Abstract
Background
Programed cell death, including apoptosis, mitochondria-mediated necrosis, and necroptosis, is critically involved in ischemic cardiac injury, pathological cardiac remodeling, and heart failure progression. Whereas apoptosis and mitochondria-mediated necrosis signaling is well established, the regulatory mechanisms of necroptosis and its significance in the pathogenesis of heart failure remain elusive.
Methods
We examined the role of Traf2 (TNF receptor-associated factor 2) in regulating myocardial necroptosis and remodeling using genetic mouse models. We also performed molecular and cellular biology studies to elucidate the mechanisms by which Traf2 regulates necroptosis signaling.
Results
We identified a critical role for Traf2 in myocardial survival and homeostasis by suppressing necroptosis. Cardiac-specific deletion of Traf2 in mice triggered necroptotic cardiac cell death, pathological remodeling, and heart failure. Plasma TNFα level was significantly elevated in Traf2-deficient mice and genetic ablation of TNFR1 largely abrogated pathological cardiac remodeling and dysfunction associated with Traf2 deletion. Mechanistically, Traf2 critically regulates RIP1-RIP3-MLKL necroptotic signaling with the adaptor protein TRADD as an upstream regulator and TAK1 as a downstream effector. Importantly, genetic deletion of RIP3 largely rescued the cardiac phenotype triggered by Traf2 deletion, validating a critical role of necroptosis in regulating pathological remodeling and heart failure propensity.
Conclusions
These results identify an important Traf2-mediated, NFκB-independent, pro-survival pathway in the heart by suppressing necroptotic signaling, which may serve as a new therapeutic target for pathological remodeling and heart failure.
Keywords: necroptosis, signal transduction, cardiomyocyte, pathological remodeling, heart failure
Introduction
Loss of functional cardiomyocytes by cell death has been implicated in ischemic myocardial injury, pathological cardiac remodeling, and heart failure. While apoptosis has been well established as a highly regulated form of cell death, necrosis was thought to be an unregulated, passive form of cell death due to overwhelming physicochemical stress. Recent advances identified a regulated type of necrosis termed programmed necrosis or necroptosis.1 Induction of necroptosis requires the activation of RIP1 and RIP3 (receptor-interacting protein 1 and 3), which forms the necroptotic cell death complex termed necrosome.2-4 The activated necrosome then engages MLKL through RIP3-dependent phosphorylation. Phosphorylated MLKL oligomerizes and binds to phospholipids to disrupt membrane integrity, resulting in necroptotic cell death. 5-6 Necroptosis has been implicated in a number of pathological conditions such as ischemic injury, neurodegenerative diseases, viral infection and inflammation. However, the regulation and significance of necroptosis in the heart remain poorly understood. Moreover, whether and how necroptosis can be targeted for the treatment of myocardial remodeling and heart failure has not been investigated.
Apoptosis and necroptosis are induced via specific death receptors such as tumor necrosis factor receptor 1 (TNFR1), among other modules. The pleiotropic nature of TNFR1 signaling results from the formation of different signaling complexes.7-8 Under normal conditions, ligation of TNFR1 induces the assembly of a plasma membrane bound signaling complex, termed complex I, which contains TNF receptor associated-protein with death domain (TRADD), TNF receptor associated protein 2 (TRAF2), receptor-interacting protein 1 (RIP1), and cellular inhibitor of apoptosis protein 1 and 2 (cIAP1 and cIAP2).9 The recruitment of TGFβ-activated kinase 1 (TAK1) and the IκB kinase (IKK) complex to complex I leads to the activation of NFκB. Under cell death inducing conditions, the TNFR1 complex internalizes and converts to a cell death-inducing complex, termed complex II, with additional recruitment of Fas-Associated protein with Death Domain (FADD) and caspase 8.9 TNFR1 signaling can also induce the necroptotic cell death complex, termed necrosome, consisting of RIP1, RIP3, and FADD, which often occurs when apoptosis is blocked.10
Traf2 is a key component of the TNFR1 signaling complex, which is recruited to TNFR1 by interacting with the adaptor protein TRADD.11 Like other TRAF proteins, Traf2 has a conserved TRAF-C domain, a coiled coil TRAF-N domain, and an N-terminal RING E3 ubiqutin ligase domain.12 Traf2 plays a pivotal role in transducing signals from receptors of the TNFR superfamily and the interleukin-1 /Toll-like receptor superfamily, regulating cell proliferation, inflammation, immune response as well as cell survival and apoptosis. In addition to transducing TNFα-induced NFκB activation, Traf2 can regulate apoptotic cell death independent its role in NFκB activation,13 but the effector mechanisms remain unclear. Traf2 also mediates mitochondrial autophagy, which confers protection against hypoxia/reoxygenation injury in cardiomyocytes.14 Importantly, recent studies indicate that Traf2 plays a role in necroptotic cell death induced by several death ligands including TNFα, Apo2L/TRAIL, and Fas/CD95L.15,16 However, the molecular mechanisms by which Traf2 regulates necroptosis remain elusive.
Genetic deletion of Traf2 in mice led to early lethality with severe developmental and immune defects and increased cellular sensitivity to TNFα killing.17 It has been shown that mice with cardiac-restricted expression of low levels of Traf2 were protected against ischemia-reperfusion injury.18 However, transgenic mice expressing high levels of Traf2 developed adverse remodeling and heart failure.19 The necessary role of Traf2 in myocardial survival and cardiac homeostasis has not been investigated by a loss-of-function approach. In this study, we identified a critical role for Traf2 in myocardial survival and homeostasis by suppressing both apoptosis and necroptosis, independent of the NFκB signaling. Cardiac-specific deletion of Traf2 in mice promoted apoptotic and necroptotic cardiac cell death, which led to pathological remodeling and heart failure. We also provide genetic evidence that ablation of TNFR1 or RIP3 largely rescued the pathological phenotype associated with Traf2 deficiency in vivo, revealing a crucial role of the TNFR1-mediated, RIP3-dependent necroptotic signaling pathway in the heart.
Methods
A detailed Methods section is available in the Online Data Supplement.
Animal models
The generation of Traf2 loxP-targeted (floxed) mice was described previously.20 Traf2fl/fl mice were crossed with αMHC-Cre21 or βMHC-Cre22. Tnfrsf1a-/- mice were obtained from the Jackson Laboratory. Ripk3-/- mice were provided by Dr. Vishva M. Dixit from Genentech, Inc. All experiments involving animals were approved by the Institutional Animal Care and Use Committees of the University of Washington and all studies were carried out in accordance with the approved guidelines.
Echocardiography, TAC, MI, and measurement of HMGB1, cTnI, and TNFα plasma levels
Echocardiography was performed with a VisualSonics Vevo 2100 imaging system as described previously.23 Transverse aortic constriction (TAC) and myocardial infarction (MI) were performed as previously described.24,25 Mouse HMGB1 plasma levels were measured using an enzyme-linked immunosorbent assay kit from Chondrex.23 cTnI plasma levels were measured using an ELISA kit from Life Diagnostics, Inc. TNFα plasma levels were measured using a TNF-alpha Quantikine ELISA kit from R&D Systems.
