Abstract
Background
Previous studies indicate that transcranial direct current stimulation (tDCS) with anode over motor cortex (M1) and cathode over contralateral supraorbital region (SO) may be effective in reducing pain, but these studies are limited in number and have not focused on older adults with osteoarthritis (OA).
Objective
To evaluate the preliminary efficacy and safety of M1-SO applied tDCS on clinical pain severity and mobility performance in adults with knee OA pain.
Methods
Forty 50- to 70-year-old community-dwelling participants with knee OA were randomly assigned to receive five daily sessions of 2 mA tDCS for 20 min (n = 20) or sham tDCS (n = 20). We measured clinical pain severity via Numeric Rating Scale, Western Ontario and McMaster Universities Osteoarthritis Index, and Short-Form McGill Pain Questionnaire. In addition, we measured mobility performance using the 6-Minute Walk Test and the Short Physical Performance Battery. Moreover, we obtained a sensation/safety questionnaire and measured cognition changes using the PROMIS-Applied Cognition-Abilities-Short Form 8a.
Results
Active tDCS over M1-SO significantly reduced Numeric Rating Scale of pain compared to sham tDCS after completion of the five daily sessions, and remained up to three weeks. No other measures were significantly different from sham. Participants tolerated tDCS over M1-SO well without serious adverse effects or cognition changes.
Conclusion
Although not consistent in all pain measurements, our findings demonstrate promising clinical efficacy for reduction in pain perception for older adults with knee OA.
Trial registration
ClinicalTrials.gov Identifier NCT02512393.
Introduction
Arthritis is a leading cause of pain, impairment of activities in daily life, and disability in people aged 45 years and above [1,2]. Of the 53 million adults diagnosed with arthritis, more than 22 million (42%) struggle with activities of daily living due to arthritis pain [3]. Osteoarthritis (OA) is the most common of the arthritic conditions, with the knee being the most commonly affected joint [2,4,5]. Patients with chronic pain, such as knee OA pain, often have insufficient pain relief [6]. Recent evidence suggests that OA pain may be characterized by generalized changes in pain and sensory processing in the central nervous system, similar to other chronic pain syndromes [7,8]. Because pharmacologic treatments are often inadequate and can lead to adverse events among older adults [9–11], there is a growing interest in non-pharmacologic interventions targeting central nervous system pain processing.
Specifically, noninvasive brain stimulation, such as transcranial direct current stimulation (tDCS), has received significant attention for the treatment of pain in chronic conditions owing to its neuromodulatory effect [12–15]. tDCS involves the application of weak direct electric current to the head in a noninvasive and painless manner, leading to the modulation of the resting membrane potentials of neurons and alteration of the endogenous excitability of the targeted brain tissue [16–18]. For pain, stimulation is typically delivered with the anode electrode placed over the primary motor cortex (M1) of the hemisphere contralateral to the pain-affected area of the body and with the cathode electrode placed over the supraorbital region (SO) ipsilateral to the affected area [15,19]. In particular, the European Chapter of the International Federation of Clinical Neurophysiology recommended that stimulation with anode over the M1 contralateral to pain side and cathode over SO contralateral to M1 placement for possible efficacy among populations with chronic pain [20]. This stimulation with anode over the M1 is believed to produce analgesic effects by modulating pain processing pathways [21,22], and recent brain imaging studies report a reliable cortical and subcortical neurophysiologic response to tDCS with anode over M1 and cathode over SO, referred to hereafter simply as M1-SO applied tDCS [23,24]. Previous studies indicated that M1-SO applied tDCS is effective in reducing pain in patients with fibromyalgia, multiple sclerosis, and traumatic spinal cord injury [12,14,25], but these studies are limited in number and have not focused on older adults or those with arthritis.
The efficacy of M1 -SO applied tDCS, for the treatment of pain in older adults remains an open question. Increased atrophy of brain gray and white matter is a hallmark of the aging process in the human brain [26–28]. Computational models suggest that differences in the cerebrospinal fluid space between the location of the electrodes on the scalp and the gray matter alters the intensity of current delivered to brain tissue [29,30]. Furthermore, changes in the structural and functional integrity of white and gray matter in aging may also affect the overall efficacy of electrical neuromodulation in older adults [31]. In addition, functional connectivity of brain networks is also thought to change with age [32–34]. These pose the possibility that M1-SO applied tDCS effects previously shown effective in younger populations may not translate to older adults. Thus, studies investigating the efficacy of M1-SO applied tDCS in older populations are needed. In the current study, we sought to evaluate the preliminary efficacy and safety of M1-SO applied tDCS to reduce clinical pain severity and improve mobility performance in older adults with knee OA.
