Abstract
Dim light at night (dLAN) disrupts circadian organization and influences adult behavior. We examined early dLAN exposure on adult affective responses. Beginning 3 (juvenile) or 5 weeks (adolescent) of age, mice were maintained in standard light-dark cycles or exposed to nightly dLAN (5 lux) for 5 weeks, then anxiety-like and fear responses were assessed. Hypothalami were collected around the clock to assess core clock genes. Exposure to dLAN at either age increased anxiety-like responses in adults. Clock and Rev-ERB expression were altered by exposure to dLAN. In contrast to adults, dLAN exposure during early life increases anxiety and fear behavior.
Keywords: light at night, anxiety, fear conditioning, circadian disruption, early life
Introduction
For the vast majority of the earth’s history, life has evolved under brightly illuminated days and dark nights. Consistent with the solar day, most organisms display endogenous ~24-hour, or circadian, rhythms entrained to this external light-dark cycle. Within the past century, however, the widespread adoption of electrical lighting has eliminated clear distinctions between day and night. The technology to provide bright light at night was adopted without a clear understanding of its implications on biology and health. Laboratory, ecological, and epidemiological studies suggest that exposure to light at night (LAN) negatively affects adult circadian (de Jong et al., 2016; Dominoni et al., 2014), metabolic (Fonken et al., 2010; McFadden et al., 2014), immune (Stevens, 2009), as well as affect (Bedrosian et al., 2013). Specifically, chronic exposure to light at night dampens amplitudes of central and peripheral molecular circadian rhythms (Fonken et al., 2013; Shuboni & Yan, 2010), decreases anxiety-like behaviors (Hogan et al., 2015), and increases depressive-like responses (Bedrosian et al., 2013; LeGates et al., 2012) in adult rodents.
In contrast to adults, little is known about the effects of light at night during development when the circadian and other physiological systems are still developing. Children are also exposed to dim light at night; watching television is a common component of many children’s bedtime routines (Owens et al., 1999) and >30% of American children have televisions in their bedrooms (Cespedes et al., 2014). Thus, the potential exists for common nighttime use of electronics by children and adolescents to affect development of the circadian system.
The suprachiasmatic nucleus (SCN) stands at the top of the circadian clock hierarchy, in that it sets the phase of peripheral clocks located throughout the body. In mice the SCN is the first tissue to stabilize the adult phase of rhythmic clock gene expression, typically by 2 weeks of age (Christ et al., 2012). Setting of adult phase in clock gene expression does not stabilize in some peripheral tissues until 8 weeks of age in most rodents (Sládek et al., 2007; Sumová et al., 2006). These rhythms are first driven mainly by maternal signals of the external lighting environment and continue to develop after weaning. Therefore, disruptions of circadian rhythms prior to weaning may have indirect maternal effects. Indeed, mice raised by Clock mutant mothers (Koizumi et al., 2013) or under non-standard lighting conditions, LAN (Borniger et al., 2014) or in short day lengths (Toki et al., 2007), develop anxiety-like behaviors in adulthood, suggesting an important role for the early life lighting environment on affective development.
The early life environment can affect various aspects of behavior and physiology well into adulthood (Gluckman et al., 2008). Maternal and early postnatal exposure to physical (Eiland et al., 2012), immune (Lin et al., 2012), and dietary (Bilbo & Tsang, 2010; Sullivan et al., 2010) stressors increase anxiety- and depressive-like responses in adulthood. These behavioral changes are accompanied by persisting changes in neuronal morphology and hypothalamic-pituitary-adrenal (HPA) reactivity (Bilbo & Tsang, 2010; Eiland et al., 2012; Koehl et al., 1999; Lin et al., 2012; Sullivan et al., 2010). Studies thus far have focused on early developmental time points despite the continued development of the brain into late adolescence (Cunningham et al., 2002; Juraska & Markham, 2004; Spear, 2000). The brains of juvenile (3–4 wks) and adolescent (5 wks) mice are responsive to stressors that subsequently increases anxiety-like behavior in adulthood suggesting a wider window of vulnerability (Boitard et al., 2015; Isgor et al., 2004; Tsoory & Richter-Levin, 2006). We predicted that circadian disruption through exposure to dLAN, beginning at a juvenile (3 wk) and adolescent (5 wk) developmental epoch would also adult anxiety and fear-like behavior in adulthood.
