Abstract
TNF plays an integral role in inflammatory bowel disease (IBD) as evidenced by the dramatic therapeutic responses in Crohn’s disease (CD) patients induced by chimeric anti-TNF mAbs. However, treatment of CD patients with etanercept, a decoy receptor that binds soluble TNF, fails to improve disease. To explore this discrepancy, we interrogated the role of TNF signaling on Wnt/β-catenin-mediated intestinal stem and progenitor cell (ISC/PC) expansion in CD patients, human cells, and preclinical mouse models. We hypothesized that TNF exerts beneficial effects on intestinal epithelial cell (IEC) responses to injury. In CD patients, ISC/PC Wnt/β-catenin signaling correlates with inflammation status. TNF-deficient (Tnf−/−) mice exhibited increased apoptosis, less IEC proliferation, and less Wnt signaling when stimulated with anti-CD3 mAb. Bone marrow chimera (BMC) mice revealed that mucosal repair depended on TNF production by BM-derived cells and TNFR expression by radioresistant IEC. WT-> Tnfr1/2−/− BMC mice given chronic DSS colitis exhibited delayed ulcer healing, more mucosal inflammation, and impaired Wnt/β-catenin signaling, consistent with the hypothesis that epithelial TNFR signaling participates in mucosal healing. The direct effect of TNF on stem cells was demonstrated by studies of TNF-induced Wnt/β-catenin target gene expression in murine enteroids and colonoid cultures and TNF-induced β-catenin activation in non-transformed human NCM460 cells (TOPFlash) and mice (TOP-GAL). Together these data support the hypothesis that TNF plays a beneficial role in enhancing Wnt/β-catenin signaling during ulcer healing in IBD. These novel findings will inform clinicians and therapeutic chemists alike as they strive to develop novel therapies for IBD patients.
Introduction
Tumor necrosis factor (TNF) is a central regulator of inflammation and has been implicated in human diseases including psoriasis, ankylosing spondylitis, rheumatoid arthritis and inflammatory bowel diseases (IBD)(1). TNF interacts with TNF receptors 1 and 2 (TNFR1 and TNFR2), which are differentially expressed on intestinal bone-marrow (BM)-derived cells (macrophage/monocytes, lymphocyte, natural killer and dendritic) as well as stromal (myofibroblasts, mesenchymal cells) and intestinal epithelial cells (IEC). In Crohn’s disease (CD) and ulcerative colitis (UC), diarrhea, mucosal ulceration and intestinal bleeding are associated with high tissue levels of TNF along with cytokines IFNγ, IL-17, and IL-12(2–5). However, the direct effects of these inflammatory molecules on epithelial cells remain uncertain.
Most biologic therapies targeting TNF have been successful at rapidly controlling inflammation in the majority of IBD patients. However, clinical data show that etanercept, a decoy receptor that binds to soluble TNF, is either ineffective or detrimental in the treatment of CD(6). Anti-TNF monoclonal antibodies (mAb) such as infliximab and adalimumab dampen inflammation by inducing apoptosis in immune cells (i.e. monocytes) expressing membrane bound TNF through reverse signaling(7). In contrast, etanercept reduces levels of soluble TNF without directly binding mucosal leukocytes. We postulate that the deleterious effects of etanercept may be due to neutralization of TNF, which is needed for epithelial response in colitis.
Examination of tissues from IBD patients reveals that inflammation affects epithelial proliferation, death and lineage commitment. Chronic inflammation associated with IBD leads to architectural distortion characterized by crypt branching and fissioning (glandular irregularity) as well as goblet cell depletion. Alterations in crypt structure and composition seen in IBD suggest that mucosal inflammation affects signaling events in intestinal stem cell (ISC) and progenitor cell (PC) populations(8). During acute ulceration, the intestinal epithelium develops hyperproliferative crypts at ulcer margins by activating ISC/PC populations(9). These proliferative cells then support migration across ulcer surfaces, re-epithelialization, and the subsequent establishment of new crypt structures.
Maintenance of ISC and PC proliferative zones requires active Wnt/β-catenin signaling(10). Noggin and epidermal growth factor (EGF) cooperate with β-catenin signaling, as the addition of these factors expands primary epithelial stem cells(11). Data from our group and others show that phosphoinositide 3-kinase (PI3K) signaling cooperates with Wnt to regulate stem cell behavior in the intestine(12, 13). Models of mutated BMPR1A(14) and deficient phosphatase and tensin homolog (PTEN)(12) signaling indicate that Akt activation enhances β-catenin signaling. Increased PI3K activation leads to Akt phosphorylation of β-catenin at serine 552 (pβ-catenin552) (12). We implicated this mechanism in mucosal inflammation by showing that T cell activation, as well as colitis in IL10−/− mice, increases nuclear p-β-catenin552 in small bowel and colonic ISC(13). These findings are consistent with the idea that inflammatory mediators released in the local environment activate relevant signaling pathways that enhance Wnt/β-catenin signaling.
Based on data from colitis patients and mouse models, we hypothesized that TNF supports Wnt/β-catenin signaling at times of severe inflammation and in ulceration. This was tested in WT and Tnf−/− mice. Whereas tissue histology and Wnt/β-catenin signaling were unperturbed in untreated Tnf−/− mice, T cell activation with anti-CD3 mAb (anti-T cell co-receptor mAb) induced widespread crypt distortion owing to a failure of Wnt/β-catenin signaling and proliferation in ISC/ crypt based columnar (CBC) and transit amplifying (TA) populations. The idea that TNF from BM-derived cells acts directly on epithelial cells was supported by data in bone-marrow chimera (BMC) and in vitro enteroid and colonoid models. BMC mice lacking epithelial TNFR1/2 demonstrated that epithelial TNFR signaling is beneficial during ulcer healing in chronic DSS colitis. These data place TNF as a critical mediator of epithelial responses to severe mucosal inflammation with implications in stem cell activation.
Materials and Methods
Animal studies
C57BL/6 (WT), Tnf−/−, TNFR1/2−/−, BAT-GAL, and TOP-GAL mice were purchased from The Jackson Laboratory (Bar Harbor, MD). All mice were used when 8–14 weeks old and housed in the Northwestern University or University of Kentucky animal care facilities under specific pathogen-free conditions. For T cell activation, anti-CD3 monoclonal antibody (145-2C11; 10mg/kg) or control hamster monoclonal antibody (UC8-IB9) was administered at 15mg/kg intraperitoneally (IP). Mice were injected IP with 0.1mg of bromodeoxyuridine (BrdU; Sigma) 2 hours before sacrifice. For anti-TNF studies, mice were injected IP with 50 mg/kg rat/mouse chimeric monoclonal IgG2a,κ antibody specific for mouse TNF (CNTO 2213 lot# 0707-05V, a gift from Dave Shealy, Johnson and Johnson pharmaceuticals, New Brunswick, NJ). For the untreated group, CNTO 1322 lot#0218-03T, a rat/mouse chimeric monoclonal IgG2a,κ negative control antibody was injected IP. For in vivo TNF injection, PBS or recombinant murine TNF (100ug/kg, Peprotech) was injected IP. For bone marrow chimera studies, recipient mice were irradiated with 9.5Gy. Bone marrow was harvested from donor femurs, red blood cells were lysed, and cells were filtered and counted on a hemocytometer. Recipient mice received 5 million donor cells by retro-orbital injection and were provided antibiotics for 14 days. Mice were given 8 weeks to allow for stable engraftment prior to experimentation. To induce chronic colitis, mice were provided 2.5% dextran sodium sulfate (DSS) in the drinking water ad libitum for 7 days, followed by 14 days of water. This was repeated for 3 cycles, and mice were euthanized 6 days following the last cycle of DSS. All studies and procedures were approved by the Animal Care and Use Committees of Northwestern University and the University of Kentucky.
NCM TOPFlash Assay
NCM460 cells (normal derived colon mucosa) were received by a cell licensing agreement with INCELL Corporation (San Antonio, TX), and were routinely propagated under standard conditions in M3:10 medium with addition of the conditional medium (30%) from previously cultured NCM460 cells. Cells were transfected with a reporter construct containing TCF/luc to evaluate β-catenin transcription. Transfected cells were treated overnight with 1ng/ml of TNF. Luciferase was detected with Luciferase Reagent (Promega, Madison, WI).
