Abstract
Background
Evidence suggests that monoclonal B-cell lymphocytosis precedes all chronic lymphocytic leukemia cases, although the molecular mechanisms responsible for disease progression are not understood. Aberrant miRNA expression may contribute to the pathogenesis of chronic lymphocytic leukemia. The objective of this study was to compare miRNA expression profiles of patients with Binet A chronic lymphocytic leukemia with those of subjects with high-count monoclonal B-cell lymphocytosis and healthy volunteers (controls).
Methods
Twenty-one chronic lymphocytic leukemia patients, 12 subjects with monoclonal B-cell lymphocytosis and ten healthy volunteers were enrolled in this study. Flow cytometry CD19+CD5+-based cell sorting was performed for the chronic lymphocytic leukemia and monoclonal B-cell lymphocytosis groups and CD19+ cells were sorted to analyze the control group. The expressions of miRNAs (miR-15a, miR-16-1, miR-29b, miR-34a, miR-181a, miR-181b and miR-155) were determined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).
Results
Significant differences between the expressions in the chronic lymphocytic leukemia and monoclonal B-cell lymphocytosis groups were restricted to the expression of miR-155, which was higher in the former group. A comparison between healthy controls and monoclonal B-cell lymphocytosis/chronic lymphocytic leukemia patients revealed higher miR-155 and miR-34a levels and lower miR-15a, miR-16-1, miR-181a and miR-181b in the latter group.
Conclusions
Our results show a progressive increase of miR-155 expression from controls to monoclonal B-cell lymphocytosis to chronic lymphocytic leukemia. The role of miR-155 in the development of overt chronic lymphocytic leukemia in individuals with monoclonal B-cell lymphocytosis must be further analyzed.
Keywords: Monoclonal B-cell lymphocytosis, Chronic lymphocytic leukemia, microRNA
Introduction
Chronic lymphocytic leukemia (CLL) is a malignant neoplasm characterized by an excess of monoclonal B lymphocytes in peripheral blood, bone marrow, the spleen and lymph nodes.1 CLL is the most common leukemia in the Western world, with an annual incidence of 5.1 cases/100,000 people.2
Over the last years, advances in multi-parameter flow cytometry allowed the identification of small populations of monoclonal B lymphocytes in the blood of apparently healthy subjects.3 The presence of monoclonal B-cells in the peripheral blood of up to 5 × 109/L is classified as monoclonal B-cell lymphocytosis (MBL) in the absence of other lymphomatous features.4, 5 This condition is found in 0.6–12% of healthy individuals.3, 6, 7
MBL is classified as high-count or low-count MBL. The first is usually diagnosed in asymptomatic subjects with mild lymphocytosis and the latter occurs in asymptomatic subjects with normal blood counts who have been submitted to flow cytometry screening.8, 9, 10 The most recent World Health Organization (WHO) classification defines low-count MBL as a peripheral blood CLL count of <0.5 × 109/L and no extramedullary disease.4 As in CLL, MBL is more common in men and in relatives of CLL patients11, 12, 13 with its frequency increasing with age.11, 12, 14, 15, 16, 17
Virtually, all CLL cases are preceded by MBL.18 Even though the mechanisms underlying CLL pathogenesis and MBL progression to CLL are still not well understood, it is speculated that the initial genetic lesion in MBL may occur in the immature bone marrow B cell compartment and afterwards repetitive antigenic stimulation may induce additional genetic lesions, eventually leading to neoplastic transformation.19
MicroRNAs (miRNA) are 19–25 nucleotide single strand RNAs responsible for gene expression and cellular metabolism regulation.20, 21 Changes in miRNA expression have been associated with solid and hematologic tumors.21 Some microRNAs have been shown to be abnormally expressed in CLL.22 Studies addressing the molecular and genetic basis of MBL may help to elucidate initial steps of CLL pathogenesis and, therefore, increase knowledge about CLL origin. Moreover, only a few studies have investigated miRNAs in MBL.9, 23 Here we hypothesized that abnormalities in some miRNA expressions may be present in MBL and may have a role in initial monoclonal B-cell expansion.
