Abstract
Background
Total parenteral nutrition (TPN) administered via central venous catheter has been identified as an independent risk factor for central line-associated bloodstream infections (CLABSIs). The aim of this study was to provide an updated description of the relationship between TPN and CLABSI and assess temporal trends in CLABSI rates for individuals who received TPN in the period 2009 to 2014, after CMS declared CLABSI a “never event”.
Methods
Using data obtained from all adult patient discharges between January 1, 2009 and December 31, 2014 from two affiliated hospitals in a large health system in New York City, univariate and multivariate analyses were performed to examine the relationship between TPN and CLABSIs as well as temporal trends.
Results
Among 38,674 patients with central lines, 3,517 developed CLABSI and 767 patients were prescribed TPN. TPN was an independent risk factor for developing CLABSI among our patients (OR, 2.65; 95% CI, 2.20-3.19). The incidence of CLABSI among patients who were prescribed TPN was not significantly different across the years of this study even after adjusting for severity of illness.
Conclusion
TPN remains a significant risk factor for CLABSIs; further work is needed to identify effective strategies to reduce rates of CLABSI among patients receiving TPN.
Introduction
Healthcare-associated infections (HAIs) have long been a concern for hospitals, both for the health and safety of patients as well as the financial burden for hospitals, insurers and patients. An estimated 1 in every 25 patients has an HAI on any given day1; approximately 722,000 HAIs occurred in the year 2011 with just over 10% of affected patients with HAIs dying during their hospitalization2. In a recent study, 14% of HAIs were identified as central line-associated bloodstream infections (CLABSIs).2 CLABSIs cost up to an estimated $56,000 per patient, and are associated with an increased hospital length of stay of about three weeks as well as an increased mortality of 14-40%.3,4
Although the incidence of CLABSI has been decreasing at a national level, in part as a result of hospital level infection control initiatives such as increased adherence to proper hand hygiene and improved catheter insertion practices4, there are still a considerable number each year.2 A 2014 report from the Centers for Disease Control and Prevention (CDC) estimated that a total of 30,100 CLABSIs still occur nationally every year.1
Total parenteral nutrition (TPN) administered via central venous catheter has been identified as an independent risk factor or developing CLABSIs.6,7,8 A previous study in a large tertiary care hospital in New York City reported that patients who received TPN from September 2007 through December 2008 had an increased risk of CLABSI (OR, 4.33; 95% CI, 2.50-7.48).8 This study was concluded just as the Centers for Medicare and Medicaid Services issued new regulations related to reimbursement for CLABSI making them a “never event”. These regulations deemed that hospitals would not receive payment for added costs of caring for CLABSI or seven other HAI, since HAIs are preventable.9 An exhaustive CLABSI prevention policy and protocol (Table 1), along with extensive education and strict monitoring has been in place at our institution since 2008. Modified as new national guidelines are issued, the policy reflects standards published by CDC and professional organizations.
Table 1.
Highlights of Procedures for CLABSI Prevention
| Insertion: |
| Practitioner notification to nurse |
| Time out performed and documented on templated note in EMR |
| Non-sterile assistant must be present |
| Central line cart in ICUs |
| Hand hygiene and sterile gloves |
| 2% chlorhexidine and 70% isopropyl alcohol site prep |
| After prep, change gloves and repeat hand hygiene |
| Sterile gowns and gloves, mask and caps (including supervisory staff) |
| Small sterile fenestrated drape over site |
| Large sterile drape covers patient head to toes |
| Ultrasonographic guidance when possible |
| After insertion remove drape and gloves |
| Repeat hand hygiene and don sterile gloves |
| Re-prep site as above |
| Sterile transparent semipermeable CHG dressing |
| Maintenance: |
| Assess and document necessity daily |
| Remove within 24 hours if inserted in emergent setting |
| Remove promptly if not needed |
| Remove if suspected to be source of infection |
| Guidewire exchanges discouraged |
| Examine dressing and site every shift |
| Change dressing weekly |
| Needleless endcaps on each lumen |
| Cap with alcohol-impregnated caps when not in use |
| Clean hub with alcohol every time accessed |
| Use dedicated catheter port for PN |
| Documentation using flowsheets and templates in EMR |
There has been no updated description of the relative risk of CLABSI among hospitalized patients receiving TPN since the national policies were instituted. Therefore, the aim of this study was to describe the relationship between TPN and CLABSI and assess temporal trends in CLABSI rates for patients who received TPN during the years 2009 through 2014.
