Abstract
IMPORTANCE
Response patterns with immunotherapy may differ from those of other treatments. This warrants further investigation because some patients may benefit from continued immunotherapy beyond Response Evaluation Criteria in Solid Tumors (RECIST)-defined first progression.
OBJECTIVE
To evaluate the safety and potential benefit of treatment with nivolumab, a programmed cell death 1 immune checkpoint inhibitor, beyond investigator-assessed first progression in patients with metastatic renal cell carcinoma (mRCC).
DESIGN, SETTING, AND PARTICIPANTS
Subgroup analysis of a blinded, randomized, multicenter, phase 2 dose-ranging trial initiated May 31, 2011, including patients with clear-cell mRCC previously treated with antiangiogenic therapy. Data cutoffs for this subgroup analysis were May 15, 2013, for progression-free survival and objective response rate and March 5, 2014, for overall survival and duration of response. In this analysis, patients treated beyond first progression received their last dose of nivolumab more than 6 weeks after RECIST-defined progression, and patients not treated beyond first progression discontinued nivolumab before or at RECIST-defined progression.
INTERVENTIONS
Nivolumab 0.3, 2, or 10 mg/kg intravenously every 3 weeks.
MAIN OUTCOMES AND MEASURES
Safety and efficacy of nivolumab treatment.
RESULTS
Of 168 patients (median [range] age, 61 [37–81] years; 72% male) randomized to nivolumab, 154 experienced progression (36 were treated beyond first progression, 26 were treated beyond first progression for ≤ 6 weeks, and 92 were not treated beyond first progression), 13 were treated and did not experience progression, and 1 was not treated. Prior to first progression, the RECIST-defined objective response rate was 14% (5 patients) and 16% (15 patients), and median progression-free survival was 4.2 (95% CI, 2.8–5.5) and 2.6 (95% CI, 1.5–3.9) months in patients treated and not treated beyond progression, respectively. Following initial progression, 25 (69%) patients treated beyond progression experienced subsequent tumor reduction or stabilization in target lesion size. The incidence of treatment-related adverse events was higher in patients treated beyond progression (n = 29 [81%]) vs those not treated beyond progression (n = 61 [66%]); however, after adjusting for length of treatment exposure, incidence was lower in patients treated beyond progression (322.9 vs 518.7 incidence rate/100 patient-years for patients treated vs not treated beyond progression).
CONCLUSIONS AND RELEVANCE
In this subgroup analysis, a proportion of patients who continued treatment beyond RECIST-defined first progression demonstrated sustained reductions in tumor burden or stabilization in the size of target lesions, with an acceptable safety profile. Further analysis will help define the clinical benefit for patients with mRCC treated with nivolumab beyond progression.
Despite significant improvements in objective response rate (ORR) and progression-free survival (PFS) seen with targeted therapies that inhibit the vascular endothelial growth factor (VEGF)-mediated and mammalian target of rapamycin-mediated pathways for the treatment of metastatic renal cell carcinoma (mRCC), the majority of patients eventually experience treatment resistance and disease progression.1,2 Only 12% of patients with mRCC are alive at 5 years3 and only 1 agent has shown an improvement in overall survival (OS) in a specific subgroup of patients,4 underscoring the need for novel treatment options that provide durable responses, improved OS for a broad range of patients, and a more manageable safety profile.1 Newer agents such as immune checkpoint inhibitors like nivolumab (recently approved by the US Food and Drug Administration for the treatment of advanced RCC in patients who have received prior antiangiogenic therapy) provide a unique mechanism of action vs currently approved targeted therapies for mRCC.3
Nivolumab is a fully human IgG4 programmed cell death 1 (PD-1) immune checkpoint inhibitor antibody that selectively blocks the interaction between PD-1 and its ligands PD-L1 and PD-L2, which is a mechanism that normally leads to immune tolerance.5–7 In the phase 3 CheckMate 025 trial, which evaluated patients (N = 821) with advanced clear-cell RCC who had received previous treatment with 1 or 2 antiangiogenic regimens, treatment with nivolumab resulted in a significant survival advantage over everolimus (25.0 vs 19.6 months, hazard ratio 0.73; P = .002).8 Grade 3 or 4 treatment-related adverse events (AEs) occurred less frequently in nivolumab-treated patients than in everolimus-treated patients (19% vs 37%).