Cell culture, Adenoviral and lentiviral vectors, and cell death analysis
Primary neonatal rat cardiomyocytes were prepared from hearts of 1- to 2-day-old Sprague-Dawley rat pups as previously described.23,24 Traf2+/+ and Traf2-/- MEFs were from Tak Mak (University Health Network, Canada). Adβgal and AdTAK1-ΔN have been described previously.26 Adenoviruses encoding mouse Traf2 were generated using the ViraPower Adenoviral Expression System (Invitrogen). Lentiviral particles encoding shRNA sequences for specific target genes were obtained from Sigma. Cell death was measured as we previously described.23
Statistics
Sample size was estimated before performing the study based on our previous experience with similar studies or by conducting pilot experiments to estimate effect size followed by power analysis (α=0.05; power=80%). Results are presented as mean ± s.e.m. Mann-Whitney U-test or Kruskal-Wallis test followed by post-hoc Mann-Whitney U-test with Bonferroni's correction was used for studies with small sample sizes. Some data were analyzed by Friedman test followed by pairwise comparisons with post hoc Wilcoxon signed-rank test. Data groups with normal distribution were evaluated by one-way ANOVA with the Bonferroni's post hoc test. P < 0.05 was considered statistically significant.
Results
Loss of Traf2 in the heart induces cardiomyocyte death, pathological cardiac remodeling, and dysfunction
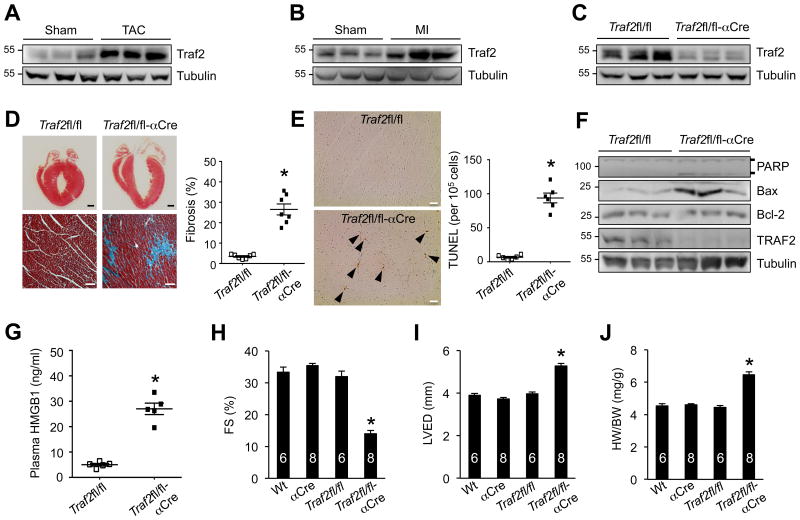
To assess the role of Traf2 in the heart, we first examined cardiac Traf2 expression following pathological stress. Intriguingly, Traf2 expression was markedly increased in the mouse heart subjected to TAC and MI injury for 2 weeks (Figure 1A,B), suggesting a potential role of Traf2 in myocardial response to pathological stress. To study the function of Traf2 in the heart in vivo, we generated mice with cardiomyocyte-specific deletion of Traf2. Mice homozygous for the Traf2-loxp (fl)-targeted allele (Traf2fl/fl)20 were crossed with cardiomyocyte-specific Cre lines, including α-myosin heavy chain α-MHC-Cre21 and β-MHC-Cre22. Western blot analysis showed efficient deletion of Traf2 (> 90%) in the heart from the Traf2fl/fl-αMHC-Cre mice (Figure 1C). Histological analysis of cardiac sections of Traf2fl/fl-αMHC-Cre mice revealed high levels of myocardial fibrosis and cardiomyocyte dropout (Figure 1D). A significant increase in TUNEL-positive cells was also detected in Traf2-deficient mice (Figure 1E). Consistent with this, Western blot analysis showed increased PAPR cleavage and up-regulated Bax expression in the Traf2-deficient heart compared with Traf2fl/fl controls, indicating increased apoptosis (Figure 1F). Importantly, increased plasma levels of high mobility group box 1 (HMGB1, Figure 1G), a new biomarker for necrotic cell death and myocardial infarction,27,28 was also detected in Traf2-deficient mice. Therefore, loss of Traf2 promoted apoptotic and necrotic cardiac cell death in vivo, suggesting an essential role for Traf2 myocardial survival and homeostasis.
Figure 1. Loss of Traf2 in the heart induces cardiomyocyte death, pathological remodeling and cardiac dysfunction.
A, Western blotting for Traf2 and a-tubulin (loading control) in cardiac extracts from 2 months old wild-type mice subjected to TAC or sham surgery for 2 weeks. B, Western blotting for Traf2 and a-tubulin in cardiac extracts from 2 months old wild-type mice subjected to MI or sham surgery for 2 weeks. C, Western blotting for Traf2 and a-tubulin in cardiac extracts from Traf2fl/fl and Traf2fl/fl-αMHC-Cre mice at 2 months of age. D, Masson's trichrome-stained, paraffin-embedded sections from the hearts of Traf2fl/fl and Traf2fl/fl-αMHC-Cre mice. Myocardial fibrosis was determined by MetaMorph software. *P < 0.01 versus Traf2fl/fl. N = 7 per group. Scale bars, top: 1 mm; bottom: 50 μm. E, TUNEL-positive nuclei of paraffin-embedded sections from the hearts of Traf2fl/fl and Traf2fl/fl-aCre mice. *P < 0.05 versus Traf2fl/fl. N = 6 per group. Scale bars, 10 μm. F, Western blotting for the indicated proteins from cardiac extracts of the indicated mice. G, Plasma HMGB1 levels from Traf2fl/fl and Traf2fl/fl-aCre mice. *P < 0.05 versus Traf2fl/fl. N = 5 per group. H and I, Echocardiographic assessment of fractional shortening (FS) and left ventricular dimension in diastole (LVED) in the indicated mice at 2 months of age. *P < 0.05 versus Wt, aCre, or Traf2fl/fl. J, Heart weight to body weight ratio (HW/BW) of the indicated mice. *P < 0.05 versus Wt, aCre, or Traf2fl/fl. Mann-Whitney U-test was used in D, E, G. Kruskal-Wallis test followed by post-hoc Mann-Whitney U-test with Bonferroni's correction was performed in H-J.
Importantly, Traf2fl/fl-αMHC-Cre mice developed significant cardiac dysfunction, ventricular dilation, and cardiac hypertrophy as compared to the littermate controls (Figure 1H-J, Supplemental Figure 1). To confirm these results, we also crossed Traf2fl/fl mice with another cardiac-specific β-MHC-Cre line (Supplemental Figure 2). Traf2fl/fl-βMHC-Cre mice again developed severe cardiac dysfunction, ventricular dilation, cardiac hypertrophy, and fibrosis (Supplemental Figure 2). These results indicate that ablation of Traf2 promotes pathological cardiac remodeling and dysfunction, further suggesting a protective role of Traf2 in the heart.
We further investigated the role of Traf2 in regulating myocardial remodeling and heart failure propensity following pathological stimulation. Here we used mice heterozygous for Traf2-loxP allele with αMHC-Cre (Traf2fl/+αCre) to evaluate the role of Traf2 in MI-induced pathological remodeling and heart failure. In contrast to the Traf2fl/fl-αCre mice, Traf2fl/+αCre mice were overtly normal at baseline, with no signs of cardiac dysfunction (Supplemental Figure 3). However, Traf2fl/+αCre mice showed a greater propensity for cardiac dysfunction and ventricular dilation following MI, which was associated with elevated myocardial cell death assessed by TUNEL staining and plasma HMGB1 levels (Supplemental Figure 3). An increase in infarct size as well as cTnI plasma levels was also detected after acute MI for 24 h (Supplemental Figure 3). Thus, reduced Traf2 expression predisposed mice to cardiac dysfunction and failure after MI, suggesting a critical cardio-protective role for Traf2 in response to pathological stress.
