score, median (IQR) |
6 (3,11) | 6 (3,11) | 6 (3,11) | 7 (3,12) |
| Substance use Smoke cigarettes, No./total No. (%) |
275/2216 (12.4) | 215/1565 (13.7) | 47/553 (8.5) | 13/98 (13.3) |
| Regular alcohol consumption (≥ 2 times/wk), No./total No. (%) |
143/2167 (6.6) | 88/1526 (5.8) | 42/543 (7.7) | 13/98 (13.3) |
| AUD symptoms, No./total No. (%) |
142/2162 (6.6) | 99/1522 (6.5) | 36/542 (6.6) | 7/98 (7.1) |
| Illicit drug use, No./total No. (%) |
94/2159 (4.4) | 66/1520 (4.3) | 21/542 (3.9) | 7/97 (7.2) |
Abbreviations: AUD, Alcohol Use Disorder; ISEL-12, 12-item Interpersonal Support Evaluation List; Laparoscopic adjustable gastric banding (LAGB); non-steroidal anti-inflammatory drugs (NSAID); Roux-en-Y gastric bypass (RYGB); SF-36, Short-Form 36-item Health Survey; WOMAC, Western Ontario and McMaster Universities Osteoarthritis index.
Data were reported as No.(%) unless otherwise indicated.
Open RYGB (n=174) and laparoscopic RYGB (n=1393) were combined because surgical approach was no related to post-surgery opioid use(Table 2).
Sleeve gastrectomy (n=51), banded Roux-en-Y gastric bypass (n=30) and biliopancreatic diversion with duodenal switch (n=17) were combined due to the low frequency of each.
Due to small numbers, the following racial groups were combined: Asian, American Indian/Alaska Native, Native Hawaiian/other Pacific Islander, multiple races.
Due to small numbers, other type, unknown, and no insurance were combined.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Change in analgesics
Change in the prevalence of opioid use and opioid dependency medication use over time did not differ by surgical procedure. Thus, these outcomes are reported among all surgical procedures (Figure 1; eTable 3 [Supplement]). There was a quadratic trend in the prevalence of opioid use from baseline to 7 years post-surgery (P<.01); the prevalence dropped from 14.7% (95% CI, 13.3–16.2) at baseline to 12.9% (95% CI, 11.5–14.4) at 6 months (P=0.04), but then increased over time and was significantly higher than baseline at year-7 (20.3%, 95% CI, 18.2–22.5; P<.001). There was not a significant linear (P=0.33) nor quadratic (P=0.67) trend in the prevalence of continued use (54.2%, 95% CI 48.6–59.8, at 6 months) across follow-up. In contrast, post-surgery initiated use increased from 5.8% (95% CI, 4.7–6.9) at 6 months to 14.2% (95%CI, 12.2–16.3) at year-7 (linear trend P<.001).
A table showing the observed prevalence of commonly prescribed opioid analgesics, use of multiple prescribed opioid analgesics, and use of prescribed nonopioid analgesics, among participants with regular opioid use, by time point, is available online (eTable 4 [Supplement]). Hydrocodone was the most commonly reported prescribed opioid analgesic at all time points (40%), followed by tramadol and oxycodone (both ≥ 20%). At any given time point, more than 10% reported multiple opioid analgesics and more than 60% reported prescribed non-opioid analgesics.
Although fewer than 2% of participants reported use of medications typically prescribed for opioid dependence at all time points, there was a significant linear trend across time (P=.01) indicating the prevalence increased from baseline (0.8%, 95% CI, 0.4–1.2) to year-7 (1.4%, 95% CI, 0.8–2.0) (eTable 3 [Supplement]).
Change in secondary outcomes over time differed by surgical procedure. Thus, these outcomes are reported by surgical procedure only (Figure 2; eTable 6 [Supplement]). Among participants who underwent RYGB (Figure 2A), the prevalence of non-opioid analgesic use increased over time (P<.001), first decreasing from baseline (22.3%, 95% CI, 20.3–24.3) to 6 months (13.5%, 95% CI, 11.8–15.2; P<.001), and then increasing over time to higher than baseline (25.8%, 95% CI, 23.0–28.6, at year-7; P=.048). The prevalence of any analgesic use followed the same pattern. The prevalence of NSAID use followed a quadratic trend (P<.001), decreasing from baseline (13.0%, 95% CI, 11.4–14.7) to 6 months (4.8%, 95% CI, 3.8–5.9; P<.001) and then increasing over time but remaining lower than baseline through year-7 (9.6%, 95% CI, 7.6–11.5; P<.001).