Histological Analysis
Ten-centimeter segments of ileum or colon were formalin-fixed, paraffin embedded, and sectioned at 5um. The following antibodies were used: anti-BrdU (MBL international, Woburn, MA), anti-Ki67 clones TEC-3 and MIB-1 (Dako, Carpenteria, CA), anti-c-Myc (N-262, Santa Cruz Biotechnology, Dallas, TX), anti-survivin (NB500-201, Novus Biologicals, Littleton, CO) and anti-cleaved caspase-3 (Cell signaling Technology, Danvers, MA). For TUNEL staining, the In Situ Cell Death Detection Kit, POD (Roche, Basel, Switzerland) was used. For BAT-GAL and TOP-GAL mice, β-galactosidase staining was performed as described (15), and the number of β-galactosidase positive cells per well-oriented crypt was counted for a minimum of eight sections. For colitis assessment, blinded evaluation was performed independently by 2 investigators (G.L and G.Y). Colitis in human biopsy specimens were scored from 0 to 4 based on increasing severity of mononuclear infiltration and epithelial ulceration with immunohistochemical analysis performed on chronically inflamed adjacent mucosa.
Real-time Semi-quantitative Reverse-Transcription PCR
Total RNA was isolated from 0.5cm segments of the intestine or cultured cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real time PCR used the AB StepOne Plus real-time PCR system and Power SYBR green PCR master mix (Applied Biosystems). Primers were designed by Primer Express software 3.0 (Applied Biosystems) based on nucleotide sequences from the National Center for Biotechnology Information data bank. For each sample, the threshold value for glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was determined and used as an internal reference. All assays were performed in triplicate and fold changes were calculated using the ΔΔCT method.
Enteroid/Colonoid Culture
Small intestine and colonic crypts were isolated and cultured in a 3D system as described previously(11, 16). Crypts were embedded in Matrigel (BD Biosciences) and seeded in a pre-warmed 24 well plate at approximately 200 crypts in 50uL. After the Matrigel solidified, 500uL d of 50% L-WRN media was overlaid(16).
Cytokine Stimulation of Enteroid/Colonoid Culture
Enteroids and colonoids were propagated for 1 week, passaged, and cultured for 24 hours in 50% L-WRN media. Media was then changed to 25% L-WRN containing 0, 1 or 10 ng/mL recombinant TNF (Peprotech). TNF concentrations were based on experiments (above) showing that 1 ng/mL is sufficient to induce TOPFlash reporter activation in NCM cells. Cells were cultured for 5 additional days and measured for size (diameter) and budding every other day. For RNA analysis, the Matrigel was washed with PBS and enteroids/colonoids were lysed directly in RLT buffer (Qiagen). RNA was isolated using the RNeasy Mini kit (Qiagen).
Human Colonic Specimens
Human colonic biopsy specimens were obtained from patients undergoing diagnostic or surveillance colonoscopy for known or suspected Crohn’s disease, receiving anti-TNF mAb alone or no therapy (untreated), and collected from Northwestern University or the University of Kentucky. All active untreated patients with CD and those that were active but refractory to anti-TNF had moderate to severe active CD (CDAI ≥200). CDAI was not significantly different between untreated CD and anti-TNF-treated patients. For comparison, biopsy specimens were obtained from healthy patients undergoing routine colon cancer surveillance as well as patients with CD currently in remission on anti-TNF therapies. Collection of all patient materials for this study was approved by Northwestern University’s Office for the Protection of Human Subjects or the University of Kentucky Institutional Review Board.
Western Blot Analysis
Human and mouse tissue were homogenized in Buffer I (50mM Tris-HCl, 100mM NaCl, 0.01% digitonin), disrupted with 26G needle, and centrifuged at 4°C at maximum speed. The supernatant was taken as the cytosolic fraction. The pellets were resuspended in Buffer II (50mM Tris-HCl, 2% Triton X-100, 100mM NaCl), incubated on ice for 30 minutes, and centrifuged as above. The supernatant was taken as the membrane/organelle fraction. To obtain nuclear fractions, the remaining pellet was dissolved in Buffer III (50mM Tris-HCl, 0.25% n-dodecyl-D-maltoside, 100mM NaCl, and 20U/mL Benzonase (Sigma)), incubated for 30 minutes at room temperature, and centrifuged. The pellet was discarded. Proteins were subjected to SDS-PAGE and transferred to Immobilon FL membrane (Millipore, Billerica, MS). Membranes were probed for actin (Sigma, St. Louis, MO), axin2 (Abcam, Cambridge, UK), pβ-cateninS552 (gift of Linheng Li, Stowers), BMP4 (Abcam), c-Myc (Cell Signaling), cyclin D1 (Santa Cruz Biotechnology) and laminB1 (Zymed, ThermoFisher, Waltham, MA).
Statistical Analysis
Statistical analyses were performed using Graph Pad Prism software (GraphPad Software). For more than two groups, data were compared by one-way analysis of variance (ANOVA) followed by a post-hoc Tukey’s test. For comparison of two groups, Student’s t-test was used. P-values ≤ 0.05 were considered statistically significant. Unless specified, values represent mean ± standard error of the mean (17).
Results
TNF mediates epithelial β-catenin signaling and proliferation in Crohn’s disease patients
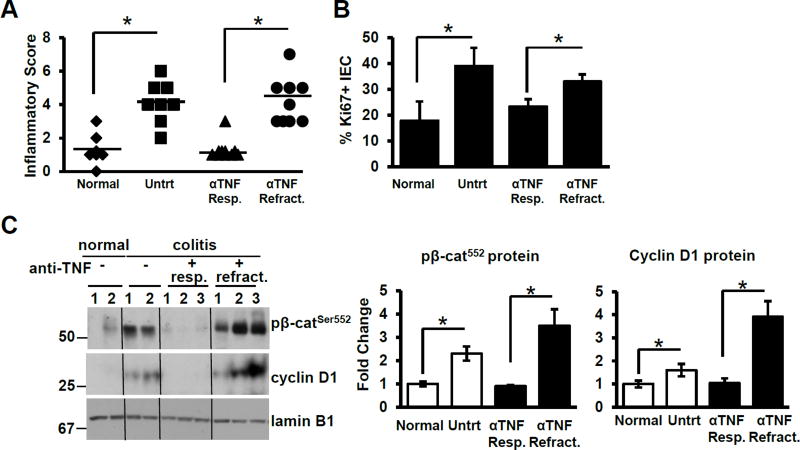
To examine whether TNF signaling affects Wnt/β-catenin signaling during mucosal inflammation, we examined the levels of epithelial nuclear p-β-catenin552 and cyclin D1 in colonic biopsy samples of healthy untreated (n=7), active untreated CD (n=8) and anti-TNF mAb-treated CD patients who were either refractory to treatment (n=9) or in remission (n=10). Both subsets of clinically active CD patients had active tissue inflammation based on histological analysis (Fig. 1A). As expected, inflammatory scores correlated with epithelial proliferation as examined by Ki67 staining (Fig. 1B). The number of Ki67-positive cells per crypt was elevated in inflamed tissues (healthy 18±7; active untreated and active anti-TNF-treated CD; 39±7.1 and 33±3) and reduced in patients on anti-TNF in remission (23±3).
Figure 1. TNF mediates epithelial β-catenin signaling and proliferation.
(A) Histological inflammatory scores for normal (healthy; n=7), untreated CD (n=8), anti-TNF responsive (n=10) and anti-TNF refractory CD (n=10) patients. Bars denote the average score of each group. (B) Ki67-stained cells per 100 IEC in normal, untreated CD, anti-TNF responsive and anti-TNF refractory CD patients. (C) Immunoblot analysis and densitometry of p-β-catenin552 and cyclin D1 in nuclear fractions of isolated human colonic epithelial cells (n = 2–3 for each group). Values represent mean ± SEM, *p<0.05.
