Abstract
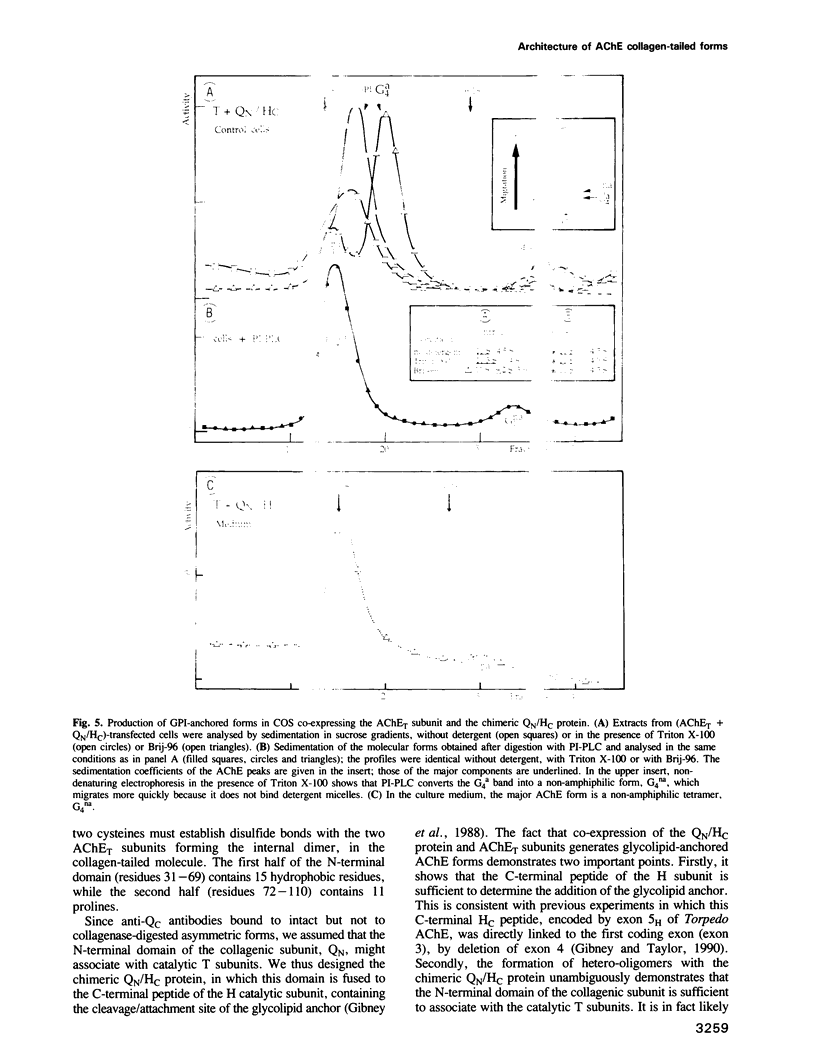
Asymmetric forms of Torpedo acetylcholinesterase (AChE) are produced in COS cells by the simultaneous expression of collagenic subunits (Q) and catalytic T subunits (AChET). Truncated AChET delta subunits, from which most of the C-terminal peptide (TC) had been deleted by mutagenesis, did not associate with Q subunits. The TC peptide is therefore necessary for the association of the AChET and Q subunits. In order to determine the orientation of the Q subunit in the collagen-tailed forms, we have developed an antiserum against its non-collagenic C-terminal domain, expressed as a fusion protein in Escherichia coli. This antiserum, which recognized the Q subunit in Western blots, was found to react with intact asymmetric forms, but not with collagenase-treated forms, from which the distal part of the tail had been cleaved, suggesting that the N-terminal non-collogenic domain (QN) is responsible for the interaction with the AChET subunits. This was confirmed by creating a chimeric subunit (QN/HC), in which QN was linked to the C-terminal peptide of the H subunit of Torpedo AChE, which contains the glycophosphatidylinositol (GPI) cleavage/attachment signal: co-expression of AChET and QN/NC produced GPI-anchored tetramers, which were sensitive to PI-PLC and largely exposed to the external surface of the cells. We thus demonstrate that: (i) the HC peptide is sufficient to determine the addition of a glycolipid anchor and (ii) the QN domain is sufficient to bind a catalytic AChET tetramer by interacting with the TC peptide.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Silman I. Molecular structure of elongated forms of electric eel acetylcholinesterase. J Mol Biol. 1978 Nov 5;125(3):293–311. doi: 10.1016/0022-2836(78)90404-7. [DOI] [PubMed] [Google Scholar]
- Bon S., Cartaud J., Massoulie J. Dumbbell-shaped associations of tailed Electrophorus acetylcholinesterase molecules. Mol Biol Rep. 1978 Feb 28;4(1):61–63. doi: 10.1007/BF00775183. [DOI] [PubMed] [Google Scholar]
- Bon S., Massoulié J. Collagenase sensitivity and aggregation properties of Electrophorus acetylcholinesterase. Eur J Biochem. 1978 Aug 15;89(1):89–94. doi: 10.1111/j.1432-1033.1978.tb20899.x. [DOI] [PubMed] [Google Scholar]
- Bon S., Toutant J. P., Méflah K., Massoulié J. Amphiphilic and nonamphiphilic forms of Torpedo cholinesterases: I. Solubility and aggregation properties. J Neurochem. 1988 Sep;51(3):776–785. doi: 10.1111/j.1471-4159.1988.tb01812.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Gennari K., Brunner J., Brodbeck U. Tetrameric detergent-soluble acetylcholinesterase from human caudate nucleus: subunit composition and number of active sites. J Neurochem. 1987 Jul;49(1):12–18. doi: 10.1111/j.1471-4159.1987.tb03386.x. [DOI] [PubMed] [Google Scholar]
- Gibney G., MacPhee-Quigley K., Thompson B., Vedvick T., Low M. G., Taylor S. S., Taylor P. Divergence in primary structure between the molecular forms of acetylcholinesterase. J Biol Chem. 1988 Jan 25;263(3):1140–1145. [PubMed] [Google Scholar]
- Gibney G., Taylor P. Biosynthesis of Torpedo acetylcholinesterase in mammalian cells. Functional expression and mutagenesis of the glycophospholipid-anchored form. J Biol Chem. 1990 Jul 25;265(21):12576–12583. [PubMed] [Google Scholar]