Figure 2.
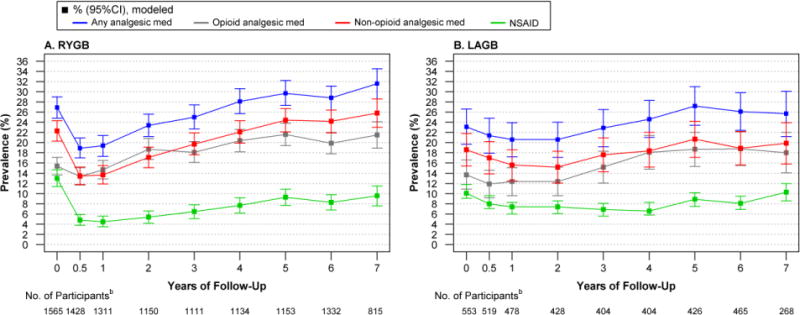
Modeled Prevalence of Prescribed Analgesic Use in Relation to Bariatric Surgery, by Surgical Procedurea
A. Among participants who underwent RYGB, the prevalence of any prescription analgesic use, and specifically non-opioid analgesic use, increased over time (P for both <.001), first decreasing from baseline to 6 months (P for both<.001) and then increasing such that year-7 prevalence was higher than baseline (P for both<0.05). The prevalence of NSAID use followed a quadratic trend (P<.001), decreasing from baseline to 6 months (P<.001) before increasing over time. However, post-surgery prevalence remained lower than baseline through year-7 (P=0.01). B. Among participants who underwent LAGB, the prevalence of any prescription analgesic use increased over time (P=0.01), but was not significantly different from baseline at 6 months (P=0.45) or year-7 (P=0.50). The prevalence of non-opioid analgesic use did not differ over time (p=0.34) or differ from baseline at 6-month (P=0.50) or 7-year (P=0.83). The prevalence of NSAID use followed a quadratic trend over time (P=0.01) but was not significantly different from baseline at 6 months (P=0.23) or year-7 (P=0.99).
Abbreviations: Laparoscopic adjustable gastric banding (LAGB); non-steroidal anti-inflammatory drugs (NSAID); Roux-en-Y gastric bypass (RYGB).
aModels were adjusted for baseline factors related to missing follow-up data (i.e., site, age, smoking status). Observed and modeled data for these and “other” procedures is reported in eTables 5 and 6 [Supplement], respectively. bData are based on observations until January 31, 2015; data collection ended before 429 RYGB and 173 LAGB participants were eligible for a 7 year assessment.
As shown in Figure 2B, among participants who underwent LAGB there was not a significant trend across time (linear trend P=0.06), nor short- or long-term differences (P=0.50 and P=0.83, respectively) in the prevalence of non-opioid analgesic use (18.6%, 95% CI, 15.4–21.8, at baseline). Although there was a significant linear trend in the prevalence of any analgesic use indicating an increase over time (P<.01), the prevalence did not significantly differ between baseline (23.1%, 95% CI, 19.7–26.6) and 6 months (21.4%, 95% CI, 17.9–24.5; P=0.45) or year-7 (25.7%, 95% CI, 21.2–30.1; P=0.50). The prevalence of NSAID use followed a quadratic trend over time (P=.01) indicating an initial drop in prevalence followed by an increase. However, the prevalence at 6 months (9.6%, 95% CI, 7.1–12.1; P=0.23) and year-7 (12.0%, 95% CI, 8.6–15.5; P=0.99) did not significantly differ from baseline (11.8%, 95% CI, 9.1–14.4).
Factors related to continuation and post-surgery initiation of opioid analgesic use
With control for baseline factors, surgical procedure was not significantly related to post-surgery continued or initiated opioid use, respectively (eTable 7 [Supplement]). In models considering pre- and post-surgery status, more pain at baseline, worsening or less of an improvement in pain following surgery, and starting or continued non-opioid analgesic use (versus no use or stopping use) were independently associated with higher risk of both continued and post-surgery initiated opioid use. Continued use of benzodiazepine (versus no use or stopping use) was also associated with higher risk of continued opioid use. Public versus private insurance, back, hip, knee or ankle surgery, a subsequent bariatric procedure and more improvement in mental health following surgery were also significantly associated with higher risk of post-surgery initiated use (Table 2).