Methods
Cell samples from normal controls, individuals with high-count MBL and CLL patients
Samples from 12 individuals with high-count MBL and 21 patients with stage A CLL according to the Binet classification were analyzed. All the patients were being monitored in a university hospital in Ribeirao Preto, Sao Paulo, Brazil. Ten healthy individuals were studied as a control group. The study was approved by the Institution Ethics Research Review Board and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki and its revisions.
Using cell sorting by flow cytometry, CD19+CD5+ lymphocytes were isolated from the peripheral blood of individuals in the two study groups, and CD19+ lymphocytes from the peripheral blood of controls as previously described.24 The mean percentage of the desired cell population after isolation was 89.48% (±8.48%).
The clinical characteristics of the enrolled subjects are summarized in Table 1.
Table 1.
Characteristics of the enrolled subjects.
| Characteristic | Control | High-count monoclonal B-cell lymphocytosis | Chronic lymphocytic leukemia |
|---|---|---|---|
| Number of subjects | 10 | 12 | 21 |
| Medium age | 38 | 78 | 70 |
| Range (years) | 25–60 | 57–97 | 58–81 |
| Gender (n) | |||
| Male | 2 | 9 | 11 |
| Female | 8 | 3 | 10 |
| Medium number of lymphocytes | 2.064 | 3.933 | 41.714 |
| Range (μL) | 1.360–3.300 | 2.900–5.900 | 7.500–147.000 |
| % CD5+B-Lymphocytes of total lymphocytes | – | 34.9 | 78.02 |
| Range (%) | – | 5.97–61.01 | 58.63–91.09 |
RNA isolation and miRNA expression
RNA was extracted from CD5+ B lymphocytes isolated from the study groups and B lymphocytes from the control group, using Trizol (Life Technologies, California, USA). Seven miRNAs known to have abnormal expressions in CLL were selected for analysis (miR-15a, miR-16-1, miR-29b, miR-34a, miR-181a, miR-181b and miR-155). miRNA expression was quantified by TaqMan miRNA quantitative reverse-transcription polymerase chain reaction (qRT-PCR) as previously described.25, 26 Briefly, 5 ng of total RNA were reverse transcribed using the microRNA reverse-transcription kit (Applied Bio-systems, California, USA) with specific stem-loop primers. qRT-PCR analysis was performed using the miRNA-specific Taqman assay (Applied Bio-systems). Expression levels of the nucleolar RNAs RNU24, RNU44 and RNU48 were similar for all groups and the geometric mean of their expression was used to normalize the expression of the miRNAs, as previously described27 using the 2−ΔCt formula. The coefficients of variation (CV) of the reactions were calculated. If the CV of a reaction was greater than 2% in two successive experiments, its value was considered unavailable. All miRNAs were studied in duplicate.
Statistical analysis
The Kolmogorov–Smirnov normality test was initially performed to compare miRNA expressions. The expressions of the miRNAs, miR-15a, miR-16-1, miR-29b, miR-181b and miR-155, did not deviate from the normal distribution and variance analysis (ANOVA) was used followed by the Bonferoni post hoc test. The Kruskal–Wallis non-parametric test (non-parametric ANOVA) was used followed by the Dunn post hoc test for the miRNAs miR-34a and miR-181a that had non-normal distributions. All tests with p-values ≤0.05 were considered statistically significant.
