Abstract
High-dietary fat intake is a major risk factor for development of metabolic and cardiovascular-renal dysfunction including obesity, coronary artery disease, hypertension, and chronic renal failure. We examined the effect of a high-fat diet on renal function and morphology in spontaneously hypertensive rats (SHR), a phenotype designed to mimic metabolic syndrome. High-fat diet induced increase (P < 0.05) in blood pressure, body weight, and renal lipid deposition in these rats. This increase in body weight was accompanied by elevations (P < 0.05) of blood glucose and low-density lipoprotein (LDL) levels, a decrease (P < 0.05) in adiponectin and increases (P < 0.05) in plasma monocyte chemotactic protein-1 (MCP-1) along with renal macrophage infiltration. These pathophysiological perturbations were attenuated (P < 0.05) by heme oxygenase-1 (HO-1) induction by treatment with cobalt protoporphyrin (CoPP). Further effects of CoPP included increased (P < 0.05) renal expression of adiponectin along with enhancement (P < 0.05) of pAKT, pAMPK, and p-eNOS in SHRs fed a high-fat diet. Prevention of such beneficial effects of CoPP by the concurrent administration of the heme-HO inhibitor stannous mesoporphyrin (SnMP) corroborates the role of HO system in mediating such effects. Taken together, our results demonstrate that high-fat diet induces a metabolic syndrome-like phenotype in hypertensive rats, which is amenable to rescue by increases in HO-1- and adiponectin-dependent pathways.
Introduction
Obesity, which affects one in three Americans, is often associated with hypertension (1). Obesity and hypertension are comorbid pathological conditions that have been identified as independent risk factors for the development of endothelial dysfunction and renal disease (1–3). Blood pressure is strongly correlated with BMI, and in the Framingham Offspring Study, ≤78% of male hypertensive cases were attributable to obesity (4). Furthermore, obesity increases the risk for chronic kidney disease (CKD) by almost fourfold independent of other risk factors (2) (reviewed in ref. 5). Obese patients with hypertension are at the greatest risk for developing CKD (6). Also, chronic metabolic abnormalities, such as obesity, are frequently associated with imbalances in cellular redox coupled with oxidative stress (5), which contributes toward long-term morbidity and mortality associated with these conditions (http://hyper.ahajournals.org/content/51/2/352.full).
The heme-HO system, comprising of HO-1 (inducible) and HO-2 (constitutive) isoforms, is one of the key defense mechanisms against oxidative stress (7). This effect of HO system is attributable, in large part, to the antioxidant and antiapoptotic properties of the heme degradation products, bilirubin/biliverdin and carbon monoxide (7). Protective effects of this system are elaborated by studies showing that increasing the expression of HO-1 resulted in decreased blood pressure in hypertensive rats (8–10) and increased adiponectin in obese and nonobese diabetic rats and mice (11–14) along with suppression of inflammatory cytokines.
Adiponectin is an adipose tissue-specific protein that has been shown to have antiatherogenic, antihypertensive and insulin-sensitizing properties (15,16). Hypoadiponectinemia was shown to be a risk factor for hypertension after adjusting for age, BMI, and total cholesterol levels (17). An inverse relationship exists between plasma adiponectin levels and systolic blood pressure as well as renal dysfunction in obese subjects and animals (15,18). A recent study has also demonstrated renoprotective effects of adiponectin administration in mouse models of renal ischemia/reperfusion (19), involving mechanism related to HO-1 upregulation. In addition, induction of HO-1 has been shown to be associated with a parallel increase in serum adiponectin and activation of AMPK-AKT signaling (11,13,20,21), which contributes to improved NO bioavailability, vascular function, glucose transport and fatty acid oxidation (22,23). Thus, alterations in the heme-HO system not only influence vascular function but also modulate metabolic and cardiovascular-renal processes which, in turn, are dependent upon activation of adiponectin/AMPK pathways.
Where protective effects of HO enzyme system in animal models of obesity are clearly defined (11), a paucity of evidence exists regarding similar effects in comorbid conditions such as hypertension and obesity. In contextual light, we proposed the present study to explore cardiovascular-renal effects of high-fat diet on spontaneously hypertensive rats (SHRs), along with an examination of effects of enhancement of the HO-adiponectin axis in this setting. We tested our hypothesis by using a well-described high-fat diet regimen (24), that does not cause atherosclerotic lesion formation in mice (25), to address the effects of a known HO-1 inducer, cobalt protoporphyrin (CoPP). To verify that the effects of CoPP were due to an increase in HO activity, we, also, treated a group of SHR concurrently with stannous mesoporphyrin (SnMP) to inhibit HO activity. Our results show that obesity exacerbates renal dysfunction in SHR and that induction of HO-1 reduced renal macrophage infiltration and lipid accumulation in SHR rats, effects associated with decreased superoxide generation and monocyte chemotactic protein-1 (MCP-1). Moreover, induction of HO-1 increased adiponectin and renal expression of the pAMPK-peNOS pathway. Thus, HO-1 appears to play a critical role in the cellular defense against obesity-induced renal dysfunction in a hypertensive animal model fed a high-fat diet. These findings may have important clinical implications in the management of patients with the metabolic syndrome.