Table 2.
Associations with Continuation and Initiation of Prescribed Opioid Analgesic Use, following Bariatric Surgery.
| Regular Opioid Use Post-Surgery
|
||||
|---|---|---|---|---|
| Continueda (N=280) | Initiatedb (N=1631) | |||
| ARR(95%CI)c | P | ARR(95%CI)c | P | |
| Pre-surgery | ||||
| Male (Ref. = Female) | 1.07(0.91–1.27) | 0.40 | 0.91(0.74–1.12) | 0.36 |
| Age, per 10 years younger | 1.04(0.97–1.11) | 0.32 | 1.00(0.92–1.08) | 0.93 |
| Race (Ref. = Black) | 0.20 | 0.40 | ||
| White | 0.91(0.73–1.13) | 1.32(0.88–1.98) | ||
| Other | 1.11(0.80–1.52) | 1.29(0.72–2.29) | ||
| Hispanic ethnicity | 1.02(0.71–1.47) | 0.92 | 0.86(0.49–1.51) | 0.60 |
| Household income <$25,000 (Ref. = ≥$25,000) | 1.00(0.85–1.18) | 0.99 | 1.04(0.85–1.28) | 0.69 |
| Insurance (Ref. = Private) | 0.60 | <0.01 | ||
| Public | 1.03(0.88–1.21) | 1.33(1.09–1.64) | ||
| Other/unknown/none | 0.91(0.71–1.16) | 1.46(1.15–1.84) | ||
| SF-36 Bodily pain, per 10 points lower (worse) | 1.14(1.03–1.25) | 0.01 | 1.55(1.38–1.74) | <.001 |
| SF-36 Mental component score, per 10 points lower (worse) | d | 0.94(0.86–1.02) | 0.14 | |
| Smoke cigarettes (Ref. = No) | 1.05(0.87–1.26) | 0.61 | 1.25(0.99–1.59) | 0.06 |
| Surgical procedure (Ref. = Laparoscopic RYGB) | 0.93 | 0.70 | ||
| Open RYGB | 0.98(0.80–1.21) | 1.02(0.81–1.29) | ||
| LAGB | 0.99(0.83–1.18) | 0.87(0.66–1.15) | ||
| Other | 0.91(0.67–1.22) | 0.88(0.61–1.28) | ||
| Post-surgery | ||||
| History of back, hip, knee or ankle surgery | 1.01(0.89–1.14) | 0.91 | 1.21(1.02–1.45) | 0.03 |
| Revision, reversal or new bariatric procedure | 1.09(0.85–1.40) | 0.48 | 1.50(1.08–2.07) | 0.01 |
| Pre- to post-surgery change | ||||
| Weight change, per −5% (loss) | 1.00(0.98–1.02) | .84 | 1.03(0.99–1.07) | 0.12 |
| SF-36 Bodily pain, per 10 points lower (worse) | 1.11(1.04–1.18) | <.01 | 1.72(1.57–1.90) | <.001 |
| SF-36 Mental component score, per 10 points lower (worse) | d | 0.91(0.85–0.97) | <0.01 | |
| Pre- and post-surgery status | ||||
| Prescribed benzodiazepine use | 0.02 | 0.24 | ||
| Started vs. never | 0.90(0.75–1.07) | 1.26(1.00–1.57) | ||
| Continued vs. stopped | 1.21(1.02–1.44) | 1.05(0.64–1.74) | ||
| Continued vs. never | 1.15(1.01–1.30) | 1.15(0.82–1.61) | ||
| Prescribed non-opioid analgesic usee | <.001 | <.001 | ||
| Started vs. never | 2.06(1.59–2.68) | 6.09(4.77–7.78) | ||
| Continued vs. stopped | 2.44(1.99–2.98) | 4.00(2.60–6.16) | ||
| Continued vs. never | 2.14(1.68–2.72) | 4.05(2.98–5.49) | ||
Abbreviations: LAGB, laparoscopic adjustable gastric banding; SF-36, Short-Form 36-item Health Survey;
RYGB, Roux-en-Y gastric bypass.
Regular use of prescribed opioids pre- and post-surgery vs. regular use pre-surgery only.
Regular use of prescribed opioids post-surgery only vs. no regular use pre- and post-surgery.
Adjusted for other variables as indicated in this table, as well as site.
The ARR (95%CI) is not reported for variables that were not retained in the model due to lack of significance (P>.05 overall).
