Abstract
Recent viral outbreaks highlight the need for reliable, yet broadly deployable diagnostics for detection of epidemic and emerging pathogens. In this study we designed and optimized methods to visually detect viral nucleic acid by isothermal amplification and SYBR dye intercalation. We designed and tested loop-mediated isothermal amplification (LAMP) primers and lyophilized reactions to optimize the detection of Zaire Ebola Virus (ZEBOV) and further evolved the LAMP platform to allow room-temperature storage for deployment in resource limited settings. Our results demonstrated excellent sensitivity and specificity for viral nucleic acid sequences with lower limits of detection of less than 100 copies. Moreover, lyophilized reaction mixtures retained activity for prolonged periods under dry conditions at room temperature. This approach offers a way for detection of emerging viruses in resource limited settings.
Keywords: Ebola, Lyophilized, Reverse-transcriptase, Nucleic-acid-amplification
Introduction
The challenges in controlling the 2014 Ebola outbreak in Western Africa and now the Zika virus in the western hemisphere highlight the need for simple, rapid diagnostic tests suitable for field use in resource limited settings (Cenciarelli et al., 2015). Potential approaches to point-of-care testing include nucleic acid amplification testing, solid phase immunoassays, and portable, cost effective platforms for existing technologies. Of these potential approaches, isothermal nucleic acid amplification tests have several attractive features, including ability to detect small amounts of pathogen, high pathogen specificity, low cost, and relative ease of assay set-up (Fakruddin et al., 2013).
Of the available nucleic acid amplification tests, reverse-transcriptase loop-mediated isothermal amplification (RT-LAMP) is promising. RT-LAMP technology uses loop-forming oligonucleotides in combination with a strand-displacing polymerase to allow rapid and robust target amplification under isothermal conditions, thus avoiding the need for a thermocycler (Notomi et al., 2000). It has been effective in the amplification of nucleic acid from a variety of human pathogens, including HIV, Dengue, H7N9 Influenza, Hantaan, and Ebola (Curtis et al., 2008; Teoh et al., 2013; Liu et al., 2014; Kurosaki et al., 2007; Hu et al., 2015). However, a significant limitation of the assay is the need for cold storage of reaction components, which is often infeasible in resources limited settings (Njiru, 2012). The ability to lyophilize reagents to promote long term storage may overcome this limitation. Additionally, the RT-LAMP oligonucleotide design requires extensive regions of target homology (Evenson et al., 2012), making detection of polymorphic genomes challenging. For instance, the previously reported RT-LAMP assay for ZEBOV includes numerous primer-template mismatches, which could negatively affect the sensitivity to divergent ZEBOV isolates (Fig. 1) (Kurosaki et al., 2007).
Fig. 1. ZEBOV RT-LAMP target region.
Positional nucleotide frequency in an alignment of 143 ZEBOV isolates is demonstrated on the y axis. Points are color coded, where green = A, red = T, black = G, and blue = C. The regions targeted by RT-LAMP oligonucleotides in the present study (A) and in a previously reported ZEBOV RT-LAMP assay (B) are depicted at the top of the plot. Red bars in panel A indicate the sites on inosine incorporation.
In the current work, we present the optimization of a RT-LAMP assay for ZEBOV with the ability to visually detect a diverse array of ZEBOV isolates. We also report the development of a method for lyophilization of the RT-LAMP reaction mixture, allowing for extended room temperature storage.
Materials and methods
2.1. Test construct pZEBOV-L design
An alignment of 143 full-length ZEBOV sequences was obtained from the Los Alamos National Laboratory (LANL, http://hfv.lanl.gov), which contains isolates from 1976, 1995, 2007, and 2014 outbreaks. In the alignment, a region of high-conservation was observed within the L gene corresponding to ZEBOV G3770.2 coordinates 13131–13601. This fragment was synthesized and cloned into pIDT-Blue, which contains a downstream T3 promoter oriented in the antisense direction (Integrated DNA Technologies (IDT), Coralville, Iowa, USA).
2.2. Ebola Zaire RNA synthesis
To create virus-like negative-sense RNA, in vitro transcription was performed using pZEBOV-L as a template. First, pZEBOV-L was digested with PvuII, giving rise to a 935 bp fragment encompassing the L fragment described above along with the downstream T3 promoter site. This plasmid fragment was gel purified and 200 ng was used as the template for in vitro transcription using the Maxiscript T3 kit according to the manufacturer’s instructions (Life Technologies Ambion, Grand Island, NY, USA). Subsequently, DNAse digestion was performed using double the enzyme amount and double the incubation time recommended by the manufacturer. RNA product was then purified using the RNeasy kit (Qiagen) and quantified using the Qubit fluorimeter (Life Technologies). Next plasmid (pCMV-3Tag-4a, T3/T7 flanked insert) containing a homologous region in the L-gene of the Marburg virus (pMARV-L) was constructed and tested to confirm EBOV specificity. First, pMARV-L was digested with BAMHI to linearize MARV-L. This template was then used for in vitro transcription to generate negative and positive strand RNA using the Maxiscript T3/T7 kit according to the manufacturer’s instructions (Life Technologies Ambion, Grand Island, NY, USA).
