Abstract
Emerging evidence suggests that increased nicotinamide phosphoribosyltransferase (NAMPT) expression is associated with the development and prognosis of many cancers, but it remains unknown regarding its role in oral squamous cell carcinoma (OSCC). In the present study, the results from tissue microarray showed that NAMPT was overexpressed in OSCC patients and its expression level was directly correlated with differential grades of cancer. Interestingly, treatment of OSCC cells with chemotherapy agent arsenic trioxide (ATO) decreased the levels of NAMPT protein and increased cellular death in an ATO dose- and time-dependent manner. Most importantly, combination of low concentration ATO with FK866 (a NAMPT inhibitor) exerted enhanced inhibitive effect on NAMPT protein and mRNA expressions, leading to synergistic cytotoxicity on cancer cells through increasing cell apoptosis and depleting intracellular nicotinamide adenine dinucleotide levels. These findings demonstrate the crucial role of NAMPT in the prognosis of OSCC and reveal inhibition of NAMPT as a novel mechanism of ATO in suppressing cancer cell growth. Our results suggest that ATO can significantly enhance therapeutic efficacy of NAMPT inhibitor, and combined treatment may be a novel and effective therapeutic strategy for OSCC patients.
Keywords: nicotinamide phosphoribosyltransferase, nicotinamide adenine dinucleotide, arsenic trioxide, FK866, squamous cell carcinoma
Introduction
Oral squamous cell carcinoma (OSCC) is a common malignant tumor, which most frequently occurs on the tongue. Despite advances in therapeutic methods, including surgery in combination with radiation and/or chemotherapy, the survival rate of this disease has not been significantly increased from 50–60% in past decades (1). Therefore, novel and more effective therapeutic strategies for patients with OSCC are urgently needed.
Nicotinamide phosphoribosyltransferase (NAMPT), also named pre-B-cell colony-enhancing factor (PBEF) or visfatin, was originally thought to be a cytokine that functions as a co-factor for B cell maturation (2). It was later identified as a crucial enzyme involved in nicotinamide adenine dinucleotide (NAD) biosynthesis (3). NAD is both an essential coenzyme involved in cellular redox reactions and a substrate for NAD-dependent enzymes such as PARP and Sirtuins (4). Cancer cells have a high capacity for glucose uptake and an increased rate of glycolysis even in the presence of oxygen, called the Warburg effect (5). This high metabolic demand requires increased NAD, which is involved in many critical processes for cancer including transcriptional regulation, cell-cycle progression, anti-apoptosis, and DNA repair (6). Since NAD is rapidly consumed by NAD-dependent enzymes, NAMPT is critically important for the replenishment of the intracellular NAD pool in cancer cells (7). Recently, it has been reported that NAMPT plays a pivotal role in malignant tumors. The development of many cancers is associated with increased NAMPT expression (8). Furthermore, higher NAMPT expression correlates with increased tumor invasion, metastatic potential, and chemotherapy resistance in some malignancies (9). NAMPT inhibitors have been evaluated in a variety of tumors, both in vitro and in nude- mouse xenografts, in which the inhibitors were shown to be able to reduce tumor growth (10–12). However, the pathological role of NAMPT in OSCC remains unknown.
Arsenic trioxide (ATO) is a chemotherapy agent that has been successfully used to treat patients with acute promyelocytic leukemia (13). Accumulating evidences from in vitro studies indicate that ATO is also a promising therapeutic drug for certain solid malignant tumors (14). The anti-cancer molecular mechanisms of ATO have been suggested to include inducing apoptosis, promoting differentiation, activating ROS generation, and inhibiting the mitochondrial permeability (15–17). However, the effect and mechanism of ATO on NAMPT expression in cancer remain unclear.
In the present study, we investigated the expressions of NAMPT in three differentiated grades of OSCC in patients, and studied the effects of ATO on NAMPT in OSCC cell lines and its relevant mechanisms.
Materials and Methods
Tissue microarray and immunohistoche mistry
The specimens of OSCC for tissue microarray (TMA) analysis were purchased from Biomax US (Rockville, MD), catalog numbers OR601a. The OR601a consists of 61 tissue samples, which contains 50 cases of OSCC and 10 cases of normal and adjacent tissue (NAT), and its details of OSCC clinical characteristics are as following: age, ≤45, 14 cases; >45, 46 cases; gender, male, 37 cases; female, 23 cases; TNM grading, stage I, 20 cases; stage II, 24 cases; stage III, 5 cases; stage IV, 1 case (The TNM classification is a cancer staging notation system with alphanumeric codes. T, the size of the original tumor and whether it has invaded nearby tissue; N, regional lymph nodes that are involved; M, distant metastasis.); Histopathologic differentiation, well-, 37 cases; moderately-, 8 cases; poorly-, 5 cases.
Immunohistochemistry was performed on all specimens to examine the NAMPT protein expression. In brief, the specimens were stained with goat anti-rat antibody against NAMPT (1:100, Santa Cruz Biotech, Santa Cruz, CA) by routine immunohistochemical assay. NAMPT immune staining was quantified and analyzed with an Image-Pro Plus 6.0 in 10 different fields in each section.
Cell culture
SCC15 and SCC25 cells were obtained from American Type Culture Collection (Manassas, VA), and were cultured in a 1:1 mixture of DMEM and Ham’s F12 medium (Thermo Fisher Scientific, Waltham, MA) with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA), 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere of 5% CO2.