Ablation of TNFR1 prevents the pathological cardiac remodeling and dysfunction in Traf2-deficient mice
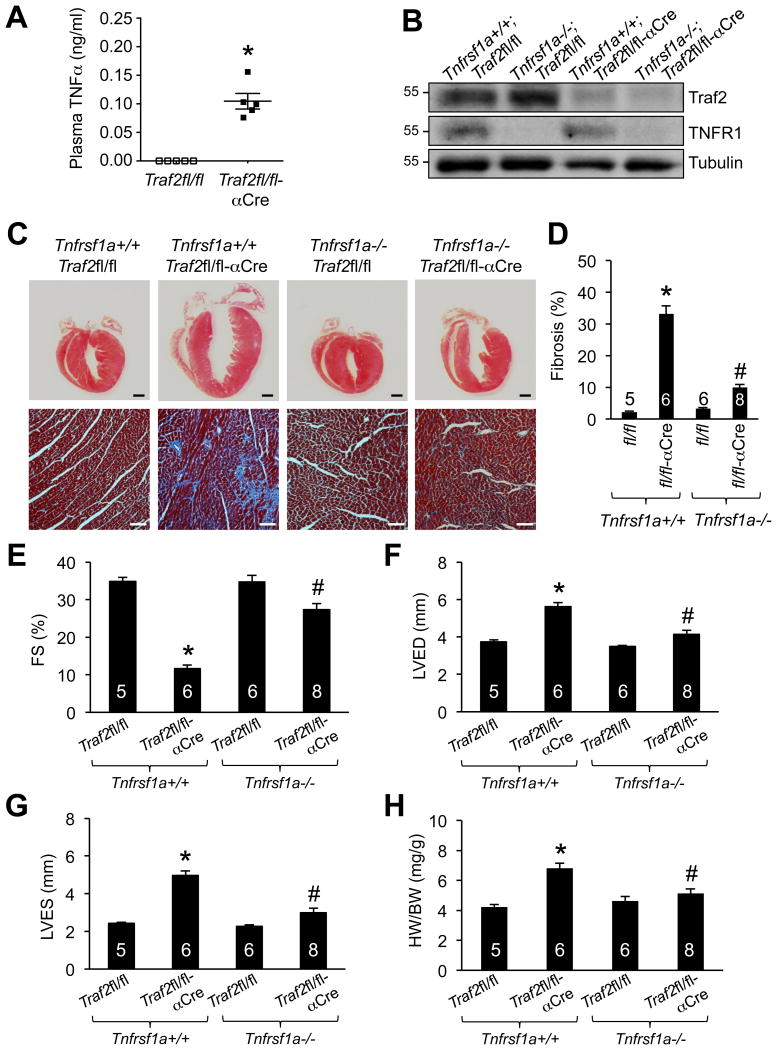
TNFR1 is an important death receptor that has been implicated in the pathogenesis of myocardial remodeling and heart failure.29 Traf2 can be recruited to the TNFR1 to form the complex I and activates downstream signaling.11 Here, we detected a significant increase in the plasma level of TNFα in Traf2-deficient mice (Figure 2A). These observations prompted us to investigate the role of TNFR1-mediated cell death pathway in pathological remodeling and heart failure in Traf2-deficient mice. To explore this, we crossed Tnfrsf1a-/- mice with Traf2fl/fl-αCre mice (Figure 2B). Strikingly, deletion of TNFR1 largely rescued the pathological phenotype of Traf2fl/fl-αCre mice by preventing cardiac fibrosis, contractile dysfunction, ventricular dilation, and cardiac hypertrophy (Figure 2C-H). Moreover, ablation of TNFR1, but not TNFR2, effectively blocked TNF-induced, RIP3-dependent cell death in Traf2-deficient cardiomyocytes in vitro (Supplemental Figure 4). These data suggest that TNFR1 signaling plays a critical role in the development of adverse cardiac remodeling and dysfunction triggered by Traf2 deficiency.
Figure 2. Ablation of TNFR1 prevents the pathological cardiac remodeling and dysfunction in Traf2-deficient mice.
A, Plasma TNFα levels from Traf2fl/fl and Traf2fl/fl-aCre mice at 2 months of age. *P < 0.05 versus Traf2fl/fl. N = 5 per group. B, Western blotting for the indicated proteins in cardiac extracts from mice of the indicated genotypes at 2 months of age. C, Masson's trichrome-stained, paraffin-embedded cardiac sections from mice of the indicated genotypes. Scale bars, top: 1 mm; bottom: 50 μm. D, Myocardial fibrosis quantified by MetaMorph software. *P < 0.01 versus Tnfrsf1a+/+Traf2fl/fl. #P < 0.05 versus Tnfrsf1a+/+Traf2fl/fl-aCre. E-G, Fractional shortening (FS), left ventricular dimension in diastole (LVED) and systole (LVES) determined by echocardiography in mice of the indicated genotypes. *P < 0.05 versus Tnfrsf1a+/+Traf2fl/fl; #P < 0.05 versus Tnfrsf1a+/+Traf2fl/fl-aCre. H, Heart weight to body weight ratio (HW/BW) of mice of the indicated genotypes. *P < 0.05 versus Tnfrsf1a+/+Traf2fl/fl; #P < 0.05 versus Tnfrsf1a+/+Traf2fl/fl-aCre. The number of mice analyzed is shown in the bars of each panel. Mann-Whitney U-test was performed in A. Kruskal-Wallis test followed by post-hoc Mann-Whitney U-test with Bonferroni's correction was performed in D-H.
Traf2 is a nodal regulator of apoptotic and necrotic cell death
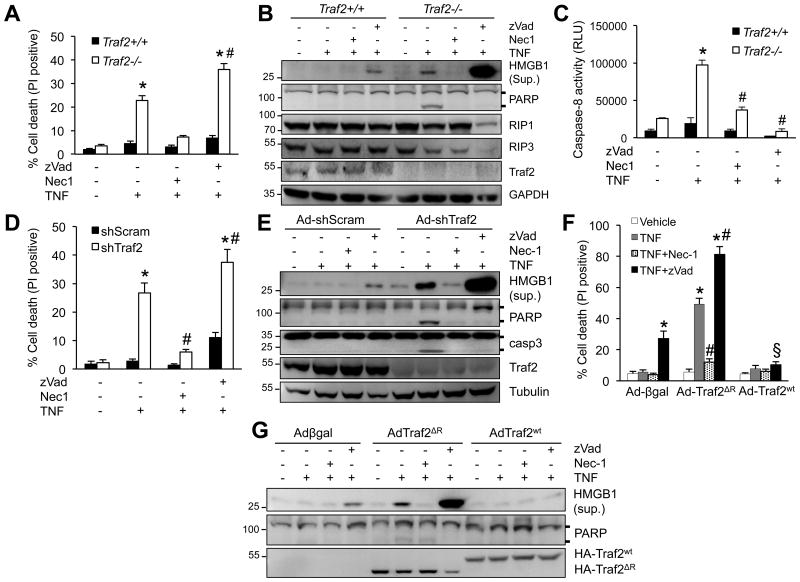
Based on data presented above, we hypothesized that deletion of Traf2 triggers adverse cardiac remodeling and heart failure by promoting TNFα-mediated cell death signaling. Indeed, deletion of Traf2 in mouse embryonic fibroblasts (MEFs) induced a rapid cell death with propidium iodide (PI) uptake upon TNFα stimulation (Figure 3A). This effect was largely blocked by the RIP1 inhibitor necrostatin-1 (Nec-1),30 but not by the pan-caspase inhibitor z-Vad-fmk (zVad), suggesting the induction of necroptosis. Moreover, HMGB1, a biomarker for necrosis, was released into the culture supernatants of Traf2-/- MEFs upon TNFα stimulation, which was further increased by the addition of zVad (Figure 3B). Deletion of Traf2 also promoted TNFα-induced PARP cleavage and caspase 8 activation, an effect that was reversed by both Nec-1 and zVad (Figures 3B,C). These results indicate that inhibition of TAK1 promotes apoptotic and necroptotic signaling through the activation of RIP1 and caspases. Next, we examined whether Traf2 regulates apoptosis and/or necroptosis in cardiomyocytes by silencing Traf2 with an adenoviral vector. Consistent with the results in Traf2-/- MEFs, ablation of Traf2 similarly promoted TNFα-induced apoptosis and necroptosis in cardiomyocytes (Figures 3D,E).
