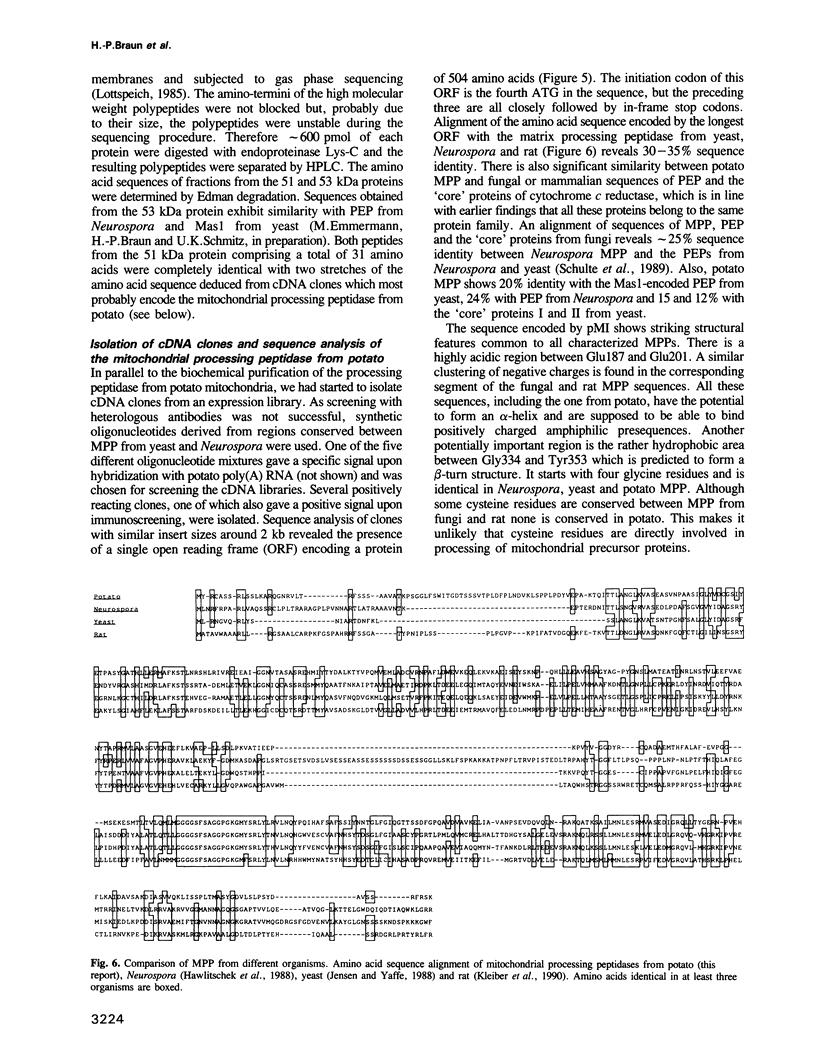
Abstract
The major mitochondrial processing activity removing presequences from nuclear encoded precursor proteins is present in the soluble fraction of fungal and mammalian mitochondria. We found that in potato, this activity resides in the inner mitochondrial membrane. Surprisingly, the proteolytic activity co-purifies with cytochrome c reductase, a protein complex of the respiratory chain. The purified complex is bifunctional, as it has the ability to transfer electrons from ubiquinol to cytochrome c and to cleave off the presequences of mitochondrial precursor proteins. In contrast to the nine subunit fungal complex, cytochrome c reductase from potato comprises 10 polypeptides. Protein sequencing of peptides from individual subunits and analysis of corresponding cDNA clones reveals that subunit III of cytochrome c reductase (51 kDa) represents the general mitochondrial processing peptidase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arretz M., Schneider H., Wienhues U., Neupert W. Processing of mitochondrial precursor proteins. Biomed Biochim Acta. 1991;50(4-6):403–412. [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Behrens M., Michaelis G., Pratje E. Mitochondrial inner membrane protease 1 of Saccharomyces cerevisiae shows sequence similarity to the Escherichia coli leader peptidase. Mol Gen Genet. 1991 Aug;228(1-2):167–176. doi: 10.1007/BF00282462. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Huang L. S., DeRose V. J. Ubiquinol-cytochrome c oxidoreductase of higher plants. Isolation and characterization of the bc1 complex from potato tuber mitochondria. J Biol Chem. 1991 May 15;266(14):9064–9077. [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., De Loose M., Van Montagu M., Inzé D. The induction of manganese superoxide dismutase in response to stress in Nicotiana plumbaginifolia. EMBO J. 1989 Jan;8(1):31–38. doi: 10.1002/j.1460-2075.1989.tb03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun H. P., Emmermann M., Kruft V., Schmitz U. K. Cytochrome c1 from potato: a protein with a presequence for targeting to the mitochondrial intermembrane space. Mol Gen Genet. 1992 Jan;231(2):217–225. doi: 10.1007/BF00279794. [DOI] [PubMed] [Google Scholar]
- Chaumont F., O'Riordan V., Boutry M. Protein transport into mitochondria is conserved between plant and yeast species. J Biol Chem. 1990 Oct 5;265(28):16856–16862. [PubMed] [Google Scholar]
- Emmermann M., Braun H. P., Schmitz U. K. The ADP/ATP translocator from potato has a long amino-terminal extension. Curr Genet. 1991 Nov;20(5):405–410. doi: 10.1007/BF00317069. [DOI] [PubMed] [Google Scholar]
- Esposti M. D., Flamini E., Zannoni D. Functional Characterization and Partial Purification of the Ubiquinol-Cytochrome c Oxidoreductase from Higher Plant Mitochondria (Helianthus tuberosus). Plant Physiol. 1985 Mar;77(3):758–764. doi: 10.1104/pp.77.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellini N. Organization and structure of the genes for the cytochrome b/c1 complex in purple photosynthetic bacteria. A phylogenetic study describing the homology of the b/c1 subunits between prokaryotes, mitochondria, and chloroplasts. J Bioenerg Biomembr. 1988 Feb;20(1):59–83. doi: 10.1007/BF00762138. [DOI] [PubMed] [Google Scholar]