Methods
Study Design and Setting
Data were obtained from all adult patient discharges between January 1, 2009 and December 31, 2014 in the two affiliated hospitals of Columbia University Medical Center, a large health system in New York City: a 745-bed tertiary-care hospital which included 6 ICUs and more than 40 operating rooms, and a 220-bed community hospital. The larger hospital averaged 44,000 discharges each year during the study period while the smaller hospital had an average of 12,000 discharges each year. The medical center has a robust hospital system-wide infection prevention and control program, which was in place during the study period. During the years of the study, there totaled approximately 200 prescriptions for TPN each year at both hospitals, combined. A nutrition support team composed of physicians, dieticians, pharmacists and nurses monitored the administration of TPN. A large compounding pharmacy was used for the outsourced compounding of TPN for both hospitals. The Institutional Review Board at Columbia University Medical Center approved this research study.
Definitions
CLABSIs were identified using previously validated computerized algorithms which search the electronic medical record. The diagnosis is based on definitions issued by the CDC and NHSN.10, 11 For this study, CLABSI was defined as a laboratory-confirmed BSI in a patient with a central line in place for at least 48 hours prior to the onset of symptoms and unrelated to an infection from another site. This definition was utilized in our previous study in which we validated by medical record review that the results were completely congruent, i.e., that patients defined with this algorithm did indeed have CLABSI.8
Data Collection
Data were obtained from the Healthcare Information Technology to Reduce Healthcare Associated Infections (HIT-HAI) electronic database (R01NR010822). The following data were collected from a number of electronic sources from discharges among 38,674 adult patients who received a central line between January 1, 2009 and December 31, 2014: age, sex, dates of admission and discharge, hospital, ICU stay (yes/no), dates and duration of catheter insertion and removal, Charlson Comorbidity Index (CCI)12, medications received, HIV status, renal disease, transplant, diabetes, malignancy, and presence of an HAI (bloodstream or pneumonia). CCI is routinely calculated for all of our hospitalized patients. TPN exposure was identified by search of electronic medical records by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure code. Dates of exposure were then obtained by manual search of the record.
Statistical Analysis
Chi-square or Student's t-tests were initially performed on multiple putative risk factors, used in our prior study, and derived from a review of relevant literature, to determine which had an association with CLABSI in our cohort. Variables with p-value <0.05 were used to build the multivariable model. Multivariable regression analysis was performed to evaluate the relationship between TPN and the occurrence of CLABSI when adjusting for variables significant in univariate analyses. To assess temporal trends, the proportion of individuals who developed a CLABSI while receiving TPN, Chi-square test was performed to detect a change in incidence year to year over the study period. To adjust for severity of illness, univariate analyses were performed on variables used as a proxy for severity of illness, including comorbid diagnoses and CCI. Variables associated with CLABSI with a p-value <0.05 were included in the multivariable model. Multivariable regression analysis was performed to evaluate year of TPN receipt as a predictor of CLABSI when adjusting for severity of illness. All statistical analyses were performed using SAS (Version 9.4; Cary, North Carolina, USA).
Results
The analysis included a total of 38,674 patients with a central line. There were 3,517 CLABSIs and 767 patients received TPN during the period of this study. Individuals who received TPN did not differ in CCI, but were younger, more likely to be female, have an ICU stay, and had a longer duration of catheterization than individuals who did not receive TPN (Table 2).
Table 2.