Figure 3. Traf2 is a nodal regulator of apoptotic and necrotic cell death.
A, Cell death assessed by propidium iodide (PI) staining of TRAF2+/+ and TRAF2-/- MEFs treated with vehicle control or 10 ng/ml TNFα for 4 h, in the presence or absence of Nec-1 (necrostatin-1; RIP1 inhibitor) or zVad (zVad-fmk; pan caspase inhibitor). *P < 0.01 versus Con; #P < 0.05 versus Traf2-/- TNF. B, Western blotting for the indicated proteins from TRAF2+/+ and TRAF2-/- MEFs treated as in A. Sup, supernatant; Lys, lysate. C, Caspase 8 activity in TRAF2+/+ and TRAF2-/- MEFs treated as in A for 2 h. *P < 0.01 versus Con; #P < 0.05 versus TNF only. D, Cell death assessed by PI staining of cardiomyocytes infected with an adenovirus expressing Traf2 shRNA (shTraf2) or a scrambled sequence (shScram), followed by 10 ng/ml TNFa or vehicle control for 4 hours, in the presence or absence of Nec-1 or zVad. *P < 0.05 versus Control; #P < 0.05 versus shTraf2 TNFa. E, Western blotting for the indicated proteins in cardiomyocytes infected with shScram or shTraf2 adenovirus, then treated as indicated for 4 h. F, Cell death in cardiomuocytes infected with adenoviruses encoding wild-type Traf2 (AdTraf2Wt), Traf2 lacking the RING domain (AdTraf2ΔR), or β-galactosidase control (Adβgal), then treated as indicated for 4 h. *P < 0.05 versus Vehicle; #P < 0.05 versus Ad-Traf2ΔR TNF; §P < 0.05 versus Ad-Traf2ΔR TNF+zVad. G, Western blotting for the indicated proteins in cardiomyocytes treated as in F. One-way ANOVA with Bonferroni's post-hoc test was used in A, C, D, F. Data were from at least 3 independent experiments with ≥ 900 cells per group analyzed for cell death. All immunoblot data represent at least three independent experiments with similar results.
To further examine the regulatory role of Traf2 in necroptosis, cardiomyocytes were infected with an adenovirus encoding wild-type Traf2 (Ad-Traf2), a dominant-negative Traf2 mutant lacking the RING E3 ubiquitin ligase domain (Ad-Traf2ΔR), or a control β-galactosidase adenovirus (Ad-βgal), followed by TNFα stimulation. Overexpression of wild-type Traf2 suppressed necroptotic cell death as well as HMGB1 release induced by TNFα plus zVad (Figure 3F,G). In contrast, overexpression of Traf2ΔR promoted TNFα-induced necroptosis, which was blocked by Nec-1 but augmented by zVad (Figure 3F,G). Thus, the RING E3 ubiquitin ligase domain of Traf2 plays a critical role in cell survival and necroptosis inhibition, probably through ubiquitination of key necroptosis signaling proteins such as TAK1 and RIP1 (see below).
Traf2 has been shown to mediate TNFα-induced NFκB activation, a transcription factor that drives the expression of pro-survival genes. However, consistent with previous studies,31,32 we observed that deletion of Traf2 in MEFs or overexpression of Traf2ΔR in cardiomyocytes had minimal effects on TNFα-induced NFκB activation, as assessed by phosphorylation and degradation of IκB (Supplemental Figure 5). To further evaluate the role of NFκB in TNFα-induced necroptosis, cardiomyocytes were infected with an adenovirus encoding the non-degradable IκBα mutant (IκBα-S32/36A; Ad-IκBαM) that completely blocked NFκB activity,33 in the presence of Ad-Traf2ΔR followed by TNFα stimulation. TNFα stimulation for 4 h failed to induce necroptosis or HMGB1 release in Ad-IκBαM-infected cells (Supplemental Figure 5). On the other hand, TNFα induced robust necroptosis and HMGB1 release in Ad-Traf2ΔR infected cells, which was not altered by NFκB inhibition (Supplemental Figure 5). These data indicate that Traf2 regulates TNF-induced necroptosis, at least in the acute phase, through an NFκB-independent mechanism.
Traf2 regulates necroptotic cell death signaling through TAK1 and TRADD
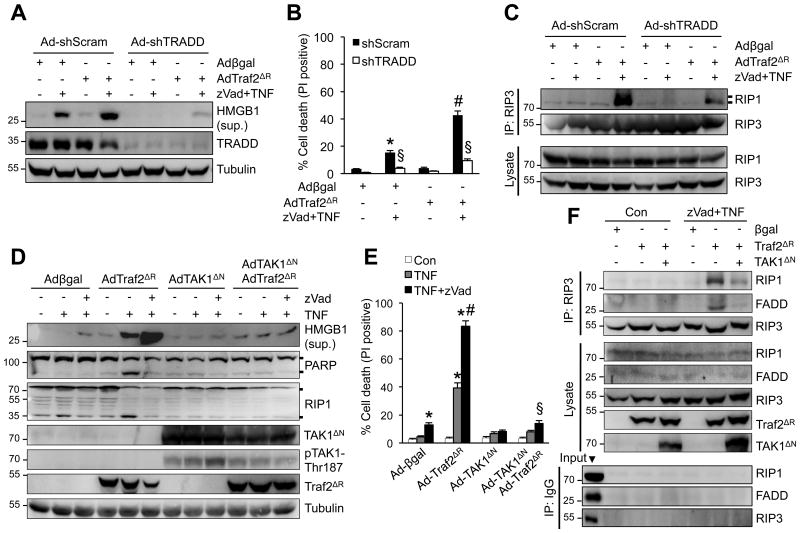
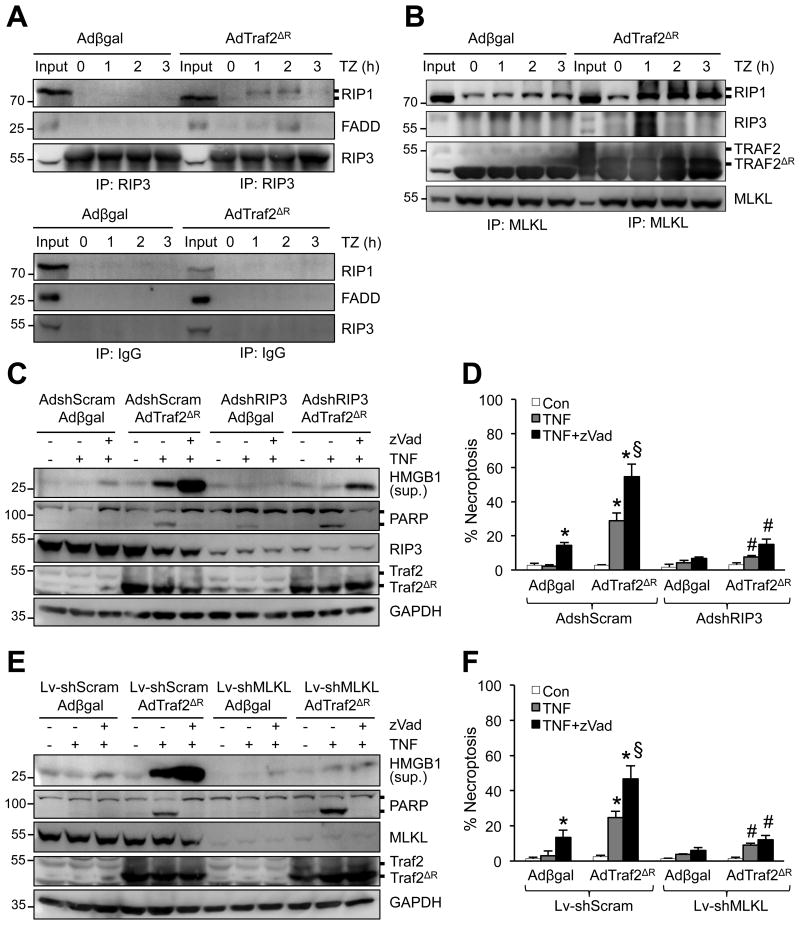
To investigate the underlying mechanisms, we examined the role of several key components of TNFR1 signaling pathway in necroptotic cell death triggered by Traf2 inhibition. The adapter protein TRADD mediates the recruitment of Traf2 to TNFR1, which is critical for the induction of downstream signaling complexes.11 However, the role of TRADD in necroptotic signaling remains unclear. Cardiomyocytes were infected with adenoviral vectors encoding TRADD shRNA (Ad-shTRADD) or a scrambled sequence (Ad-shScram) along with Adβgal or AdTraf2ΔR, followed by stimulation with TNFα plus zVad. Importantly, ablation of TRADD largely blocked necroptotic cell death as well as HMGB1 release induced by TNFα plus zVad in Ad-Traf2ΔR-infected cells (Figure 4A,B). In addition, knockdown of TRADD also largely abrogated the RIP1-RIP3 necrosome formation (Figure 4C). Therefore, these data indicate an indispensable role for the adaptor protein TRADD in necroptotic signaling upstream of Traf2.
