Abstract
Objective: Cerebral photobiomodulation (PBM) improves mood and cognition. Cerebral metabolic enhancement is a mechanism proposed to underlie PBM effects. No PBM studies to date have applied phosphorus magnetic resonance spectroscopy (31P MRS), which can be used to assess metabolic intermediates such as phosphocreatine (PCr) and adenosine triphosphate, the latter of which is elevated by PBM. Accordingly, we used 9.4 Tesla 31P MRS to characterize effects of single and repeat cerebral PBM treatments on metabolism. PBM was delivered to healthy adult beagles in the form of transcranial laser treatment (TLT) at a wavelength of 808 nm, which passes safely through the skull and activates cytochrome C oxidase, a mitochondrial respiratory chain enzyme. Methods: Isoflurane-anesthetized subjects (n = 4) underwent a baseline 31P MRS scan followed by TLT applied sequentially for 2 min each to anterior and posterior cranium midline locations, to irradiate the dorsal cortex. Subjects then underwent 31P MRS scans for 2 h to assess acute TLT effects. After 2 weeks of repeat TLT (3 times/week), subjects were scanned again with 31P MRS to characterize effects of repeat TLT. Results: TLT did not induce acute 31P MRS changes over the course of 2 h in either scan session. However, after repeat TLT, the baseline PCr/β-nucleoside triphosphate ratio was higher than the scan 1 baseline (p < 0.0001), an effect attributable to increased PCr level (p < 0.0001). Conclusions: Our findings are consistent with reports that bioenergetic effects of PBM can take several hours to evolve. Thus, in vivo 31P MRS may be useful for characterizing bioenergetic effects of PBM in brain and other tissues.
Keywords: : adenosine triphosphate, cerebral metabolism, cytochrome C oxidase, low-level laser therapy, major depression, near-infrared light, phosphocreatine, phosphorus magnetic resonance spectroscopy, photobiomodulation
Introduction
Photobiomodulation (PBM) activates and upregulates cytochrome C oxidase (COX, Complex IV), a photoacceptor and key enzyme in the mitochondrial respiratory chain enzyme.1,2 PBM evokes a wide range of biological effects in multiple tissue types; it increases mitochondrial respiration and adenosine triphosphate (ATP) levels,3–8 increases oxygen consumption,9,10 increases tissue perfusion,11–13 alters gene transcription patterns, messenger RNA levels, and protein synthesis,14–16 and reduces motor cortex excitability.17 The effects of PBM appear to be beneficial in several pre-clinical and human disease models.18–20
Accumulating evidence also suggests that PBM can improve cognition and mood. In pre-clinical studies, cortical PBM improved working memory and spatial navigation in middle-aged CD-1 mice,21 enhanced fear extinction learning among rats,10 and reduced depressive behaviors in mice with experimental traumatic brain injury (TBI) and in rats subjected to chronic mild stress.22,23 In clinical studies, cortical PBM improved depression and anxiety ratings,24,25 verbal learning and memory, executive function, social and occupational function, and mood, in mild TBI patients.26 In healthy humans, cortical PBM improved reaction times, short-term memory, overall affect, and executive function.27,28
Human PBM studies have demonstrated cortical perfusion increases in subjects with major depression,24 improved cerebral perfusion in a persistent vegetative state patient,29 and improved large cerebral artery flow dynamics in healthy elderly women.30 However, to our knowledge, no study to date has assessed cerebral metabolic effects of PBM in vivo.