Treatment with immunomodulatory agents such as nivolumab in various tumor types has sometimes been associated with “tumor flare.”9–15 Tumor flare refers to the apparent increase in tumor burden or the occurrence of new lesions that sometimes precedes clinical responses in patients receiving immunotherapy.16 Tumor flare is believed to be due to transient immune cell infiltration into the tumor or continued tumor growth that can occur while the immune system is priming for an antitumor response (eFigure 1 in Supplement 1).16 Therefore, the time required to establish an effective immune response to active immunotherapy may exceed what is expected based on typical response times to targeted therapies.17
Historically, the Response Evaluation Criteria in Solid Tumors (RECIST) Working Group has provided standard guidelines to define tumor response to therapy.18 By RECIST criteria, a significant (≥20%) increase in the size of tumor lesions and/or the development of new lesions is considered unequivocal evidence of disease progression. Thus, when assessed by RECIST criteria, tumor flare occurring with immunotherapy will be viewed as disease progression and may lead to discontinuation of treatment before the potential clinical benefit of the treatment is fully realized.19 It is therefore of interest to understand whether patients receiving immunotherapy may derive continued clinical benefit if treated beyond RECIST-defined progression.
Treatment beyond first RECIST-defined progression was allowed in a phase 2 dose-ranging trial of nivolumab (0.3, 2, and 10 mg/kg intravenously once every 3 weeks) in patients with mRCC (N = 168) previously treated with VEGF pathway inhibitors.20 In the overall study population, ORR ranged from 20% to 22%, median PFS ranged from 2.7 to 4.2 months, and median OS ranged from 18.2 to 25.5 months. The most common treatment-related AE was fatigue, experienced by 22% to 35% of patients. Treatment beyond first progression was allowed for patients who tolerated nivolumab and exhibited investigator-assessed clinical benefit. Initial assessment of the subgroup of patients treated beyond first progression demonstrated that some patients had sustained reductions or stabilization in the size of their target lesions.20 The objective of this subgroup analysis was to further evaluate the potential clinical benefit of nivolumab in patients with mRCC treated beyond investigator-assessed first progression.
Methods
Study Design and Treatment
This was a subgroup analysis of a blinded, randomized, multicenter, phase 2 dose-ranging trial conducted at academic centers in the United States, Canada, Finland, and Italy (see study protocol in Supplement 2).20 Patients were randomized 1:1:1 to receive nivolumab 0.3, 2, or 10 mg/kg every 3 weeks as a 60-minute intravenous infusion on day 1 of each treatment cycle. Treatment continued until disease progression defined by RE-CIST version 1.1 (growth of existing lesions defined as ≥20% increase in the sum of diameters of target lesions, taking as reference the smallest sum during study participation, and absolute increase of ≥5 mm or appearance of new lesions), intolerance to treatment, or until stopped for other protocol-defined reasons. Per protocol, treatment beyond first progression was allowed in patients continuing to tolerate nivolumab and exhibiting investigator-assessed clinical benefit (eg, immune-related partial response; immune-related stable disease) at the time of first progression. Immune-related partial response was defined as at least 30% decrease in the sum of diameters of target lesions and all new measurable lesions, taking as reference the baseline sum diameters. Immune-related stable disease was defined as neither sufficient shrinkage to qualify for immune-related partial response nor sufficient increase to qualify for immune-related progressive disease, taking as reference the smallest sum diameters during study participation. The study was approved by the institutional review board or independent ethics committee of each center. All patients provided written informed consent.
Patients
Patients eligible for study inclusion had histologic confirmation of RCC with a clear-cell component and measurable disease by RECIST version 1.1, had received prior treatment with at least 1 antiangiogenic therapy in the metastatic setting, experienced disease progression within 6 months of enrollment, and had a Karnofsky performance status (KPS) of at least 70. Patients with active central nervous system metastases, autoimmune disease, or who had received more than 3 prior treatment regimens in the metastatic setting were excluded.