PrestoBlue cell viability assay
After incubation with varying concentrations of ATO (1, 2, 4, and 8 μM) or FK866 (NAMPT inhibitor, 10 nM), survival rate of SCC15 and SCC25 cells was measured using Prestoblue™ cell viability reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The OD570/600 was recorded on a SpectraMAX 190 microplate reader (Molecular Devices, Sunnyvale, CA).
Cell immunofluorescence
For immunofluorescence staining, SCC15 and SCC25 cells were cultured in a glass bottom cell culture dish (Nest, Wuxi, China). After treatment with varying concentrations of ATO (1, 2, 4, and 8 μM) or/and FK866 (10 nM) for 48 h, the cells were incubated with NAMPT antibody (1:50) at 37 °C for 1 h and 4 °C overnight, and followed by Cy3- labeled secondary antibody (Thermo Fisher Scientific, Waltham, MA) for 1 h. The cells were then counterstained with Hoechst (Thermo Fisher Scientific, Waltham, MA). Images were subsequently captured using an inverted fluorescence microscope (Leica DMi3000 B, Mannheim, Germany). The positive staining was shown as red fluorescence.
TUNEL assay
To evaluate apoptosis induced by ATO and FK866, SCC15 and SCC25 cells were stained with terminal deoxynucleotidyl transferase- mediated deoxyuridine triphosphate-biotin nick end labelling (TUNEL, In Situ Cell Death Detection Kit, Roche, Penzberg, Germany). The detection procedure was performed according to the manufacturer’s instructions. OSCC cells were observed using an inverted fluorescence microscope (Leica DMi3000 B, Mannheim, Germany). The nuclei of apoptotic cells were stained green fluorescence with FITC and quantitated by counting the number of TUNEL-positive cells in 10 random microscopic fields at X400 magnification.
Semi-quantitative RT-PCR
Total RNA was extracted and purified by using a RNA Prep Pure Cell kit (Tiangen, Beijing, China). Each 2 μg RNA sample was reverse transcribed into cDNA with an RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). The primers are as the following: NAMPT, 5′-AAT ATC CAC CCA ACA CAA GCA AA-3′ (sense) and 5′-TGA CAA AGC CCT CAG GAA CAG-3′ (antisense); β-actin, used as an internal control, were 5′-CTG ACG GCC AGG TCA TCA C-3′ (sense) and 5′-CTG CTT GCT GAT CCA CAT CTG-3′ (antisense). The band intensity were quantitated by G:BOX-F3 fluorescence imaging system (Syngene, Cambridge, United Kingdom).
Measurement of NAD+/NADH
Following exposure to 2 μM ATO and in absence or presence of 10 nM FK866 for 48 h, relative NAD levels in SCC15 and SCC25 cells was analyzed by NAD+/NADH Quantification kit (Sigma Aldrich, St. Louis) as specified by the manufacturer.
Statistical analysis
All data are expressed as mean ± SEM. Differences between the groups were evaluated by one-way analysis of variance (ANOVA), followed by the Bonferroni test. Statistical significance threshold was set at P < 0.05.
Results
NAMPT was overexpressed in OSCC and was highly associated with the grades of differentiation
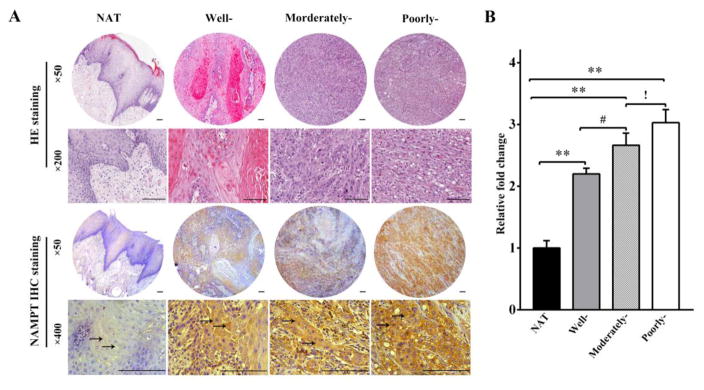
TMA analysis was carried out to examine the NAMPT protein expression in OSCC patients. As shown in Figure 1A, the brown staining of NAMPT protein expression was displayed, which was mainly distributed in the cytoplasm of all three differentiated grades of OSCC (indicated by arrow). Comparing with NAT tissue, the protein expression of NAMPT increased by 2.2, 2.7, and 3.0 folds in well-, moderately-, and poorly-differentiated OSCC, respectively, in a histopathologic grade-dependent manner (Figure 1B). These data demonstrated a strong positive correlation between the overexpression of NAMPT and the degree of differentiation of cancer. These findings indicate that NAMPT may be critically involved in the progression and development of OSCC.
Figure 1. Increased NAMPT expression was observed in OSCC and it was highly associated with the grades of differentiation.
NAMPT expression was analyzed with tissue microarray (TMA). Positive immunostaining for NAMPT was brown and indicated by arrow. (A) Representative immunohistochemical staining of NAMPT expression. (B) Relative intensity fold change of NAMPT expression in normal and adjacent tissue (NAT) and OSCC tissues of three grades of differentiation. **p<0.01 vs. NAT; #p<0.05 vs. well differentiated OSCC; !p<0.05 vs. moderately differentiated OSCC. Bar=100μm.