Methods
Design
We conducted a single-center, experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study at the University of Florida Institute on Aging to evaluate the efficacy of five daily sessions of M1-SO applied tDCS on clinical pain severity and mobility performance in older persons with knee OA. The study included a total of 6 study visits (baseline evaluation and 5 consecutive daily sessions) and 3 weekly follow-up assessments. After undergoing a telephone screening for eligibility assessment, participants were scheduled for a baseline evaluation, which included the following: acquisition of written informed consent; determination of OA using the American College of Rheumatology criteria [35,36]; and a baseline evaluation of demographic and clinical characteristics including medications. Weight-bearing radiographs of both knees were taken for all participants, and the OA severity was determined using the Kellgren-Lawrence grading system [37] (Fig. 1). We chose a 3-week follow-up because M1-SO applied tDCS has been shown to induce modulatory effects for up to 3 weeks after the end of five daily stimulation sessions [12]. All procedures were approved by the Institutional Review Board of the affiliated university, and written informed consent was obtained from all participants before participation.
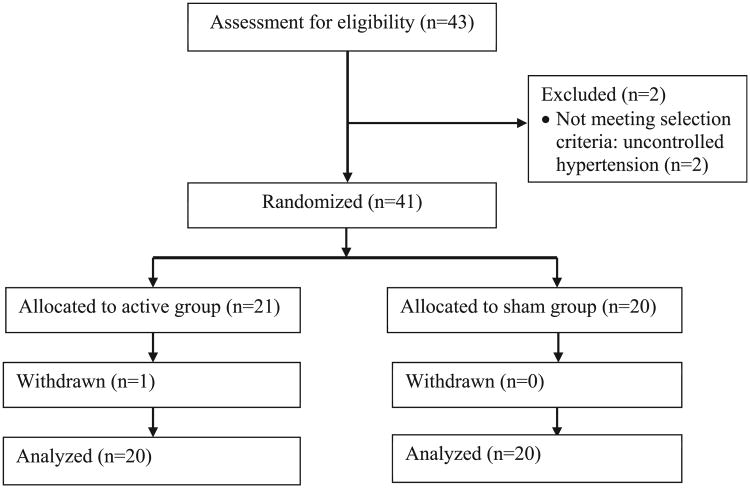
Fig. 1.
Participant flow diagram.
Randomization and blinding
Participants who met eligibility criteria were randomly assigned with a ratio of 1 to 1 to either the active tDCS (n = 20) or sham tDCS group (n = 20) using a covariate adaptive randomization procedure so that the two groups had approximately equal distribution regarding age, gender and race. Allocation concealment was ensured as the randomization codes were released only after all the interventions and assessments were completed.
We used a Soterix CT direct current stimulator (Soterix Medical Inc., NY) to deliver experimenter- and participant-blinded tDCS. The experimenter was blind to the condition, and entered a 6-digit code into the device to deliver stimulation. The participants were blinded with regard to the type of tDCS and they were aware of the fact that they could receive either sham or active stimulation. Only the statistician with no clinical involvement in this trial was able to unblind data at the completion of the study.
Study participants
Participants with knee OA pain were recruited in North Central Florida between September 2015 and August 2016 using advertisements in local institutions and communities. Participants who were 50–70 years old were considered eligible if they had self-reported unilateral or bilateral knee OA pain, according to American College of Rheumatology criteria [35,36]; could speak and read English; were willing to be randomly assigned to either the intervention or control group; were available for five consecutive daily sessions and for a follow-up phone assessment each week for three weeks; had no plan to change medication regimens for pain throughout the trial; and were willing and able to provide written informed consent prior to enrollment. Participants were excluded if they had concurrent medical conditions that could confound symptomatic OA-related outcome measures or coexisting diseases that could hinder the completion of the protocol, including: (1) prosthetic knee replacement or non-arthroscopic surgery to the affected knee, (2) serious medical illness, such as uncontrolled hypertension, heart failure, or history of acute myocardial infarction, (3) peripheral neuropathy, (4) systemic rheumatic disorders, including rheumatoid arthritis, systemic lupus erythematosus, and fibromyalgia, (5) alcohol/substance abuse, (6) cognitive impairment (i.e., Mini-Mental Status Exam score ≤ 23), (7) history of brain surgery, tumor, seizure, stroke, or intracranial metal implantation, (8) pregnancy or lactation, and (9) hospitalization within the preceding year for psychiatric illness.
tDCS intervention
tDCS with a constant current of 2 mA intensity was applied for 20 min once a day for five consecutive days using a pair of thick (0.3 cm) rectangular surface sponge electrodes (5 cm × 7 cm) saturated with 10 cc of saline. Electrical current was ramped up and ramped down over ten 10 s at the beginning and end of the stimulation period, respectively. The anode electrode was placed over the M1 (C3 or C4 according to the 10–20 system for electroen-cephalography electrode placement) of the hemisphere contralateral to the affected knee, and the cathode electrode was placed over the SO ipsilateral to the affected knee (M1-SO montage). This stimulation paradigm was chosen because it is an effective treatment for other causes of chronic pain and provides a broad pattern of stimulation to motor, somatosensory, and frontal cortices implicated in pain sensitivity [12,38,39].