Accordingly, we utilized phosphorus magnetic resonance spectroscopy (31P MRS), a noninvasive imaging method capable of assessing cerebral metabolism, to study effects of acute and repeated cortical infrared (808 nm) PBM in healthy adult beagle dogs. Bioenergetic compounds such as phosphocreatine (PCr), the main high-energy phosphorus storage form in brain, can be measured with 31P MRS, as can ATP, which supplies most of the brain's energy needs; the 31P MRS β-nucleoside triphosphate (β-NTP) resonance typically is used as a measure of ATP levels. 31P MRS has been used to detect tissue effects of metabolic challenges such as prolonged sensory stimulation,31 exhaustive exercise,32 and to characterize cerebral metabolic abnormalities in disorders such as Alzheimer's disease,33 major depression,25,34 and substance abuse.35 31P MRS also has been used to characterize cerebral metabolic effects of treatments that enhance metabolism, including creatine supplementation36–38 and thyroid hormone administration.34 Creatine supplementation in mice has been associated with increased muscle COX expression,39 suggesting that COX is a common target for creatine and PBM. Accordingly, and in light of the 31P MRS report that creatine supplementation in healthy adults increased brain PCr/β-NTP ratios,38 we hypothesized that acute and repeated PBM, administered in this study using 808 nm transcranial laser treatment (TLT), would increase cortical PCr/β-NTP ratios.
Materials and Methods
Subjects
Subjects in this study were four healthy adult female beagle dogs (Canis lupus familiaris) aged 49–64 months weighing 12.4–14.2 kg. Blood work (complete blood count and clinical chemistry) for all subjects was normal at the beginning and at the end of the study. Subjects were cohoused and maintained at Toxikon Corporation (Bedford, MA) on a 12-h light–12-h dark cycle and were provided ad libitum water and food (Teklad 8755 Dog Food; Harlan Laboratories, Madison, WI). Before each of two 31P MRS imaging sessions, subjects were fasted overnight to prevent anesthesia-induced food regurgitation/aspiration. Subjects were transported by a climate-controlled van to McLean Hospital (Belmont, MA) for imaging studies. The distance between the institutions is about 10 miles and one-way transport time was less than 30 min. After scan sessions and recovery from anesthesia, subjects were returned to Toxikon by the climate-controlled van. Subjects were observed daily throughout the study to assess whether study procedures induced adverse or other effects. All research procedures conformed with the NIH Guide for Care and Use of Laboratory Animals, National Research Council (2011). The study was conducted under Institutional Animal Care and Use Committee approvals at McLean Hospital and at Toxikon Corporation.
Magnetic resonance imaging and spectroscopy
For imaging and spectroscopy sessions, subjects were sedated with acepromazine (0.025 mg/kg) followed by butorphanol (0.5–1 mg/kg), then anesthetized with propofol (5 mg/kg), intubated, and then maintained initially with a constant rate of propofol infusion [0.1–0.4 mg/(kg·min)] during scan setup. Subjects then were switched over to isoflurane (1–2%) and breathable air (1.0 L/min) for the remainder of the scan sessions. Magnetic resonance imaging (MRI) and 31P MRS were conducted using a 9.4 Tesla system with a 29-cm horizontal bore and a 10 G/cm gradient (Varian/Agilent Direct Drive scanner). A half-volume surface coil of 10 cm active length was used to acquire MRI in three planes for MRS voxel localization. The 31P MRS voxel (8 × 10 × 12 mm, nominal voxel volume = 0.96 cm3) was positioned over the dorsal anterior cingulate cortex, directly under the cranial area, to which TLT was applied (Fig. 1). 31P MRS scans were acquired using the ISIS (Image-Selected In vivo Spectroscopy) single-voxel spectroscopy pulse sequence.4031P MRS parameters were repetition time (TR) = 3000 ms, mixing time (TM) = 10 ms, excitation: 150 μs 90° hard pulse (8 kHz excitation bandwidth) centered on the γ-NTP peak, 8 kHz acquisition bandwidth, 8192 complex points, 128 averages (16 phase cycles), and total acquisition time per scan ∼6½ min. Hyperbolic secant 180° pulses (3000 μs) were used for voxel localization. A typical 31P MRS spectrum is shown in Fig. 2. A pre-TLT baseline 31P MRS spectrum was acquired. Then, anesthetized subjects were removed from the scanner to an adjacent treatment room and administered a TLT as described below. Next, subjects were repositioned in the scanner and underwent three additional 31P MRS scans over a 2-h interval (anesthesia time limit), to determine whether TLT administration acutely altered 31P MRS measures. Diligent care was taken when repositioning subjects for all scans to maintain head orientations, using internal landmark serial alignment procedures analogous to those we have used in humans,41 to maximize 31P MRS cortical voxel overlap between scans. The complete staging of scans and TLTs in this study is illustrated in Fig. 3.