ATO inhibited cell growth and NAMPT levels in OSCC cells
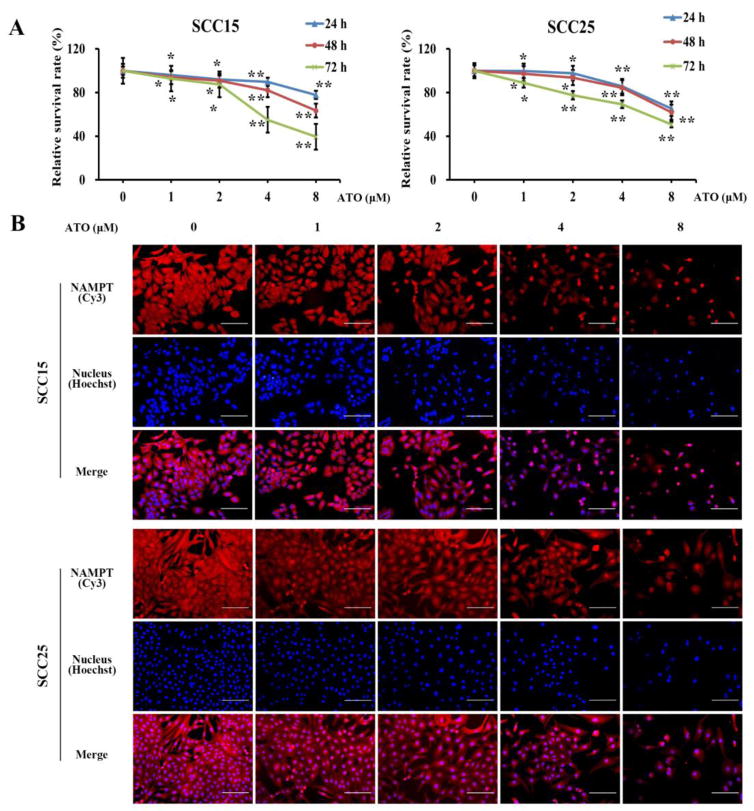
SCC15 and SCC25 cell lines were used to examine the effect of ATO on OSCC cell growth and the underlying mechanism. SCC15 and SCC25 cells were exposed to ATO at a dose of 1, 2, 4, or 8 μM for 24, 48 or 72 h. The cellular growth inhibition was measured with PrestoBlue cell viability assay. Treatment with ATO significantly reduced the cell survival rate in a concentration- and time-dependent manner in both cell lines (Figure 2A).
Figure 2. ATO s uppressed the cell survival and inhibited the protein expression of NAMPT in OSCC cells.
After incubation with varying concentrations of ATO (1, 2, 4, and 8 μM) at 24, 48, and 72 h, (A) the cell survival rate of SCC15 and SCC25 was determined by PrestoBlue cell viability assay; (B) NAMPT protein expression was determined by immunofluorescence. *p<0.05 and **p<0.01vs. untreated cells. Bar=100μm.
NAMPT is a pivotal factor in malignant tumor development. Immunofluorescence was conducted to determine whether ATO might inhibit NAMPT level in OSCC cells to reduce cell survival. SCC15 and SCC25 cells were incubated with ATO of above concentrations for 48 h. Immunofluorescence staining of NAMPT protein was dramatically diminished along with increasing ATO concentrations (Figure 2B). Taken together, these findings suggest that NAMPT may be involved in the inhibition of the survival of cancer cells by ATO.
Synergistic inhibitive effect of ATO combination with FK866 on OSCC cells
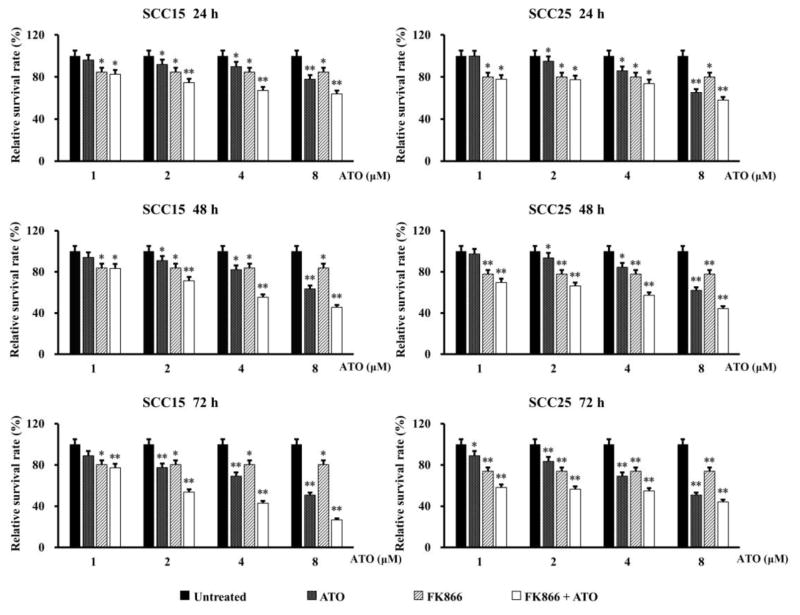
FK866, also named APO866, is a highly specific noncompetitive inhibitor of NAMPT. To evaluate the potential of ATO in promoting the inhibitive efficacy of FK866 on OSCC cells, PrestoBlue cell viability assay was used to measure the cell survival rate after treatment with varying concentrations of ATO (1–8 μM) or/and 10 nM FK866 for 24, 48 and 72 h. FK866 concentration was selected according to manufacturer’s instruction, which can induce depletion of intracellular NAD and cell death in various hematologic malignancies (18).