Characteristics of patients with and without TPN who had a central line
| Characteristics | TPN (n=767) | No TPN (n= 37,907) | p-value |
|---|---|---|---|
| Age, mean (SD), y | 58.5 (17.5) | 62.1 (16.9) | <.0001 |
| Charlson Comorbidity Index, mean (SD) | 3.0 (3.1) | 2.8 (2.5) | 0.12 |
| Duration of catheterization, mean (SD), d | 19.4 (26.2) | 7.6 (13.1) | <.0001 |
| Male sex % | 48 | 53.7 | 0.002 |
| Intensive care unit stay % | 46.4 | 55.2 | <.0001 |
TPN, total parenteral nutrition; SD, standard deviation
In univariate analyses, TPN, duration of catheterization, malignancy, diabetes, HIV, renal failure, history of transplant, ICU stay and pneumonia were associated with CLABSI (Table 3). Multivariable regression analysis demonstrated that TPN (OR, 2.65; 95% CI, 2.20-3.19), catheterization duration (OR, 1.03; 95% CI, 1.02-1.03), malignancy (OR, 1.41; 95% CI, 1.27-1.57), diabetes (OR, 1.11; 95% CI, 1.02-1.21), HIV (OR, 2.27; 95% CI, 1.76-2.93), renal failure (OR, 2.45; 95% CI, 2.26-2.67), ICU stay (OR, 1.20; 95% CI, 1.11-1.30), and pneumonia (OR, 1.35; 95% CI, 1.19-1.54) were significant risk factors for the occurrence of CLABSI. History of transplant (OR, 0.41; 95% CI, 0.33-0.50) was associated with a decreased risk of CLABSI (Table 4).
Table 3.
Characteristics of patients with and without CLABSI
| Risk Factors | CLABSI (n=3517) | No CLABSI (n=35,157) | p-value |
|---|---|---|---|
| Age, mean (SD), y | 62.1 | 62.0 | 0.92 |
| Charlson comorbidity index, mean (SD) | 3.4 (2.6) | 2.7 (2.5) | <.0001 |
| Catheterization duration, mean (SD), d | 18.4 (26.6) | 6.8 (11.0) | <.0001 |
| TPN, % | 5.67 | 1.61 | <.0001 |
| Malignancy, % | 22.7 | 19.7 | <.0001 |
| Diabetes, % | 33.4 | 28.3 | <.0001 |
| HIV, % | 2.9 | 1.1 | <.0001 |
| Renal failure, % | 64.5 | 37.6 | <.0001 |
| History of transplant, % | 3.4 | 4.7 | 0.0008 |
| ICU stay, % | 65.4 | 53.9 | <.0001 |
| Immunodeficiency, % | 0.5 | 0.4 | 0.08 |
| Pneumonia, % | 12.4 | 5.1 | <.0001 |
TPN, total parenteral nutrition; CLABSI, central line-associated bloodstream infections; HIV, human immunodeficiency virus; ICU, intensive care unit
Table 4.
Risk factors for CLABSI, multivariate analysis
| Risk Factors | OR | 95% CI | p-value |
|---|---|---|---|
| TPN | 2.65 | 2.20-3.19 | <.0001 |
| Catheter duration | 1.03 | 1.02-1.03 | <.0001 |
| Malignancy | 1.41 | 1.27-1.57 | <.0001 |
| Diabetes | 1.11 | 1.02-1.21 | 0.015 |
| HIV | 2.27 | 1.76-2.93 | <.0001 |
| Renal failure | 2.45 | 2.26-2.67 | <.0001 |
| History of transplant | 0.41 | 0.33-0.50 | <.0001 |
| ICU stay | 1.20 | 1.11-1.30 | <.0001 |
| Pneumonia | 1.35 | 1.19-1.54 | <.0001 |
CLABSI, central line-associated bloodstream infection; TPN, total parenteral nutrition; HIV, human immunodeficiency virus; ICU, intensive care unit
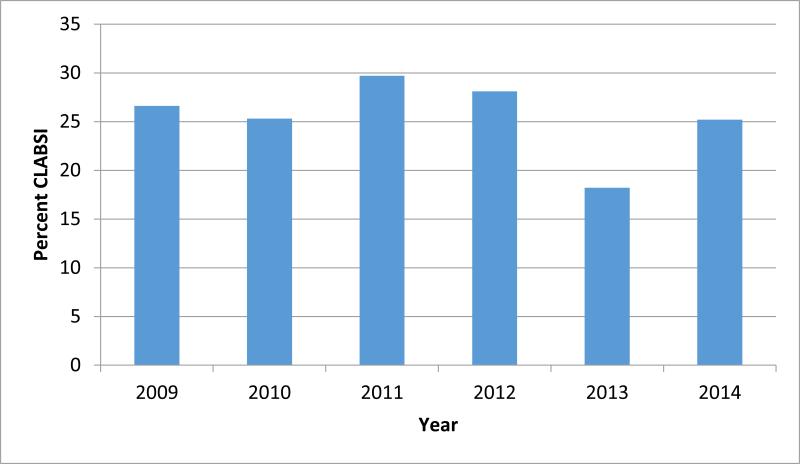
The incidence of CLABSI among individuals who were prescribed TPN was not significantly different across the years in this study (Chi-square p-value= 0.51) (Figure 1). Additionally, after adjusting for severity of illness, the year of TPN receipt was not a significant predictor of the occurrence of CLABSI.