For sham stimulation, the electrodes were placed in the same positions with anode over M1 and cathode over contralateral SO, but the stimulator delivered 2 mA of current for only 30 s, with the same ramp up and down period of 10 s. This sham stimulation method has been shown to be reliable and indistinguishable from active treatment [12,40]. To minimize risk and maintain consistency, all the sessions were administered by one experimenter who had tDCS implementation training by Dr. Woods at the University of Florida Center for Cognitive Aging and Memory. Both experimenters and participants were blinded to the stimulation condition assigned to participants. As described above, blinding was achieved using a Soterix Clinical Trial tDCS device that requires input of a 6 digit blinded code for initiation of stimulation.
Clinical assessment
Clinical pain severity was measured via the (1) Numeric Rating Scale (NRS) for current knee pain, (2) Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) for OA-related pain symptoms [41], and (3) Short-Form McGill Pain Questionnaire (SF-MPQ-2) for comprehensive assessment and characterization of pain symptoms [42]. The NRS score was determined by asking participants to rate their current knee pain from 0 (no pain) to 100 (worst pain imaginable). This scale has been widely used in clinical studies that evaluated pain, and both validity and reproducibility have been demonstrated. The WOMAC consists of 3 subscales relating to pain during activities (range 0–20), stiffness during the day (range 0–8), and impairments of physical function (range 0–68), with higher scores indicating worse pain, stiffness, and impairments of physical function. The SF-MPQ-2 [43] consists of 4 subscales, including continuous pain (6 items), intermittent pain (6 items), neuropathic pain (6 items), and affective description of pain (4 items). Each subscale of the SF-MPQ-2 was scored with a range of 0–10, with higher scores indicating worse continuous pain, intermittent pain, neuropathic pain, and affective description of pain. The SF-MPQ-2 has been widely used to assess multiple aspects of pain, and its psychometric properties have been established [43]. The NRS was obtained at 11 time points during the treatment period (baseline and before and after each treatment session) as well as 3 time points during 3 weekly follow-up periods (once a week). The WOMAC and SF-MPQ-2 were administered twice (baseline and at the end of the treatment week).
In addition, mobility performance was measured using the 6-Minute Walk Test (6MWT) [44] and the Short Physical Performance Battery (SPPB). The 6MWT measures the total distance in meters that a participant can quickly walk on a flat, hard surface in a period of 6 min [44]. The SPPB consists of 3 measured components of lower-extremity function (standing balance, 4-m walking speed, and ability to rise from a chair), each scored on a 0–4 ordinal scale and summed to yield an overall integer score with a range of 0–12. These mobility performance measures were obtained twice (at baseline and at the end of the treatment week).
Safety
We monitored possible side effects of treatment by asking whether participants experienced the symptoms of tingling, itching sensation, burning sensation, pain at the stimulation site, fatigue, nervousness, headache, difficulty concentrating, mood change, and changes in vision or visual perception [45]. After each session of stimulation, participants completed a questionnaire rating the severity of these symptoms from 0 (not at all) to 10 (highest degree). In addition, since tDCS was applied directly to participant's head and it might impair cognitive function, which could contribute to changes in pain, we measured cognitive function. At baseline and the end of the treatment week, we administered the PROMIS version 1.0-Applied Cognition-Abilities-Short Form 8a [46], which consists of 8 items that are scored from 1 (not at all) to 5 (very much).
Statistical analyses
Initially, the baseline demographic characteristics, clinical pain scores, and OA severity were compared between the sham and tDCS group using Student's t-test for continuous variables and Fisher's exact test for categorical variables. The change from baseline in NRS scores was evaluated based on repeated measure analysis of variance using a linear mixed model with random subject effects and controlling for other fixed effects including sequence of the sessions, age, gender, race, baseline NRS score, OA severity, and other interactions. The appropriate model was selected based on Akaike's information criterion. Linear contrasts were employed to compare the treatment groups at each follow-up visit. Furthermore, WOMAC and SF-MPQ-2 scores and mobility performance (6WMT and SPPB) were compared for the two treatment groups (active and sham tDCS) using Student's t-tests. Finally, descriptive statistics were provided and Fisher's exact tests were conducted to compare the two groups on the safety outcomes. Unless otherwise stated, all the results are presented as means and standard deviation, and each P-value corresponds to a two-sided alternative hypothesis. Statistical significance was established based on P < 0.05.
Results
Participants
The participant flow diagram is shown in Fig. 1. Our goal was to complete data collection in 40 participants for this pilot trial. Forty-three participants were assessed for eligibility, and of those, 2 participants were excluded because they did not meet selection criteria owing to uncontrolled hypertension. Therefore, 41 participants were randomly assigned to receive either active tDCS or sham tDCS. Among them, one participant withdrew from the study because the patient could not attend the remaining sessions for personal reasons. All 40 participants who continued in the study completed all sessions and assessments. Twenty participants received active tDCS and the other 20 participants received sham tDCS. Table 1 presents the demographic and clinical characteristics at baseline for the active and sham tDCS groups. The average baseline scores on the NRS and the WOMAC subscale related to impairments of physical function were different between the groups (P = 0.04 and P = 0.03, respectively). The baseline differences in the clinical pain scores were adjusted by including baseline scores as a covariate in the linear mixed model.