As shown in Figure 3, low concentration of 2 μM ATO significantly exerted the similar cytotoxicity to FK866 on SCC15 and SCC25 cells at every time point. Most interestingly, a synergistic inhibitive effect was observed in SCC15 and SCC25 cells even at low concentration of 2 μM ATO combined with FK866. To more clearly display the synergistic effect on cytotoxicity, the survival rates of SCC15 and SCC25 cells treated with 2 μM ATO and/or FK866 at each measured time point were shown in Table 1. Our results indicate that combination of ATO with the inhibitor of NAMPT may play a synergistic role on suppressing cancer cell survival.
Figure 3. Cytotoxicity of combined treatment of ATO with FK866 in OSCC cells.
SCC15 and SCC25 cells were exposed to varying concentrations of ATO (1–8 μM) and/or FK866 (10 nM) for 24, 48, and 72 h, respectively. Cell survival rate was measured with PrestoBlue cell viability assay. *p<0.05 and **p<0.01 vs. untreated cells.
Table 1.
Synergistic cytotoxic effect of 2 μM ATO combination with FK866 on OSCC cells.
| Time (h) | Decreased cell survival rate (%) | |||||
|---|---|---|---|---|---|---|
| SCC15 | SCC25 | |||||
| ATO | FK866 | ATO + FK866 | ATO | FK866 | ATO+ FK866 | |
| 24 | 8.1 ± 0.4 * | 15.4 ± 0.8 * | 25.4 ± 1.3 ** | 5.2 ± 0.3 * | 19.9 ± 1.0 * | 22.6 ± 1.1* |
| 48 | 9.1 ± 0.5 * | 16.3 ± 0.8 * | 28.6 ± 1.4 ** | 6.4 ± 0.3 * | 22.3 ± 1.1 ** | 33.7 ± 1.7 ** |
| 72 | 22.5 ± 1.1 ** | 16.6 ± 0.8 ** | 46.3 ± 2.3 ** | 26.2 ± 1.3 ** | 16.5 ± 0.8 ** | 53.5 ± 2.7 ** |
After exposure to 2 μM ATO and/or 10 nM FK866 for 24, 48, and 72 h, respectively, the cell survival rate of SCC15 and SCC25 cells was examined. Data represent mean ± SEM.
p < 0.05 and
p < 0.01 compared with untreated cells.
Induction of apoptosis of OSCC cells by ATO and FK866 treatment
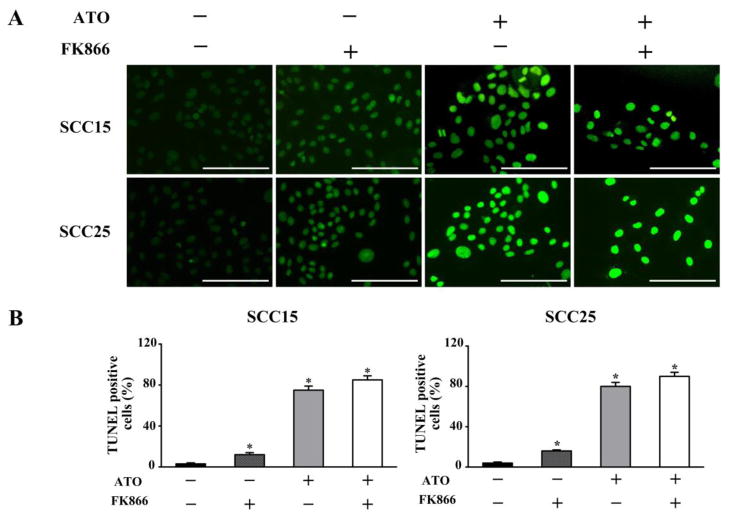
To understand the possible mechanism of ATO inhibiting the cell survival of OSCC, we performed TUNEL staining to investigate cell apoptosis after incubation with 2 μM ATO or/and 10 nM FK866 in SCC15 and SCC25 cells at 48 h. Upregulation of cell apoptosis was found in both cell lines. TUNEL staining of OSCC cells was stained green fluorescence, which exhibited typical apoptosis-like changes with condensation of chromatin and segmentation of the nucleus in TUNEL-positive cells. ATO significantly increased TUNEL-positive cell both in SCC15 and SCC25 cells, which was further enhanced by ATO combination with FK866 (Figure 4). These data suggest that induction of cell apoptosis is an important mechanism of ATO inhibiting OSCC cell viability.
Figure 4. Elevated apoptosis induction by ATO combined with FK866 on OSCC cells.
After exposure with ATO (2 μM) or/and FK866 (10 nM) for 48 h in SCC15 and SCC25 cells, TUNEL staining was performed to investigate cell apoptosis. (A) Apoptotic cells were stained with green fluorescence. (B) Quantification for TUNEL-positive cells in 10 random microscopic fields at x400 magnification. *p<0.05 vs. untreated cells. Bar=100μm.