Methods and Procedures
Animal treatment
All animal studies were approved by the New York Medical College Animal Care and Use Committee in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals. Fifty-eight seven-week–old male SHRs were purchased from Charles River Laboratories and were divided into four groups: (i) SHR control, (ii) SHR-fat, (iii) SHR-fat, and CoPP treatment, (iv) SHR-fat and CoPP and SnMP treatment. SHR rats were fed ad libitum either with a normal diet (group A) containing 11% fat, 62% carbohydrate, and 27.0% protein total, 12.6 kJ/g or a high-fat diet (groups B, C, D) containing 58% fat from lard, 25.6% carbohydrate, and 16.4% protein yielding 23.4 kJ/g (Bio-SERV, Frenchtown, NJ) for 15 weeks (24,26). The diet used is distinct from the so-called “Western” or “atherosclerotic” diet which contains, in addition to high fat, cholesterol, and bile acids. While the high-fat diet used in the present study results in obesity, it does not cause atherosclerotic lesion formation in mice (25). During the first week of the high-fat diet, rats ate less than those fed the normal diet. Subsequently, there was no difference in food intake between the groups. After 4 weeks of high-fat diet, CoPP, an inducer of HO-1, was administered intraperitoneally once a week (3 mg/kg) for 11 weeks to SHR rats maintained on a high-fat diet. Some of the SHR treated with CoPP were concurrently treated with tin mesoporphyrin IX dichloride (SnMP), to inhibit HO activity, which was administered intraperitoneally three times a week (20 mg/kg) (9) to ascertain that any effects of CoPP treatment were related to increased HO activity. The untreated SHR rats maintained on the high-fat diet were administered the vehicle for CoPP and SnMP once a week and three times a week respectively (0.1 mmol/l sodium citrate buffer pH 7.8) for 11 weeks.
Rats were weighed every 7 days and systolic blood pressure was determined weekly by the tail-cuff method
During the 48-h period before sacrifice, rats were housed in metabolic cages for urine collection. After a 6-h fast, rats were anesthetized with sodium pentobarbital (65 mg/kg, intraperitoneal) and blood was obtained from a tail vein for glucose measurement using a glucometer (Lifescan, Miligitas, CA). Blood samples and renal cortex were then obtained and prepared as previously described (9,11).
Morphology and immunohistochemistry
Kidneys were harvested and a portion was fixed in formalin, embedded in paraffin, and processed for histology. Tissue sections were cut to 3–4-μm thickness and stained with hematoxylin and eosin to examine morphological changes. Cryosections were stained with Oil Red O and counterstained with hematoxylin and eosin to determine the presence of lipid droplets, which were quantitated by integrated optical density measurements. Sections from kidneys also were incubated with a CD68 antibody (Serotec, Raleigh, NC); that targets the macrophage population. Staining intensity was computed as the integrated optical density as previously described (14). Digitally fixed images at ×20 magnification were analyzed using an optical microscope (Olympus, Milan, Italy) equipped with an image analyzer (Image ProPlus). For quantitative analyses, integrated optical density was calculated for arbitrary areas where three fields with the same area were measured in each section.
Plasma adiponectin and MCP-1 measurements
The high-molecular weight form of adiponectin and MCP-1 in plasma were determined using an enzyme-linked immunosorbent assay assay (Pierce Biotechnology, Woburn, MA) as previously described (11).
Western blot analysis of renal tissue for HO, eNOS, p-eNOS, iNOS, AMPK, pAMPK, AKT, pAKT, and adiponectin
At the time of sacrifice, kidneys were harvested, and stored at −140 °C. Frozen kidneys were pulverized under liquid nitrogen and placed in a homogenization buffer prior to immunoblotting with antibodies against HO-1, and HO-2 (Stressgen Biotechnologies, Victoria, BC), AKT, AMPK, pAMPK, pAKT, and adiponectin (Cell Signaling Technology, Beverly, MA) and endothelial nitric oxide synthase (eNOS), p-eNOS, and inducible nitric oxide synthase (iNOS) (Santa Cruz Biotechnology, Santa Cruz, CA).
Immunoprecipitation of renal tissue for c-Src
Whole cell lysates were prepared with Nonidet P-40 buffer-containing 1% Nonidet P-40, sodium deoxycholate 0.25%, 50 mmol/l NaCl, 50 mmol/l HEPES, glycerol 10%, (pH 7.4), 1 mmol/l sodium vanadate, 0.5 mmol/l sodium fluoride, and complete protease inhibitor mixture (Sigma, St Louis, MO). After clarification, 500 μg of total protein was immunoprecipitated with antibody against c-Src (Santa Cruz Biotechnology) and protein G-agarose beads (Millipore, Billerica, MA), and then eluted with ×2 Laemmli buffer for immunoblotting. After immunoblotting for phospho-c-Src (polyclonal anti-Src (pY418) phosphospecific antibody; Invitrogen, Camarillo, CA), the same membrane was stripped and immunoblotted for c-Src (total c-Src). The activation of c-Src was expressed as ratio of phospho-c-Src to total c-Src.
Measurement of HO activity
HO activity was assayed as described previously (13) using a technique in which bilirubin, the end product of heme degradation, was extracted with chloroform, and its concentration was determined spectrophotometrically (dual UV-visible beam spectrophotometer Lambda 25; PerkinElmer Life and Analytical Sciences, Waltham, MA) using the difference in absorbance at a wavelength from 460 to 530 nm, with an extinction coefficient of 40 mmol/l−1 cm−1.
Measurements of LDL, O2− production, creatinine, and urinary protein levels
The 24-h urinary albumin excretion was measured by immunoassay (Bayer, Elkhart, IN). Serum and urinary creatinine were measured by enzyme-linked immunosorbent assay (Cayman, Ann Arbor, MI). Low-density lipoprotein (LDL) was measured in serum using a LDL Quantification Kit (Biovision, Mountainview, CA) according to the manufacturer's instructions. For the detection of O2−, homogenized kidneys were placed in plastic scintillation vials containing 5 μmol/l lucigenin in a final volume of 1 ml of air-equilibrated Krebs solution as described previously (11).
Statistical analysis
The data are presented as mean ± s.e.m. where n = 6/group for the results. For comparison between treatment groups, the Null hypothesis was tested by a single factor ANOVA for multiple groups or unpaired t-test for two groups. Statistical significance (P < 0.05) between the experimental groups was determined by the Fisher method of analysis for multiple comparisons.