TNF mediates epithelial β-catenin signaling in human and mouse IEC
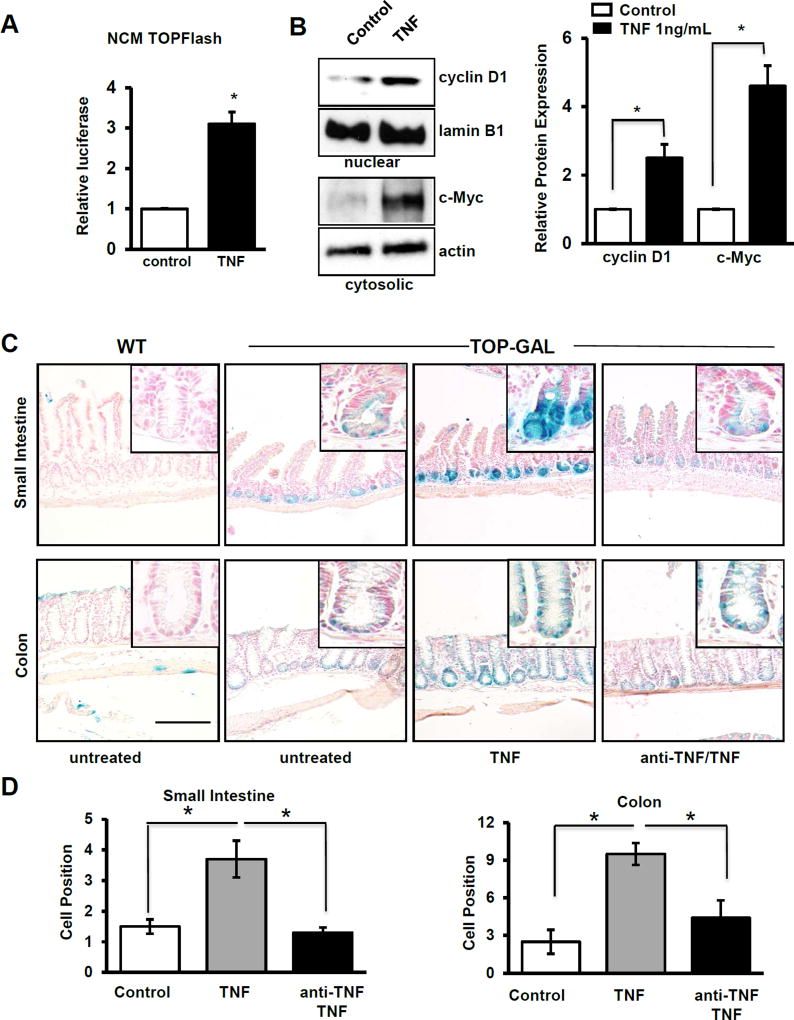
To examine whether TNF directly promotes Wnt/β-catenin signaling in human cells, NCM460 nontransformed human colonic epithelial cells were transfected with the TOPFlash construct, which reports TCF/LEF transcriptional activation. TNF treatment increased β-catenin/TCF luciferase reporter activity, consistent with enhanced transcriptional activation of this promoter (Fig 2A). These findings were supported by western blot data in NCM460 cells in which TNF significantly increased cyclin D1 and c-Myc, both products of β-catenin target gene expression (Fig. 2B). Based on these data, we speculate that TNF induces epithelial Wnt/β-catenin activation and proliferation during mucosal inflammation.
Figure 2. TNF and inflammation activate epithelial Wnt signaling.
(A) TCF/LEF promoter activity measured as mean relative luciferase units in control and TNF-treated human, nontransformed colonic NCM460 cells. (B) Western blot and corresponding densitometries of cytoplasmic and nuclear levels of cyclinD1 and c-Myc normalized to lamin B1 and actin, respectively, in control and TNF-treated NCM cells. Independent cell culture experiments were repeated four times (3–6 wells of each treatment per experiment). (C) β-galactosidase staining of control and TNF-treated (24hr) TOP-GAL mice with and without anti-TNF treatment. The scale bar represents 100 µm. (D) Quantification of the highest cell position with positive LacZ+ staining in ileum and colon. A minimum of 20 well-oriented crypts were counted per mouse; n=3 control mice, 4 treated with TNF, and 4 treated with both anti-TNF and TNF. Values represent mean ± SEM, *p<0.05.
To determine whether TNF can induce β-catenin signaling in vivo, TOP-GAL mice, which report LEF1/TCF signaling and activated β-catenin through expression of β-galactosidase, were utilized. Mice were injected with recombinant TNF and cell positions analyzed. As a control, one cohort of mice was pre-treated with anti-TNF mAb 3 hours prior to TNF injection. Untreated mice had β-galactosidase expression restricted to the crypt base in both small intestine and colon (Fig 2C). Treatment with TNF increased the number of cells with active β-catenin signaling in the small intestine (untreated and TNF-treated; 1.5±0.2 and 3.7±0.6; p<0.05) and colon (2.5±0.9 and 9.5±0.9; p<0.05). Pre-treatment with anti-TNF abolished this response (Fig 2D).
TNF induces ISC gene expression downstream of β-catenin
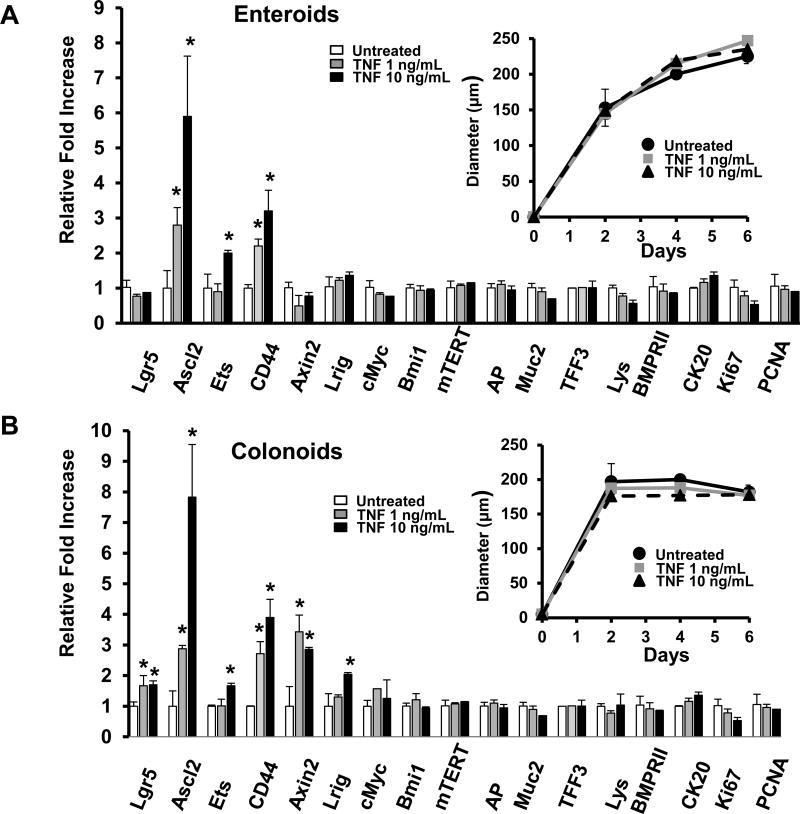
Wnt/β-catenin signaling plays a crucial role in maintaining intestinal stem and progenitor cells(18). Therefore, we next examined the role of TNF in Wnt/β-catenin signaling in ISC and PC cells using primary small intestinal enteroid (Fig 3A) and colonoid (Fig 3B) cultures. Data indicate that 5 days of TNF-treatment induced several Wnt target genes in enteroids. Importantly, TNF significantly upregulated the expression of Ascl2, a Wnt target gene and key ISC transcription factor(19) in a dose-dependent manner (Fig 3A). TNF also upregulated the Ascl2 target gene Ets2(20) and Wnt-target CD44, both of which are implicated in stem cell maintenance and suppression of differentiation. TNF treatment did not alter the mRNA expression of differentiation markers (AP, Muc2, TFF3, Lys, CK20) or markers of proliferation (Ki67 and PCNA). TNF did not alter the size of the enteroids (Fig 3A), the percent of enteroids that budded, or the number of buds per enteroid (Suppl Fig 1A). In colonoids, the stem cell markers Lgr5, Ascl2, Lrig(21) and Axin2 were upregulated by TNF, as was the β-catenin target gene CD44 (Fig 3B). Markers of differentiation and proliferation were not significantly changed, nor were the size (Fig 3B) or budding characteristics of the colonoids (Suppl Fig 1B). Acute TNF treatment (4 and 24 hours) did not induce any significant changes in Wnt target genes (data not shown). Taken together, these data suggest that TNF cooperates with Wnt signaling to enhance Wnt target gene expression needed for maintenance of intestinal ISC populations.