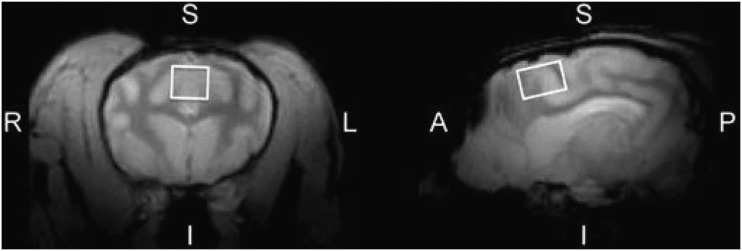
FIG. 1.
Representative magnetic resonance spectroscopy voxel placement in the beagle anterior cingulate cortex. Left panel: Coronal MRI slice (R, right; L, left; S, superior; I, inferior). Right panel: Sagittal MRI slice (A, anterior; P, posterior). The voxel placement is illustrated as a white box. MRI, magnetic resonance imaging.
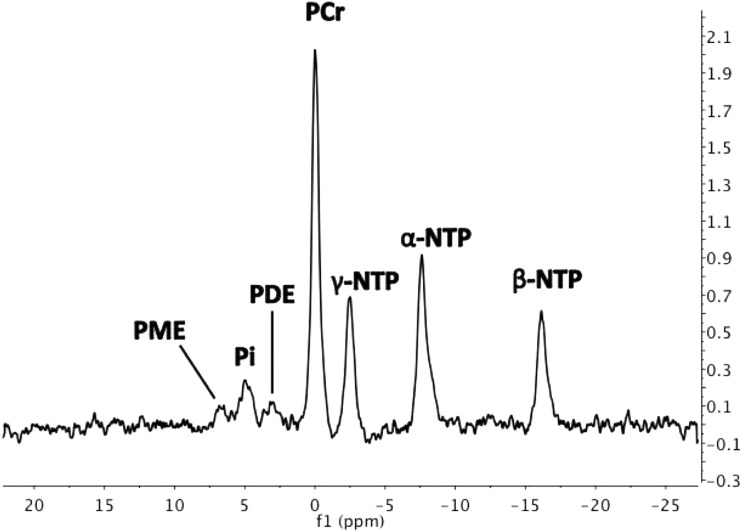
FIG. 2.
Representative 9.4 Tesla anterior cingulate cortex 31P MRS spectrum with peaks for PME and PDE, Pi, PCr, β-NTP (primarily adenosine triphosphate), γ-NTP, and α-NTP (respectively). 31P MRS, phosphorus magnetic resonance spectroscopy; α-NTP, α-nucleoside triphosphate; β-NTP, β-nucleoside triphosphate; γ-NTP, γ-nucleoside triphosphate; PCr, phosphocreatine; PDE, phosphodiesters; Pi, inorganic phosphate; PME, phosphomonoesters.
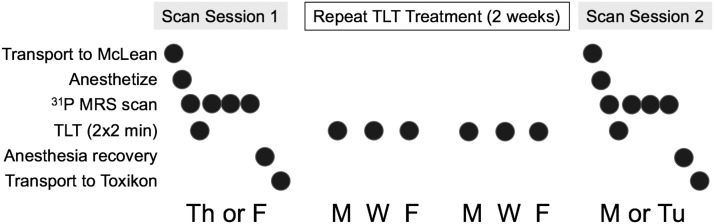
FIG. 3.