Figure 1.
Percent of patients who developed CLABSI among all patients with a central line receiving TPN (chi-square p-value = 0.51).
CLABSI, central line associated blood stream infections
TPN, total parenteral nutrition
Discussion
This study confirms what others have reported, that TPN is a significant risk factor for the occurrence of CLABSI.6,8 Beghetto et al. reported that individuals in their study population who received TPN had a higher risk of developing CLABSI than patients who did not receive TPN (RR, 3.30; 95% CI, 1.30-8.34).6 Rates of CLABSI, in general, are decreasing across the nation.5 In the same hospitals included in our study during previous years (2006-8), Ippolito et al. found that individuals who received TPN had higher odds of developing a CLABSI compared to patients who did not receive TPN (OR, 4.33; 95% CI, 2.50-7.48).8
While rates of TPN-related CLABSI did not decrease in our cohort during the time period studied, the odds ratio reported in this study (OR, 2.65; CI, 2.20-3.19) is lower by approximately half those previously reported. A lack of decrease during the period may be due to a small sample size, given the relatively low volume of TPN in our institution, or may be the result of a drop in rates due to proactive measures taken in anticipation to the Centers for Medicare and Medicaid (CMS) Hospital-Acquired Conditions Initiative.9 Several studies have evaluated the role of the CMS Initiative in the reduction of CLABSIs. Waters et al. observed a decrease in CLABSI rate of 11% associated with the implementation of the CMS initiative.13 Lee et al. also observed a decrease in CLABSIs after the CMS initiative.14 Contrary to these results, Vaz et al. reported no difference in the rate of CLABSI before and after the CMS initiative.15
The purpose of the current study was to assess CLABSI incidence associated with TPN. In this study setting, TPN is ordered only by the nutrition support service and there is an extremely low TPN usage rate (average 5 per day in 1000 quaternary care hospital beds). Hence the indications for TPN were stringently evaluated by the same team over the entire study period. Despite finding a reduction in the relative contribution of TPN to CLABSI as compared to our prior study, these data are observational. Until randomized studies demonstrate that the safety and efficacy of TPN exceeds that of no intervention and/or enteral nutrition, it is important in any setting to consider alternative methods of nutrition support and strategies to reduce adverse events associated with TPN.
Limitations of this study include the use of electronic medical records, which may have missing or inaccurately recorded information. Further, we were able to study only diagnoses and comorbid conditions available in our data bases. This is of particular concern since electronic diagnosis and procedure codes are primarily used for billing purposes.16 No causal analysis between TPN and CLABSI was performed in this study, so causality between TPN and CLABSIs cannot be inferred. Additionally, the results of this study cannot be generalized to populations outside of the population of patients included in this study.
Conclusions
Policy changes such as the CMS HAI Initiative have increased awareness of HAIs and pushed hospitals to make changes to reduce their occurrence. Identifying patient populations at higher risk for developing HAIs can aid hospitals to target preventive measures at those patients. Because the results of this study indicate that TPN remains a significant predictor of CLABSI and the rates of CLABSI, additional preventive measures may be necessary to reduce the incidence of CLABSIs among the population of patients receiving TPN.