Figure 4. Traf2 regulates necroptotic cell death signaling through TAK1 and TRADD.
A, Western blotting for the indicated proteins from cardiomyocytes infected with adenoviruses encoding TRADD shRNA (Ad-shTRADD) or a scrambled sequence (Ad-shScram) along with Adβgal or AdTraf2ΔR then treated with zVad plus TNF for 4 h. B, Cell death assessed by PI staining of cardiomyocytes treated as in A for 4 h. *P < 0.05 versus vehicle control; #P < 0.05 versus Adβgal zVad+TNF; §P < 0.05 versus shScram zVad+TNF in the corresponding group. C, Western blotting following IP with an anti-RIP3 antibody from extracts of cardiomyocytes treated as in A for 1 h. D, Western blotting for the indicated proteins from cardiomyocytes infected with Adβgal or AdTraf2ΔR along with an adenovirus expressing a constitutively active TAK1 mutant (AdTAK1ΔN), then treated with vehicle control or 10 ng/ml TNFa for 4 h with or without zVad. E, Cell death in cardiomyocytes treated as in D. *P < 0.05 versus vehicle control (Con); #P < 0.05 versus AdTraf2ΔR TNF; §P < 0.05 versus Ad-Traf2ΔR TNF+zVad. F, Western blotting following IP with an anti-RIP3 antibody (top) or pre-immune IgG control (bottom) from extracts of cardiomyocytes infected with Adβgal, AdTraf2ΔR, or AdTAK1ΔN, then treated with vehicle control or zVad plus TNFa for 1 h. One-way ANOVA with Bonferroni's post-hoc test was used in B and E. Data were from at least 3 independent experiments with ≥ 900 cells per group analyzed for cell death.
Traf2 has been shown to critically regulate polyubiquitination and activation of TAK1,34-36 an important regulator of necroptosis.23 To determine if TAK1 acts as an effector downstream of Traf2 in necroptotic signaling, we examined if forced activation could affect necroptotic signaling in Traf2-deficient cells. Cardiomyocytes were infected with an adenovirus encoding a constitutively active TAK1 mutant (Ad-TAK1ΔN) in the presence or absence of AdTraf2ΔR, followed by stimulation with TNFα and zVad. Indeed, TAK1ΔN overexpression largely blocked necroptotic cell death and HMGB1 release induced by TNFα plus zVad in AdTraf2ΔR-infected cells (Figure 4D,E). TAK1ΔN overexpression was associated with auto-phosphorylation on Thr187, indicating kinase activation.26 Forced activation of TAK1 also inhibited the necrosome formation by inhibiting RIP1-RIP3-FADD interaction (Figure 4F). Therefore, TAK1 activation is sufficient to inhibit necroptotic signaling triggered by Traf2 inhibition, suggesting that TAK1 may act downstream of Traf2 in necroptotic signaling.
CYLD is a deubiquitinating enzyme that has be shown to counteract with the effect of Traf2 in regulating protein ubiquitination such as TAK1 and RIP1.35,37 To examine if and how CYLD affects necroptotic signaling triggered by Traf2 inhibition, cardiomycytes were infected with an adenovirus expressing CYLD shRNA in the presence or absence of AdTraf2ΔR, followed by stimulation with TNFα and zVad. Surprisingly, ablation of CYLD had no significant effect on necroptotic cell death or HMGB1 release induced by Traf2 inhibition (Supplemental Figure 6). Moreover, overexpression of wild-type CYLD by adenoviral vectors didn't affect necroptosis induced by Traf2 inactivation (Guo X, et al., unpublished data). These data suggest that CYLD is dispensable for necroptotic signaling in the setting of Traf2 inhibition.
Loss of Traf2 promotes necroptotic signaling through RIP1-RIP3-MLKL
To investigate the molecular mechanism by which Traf2 regulates necroptosis, we assessed whether Traf2 exerts its effects on necroptotic signaling by regulating the RIP1-RIP3-FADD necrosome formation. Inactivation of Traf2 by Ad-Traf2ΔR markedly promoted the interaction of RIP3 with RIP1 and FADD (Figure 5A). It has been suggested that the activated necrosome further engages MLKL, which oligomerizes and forms pores in the plasma membrane to initiate necroptotic cell death.5,6 Indeed, overexpression of Traf2ΔR also promoted the RIP1-RIP3-MLKL interaction induced by TNFα plus zVad (Figure 5B). Of note, both endogenous Traf2 and Traf2ΔR mutant constitutively interact with MLKL, which was not altered by TNFα plus zVad (Figure 5B).
Figure 5. Loss of Traf2 promotes necroptotic signaling through RIP1-RIP3-MLKL.
A and B, Western blotting for the indicated proteins following IP with anti-RIP3 (A, top panel), anti-MLKL (B), or pre-immune IgG control (A, bottom panel) from extracts of cardiomyocytes infected with Adβgal or AdTraf2ΔR, then treated with TNFa plus zVad for 0-3 h. C, Western blotting for the indicated proteins from cardiomyocytes infected with the indicated adenoviral vectors then treated with vehicle control, TNF, or TNF+zVad for 4 h. D, Quantification of necroptosis (PI positive without chromatin condensation) from cells treated as in C. *P < 0.05 versus vehicle control; #P < 0.05 versus Ad-shScram in the corresponding group; §P < 0.05 versus Ad-shScram Adβgal TNF+zVad. E, Western blotting for the indicated proteins from cardiomyocytes infected with the indicated adenoviral and lentiviral vectors then treated with vehicle control, TNF, or TNF+zVad for 4 h. F, Quantification of necroptosis (PI positive without chromatin condensation) from cells treated as in E. *P < 0.05 versus vehicle control; #P < 0.05 versus Lv-shScram in the corresponding group; §P < 0.05 versus Ad-shScram Adβgal TNF+zVad. Data were from at least 3 independent experiments with ≥ 900 cells per group analyzed for cell death. One-way ANOVA with Bonferroni's post-hoc test was performed in D and F.