- Graack H. R., Grohmann L., Kitakawa M. The nuclear coded mitoribosomal proteins YmL27 and YmL31 are both essential for mitochondrial function in yeast. Biochimie. 1991 Jun;73(6):837–844. doi: 10.1016/0300-9084(91)90063-7. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Huang J., Hack E., Thornburg R. W., Myers A. M. A yeast mitochondrial leader peptide functions in vivo as a dual targeting signal for both chloroplasts and mitochondria. Plant Cell. 1990 Dec;2(12):1249–1260. doi: 10.1105/tpc.2.12.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaya G., Kalousek F., Fenton W. A., Rosenberg L. E. Cleavage of precursors by the mitochondrial processing peptidase requires a compatible mature protein or an intermediate octapeptide. J Cell Biol. 1991 Apr;113(1):65–76. doi: 10.1083/jcb.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. E., Yaffe M. P. Import of proteins into yeast mitochondria: the nuclear MAS2 gene encodes a component of the processing protease that is homologous to the MAS1-encoded subunit. EMBO J. 1988 Dec 1;7(12):3863–3871. doi: 10.1002/j.1460-2075.1988.tb03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Hendrick J. P., Rosenberg L. E. Two mitochondrial matrix proteases act sequentially in the processing of mammalian matrix enzymes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7536–7540. doi: 10.1073/pnas.85.20.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber J., Kalousek F., Swaroop M., Rosenberg L. E. The general mitochondrial matrix processing protease from rat liver: structural characterization of the catalytic subunit. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7978–7982. doi: 10.1073/pnas.87.20.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Ito A., Okazaki H., Omura T. Purification and characterization of a processing protease from rat liver mitochondria. EMBO J. 1989 Sep;8(9):2605–2612. doi: 10.1002/j.1460-2075.1989.tb08400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Söllner T., Neupert W. Mitochondrial import receptors for precursor proteins. Trends Biochem Sci. 1991 Feb;16(2):63–67. doi: 10.1016/0968-0004(91)90026-r. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Hartl F. U., Cheng M. Y., Ostermann J., Horwich A., Neupert W. The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. EMBO J. 1988 Nov;7(11):3493–3500. doi: 10.1002/j.1460-2075.1988.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Guiard B. One nuclear gene controls the removal of transient pre-sequences from two yeast proteins: one encoded by the nuclear the other by the mitochondrial genome. EMBO J. 1986 Jun;5(6):1313–1317. doi: 10.1002/j.1460-2075.1986.tb04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Homologues of insulinase, a new superfamily of metalloendopeptidases. Biochem J. 1991 Apr 15;275(Pt 2):389–391. doi: 10.1042/bj2750389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Theiler F., Horvath S. J., Tomich J. M., Richards J. H., Allison D. S., Schatz G. Amphiphilicity is essential for mitochondrial presequence function. EMBO J. 1988 Mar;7(3):649–653. doi: 10.1002/j.1460-2075.1988.tb02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlen D. A., Hoffmann J., van der Pas J. C., Nehls U., Preis D., Sackmann U., Weiss H. Relationship between a subunit of NADH dehydrogenase (complex I) and a protein family including subunits of cytochrome reductase and processing protease of mitochondria. FEBS Lett. 1991 Jan 14;278(1):75–78. doi: 10.1016/0014-5793(91)80087-j. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz U. K., Lonsdale D. M. A yeast mitochondrial presequence functions as a signal for targeting to plant mitochondria in vivo. Plant Cell. 1989 Aug;1(8):783–791. doi: 10.1105/tpc.1.8.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Behrens M., Scherer P., Pratje E., Michaelis G., Schatz G. Inner membrane protease I, an enzyme mediating intramitochondrial protein sorting in yeast. EMBO J. 1991 Feb;10(2):247–254. doi: 10.1002/j.1460-2075.1991.tb07944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Arretz M., Wachter E., Neupert W. Matrix processing peptidase of mitochondria. Structure-function relationships. J Biol Chem. 1990 Jun 15;265(17):9881–9887. [PubMed] [Google Scholar]
- Schulte U., Arretz M., Schneider H., Tropschug M., Wachter E., Neupert W., Weiss H. A family of mitochondrial proteins involved in bioenergetICS and biogenesis. Nature. 1989 May 11;339(6220):147–149. doi: 10.1038/339147a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., Link T. A., Engel W. D., von Jagow G. Isolation of the eleven protein subunits of the bc1 complex from beef heart. Methods Enzymol. 1986;126:224–237. doi: 10.1016/s0076-6879(86)26024-3. [DOI] [PubMed] [Google Scholar]
- Siedow J. N., Power S., de la Rosa F. F., Palmer G. The preparation and characterization of highly purified, enzymically active complex III from baker's yeast. J Biol Chem. 1978 Apr 10;253(7):2392–2399. [PubMed] [Google Scholar]
- Trumpower B. L. Cytochrome bc1 complexes of microorganisms. Microbiol Rev. 1990 Jun;54(2):101–129. doi: 10.1128/mr.54.2.101-129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Miyada C. G. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 1987;152:432–442. doi: 10.1016/0076-6879(87)52050-x. [DOI] [PubMed] [Google Scholar]
- Weiss H., Juchs B. Isolation of a multiprotein complex containing cytochrome b and c1 from Neurospora crassa mitochondria by affinity chromatography on immobilized cytochrome c. Difference in the binding between ferricytochrome c and ferrocytochrome c to the multiprotein complex. Eur J Biochem. 1978 Jul 17;88(1):17–28. doi: 10.1111/j.1432-1033.1978.tb12418.x. [DOI] [PubMed] [Google Scholar]
- Weiss H., Kolb H. J. Isolation of mitochondrial succinate: ubiquinone reductase, cytochrome c reductase and cytochrome c oxidase from Neurospora crassa using nonionic detergent. Eur J Biochem. 1979 Aug 15;99(1):139–149. doi: 10.1111/j.1432-1033.1979.tb13240.x. [DOI] [PubMed] [Google Scholar]
- Weiss H., Leonard K., Neupert W. Puzzling subunits of mitochondrial cytochrome reductase. Trends Biochem Sci. 1990 May;15(5):178–180. doi: 10.1016/0968-0004(90)90155-5. [DOI] [PubMed] [Google Scholar]
- White J. A., Scandalios J. G. Deletion analysis of the maize mitochondrial superoxide dismutase transit peptide. Proc Natl Acad Sci U S A. 1989 May;86(10):3534–3538. doi: 10.1073/pnas.86.10.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]