Enhance d inhibition on the expressions of protein and mRNA of NAMPT in OSCC cells induced by ATO in combination with FK866
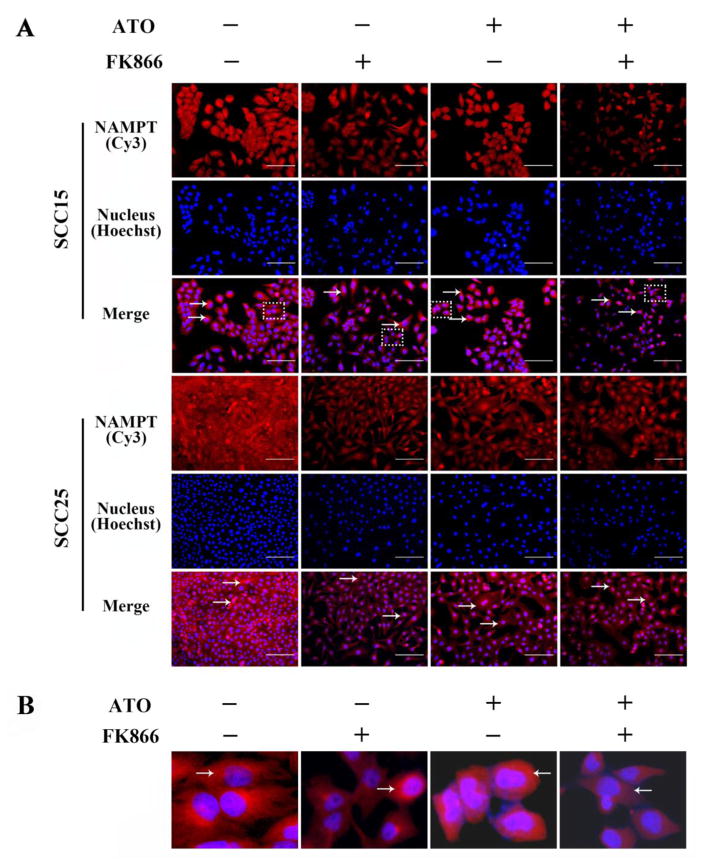
To further investigate the underlying mechanism of enhanced cytotoxicity of ATO combination with FK866 in OSCC cells, the protein and mRNA levels of NAMPT were examined after SCC15 and SCC25 cells were incubated with 2 μM ATO or/and 10 nM FK866 for 48 h. As shown in Figure 5, NAMPT protein expression was stained with red fluorescence and indicated by arrow. A marked decrease in NAMPT expression was found in ATO alone and FK866 alone cells. Importantly, NAMPT protein expression was further inhibited by ATO combined with FK866. In order to display the cytoplasmic distribution of NAMPT, selected areas as marked by dotted boxes in merged images of SCC15 are enlarged to show as Fig 5B.
Figure 5. Enhanced inhibition on the expressions of NAMPT protein in OSCC cells induced by ATO combined with FK866.
After incubation with ATO (2 μM) and FK866 (10 nM) for 48 h in SCC15 and SCC25 cells, NAMPT protein expression was detected by immunofluorescence, which was stained with red fluorescence and indicated by arrow (A), and enlarged areas marked by dotted boxes were shown in (B) to display the cytoplasmic distribution of NAMPT. Bar=100μm.
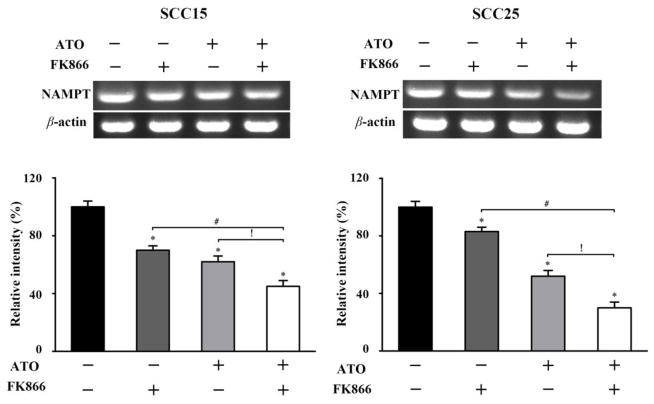
Similar inhibitive efficacy of NAMPT mRNA expression induced by ATO combined with FK866 was observed. After incubation with ATO alone, FK866 alone, or two drugs combination for 48 h, the mRNA expression of NAMPT in both SCC15 and SCC25 cells was markedly reduced with the combination having the greatest effect (Figure 6). These results further suggest that the underlying mechanism of combined ATO with the inhibitor of NAMPT in inducing cancer cell death may be closely correlated with the decreased NAMPT expressions both in the protein and mRNA levels, indicating that NAMPT inhibition may be one of the mechanisms of ATO on suppressing cancer cell survival.
Figure 6. Increased inhibition on the expressions of NAMPT mRNA in OSCC cells by ATO-FK866.
After incubation with ATO (2 μM) and FK866 (10 nM) for 48 h in SCC15 and SCC25 cells, NAMPT mRNA expression was determined by RT-PCR. *p<0.05 vs. untreated cells, #p<0.05 vs. FK866 treated cells, !p<0.05 vs. ATO treated cells.