Consistent with the data above, ablation of RIP3 largely abolished HMGB1 release as well as necroptotic cell death triggered by Traf2 inhibition (Figure 5C,D). Interestingly, ablation of RIP3 induced a mild increase in PARP cleavage and apoptotic cell death, which was blocked by zVad (Figure 5C, Supplemental Figure 7). On the other hand, zVad further increased HMGB1 release and necroptosis that was blocked by RIP3 knockdown (Figure 5C,D). Similarly, silencing of MLKL blocked TNFα-induced HMGB1 release and necroptotic cell death in Ad-Traf2ΔR-infected cells (Figure 5E,F). Deletion of MLKL also mildly increased PARP cleavage and apoptotic cell death (Figure 5E, Supplemental Figure 7). These data suggest a crosstalk between necroptosis and apoptosis that is delicately regulated by RIP3/MLKL and caspases.
Ablation of RIP3 rescues pathological cardiac remodeling and dysfunction in Traf2-deficient mice by inhibiting necroptosis in vivo
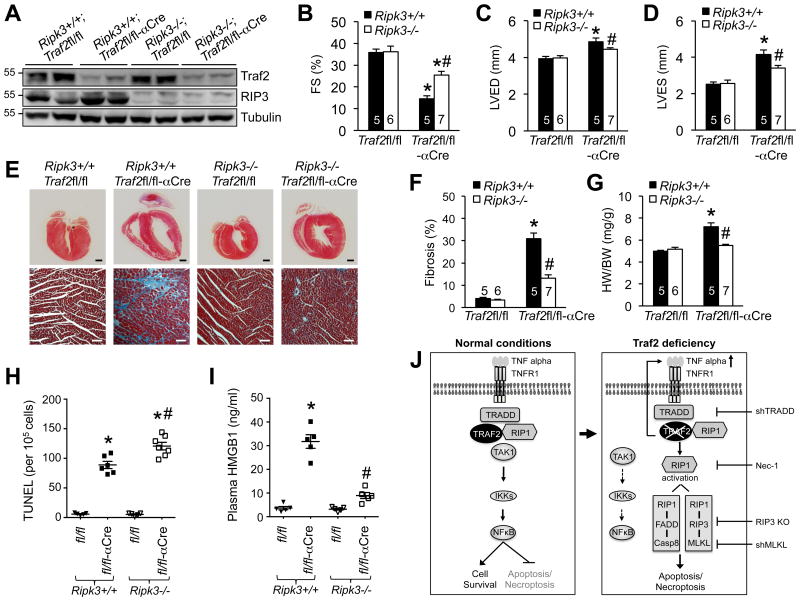
To directly assess whether necroptosis contributes to cardiac cell death and pathological remodeling in Traf2-deficient mice in vivo, we generated cardiac-specific Traf2 knockout mice on a Ripk3-/- background, by crossing Traf2fl/fl-αMHC-Cre mice with Ripk3-/- mice. Mice of different genotypes were confirmed by Western blot analysis of RIP3 and Traf2 expression in the heart (Figure 6A). Traf2fl/fl-αMHC-Cre mice on the Ripk3+/+ background again developed severe contractile dysfunction, ventricular dilation, fibrosis, and hypertrophy (Figure 6B-G). This was associated with a marked increase in TUNEL positive cells as well as plasma HMGB1, indicating elevated cardiac cell death (Figure 6H,I). Importantly, ablation of RIP3 largely reversed cardiac dysfunction, ventricular dilation and fibrosis, as well as hypertrophy in Traf2-deficient mice (Figure 6B-G). As expected, necrotic HMGB1 release was also abolished by RIP3 ablation (Figure 6I). Intriguingly, a mild increase of myocardial TUNEL staining was observed in Traf2-deficient mice on the Ripk3-/- background compared with those on the Ripk3+/+ background (Figure 6H), suggesting that blockade of necroptosis was associated a mild but significant increase in apoptosis. Moreover, ablation of RIP3 in cardiomyocytes also largely blocked cell death triggered by Traf2 deletion or inactivation (Supplemental Figure 4C; Figure 5C,D). Taken together, these data reveal a critical role of myocardial necroptosis in the development of cardiac remodeling and dysfunction in Traf2-deficient mice. These results also validate RIP3 as a therapeutic target for heart failure since its ablation prevented pathological remodeling and heart failure in a mouse model of myocardial necroptosis induced by Traf2 ablation.
Figure 6. Ablation of RIP3 rescues pathological cardiac remodeling and dysfunction in Traf2-deficient mice by inhibiting necroptosis.
A, Western blots for the indicated proteins in cardiac extracts from mice of indicated genotypes at 2 months of age. B-D, FS, LVED, and LVES in 2 months old mice of the indicated genotypes. *P < 0.01 versus Traf2fl/fl; #P < 0.05 versus Ripk3+/+Traf2fl/fl-αCre. E, Masson's trichrome-stained cardiac sections from mice indicated in B, C, and D. Scale bars, top: 1 mm; bottom: 50 μm. F, Myocardial fibrosis quantified by MetaMorph software. *P < 0.01 versus Traf2fl/fl; #P < 0.05 versus Ripk3+/+Traf2fl/fl-αCre. G, Heart weight to body weight ratios (HW/BW) in mice of the indicated genotypes. *P < 0.05 versus Traf2fl/fl; #P < 0.05 versus Ripk3+/+Traf2fl/fl-αCre. H, TUNEL positive myocytes in cardiac sections from mice indicated in B, C, and D. *P < 0.05 versus Traf2fl/fl; #P < 0.05 versus Ripk3+/+Traf2fl/fl-αCre. I, Plasma HMGB1 levels from mice indicated in B, C, and D. *P < 0.01 versus Traf2fl/fl; #P < 0.05 versus Ripk3+/+Traf2fl/fl-αCre. J, Proposed model: Traf2 functions as a nodal regulator in TNFR1-mediated apoptosis and necroptosis. Kruskal-Wallis test followed by post-hoc Mann-Whitney U-test with Bonferroni's correction was performed in B-I.
Discussion
This study identified a critical role for Traf2 in myocardial survival and homeostasis by suppressing apoptosis and necroptosis. We showed that mice with cardiac-specific deletion of Traf2 developed pathological remodeling and heart failure through the induction of apoptotic and necroptotic cardiac cell death. We also provided genetic evidence identifying TNFR1-mediated, RIP3-dependent necroptosis in the pathogenesis of adverse cardiac remodeling and heart failure in Traf2-deficient mice. Our results support a model that ablation of Traf2 promotes TNFR1-mediated apoptotic and necroptotic signaling via a feed-forward mechanism through increased TNFα production (Figure 6J). Inhibition of Traf2 promotes necroptotic cell death through an NFκB-independent, but RIP1-RIP3-MLKL dependent mechanism (Figure 6J). Mechanistically, the adaptor protein TRADD is required in TNFα-induced necroptosis signaling triggered by Traf2 ablation. TAK1 acts downstream of Traf2 to regulate RIP1-mediated cell death complex formation and apoptosis/necroptosis.