In this analysis, patients considered to be treated beyond first progression were defined as having received their last available dose of nivolumab more than 6 weeks after initial progression date, and discontinued therapy after the next documented progression. Patients defined as not treated beyond first progression discontinued study treatment before or on the date of progression. Patients defined as treated briefly beyond progression received their last available dose of nivolumab no more than 6 weeks after the date of progression.
Efficacy and Safety Assessments
The primary end point was dose response by PFS. Progression-free survival was defined as time from randomization to investigator-assessed first clinical or radiographic RECIST progression, or death. Secondary end points included ORR based on investigator RECIST assessment, time to response, duration of response defined as time from complete response or partial response to first disease progression, and OS beginning 6 weeks from first progression in evaluable patients (those who were alive, continuing in the study, or had <6 weeks between progression and data cutoff) to death. Best overall response was defined as best tumor response (complete or partial response) from randomization to first disease progression or treatment discontinuation. Tumor burden was estimated by measurable disease (presence of ≥1 measurable tumor lesion). Assessments were performed at baseline and every 6 weeks from and every 12 weeks thereafter, until disease progression or treatment discontinuation (whichever occurred later). After treatment discontinuation, patients were evaluated every 3 months for survival and safety.
Safety was assessed at every clinic visit. Adverse events were graded for severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.21 Patients were observed for AEs until events resolved, returned to baseline, or were deemed irreversible.
Statistical Analyses
Progression-free survival, OS, and time to objective response were estimated using Kaplan-Meier methodology.22 Two-sided 95% confidence intervals (CIs) for median PFS, OS, and duration were computed by the Brookmeyer and Crowley method.23 The ORR analysis was based on best overall response, as defined in the Methods section, and 2-sided, 95% CIs for the response rate were computed by the Clopper and Pearson method.24 Time to objective response was summarized using descriptive statistics. Tumor burden change (sum of diameters of target lesions) over time for each patient was displayed graphically. Data cutoffs were May 15, 2013, for PFS and ORR analyses and March 5, 2014, for OS and duration of response.
Results
Patient Population
Between May 2011 and January 2012, 168 patients were randomized to 1 of 3 nivolumab doses as previously described.20 The median age of patients was 61 years (range, 37–81) and 72% were male. Of the randomized patients, 154 experienced progression, 13 were treated and did not experience progression, and 1 was not treated. Of the 154 patients who experienced progression, 36 (n = 10, 12, and 14 for the 0.3-, 2-, and 10-mg/kg doses, respectively) were treated beyond first progression for more than 6 weeks after their initial progression date, 26 were treated briefly beyond first progression for 6 weeks or less after their initial progression date (n = 11, 7, and 8 for the 0.3-, 2-, and 10-mg/kg doses), and 92 (n = 31, 32, and 29 for the 0.3-, 2-, and 10-mg/kg doses) were not treated beyond first progression (Figure 1). A summary of characteristics and dosing in patients treated briefly beyond progression can be found in eTable 1 in Supplement 1.
Figure 1.