Figure 3. TNF promotes ISC activation.
mRNA expression levels of stem cell markers and Wnt target genes (Lgr5, Ascl2, Ets, CD44, Axin2, Lrig, c-Myc and mTERT), differentitation markers (AP, Muc2, TFF3, Lys and CK20), and proliferation markers (Ki67 and PCMA) in (A) mouse small intestinal enteroids and (B) mouse colonoids cultured in the presence or absence of recombinant TNF for 5 days. The diameter (µm) of enteroids and colonoids are shown as insets. Enteroids and colonoids were cultured in individual experiments from 5 separate mice. For each experiment, 3–6 wells were treated for each condition. Values represent mean ± SEM, *p<0.01.
Loss of TNF impairs Wnt signaling, ISC activation and IEC expansion during T cell activation
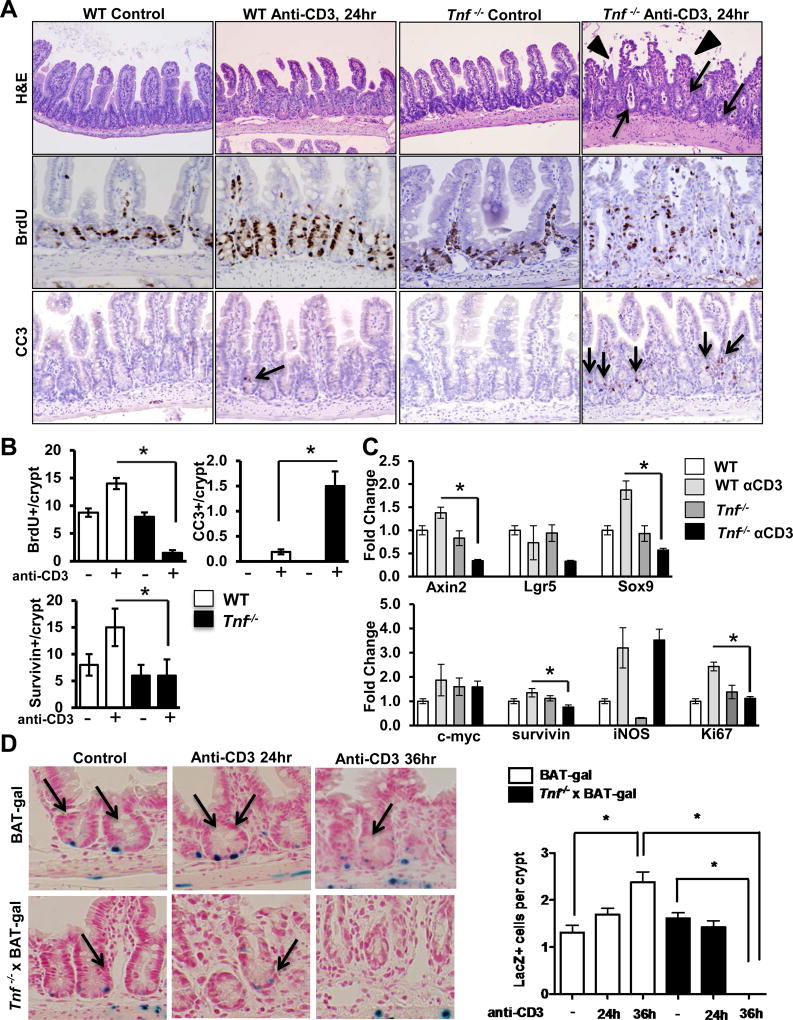
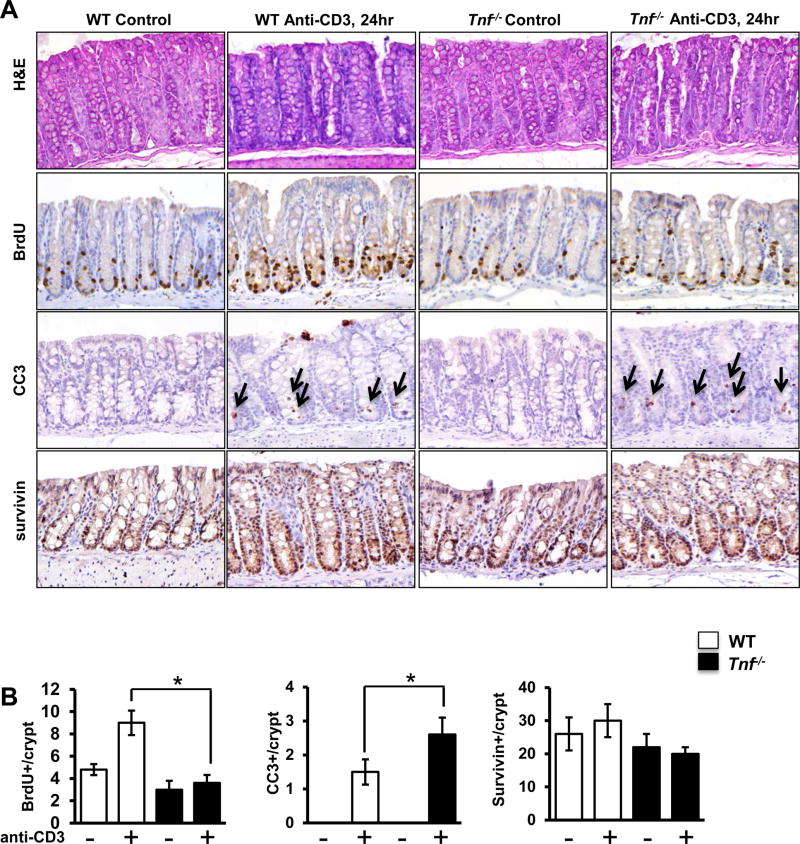
We next examined whether TNF signaling is required to induce mitogenic Wnt/β-catenin signaling during T cell activation. WT and Tnf−/− mice were treated with T cell activating anti-CD3 mAb and IEC responses examined. Histology in WT mice revealed that T cell activation induced crypt elongation and mild villus blunting as previously reported(22, 23). In contrast, T cell activation in Tnf−/− mice induced profound crypt dilation and exaggerated villus blunting, which was most pronounced 24 hours following anti-CD3 treatment (Fig 4A). Thus, data suggest TNF mediates physiologic IEC responses to T cell activation.
Figure 4. TNF mediates T cell-induced ISC and PC IEC activation in the small intestine.
(A) Representative hematoxylin and eosin (H&E) staining of control and anti-CD3 mAb-treated WT and Tnf−/− mice at 24 hours. Arrows show crypt dilation with cellular debris, arrowheads show villus blunting. Representative images of BrdU and cleaved caspase-3 staining. Survivin staining is shown in Suppl Fig 3. In panels with cleaved caspase 3, arrows identify apoptotic cells. (B) Numbers of BrdU-, cleaved caspase-3, and survivin-positive cells detected per crypt. Positive cells were counted in at least 20 well-oriented crypt/villus axes per section. Values represent mean ± SEM, *p<0.05. (C) mRNA expression levels of stem cell markers (Axin2, Lgr5, Sox9), progenitor cell markers (c-Myc, survivin), Ki67, and iNOS in ileum of WT and Tnf−/− mice. Anti-CD3 experiments in Tnf−/− mice were repeated twice; n = 6–8 mice per group. (D) β-galactosidase staining of control and anti-CD3 mAb-treated BAT-GAL and Tnf−/− × BAT-GAL mice, and quantification of nuclear β-galactosidase-positive IEC per well-oriented crypt. Positive cells were counted in at least 20 well-oriented crypts per section; n=3 mice per group. Values represent mean ± SEM, *p<0.05.