Results
Effect of a high-fat diet on body weight and metabolic response
Figure 1a shows the % change in body weight over its baselinevalues in the four groups. In untreated SHR rats body weight increased 54% ± 5.5 on a normal diet over a period of 15 weeks, whereas in rats fed a high-fat diet body weight increased 79% ± 3.7 (P < 0.05). We, also, examined the effect of long-term CoPP treatment on body weight gain in response to a high-fat diet. Weekly treatment with CoPP was started 4 weeks after the initiation of the high-fat diet and was well tolerated by the SHR (n = 14/group); activity and grooming were maintained during CoPP treatment. Rats fed a high-fat diet and concurrently exposed to CoPP, showed reduction in body weight as compared to SHR rats on high-fat diet, 68% ± 2.4 (P < 0.05). A significant increase in body weight was seen when animals fed a high-fat diet were exposed to CoPP + SnMP. The weight gain was 75% ± 4.9 and was not significantly different from animals fed a high-fat diet.
Figure 1.
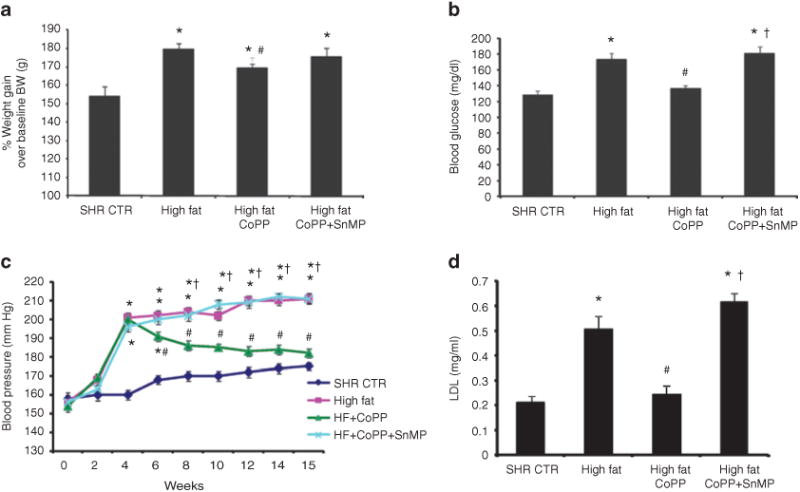
Effect of a high-fat diet and treatment with cobalt protoporphyrin (CoPP) and CoPP + stannous mesoporphyrin (SnMP) in spontaneously hypertensive rat (SHR) (n = 14 per group) on (a) % of body weight gain. *P < 0.01 vs. control, #P < 0.05 vs. high fat. (b) Blood glucose in samples obtained after a 6-h fast. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. SHR high-fat CoPP. (c) Blood pressure, which was measured by tail-cuff method. *P < 0.01 vs. control, #P < 0.01 vs. high fat, †P < 0.01 vs. high-fat CoPP. (d) Plasma low-density lipoprotein (LDL) levels, n = 6 for each group. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP.
The mean blood glucose level in the SHR rats maintained on a normal diet was 128 ± 4 mg/dl, and was increased to 173 ± 14 mg/dl by a high-fat diet (P < 0.05; n = 6/group) (Figure 1b). This increase in blood glucose levels was attenuated by CoPP treatment in SHR rats fed a high-fat diet (P < 0.05) and this effect was reversed by treatment with SnMP. Systolic blood pressure was increased over the 15-week period in SHR rats (Figure 1c; n = 6/group). The systolic blood pressure was 175 ± 11 mm Hg in the SHR control and was significantly increased in the rats fed a high-fat diet, 211 ± 9 mm Hg (P < 0.05). The elevation in systolic pressure was attenuated by CoPP treatment in SHR fed a high-fat diet whereas SnMP treatment nullified the antihypertensive effect of CoPP in SHR fed a high-fat diet (Figure 1c). Plasma LDL levels were significantly (P < 0.05) elevated in SHR fed a high-fat diet (Figure 1d, n = 6/group). CoPP treatment prevented the increase in LDL in SHR while concomitant treatment with SnMP blocked the effect of CoPP.
Effect of a high-fat diet on renal damage and oxidative stress
A high-fat diet was associated with histological evidence of the development of renal damage that was greatly reduced by CoPP treatment (Figure 2a). Oil Red O staining was increased in SHR rats fed a high-fat diet and this increase in lipid deposition was prevented by CoPP treatment (Figure 2b,c) an effect reversed by concomitant treatment with SnMP.
Figure 2.
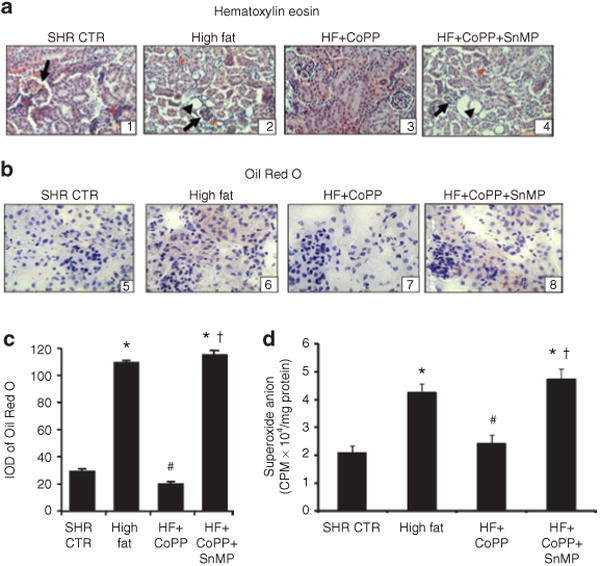
Effects of CoPP on histopathological abnormalities and renal redox status in rats on a high-fat diet. (a) Representative images of renal tissue of spontaneously hypertensive rat (SHR) stained with hematoxylin and eosin. SHR (A1) displayed disorganization of the renal cytoarchitecture, glomerular sclerotic lesions (arrow) and inflammatory cells (asterisk). In SHR fed a high-fat diet (A2), dilated distal tubules (head of arrow), focal glomerular sclerotic lesions (arrow) and the presence of inflammatory cells (asterisk) were seen. In SHR fed a high-fat diet and treated with cobalt protoporphyrin (CoPP) (A3) tissue damage was abrogated while the tissue damage was aggravated with stannous mesoporphyrin (SnMP)+CoPP treatment of SHR fed a high-fat diet (A4). (b) Cryosections from kidneys were used for Oil Red O staining, and the sections were then counterstained with hematoxylin and eosin. Lipid droplets appeared as red spots and were increased after a high-fat diet in SHR (B6). The increase was prevented by treatment with CoPP (B7) and increased lipid droplets were seen in group treated with SnMP (B8). (c) Integrated optical density (IOD) of Oil Red O staining. *P < 0.01 vs. control, #P < 0.01 vs. high fat, †P < 0.05 vs. high-fat CoPP. (d) Renal O2- production of SHR rats and those fed a high-fat diet and treated with CoPP or CoPP + SnMP. n = 6 for each group. *P < 0.01 vs. control, #P < 0.01 vs. high fat, †P < 0.01 vs. high-fat CoPP.