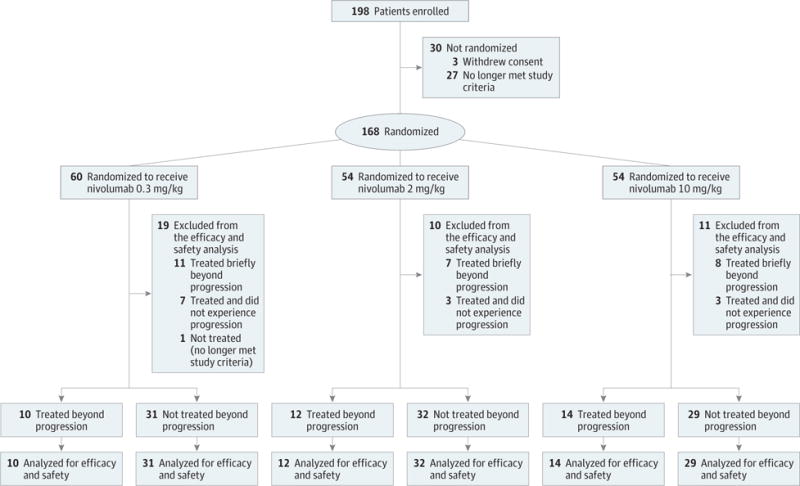
Patient Disposition (as of May 15, 2013, Data Cutoff)
Demographic and baseline characteristics at study entry for patients treated and not treated beyond first progression are summarized in Table 1. A similar proportion of patients treated and not treated beyond first progression met Memorial Sloan Kettering Cancer Center poor-risk criteria (8 [22%] vs 24 [26%]), had a baseline KPS of at least 90 (21 [58%] vs 46 [50%]), and had 1 prior systemic antiangiogenic regimen in the metastatic setting (26 [72%] vs 60 [65%]). Fewer patients treated beyond first progression (25 [69%]) had at least 2 evaluable sites vs those not treated beyond first progression (80[87%]). Of 36 patients treated beyond progression, 32 patients discontinued treatment; the most common reason for discontinuation was progressive disease (n = 28) (eTable 2 in Supplement 1).
Table 1.
Demographic and Baseline Patient Characteristics at Study Entry
| Characteristic | Patients Treated Beyond Progression (n = 36) |
Patients Not Treated Beyond Progression (n = 92)a |
|---|---|---|
| Median age (range), y | 60.0 (43.0–78.0) | 61.0 (38.0–81.0) |
| Sex, No. (%) | ||
| Male | 32 (89) | 61 (66) |
| Female | 4 (11) | 31 (34) |
| Memorial Sloan Kettering Cancer Center risk group, No. (%) | ||
| Favorable | 8 (22) | 33 (36) |
| Intermediate | 20 (56) | 33 (36) |
| Poor | 8 (22) | 24 (26) |
| Not reported | 0 | 2 (2) |
| Karnofsky performance status, No. (%) | ||
| 70 or 80 | 14 (39) | 46 (50) |
| ≥90 | 21 (58) | 46 (50) |
| No. of evaluable sites, No. (%)b | ||
| 1 | 11 (31) | 12 (13) |
| ≥2 | 25 (69) | 80 (87) |
| Prior radiotherapy, No. (%) | 12 (33) | 36 (39) |
| No. of prior systemic antiangiogenic regimens in the metastatic setting, No. (%) | ||
| 1 | 26 (72) | 60 (65) |
| 2 | 9 (25) | 28 (30) |
| ≥3 | 1 (3) | 4 (4) |
Percentages may not sum to 100 because of rounding.
Including target and nontarget lesions.
At the time of disease progression, a higher proportion of patients treated beyond first progression (21 [58%]) had a KPS of at least 90 vs those not treated beyond first progression (37 [40%]) (eTable 3 in Supplement 1). At the time of disease progression, a higher proportion of patients with new lesions were treated beyond first progression (22 [61%]) vs those not treated beyond first progression (41 [45%]), while a similar proportion of patients with an increase in target lesions were treated (13 [36%]) and not treated beyond first progression (34 [37%]) (eTable 3 in Supplement 1).
Efficacy
A summary of RECIST-based efficacy results before first progression is presented in Table 2. A similar proportion of patients treated and not treated beyond first progression achieved an objective response (5 [14%] and 15 [16%]). Median (range) time to objective response was longer in patients treated beyond first progression (4.2 [1.4–6.9] months) vs those not treated beyond first progression (2.6 [1.2–5.6] months). Of note, 9 (25%) and 38 (41%) patients treated and not treated beyond first progression, respectively, had progressive disease as their best response. Prior to first progression, patients treated beyond first progression had a longer median PFS (4.2 [95% CI, 2.8–5.5] months) than those not treated beyond first progression (2.6 [95% CI, 1.5–3.9] months).
Table 2.