When prescribed non-steroidal anti-inflammatory drug (NSAID) use replaced prescribed non-opioid analgesic use, NSAID use was not significantly associated with risk of continued opioid use (P=0.69; started vs. never ARR=1.03, 95%CI, 0.88–1.20; continued vs. stopped ARR=1.09, 95%CI, 0.91–1.31; continued vs. never ARR=1.12, 95%CI, 0.91–1.37). However, compared to not using NSAIDs pre- and post-surgery, starting (ARR 1.63, 95%CI, 1.29–2.06) or continuing (ARR 1.51, 95%CI, 1.04–2.19) NSAID use were associated with increased risk of post-surgery initiated opioid use (P<.001). The risk of post-surgery initiated opioid use did not significantly differ by continued vs. stopped NSAID use (ARR=1.29, 95%CI, 0.86–1.94).
When NSAID use replaced non-opioid analgesic use in the multivariable models, associations with post-surgery initiated opioid use were similar, although less pronounced (i.e., compared to no use of NSAIDs, ARR=1.63, 95%CI, 1.29–2.06, for starting NSAIDs, and ARR=1.51, 95%CI, 1.04–2.19, for continuing NSAIDs; P<.001). In contrast, continued use of NSAIDs was not significantly associated with continued opioid use (P=0.69; Table 2). None of the other factors evaluated in the models considering pre- and post-surgery status, including percent weight change, were significantly related to post-surgery continued or initiated opioid use (Table 2).
Sensitivity analysis of frequency threshold
Findings with respect to change in the prevalence of opioid use over time were similar as reported above when daily opioid use was examined (eTable 3 [Supplement]). There was a linear trend from baseline to 7 years post-surgery (P=.01). Prevalence initially dropped following surgery from 8.2% (95%CI, 7.0–9.3) at baseline to 6.4% (95%CI, 5.4–7.4) at 6 months (P<.01), but then increased to higher than baseline levels as time progressed (11.4%, 95%CI, 9.7–13.1, at year-7; P<.001). Most of the same factors reported above were related to continuation and initiation of daily opioid analgesic use (eTable 8 [Supplement]). Additionally, weekly or as needed opioid use pre-surgery was associated with a higher risk of post-surgery initiated daily opioid use (ARR=1.63, 95%CI, 1.19–2.24; P<.01).
Discussion
In this large multi-site observational study of adults who underwent bariatric surgery, following an initial decrease in the first six months post-surgery, the percentage of participants reporting regular or daily use of prescribed opioid analgesics increased, such that the prevalence appeared higher by the third year post-surgery versus baseline, and continued to increase through year-7. Almost half of the patients reporting regular or daily opioid use at baseline reported no such use at post-surgery assessments. However, among the much larger group of opioid-naive patients (i.e., those who did not report regular or daily opioid use at baseline), opioid use gradually increased throughout the post-surgery follow-up. Thus, post-surgery initiation of opioid use explains this phenomenon.
Although there is a dearth of research investigating opioid use following bariatric surgery, two reports by Raebel et al. provide comparison data through the first post-operative year(15;16). Among 11719 adults who underwent surgery from 2005–2009, 8% had chronic opioid use in the year prior to surgery, 77% of whom had chronic opioid use in the year following surgery(15), whereas 4% of patients without chronic use pre-surgery developed chronic opioid use within one year post-surgery(16). Despite differences in assessment methods, two of our prevalence estimates are nearly identical (i.e., 8% daily use pre-surgery; 4% post-surgery initiated daily use at year-1). In contrast, our prevalence estimate of continued daily use 1-year post-surgery (i.e., 53%) is lower.
Reports of opioid use following bariatric surgery beyond the first post-operative year are lacking. However, the Swedish Obesity Study compared the six-year pharmaceutical cost of prescribed analgesic medications (i.e. opioid and non-opioid) in adults who underwent bariatric surgery versus a non-surgical control group(35). Despite a mean weight loss of 16% in the surgical group versus a mean weight gain of 1% in the control group, among patients taking analgesic medications at baseline, there was no difference in six-year analgesic medication cost between groups. Furthermore, among patients who were not taking analgesic medications pre-surgery, the surgical group had significantly higher analgesic medication costs, suggesting, like our data, that use of analgesic medication may increase following bariatric surgery.