Given the rapid crypt disruption and villus blunting in T cell-stimulated Tnf−/− mice, BrdU labeling was examined in WT and Tnf−/− mice (Fig 4A, B). T cell activation increased BrdU labeling by 55% in WT IEC (control and anti-CD3-treated mice; 8.9±0.7 and 14.3±0.9) (Fig 4B). By comparison, IEC proliferative responses decreased by over 80% in anti-CD3-treated Tnf−/− mice compared to unstimulated controls (control and anti-CD3-treated mice; 8.1±0.6 and 1.3±0.3). These data suggest that TNF is required to sustain T cell mediated proliferative responses in intestinal crypts.
In addition to proliferative responses, anti-CD3 treatment has been shown to induce a biphasic wave of apoptosis, with acute apoptosis of villus tips occurring in the first 4 hours and IEC death in crypts peaking around 24 hours(24, 25). At 24 hours, cleaved caspase-3 staining demonstrated a significant increase in crypt apoptosis in WT mice treated with anti-CD3 (WT untreated and WT anti-CD3; undetectable and 0.2±0.1, p<0.05). In Tnf−/− mice the response to anti-CD3 was exacerbated, and the number of apoptotic cells per crypt increased by 7-fold (1.5±0.3) (Fig 4A,B).
Survivin, a member of the inhibitor of apoptosis (IAP) family, is a downstream target of the Wnt/β-catenin pathway and is often induced as an adaptive response to epithelial damage(26–28). Additionally, survivin identifies PC cell populations in the gut (29, 30). Survivin staining (Suppl Fig 3) demonstrated a significant increase in survivin-positive cells in T cell-stimulated WT mice (WT untreated and WT anti-CD3; 8±2 and 15±3, p<0.05). In contrast, Tnf−/− mice failed to respond (Tnf−/− untreated and Tnf−/− anti-CD3; 6±2 and 6±3, NS) (Fig 4B) indicating that TNF promotes survivin expression in mucosal inflammation.
Real-time PCR analysis of tissue from WT and Tnf−/− small intestine confirmed that Tnf−/− mice have a significant down-regulation of mRNA encoding the stem cell markers Axin2, Lgr5 and Sox9 in response to T cell activation (Fig 4C). Gene expression levels of PC markers c-Myc and survivin were modestly upregulated by anti-CD3 treatment; however; TNF-deficiency did not affect c-Myc expression and only slightly reduced survivin levels in response to anti-CD3. Interestingly, levels of inducible nitric oxide synthase (iNOS) were induced by anti-CD3 equally in WT and Tnf−/− mice. T cell-induced Ki67 mRNA induction was aborted in Tnf−/− mice (Fig 4C).
We analyzed WT and Tnf−/− mice 24 hours following anti-CD3 based on previous reports and our own studies demonstrating profound differences in proliferation and crypt apoptosis at that time point. However, differences in mucosal cytokine expression between WT and Tnf−/− mice were observed as early as 3 hours following anti-CD3 treatment, even though tissues appeared histologically normal (Suppl Fig 2). Proinflammatory cytokines IL-2, IL-6, and IL-1β were significantly upregulated in treated Tnf−/− mice at 3 hours. At 18–24 hours post-treatment, Tnf−/− mice dysplayed severe villus blunting and architectural distortion (Suppl Fig 2 and Fig 4). Crypt apoptosis and impaired proliferation persisted to 36 hours post-treatment (data not shown). However, Tnf−/− mice exhibited increased proliferation with normalization see at 96 hours (Suppl Fig 2).
To determine if anti-CD3 treatment regulates β-catenin signaling in the stem cell niche, BAT-GAL mice were utilized. Like TOP-GAL mice, BAT-GAL mice express the lacZ gene under the control of β-catenin/T cell factor response elements and are considered a virtuous readout of Wnt signaling in a variety of tissues(31). Given that β-galactosidase staining in BAT-GAL mice is an in vivo indicator of Wnt/β-catenin signaling, we examined BAT-GAL and Tnf−/− × BAT-GAL mice after T cell activation. T cell activation doubled numbers of IEC with BAT-GAL expression mostly in CBCs (high magnification in Fig 4D, and low magnification in Suppl Fig 2D). By comparison BAT-GAL expression in CBCs was dramatically reduced in T cell-stimulated TNF-deficient mice. Similarly, examination of c-Myc, a Wnt/ β-catenin target gene, demonstrated parallel responses upon T cell activation in WT and Tnf−/− mice (Suppl Fig 2C).
To determine if TNF regulates IEC mitogenic responses in the colon, WT and Tnf−/− distal colon tissue was analyzed for proliferation and apoptosis at baseline and following T cell stimulation. Similar to small intestine, T cell activation induced robust increases in proliferation (BrdU incorporation) in WT mice but not Tnf−/− mice (Fig 5A,B). Tnf−/− mice also exhibited a 1.7-fold increase in the number of apoptotic cells per crypt in response to anti-CD3. In the colon, survivin was not significantly altered by anti-CD3 treatment in either WT or Tnf−/− mice (Fig 5A,B).
Figure 5. TNF mediates T cell-induced IEC activation in the colon.
(A) Representative images of H&E, BrdU, cleaved caspase-3, and survivin staining of control and anti-CD3 mAb-treated WT and Tnf−/− colon at 24 hours. (B) Quantification of BrdU-, cleaved caspase-3- and survivin-positive IEC detected per crypt. Positive cells were counted in at least 20 well-oriented crypts per section; n=6–8 mice per group. Values represent mean ± SEM, *p<0.05.
Bone marrow-derived TNF and epithelial TNF receptors are crucial for maintaining epithelial proliferative response upon T cell activation
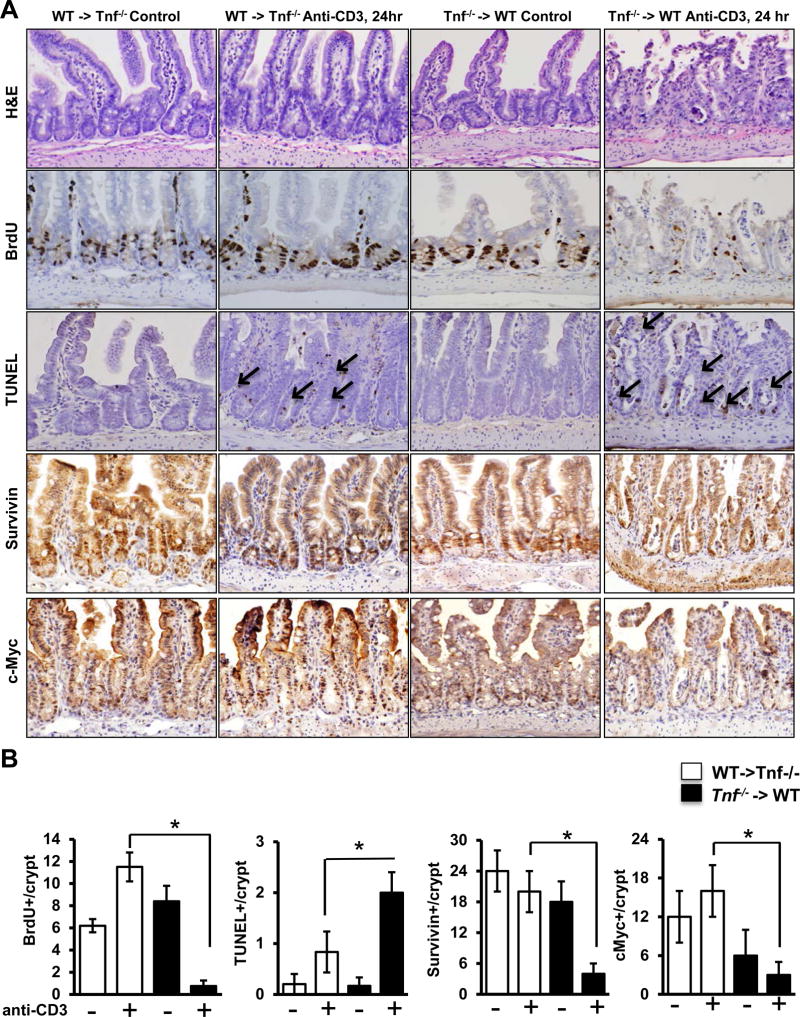
To determine the source of TNF required for T cell-induced IEC responses, bone marrow chimeric (BMC) mice were constructed using WT and Tnf−/− mice. T cell-induced epithelial responses (IEC BrdU incorporation and apoptosis) in WT →WT (donor→recipient, data not shown) and WT→Tnf−/− BMC closely resembled those detected in intact WT mice (Fig 6; Suppl Fig 4). By comparison, IEC responses in Tnf−/−→WT and Tnf−/−→Tnf−/− BMC reflected changes seen in Tnf−/− mice. In particular, changes in BrdU staining in chimeric mice reconstituted with TNF-deficient BM closely mirrored IEC responses in intact Tnf−/− mice (Fig 6A,B). TUNEL staining demonstrated that Tnf−/−→WT have a 2-fold increase in apoptotic cells (WT-> Tnf−/− anti-CD3 and Tnf−/− -> WT anti-CD3; 0.9±0.3 and 2.1±0.3, p<0.05). Epithelial survivin and c-Myc staining were also abrogated in mice with TNF-deficient BM, suggesting impaired epithelial Wnt/β-catenin signaling in Tnf−/−→WT mice (Fig 6A,B). These responses were recapitulated in colonic tissue of BMC mice (Suppl Fig 4). Together, the data suggest that TNF produced by BM-derived populations (e.g. macrophages, T cells, mast cells) mediate ISC and PC activation during mucosal immune responses.