Summary of Efficacy Results Before First Progression
| Measure | Patients Treated Beyond Progression (n = 36) |
Patients Not Treated Beyond Progression (n = 92)a |
|---|---|---|
| Objective response rate, No. (% [95% CI]) | 5 (14 [5–30]) | 15 (16 [9–26]) |
| Time to objective response, median (range), mo | 4.2 (1.4–6.9) | 2.6 (1.2–5.6) |
| Best overall response, No. (%) | ||
| Complete response | 0 | 1 (1) |
| Partial response | 5 (14) | 14 (15) |
| Stable disease | 21 (58) | 35 (38) |
| Progressive disease | 9 (25) | 38 (41) |
| Other | 1 (3) | 4 (4) |
| Progression-free survival, median (95% CI), mo | 4.2 (2.8–5.5) | 2.6 (1.5–3.9) |
Percentages may not sum to 100 because of rounding.
Duration of treatment and survival of patients treated beyond first progression are shown in Figure 2. At the time of analysis, 89%(n = 32) of patients treated beyond first progression had dis-continued treatment. Of the patients who discontinued treatment, 23 discontinued and died and 9 discontinued. At the time of analysis, 4 (11%) were still receiving treatment more than 3 years after their first progression. Median (range) duration of treatment from time of first progression to last dose or death for patients who discontinued treatment (n = 32) was 4.2 (1.5–32.2) months and the median (range) duration of treatment for those still receiving treatment (n = 4) was 37.4 (33.2–37.8) months.
Figure 2. Duration of Treatment and Survival in Patients Treated Beyond Progression.
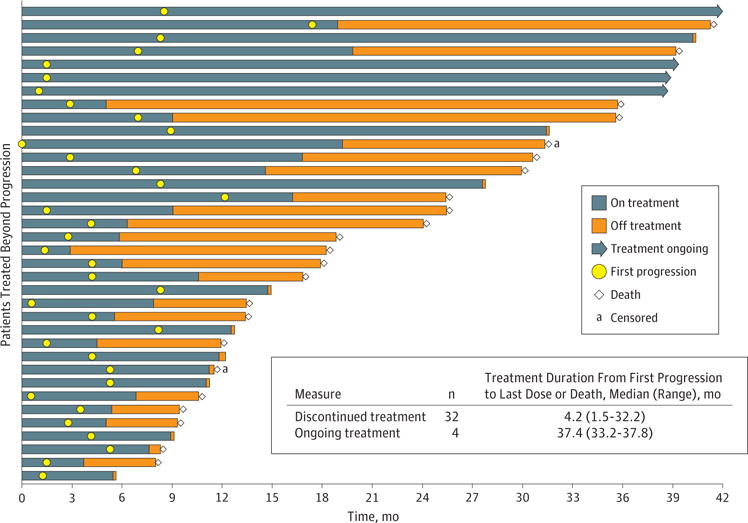
Patients were censored for progression-free survival if they received subsequent anticancer therapy, radiotherapy, or surgery, without progression being reported prior to this or on the same day. One patient had only 1 day of tumor progression so is not visible in the Figure.
Change in tumor burden after first progression in patients treated beyond first progression is shown in Figure 3. Following initial progression, 25 (69%) patients treated beyond first progression experienced subsequent tumor reduction in target lesions(Figure 3). Of patients treated beyond first progression who had at least a 20% increase in tumor burden by the time of first progression, half experienced a subsequent tumor reduction (Figure 3). Patterns of change in tumor burden over time (before and after progression) are shown in eFigure 2 in Supplement 1. A total of 12 patients had a change from baseline in tumor burden that exceeded the 30% reduction consistent with a RECIST 1.1 response (eFigure 2 in Supplement 1). Radiographic scans of 2 patients treated beyond first progression are shown in eFigure 3 in Supplement 1.
Figure 3. Tumor Burden Change From First Progression.
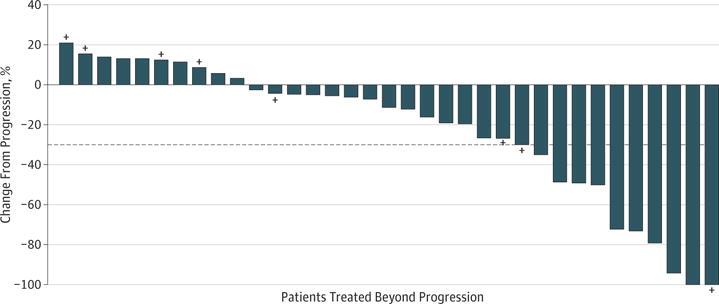
Tumor burden change in target lesions from first progression in patients treated beyond progression. The horizontal dashed reference line indicates the 30% reduction consistent with a Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 response. Plus signs indicate patients who had at least 20% increase in target lesions at time of first progression. One patient did not have tumor sum of diameters values available prior to progression because 1 of the target lesions became nonmeasurable, and therefore is not shown in the Figure.