Low concentration ATO decreased NAD levels and promoted the inhibitive efficacy of FK866 in OSCC cells
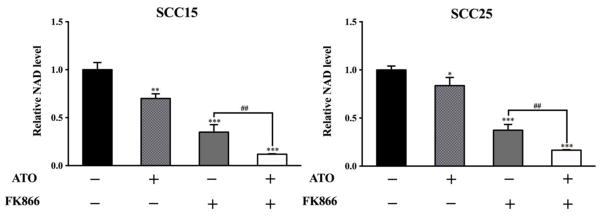
Since NAMPT is critically involved in NAD biosynthesis, we next investigated the effect of ATO-mediated NAMPT inhibition on NAD levels. Intracellular NAD+/NADH content was examined in SCC15 and SCC25 cells after incubation with 2 μM ATO or/and 10 nM FK866 for 48 h. As shown in Figure 7, compared with untreated cells, both ATO and FK866 induced a marked reduction in relative NAD levels with the combination having the most dramatic effect. These data demonstrated that low concentration of ATO could significantly reduce NAD levels in cancer cells and ATO combined with NAMPT inhibitor could further accentuate NAD depletion.
Figure 7. Low concentration ATO reduced NAD levels and accentuated NAD depletion induced by FK866 in OSCC cells.
After incubation with ATO (2 μM) and/or FK866 (10 nM) for 48 h in SCC15 and SCC25 cells, relative NAD levels was detected by NAD+/NADH Quantification Kit. **p<0.01 and ***p<0.001 vs. untreated cells; ##p<0.01 vs. FK866 treated alone cells.
Discussion
NAMPT is expressed in almost all organs, tissues, and cells, and mainly localized in the cytoplasm and nucleus as well as extracellularly (2, 19). In the present study, the protein expression of NAMPT markedly increased in cytoplasm in all three differential grades of OSCC when compared with NAT tissues. It is important to note that the NAMPT protein level was the highest in poorly-differentiated OSCC among three differentiated grades. The results demonstrated a strong positive correlation between the expression of NAMPT and the grades of differentiation of OSCC, implying that NAMPT overexpression might play an important role in development, progression, and prognosis of OSCC.
ATO significantly reduced the cell survival rate of OSCC in a concentration- and time-dependent manner, which is consistent with the literature reports that ATO treatment stimulated a dose-dependent inhibition of cancer cell survival (20). Furthermore, our data showed that NAMPT protein expression was dramatically decreased along with increasing ATO concentrations, suggesting that NAMPT inhibition might be involved in the mechanism of ATO-induced cancer cell death. Similar to this finding, a recent study showed that ATO greatly impacted several metabolomics pathways on gastric carcinoma cells, suggesting that these metabolomics changes could help elucidate the antitumor mechanism of ATO (21). Generally, NAMPT expression is positively regulated by various transcription factors, such as FoxO1 and NF-κB (22, 23). For instance, Cagnetta et al showed that NAMPT inhibition by FK866 induced multiple myeloma cell death, which was associated with the downregulation of NF-κB signaling (23). Arsenite has also been shown to trigger FoxO phosphorylation and inactivation in HaCaT human keratinocytes, and inhibit NF-κB translocation and other NF-κB dependent gene transcription in human bronchial epithelial and embryonic kidney cells (24, 25). Similarly, Mathas et al showed that arsenite inhibited NF-κB activation, and thereby induced cell apoptosis in Hodgkin/Reed-Sternberg cell lines (26). Ganapathy et al also reported that low dose arsenic induced a metabolic shift to glycolysis in human fibroblast, in which NF-κB initiated the glycolytic pathway via upregulation of HIF1α, a transcription factor that is involved in the glycolytic pathway and plays a pivotal role in the Warburg effect in cancer cells for energy metabolism (27, 28). Therefore, it is possible that the mechanisms of ATO inhibiting NAMPT expression might be related to the suppression of FoxO1, NF-κB and HIF1α signaling pathways. Our findings provide direct evidence that NAMPT plays a critical role in cytotoxicity of OSCC induced by ATO, which facilitates the understanding of the efficacy mechanism of ATO on suppressing cancer cells.
NAMPT inhibitors as monotherapy (such as FK866 and GMX1777) have been put into clinical trials, but, up to date, have failed to show significant anti-tumor action (4, 29). Notably, it has been reported that the tumor cells’ sensitivity to NAMPT inhibitors is inversely proportional to the level of NAMPT expression (30, 31). Combined with our results that poorly differentiated OSCC expressed the highest level of NAMPT, it indicates that poorly differentiated cancer cells might be the least sensitive to NAMPT inhibitors. Hence, it would be highly desirable to enhance the efficacy of NAMPT inhibitors on OSCC with other drugs. We hypothesized that combining with ATO might improve the therapeutic efficacy of FK866 in OSCC treatment. In this study, our findings showed that 2 μM ATO exhibited the similar cytotoxicity to 10 nM FK866 in both cancer cell lines. Most noticeably, the regimen of ATO-FK866 combination showed synergistic cytotoxicity even at very low concentration of 2 μM ATO (2–50 μM represents the ATO dosage used in clinics for cancer chemotherapy (32). Meanwhile, cell apoptosis was dramatically increased in both OSCC cell lines after treatment with ATO or ATO-FK866 combination, which underlines the mechanism of the inhibition of cell viability by ATO.