Methods
Mice
Adult male and female Swiss-Webster mice (7 weeks of age) were obtained from Charles River Laboratories to serve as breeding pairs. Mice were housed in heterosexual pairs in polypropylene cages (30 × 15 × 14 cm) at an ambient temperature of 22 ± 1°C, relative humidity of 25± 10%, and 14:10 h light dark cycles with lights on at 2:00 h and lights off at 16:00 h Eastern Time. Regular chow (Harlan Teklad 8640; Madison, WI) and filtered tap water were available ad libitum. Pups obtained from these pairings were housed individually after weaning. All experimental procedures were approved by The Ohio State University Institutional Animal Care and Use Committee, and animals were maintained in accordance with the recommendations of the National Institutes of Health and The Guide for the Care and Use of Laboratory Animals.
Experiment 1. Effect of juvenile and adolescent exposure to light at night on fear behavior
Male and female offspring were reared in standard lighting conditions (LD: 16: 8 h ~130 lux/0 lux) until 3 (n = 64) and 5 (n = 59) weeks of age, at which point they were randomly separated into LD (n = 60) and dim LAN (n = 63; dLAN, 16:8 h ~130 lux/5 lux) conditions. After 5 weeks in their respective lighting conditions mice underwent behavioral testing consisting of open field, elevated plus, and fear conditioning over the course of 5 days. Day 1 consisted of open field testing, day 2 elevated plus, no testing occurred on day 3, day 4–5 fear conditioning. The acquisition and context trials of fear conditioning were conducted during the mid- light phase (0900–1300 h): all other tests were conducted during the early dark phase (1500- 1900 h). Animals were acclimated to the testing room for a minimum of 20 minutes prior to testing. The open field and fear conditioning tests were scored automatically with computerized software as described below. Elevated plus was scored via video by an observer unaware of experimental conditions using the Observer XT 8.0 software (Noldus Information Technology, Leesburg, VA, USA).
Experiment 2. Early life exposure to light at night on clock gene expression
Male and female offspring were reared in standard lighting conditions until 3 and 5 weeks of age and separated into LD and dLAN lighting conditions as described above. After 6 weeks in their respective lighting conditions, mice were killed at 4 time points around the clock, Zeitgeber Time (ZT) 2, 8, 14, and 20. Mice were anesthetized with isoflurane vapors and rapidly decapitated. Brains were collected and placed in RNAlater to facilitate subsequent dissection of the hypothalamus for analysis of clock gene expression using qPCR.
Open Field
The light- and sound- controlled open field chambers contain a 40×40 cm acrylic box surrounded by stacked infrared beam emitter/detectors (San Diego Instruments, Inc., San Diego, CA, USA) to distinguish between horizontal and vertical movement. Mice were placed in the center of acrylic boxes and locomotor activity was recorded for 20 minutes using Photobeam Activity System (PAS) software (San Diego Instruments, San Diego, CA, USA). Activity counts are defined as interruptions in the infrared light sources by the mouse. Total activity, amount of activity in the center of the arena, and number of rears were analyzed.
Elevated Plus Maze
The elevated plus maze apparatus consists of 2 exposed and 2 enclosed (15cm high) arms (67 × 5.5 cm). Mice were placed in the center of the platform facing an open arm and behavior was videotaped for 5 minutes. Recordings were analyzed for latency to enter open arm, number of visits to open arms, and total time spent in open arms.