In a landmark analysis of evaluable patients beginning 6 weeks from first progression, median OS for patients treated beyond first progression was 22.5 (95% CI, 12.3–31.3) months and for those not treated beyond first progression was 12.3 (95% CI, 8.0–17.1) months (eFigure 4 in Supplement 1).
Safety
The overall median (range) number of doses received from the start of the study was 14.5 (5–61) and 3.0 (1–54) in patients treated and not treated beyond first progression, respectively. Patients treated beyond progression received a median (range) of 6.5 (1–53) doses beyond first progression. Consistent with differences in drug exposure between patient groups, the incidence of any-grade treatment-related AEs was higher in patients treated beyond first progression vs those not treated beyond first progression; however, patients treated beyond first progression had a lower incidence of grade 3 or 4 AEs (eTable 4 in Supplement 1). When adjusted for duration of treatment exposure, the incidence of all-grade treatment-related AEs was lower in patients treated beyond first progression vs those not treated beyond first progression (eTable 5 in Supplement 1). In addition, types of treatment-related AEs occurring before and after first progression were similar in those patients treated beyond progression (eTable 6 in Supplement 1).
Discussion
In this subgroup analysis, a proportion of patients who continued treatment beyond first progression based on investigator-assessed clinical benefit demonstrated sustained reductions in tumor burden or stabilization in the size of target lesions. Consistent with the overall population of this study,20 the safety profile of nivolumab in patients treated beyond first progression was acceptable, indicating that safe treatment with nivolumab beyond first progression is feasible for patients with previously treated mRCC.
Two prevailing hypotheses have been proposed to explain the initial appearance of progressive disease prior to subsequent clinical benefit that is sometimes observed during immunotherapy.16 One hypothesis suggests that immunologic treatment may induce infiltration of immune cells and inflammation of the tumor, which results in increased tumor size by objective measures (eg, by imaging). Alternatively, the growth of preexisting lesions or the appearance of new lesions can occur after administration of immunotherapy, as the process of immune activation may potentially be delayed. The tumor may grow transiently during the period of immune activation and before an effective antitumor response occurs.19 These characteristic effects of immunomodulatory agents may result in the detection of transient progression, which would decrease RECIST-defined PFS, but not necessarily OS. Such phenomena are not uncommon in immunotherapy studies in which treatment is associated with an initial tumor flare prior to reduced tumor burden and shrinkage. For example, studies of nivolumab therapy in melanoma13,15 and non-small-cell lung cancer14 demonstrated that a subgroup of patients treated beyond RECIST-defined first progression showed a nonconventional pattern of benefit that included a reduced tumor burden relative to patients who were not treated beyond first progression. Similar findings were also reported in patients with melanoma who were treated with the anti-cytotoxic T-lymphocyte antigen4 (CTLA-4) antibody ipilimumab9,11 or the anti-PD-1 antibody pembrolizumab.12 It is not known, however, how T-cell receptor occupancy of drugs such as nivolumab affects tumor response and whether continued treatment is absolutely required for these late responses.