We observed that Traf2 expression in the heart was significantly up-regulated following pathological stress including pressure overload and myocardial infarction. Intriguingly, other cellular stress, such as UV irradiation or translational inhibition, promotes Traf2 degradation.38 Up-regulation of Traf2 may represent an important cardio-protective mechanism upon pathological stress. Indeed, it has been shown that mild overexpression of Traf2 provided protection against cardiac ischemic injury in transgenic mice.18 However, cardiac-specific overexpression of high levels of Traf2 provoked adverse remodeling and heart failure.19 Loss-of-function approaches may have greater biological relevance in elucidating the function of Traf2 signaling under physiological and pathological conditions. In this case, we showed that Traf2-deficient mice spontaneously developed dilated cardiomyopathy and heart failure with elevated cardiac cell death, thus validating a protective role of Traf2 in the heart. It has been shown that Traf2 and Traf5 play redundant roles in TNFα-induced NFκB activation.39 However, unlike Traf2, deletion of Traf5 has no effects on TNFα-induced cardiac cell death (Chen Y et al., unpublished data). Consistent with this, Traf5-/- mice showed no obvious abnormalities in contrast to early lethality and tissue necrosis found in Traf2-/- mice.17,40 Moreover, Traf3-/- and Traf6-/- mice showed distinct phenotypes compared with Traf2-/- mice, indicating Traf3 or Traf6 is not essential for cell survival.41,42 Thus, unlike other TRAF proteins, Traf2 is a dedicated regulator of cardiac cell survival/death and heart failure propensity.
We detected elevated plasma levels of TNFα in our cardiac-specific Traf2-deficient mice in vivo, consistent with a previous study using the global Traf2 knockout mice.17 It has been shown that deletion of Traf2 led to increased TNFα production in L929 cells in vitro, through a RIP1- and EDD-dependent mechanism.43 Elevated TNFα production may represent an important feed-forward mechanism to promote cell death in Traf2-deficient cells in vivo and in vitro. Importantly, ablation of TNFR1 largely blocked adverse remodeling and heart failure in Traf2-deficient mice, further indicating the dysregulated TNFα signaling contributes to the pathological cardiac phenotype induced by Traf2 deficiency. Of note, genetic deletion of TNFR1 didn't fully rescue the pathological and functional defects in Traf2-deficient mice, suggesting the existence of additional TNFR1-independent mechanisms for cardiac cell death and disease development. It is possible that certain aspects of the pathological phenotype in Traf2-deficient mice could be mediated by other receptors (such as DR3 and DR6) or other pathological drivers (such as death ligands FasL or Apo2L/TRAIL).
We showed that Traf2 inhibition promoted TNFα-induced caspase activation and apoptosis, which were blocked by Nec-1, indicating the induction of RIP1-dependent apoptosis. The underlying mechanism probably involves RIP1-dependent induction of a caspse 8 activating complex consisting RIP1-FADD-caspase 833. Moreover, Traf2 inhibition also promoted necroptosis by inducing the RIP1-RIP3 necrosome. A significant decrease in RIP1 ubiquitination has been detected in the TNFR1 complex from Traf2-/- cells upon TNFα stimulation,32 which was reversed by reconstituting with wild-type, but not the RING domain-deleted form of Traf2. Decreased RIP1 ubiquitination, which promotes RIP1 activation and RIP1-RIP3 interaction,4,44 may account for the enhanced necrosome formation in Traf2-deficient cells. In line with this, we showed that overexpression of wild-type Traf2 inhibited, but Traf2 lacking RING domain (Traf2ΔR) promoted, TNFα-induced necroptosis in cardiomyocytes. However, Petersen et al.15 reported that transfection of Traf2-/- MEFs with Traf2ΔR suppressed TNFα-induced necroptosis as effectively as reconstitution with wild-type Traf2. The reason for this obvious discrepancy is unclear, potentially caused by different cell lines used (cardiomyocytes vs. MEFs) and different necroptosis-inducing conditions (TNF+zVad vs. TNF+cylcoheximide+zVad). Nonetheless, we provided further evidence showing that the RING domain of Traf2 is critical in suppressing necrosome formation and necroptotic cell death through a RIP1-RIP3-MLKL dependent mechanism. Also of note, although ablation of MLKL abrogated necroptosis in Traf2-deficient cells, we observed a constitutive interaction between MLKL and Traf2 (both wild-type and ΔR), which was not altered by necroptosis induced by TNFα plus zVAD (without cycloheximide). Thus, our data didn't support the model proposed by Petersen et al.15 where TNFα-induced necroptosis disrupts Traf2-MLKL interaction to promote RIP3-MLKL binding. Those effects observed by Petersen et al.15 could be caused by enhanced degradation Traf2 induced by TNFα and cycloheximide treatment and thus decreased Traf2-MLKL association.38 Our data clearly indicate that Traf2-MLKL dissociation is dispensable for necroptotic signaling under certain necroptosis-inducing conditions (e.g., without cycloheximide).
Ablation of Traf2 in the heart led to pathological myocardial remodeling and heart failure, which was largely rescued by genetic deletion of Ripk3, suggesting that unchecked myocardial necroptosis contributed significantly to the observed the pathological phenotype in Traf2-deficient mice. However, the rescue of the pathological and functional cardiac defects in Traf2-deficient mice by Ripk3 deletion was not complete. Actually, Ripk3 deletion largely blocked necroptosis but mildly increased apoptosis in the Traf2-deficient heart. These results highlight the complexity of cell death regulation involving the crosstalk between apoptotic and necroptotic pathways. Thus, therapies targeting both apoptotic and necroptotic pathways may prove to be more effective under certain pathological conditions.
Our results also defined the roles of several key components of the TNFR1 signaling pathway, including TRADD, TAK1, and CYLD, in necroptotic cell death in the setting of Traf2 inhibition. TRADD binds directly to the death domain of TNFR1 and transduces signals both for NFκB activation and apoptosis,11 by recruiting distinct signaling proteins of the TNFR1 complex, including Traf2, RIP1, or FADD.11,45 Here we showed that deletion of TRADD prevented TNFα-induced necrosome formation and necroptotic cell death in the setting of Traf2 inhibition, thus revealing an essential role of TRADD in necroptotic signaling. Deletion of TRADD may inhibit necroptotic signaling by preventing the recruitment of RIP1 to TNFR1 complex I. Indeed, it has been shown that recruitment of RIP1 to the TNFR1 complex is required for its ability to subsequently interact with FADD or RIP3 to induce cell death complexes.46 These data indicate that TRADD, which acts upstream of Traf2, functions as a critical regulator of necroptotic signaling, in addition to its known role in NFκB activation and apoptosis. Moreover, Traf2 has been shown to regulate TAK1 activation through Lys63-linked ubiquitination in vitro and in vivo.35 Inhibition of Traf2 may cause defective TAK1 signaling that in turn promotes necroptosis. In supporting this, forced activation of TAK1 blocked necrosome formation and necroptotic cell death in Traf2-deficient cells, indicating that TAK1 exerts its anti-necroptotic effect by acting downstream of Traf2. CYLD has also been shown to function as a deubiquitinating enzyme for TAK1, RIP1, and Traf2.15,47,48 CYLD and Traf2 may play opposite roles in regulating protein ubiquitination as well as necroptotic signaling. In contrast to a previous study,15 our data showed that deletion of CYLD failed to block TNFα-induced necroptosis in Traf2-deficient cells. By comparison, we observed that CYLD is required for RIP1 kinase activation and subsequent necrosome formation in the setting of TAK1 inhibition.33 Therefore, CYLD doesn't seem to be critical in transducing necroptosis in the setting of Traf2 inhibition, although it is required for necroptotic signaling under certain conditions.