Fear Conditioning
The fear conditioning apparatus (MedAssociates, Inc., Georgia, VT, USA) consists of a light- and sound- controlled Plexiglas chamber with a metal grid floor, through which a mild electric shock can be administered. A video camera is mounted to the door of the chamber to record animal movement. “Freezing” behavior was analyzed and quantified using VideoFreeze software (Med Associates). Day 1, following 3 minutes of acclimation to the chamber, animals were presented with a series of 7 tones (3500 Hz, 80 dB) for 20 seconds (conditioned stimulus). During the last two seconds of the stimuli, a 0.6 mA foot shock was administered (unconditioned stimulus). After the last tone, animals were left in the chamber for an additional 60 seconds before being returned to their home cage. Day 2, animals were tested for context-dependent fear behavior by recording freezing behavior in response to the testing chamber in the absence of any tone or shock for three minutes. During the dark phase of that same day, animals were tested for amygdala dependent cue conditioning in a novel environment in the absence of a foot shock. The chamber shape was changed into a semi-circle using a white insert and the metal grid flooring was covered by a white panel. Additionally, white lighting was eliminated and a vanilla scent was added to the environment. The tone paradigm is identical to day 1 with the exception of the shock. The entire test is programmed and controlled by VideoFreeze.
qPCR
Collected tissues were homogenized and total RNA extracted using Trizol. RNA was reverse transcribed into cDNA using M-MLV Reverse Transcriptase enzyme (Invitrogen, Carlsbad, CA, USA). Clock gene expression (Clock, Bmal1, Per1, Cry2, and Rev-ERB) were assessed in the whole hypothalamus.
Statistical Analyses
Comparisons of central tendency, time spent in open arms between groups were conducted using a two-way ANOVA with diet, lighting condition, and sex as between subject factors. Freezing behavior was analyzed using Student’s t-tests. Unevenly distributed data were assessed for main effects using Mann-Whitney U tests and interactions using Kruskal-Wallis tests. Post hoc tests of statistically significant interactions were performed using Tukey’s HSD test for two-way ANOVA. Data regarding clock gene expression in the hypothalamus were analyzed using a mixed model with random plate effect to account for the within-plate correlation. Analyses were completed with SPSS software (version 21.0.0) and SAS (version 9.3, Cary, NC). A two-sided significance level of α ≤ 0.05 was used for all tests.
Results
Experiment 1
Open Field
Mice exposed to dLAN during development spent less time in the center of the open field (F1,115 = 27.15, p<0.001; Fig 1A, 1D). Females showed less central tendency than males (F1,115 = 31.68, p<0.001). There was no effect of timing; i.e., juvenile and adolescent exposure to dLAN elicited the same anxiety-like response (p>0.05).
Figure 1.
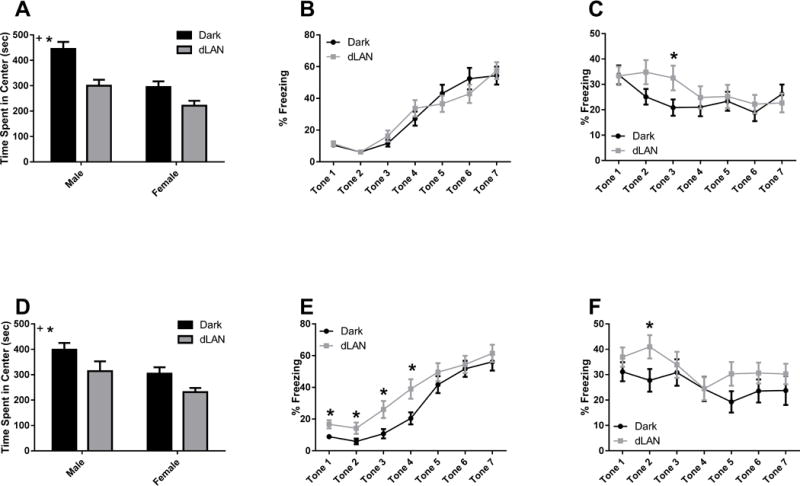
Early life exposure to dLAN increase anxiety-and fear-like behavior in adulthood. Data are presented as mean ± SEM. n ≥ 14/group. *p < 0.05 in DARK vs. dLAN; +p < 0.05 in Male vs. Female.
Elevated Plus
Exposure to dLAN did not affect behavior on the elevated plus maze at either time point (p>0.05).