Although Raebel et al. reported no difference in continued chronic opioid use in the year following surgery by pre-surgery chronic pain status(15), in the current investigation, more pain pre-surgery, as well as less improvement or worsening of pain pre-to-post-surgery, were associated with higher risk of both continued and post-surgery initiated opioid use. Like previous studies in different populations(4;6;18;19), having public versus private insurance, and having additional surgeries (i.e., back, hip, knee or ankle surgery, or a subsequent bariatric procedure) were related to increased risk of initiating opioid use following bariatric surgery, suggesting that surgical pain management(6;38) and new-onset pain following surgery(39) may have contributed to the increase in opioid use.
Contray to the proposed mechanism that opioid use increases post-surgery due to discontinuation of NSAIDs, starting NSAID use post-surgery was associated with higher risk of post-surgery initiated opioid use, while stopping NSAID was associated with lower risk. Starting use of non-opioid analgesics was also associated with higher risk of both continued and post-surgery initiated opioid use, while stopping non-opioid analgesic use post-surgery was associated with lower risks of both. These findings likely reflect that opioid and non-opioid analgesic medications are often used in tandem, versus as alternatives to each other.
Most factors related to substance use disorder that were examined (i.e., sex, age, income, social support, depressive symptoms, mental quality of life, psychiatric hospitalization, antidepressant medication, smoking, alcohol consumption, illicit drug use and surgical procedure(7;25)) were not related to continued or post-surgery initiated opioid use. An exception was that continued benzodiazepine use, which may be suggestive of addictive behavior(36) and increase risk of opioid overdose(37), was associated with an increased risk of continued opioid use. There was also an increase over time in the use of medications typically prescribed for opioid dependence, although use of such medications remained rare throughout follow-up (<2%). Given our findings, as well as a report by Raebel et al., which identified younger age and laparoscopic RYGB versus LAGB as risk factors for post-surgery initiated chronic opioid use(16), additional work is needed to clarify the risk of opioid abuse and dependence following bariatric surgery.
Major strengths of this study are its large, geographically diverse sample, standardized and detailed data collection, which allowed us to evaluate many potential risk factors, long-term follow-up and high retention(40). Analyses controlled for baseline factors related to missing follow-up data and the sensitivity analysis indicated the missing data were not related to opioid use, and thus should not bias the results. Additionally, the initial sample size and retention rate ensured sufficient statistical power.
Study limitations should also be noted. First, the LABS-2 study did not have a non-surgical control group, thus the findings cannot necessarily be attributed to the surgery itself. Although the LABS-2 data did not provide evidence of a secular trend in opioid use across follow-up, secular trends in pain management have been described. For instance, the number of opioid prescriptions dispensed by US retail pharmacies increased steadily from 2006–2011, before declining from 2011–2013(4). Additionally, a report based on the National Ambulatory Medical Care Survey found an increase in the proportion of patients prescribed opioids at clinic visits for musculoskeletal pain from 2000–2010(3). However, the trend was less clear during the timeframe that overlapped with LABS-2 data collection (i.e., 16.6%, 20.2%, 18.5%, 21.2%, and 19.6% in years 2006–2010, respectively). Second, this study relied on self-reported medication use, the accuracy of which is unmeasured in this sample. However, self-report of prescription opioid use in a 90-day fixed-time window has had good agreement with pharmacy records in a population-based study (K=.62; 95%CI .58–.67), and due to medication nonadherence, may better reflect actual use than pharmacy records(41). Furthermore, there is no reason to suspect misclassification of medication use varied by time point, surgical procedure, or patient characteristics. Still, our methodology led to gaps in outcome assessment, and like pharmacy records, may not have captured medications that were only given during inpatient care. Third, LABS-2 did not measure indication for, or dose of, medications, so we could not identify non-opioid prescription medications used for pain that have other common indications (e.g., anticonvulsant medications, antidepressant medications), determine whether opioid and non-opioid analgesic medication use was for acute or chronic pain, nor determine reduction or escalation in dosage. Finally, LABS-2 did not assess use of all over the counter analgesics, non-prescribed opioids (e.g., heroin), symptoms of opioid use disorder, or all covariates of interest (i.e., lifetime psychiatric and substance use history). Despite these limitations, this study’s prospective assessment for 6–7 years distinguish it from previous studies that have relied on pharmacy records or chart reviews for a relatively short timeframe to study use of prescribed opioid medications following surgery(6–8;19;34;36).