In conclusion, our results demonstrated that Traf2 functions as a critical pro-survival factor in the heart by suppressing both myocardial apoptosis and necroptosis. We provide novel evidence that inhibition of necroptosis by Traf2 is critical for myocardial homeostasis and the prevention of pathological remodeling and heart failure progression. Our data also identify several signaling proteins in the TNFR1 pathway (e.g., TRADD, Traf2, and TAK1) as critical regulators of necroptosis in cardiac myocytes. Targeting key components of the necroptotic signaling may represent a valid therapeutic strategy for pathological cardiac remodeling and heart failure.
Supplementary Material
Clinical Perspective.
What is new?
This study identified an important Traf2-mediated, NFκB-independent, pro-survival pathway in the heart by suppressing apoptosis and necroptosis.
This study defined novel molecular mechanisms whereby Traf2 suppresses TNFR1-mediated, RIP3-dependent necroptosis, which is critical for myocardial survival and homeostasis.
What are the clinical implications?
These findings suggest that the necroptosis-suppressing Traf2 signaling pathway and its effectors may serve as novel therapeutic targets for pathological cardiac remodeling and heart failure.
Acknowledgments
The authors want to thank Dr. Robert Brink from Centenary Institute of Cancer Medicine and Cell Biology, Australia, for providing Traf2fl/fl mice. We thank Dr. Jeffery D. Molkentin at Cincinnati Children's Hospital for providing αMHC-Cre and βMHC-Cre mice.
Sources of Funding: This work was supported by grants from the National Institutes of Health (R00HL0908076 and R01HL116507 to Dr Liu; T32HL007828 to Dr Yin).
Footnotes
Disclosures: None.
References
- 1.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 2.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 4.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 8.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 10.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 12.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 13.Natoli G, Costanzo A, Guido F, Moretti F, Bernardo A, Burgio VL, Agresti C, Levrero M. Nuclear factor kB-independent cytoprotective pathways originating at tumor necrosis factor receptor-associated factor 2. J Biol Chem. 1998;273:31262–31272. doi: 10.1074/jbc.273.47.31262. [DOI] [PubMed] [Google Scholar]
- 14.Yang KC, Ma X, Liu H, Murphy J, Barger PM, Mann DL, Diwan A. Tumor necrosis factor receptor-associated factor 2 mediates mitochondrial autophagy. Circ Heart Fail. 2015;8:175–187. doi: 10.1161/CIRCHEARTFAILURE.114.001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen SL, Chen TT, Lawrence DA, Marsters SA, Gonzalvez F, Ashkenazi A. TRAF2 is a biologically important necroptosis suppressor. Cell Death Differ. 2015;22:1846–1857. doi: 10.1038/cdd.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karl I, Jossberger-Werner M, Schmidt N, Horn S, 2, Goebeler M, Leverkus M, Wajant H, Giner T. TRAF2 inhibits TRAIL- and CD95L-induced apoptosis and necroptosis. Cell Death Dis. 2014;5:e1444. doi: 10.1038/cddis.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 18.Burchfield JS, Dong JW, Sakata Y, Gao F, Tzeng HP, Topkara VK, Entman ML, Sivasubramanian N, Mann DL. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail. 2010;3:157–164. doi: 10.1161/CIRCHEARTFAILURE.109.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Divakaran VG, Evans S, Topkara VK, Diwan A, Burchfield J, Gao F, Dong J, Tzeng HP, Sivasubramanian N, Barger PM, Mann DL. Tumor necrosis factor receptor-associated factor 2 signaling provokes adverse cardiac remodeling in the adult mammalian heart. Circ Heart Fail. 2013;6:535–543. doi: 10.1161/CIRCHEARTFAILURE.112.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem. 2004;279:26192–261200. doi: 10.1074/jbc.M313800200. [DOI] [PubMed] [Google Scholar]
- 22.Palermo J, Gulick J, Colbert M, Fewell J, Robbins J. Transgenic remodeling of the contractile apparatus in the mammalian heart. Circ Res. 1996;78:504–509. doi: 10.1161/01.res.78.3.504. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Chen Y, Doan J, Murray J, Molkentin JD, Liu Q. Transforming growth factor β-activated kinase 1 signaling pathway critically regulates myocardial survival and remodeling. Circulation. 2014;130:2162–2172. doi: 10.1161/CIRCULATIONAHA.114.011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Chen Y, Auger-Messier M, Molkentin JD. Interaction between NFκB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res. 2012;110:1077–1086. doi: 10.1161/CIRCRESAHA.111.260729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Chen Y, Li J, Yin H, Guo X, Doan J, Molkentin JD, Liu Q. TAK1 Regulates Myocardial Response to Pathological Stress via NFAT, NFκB, and Bnip3 Pathways. Sci Rep. 2015;5:16626. doi: 10.1038/srep16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Busby JC, Molkentin JD. Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat Cell Biol. 2009;11:154–161. doi: 10.1038/ncb1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 28.Andrassy M, Volz HC, Riedle N, Gitsioudis G, Seidel C, Laohachewin D, Zankl AR, Kaya Z, Bierhaus A, Giannitsis E, Katus HA, Korosoglou G. HMGB1 as a predictor of infarct transmurality and functional recovery in patients with myocardial infarction. J Intern Med. 2011;270:245–253. doi: 10.1111/j.1365-2796.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 29.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Blackwell K, Shi Z, Habelhah H. The RING domain of TRAF2 plays an essential role in the inhibition of TNFalpha-induced cell death but not in the activation of NF-kappaB. J Mol Biol. 2010;396:528–539. doi: 10.1016/j.jmb.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Yin h, Chen Y, Li L, Li J, Liu Q. TAK1 Regulates Caspase 8 Activation and Necroptotic Signaling via Multiple Cell Death Checkpoints. Cell Death Dis. 2016;7:e2381. doi: 10.1038/cddis.2016.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- 35.Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, Zhang H, Fu S, Qin J, Yang J. Lysine 63-linked Polyubiquitination of TAK1 at Lysine 158 Is Required for Tumor Necrosis Factor α- and Interleukin-1β-induced IKK/NF-κB and JNK/AP-1 Activation. J Biol Chem. 2010;285:5347–5360. doi: 10.1074/jbc.M109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 2003;22:6277–6288. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Habelhah H, Frew IJ, Laine A, Janes PW, Relaix F, Sassoon D, Bowtell DD, Ronai Z. Stress-induced decrease in TRAF2 stability is mediated by Siah2. EMBO J. 2002;21:5756–5765. doi: 10.1093/emboj/cdf576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, Yeh WC, Nakano H. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem. 2001;276:36530–16534. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- 40.Nakano H, Sakon S, Koseki H, Takemori T, Tada K, Matsumoto M, Munechika E, Sakai T, Shirasawa T, Akiba H, Kobata T, Santee SM, Ware CF, Rennert PD, Taniguchi M, Yagita H, Okumura K. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc Natl Acad Sci USA. 1999;96:9803–9808. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 42.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christofferson DE, Li Y, Hitomi J, Zhou W, Upperman C, Zhu H, Gerber SA, Gygi S, Yuan J. A novel role for RIP1 kinase in mediating TNFα production. Cell Death Dis. 2012;3:e320. doi: 10.1038/cddis.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K, Vucic D, Fulda S, Vandenabeele P, Bertrand MJ. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18:656–665. doi: 10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanlangenakker N, Bertrand MJ, Bogaert P, Vandenabeele P, Vanden Berghe T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2011;2:e230. doi: 10.1038/cddis.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, Leonard TO, Norbury CC, Fitzpatrick L, Zhang M, Sun SC. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.