Experiment staging. Subjects were transported to McLean Hospital on a Th or F for the first imaging session, during which subjects were anesthetized, underwent a baseline 31P MRS scan, were administered TLTs, and then underwent three additional post-TLT 31P MRS scans. Then, subjects were recovered from anesthesia and transported to their housing location at Toxikon. Over the next 2 weeks, awake subjects were administered identical TLTs on M, W, and F, and then were transported to McLean Hospital to undergo a second scan session identical to the first session on M or Tu. F, Friday; M, Monday; Th, Thursday; TLT, transcranial laser treatment; Tu, Tuesday; W, Wednesday.
Laser photobiomodulation treatment
Before each laser treatment, subjects had their dorsal scalp hair shaved using a #30 blade clippers. After baseline scans, the PhotoThera Research Laser System (RD0010, Carlsbad, CA) was used to deliver continuous wave laser output (808 ± 10 nm, near-infrared wavelength) using a laser diode with power output ranging between 4.2 and 5.8 W. The nominal beam diameter was 22 mm for 2-min exposures each at the anterior and posterior cranium midline locations, for a total of 4 min of treatment. This dosage has been estimated to deliver 4–10 mW/cm2 to the dura mater, with the total dose administered per single two-site treatment ranging from 3.6 to 9.1 J. The cumulative dose of all two-site treatments (n = 8/subject) ranged from 29.2 to 73.0 J. The PhotoThera Research Laser System is similar to the system used in human stroke trials and by Cassano et al. to document TLT-induced clinical improvements in humans with major depression.25 TLT administered with an 808 nm near infrared (NIR) wavelength has been demonstrated to penetrate up to 4 cm in human cadaver brain tissue.42 Thus, this protocol provided nearly complete cortical and at least partial subcortical PBM in the beagle brain. Subjects and staff wore infrared light-filtering goggles during TLT to protect against eye damage.
After completing the first scan session, subjects were transported back to Toxikon, where they underwent six repeat TLTs (one treatment each on Mondays, Wednesdays, and Fridays for 2 weeks). Repeat TLTs utilized the same procedures and instruments as described above, except that subjects were not anesthetized for these TLTs. After repeat TLTs were completed, subjects were brought back to McLean Hospital for a second 31P MRS scan session, using identical anesthesia and imaging procedures as in the first scan. The baseline 31P MRS spectrum acquisitions began on average (standard deviation, SD) 80.0 ± 12.9 h (range 67–93 h) after the last repeated TLT. TLT power output averaged (SD) 5.23 ± 0.036 and 5.20 ± 0.042 W on the first and second scan sessions, respectively, and averaged 5.18 ± 0.145 W during the six repeat TLT sessions.
MRS processing and statistical analyses
31P MRS spectra were phased, baselined, and fitted in MestReNova using the Levenberg–Marquardt algorithm. The modified Henderson–Hasselbalch formula was used to calculate pH.43 31P MRS data were analyzed with two-way analysis of variance, treating scan session (session 1 versus session 2) and within session (serial MRS acquisitions) as factors. The PCr/β-NTP ratio was the primary dependent variable. In addition, PCr, β-NTP, and pH also were examined individually post hoc to test for within- and between-scan session differences in these measures. Statistical analyses were carried out in STATA 14.
Results
We observed no adverse or other effects of TLT when administered to anesthetized or awake subjects either during or after treatments.