Because RECIST-defined progression during treatment with immunotherapy may not necessarily indicate biologic disease progression, at least in the initial phase, the development of immune-related response criteria was undertaken to better monitorpatients treated with immunotherapy.16,25 Based on clinical experience with ipilimumab, these criteria were formed to systematically characterize additional patterns of response observed with immunotherapies.16 Although the concept of immune-related response criteria is important, the criteria are limited in scope because they might not fully address all relevant patterns of clinical activity,16 and therefore new approaches are being considered for patients treated with immunotherapy. For example, composite end points, biomarkers, time to symptomatic progression, and quality of life have all been considered as potential measures to gauge efficacy and/or serve as clinical trial end points for studies evaluating immunotherapy.10,26–28 It is important to note that the whole clinical picture-and not just response criteria-should be taken into account when deciding which patients to treat beyond first progression. Although physicians in this study were not directly asked how they chose which patients to treat beyond first progression, based on a retrospective assessment of the patient characteristics, those with better KPS, longer time to progression, higher likelihood of disease control, and progression associated with the appearance of new lesions were chosen to continue treatment. Differences seen in duration of treatment and tumor reduction highlight the importance of an individualized approach to decision making when considering treatment with nivolumab beyond first progression. It is likely that patients selected to be treated beyond first progression based on clinical benefit and treatment tolerability would live longer than those who died shortly after starting study treatment and, hence, would not be treated beyond progression. To overcome this limitation, we performed a landmark analysis of patients beginning 6 weeks from first progression and identified differences in OS in patients treated beyond first progression and those not treated beyond first progression.
The following limitations of this analysis should be taken into consideration. The current exploratory analysis comprised a relatively small number of patients treated beyond RECIST-defined first progression. In addition, tumor assessments were conducted every 6 weeks; it is possible that if tumor assessments were conducted less often (eg, 8–12 weeks, as in some other studies of immunotherapies),8,12 the treating physician may not have observed the phenomenon of tumor flare (evidenced as an initial RECIST-defined progression) and would thus have had no reason to decide whether to continue ordiscontinue treatment. Additionally, although no new or unexpected AEs were seen in patients treated beyond first progression, it should be restated that this group was preselected per protocol for those who were tolerating therapy.
Conclusions
This analysis demonstrated that sustained reductions in tumor burden or stabilization in the size of target lesions may be possible with continued nivolumab treatment following initial disease progression in mRCC. Furthermore, the present findings suggest that some patients with RECIST-defined progression can safely continue nivolumab treatment if deemed feasible by the treating physician. On the basis of these findings, larger-scale analyses and/or clinical studies of nivolumab treatment beyond RECIST-defined first progression in patients with mRCC are warranted. Additional data may aid in the development of guidelines to help ensure optimal use of the new class of immunotherapy in RCC.
Supplementary Material
Key Points.
Question
Can patients with previously treated metastatic renal cell carcinoma benefit from continued nivolumab treatment beyond Response Evaluation Criteria in Solid Tumors (RECIST)-defined first progression?
Findings
In this analysis of 36 patients who continued nivolumab treatment beyond RECIST-defined first progression in a randomized clinical trial, 25 demonstrated reductions in tumor burden or stabilization in the size of target lesions after first progression.
Meaning
The potential for reduction in tumor burden after RECIST-defined first progression suggests that some patients may derive benefit from nivolumab therapy beyond when they would have traditionally discontinued treatment.
Acknowledgments
Funding/Support: This study was funded by Bristol-Myers Squibb (Lawrenceville, New Jersey).
Role of the Funder/Sponsor: Bristol-Myers Squibb provided the study drug and worked with investigators to design and conduct the study; and collect, manage, analyze, and interpret the data. Bristol-Myers Squibb, in collaboration with the authors, contributed to the preparation, review, and approval of the manuscript and the decision to submit the manuscript for publication.
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01354431
Author Contributions: Dr George had full access to all ofthe data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: George, Motzer, Hammers, Kuzel, Jiang, Waxman, Rini.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: George, Motzer, Hammers, Tykodi, Plimack, Jiang, Waxman, Rini.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Jiang, Waxman.
Administrative, technical, or material support: Motzer, Hammers, Tykodi, Waxman.
Study supervision: George, Motzer, Kuzel, Waxman, Rini.