We further investigated the potential mechanism of ATO enhancement of FK866 efficacy by examining their combined effects on the protein and mRNA levels of NAMPT. Both NAMPT protein and mRNA expressions were greatly reduced by ATO combined with FK866, more than each drug alone. Furthermore, ATO induced a significant decline of NAD levels both in SCC15 and SCC25 cells, which was further aggravated by the ATO-FK866 combination. Taken together, our results indicated that inhibition of NAD+ salvage pathway might be an important underlying mechanism of ATO in suppressing cancer cell survival. Moreover, it is interesting to note that inhibition of NAMPT protein and mRNA expressions induced by the ATO-FK866 combination did not exhibit significant synergistic efficacy as that was clearly shown in cytotoxicity, the possible explanation of which may involve the regulation of other signal pathways stimulated by ATO.
Though NAMPT inhibition represents a promising therapeutic approach for cancer, however, NAMPT inhibitors may cause significant toxicity in healthy cells and tissues (29). Besides, use of NAMPT inhibitors has been reported to lead to cancer cell resistance in several tumor cell lines (33). Therefore, combination regimens could be an effective approach to reduce NAMPT inhibitor side effects and resistance. To date, some drug combination strategies relating to NAMPT inhibitors have been reported. Combination with drugs that can produce DNA damage or inhibit DNA damage repair would be a promising strategy (34). Our previous research suggested that ATO inhibits oxidative DNA damage repair in human keratinocytes (35). Although it has been known that ATO (as a single agent) has strict limitation in clinical patients who are diagnosed with malignant solid tumors (such as OSCC) because of its side effects, recent studies have reported that therapeutic dose of ATO is clinically tolerable in advanced head and neck squamous cell carcinoma patients (36). In the present study, we demonstrated the possibility that ATO in combination with NAMPT inhibitor could increase the therapeutic effect for OSCC. Consequently, we suggest that the combination of low concentration ATO with the inhibitors of NAMPT could be a novel therapeutic strategy, which might provide new opportunities to achieve effective treatment in the clinic.
In conclusion, our findings indicate that NAMPT is overexpressed in OSCC when compared with normal and adjacent tissues, and increased NAMPT expression is highly associated with cellular differentiation grades of OSCC, indicating that NAMPT may participate in the development, progression and prognosis of OSCC. These findings may have high implications for exploring the NAMPT pathway for OSCC treatment in clinic. Moreover, ATO can inhibit cell viability via depressing NAMPT transcription and translation and interfering NAD biosynthesis in OSCC. The identification of NAMPT as an important target of ATO opens new perspectives for optimizing the use of this ancient remedy in future OSCC therapies. More importantly, synergistic inhibitive efficacy of ATO combined with FK866 may provide a novel therapeutic strategy for OSCC patients and possibility in overcoming NAMPT inhibitor mediated therapeutic resistance and toxicity, or as a valuable adjuvant therapeutic modality for advanced OSCC refractory to other treatments. Further studies to explore this hypothesis are warranted.
Highlights.
Level of overexpressed NAMPT is associated with differential grade of OSCC patients
Arsenic trioxide (ATO) exerts anti-cancer effects through NAMPT inhibition
ATO enhances tumor cell killing efficacy of NAMPT inhibitor through depleting NAD
Combined ATO/NAMPT inhibitor may be a novel therapy for advanced OSCC patients
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (81371161) and a grant from Science and Technology Plan Foundation of Liaoning Province (2014225003).
Footnotes
Conflict of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8:11884–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–7. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:67–76. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 6.Chiarugi A, Dölle C, Felici R, Ziegler M. The NAD metabolome--a key determinant of cancer cell biology. Nat Rev Cancer. 2012;12:741–52. doi: 10.1038/nrc3340. [DOI] [PubMed] [Google Scholar]
- 7.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–42. [PubMed] [Google Scholar]
- 8.Galli U, Travelli C, Massarotti A, Fakhfouri G, Rahimian R, Tron GC, et al. Medicinal chemistry of nicotinamide phosphoribosyltransferase (NAMPT) inhibitors. J Med Chem. 2013;56:6279–96. doi: 10.1021/jm4001049. [DOI] [PubMed] [Google Scholar]
- 9.Vora M, Ansari J, Shanti RM, Veillon D, Cotelingam J, Coppola D, et al. Increased nicotinamide phosphoribosyltransferase in rhabdomyosarcomas and leiomyosarcomas compared to skeletal and smooth muscle tissue. Anticancer Res. 2016;36:503–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Bi TQ, Che XM, Liao XH, Zhang DJ, Long HL, Li HJ, et al. Overexpression of Nampt in gastric cancer and chemopotentiating effects of the Nampt inhibitor FK866 in combination with fluorouracil. Oncol Rep. 2011;26:1251–7. doi: 10.3892/or.2011.1378. [DOI] [PubMed] [Google Scholar]
- 11.Gehrke I, Bouchard ED, Beiggi S, Poeppl AG, Johnston JB, Gibson SB, et al. Clin. Cancer Res. 2014;20:4861–72. doi: 10.1158/1078-0432.CCR-14-0624. [DOI] [PubMed] [Google Scholar]