Table 1.
Baseline demographic and clinical characteristics of the participants.
| Variables | Sham tDCS (N = 20) | active tDCS (N = 20) | p-value |
|---|---|---|---|
| Age, years, M ± SD | 59.30 ± 8.60 | 60.60 ± 9.80 | 0.67 |
| Gender, n (%) | 0.75 | ||
| Male | 9 (45%) | 10 (50%) | |
| Female | 11 (55%) | 10 (50%) | |
| Race, n (%) | 1.00 | ||
| Non-Hispanic White | 10 (50%) | 10 (50%) | |
| Others (Asian) | 10 (50%) | 10 (50%) | |
| BMI, kg/m2, M (SD) | 26.00 ± 4.10 | 27.00 ± 3.30 | 0.36 |
| NRS, M (SD) | 19.00 ± 7.70 | 27.30 ± 15.00 | 0.04 |
| WOMAC, M (SD) | |||
| Pain | 3.90 ± 2.00 | 5.40 ± 3.00 | 0.07 |
| Stiffness | 2.60 ± 1.20 | 2.90 ± 1.50 | 0.42 |
| Functional impairments | 11.80 ± 6.80 | 18.40 ± 11.50 | 0.03 |
| SF-MPQ-2, M ± SD | |||
| Continuous pain | 1.50 ± 1.00 | 2.30 ± 2.10 | 0.17 |
| Intermittent pain | 1.00 ± 1.30 | 1.80 ± 2.60 | 0.24 |
| Neuropathic pain | 0.80 ± 0.90 | 0.90 ± 1.30 | 0.71 |
| Affective description | 0.70 ± 0.90 | 1.60 ± 2.30 | 0.11 |
| SPPB, M ± SD | 11.10 ± 1.50 | 11.10 ± 1.20 | 0.91 |
| 6WMT, M ± SD | 490.70 ± 81.70 | 478.20 ± 73.60 | 0.61 |
| K/L radiographic score, n (%) | 0.70 | ||
| Grade 0 | 8 (40.0%) | 5 (25.0%) | |
| Grade 1 | 3 (15.0%) | 5 (25.0%) | |
| Grade 2 | 5 (25.0%) | 5 (25.0%) | |
| Grade 3 | 4 (20.0%) | 4 (20.0%) | |
| Grade 4 | 0 (00.0%) | 1 (5.0%) |
Note. M = Mean; SD = Standard Deviation; BMI = Body Mass Index; NRS = Numeric Rating Scale of Pain; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; SF-MPQ-2 = Short-Form McGill Pain Questionnaire-2, SPPB = Short Physical Performance Battery; 6MWT = 6-Minute Walk Test; K/L = Kellgren/Lawrence.
Clinical pain severity
Table 2 presents comparison results between the two groups on the changes from baseline for different clinical pain scores and mobility performance, and Table 3 presents individual data on the changes from baseline for NRS scores. Based on the linear mixed model, the type-3 fixed-effect test revealed a significant difference in the NRS score between groups (F [1146] = 4.60, P = 0.03), after adjusting for the baseline scores and other covariates. Specifically, participants in the active tDCS group had a significantly greater reduction in knee pain than the sham tDCS group across the daily stimulation visits (Cohen's d = 0.75, P = 0.02 at visit 2; d = 0.77, P = 0.02 at visit 4; d = 0.89, P = 0.01 at visit 6). Also, participants in the active tDCS group had a significantly greater reduction in knee pain based on the NRS score than the sham tDCS group across all follow-up assessments (d = 0.99, P < 0.01 at 1-week follow-up; d = 0.77, P = 0.02 at 2-week follow-up; d = 0.79, P = 0.02 at 3-week follow-up).
Table 2.
Comparison between groups on changes from baseline in clinical pain variables.