Conflict of Interest Disclosures: Dr George has received grants from Acceleron, Agensys, Bayer, Bristol-Myers Squibb, Merck, Novartis, and Pfizer; and personal fees from Astellas, Bayer, Bristol-Myers Squibb, Novartis, Onclive, Sanofi, and Xcenda. Dr Motzer has received grants from Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, Novartis, and Pfizer; and personal fees from Bristol-Myers Squibb, Eisai, Novartis, and Pfizer. Dr Hammers has received grants from Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, and Exelixis; and personal fees from Bristol-Myers Squibb and Ono Pharmaceutical Company. Dr Redman has received grants and personal fees from Bristol-Myers Squibb. Dr Kuzel has received grants from Bayer, Bristol-Myers Squibb, CureTech, Eisai, Genentech, MedImmune, Merck, and Millennium Takeda; and personal fees from Amgen, Argos Therapeutics, Astellas, Bayer, Bionomics, Celgene, Eisai, Genentech, and Janssen Pharmaceuticals. Dr Tykodi has received grants from Argos Therapeutics, Bristol-Myers Squibb, Exelixis, Immatics Biotechnologies, Novartis, and Prometheus; and personal fees from Amgen and Prometheus. Dr Plimack has received grants from Acceleron Pharma, AstraZeneca, Bristol-Myers Squibb, Dendreon, Eli Lilly, GlaxoSmithKline, Merck, and Pfizer; and personal fees from Acceleron Pharma, Bristol-Myers Squibb, Genentech, Novartis, Pfizer, and Roche. Drs Jiang and Waxman are employees/stockholders of Bristol-Myers Squibb. Dr Rini has received grants from Bristol-Myers Squibb, GlaxoSmithKline, Immatics Biotechnologies, Millennium Pharmaceuticals, Pfizer, and Roche/Genentech; and personal fees from Bristol-Myers Squibb. No other disclosures are reported.
Additional Contributions: We thank the patients and their families; research colleagues and clinical teams; and Bristol-Myers Squibb (Lawrenceville, New Jersey)/Ono Pharmaceutical Company Limited (Osaka City, Japan) for supporting this work. Writing and editorial support was provided by Payal Gandhi, PhD, of PPSI (a PAREXEL company), and was funded by Bristol-Myers Squibb. Dr Gandhi was not compensated beyond her salary for this work.
References
- 1.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: kidney cancer. doi: 10.6004/jnccn.2009.0043. Version 1.2016. NCCN Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed September 16, 2016. [DOI] [PubMed]
- 2.Escudier B, Porta C, Schmidinger M, et al. ESMO Guidelines Working Group Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii49–iii56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 3.Raman R, Vaena D. Immunotherapy in metastatic renal cell carcinoma: a comprehensive review. Biomed Res Int. 2015;2015:367354. doi: 10.1155/2015/367354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudes G, Carducci M, Tomczak P, et al. Global ARCC Trial Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 5.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther. 2013;13(6):847–861. doi: 10.1517/14712598.2013.770836. [DOI] [PubMed] [Google Scholar]
- 7.Nurieva RI, Liu X, Dong C. Molecular mechanisms of T-cell tolerance. Immunol Rev. 2011;241(1):133–144. doi: 10.1111/j.1600-065X.2011.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, McDermott DF, et al. CheckMate 025 Investigators Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 10.Hales RK, Banchereau J, Ribas A, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010;21(10):1944–1951. doi: 10.1093/annonc/mdq048. [DOI] [PubMed] [Google Scholar]
- 11.Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopoten tiator for metastatic melanoma: how different are these from conventional treatment responses? Am J Clin Oncol. 2012;35(6):606–611. doi: 10.1097/COC.0b013e318209cda9. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, Ribas A, Daud A, et al. Patterns of response in patients with advanced melanoma treated with pembrolizumab (MK-3475) and evaluation of immune-related response criteria (irRC) J Immunother Cancer. 2014;2(suppl 3) poster P103. [Google Scholar]
- 13.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 16.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 17.Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies—outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC) Vaccine. 2007;25(suppl 2):B97–B109. doi: 10.1016/j.vaccine.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–24. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33(31):3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430–143. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) 2009 version 4.0. NCI Website. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_85x11.pdf Accessed September 16, 2015.
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 23.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29–41. [Google Scholar]
- 24.Clopper CJ, Pearson ES. The use of confidence of fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 25.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 27.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17(8):2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 28.Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17(6):1654–1663. doi: 10.1200/JCO.1999.17.6.1654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