Results
miRNA expression
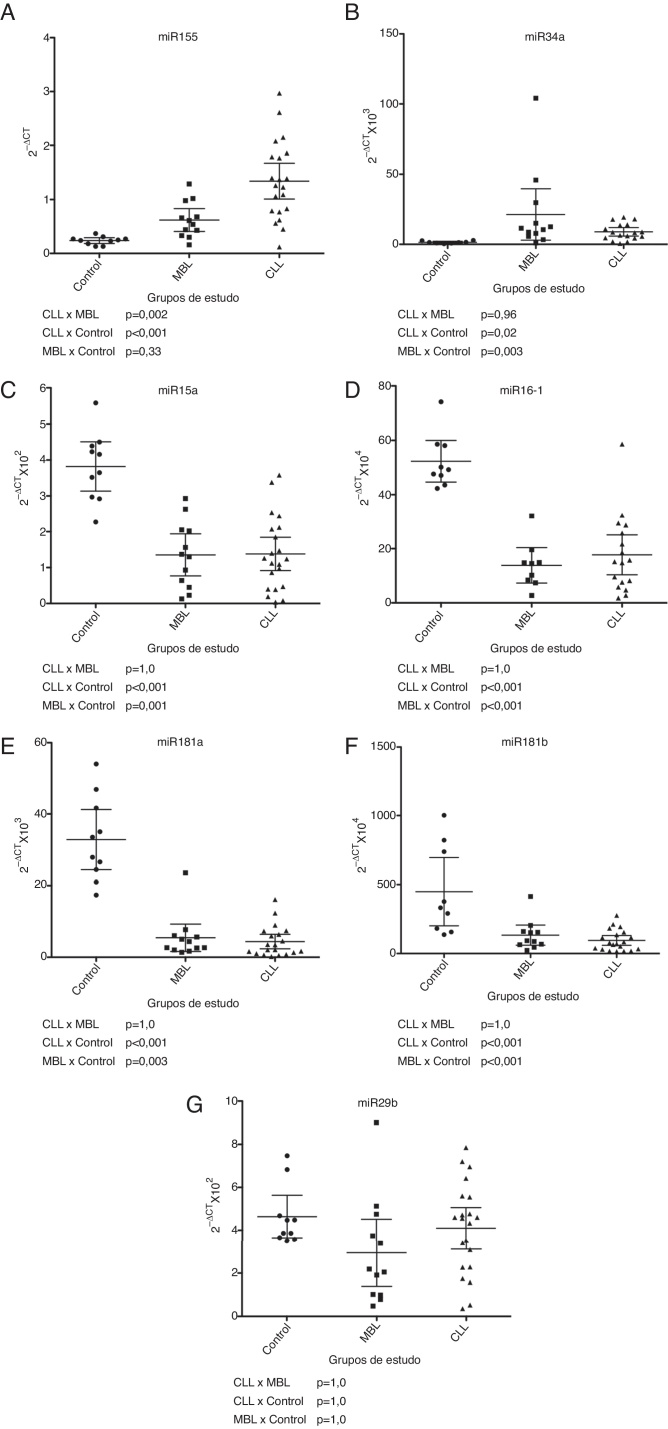
Of the seven miRNAs deemed relevant for CLL pathogenesis, miRNA-155 and miR-34a were differentially expressed in the CLL and high-count MBL groups compared to the controls (p-value = 0.33 and p-value = 0.003 for MBL and controls and p-value <0.001 and p-value = 0.02 for CLL and controls, respectively) with the highest values detected in CLL (Figure 1). miRNA-155 expression in high-count MBL had a tendency to be greater than in healthy subjects, but this difference was not statistically significant, maybe due to the small number of samples studied (Figure 1). miR-34a expression was not statistically different between CLL and MBL (Figure 1). miR-15a, miR-16-1, miR-181a and miR-181b were down-regulated in CLL and MBL. Their expression was lower in CLL patients when compared to controls (p-value < 0.001 for all miRNAs). High-count MBL subjects also had lower expression than controls for miR-15a, miR-16-1 and miR-181b (p-value = 0.003) and for miR-181a (p-value < 0.001) (Figure 1).
Figure 1.
MicroRNA expression of control, monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia groups: (A) miR-155; (B) miR-34a; (C) miR-15a; (D) miR-16-1; (E) miR-181a; (F) miR-181b; (G) miR-29b.
Expression of miR-29b was similar for all three groups (Figure 1).
Discussion
This study with a Binet A CLL group and a high-count MBL group demonstrated new molecular differences between these two entities.
Statistical similarities have been identified regarding the frequencies of immunoglobulin heavy chain variable (IGHV) genes between high-count MBL and the initial stages of CLL, although findings from low-count MBL were different between these groups.28, 29 Other authors also found biological similarities between these three entities. Not only high-count MBL but also low-count MBL bear cytogenetic abnormalities common in CLL, including 13q-, 17p- and trisomy 12.6, 30, 31, 32
Aiming to better understand genetic alterations in CLL, this study compared the expressions of miRNAs previously described as altered in this disease with their expression in MBL. Knowledge of miRNA expression in different monoclonal proliferation stages may improve the comprehension of CLL pathophysiology.