| Variables | sham group (n = 20) | active group (n = 20) | Effect size (d) | p-value |
|---|---|---|---|---|
| NRS change at visit2 | −5.60 ± 12.80 | −14.90 ± 11.90 | 0.75 | 0.02 |
| NRS change at visit3 | −7.40 ± 10.00 | −16.10 ± 16.80 | 0.63 | 0.05 |
| NRS change at visit4 | −4.50 ± 13.20 | −16.50 ± 17.60 | 0.77 | 0.02 |
| NRS change at visit5 | −8.20 ± 11.80 | −15.20 ± 19.20 | 0.44 | 0.17 |
| NRS change at visit6 | −6.50 ± 10.10 | −18.50 ± 16.10 | 0.89 | 0.01 |
| NRS change at follow-up 1 | 1.00 ± 16.60 | −16.40 ± 18.60 | 0.99 | 0.00 |
| NRS change at follow-up 2 | −1.50 ± 14.20 | −13.50 ± 16.90 | 0.77 | 0.02 |
| NRS change at follow-up 3 | −3.80 ± 11.80 | −15.60 ± 17.50 | 0.79 | 0.02 |
| WOMAC change at visit 6 | ||||
| Pain | −0.60 ± 2.10 | −1.30 ± 3.10 | 0.26 | 0.45 |
| Stiffness | −0.20 ± 0.80 | −0.60 ± 1.40 | 0.35 | 0.33 |
| Functional impairment | 0.10 ± 7.30 | −2.40 ± 10.40 | 0.28 | 0.39 |
| SF-MPQ-2 change at visit 6 | ||||
| Continuous pain | 0.10 ± 1.40 | −1.00 ± 2.00 | 0.64 | 0.07 |
| Intermittent pain | 0.20 ± 1.40 | −0.80 ± 2.10 | 0.56 | 0.09 |
| Neuropathic pain | −0.10 ± 0.70 | −0.40 ± 1.20 | 0.31 | 0.41 |
| Affective description | 0.10 ± 1.10 | −1.00 ± 2.30 | 0.61 | 0.06 |
| SPPB change at visit 6 | −0.10 ± 1.20 | 0.20 ± 0.70 | 0.26 | 0.41 |
| 6WMT change at visit 6 | 3.60 ± 33.30 | 19.30 ± 25.60 | 0.53 | 0.10 |
Note. Mean ± Standard Deviation were presented in the first two columns. NRS = Numeric Rating Scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; SF-MPQ = Short-Form McGill Pain Questionnaire; SPPB = Short Physical Performance Battery; 6MWT = 6-Minute Walk Test.
Table 3.
Individual data on the changes from baseline for Numeric Rating Scale scores.
| Number | Baseline NRS | Numeric Rating Scale (NRS) change | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Follow-up 1 | Follow-up 2 | Follow-up 3 | ||
| Sham 1 | 30 | −25 | −20 | −25 | −30 | −25 | −5 | 5 | 0 |
| Sham 2 | 15 | 0 | 0 | 0 | 5 | 5 | 5 | −5 | −5 |
| Sham 3 | 20 | −20 | −15 | −10 | −20 | −20 | −15 | −20 | −20 |
| Sham 4 | 30 | 0 | 0 | 0 | 10 | 0 | 10 | 10 | 10 |
| Sham 5 | 20 | 15 | −10 | 0 | −15 | −15 | 50 | 20 | 10 |
| Sham 6 | 30 | 20 | 20 | 20 | 20 | 20 | 20 | 25 | 10 |
| Sham 7 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sham 8 | 20 | −10 | 0 | 0 | −5 | −5 | 5 | 5 | 0 |
| Sham 9 | 30 | −20 | −10 | −10 | −10 | 0 | 20 | 0 | 0 |
| Sham 10 | 15 | −13 | −12 | −12 | −13 | −12 | −5 | −10 | −5 |
| Sham 11 | 30 | −30 | −30 | −30 | −30 | −20 | −30 | −30 | −20 |
| Sham 12 | 10 | 0 | −10 | −10 | −10 | −10 | −10 | −10 | −10 |
| Sham 13 | 20 | 10 | −10 | 30 | −10 | 0 | 0 | 5 | 5 |
| Sham 14 | 10 | −10 | −10 | −10 | −10 | 0 | −10 | −10 | −10 |
| Sham 15 | 20 | 2 | 0 | −2 | −5 | −5 | −5 | −10 | −15 |
| Sham 16 | 20 | 0 | 0 | 5 | 0 | −2 | −5 | 0 | −20 |
| Sham 17 | 10 | 0 | −10 | −10 | −10 | −10 | −10 | −10 | −10 |
| Sham 18 | 10 | −10 | −10 | −10 | −10 | −10 | 15 | 25 | 25 |
| Sham 19 | 10 | −10 | −10 | −5 | −10 | −10 | −8 | −10 | −10 |
| Sham 20 | 10 | −10 | −10 | −10 | −10 | −10 | −2 | −10 | −10 |
| Active 1 | 25 | −15 | −10 | −5 | −5 | −10 | −5 | −10 | −15 |
| Active 2 | 75 | −35 | −65 | −67 | −69 | −67 | −70 | −69 | −70 |
| Active 3 | 20 | −10 | 10 | 10 | 5 | 0 | 0 | −5 | −10 |
| Active 4 | 30 | −20 | −30 | −20 | −30 | −30 | −30 | 0 | −30 |
| Active 5 | 20 | −10 | 0 | 0 | 10 | −10 | 0 | −10 | 0 |
| Active 6 | 20 | 0 | 0 | 0 | 0 | 0 | 20 | 10 | 10 |
| Active 7 | 30 | −25 | −30 | −30 | −30 | −30 | −25 | −25 | −25 |
| Active 8 | 60 | −40 | −40 | −40 | −40 | −40 | −40 | −30 | −40 |
| Active 9 | 25 | −15 | −15 | −20 | −25 | −25 | −15 | −5 | −15 |
| Active 10 | 10 | −10 | −10 | 0 | 0 | 0 | −10 | −10 | 0 |
| Active 11 | 30 | −25 | −25 | −30 | −20 | −20 | −20 | −15 | −20 |
| Active 12 | 25 | −10 | −20 | −24 | −25 | −25 | −25 | −15 | −20 |