Renal oxidative stress was increased as cortical superoxide generation was greater in SHR fed a high-fat diet compared with rats fed a normal diet (Figure 2d where n = 6/group), (P < 0.05). CoPP treatment prevented the increase in renal O2− generation in SHR maintained on a high-fat diet (P < 0.01), an effect abolished by concurrent administration of SnMP.
An increase in macrophage infiltration was observed in SHR fed a high-fat diet for 15 weeks (P < 0.05) as measured by quantitative analysis of immunostaining to CD68 (Figure 3a,b). CoPP administration prevented the increase in macrophage infiltration in the kidneys of SHR and this effect was reversed by SnMP treatment (Figure 3a,b).
Figure 3.
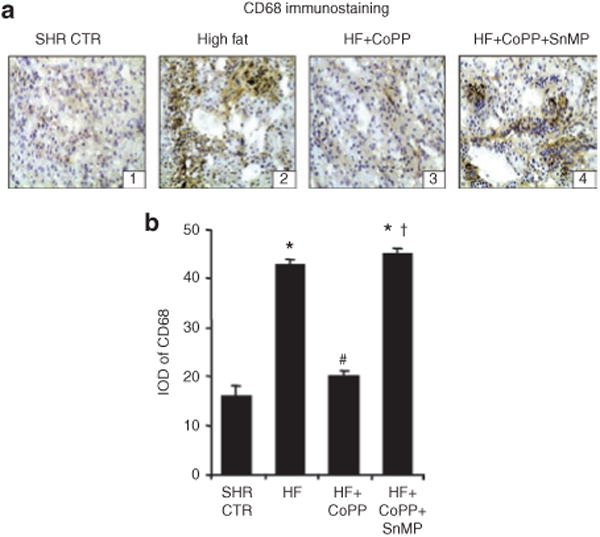
Renal macrophage infiltration in spontaneously hypertensive rat (SHR) fed a high-fat diet and treated with cobalt protoporphyrin (CoPP) and CoPP + stannous mesoporphyrin (SnMP). (a) Representative images of renal sections stained for CD68. (b) Integrated optical density (IOD) of CD68 staining. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP.
Effect of a high-fat diet on renal function
Urinary albumin excretion and plasma creatinine levels were increased in obese SHR compared to control animals (P < 0.05, Figure 4a,b). CoPP treatment prevented the increase in urinary albumin excretion and serum creatinine levels in SHR (P < 0.05). A high-fat diet decreased creatinine clearance in SHR and the impairment of renal function was prevented by CoPP. Concurrent treatment of SHR with CoPP and SnMP prevented the beneficial effects of CoPP on renal function as urinary protein excretion was increased and creatinine clearance decreased (Figure 4a,c). When creatinine clearance was expressed relative to body weight, the value for SHR was 0.36 ml/min/100 g. However, a high-fat diet for 15 weeks to SHR significantly reduced creatinine clearance 0.167 ml/min/100 g (P < 0.05).
Figure 4.
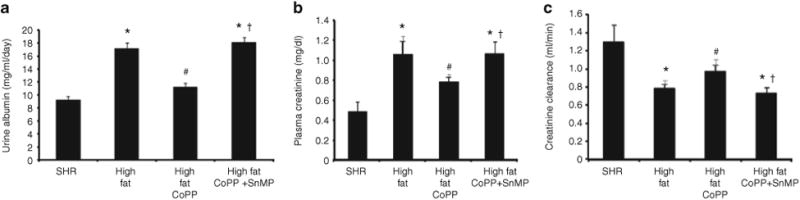
Effect of a high-fat diet and treatment of spontaneously hypertensive rat (SHR) with cobalt protoporphyrin (CoPP) and CoPP + stannous mesoporphyrin (SnMP) on (a) Urinary albumin excretion. n = 6 for each group. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP; (b) plasma creatinine, n = 6 for each group. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high fat CoPP, and (c) creatinine clearance. n = 6 for each group. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP.
Effect of a high-fat diet on renal cortical HO-1 and HO-2 expression
First, we confirmed that CoPP treatment for 11 weeks resulted in upregulation of HO-1. HO-1 protein in the kidneys of SHR fed a high-fat diet was significantly less than that of the respective control group (Figure 5a where n = 6/group) when the latter was fed a normal diet (P < 0.05). Treatment with CoPP resulted in a significant increase in HO-1 levels in SHR fed a high-fat diet. Although SnMP treatment showed a significant increase in HO-1 expression (Figure 5d), it is a potent inhibitor of HO activity as shown previously (8–10) and thus prevents heme degradation and inhibits formation of carbon monoxide and biliverdin. HO-2 levels were unaffected either by high-fat diet or by CoPP treatment (Figure 5a). Consistent with protein expression, HO activity was modestly decreased in obese SHR compared to the control group (Figure 5b). CoPP treatment significantly increased HO activity in SHR fed a high-fat diet, 1.59 ± 0.23 nmol bilirubin/mg/h compared to 0.48 + 0.09 nmol bilirubin/mg/h in untreated SHR fed a high-fat diet (P < 0.001).