Several studies using different methodologies have identified overexpression of miR-155 in CLL when compared to normal controls.23, 33, 34, 35, 36, 37 Our results confirmed the seminal findings of Ferrajoli et al. who showed that the expression of miR-155 is greater in Binet A CLL than in high-count MBL and that its expression in these individuals is greater than in normal controls.23 Thus, our findings suggest that miR-155 may be used as a progression marker for MBL individuals, but this must be confirmed by further specific studies.
As is usual for miRNAs, miR-155 has distinct functions in different cell types. In B-lymphocytes, it represses the expression of PU.1 transcription factor and the inositol 5-phosphatase Src homology-2 domain-containing inositol 5-phosphatase 1 (SHIP1).38 As an antagonist of the phosphatidylinositol 3-kinase (PI3K) pathway, SHIP1 is a negative regulator of B cell receptor (BCR) signaling and it was identified as a target of miR-155 in diffuse B-cell lymphoma.39 In CLL, the regulation of miR-155 and its target genes are not well understood. Its expression may be regulated by transcription factors that have important roles in its evolution. Furthermore, miR-155 may directly regulate coding genes that are important for the transition of MBL to CLL.23
This study also identified miR-34a to be overexpressed in CLL when compared to healthy controls.35, 40, 41 This miRNA has been associated to the regulation of the tumor protein p53 (TP53) pathway41, 42, 43, 44, 45 and may be related to fludarabine resistance in CLL.44
The study that first demonstrated down-regulation of miR-15a and miR-16-1 in CLL patients was also the first to demonstrate abnormal expressions of miRNAs in cancer.46 Subsequent studies using different techniques have confirmed this finding, particularly in patients with del 13q14.47, 48, 49 Other authors found similar expressions of these miRNAs in CLL and normal controls using microarrays35 and RT-qPCR.33 Klein et al. demonstrated that mouse models that have the chromosome area (13q14) responsible for the transcription of these miRNAs deleted developed monoclonal expansion of lymphocytes in blood.50
In this study, the miRNAs miR-181a and miR-181b were down-regulated in CLL when compared to normal controls. Other authors found similar results in studies using RT-qPCR,36, 48 Northern Blot34 and microarrays.35 Visone et al. demonstrated that down-regulation of miR-181b is a better marker for worse prognosis in CLL than the IGHV mutation status and ZAP-70 expression, and suggest that its expression should be monitored in CLL patients.51
The pattern of miR-29b expression in CLL is not well understood. Here the results for CLL Binet A, high-count MBL and normal controls were similar. Sampath et al. found that 70% of the CLL patients have a similar expression to normal controls.49 Fulci et al. also had results similar to this study.33 However, Santanam et al. found this miRNA to be overexpressed in CLL, and Zhu et al. found it to be down-regulated in CLL.48, 52 All these studies have heterogeneous groups of CLL patients regarding the stage of the disease. These different results suggest that miR-29b expression may change according to disease stage. However, this hypothesis should be confirmed in future studies.
Apart from the study of Ferrajoli et al. on miR-155, only one other group has evaluated miRNA expression in high-count MBL.23 Thus, Morabito et al. used microarrays to study individuals previously diagnosed with CLL before the change of diagnosis criteria proposed by the International Workshop on Chronic Lymphocytic Leukemia in 2008.9, 53 They found miR-130a was the only one with different expressions between CLL and high-count MBL. Using another technique and a more heterogeneous high-count MBL population, this study found the expressions of the miR-34a, miR-15a, miR-16-1, miR-181a, miR-181b and miR-29b to be similar in CLL and high-count MBL.
The results reported here should be confirmed by further studies, since the groups were small and more individuals must be studied to allow robust conclusions. Moreover, although the CLL and MBL groups were composed mainly of older individuals and the MBL group mainly of males, our control group was composed mainly of females and the medium age for this group was lower than for the study groups, although there are no data suggesting that the levels of these miRNAs change with sex and age.
In conclusion, the finding that some miRNAs have abnormal expressions in MBL and that this condition is also present in CLL suggests that these genetic changes may be part of the initial events responsible for monoclonal CD5+ B-cell proliferation. The differential expression of miR-155 in MBL and CLL suggests its role in signaling pathways that are important to the development of the disease.