| Active 13 | 15 | 5 | −5 | −5 | −10 | −10 | −10 | −10 | −10 |
| Active 14 | 20 | −5 | −8 | −10 | −5 | −10 | −5 | −10 | −5 |
| Active 15 | 20 | −5 | −5 | −5 | −5 | −5 | −5 | −5 | −5 |
| Active 16 | 30 | −30 | −20 | −25 | −25 | −30 | −30 | −20 | −15 |
| Active 17 | 20 | 0 | 0 | 0 | 0 | −10 | 0 | 15 | 5 |
| Active 18 | 20 | −10 | −10 | −20 | −20 | −10 | −20 | −19 | −20 |
| Active 19 | 30 | −20 | −20 | −20 | 10 | −20 | −20 | −20 | −10 |
| Active 20 | 20 | −18 | −18 | −18 | −19 | −18 | −17 | −17 | −17 |
We did not find statistically significant differences in WOMAC and SF-MPQ-2 subscale score changes between groups, but we present effect sizes because of the relatively small sample size. Effect sizes (Cohen's d) were d = 0.26 for the WOMAC pain subscale, d = 0.35 for the WOMAC stiffness subscale, and d = 0.27 for the WOMAC functional impairment subscale. Effect sizes (Cohen's d) were d = 0.64 for the SF-MPQ-2 continuous pain subscale, d = 0.56 for the SF-MPQ-2 intermittent pain subscale, d = 0.31 for the SF-MPQ-2 neuropathic pain subscale, and d = 0.61 for the SF-MPQ-2 affective description subscale.
Mobility performance
After five daily sessions, the average increase in the total SPPB score was 0.20 ± 0.70 for the active group, while the average decrease in the total SPPB score in the sham group was 0.10 ± 1.20. Also, patients in the active tDCS group walked on average 19.30 ± 25.60 m more at visit 6 than at baseline, whereas patients in the sham tDCS group walked on average only 3.60 ± 33.30 m more at visit 6 than at baseline. Effect sizes (Cohen's d) were d = 0.26 for SPPB and d = 0.53 for 6WMT. However, we did not find statistically significant differences in total SPPB and 6MWT.
Safety
All participants tolerated M1-SO applied tDCS well without experiencing any serious adverse effects. No participants complained about fatigue, nervousness, headache, difficulty concentrating, mood change, or vision changes during tDCS sessions. Twenty-seven incidents occurred during tDCS sessions, specifically pain at the stimulation site (6 incidents: 2 in the sham group and 4 in the active group, P = 0.66) and changes in visual perception (1 in the active group, P = 1.00). However, the severity of these symptoms was less than or equal to 2 of 10, and participants wanted to complete the tDCS session and did not complain about these symptoms after completing the tDCS sessions.
There were differences in the instances of tingling sensation between the sham and active tDCS groups (3 instances in the sham group and 10 instances in the active group, P = 0.04), but no differences in itching sensation (4 instances: 2 in the sham group and 2 in the active group, P = 1.00) or burning sensation (3 in the active group, P = 0.23).
In addition, changes in cognition from baseline were similar for the active tDCS group (0.40 ± 3.70) and sham tDCS group (0.40 ± 5.20) on the PROMIS version 1.0-Applied Cognition-Abilities based on Student's t tests (P = 1.00).
Discussion
This study was one of the first to test the efficacy of tDCS with anode over M1 and cathode over contralateral SO, in older adults with knee OA using an experimenter- and participant-blinded, randomized, sham-controlled design. We observed significant reductions in NRS-rated knee pain in the active tDCS group compared to the sham tDCS group. Also, although there was no statistical significance, we observed marginally greater reductions in other clinical pain measures (WOMAC, SF-MPQ-2). Similarly, nonsignificant improvements in mobility performance (SPPB and 6WMT) were observed in the active tDCS group compared to the sham tDCS group, with moderate effect sizes. Finally, active tDCS did not cause any significant adverse effects or cognitive impairment.