Conclusion
Among a cohort of adults who underwent bariatric surgery, following an initial decrease, the percentage of patients reporting use of prescribed opioid analgesics, at the regular or daily threshold, increased throughout seven years of follow-up, surpassing the baseline prevalence. This was due to an increase in post-surgery initiated use. Surgical procedure type and amount of weight loss were not associated with risk of post-surgery continued or initiated opioid use, whereas severity of pain was. In the context of the Center for Disease Control and Prevention’s recent evidence-based conclusion that opioids should not be routinely used to manage chronic pain(42), these findings highlight the need for alternative long-term pain management approaches following bariatric surgery, which may include nonopioid analgesic and nonpharmacological options(43;44).
Supplementary Material
Acknowledgments
Source of support:
LABS-2 was funded by a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: Data Coordinating Center -U01 DK066557; Columbia-Presbyterian - U01-DK66667 (in collaboration with Cornell University Medical Center CTSC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: All authors will complete the ICMJE Form for Disclosure of Potential Conflicts of Interest. Authors have no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work other than the following: Dr. A has received research grants from Allergan Pfizer, Covidien, EndoGastric Solutions, Nutrisystem and is on the Scientific Advisory Board of Ethicon J & J Healthcare System. Dr. B has an advisor role with Pacira Pharmaceuticals. Dr. C is a Consultant and Advisor for Crospon and has received a research grant from Enteromedics. Dr. D has research support from Sanford Profile. Drs. E-Z have no disclosures to report.
Trial Registration. ClinicalTrials.govIdentifier: NCT00465829
Reference List
- 1.Quality improvement guidelines for the treatment of acute pain and cancer pain. American Pain Society Quality of Care Committee. JAMA. 1995;274(23):1874–1880. doi: 10.1001/jama.1995.03530230060032. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Agency for Health Care Policy and Research. Acute Pain Management: Operative or Medical Procedures and Trauma. Rockville, MD: U.S. Department of Health and Human Services; 1992. (AHCPR Pub. No. 92-0032). [Google Scholar]
- 3.Daubresse M, Chang HY, Yu Y, Viswanathan S, Shah ND, Stafford RS, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med Care. 2013;51(10):870–878. doi: 10.1097/MLR.0b013e3182a95d86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute of Drug Abuse. Prescription Opioid and Heroin Abuse. Accessed online Feb 2, 2017 at https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/prescription-opioid-heroin-abuse.
- 5.Califf RM, Woodcock J, Ostroff S. A Proactive Response to Prescription Opioid Abuse. N Engl J Med. 2016;374(15):1480–1485. doi: 10.1056/NEJMsr1601307. [DOI] [PubMed] [Google Scholar]
- 6.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286–1293. doi: 10.1001/jamainternmed.2016.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Wu LT. Substance use after bariatric surgery: A review. J Psychiatr Res. 2016;76:16–29. doi: 10.1016/j.jpsychires.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. doi: 10.2147/JPR.S55598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novack V, Fuchs L, Lantsberg L, Kama S, Lahoud U, Horev A, et al. Changes in headache frequency in premenopausal obese women with migraine after bariatric surgery: a case series. Cephalalgia. 2011;31(13):1336–1342. doi: 10.1177/0333102411413162. [DOI] [PubMed] [Google Scholar]
- 12.Gill RS, Al Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev. 2011;12(12):1083–1089. doi: 10.1111/j.1467-789X.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 13.King WC, Chen JY, Belle SH, Courcoulas AP, Dakin GF, Elder KA, et al. Change in Pain and Physical Function Following Bariatric Surgery for Severe Obesity. JAMA. 2016;315(13):1362–1371. doi: 10.1001/jama.2016.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speck RM, Bond DS, Sarwer DB, Farrar JT. A systematic review of musculoskeletal pain among bariatric surgery patients: implications for physical activity and exercise. Surg Obes Relat Dis. 2014;10(1):161–170. doi: 10.1016/j.soard.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Raebel MA, Newcomer SR, Reifler LM, Boudreau D, Elliott TE, DeBar L, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369–1376. doi: 10.1001/jama.2013.278344. [DOI] [PubMed] [Google Scholar]
- 16.Raebel MA, Newcomer SR, Bayliss EA, Boudreau D, DeBar L, Elliott TE, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf. 2014;23(12):1247–1257. doi: 10.1002/pds.3625. [DOI] [PubMed] [Google Scholar]