Figure 6. Bone marrow-derived TNF is crucial for maintaining epithelial proliferative responses upon T cell activation.
(A) Representative images of H&E staining, BrdU, TUNEL, survivin, and c-Myc staining of ileal tissue of control- and anti-CD3-treated (24 hours) WT→Tnf−/− and Tnf−/−→WT BMC mice. Arrows indicate TUNEL-positive IEC. (B) Quantification of BrdU-, TUNEL, survivin, and c-myc- positive cells per crypt. Positive cells were counted in at least 20 well-oriented crypt/villus axes per section; n=6–8 mice per group. Values represent mean ± SEM, *p<0.05.
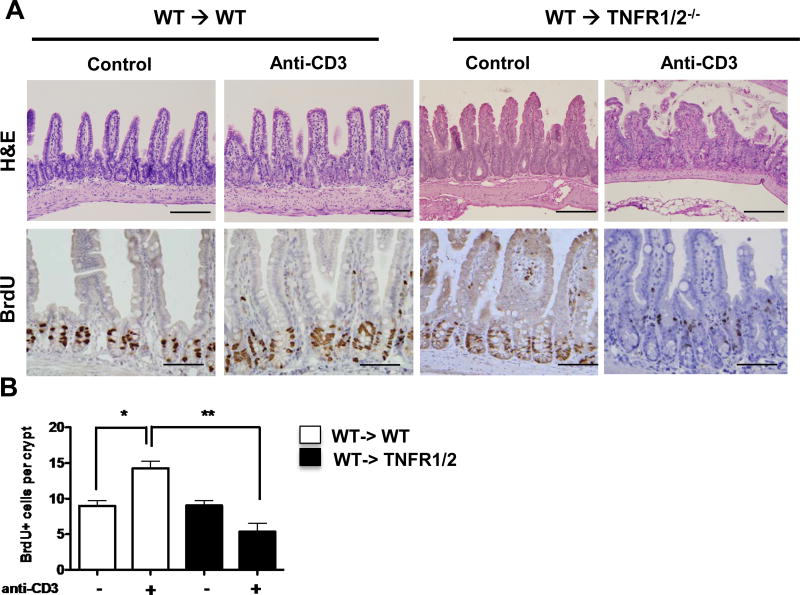
Given data indicating that TNF produced by BM-derived cells stimulates Wnt/β-catenin signaling in IEC, we next examined the requirements for TNFR expression in radiation-resistant epithelial cells in Wnt signaling. To determine the role of IEC TNFR expression in T cell-induced ISC and PC responses, BMC mice were generated with WT BM reconstitution of Tnfrsf1a/b−/− recipient (WT→Tnfrsf1a/b−/−; herein TNFR1/2−/− ). Data show that T cell-induced architectural changes and BrdU incorporation (Fig 7A,B) in WT→WT BMC resembled intact WT mice. In contrast, increases in epithelial proliferation were abrogated in WT→TNFR1/2−/− BMC mice (data not shown). The results of BMC mice indicate that TNF production by BM-derived cells induces epithelial TNFR-mediated responses.
Figure 7. TNFR signaling mediates T cell-mediated IEC response.
(A) Representative images of H&E and BrdU staining for ileal tissue of control- and anti-CD3-treated bone marrow chimera mice. Scale bars represent 150µm. (B) Quantification of BrdU-positive cells per crypt. Positive cells were counted in at least 20 well-oriented crypt/villus axes per section; n=4 mice per group. Values represent mean ± SEM *p<0.01, **p<0.001.
Epithelial TNFR signaling is crucial in promoting mucosal healing in chronic colitis
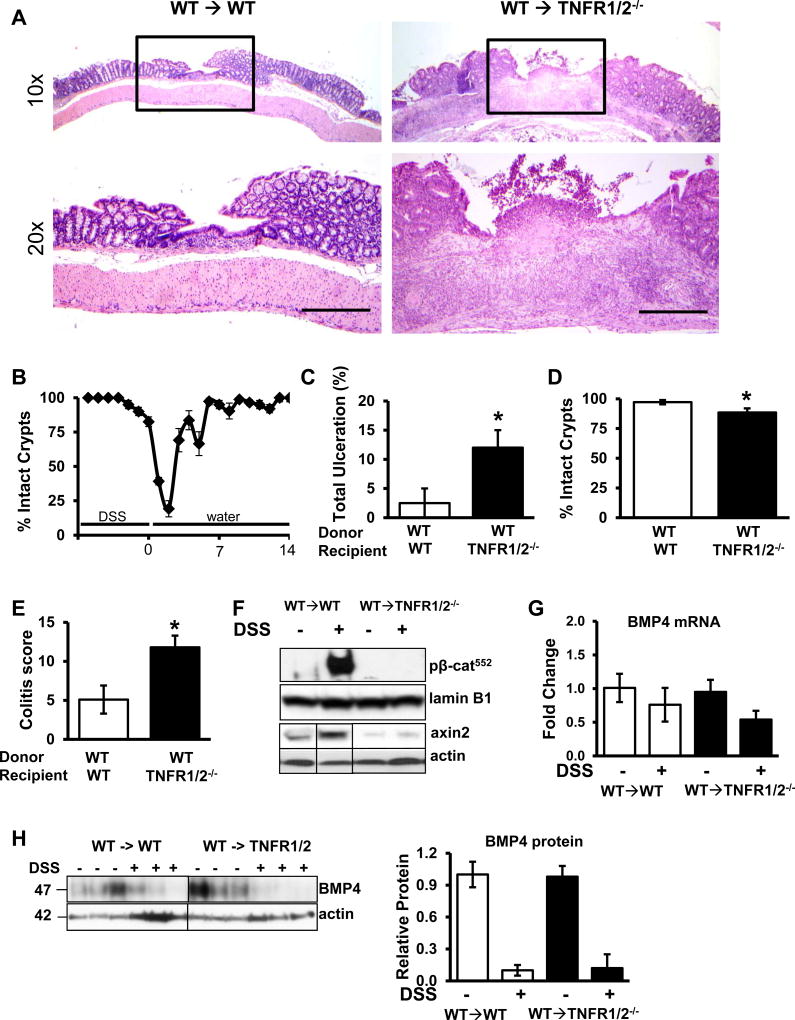
Given our findings that suggest that IEC TNF receptor signaling is critical for T cell-induced IEC responses in the colon, we examined the requirement for TNFR signaling in mediating colonic IEC responses to chronic colitis (Fig 8). Following 3 cycles of 2.5% DSS, mice develop a highly reproducible course of colitis characterized by ulceration, inflammatory infiltrate, hyperplasia of epithelium, and crypt branching. In WT mice, severe ulceration (60–80% denuded epithelium) peaks 3 days following withdraw of DSS and the mice completely recover in the following 11 days. Healing can be measured as the percent of colonic surface with intact crypts (Fig 8B) and in order to observe both damaged and healing epithelium, mice are analyzed 6 days following withdrawal of DSS. Compared to WT→WT BMC, BMC with deficient TNFR1/2 expression (WT→TNFR1/2−/−) exhibited a more severe response to colitis, as measured by increased total ulceration (Fig 8C), reduced surface area with intact crypts (Fig 8D), and an increased colitis score (Fig 8E). β-catenin signaling was measured by WB analysis of epithelial protein isolates demonstrating that increased nuclear p-β-catenin552 and cytosolic axin2 seen in WT→WT mice with colitis was abrogated in WT→TNFR1/2−/− BMC (Fig 8F). Interestingly, although healing was delayed in WT→TNFR1/2−/− BMC mice, complete re-epithelialization was completed by 14 days following DSS (relative to complete healing at 7 days in WT->WT mice, data not shown). It is possible that non-epithelial radio-resistant populations of cells (e.g. tissue macrophages, fibroblasts, etc) contribute to the healing response in this model. Although we cannot rule out this contribution, we analyzed tissue samples for BMP4 mRNA expression (Fig 8G) and protein levels (Fig 8H). DSS treatment reduced BMP4 levels to the same degree in both WT->WT and WT->TNFR1/2 mice. Together these data suggest that epithelial TNF receptor signaling is crucial for promoting mucosal healing by mediating ISC/PC activation during chronic colitis.