Figure 5.
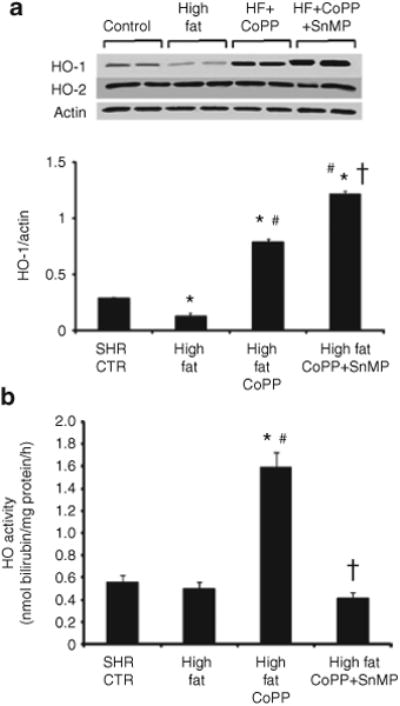
Effect of a high-fat diet on renal HO-1 expression and activity. (a) Western blot and densitometry analysis of heme oxygenase-1 (HO)-1 and HO-2 proteins in kidneys from spontaneously hypertensive rat (SHR). Rats were fed a high-fat diet and treated with cobalt protoporphyrin (CoPP) or CoPP + stannous mesoporphyrin (SnMP). Immunoblots were performed with antibodies against rat HO-1 and HO-2. Data are shown as mean band density normalized relative to β-actin. n = 6, *P < 0.01 vs. control, #P < 0.01 vs. high fat, †P < 0.01 vs. high-fat CoPP. (b) Renal HO activity of SHR and those fed a high-fat diet and treated with CoPP or CoPP + SnMP. n = 6 for each group. *P < 0.01 vs. control, #P < 0.01 vs. high fat, †P < 0.01 vs. high-fat CoPP.
Effect of a high-fat diet on plasma adiponectin and inflammatory cytokines levels
Plasma adiponectin levels were lower in rats fed a high-fat diet when compared to control animals fed a normal diet (P < 0.05; n = 6/group) (Figure 6a). This effect was reversed when rats were treated with CoPP (P < 0.05). Indeed, in SHR rats maintained on a high-fat diet and treated with CoPP, plasma adiponectin levels were higher than those in the respective control groups (P < 0.05). Concurrent administration of SnMP with CoPP in the SHR fed a high-fat diet prevented the increase in adiponectin, so that the levels of this protein were not different from those in the untreated SHR.
Figure 6.
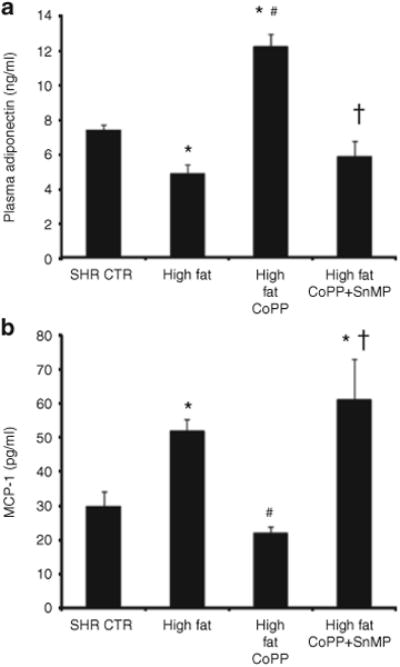
Plasma adiponectin and monocyte chemotactic protein-1 (MCP-1) levels in spontaneously hypertensive rat (SHR). Rats were fed a high-fat diet and treated with cobalt protoporphyrin (CoPP) or CoPP + SnMP. (a) Plasma adiponectin levels where n = 6 for each group. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high fat CoPP and (b) Plasma MCP-1 levels where n = 6 for each group. *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP.
Contrary to the pattern observed with adiponectin levels, plasma MCP-1 levels were greater in SHR fed a high-fat diet compared to rats fed a normal diet (Figure 6b; n = 6/group) (P < 0.05). Increasing HO-1 by CoPP administration significantly decreased plasma MCP-1 levels and this effect was prevented by concurrent SnMP treatment (P < 0.01, Figure 6b).
Effect of high-fat diet on renal adiponectin, pAMPK, and pAKT signaling
Renal adiponectin levels, normalized against β-actin, exhibited a similar pattern to plasma adiponectin levels. Thus, feeding SHR a high-fat diet for 15 weeks resulted in a decrease in adiponectin compared to untreated SHR (Figure 7a; n = 6/group). Induction of HO with CoPP increased renal adiponectin levels in hypertensive rats (P < 0.01) and the increase in SHR was prevented and reversed to a decrease when the rats were, also, treated with SnMP to inhibit HO (Figure 7a).A high-fat diet resulted in significant decreases in pAMPK and pAKT expression in kidneys from SHR (P < 0.05; n = 6/group) (Figure 7b,c). CoPP administration caused a significant increase in the expression of pAKT and pAMPK in the rats fed a high-fat diet (P < 0.05) compared to untreated rats fed a high-fat diet. The changes in expression of pAMPK and pAKT paralleled those seen with HO-1 protein expression. In SHR maintained on a high-fat diet and treated with CoPP, the concurrent administration of SnMP prevented the increase in pAKT and pAMPK; indeed, the expression of both pAKT and pAMPK was reduced to levels lower than those seen in SHR on the high-fat diet alone (P < 0.01).