Author contributions
FMF, DLZ, RTCSR, EMR, DMM and RPF designed the research protocols. FMF, PSS and BS performed the research. FMF, BS, DMM and RPF analyzed the data. FMF, RTCSR, EMR, DMM and RPF wrote the manuscript.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPQ) grant 573.754/2008-0.
References
- 1.Müller-Hermelink H.K., Montserrat E., Catovsky D., Campo E., Harris N.L., Stein H. Chonic lymphocytic leukaemia/small lymphocytic lymphoma. In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., editors. WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. World Health Organization Classification of Tumors. International Agency for Research on Cancer; Lyon: 2008. pp. 180–182. [Google Scholar]
- 2.Dores G.M., Anderson W.F., Curtis R.E., Landgren O., Ostroumova E., Bluhm E.C. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol. 2007;139(5):809–819. doi: 10.1111/j.1365-2141.2007.06856.x. [DOI] [PubMed] [Google Scholar]
- 3.Rawstron A.C., Green M.J., Kuzmicki A., Kennedy B., Fenton J.A., Evans P.A. Monoclonal B lymphocytes with the characteristics of indolent chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood. 2002;100(2):635–639. doi: 10.1182/blood.v100.2.635. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marti G.E., Rawstron A.C., Ghia P., Hillmen P., Houlston R.S., Kay N. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130(3):325–332. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 6.Rawstron A.C., Bennett F.L., O’Connor S.J., Kwok M., Fenton J.A., Plummer M. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 7.D’Arena G., Musto P. Monoclonal B-cell lymphocytosis. Transl Med UniSa. 2014;8:75–79. [PMC free article] [PubMed] [Google Scholar]
- 8.Ghia P., Caligaris-Cappio F. Monoclonal B-cell lymphocytosis: right track or red herring? Blood. 2012;119(19):4358–4362. doi: 10.1182/blood-2012-01-404681. [DOI] [PubMed] [Google Scholar]
- 9.Morabito F., Mosca L., Cutrona G., Agnelli L., Tuana G., Ferracin M. Clinical monoclonal B lymphocytosis versus Rai 0 chronic lymphocytic leukemia: a comparison of cellular, cytogenetic, molecular, and clinical features. Clin Cancer Res. 2013;19(21):5890–5900. doi: 10.1158/1078-0432.CCR-13-0622. [DOI] [PubMed] [Google Scholar]
- 10.Rawstron A.C. Monoclonal B cell lymphocytosis – what does it really mean? Curr Hematol Malig Rep. 2013;8(1):52–59. doi: 10.1007/s11899-012-0144-z. [DOI] [PubMed] [Google Scholar]
- 11.Rawstron A.C., Yuille M.R., Fuller J., Cullen M., Kennedy B., Richards S.J. Inherited predisposition to CLL is detectable as subclinical monoclonal B-lymphocyte expansion. Blood. 2002;100(7):2289–2290. doi: 10.1182/blood-2002-03-0892. [DOI] [PubMed] [Google Scholar]
- 12.Matos D.M., Ismael S.J., Scrideli C.A., de Oliveira F.M., Rego E.M., Falcao R.P. Monoclonal B-cell lymphocytosis in first-degree relatives of patients with sporadic (non-familial) chronic lymphocytic leukaemia. Br J Haematol. 2009;147(3):339–346. doi: 10.1111/j.1365-2141.2009.07861.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldin L.R., Lanasa M.C., Slager S.L., Cerhan J.R., Vachon C.M., Strom S.S. Common occurrence of monoclonal B-cell lymphocytosis among members of high-risk CLL families. Br J Haematol. 2010;151(2):152–158. doi: 10.1111/j.1365-2141.2010.08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanafelt T.D., Kay N.E., Jenkins G., Call T.G., Zent C.S., Jelinek D.F. B-cell count and survival: differentiating chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis based on clinical outcome. Blood. 2009;113(18):4188–4196. doi: 10.1182/blood-2008-09-176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghia P., Prato G., Scielzo C., Stella S., Geuna M., Guida G. Monoclonal CD5+ and CD5− B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004;103(6):2337–2342. doi: 10.1182/blood-2003-09-3277. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan C.S., Thomas M.E., Mulligan S.P. Monoclonal B-lymphocytosis: demographics, nature and subclassification in 414 community patients. Leuk Lymphoma. 2011;52(12):2293–2298. doi: 10.3109/10428194.2011.598250. [DOI] [PubMed] [Google Scholar]