Fear Conditioning
Acquisition
Adolescent exposure to dLAN increased freezing response during acquisition at tones 1–4 (Tone 1 t(39) = −2.71, p<0.05; Tone 2 t(41) = −2.04, p<0.05; Tone 3 t(43) = −2.49, p<0.05; Tone 4 t(47) = −2.57, p<0.05; Fig 1E). Juvenile exposure to dLAN had no effect on freezing behavior during acquisition (p>0.05; Fig 1B).
Context
dLAN exposure during development had no effect on freezing behavior during contextual fear testing (p>0.05).
Retention
Exposure to dLAN starting at 3 weeks and 5 weeks of age impaired extinction of fear memories at tone 3 (t(53) = −1.99, p = 0.05; Fig 1C) and tone 2 (t(54) = −2.05, p < 0.05; Fig 1F), respectively.
Experiment 2
Exposure to dLAN altered Clock expression in the hypothalamus in a sex dependent manner (F1,150= 4.44, p<0.05). Both male and female mice exposed to dLAN during development had increased amplitude of Clock gene expression relative to their dark-night exposed counterparts (F2,150 = 4.3, p<0.05 and F2,150 = 7.5, p<0.001, respectively). Conversely, exposure to dLAN decreased amplitude of Rev-ERB gene expression in the hypothalamus (F2,152 = 8.38, p<0.001).
Discussion
Chronic exposure to dim light at night during pre-adolescent development induces anxiety and fear-like behavior in adulthood. Five weeks of dLAN decreased central tendency in the open field, but did not alter behavior in the elevated plus maze. Adolescent dLAN animals also increased freezing response to a fearful stimulus (foot shock). Mice exposed to dLAN during early life required longer to decrease freezing behavior in response to a tone than mice housed in dark nights. Clock gene expression in the whole hypothalamus increased amplitude in young dLAN exposed mice whereas amplitude of Rev-ERB gene expression decreased. This alteration in clock genes along with behavioral alterations demonstrates that dLAN has developmentally-staged effects on the circadian system.
In common with other early life disruption models, juvenile and adolescent exposure to dLAN increased anxiety-like behaviors. These data confirm and extend the window of behavioral vulnerability to dLAN proposed by previous studies (Borniger et al., 2014). In contrast to previous data in adults, the present results suggest that exposure to dLAN starting as late as 5 weeks of age induces anxiety-like behaviors, whereas after 8 weeks of age anxiety-like behaviors are reduced. This may reflect the opposing effects of dLAN on Clock gene expression in the hypothalamus. In adult mice, Clock expression is unaffected by exposure to dLAN, but increases amplitude in pre-adolescent animals exposed to dLAN. Clock amplitude increases in the nucleus accumbens in response to chronic stress, along with increased anxiety- and depressive-like behaviors (Logan et al., 2015). These observations suggest that magnitude and direction of clock disruption may be region specific in anxiety.
The amplitude of the daily rhythm of expression for many clock genes decrease in adult animals exposed to dLAN (Fonken et al., 2013). We observed that young animals exposed to dLAN decreased amplitude of Rev-ERB gene expression in the hypothalamus. Although amplitude of daily Rev-ERB gene expression was reported flattened in the liver and white adipose in adult mice, hypothalamic Rev-ERB gene expression remained unchanged. Unlike adults, young animals increased anxiety-like behavioral responses in response to dLAN. Alterations in Rev-ERB function have been associated with bipolar disorder (Kripke et al., 2009; Severino et al., 2009), which is comorbid with anxiety (Freeman et al., 2002). Elimination of Rev-ERB in the midbrain increases manic behavior, decreased anxiety- and depressive-like behaviors in adult mice, due to a hyperdopaminergic state (Chung et al., 2014). It is possible that alterations to Rev-ERB have differential effects on dopaminergic tone early in life. This is supported by the observation that dopamine negative feedback becomes regulated during the periadolescent period (Spear & Brake, 1983) and may be the underlying difference between the fear responses in the juvenile versus adolescent exposed animals.