- 17.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21(Suppl 1):S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 19.Soneji N, Clarke HA, Ko DT, Wijeysundera DN. Risks of Developing Persistent Opioid Use After Major Surgery. JAMA Surg. 2016 doi: 10.1001/jamasurg.2016.1681. [DOI] [PubMed] [Google Scholar]
- 20.Lloret-Linares C, Lopes A, Decleves X, Serrie A, Mouly S, Bergmann JF, et al. Challenges in the optimisation of post-operative pain management with opioids in obese patients: a literature review. Obes Surg. 2013;23(9):1458–1475. doi: 10.1007/s11695-013-0998-8. [DOI] [PubMed] [Google Scholar]
- 21.Biegler JM, Freet CS, Horvath N, Rogers AM, Hajnal A. Increased intravenous morphine self-administration following Roux-en-Y gastric bypass in dietary obese rats. Brain Res Bull. 2016;123:47–52. doi: 10.1016/j.brainresbull.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926–935. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3(2):116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell JE, King WC, Courcoulas A, Dakin G, Elder K, Engel S, et al. Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Disord. 2015;48(2):215–222. doi: 10.1002/eat.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King WC, Chen JY, Courcoulas AP, Dakin G, Engel SD, Flum DR, et al. Alcohol and Other Substance Use after Bariatric Surgery: Prospective Evidence from a US Multicenter Cohort Study. SOARD. doi: 10.1016/j.soard.2017.03.021. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frendl DM, Ware JE., Jr Patient-reported functional health and well-being outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Med Care. 2014;52(5):439–445. doi: 10.1097/MLR.000000000000010311. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Kosinski M, Keller SK. SF-36® physical and mental health summary scales: a user’s manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 28.Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S148–S153. [PubMed] [Google Scholar]
- 29.Brookings JB, Bolton B. Confirmatory factor analysis of the Interpersonal Support Evaluation List. Am J Community Psychol. 1988;16(1):137–147. doi: 10.1007/BF00906076. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1998;8:77–100. [Google Scholar]
- 31.King WC, Bond DS. The importance of pre and postoperative physical activity counseling in bariatric surgery. Exerc Sport Sci Rev. 2013;41(1):26–35. doi: 10.1097/JES.0b013e31826444e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43(4):913–928. [PubMed] [Google Scholar]
- 33.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weingarten TN, Sprung J, Flores A, Baena AM, Schroeder DR, Warner DO. Opioid requirements after laparoscopic bariatric surgery. Obes Surg. 2011;21(9):1407–1412. doi: 10.1007/s11695-010-0217-9. [DOI] [PubMed] [Google Scholar]
- 35.Narbro K, Agren G, Jonsson E, Naslund I, Sjostrom L, Peltonen M. Pharmaceutical costs in obese individuals: comparison with a randomly selected population sample and long-term changes after conventional and surgical treatment: the SOS intervention study. Arch Intern Med. 2002;162(18):2061–2069. doi: 10.1001/archinte.162.18.2061. [DOI] [PubMed] [Google Scholar]
- 36.Hojsted J, Ekholm O, Kurita GP, Juel K, Sjogren P. Addictive behaviors related to opioid use for chronic pain: a population-based study. Pain. 2013;154(12):2677–2683. doi: 10.1016/j.pain.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids Prescribed After Low-Risk Surgical Procedures in the United States, 2004–2012. JAMA. 2016;315(15):1654–1657. doi: 10.1001/jama.2016.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CW, Kelly JJ, Wassef WY. Complications of bariatric surgery. Curr Opin Gastroenterol. 2007;23(6):636–643. doi: 10.1097/MOG.0b013e3282f094b5. [DOI] [PubMed] [Google Scholar]
- 40.Puzziferri N, Roshek TB, III, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neilsen MW, Søndergaard B, Kjøller M, Hansen EH. Agreement between self-reported data on medicine use and prescription records vary according to method of analysis and therapeutic group. J Clin Epidemiol. 2008;61(9):919–24. doi: 10.1016/j.jclinepi.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Dowell D, Haegerich TM. Using the CDC Guideline and Tools for Opioid Prescribing in Patients with Chronic Pain. Am Fam Physician. 2016;93(12):970–972. [PMC free article] [PubMed] [Google Scholar]
- 43.Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med. 2016;374(13):1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 44.Zdziarski LA, Wasser JG, Vincent HK. Chronic pain management in the obese patient: a focused review of key challenges and potential exercise solutions. J Pain Res. 2015;8:63–77. doi: 10.2147/JPR.S55360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