- 17.Kern W., Bacher U., Haferlach C., Dicker F., Alpermann T., Schnittger S. Monoclonal B-cell lymphocytosis is closely related to chronic lymphocytic leukaemia and may be better classified as early-stage CLL. Br J Haematol. 2012;157(1):86–96. doi: 10.1111/j.1365-2141.2011.09010.x. [DOI] [PubMed] [Google Scholar]
- 18.Landgren O., Albitar M., Ma W., Abbasi F., Hayes R.B., Ghia P. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360(7):659–667. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiorazzi N., Rai K.R., Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Garcia I., Miska E.A. MicroRNA functions in animal development and human disease. Development. 2005;132(21):4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 21.Chen B., Li H., Zeng X., Yang P., Liu X., Zhao X. Roles of microRNA on cancer cell metabolism. J Transl Med. 2012;10:228. doi: 10.1186/1479-5876-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward B.P., Tsongalis G.J., Kaur P. MicroRNAs in chronic lymphocytic leukemia. Exp Mol Pathol. 2011;90(2):173–178. doi: 10.1016/j.yexmp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Ferrajoli A., Shanafelt T.D., Ivan C., Shimizu M., Rabe K.G., Nouraee N. Prognostic value of miR-155 in individuals with monoclonal B-cell lymphocytosis and patients with B chronic lymphocytic leukemia. Blood. 2013;122(11):1891–1899. doi: 10.1182/blood-2013-01-478222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S., Campbell H.M., Dittel B.N., Ray A. Purification of specific cell population by fluorescence activated cell sorting (FACS) J Vis Exp. 2010;41 doi: 10.3791/1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C., Ridzon D.A., Broomer A.J., Zhou Z., Lee D.H., Nguyen J.T. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33(20):e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmittgen T.D., Lee E.J., Jiang J., Sarkar A., Yang L., Elton T.S. Real-time PCR quantification of precursor and mature microRNA. Methods. 2008;44(1):31–38. doi: 10.1016/j.ymeth.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. Research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dagklis A., Fazi C., Sala C., Cantarelli V., Scielzo C., Massacane R. The immunoglobulin gene repertoire of low-count chronic lymphocytic leukemia (CLL)-like monoclonal B lymphocytosis is different from CLL: diagnostic implications for clinical monitoring. Blood. 2009;114(1):26–32. doi: 10.1182/blood-2008-09-176933. [DOI] [PubMed] [Google Scholar]
- 29.Vardi A., Dagklis A., Scarfo L., Jelinek D., Newton D., Bennett F. Immunogenetics shows that not all MBL are equal: the larger the clone, the more similar to CLL. Blood. 2013;121(22):4521–4528. doi: 10.1182/blood-2012-12-471698. [DOI] [PubMed] [Google Scholar]
- 30.Nieto W.G., Almeida J., Romero A., Teodosio C., Lopez A., Henriques A.F. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114(1):33–37. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 31.Rossi D., Sozzi E., Puma A., De Paoli L., Rasi S., Spina V. The prognosis of clinical monoclonal B cell lymphocytosis differs from prognosis of Rai 0 chronic lymphocytic leukaemia and is recapitulated by biological risk factors. Br J Haematol. 2009;146(1):64–75. doi: 10.1111/j.1365-2141.2009.07711.x. [DOI] [PubMed] [Google Scholar]
- 32.Fazi C., Scarfo L., Pecciarini L., Cottini F., Dagklis A., Janus A. General population low-count CLL-like MBL persists over time without clinical progression, although carrying the same cytogenetic abnormalities of CLL. Blood. 2011;118(25):6618–6625. doi: 10.1182/blood-2011-05-357251. [DOI] [PubMed] [Google Scholar]
- 33.Fulci V., Chiaretti S., Goldoni M., Azzalin G., Carucci N., Tavolaro S. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109(11):4944–4951. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- 34.Marton S., Garcia M.R., Robello C., Persson H., Trajtenberg F., Pritsch O. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia. 2008;22(2):330–338. doi: 10.1038/sj.leu.2405022. [DOI] [PubMed] [Google Scholar]