Although mice in this study were exposed to dLAN prior to puberty, a sex difference in anxiety-like behavior was observed suggesting activational effects of steroid hormones on the clock-influenced development of affective responses. Young female mice exposed to dLAN increased anxiety-like behavior relative to males. Maternal high fat diet, maternal separation, and neonatal LPS are anxiogenic in male offspring, but have little or even anxiolytic effects in females (Bilbo & Tsang, 2010; Romeo et al., 2003; Tenk et al., 2013; Wigger & Neumann, 1999). The phenotype observed in the present study is reminiscent of nutritional, immune, or affective manipulations during adolescence that preferentially increase anxiety-like behaviors in females (Eiland et al., 2012; C.M. McCormick et al., 2008; Pohl et al., 2007; Weintraub et al., 2010). These anxiety-like behaviors are all associated with disruptions in the development of neurotransmitter balance and the HPA axis (Donner & Lowry, 2013). The circadian system regulates both the HPA axis and a variety of neurotransmitter systems; hypothalamic clock gene disruptions observed in dLAN exposed animals may have downstream effects on integration of a stressful/fearful stimulus.
Adolescence is a time of rapid brain development and stabilization of both endocrine and neurotransmitter systems (Chambers et al., 2003; He & Hodge, 2007; McCormick & Mathews, 2007). There are circadian rhythms in neurotransmitters involved in regulating anxiety state and their receptors, specifically serotonin, GABA, and glutamate (Castañeda et al., 2004; Spanagel et al., 2005; Weiner et al., 1992; Wesemann & Weiner, 1990). Central disruption of these neurotransmitters is associated with anxiety and mood disorders (Donner & Lowry, 2013; McClung, 2007). Although GR abundance reaches adult levels within the first two weeks of age (Meaney et al., 1985), the Clock:Bmal1 heterodimer is able to regulate transcriptional activity of the glucocorticoid receptor (Nader et al., 2009). Sex and dLAN-induced differences in Clock gene expression may be implicated in the functional integration of a stressful stimulus, contributing to the divergent behavioral outcomes in young versus adult animals exposed dLAN. Affective disorders have been associated with disruptions in circadian rhythms (Frank et al., 2014; Sipilä et al., 2010). Children with anxiety-disorders also display altered circadian profiles (Chase & Pincus, 2011; Feder et al., 2004). Exposure to nighttime environmental lighting alters chronotype in adolescents (Vollmer et al., 2012), suggesting some effects of light at night on the adolescent circadian system. This may be cause for concern when exposure to light at night is so prevalent.
Taken together, these data suggest that early life exposure to dLAN alters molecular circadian rhythms and anxiety-like behavior later in life. This response may reflect a developmental vulnerability to dLAN that may be driving the differential effects during early life as opposed to adulthood on behavioral outcomes. Future studies should expand on the downstream effects of these changes in Clock and Rev-ERB gene expression in the hypothalamus and the neuroendocrine balance.
Figure 2.
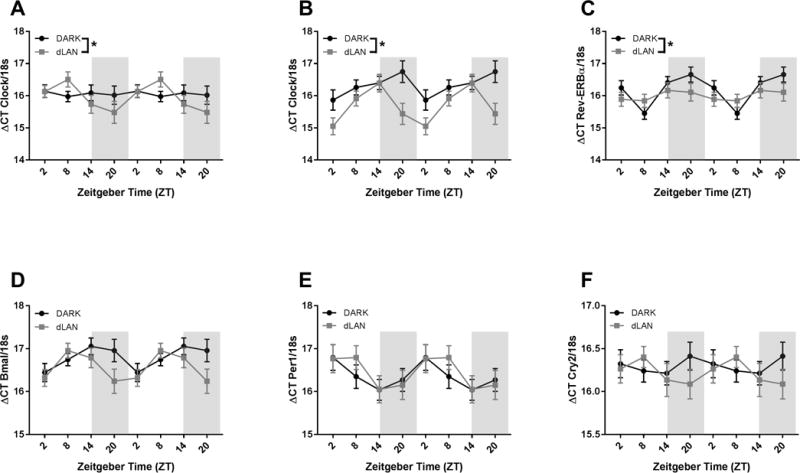
Early life exposure to dLAN alters hypothalamic Clock and Rev-ERB gene expression. Data are double plotted and presented as mean ΔCT± SEM. n >7 mice per group per sex per time point for Clock, n > 19 mice per group per time point for all other genes. Grey bars represent night. *p < 0.05 in DARK vs. dLAN.