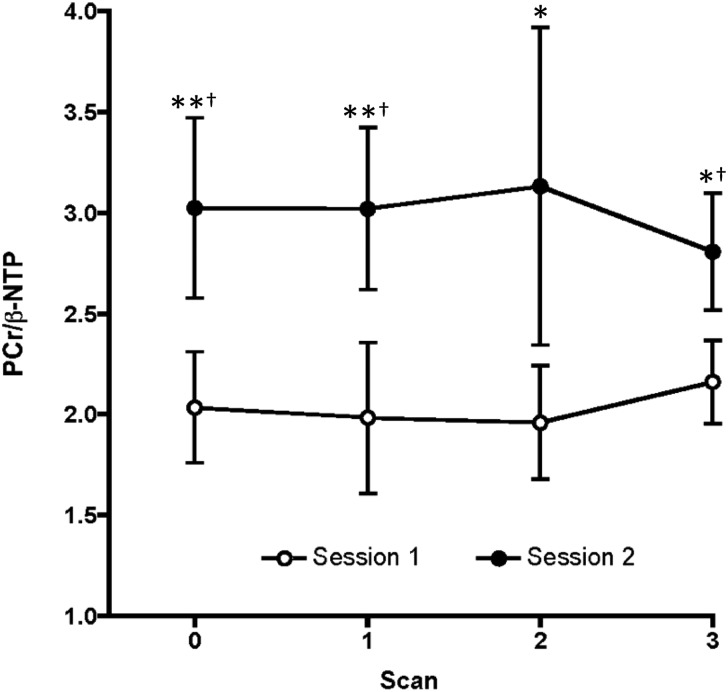
We did not detect any acute effects of TLT on PCr/β-NTP ratios or on any other 31P MRS measure in either scan session (Fig. 4). By contrast, following repeat TLTs, we detected higher PCr/β-NTP ratios during scan session 2 (F(1, 24) = 56.96, p < 0.0001 [F-test]; Fig. 4). Post hoc testing indicated significantly higher PCr/β-NTP ratios at all session 2 time points when compared with session 1 measures (Fig. 4). The between-session PCr/β-NTP effect was driven by a PCr increase (F(1, 24) = 101.96, p < 0.0001), with no change in β-NTP level.
FIG. 4.
PCr/β-NTP ratios during both scan sessions. Shown are means and standard deviations of measurements made at each scan in all four subjects. *p < 0.05 in post hoc comparison of session 2 versus session 1 scan values. **p < 0.01 in post hoc comparison of session 2 versus session 1 scan values. †Statistically significant session difference after Bonferroni correction.
Discussion
We found an increased cortical PCr/β-NTP ratio after 2 weeks of repeated TLT, an effect driven by elevated PCr levels. To our knowledge, this is the first report of PBM effects using in vivo 31P MRS methods. In muscle, elevated PCr levels increase tissue energy efficiency by depressing resting-state mitochondrial respiration44 and by enabling increased muscle performance on demand.45 A similar effect also has been reported in several PBM studies.46 Since the brain evolved to support robust metabolic responses in active neurons and circuits while minimizing resting global energy expenditure,47 a PBM-induced increase in brain PCr levels could help supply the on-demand energy necessary for rapid circuit activation, thereby improving brain function, as reported previously.10,21–28 PCr elevations in the heart and brain have been associated with preconditioning stimuli,48–50 and preconditioning effects of PBM have been described in multiple tissues.1,13,46 Accordingly, it is possible that PBM-associated elevations in the PCr/β-NTP ratio and PCr we detected at the scan session 2 baseline could reflect a cortical preconditioning effect of TLT.
Oral creatine supplementation in humans also has been associated with a PCr/β-NTP ratio increase,38 however, in that study, the increase was attributed to decreased β-NTP (ATP) levels with no PCr change. A prior creatine supplementation study also reported β-NTP reductions and marginal PCr increases.37 Thyroid hormone also targets COX and enhances its activity by displacing inhibitory ATP from regulatory subunit Va,51,52 and it lowered PCr levels among depressed subjects who responded to treatment.34 Thus, several treatments that target COX appear to differentially modulate ATP and PCr levels.