Figure 7.
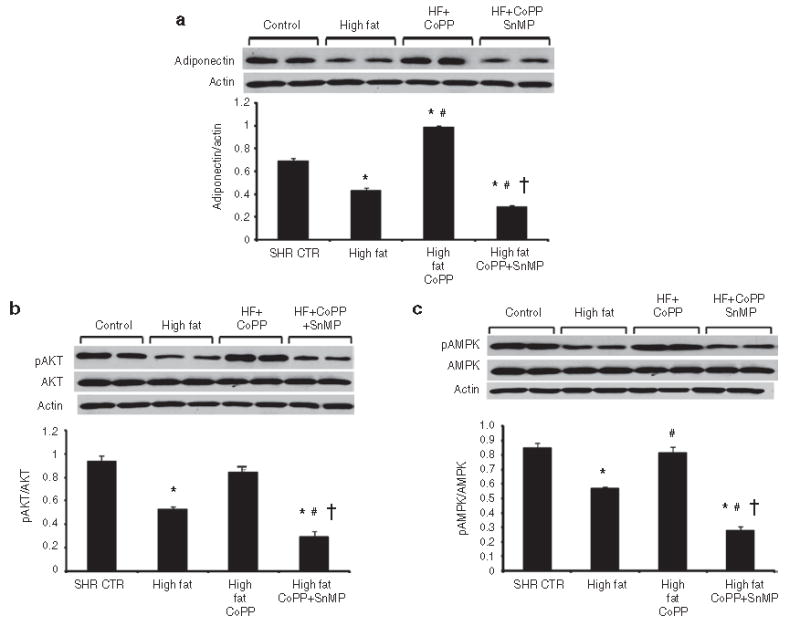
Western blot and densitometry analysis of (a) adiponectin, (b) AKT and pAKT, and (c) AMPK and pAMPK in kidneys obtained from spontaneously hypertensive rat (SHR). Rats were fed a high-fat diet and treated with cobalt protoporphyrin (CoPP) and CoPP + stannous mesoporphyrin (SnMP). Data are shown as the adiponectin/actin ratio, pAKT/AKT ratio, and pAMPK/AMPK ratio, respectively. n = 6, *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP.
Effect of a high-fat diet on renal eNOS and p-eNOS levels
SHR fed a high-fat diet had lower levels of eNOS and p-eNOS protein compared with animals fed a normal diet (P < 0.05) (Figure 8a). CoPP treatment resulted in increased expression of both eNOS and p-eNOS protein in SHR fed a high-fat diet (P < 0.05) compared with untreated rats maintained on a high-fat diet. SnMP treatment of CoPP-treated SHR fed a high-fat diet markedly suppressed eNOS and p-eNOS levels (P < 0.01) (Figure 8a). In contrast to eNOS, iNOS levels significantly increased in SHR fed a high-fat diet (Figure 8b). CoPP administration prevented the increase in iNOS expression (P < 0.5).
Figure 8.
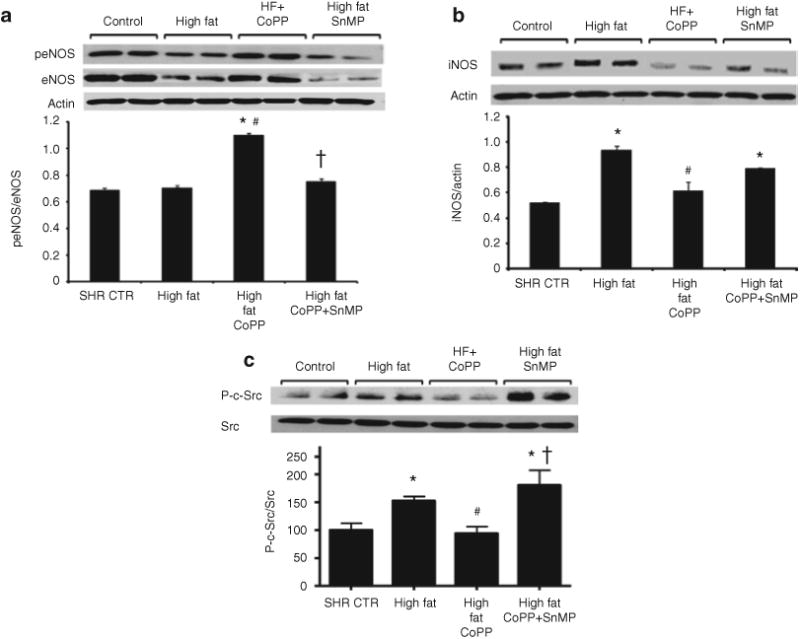
Western blot and densitometry analysis of eNOS, p-eNOS, iNOS, and c-Src in the renal cortex from spontaneously hypertensive rat (SHR). Rats were fed a high-fat diet and treated with cobalt protoporphyrin (CoPP) and CoPP + stannous mesoporphyrin (SnMP). Data are shown as mean band density normalized to β-actin or p-eNOS/eNOS ratio. (a) eNOS and p-eNOS. n = 6, *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP and (b) iNOS. n = 6, *P < 0.05 vs. control, #P < 0.05 vs. high fat. (c) c-Src. Phosphorylated c-Src was determined by immunoprecipitation followed by immunoblotting. Data are shown as ratio of phospho-c-Src to total c-Src. Values are mean ± SE and are expressed relative to a control value of 100. n = 6, *P < 0.05 vs. control, #P < 0.05 vs. high fat, †P < 0.05 vs. high-fat CoPP.