Acknowledgments
The authors thank Anupama Suresh, Tial KaiKai TinKai, Adam Weiss, Evan Thomas, and Elise Lemanski for technical assistance.
Declaration of interest
This work was supported by the National Science Foundation grant IOS 11-18792 (RJN) and National Institutes of Health grant P30NS045758. YMC was supported by NIDCR T32DE014320.
References
- Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry. 2013;18(8):930–6. doi: 10.1038/mp.2012.96. http://doi.org/10.1038/mp.2012.96. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24(6):2104–15. doi: 10.1096/fj.09-144014. http://doi.org/10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Boitard C, Maroun M, Tantot F, Cavaroc A, Sauvant J, Marchand A, Ferreira G. Juvenile obesity enhances emotional memory and amygdala plasticity through glucocorticoids. J Neurosci. 2015;35(9):4092–103. doi: 10.1523/JNEUROSCI.3122-14.2015. http://doi.org/10.1523/JNEUROSCI.3122-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borniger JC, McHenry ZD, Abi Salloum BA, Nelson RJ. Exposure to dim light at night during early development increases adult anxiety-like responses. Physiol Behav. 2014;133:99–106. doi: 10.1016/j.physbeh.2014.05.012. http://doi.org/10.1016/j.physbeh.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Castañeda TR, Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36(3):177–185. doi: 10.1046/j.1600-079x.2003.00114.x. http://doi.org/10.1046/j.1600-079X.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Cespedes EM, Gillman MW, Kleinman K, Rifas-Shiman SL, Redline S, Taveras EM. Television viewing, bedroom television, and sleep duration from infancy to mid-childhood. Pediatrics. 2014;133(5):e1163–1171. doi: 10.1542/peds.2013-3998. http://doi.org/10.1542/peds.2013-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;1606(160):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase RM, Pincus DB. Sleep-related problems in children and adolescents with anxiety disorders. Behav Sleep Med. 2011;9(4):224–236. doi: 10.1080/15402002.2011.606768. http://doi.org/http://dx.doi.org/10.1080/15402002.2011.606768. [DOI] [PubMed] [Google Scholar]
- Christ E, Korf HW, von Gall C. When does it start ticking? Ontogenetic development of the mammalian circadian system. Prog Brain Res. 2012;199:105–18. doi: 10.1016/B978-0-444-59427-3.00006-X. http://doi.org/10.1016/B978-0-444-59427-3.00006-X. [DOI] [PubMed] [Google Scholar]
- Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, Kim K. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell. 2014;157(4):858–868. doi: 10.1016/j.cell.2014.03.039. http://doi.org/10.1016/j.cell.2014.03.039. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. http://doi.org/10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- de Jong M, Jeninga L, Ouyang JQ, van Oers K, Spoelstra K, Visser ME. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol Behav. 2016;155:172–179. doi: 10.1016/j.physbeh.2015.12.012. http://doi.org/10.1016/j.physbeh.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Dominoni DM, Carmona-Wagner EO, Hofmann M, Kranstauber B, Partecke J. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J Anim Ecol. 2014;83(3):681–692. doi: 10.1111/1365-2656.12150. http://doi.org/10.1111/1365-2656.12150. [DOI] [PubMed] [Google Scholar]
- Donner NC, Lowry CA. Sex differences in anxiety and emotional behavior. Pflügers Arch – Eur J Physiol. 2013;465(5):601–626. doi: 10.1007/s00424-013-1271-7. http://doi.org/10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37(1):39–47. doi: 10.1016/j.psyneuen.2011.04.015. http://doi.org/10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Coplan JD, Goetz RR, Mathew SJ, Pine DS, Dahl RE, Weissman MM. Twenty-four-hour cortisol secretion patterns in prepubertal children with anxiety or depressive disorders. Biol Psychiatry. 2004;56(3):198–204. doi: 10.1016/j.biopsych.2004.05.005. http://doi.org/10.1016/j.biopsych.