Figure 8.
Epithelial TNF receptor signaling is required for mucosal healing after DSS treatment. (A) Representative histology (top: 4X images, bottom: 20X images) of colons from WT→WT and WT→TNFR1/2−/− bone marrow chimeric mice 6 days following 3 cycles of 2.5% DSS. Scale bars represent 1.5mm. (B) Graphical representation of the healing timecourse in WT mice treated with DSS (n = 3–6 mice at each timepoint). The percent of colonic surface area containing intact crypts during and after the last cycle of DSS is shown. (C) The percent of ulcerated area and (D) percent of area with intact crypts 6 days following DSS withdrawal. (E) Colitis scores for WT→WT and WT→TNFR1/2−/− bone marrow chimeras treated with DSS. (F) Western blot of nuclear p-β-catenin552 and axin2 from isolated IEC from WT→WT and WT→TNFR1/2 colon. (G and H) mRNA and protein levels of BMP4 from colonic tissue isolated from WT→WT and WT→TNFR1/2 colon. n=5 mice for each group. Values represent mean ± SEM, *p<0.05.
Discussion
Canonical Wnt/β-catenin plays a crucial role in mucosal wound healing (32, 33). Stappenbeck and colleagues presented strong evidence that noncanonical Wnt (e.g. Wnt5a) contributes to wound healing by allowing expanding crypts to form clefts at wound edges(34). The demonstration that noncanonical Wnt signaling opposes canonical signals(9) raises the attractive hypothesis that ulcer healing in IBD occurs as an outcome of factors that both promote and oppose Wnt signaling(35). Data presented here suggest that TNF may be a positive signal that promotes Wnt signaling. The downstream mechanisms for TNF actions include both NFκB and PI3K signaling, which have been shown to cooperate with Wnt to promote β-catenin activation(12, 14, 35). We further propose that TNF regulates ISC and PC populations and contributes to the robust mitogenic crypt responses to mucosal inflammation (as in IBD). As effects of TNF signaling deficiencies were most apparent when mice were stimulated with anti-CD3 mAb, we conclude that TNF primarily mediates inflammation-induced IEC Wnt/β-catenin signaling.
These data reveal that TNF directly activates ISC and PC populations during mucosal immune responses and ulcer healing. Comparisons between WT and Tnf−/− IEC responses to T cell activation suggest that TNF induces nuclear p-β-catenin552 signaling and proliferation of CBC and PC populations. The role of TNF in mediating ISC and PC responses was seen in WT mice where exogenous TNF induced β-catenin signaling, BrdU incorporation and BAT-GAL/TOP-GAL expression in crypt IEC. The induction of BAT-GAL in crypt IEC provides direct evidence for the effect of TNF on Wnt/β-catenin signaling(31). The effect of TNF on ISC/PC populations was further supported in enteroids and colonoid cultures in which TNF induced Wnt/β-catenin target genes (Ascl2, Lgr5, Axin2, CD44 and Lrig). Enhanced TOPFlash activity in NCM460 cells provided further evidence that TNF directly activates epithelial Wnt/β-catenin transcriptional activity. Together, these data suggest that effects of TNF on Wnt/β-catenin signaling in IEC (ISC/PC) support mucosal healing in colitis.
In addition to increasing Wnt/β-catenin signaling and activating mitogenic responses in ISC/PC, these data also suggest that bone marrow-derived TNF is protective against epithelial apoptosis during inflammation. Previous studies have shown that T cell activation induces epithelial apoptosis in a biphasic manner, differentially affecting villus tip (acute) and crypt apoptosis (>24 hours)(24). In mice lacking bone marrow-derived TNF, crypt apoptosis occurred earlier and more severely than in WT mice. T cell-induced apoptosis has been shown to occur by a variety of mechanisms, including those mediated by TNF, perforin and Fas/FasL interactions(24, 36). Our data demonstrate that in the absence of BM-derived TNF, apoptotic responses escalate. In other tissues, enhanced levels of IFNγ seen in TNF-deficient mice have been proposed to increase programmed cell death(37). Thus, TNF plays an additional regulatory role in the mucosal immune compartment by regulating IFNγ production and thereby curtailing epithelial cell death. The precise nature of the pro- and anti-apoptotic impact of TNF on IECs has been observed previously where authors propose that lower TNF levels promote survival signals and higher levels impart pro-apoptotic effects(38).
A question raised by these studies is how TNF plays dual roles in mucosal inflammation. Clinical data indicate that TNF blockade induces mucosal healing(39) whereas blockade of soluble TNF may make some parameters of colitis worse(6). In WT mice TNF activates mitogenic ISC and TA responses that aide in ulcer healing. We speculate that the mucosal healing induced by anti-TNF mAb is likely a secondary response to anti-inflammatory depletion of pathogenic effector cells. Anti-TNF induces apoptosis in pathogenic T cells by blocking their interaction with membrane-bound TNF expressing monocytes in a TNFR-dependent manner(39). As demonstrated in Tnf−/− mice, the need for epithelial TNFR signaling becomes more evident in the setting of severe inflammation with high type 1 cytokine levels. In WT →TNFR1/2−/− BMC mice, the loss of IEC TNFR signaling attenuates IEC proliferation and p-β-catenin552 levels and worsens mucosal inflammation. However, deficient IEC responses do not cause crypt disruption and loss of mucosal integrity as they do in Tnf−/− mice. We suspect that the explanation for these differences relates to the absence of severe tissue inflammation in TNFR1/2−/− BMC mice. Thus, anti-TNF likely reduces the need for TNF in ISC and PC activation. A concern raised by data here is that anti-TNF mAb therapy may reduce ISC/PC activation in patients with persistent mucosal inflammation (as in Fig 1). Continuation of ineffective TNF blockade in these patients may impair mucosal healing rather than have neutral clinical effects. Interestingly, clinical studies with etanercept, a soluble TNFR:Fc fusion protein, have been uniformly negative(6). Given that etanercept does not induce effector cell apoptosis, we speculate that TNF neutralization in these patients may impair IEC TNF signaling without sufficiently inhibiting mucosal inflammation. Thus, reduced TNF available to IEC may have contributed to the negative clinical outcomes observed.