Effect of high-fat diet on renal c-Src levels
Since c-Src is known to activate oxidative stress pathways and iNOS, we measured the expression of phosphorylated c-Src. A high-fat diet resulted in a significant increase in c-Src phosphorylation in kidneys isolated from SHR (P < 0.05; n = 6/group) (Figure 8c). While CoPP administration abolished high fat-stimulated c-Src phosphorylation (P < 0.05 vs. high fat), SnMP administration appeared to enhance high fat-stimulated c-Src phosphorylation (P < 0.05 vs. control and high fat).
Discussion
The results of the present study demonstrate that SHR fed a high-fat diet develop pathophysiological abnormalities similar to that observed in metabolic syndrome. This phenotype is characterized by increased levels of blood lipids, blood glucose and blood pressure along with an accelerated decline in renal function when compared to SHR maintained on a normal diet. We, also, demonstrated that renal HO-1 induction, accompanied by increased plasma and tissue adiponectin levels, attenuated the increases in blood pressure, systemic and local infammation and improve renal function in this model. To the best of our knowledge, this is the first report showing a protective effect of HO-adiponectin axis in a comorbid condition where a pre-existing cardiovascular-renal pathology is further aggravated by addition of a high-fat diet.
High-fat intake increased body weight, serum LDL and blood glucose levels in SHR and these changes in metabolic indices were associated with renal lipid accumulation and macrophage infiltration in these animals. Previous studies have shown that HO-1 induction decreases obesity, reduces levels of visceral and subcutaneous fat and normalizes the metabolic profile in obese rats and mice (11,13). In contrast, in the current study we induced a metabolic syndrome-like phenotype in hypertensive animals. SHR demonstrate chronic hypertension, oxidative stress and renal damage. All of these parameters were worsened by the addition of high-fat diet, strengthening our hypothesis that obesity and the associated metabolic abnormalities accelerate pathological pre-existing cardiovascular changes. Reversal of these pathophysiological abnormalities by HO-1 induction corroborates the protective effects of the HO system in such a setting.
One of the hallmark pathological abnormalities consistently observed in SHRs is renal damage with resultant proteinuria (27). Our findings, presented here, demonstrate that addition of a high-fat diet in these animals not only alters their metabolic homeostasis but also exacerbates renal damage, as evidenced by increased macrophage infiltration and deterioration in renal function parameters. These observations are not surprising as obesity-induced renal dysfunction is well established in the literature (5). In addition to increased inflammation, renal tissues from rats on high-fat diet also exhibited enhanced lipid deposition; both of which were significantly attenuated, along with improvement of renal function, in rats concurrently exposed to CoPP. These protective of HO-1 induction on renal structure and function may involve, in whole or in part, anti-oxidant-antiapoptotic properties of heme degradation products i.e., carbon monoxide and BV. Antihypertensive effects of HO-1 induction, shown here, may also contribute toward abatement of renal dysfunction in SHRs on high-fat diet. This antihypertensive effect of HO-1 induction is probably multifactorial emerging form interplay of one the various mechanisms including, carbon monoxide generation, HO-1-induced increase in eNOS expression and increased NO bioavailability due to an increase in cellular antioxidants.
Apart from effects on heme degradation products, HO-1 upregulation was associated with increased tissue and plasma levels of adiponectin. This causality between HO activity and adiponectin release was strengthened by the inhibitory effects of SnMP on both HO activity and adiponectin levels. It has been recently shown that the beneficial effects of heme-HO system in established cardiovascular-metabolic disorders is mediated, at least in part, via its effect on adiponectin-dependent pathways (11,28,29). Results presented in the current study support and advance our hypothesis that, in addition to its antioxidant properties, the HO system enhances the adiponectin axis which, in turn, modulates multiple physiological processes and may contribute toward HO-mediated attenuation of renal dysfunction (30).
The HO-1-mediated increase in adiponectin was associated with an increase in pAMPK-pAKT signaling and pAMPK and pAKT levels appear to correlate with HO-1 and adiponectin levels (12,14,21,31). Activation of AMPK is important in cellular energy homeostasis through the stimulation of glucose transport, the switching off of energy consumption by decreasing lipogenesis and by increasing fatty acid oxidation and ATP levels(32,33). An increase in AMPK-AKT signaling is considered an important metabolic response that is necessary for the attenuation of ROS-mediated endothelial dysfunction (34) and both pAMPK and pAKT use eNOS as a substrate and enhance the levels of p-eNOS (7,35,36). The results of this study support this link as induction of HO-1, also, increased p-eNOS expression in the kidney of SHR. In agreement with these results, Schulz et al. showed that dysfunction of the vascular endothelium can be prevented by pAMPK (34). The seminal role of increased HO-1 expression and HO activity in renal protection is further strengthened by the results obtained when SnMP was concurrently administered with CoPP; the inhibition of HO activity prevented the beneficial effects of HO-1 induction in obese SHR with regard to blood pressure, adiponectin, pAKT, and pAMPK. The effects of inhibition of HO activity by SnMP is in agreement with the report that human HO-1 defciency causes renal and vascular dysfunction (37)
Apart from attenuating the increases in the circulating levels of inflammatory cytokines (MCP-1), HO-1 induction via CoPP was found to inhibit transactivation of Src-kinase. Src belongs to a family of nonreceptor tyrosine kinases which regulate cellular events ranging from cellular proliferation to cell survival, motility, and invasiveness (38). Dysfunctional regulation of Src expression and activity has been linked to chronic inflammatory conditions such as diabetes (39). Phosphorylation of tyr418 on the SH2 domain leads to Src activation with resultant downstream activation of signaling cascades including EGFR, Bcl-xl and iNOS (40,41). A high-fat diet enhances Src activity in SHR and is accompanied by exacerbation of chronic inflammatory pathways as exemplified by increased levels of circulating cytokines and of renal iNOS expression. HO-1 induction attenuates Src activation and restores metabolic homeostasis associated with reductions in inflammatory cytokines and renal iNOS expression. These observations corroborate the anti-inflammatory and antioxidant properties of the heme-HO pathway, which may involve the restoration of Src activity and the attenuation of Src-dependent signaling pathways.