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28(4):262–71. doi: 10.1177/0748730413493862. http://doi.org/10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107(43):18664–9. doi: 10.1073/pnas.1008734107. http://doi.org/10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Benabou M, Bentzley B, Bianchi M, Goldstein T, Konopka G, Thomas J. Influencing circadian and sleep-wake regulation for prevention and intervention in mood and anxiety disorders: what makes a good homeostat? Ann N Y Acad Sci. 2014;1334(1):1–25. doi: 10.1111/nyas.12600. http://doi.org/10.1111/nyas.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Freeman SA, McElroy SL. The comorbidity of bipolar and anxiety disorders: prevalence, psychobiology, and treatment issues. J Affect Disord. 2002;68(1):1–23. doi: 10.1016/s0165-0327(00)00299-8. http://doi.org/10.1016/S0165-0327(00)00299-8. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. http://doi.org/10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. http://doi.org/10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Hogan MK, Kovalycsik T, Sun Q, Rajagopalan S, Nelson RJ. Combined effects of exposure to dim light at night and fine particulate matter on C3H/HeNHsd mice. Behav Brain Res. 2015;294:81–88. doi: 10.1016/j.bbr.2015.07.033. http://doi.org/10.1016/j.bbr.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14(5):636–648. doi: 10.1002/hipo.10207. http://doi.org/10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Markham JA. The cellular basis for volume changes in the rat cortex during puberty: white and gray matter. Ann N Y Acad Sci. 2004;1021(1):431–435. doi: 10.1196/annals.1308.058. http://doi.org/10.1196/annals.1308.058. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40(3):302–315. http://doi.org/10.1002/(SICI)1097-4695(19990905)40:3<302::AID-NEU3>3.0.CO;2-7. [PubMed] [Google Scholar]
- Koizumi H, Kurabayashi N, Watanabe Y, Sanada K, Kendler K, Neale M, D’Souza R. Increased anxiety in offspring reared by circadian clock mutant mice. PLoS One. 2013;8(6):e66021. doi: 10.1371/journal.pone.0066021. http://doi.org/10.1371/journal.pone.0066021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. http://doi.org/10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673. http://doi.org/10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Lin SY, Wang S. Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain Behav Immun. 2012;26(3):459–468. doi: 10.1016/j.bbi.2011.12.003. http://doi.org/10.1016/j.bbi.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol Psychiatry. 2015;78(4):249–258. doi: 10.1016/j.biopsych.2015.01.011. http://doi.org/10.1016/j.biopsych.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114(2):222–232. doi: 10.1016/j.pharmthera.2007.02.003. http://doi.org/10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–233. doi: 10.1016/j.pbb.2006.07.012. http://doi.org/10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187(2):228–238. doi: 10.1016/j.bbr.2007.09.005. http://doi.org/10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am J Epidemiol. 2014;180(3):245–50. doi: 10.1093/aje/kwu117. http://doi.org/10.1093/aje/kwu117. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I Ontogeny and autoregulation. Dev Brain Res. 1985;18(1):159–164. doi: 10.1016/0165-3806(85)90259-7. http://doi.org/10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23(5):1572–1583. doi: 10.1096/fj.08-117697. http://doi.org/10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Maxim R, McGuinn M, Nobile C, Msall M, Alario A. Television-viewing habits and sleep disturbance in school children. Pediatrics. 1999;104(3):e27. doi: 10.1542/peds.104.3.e27. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10469810. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci. 2007;121(3):462–474. doi: 10.1037/0735-7044.121.3.462. http://doi.org/10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43(5):561–567. doi: 10.1016/s0018-506x(03)00063-1. http://doi.org/10.1016/S0018-506X(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Severino G, Manchia M, Contu P, Squassina A, Lampus S, Ardau R, Del Zompo M. Association study in a Sardinian sample between bipolar disorder and the nuclear receptor REV-ERBα gene, a critical component of the circadian clock system. Bipolar Disord. 