- Inestrosa N. C., Roberts W. L., Marshall T. L., Rosenberry T. L. Acetylcholinesterase from bovine caudate nucleus is attached to membranes by a novel subunit distinct from those of acetylcholinesterases in other tissues. J Biol Chem. 1987 Apr 5;262(10):4441–4444. [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Krejci E., Coussen F., Duval N., Chatel J. M., Legay C., Puype M., Vandekerckhove J., Cartaud J., Bon S., Massoulié J. Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO J. 1991 May;10(5):1285–1293. doi: 10.1002/j.1460-2075.1991.tb08070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. L., Heinemann S., Taylor P. Structural characterization of the asymmetric (17 + 13) S forms of acetylcholinesterase from Torpedo. I. Analysis of subunit composition. J Biol Chem. 1982 Oct 25;257(20):12282–12291. [PubMed] [Google Scholar]
- Lee S. L., Taylor P. Structural characterization of the asymmetric (17 + 13) S species of acetylcholinesterase from Torpedo. II. Component peptides obtained by selective proteolysis and disulfide bond reduction. J Biol Chem. 1982 Oct 25;257(20):12292–12301. [PubMed] [Google Scholar]
- MacPhee-Quigley K., Vedvick T. S., Taylor P., Taylor S. S. Profile of the disulfide bonds in acetylcholinesterase. J Biol Chem. 1986 Oct 15;261(29):13565–13570. [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset F., Frobert Y., Grassi J., Vigny M., Boulla G., Bon S., Massoulié J. Monoclonal antibodies against acetylcholinesterase from electric organs of Electrophorus and Torpedo. Biochimie. 1987 Feb;69(2):147–156. doi: 10.1016/0300-9084(87)90247-1. [DOI] [PubMed] [Google Scholar]
- Roberts W. L., Doctor B. P., Foster J. D., Rosenberry T. L. Bovine brain acetylcholinesterase primary sequence involved in intersubunit disulfide linkages. J Biol Chem. 1991 Apr 25;266(12):7481–7487. [PubMed] [Google Scholar]
- Rosenberry T. L., Richardson J. M. Structure of 18S and 14S acetylcholinesterase. Identification of collagen-like subunits that are linked by disulfide bonds to catalytic subunits. Biochemistry. 1977 Aug 9;16(16):3550–3558. doi: 10.1021/bi00635a008. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorav J. L., Duval N., Anselmet A., Bon S., Krejci E., Legay C., Osterlund M., Reimund B., Massoulié J. Complex alternative splicing of acetylcholinesterase transcripts in Torpedo electric organ; primary structure of the precursor of the glycolipid-anchored dimeric form. EMBO J. 1988 Oct;7(10):2983–2993. doi: 10.1002/j.1460-2075.1988.tb03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorav J. L., Krejci E., Massoulié J. cDNA sequences of Torpedo marmorata acetylcholinesterase: primary structure of the precursor of a catalytic subunit; existence of multiple 5'-untranslated regions. EMBO J. 1987 Jul;6(7):1865–1873. doi: 10.1002/j.1460-2075.1987.tb02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velan B., Grosfeld H., Kronman C., Leitner M., Gozes Y., Lazar A., Flashner Y., Marcus D., Cohen S., Shafferman A. The effect of elimination of intersubunit disulfide bonds on the activity, assembly, and secretion of recombinant human acetylcholinesterase. Expression of acetylcholinesterase Cys-580----Ala mutant. J Biol Chem. 1991 Dec 15;266(35):23977–23984. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]