In conclusion, high-fat intake increased body weight gain and blood pressure and decreased adiponectin, pAMPK, and pAKT in SHR. The pharmacological enhancement of HO-1 expression, resulting in a phenotype resistant to injurious stimuli, permits the kidney to initiate a crucial and immediate defense against the events associated with the metabolic syndrome, thereby preventing the continued deterioration in renal function associated with this disease.
To demonstrate the contribution of normotensive rats in our study, we have included results and figures of WKY rats in Supplementary Materials and Methods and Figures S1–S4 online.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (DK068134, HL55601 and HL34300 to N.G.a.) and Beatrice Renfield Foundation (A.K.). all authors had full access to the data and take responsibility for its integrity. all authors have read and agree with the manuscript as written. We thank Jennifer Brown for her outstanding editorial assistance in the preparation of the manuscript.
Footnotes
Supplementary Material: Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
Disclosure: The authors declared no conflict of interest.
References
- 1.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 2.Kramer H. Obesity and chronic kidney disease. Contrib Nephrol. 2006;151:1–18. doi: 10.1159/000095315. [DOI] [PubMed] [Google Scholar]
- 3.Knight SF, Quigley JE, Yuan J, et al. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51:352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 5.Kalaitzidis RG, Siamopoulos KC. The role of obesity in kidney disease: recent findings and potential mechanisms. Int Urol Nephrol. 2011;43:771–784. doi: 10.1007/s11255-011-9974-1. [DOI] [PubMed] [Google Scholar]
- 6.Kramer H, Luke A, Bidani A, et al. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46:587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 8.Sacerdoti D, Escalante B, Abraham NG, et al. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- 9.Botros FT, Schwartzman ML, Stier CT, Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005;68:2745–2755. doi: 10.1111/j.1523-1755.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 10.Sabaawy HE, Zhang F, Nguyen X, et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Kim DH, Tsenovoy PL, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Peterson S, Husney D, et al. Interdiction of the diabetic state in NOD mice by sustained induction of heme oxygenase: possible role of carbon monoxide and bilirubin. Antioxid Redox Signal. 2007;9:855–863. doi: 10.1089/ars.2007.1568. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Burgess AP, Li M, et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 14.Nicolai A, Li M, Kim DH, et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasa Y, Otsubo S, Ishizuka T, Uchida K, Nitta K. Influence of serum high-molecular-weight and total adiponectin on arteriosclerosis in IgA nephropathy patients. Nephron Clin Pract. 2008;108:c226–c232. doi: 10.1159/000119717. [DOI] [PubMed] [Google Scholar]
- 16.Huang KC, Chen CL, Chuang LM, et al. Plasma adiponectin levels and blood pressures in nondiabetic adolescent females. J Clin Endocrinol Metab. 2003;88:4130–4134. doi: 10.1210/jc.2003-030158. [DOI] [PubMed] [Google Scholar]
- 17.Iwashima Y, Katsuya T, Ishikawa K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 18.Abraham NG, Kruger A, Peterson S. High serum levels of adiponectin in HO-1 preconditioning in mice and rats with type 2 diabetes improve vascular function. American Heart Association Abstract 642, Scientific Sessions 2007 Circulation. 2007;114:11–106. [Google Scholar]
- 19.Cheng CF, Lian WS, Chen SH, et al. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARa-heme oxygenase-1 signaling pathway. J Cell Physiol. 2012;227:239–249. doi: 10.1002/jcp.22726. [DOI] [PubMed] [Google Scholar]
- 20.Peterson SJ, Drummond G, Kim DH, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson SJ, Kim DH, Li M, et al. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998;136:17–24. doi: 10.1016/s0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 25.Molnar J, Yu S, Mzhavia N, et al. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 26.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 27.Feld LG, Van Liew JB, Brentjens JR, Boylan JW. Renal lesions and proteinuria in the spontaneously hypertensive rat made normotensive by treatment. Kidney Int. 1981;20:606–614. doi: 10.1038/ki.1981.183. [DOI] [PubMed] [Google Scholar]
- 28.Mathew AV, Okada S, Sharma K. Obesity related kidney disease. Curr Diabetes Rev. 2011;7:41–49. doi: 10.2174/157339911794273928. [DOI] [PubMed] [Google Scholar]
- 29.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–412. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran J, Xiong X, Liu W, et al. Increased plasma adiponectin closely associates with vascular endothelial dysfunction in type 2 diabetic patients with diabetic nephropathy. Diabetes Res Clin Pract. 2010;88:177–183. doi: 10.1016/j.diabres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Sambuceti G, Morbelli S, Vanella L, et al. Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage, reversed by increases in pAMPK, heme oxygenase-1, and adiponectin. Stem Cells. 2009;27:399–407. doi: 10.1634/stemcells.2008-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomas E, Tsao TS, Saha AK, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol (Lond) 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz E, Dopheide J, Schuhmacher S, et al. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 36.Chen ZP, Mitchelhill KI, Michell BJ, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 37.Radhakrishnan N, Yadav SP, Sachdeva A, et al. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol. 2011;33:74–78. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 38.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Page TH, Smolinska M, Gillespie J, Urbaniak AM, Foxwell BM. Tyrosine kinases and inflammatory signalling. Curr Mol Med. 2009;9:69–85. doi: 10.2174/156652409787314507. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Kalyankrishna S, Wislez M, et al. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyryshkin A, Gorgun FM, Abdel Fattah E, et al. Src kinase-mediated phosphorylation stabilizes inducible nitric-oxide synthase in normal cells and cancer cells. J Biol Chem. 2010;285:784–792. doi: 10.1074/jbc.M109.055038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