- 35.Pallasch C.P., Patz M., Park Y.J., Hagist S., Eggle D., Claus R. miRNA deregulation by epigenetic silencing disrupts suppression of the oncogene PLAG1 in chronic lymphocytic leukemia. Blood. 2009;114(15):3255–3264. doi: 10.1182/blood-2009-06-229898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S., Moffett H.F., Lu J., Werner L., Zhang H., Ritz J. MicroRNA expression profiling identifies activated B cell status in chronic lymphocytic leukemia cells. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vargova K., Curik N., Burda P., Basova P., Kulvait V., Pospisil V. MYB transcriptionally regulates the miR-155 host gene in chronic lymphocytic leukemia. Blood. 2011;117(14):3816–3825. doi: 10.1182/blood-2010-05-285064. [DOI] [PubMed] [Google Scholar]
- 38.Danger R., Braza F., Giral M., Soulillou J.P., Brouard S. MicroRNAs major players in B cells homeostasis and function. Front Immunol. 2014;5:98. doi: 10.3389/fimmu.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schott J., Stoecklin G. Networks controlling mRNA decay in the immune system. Wiley Interdiscip Rev RNA. 2010;1(3):432–456. doi: 10.1002/wrna.13. [DOI] [PubMed] [Google Scholar]
- 40.Zanette D.L., Rivadavia F., Molfetta G.A., Barbuzano F.G., Proto-Siqueira R., Silva-Jr W.A. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res. 2007;40(11):1435–1440. doi: 10.1590/s0100-879x2007001100003. [DOI] [PubMed] [Google Scholar]
- 41.Asslaber D., Pinon J.D., Seyfried I., Desch P., Stocher M., Tinhofer I. microRNA-34a expression correlates with MDM2 SNP309 polymorphism and treatment-free survival in chronic lymphocytic leukemia. Blood. 2010;115(21):4191–4197. doi: 10.1182/blood-2009-07-234823. [DOI] [PubMed] [Google Scholar]
- 42.Bommer G.T., Gerin I., Feng Y., Kaczorowski A.J., Kuick R., Love R.E. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17(15):1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 43.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17(2):193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 44.Merkel O., Asslaber D., Pinon J.D., Egle A., Greil R. Interdependent regulation of p53 and miR-34a in chronic lymphocytic leukemia. Cell Cycle. 2010;9(14):2764–2768. [PubMed] [Google Scholar]
- 45.Dufour A., Palermo G., Zellmeier E., Mellert G., Duchateau-Nguyen G., Schneider S. Inactivation of TP53 correlates with disease progression and low miR-34a expression in previously treated chronic lymphocytic leukemia patients. Blood. 2013;121(18):3650–3657. doi: 10.1182/blood-2012-10-458695. [DOI] [PubMed] [Google Scholar]
- 46.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calin G.A., Liu C.G., Sevignani C., Ferracin M., Felli N., Dumitru C.D. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101(32):11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu D.X., Miao K.R., Fang C., Fan L., Zhu W., Zhu H.Y. Aberrant microRNA expression in Chinese patients with chronic lymphocytic leukemia. Leuk Res. 2011;35(6):730–734. doi: 10.1016/j.leukres.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Sampath D., Liu C., Vasan K., Sulda M., Puduvalli V.K., Wierda W.G. Histone deacetylases mediate the silencing of miR-15a, miR-16, and miR-29b in chronic lymphocytic leukemia. Blood. 2012;119(5):1162–1172. doi: 10.1182/blood-2011-05-351510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein U., Lia M., Crespo M., Siegel R., Shen Q., Mo T. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17(1):28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Visone R., Veronese A., Rassenti L.Z., Balatti V., Pearl D.K., Acunzo M. miR-181b is a biomarker of disease progression in chronic lymphocytic leukemia. Blood. 2011;118(11):3072–3079. doi: 10.1182/blood-2011-01-333484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santanam U., Zanesi N., Efanov A., Costinean S., Palamarchuk A., Hagan J.P. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci U S A. 2010;107(27):12210–12215. doi: 10.1073/pnas.1007186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallek M., Cheson B.D., Catovsky D., Caligaris-Cappio F., Dighiero G., Dohner H. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]