Our inability to detect acute 31P MRS changes in either scan session after TLT may be attributable to the relatively brief time frame, 2 h, over which we assessed TLT effects. While few time course studies of PBM effects exist, published studies in cultured muscle cells and intact muscle fibers first reported significant ATP increases at 3 h after brief (90 sec) PBM, with ATP increases peaking at 6 h poststimulation and remaining elevated for up to 24 h.7,8 Thus, TLT-induced cortical 31P MRS changes may require more than 2 h to evolve. If the 31P MRS changes we observed are mediated by COX protein synthesis, which involves bigenomic coordination of transcription, translation, and coordinated assembly of 3 mitochondrial genome-encoded and 10 nuclear genome-encoded protein subunits53,54 that could contribute to a slow evolution of metabolite changes. Our finding of increased PCr/β-NTP ratios and PCr levels in the baseline scan of scan session 2, more than 65 h after the last repeat TLT, could indicate that TLT effects persist well beyond the 24-h time frame reported previously.7,8 Prolonged beneficial effects of a single acute PBM treatment on general affect and/or mood and anxiety have been reported for up to 2 weeks in healthy human subjects27 and for up to 4 weeks in depressed subjects,24 consistent with the possibility that TLT brain effects persist. Alternatively, repeated TLT, which in depressed subjects has been associated with mood and anxiety improvements for up to 4 weeks25 and with improved executive function and learning and memory up to 8 weeks in TBI patients,26 may have persisting beneficial effects.
Recent studies in transgenic mice either with global COX knockouts55 or targeted COX knockdown within highly energized fast-spiking inhibitory interneurons56 report impairments in stress responsivity,55 sociability, and sensory gating.56 Accordingly, treatments capable of increasing COX expression or function such as PBM may not only improve mitochondrial function but also may blunt stress responsivity, which could contribute to PBM's apparently beneficial effects in people with stress-sensitive disorders such as major depression.24,25
Our study has some limitations, including its small sample size. This limitation is, in part, mitigated by our use of a within-subjects repeated measures design, which increases power to detect biological effects. In addition, this initial study did not include a sham TLT group as a placebo condition.
Conclusions and Summary
Our findings are consistent with a number of literature reports of PBM effects and with known COX control and bioenergetic regulatory mechanisms, suggesting that repeat TLT can increase cortical PCr/β-NTP ratios and PCr levels, which could be beneficial in a number of brain disorders. Because 31P MRS is noninvasive, it may be useful in future animal and human studies aiming to further characterize the bioenergetic effects of PBM in the brain and in other tissues.
Acknowledgments
This study was supported by a research grant from PhotoThera, Inc. (Carlsbad, CA). Support also was provided, in part, by the NIH grant S10 RR019356 and by the Counter-Drug Technology Assessment Center, an office within the Office of National Drug Control Policy, via Contract Number DBK39-03-C-0075 awarded by the Army Contracting Agency. The content of the information does not necessarily reflect the position or the policy of the Government and no official endorsement should be inferred. We thank Scott Delapp for technical contributions to this study.
Author Disclosure Statement
Dr. Mintzopoulos and Mr. Gillis declare that no competing financial interests exist. Dr. Kaufman received a sponsored research agreement from PhotoThera, Inc., to conduct this study. Dr. Tedford was an employee of PhotoThera, Inc., at the time this study was conducted, and now is an employee of LumiThera, Inc.