Previous studies reported that M1-SO applied tDCS might reduce pain by modulating activity in brain areas involved in pain processing and by facilitating descending pain inhibitory mechanisms [47,48]. We proposed M1-SO tDCS as a possible treatment strategy for this population because this stimulation is believed to mediate analgesic effects by modulating M1-thalamic inhibitory connections involved in pain processing pathways [21,22]. This stimulation paradigm was also chosen because it has shown efficacy in treatment of other causes of chronic pain and provides a broad pattern of stimulation to motor, somatosensory, and frontal cortices implicated in pain sensitivity [12]. OA has previously been conceptualized as a regional pain condition whose symptoms are driven by peripheral pathophysiology, but the poor correspondence between measures of disease severity and clinical pain symptoms suggests that factors beyond peripheral tissue damage likely contribute to OA-related pain [49]. Indeed, individuals with knee OA have been shown to have generalized alterations in central pain processing [50]. Moreover, functional magnetic resonance imaging in individuals with knee OA has revealed increased regional cerebral blood flow in response to mechanical pain stimulation in multiple brain regions, including the cingulate, insula, somatosensory, orbitofrontal, and prefrontal cortices, thalamus, amygdala, hippocampus, and putamen [51,52].
The findings of our study are similar to those of previous clinical studies in which tDCS was applied with anode over the M1 and cathode over the contralateral SO was efficacious in reducing clinical pain intensity in chronic painful conditions. For example, Fregni et al. (2006) [12] reported that tDCS with the anode electrode over the C3 position (using the 10/20 system of electrode placement) and the cathode electrode over the contralateral SO in female patients with fibromyalgia induced significantly greater pain improvement as measured by a visual analog scale up to 3 weeks after the stimulation compared with sham stimulation and stimulation over the dorsolateral prefrontal cortex. In addition, Mori et al. (2010) [14] demonstrated that tDCS with the anode electrode over the M1 contralateral to the painful somatic area and cathode electrode over the SO contralateral to the stimulated motor cortex in adults with multiple sclerosis produced significant pain improvement as measured by a visual analog scale and SF-MPQ-2. In contrast, some studies found that M1-SO applied tDCS does not improve clinical pain outcomes [53]. Five sessions could be underdosing and may have limited treatment effects [54]. However, it is important to note that the efficacy of M1-SO tDCS depends on a number of factors, including the intensity and duration of stimulation, the polarity of the electrode, the target brain area, electrode preparation methods, and the target population [18]. Collectively, findings from these studies of adults with chronic painful conditions indicate the efficacy of M1-SO tDCS, and our study extends these findings to older adults with knee OA.
Our findings showing limited adverse effects are consistent with other clinical tDCS studies [17]. For example, in prior research, no serious adverse effects or cognitive impairments have been reported [12,14,17]. In our project, a small number of participants reported mild symptoms of non-serious adverse events during tDCS, and no one complained about those symptoms after completing the tDCS sessions.
Our findings should be interpreted in light of the study's limitations. First, we included a small sample of adults with knee OA. This small sample size might not have been large enough to detect some characteristics associated with a positive effect of M1-SO applied tDCS, and therefore, this study may have produced nonsignificant results in some measures in this study. Second, participants in the active group had less pain and functional impairment at baseline than the sham group; however, the reductions in NRS-rated knee pain in the active tDCS group compared to the sham tDCS group remained significant after adjusting the baseline scores. We considered age, race, and gender-balanced randomization, which might have contributed to the differences in some clinical characteristics. Third, due to the short-term follow-up evaluations, long-term efficacy of M1-SO applied tDCS cannot be established.
In conclusion, we demonstrated that M1-SO applied tDCS with a constant direct current of 2 mA intensity for 20 min once a day for five consecutive days was efficacious in reducing participants' perception of clinical pain. While some measures of pain symptoms were not statistically different, we still found moderate effect sizes between the active and sham groups. Future studies with larger samples and longer-term follow-up evaluations are needed to replicate and extend these findings. Also, different parameters of brain stimulation, such as the duration or frequency of tDCS, as well as combined therapy with other interventions are needed to refine this novel approach for pain treatment.
Acknowledgments
The authors thank the staff at the University of Florida Pain Research and Intervention Center of Excellence and Institute on Aging for their work on this project.
Funding: This research was supported in part by the Claude D. Pepper Older American's Independence Center (P30 AG028740), the University of Florida Center for Cognitive Aging and Memory, and NIA Grants K07AG04637 and K01AG050707, and R01AG054077. This work was also partially supported by VA HSR&D Houston Center for Innovations in Quality, Effectiveness and Safety (CIN# 13-413), Michael E. DeBakey VA Medical Center, Houston, TX.