In summary, these data present the novel idea that TNF promotes mucosal healing in colitis through actions on colonic epithelial stem and progenitor cell populations. Schwitalla et al previously suggested that TNF may enhance Wnt/β-catenin activation though NFkB signaling(40). Here we suggest that this pathway may be active (and helpful) in mucosal healing responses during colitis. Abundant data indicate that TNF is crucial to normal host defenses to enteric pathogens(41). In IBD, the same mechanisms are called into play as the mucosa heals ulcers caused by dysfunctional inflammation. Future studies are needed to determine if this pathway can be usurped at the epithelial level to aide mucosal repair in patients refractory to standard therapies.
Supplementary Material
Acknowledgments
The authors would like to thank L. Li for generously providing us with p-β-catenin552 antibody and D. Shealy (Janssen R&D) for control and anti-TNF murine chimeric mAbs.
Support
Support was provided by a Veterans Affairs Merit Award (I01CX001353-0141 to TAB); National Institutes of Health (R01AI061701 and R01DK-095662 to TAB); the Training Program in Oncogenesis and Developmental Biology through the National Cancer Institute [NCI T32 CA080621, to support EMB]; and an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health [8 P20GM103527-05, to support tissue processing]. Imaging work was also performed at the Northwestern Cell Imaging Facility supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center.
Footnotes
Disclosures:
T. A. Barrett was an honorary speaker for Janssen Research and Development and AbbVie Pharmaceuticals. D.J. Shealy is an employee of Janssen Research and Development. The other authors have no conflict of interest to declare.
References
- 1.Bradley JR. TNF-mediated inflammatory disease. The Journal of pathology. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 2.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. Journal of immunology. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 4.Neurath MF. Cytokines in inflammatory bowel disease. Nature reviews. Immunology. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 5.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World journal of surgery. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, Tremaine WJ, Johnson T, Diehl NN, Zinsmeister AR. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088–1094. doi: 10.1053/gast.2001.28674. [DOI] [PubMed] [Google Scholar]
- 7.Waetzig GH, Rosenstiel P, Arlt A, Till A, Brautigam K, Schafer H, Rose-John S, Seegert D, Schreiber S. Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-beta1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:91–93. doi: 10.1096/fj.04-2073fje. [DOI] [PubMed] [Google Scholar]
- 8.Bressenot AM, Peyrin-Biroulet L. Letter: histological assessment of disease activity in ulcerative colitis--the problem of score evaluation and validation; authors’ reply. Alimentary pharmacology & therapeutics. 2016;43:439. doi: 10.1111/apt.13489. [DOI] [PubMed] [Google Scholar]
- 9.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. The Journal of cell biology. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes & development. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 12.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nature genetics. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, Weber CR, Turner JR, He XC, Katzman RB, Li L, Barrett TA. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. 881, e861–869. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nature genetics. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nature protocols. 2013;8:2471–2482. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu W, Hu Y, Andersen TE, Jafari A, Li N, Chen W, Kassem M. Tumor necrosis factor receptor superfamily member 19 (TNFRSF19) regulates differentiation fate of human mesenchymal (stromal) stem cells through canonical Wnt signaling and C/EBP. The Journal of biological chemistry. 2010;285:14438–14449. doi: 10.1074/jbc.M109.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Molecular and cellular biology. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Flavin P, Redmond A, McBryan J, Cocchiglia S, Tibbitts P, Fahy-Browne P, Kay E, Treumann A, Perrem K, McIlroy M, Hill AD, Young LS. RuvBl2 cooperates with Ets2 to transcriptionally regulate hTERT in colon cancer. FEBS letters. 2011;585:2537–2544. doi: 10.1016/j.febslet.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. The Journal of clinical investigation. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Clayburgh DR, Mittal N, Goretsky T, Dirisina R, Zhang Z, Kron M, Ivancic D, Katzman RB, Grimm G, Lee G, Fryer J, Nusrat A, Turner JR, Barrett TA. Epithelial NF-kappaB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. The American journal of pathology. 2010;176:158–167. doi: 10.2353/ajpath.2010.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura N, Yamamoto M, Fukutake M, Ohtake N, Iizuka S, Ishige A, Sasaki H, Fukuda K, Yamamoto T, Hayakawa S. Anti-CD3 induces bi-phasic apoptosis in murine intestinal epithelial cells: possible involvement of the Fas/Fas ligand system in different T cell compartments. International immunology. 2005;17:513–522. doi: 10.1093/intimm/dxh231. [DOI] [PubMed] [Google Scholar]
- 25.Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Posca D, Ryu H, Katzman RB, Barrett TA. p53 mediates TNF-induced epithelial cell apoptosis in IBD. The American journal of pathology. 2012;181:1306–1315. doi: 10.1016/j.ajpath.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Church DN, Talbot DC. Survivin in solid tumors: rationale for development of inhibitors. Current oncology reports. 2012;14:120–128. doi: 10.1007/s11912-012-0215-2. [DOI] [PubMed] [Google Scholar]
- 27.Cohran V, Managlia E, Bradford EM, Goretsky T, Li T, Katzman RB, Cheresh P, Brown JB, Hawkins J, Liu SX, De Plaen IG, Weitkamp JH, Helmrath M, Zhang Z, Barrett TA. Epithelial PIK3R1 (p85) and TP53 Regulate Survivin Expression during Adaptation to Ileocecal Resection. The American journal of pathology. 2016;186:1837–1846. doi: 10.1016/j.ajpath.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raj D, Liu T, Samadashwily G, Li F, Grossman D. Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis. 2008;29:194–201. doi: 10.1093/carcin/bgm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martini E, Schneider E, Neufert C, Neurath MF, Becker C. Survivin is a guardian of the intestinal stem cell niche and its expression is regulated by TGF-beta. Cell cycle. 2016;15:2875–2881. doi: 10.1080/15384101.2016.1231286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martini E, Wittkopf N, Gunther C, Leppkes M, Okada H, Watson AJ, Podstawa E, Backert I, Amann K, Neurath MF, Becker C. Loss of Survivin in Intestinal Epithelial Progenitor Cells Leads to Mitotic Catastrophe and Breakdown of Gut Immune Homeostasis. Cell reports. 2016;14:1062–1073. doi: 10.1016/j.celrep.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch S, Nava P, Addis C, Kim W, Denning TL, Li L, Parkos CA, Nusrat A. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology. 2011;141:259–268. 268, e251–258. doi: 10.1053/j.gastro.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, Skerra A, Li L, Parkos CA, Nusrat A. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett TA. Developmental biology. Intestinal wound healing requires a Wnt balancing act. Science. 2012;338:51–52. doi: 10.1126/science.1229414. [DOI] [PubMed] [Google Scholar]
- 36.Merger M, Viney JL, Borojevic R, Steele-Norwood D, Zhou P, Clark DA, Riddell R, Maric R, Podack ER, Croitoru K. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut. 2002;51:155–163. doi: 10.1136/gut.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, Alexander S, Gicquel B, Wan Y, Bramson J, Inman M, Xing Z. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. The Journal of clinical investigation. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leppkes M, Roulis M, Neurath MF, Kollias G, Becker C. Pleiotropic functions of TNF-alpha in the regulation of the intestinal epithelial response to inflammation. International immunology. 2014;26:509–515. doi: 10.1093/intimm/dxu051. [DOI] [PubMed] [Google Scholar]
- 39.Atreya R, Zimmer M, Bartsch B, Waldner MJ, Atreya I, Neumann H, Hildner K, Hoffman A, Kiesslich R, Rink AD, Rau TT, Rose-John S, Kessler H, Schmidt J, Neurath MF. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14(+) macrophages. Gastroenterology. 2011;141:2026–2038. doi: 10.1053/j.gastro.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Beutler B, Grau GE. Tumor necrosis factor in the pathogenesis of infectious diseases. Critical care medicine. 1993;21:S423–435. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.