References
- 1.Wong-Riley MT, Liang HL, Eells JT, et al. . Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 2005;280:4761–4771 [DOI] [PubMed] [Google Scholar]
- 2.Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez-Lima F. In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochem Photobiol 2010;86:673–680 [DOI] [PubMed] [Google Scholar]
- 3.Passarella S, Casamassima E, Molinari S, et al. . Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett 1984;175:95–99 [DOI] [PubMed] [Google Scholar]
- 4.Karu T, Pyatibrat L, Kalendo G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 1995;27:219–223 [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett 2002;323:207–210 [DOI] [PubMed] [Google Scholar]
- 6.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res 2010;1306:100–105 [DOI] [PubMed] [Google Scholar]
- 7.Ferraresi C, de Sousa MV, Huang YY, Bagnato VS, Parizotto NA, Hamblin MR. Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med Sci 2015;30:1259–1267 [DOI] [PubMed] [Google Scholar]
- 8.Ferraresi C, Kaippert B, Avci P, et al. . Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochem Photobiol 2015;91:411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Taboada L, Yu J, El-Amouri S, et al. . Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J Alzheimers Dis 2011;23:521–535 [DOI] [PubMed] [Google Scholar]
- 10.Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis 2012;32:741–752 [DOI] [PubMed] [Google Scholar]
- 11.Kubota J. Effects of diode laser therapy on blood flow in axial pattern flaps in the rat model. Lasers Med Sci 2002;17:146–153 [DOI] [PubMed] [Google Scholar]
- 12.Mii S, Kim C, Matsui H, et al. . Increases in central retinal artery blood flow in humans following carotid artery and stellate ganglion irradiation with 0.6 to 1.6 microm irradiation. J Nippon Med Sch 2007;74:23–29 [DOI] [PubMed] [Google Scholar]
- 13.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med 2010;42:566–576 [DOI] [PubMed] [Google Scholar]
- 14.Greco M, Guida G, Perlino E, Marra E, Quagliariello E. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun 1989;163:1428–1434 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Song S, Fong CC, Tsang CH, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol 2003;120:849–857 [DOI] [PubMed] [Google Scholar]
- 16.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 2005;23:355–361 [DOI] [PubMed] [Google Scholar]
- 17.Konstantinović LM, Jelić MB, Jeremić A, Stevanović VB, Milanović SD, Filipović SR. Transcranial application of near-infrared low-level laser can modulate cortical excitability. Lasers Surg Med 2013;45:648–653 [DOI] [PubMed] [Google Scholar]
- 18.Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. Role of low-level laser therapy in neurorehabilitation. PM R 2010;2(Suppl 2):S292–S305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 2012;40:516–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol 2013;86:447–457 [DOI] [PubMed] [Google Scholar]
- 21.Michalikova S, Ennaceur A, van Rensburg R, Chazot PL. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol Learn Mem 2008;89:480–488 [DOI] [PubMed] [Google Scholar]
- 22.Ando T, Xuan W, Xu T, et al. . Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One 2011;6:e26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Alberico SL, Moges H, De Taboada L, Tedford CE, Anders JJ. Pulsed light irradiation improves behavioral outcome in a rat model of chronic mild stress. Lasers Surg Med 2012;44:227–232 [DOI] [PubMed] [Google Scholar]
- 24.Schiffer F, Johnston AL, Ravichandran C, et al. . Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 2009;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassano P, Cusin C, Mischoulon D, et al. . Near-infrared transcranial radiation for major depressive disorder: proof of concept study. Psychiatry J 2015;2015:352979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naeser MA, Zafonte R, Krengel MH, et al. . Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma 2014;31:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013;230:13–23 [DOI] [PubMed] [Google Scholar]
- 28.Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol (in press) doi: 10.1111/jnp.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed Laser Surg 2012;30:231–233 [DOI] [PubMed] [Google Scholar]
- 30.Salgado AS, Zângaro RA, Parreira RB, Kerppers II. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med Sci 2015;30:339–346 [DOI] [PubMed] [Google Scholar]