Abbreviations
- tDCS
transcranial direct current stimulation
- OA
osteoarthritis
- M1
primary motor cortex
- SO
supraorbital region
- NRS
Numeric Rating Scale
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- SF-MPQ-2
Short-Form McGill Pain Questionnaire-2
- 6MWT
6-Minute Walk Test
- SPPB
Short Physical Performance Battery
Footnotes
Some results were presented at the NYC Neuromodulation 2017 Conference, New York, NY.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am. 2008;34(3):623–43. doi: 10.1016/j.rdc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour KE, Helmick CG, Theis KA, Murphy LB, Hootman JM, Brady TJ, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation-United States, 2010-2012. Morb Mortal Wkly Rep. 2013;62(14):869–73. [Google Scholar]
- 4.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 5.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston county osteoarthritis project. J Rheumatol. 2007;34(1):172–80. [PubMed] [Google Scholar]
- 6.Institute of Medicine Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 7.Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol. 2013;8(3):518–34. doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- 8.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10(6):331–42. doi: 10.1016/j.amjopharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170(22):1968–76. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 11.Reid MC, Henderson CR, Jr, Papaleontiou M, Amanfo L, Olkhovskaya Y, Moore AA, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11(7):1063–71. doi: 10.1111/j.1526-4637.2010.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54(12):3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 13.Simis M, Reidler JS, Duarte Macea D, Moreno Duarte I, Wang X, Lenkinski R, et al. Investigation of central nervous system dysfunction in chronic pelvic pain using magnetic resonance spectroscopy and noninvasive brain stimulation. Pain Prac. 2014;15(5):423–32. doi: 10.1111/papr.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori F, Codeca C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010;11(5):436–42. doi: 10.1016/j.jpain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Antal A, Terney D, Kuhnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage. 2010;39(5):890–903. doi: 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stim. 2008;1(3):206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stim. 2016;9(5):641–61. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–48. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012;52(8):1283–95. doi: 10.1111/j.1526-4610.2012.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2017;128(1):56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 21.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383–93. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- 22.Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2(3):353–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamocortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. 2012;33(10):2499–508. doi: 10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antal A, Polania R, Schmidt-Samoa C, Dechent P, Paulus W. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage. 2011;55(2):590–6. doi: 10.1016/j.neuroimage.2010.11.085. [DOI] [PubMed] [Google Scholar]
- 25.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122(1–2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, et al. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev. 2015;24(Pt B):304–27. doi: 10.1016/j.arr.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seider TR, Fieo RA, O'Shea A, Porges EC, Woods AJ, Cohen RA. Cognitively engaging activity is associated with greater cortical and subcortical volumes. Front Aging Neurosci. 2016;8:94. doi: 10.3389/fnagi.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dotson VM, Szymkowicz SM, Sozda CN, Kirton JW, Green ML, O'Shea A, et al. Age differences in prefrontal surface area and thickness in middle aged to older adults. Front Aging Neurosci. 2015;7:250. doi: 10.3389/fnagi.2015.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PloS one. 2013;8(9):e76112. doi: 10.1371/journal.pone.0076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK. Transcranial direct current stimulation in pediatric brain: a computational modeling study. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:859–62. doi: 10.1109/EMBC.2012.6346067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Ann Neurol. 2013;73(1):10–5. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37(3):384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Meinzer M, Lindenberg R, Antonenko D, Flaisch T, Floel A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J Neurosci. 2013;33(30):12470–8. doi: 10.1523/JNEUROSCI.5743-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 35.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 36.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34(5):505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 37.Scott WW, Jr, Lethbridge-Cejku M, Reichle R, Wigley FM, Tobin JD, Hochberg MC. Reliability of grading scales for individual radiographic features of osteoarthritis of the knee. The Baltimore longitudinal study of aging atlas of knee osteoarthritis. Invest Radiol. 1993;28(6):497–501. [PubMed] [Google Scholar]
- 38.Luedtke K, Rushton A, Wright C, Geiss B, Juergens TP, May A. Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta-analysis. Clin J Pain. 2012;28(5):452–61. doi: 10.1097/AJP.0b013e31823853e3. [DOI] [PubMed] [Google Scholar]
- 39.O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;4:CD008208. doi: 10.1002/14651858.CD008208.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 42.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 43.Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce-Sandner S, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2) Pain. 2009;144(1–2):35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–98. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 45.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72(4–6):208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Becker H, Stuifbergen A, Lee H, Kullberg V. Reliability and validity of PROMIS cognitive abilities and cognitive concerns scales among people with multiple sclerosis. Int J MS Care. 2014;16(1):1–8. doi: 10.7224/1537-2073.2012-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007;69(9):827–34. doi: 10.1212/01.wnl.0000269783.86997.37. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage. 2007;37(Suppl 1):S71–9. doi: 10.1016/j.neuroimage.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 49.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum. 2013;65(2):291–302. doi: 10.1002/art.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, et al. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PloS one. 2013;8(11):e80440. doi: 10.1371/journal.pone.0080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain. 2008;4:47. doi: 10.1186/1744-8069-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61(9):1226–34. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 53.Souto G, Borges IC, Goes BT, de Mendonca ME, Goncalves RG, Garcia LB, et al. Effects of tDCS-induced motor cortex modulation on pain in HTLV-1: a blind randomized clinical trial. Clin J Pain. 2014;30(9):809–15. doi: 10.1097/AJP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 54.Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain. 2016;17(1):14–26. doi: 10.1016/j.jpain.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]