Clinical Relevancy Statement.
Our study found that adult patients receiving total parenteral nutrition (TPN) experienced an increased risk for developing a central line-associated bloodstream infection (CLABSI). This result was consistent with a previous study performed at the same hospital, and spanned a much larger and more recent period of time. The results of this study demonstrate the need for additional guidelines and practices to help reduce CLABSIs in this population of patients.
Acknowledgements
Financial support. This work was supported by the National Institute of Nursing Research (grant R01NR010822) and the National Heart, Lung and Blood Institute (Burgermaster – training grant T32 HL 7343-37).
The authors would like to thank Fred Mis, Bevin Cohen and Jianfang Liu for assistance with data acquisition.
References
- 1.CDC National and State Healthcare-Associated Infections Progress Report. published March 2014, available at http://www.cdc.gov/HAI/pdfs/progress-report/hai-progress-report.pdf.
- 2.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens V, et al. Inpatient costs, mortality and 30-day re-admission in patients with central-line associated bloodstream infections. Clin Microbiol Infect. 2014;20:O318–24. doi: 10.1111/1469-0691.12407. [DOI] [PubMed] [Google Scholar]
- 4.Goudie A, Dynan L, Brady PW, Rettiganti M. Attributable cost and length of stay for central line-associated bloodstream infections. Pediatrics. 2014;133:e1525–32. doi: 10.1542/peds.2013-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beghetto MG, Victorino J, Teixeira L, de Azevedo MJ. Parenteral nutrition as a risk factor for central venous catheter-related infection. JPEN. Journal of parenteral and enteral nutrition. 2005;29(5):367–373. doi: 10.1177/0148607105029005367. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz G, Koksal I, Aydin K, et al. Risk factors of catheter-related bloodstream infections in parenteral nutrition catheterization. JPEN. Journal of parenteral and enteral nutrition. 2007;31(4):284–287. doi: 10.1177/0148607107031004284. [DOI] [PubMed] [Google Scholar]
- 8.Ippolito P, Larson EL, Furuya EY, et al. Utility of Electronic Medical Records to Assess the Relationship Between Parenteral Nutrition and Central Line–Associated Bloodstream Infections in Adult Hospitalized Patients. Journal of Parenteral and Enteral Nutrition. :0148607114536580. doi: 10.1177/0148607114536580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for M, Medicaid Services HHS Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Federal register. 2007;72(162):47129–48175. [PubMed] [Google Scholar]
- 10.National Healthcare Safety Network (NHSN) Device-associated module: CLABSI. http://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf.
- 11.Apte M, Neidell M, Furuya EY, Caplan D, Giled S, Larson E. Using electronically available inpatient hospital data for research. Clin Transl Sci. 2011;4:338–345. doi: 10.1111/j.1752-8062.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson M, Szatrowski TP, Petersn J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 13.Waters TM, Daniels MJ, Bazzoli GJ, et al. Effect of Medicare's nonpayment for Hospital-Acquired Conditions: lessons for future policy. JAMA internal medicine. 2015 Mar;175(3):347–354. doi: 10.1001/jamainternmed.2014.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in U.S. hospitals. The New England journal of medicine. 2012;367(15):1428–1437. doi: 10.1056/NEJMsa1202419. [DOI] [PubMed] [Google Scholar]
- 15.Vaz LE, Kleinman KP, Kawai AT, et al. Impact of Medicare's Hospital-Acquired Condition policy on infections in safety net and non-safety net hospitals. Infection control and hospital epidemiology. 2015;36(6):649–655. doi: 10.1017/ice.2015.38. [DOI] [PubMed] [Google Scholar]
- 16.Sant VR, Arnell TD, Slattery E. Seres DS Gastrostomy Complications: An Audit of Diagnostic Codes As a Poor Measure of Incidence. JPEN J Parenter Enteral Nutr. 2015 Feb;39(2):231–256. abstract S-48. [Google Scholar]