2009;11(2):215–220. doi: 10.1111/j.1399-5618.2009.00667.x. http://doi.org/10.1111/j.1399-5618.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- Shuboni D, Yan L. Nighttime dim light exposure alters the responses of the circadian system. Neuroscience. 2010;170(4):1172–1178. doi: 10.1016/j.neuroscience.2010.08.009. http://doi.org/10.1016/j.neuroscience.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Sipilä T, Kananen L, Greco D, Donner J, Silander K, Terwilliger JD, Hovatta I. An association analysis of circadian genes in anxiety disorders. Biol Psychiatry. 2010;67(12):1163–1170. doi: 10.1016/j.biopsych.2009.12.011. http://doi.org/10.1016/j.biopsych.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Sládek M, Jindráková Z, Bendová Z, Sumová A. Postnatal ontogenesis of the circadian clock within the rat liver. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1224–9. doi: 10.1152/ajpregu.00184.2006. http://doi.org/10.1152/ajpregu.00184.2006. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11(1):35–42. doi: 10.1038/nm1163. http://doi.org/10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. http://doi.org/10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16(2):83–109. doi: 10.1002/dev.420160203. http://doi.org/10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stevens RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38(4):963–70. doi: 10.1093/ije/dyp178. http://doi.org/10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Grove KL. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30(10):3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. http://doi.org/10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumová A, Bendová Z, Sládek M, El-Hennamy R, Laurinová K, Jindráková Z, Illnerová H. Setting the biological time in central and peripheral clocks during ontogenesis. FEBS Lett. 2006;580(12):2836–42. doi: 10.1016/j.febslet.2006.03.023. http://doi.org/10.1016/j.febslet.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Kavaliers M, Ossenkopp KP. Neonatal treatment with lipopolysaccharide differentially affects adult anxiety responses in the light–dark test and taste neophobia test in male and female rats. Int J Dev Neurosci. 2013;31(3):171–180. doi: 10.1016/j.ijdevneu.2012.12.004. http://doi.org/10.1016/j.ijdevneu.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Toki S, Morinobu S, Imanaka A, Yamamoto S, Yamawaki S, Honma K. Importance of early lighting conditions in maternal care by dam as well as anxiety and memory later in life of offspring. Eur J Neurosci. 2007;25(3):815–829. doi: 10.1111/j.1460-9568.2007.05288.x. http://doi.org/10.1111/j.1460-9568.2007.05288.x. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Richter-Levin G. Learning under stress in the adult rat is differentially affected by “juvenile” or “adolescent” stress. Int J Neuropsychopharmacol. 2006;9(6):713–28. doi: 10.1017/S1461145705006255. http://doi.org/10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- Vollmer C, Michel U, Randler C. Outdoor light at night (LAN) is correlated with eveningness in adolescents. Chronobiol Int. 2012;29(4):502–508. doi: 10.3109/07420528.2011.635232. http://doi.org/10.3109/07420528.2011.635232. [DOI] [PubMed] [Google Scholar]
- Weiner N, Clement HW, Gemsa D, Wesemann W. Circadian and seasonal rhythms of 5-HT receptor subtypes, membrane anisotropy and 5-HT release in hippocampus and cortex of the rat. Neurochem Int. 1992;21(1):7–14. doi: 10.1016/0197-0186(92)90062-v. http://doi.org/10.1016/0197-0186(92)90062-V. [DOI] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. http://doi.org/10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Wesemann W, Weiner N. Circadian rhythm of serotonin binding in rat brain. Prog Neurobiol. 1990;35(6):405–428. doi: 10.1016/0301-0082(90)90029-g. http://doi.org/10.1016/0301-0082(90)90029-G. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66(2):293–302. doi: 10.1016/s0031-9384(98)00300-x. http://doi.org/10.1016/S0031-9384(98)00300-X. [DOI] [PubMed] [Google Scholar]