- 12.Soncini D, Caffa I, Zoppoli G, Cea M, Cagnetta A, Passalacqua M, et al. Nicotinamide phosphoribosyltransferase promotes epithelial- to-mesenchymal transition as a soluble factor independent of its enzymatic activity. J Biol Chem. 2014;289:34189–204. doi: 10.1074/jbc.M114.594721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–60. [PubMed] [Google Scholar]
- 14.Uslu R, Sanli UA, Sezgin C, Karabulut B, Terzioglu E, Omay SB, et al. Arsenic trioxide- mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin Cancer Res. 2000;6:4957–64. [PubMed] [Google Scholar]
- 15.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–61. [PubMed] [Google Scholar]
- 16.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite- induced apoptosis. J Cell Physiol. 1998;177:324–33. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, et al. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–8. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 18.Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV, et al. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood. 2009;113:3276–86. doi: 10.1182/blood-2008-08-173369. [DOI] [PubMed] [Google Scholar]
- 19.Kitani T, Okuno S, Fujisawa H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor 1. FEBS Lett. 2003;544:74–8. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Gao Q, Ning Y, Wang Z, Krebsbach PH, Polverini PJ. Arsenic trioxide enhances the therapeutic efficacy of radiation treatment of oral squamous carcinoma while protecting bone. Mol Cancer Ther. 2008;7:2060–9. doi: 10.1158/1535-7163.MCT-08-0287. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Zhang H, Yang L, Jiang H, Guo S, Li Y, et al. Construction of a metabolomics profile of arsenic trioxide effect in gastric carcinoma cell line SGC7901. Acta Biochim Biophys Sin. 2016;48:474–81. doi: 10.1093/abbs/gmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao R, Wei D, Gao H, Liu Y, DePinho RA, Dong XC. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem. 2011;286:14681–90. doi: 10.1074/jbc.M110.201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cagnetta A, Cea M, Calimeri T, Acharya C, Fulciniti M, Tai YT, et al. Intracellular NAD depletion enhances bortezomib- induced anti- myeloma activity. Blood. 2013;122:1243–55. doi: 10.1182/blood-2013-02-483511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamann I, Klotz LO. Arsenite- induced stress signaling: modulation of the phosphoinositide 3′-kinase/Akt/FoxO signaling cascade. Redox Biol. 2013;1:104–9. doi: 10.1016/j.redox.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roussel RR, Barchowsky A. Arsenic inhibits NF-κB-mediated gene transcription by blocking IκB kinase activity and IκBalpha phosphorylation and degradation. Arch Biochem Biophys. 2000;377:204–12. doi: 10.1006/abbi.2000.1770. [DOI] [PubMed] [Google Scholar]
- 26.Mathas S, Lietz A, Janz M, Hinz M, Jundt F, Scheidereit C, et al. Inhibition of NF-kappaB essentially contributes to arsenic- induced apoptosis. Blood. 2003;102:1028–34. doi: 10.1182/blood-2002-04-1154. [DOI] [PubMed] [Google Scholar]
- 27.Ganapathy S, Xiao S, Seo SJ, Lall R, Yang M, Xu T, et al. Low-dose arsenic induces chemotherapy protection via p53/NF-κB- mediated metabolic regulation. Oncogene. 2014;33:1359–66. doi: 10.1038/onc.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 29.von Heideman A, Berglund A, Larsson R, Nygren P. Safety and efficacy of NAD depleting cancer drugs: results of a phase I clinical trial of CHS 828 and overview of published data. Cancer Chemother Pharmacol. 2010;65:1165–72. doi: 10.1007/s00280-009-1125-3. [DOI] [PubMed] [Google Scholar]
- 30.Olesen UH, Hastrup N, Sehested M. Expression patterns of nicotinamide phosphoribosyltransferase and nicotinic acid phosphoribosyltransferase in human malignant lymphomas. APMIS. 2011;119:296–303. doi: 10.1111/j.1600-0463.2011.02733.x. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Y, Elkins K, Durieux JK, Lee L, Oeh J, Yang LX, et al. Dependence of tumor cell lines and patient-derived tumors on the NAD salvage pathway renders them sensitive to NAMPT inhibition with GNE-618. Neoplasia. 2013;15:1151–60. doi: 10.1593/neo.131304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo Q, Li Y, Lai Y, Zhang Z. The role of NF-κB in PARP- inhibitor- mediated sensitization and detoxification of arsenic trioxide in hepatocellular carcinoma cells. J Toxicol Sci. 2015;40:349–63. doi: 10.2131/jts.40.349. [DOI] [PubMed] [Google Scholar]
- 33.Olesen UH, Petersen JG, Garten A, Kiess W, Yoshino J, Imai S, et al. Target enzyme mutations are the molecular basis for resistance towards pharmacological inhibition of nicotinamide phosphoribosyltransferase. BMC Cancer. 2010;10:1–13. doi: 10.1186/1471-2407-10-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jieyu H, Chao T, Mengjun L, Shalong W, Xiaomei G, Jianfeng L, et al. Nampt/Visfatin/PBEF: a functionally multi- faceted protein with a pivotal role in malignant tumors. Curr Pharm Des. 2012;18:6123–32. doi: 10.2174/138161212803582531. [DOI] [PubMed] [Google Scholar]
- 35.Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, et al. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem. 2009;284:6809–17. doi: 10.1074/jbc.M805566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huilgol NG. A phase I study to study arsenic trioxide with radiation and hyperthermia in advanced head and neck cancer. Int J Hyperthermia. 2006;22:391–7. doi: 10.1080/02656730600722685. [DOI] [PubMed] [Google Scholar]