- 31.Kato T, Murashita J, Shioiri T, Hamakawa H, Inubushi T. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996;8:417–422 [DOI] [PubMed] [Google Scholar]
- 32.Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 2008;294:R585–R593 [DOI] [PubMed] [Google Scholar]
- 33.Mandal PK, Akolkar H, Tripathi M. Mapping of hippocampal pH and neurochemicals from in vivo multi-voxel 31P study in healthy normal young male/female, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis 2012;31(Suppl 3):S75–S86 [DOI] [PubMed] [Google Scholar]
- 34.Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry 2008;63:1127–1134 [DOI] [PubMed] [Google Scholar]
- 35.Kaufman MJ, Pollack MH, Villafuerte RA, et al. . Cerebral phosphorus metabolite abnormalities in opiate-dependent polydrug abusers in methadone maintenance. Psychiatry Res 1999;90:143–152 [DOI] [PubMed] [Google Scholar]
- 36.Kondo DG, Sung YH, Hellem TL, et al. . Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord 2011;135:354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyoo IK, Kong SW, Sung SM, et al. . Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res 2003;123:87–100 [DOI] [PubMed] [Google Scholar]
- 38.Pan JW, Takahashi K. Cerebral energetic effects of creatine supplementation in humans. Am J Physiol Regul Integr Comp Physiol 2007;292:R1745–R1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogborn DI, Smith KJ, Crane JD, et al. . Effects of creatine and exercise on skeletal muscle of FRG1-transgenic mice. Can J Neurol Sci 2012;39:225–231 [DOI] [PubMed] [Google Scholar]
- 40.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci 1987;508:333–348 [DOI] [PubMed] [Google Scholar]
- 41.Kaufman MJ, Henry ME, Frederick BdeB, et al. . Selective serotonin reuptake inhibitor discontinuation syndrome is associated with a rostral anterior cingulate choline metabolite decrease: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry 2003;54:534–539 [DOI] [PubMed] [Google Scholar]
- 42.Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med 2015;47:312–322 [DOI] [PubMed] [Google Scholar]
- 43.de Graaf RA. In Vivo NMR Spectroscopy, 2nd ed. New York: Wiley-Interscience, 2007; pp. 80–82 [Google Scholar]
- 44.Walsh B, Tonkonogi M, Söderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 2001;537:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiedermann D, Schneider J, Fromme A, Thorwesten L, Möller HE. Creatine loading and resting skeletal muscle phosphocreatine flux: a saturation-transfer NMR study. MAGMA 2001;13:118–126 [DOI] [PubMed] [Google Scholar]
- 46.Agrawal T, Gupta GK, Rai V, Carroll JD, Hamblin MR. Pre-conditioning with low-level laser (light) therapy: light before the storm. Dose Response 2014;12:619–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001;21:1133–1145 [DOI] [PubMed] [Google Scholar]
- 48.Kobara M, Tatsumi T, Matoba S, et al. . Effect of ischemic preconditioning on mitochondrial oxidative phosphorylation and high energy phosphates in rat hearts. J Mol Cell Cardiol 1996;28:417–428 [DOI] [PubMed] [Google Scholar]
- 49.Iwata O, Iwata S, Bainbridge A, et al. . Supra- and sub-baseline phosphocreatine recovery in developing brain after transient hypoxia-ischaemia: relation to baseline energetics, insult severity and outcome. Brain 2008;131:2220–2226 [DOI] [PubMed] [Google Scholar]
- 50.Bravo C, Kudej RK, Yuan C, et al. . Metabolomic analysis of two different models of delayed preconditioning. J Mol Cell Cardiol 2013;55:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold S, Goglia F, Kadenbach B. 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur J Biochem 1998;252:325–330 [DOI] [PubMed] [Google Scholar]
- 52.Kadenbach B, Hüttemann M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion 2015;24:64–76 [DOI] [PubMed] [Google Scholar]
- 53.Wong-Riley MT. Bigenomic regulation of cytochrome c oxidase in neurons and the tight coupling between neuronal activity and energy metabolism. Adv Exp Med Biol 2012;748:283–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhar SS, Johar K, Wong-Riley MT. Bigenomic transcriptional regulation of all thirteen cytochrome c oxidase subunit genes by specificity protein 1. Open Biol 2013;3:120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Picard M, McManus MJ, Gray JD, et al. . Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A 2015;112:E6614–E6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inan M, Zhao M, Manuszak M, et al. . Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis 2016;93:35–46 [DOI] [PubMed] [Google Scholar]