2.3. Primer design
LAMP primers were designed using PrimerExplorer v4 (https://primerexplorer.jp) and the target sequence described above. Three LAMP primer sets were generated, designated 1, 18 and 29. Primer set 1 was able to accommodate a single loop primer, whereas 18 and 29 could accommodate two. The primer sets were compared against the ZEBOV alignment noted above, and any nucleotide that was divergent from the primer sequence in >1/143 sequences was replaced with an inosine residue. The resulting primer sequences are found in Table 1. Primers were synthesized by Integrated DNA Technologies; FIP and BIP primers were PAGE purified to minimize truncated oligonucleotides.
Table 1.
LAMP primer sequences.
| Primer name | Sequence |
|---|---|
| F3-1 | AGTATCTACTACCACAATATCGG |
| B3-1 | CCATGATGCTTGATGCAATA |
| FIP-1 | AGTCGGATAAGGCAATTTTCCGTCTCATTGAAAGAGAAAGAGTTG |
| BIP-1 | GTTCAAACACTTTGTGAAGCTCTGCACGTTCCGTAACTACCAT |
| Loop-B | GGTCTTGCTAAAGCATTTCCTAGC |
| F3-18 | ACITTCGGAAAATTGCCTTA |
| B3-18 | ICTAAATGCAAGATTGTATTTCTCT |
| FIP-18 | TACCATCATATTGCTAGGAAATGCTCCGACTCGCAATGTTCAA |
| BIP-18 | GAAAGCTTATTGCATCAAGCATCAAICTACTCCCTCTAACTGT |
| LoopA-18 | GCIAACAGAGCTTCACAAAGTGT |
| LoopB-18 | TGGCACCACICAIGTGATGA |
| F3-29 | TTATCCGACTCGCAATGT |
| B3-29 | ICTAAATGCAAGATTGTATTTCTCT |
| FIP-29 | CCGTAACTACCATCATATTGCTAGGCAAACACTTTGTGAAGCTCTG |
| BIP-29 | CGTGAICAAAAAGAAAGCTTATTGCAICTACTCCCTCTAACTGT |
| LoopA-29 | GCTTTAGCAAGACCATCAGCIAA |
| LoopB-29 | ATCAAGCATCATGGCACCAC |
Primer sets compared against the ZEBOV alignment, any nucleotide that was divergent from the primer sequence in >1/143 sequences was replaced with an inosine residue. 3 primer sets with perfect identity to >99% of the ZEBOV isolates in the LANL alignment.
2.4. RT-LAMP reaction
Reactions were performed in 25 µL volume unless otherwise indicated in the text, and were comprised of 20 mM Tris-HCl, 10 mM (NH4)2SO4, 10 mM KCl, 8 mM MgSO4, 0.1% Triton X-100, 0.8 M betaine, 1.4 mM dNTPs, 1.6 µM each FIP/BIP primers (PAGE purified), 0.2 µM each F3/B3 primers, 0.4 µM each LoopF/LoopB primers, 0.32U/µL BST2.0 (New England Biosciences (NEB), Ipswich, MA, USA), 0.6U/µL RTx (NEB). Reactions were incubated at 63–65 °C for the indicated time, 60 min for most experiments. For electrophoresis detection of LAMP products, 2 µL of the reaction volume was resolved on 2.5% agarose with ethidium bromide. For UV-lamp detection of positive reactions, SybrSafe dye (Life Technologies, (Z)-4-((3-Methylbenzo[d]thiazol-2(3H)-ylidene)methyl)-1-propylquinolin-1-ium 4-methylbenzenesulfonate) was added to the reaction mix during setup, to a final concentration of 10 × (a 1:2500 dilution of the manufacturer’s stock solution) (Gill and Ghaemi, 2008). After incubation, tubes were trans-illuminated with a hand-held UV lamp. For real time detection of LAMP products, SybrSafe dye was added during reaction setup as above, and reactions were then run on an ABI 7500 real-time thermocycler at 64 °C for 60 min with fluorescence readings taken every 2 min.
2.5. Lyophilized RT-LAMP reaction
LAMP reaction mix for lyophilization was comprised of 20 mM Tris-HCl, 10 mM (NH4)2SO4, 50 mM KCl, 8 mM MgSO4, 0.1% Tween-20, 0.8 M betaine, 1.4 mM dNTPs, 1.6 µM each FIP/BIP primers (PAGE purified), 0.2 µM each F3/B3 primers, 0.4 µM each LoopF/LoopB primers, 0.32U/µL BST2.0 (NEB), 0.6U/µL RTx (NEB), 0.4U/µL SUPERase in RNase inhibitor (Life Technologies), to which 50% D-trehalose was added to a final concentration of 10%. This master mix was aliquoted into 50 or 75 µL aliquots and frozen at −20 °C. Prior to freeze drying, tubes containing reaction mix were cooled in liquid nitrogen. Tubes were then freeze-dried at −105 °C at a pressure of <0.02m Bar for 2 h. Freeze dried reaction mix was stored at room temperature in desiccant-containing vessels. At the indicated time intervals, freeze-dried reaction mix was reconstituted in water and RT-LAMP reaction was carried out as described at −105 °C at a pressure of <0.02m Bar for 2 h. Freeze dried reaction mix was stored at room temperature in desiccant-containing vessels. At the indicated time intervals, freeze-dried reaction mix was reconstituted in water and RT-LAMP reaction was carried out as described above, using 106 RNA template copies.
3. Results
3.1. RT-LAMP assay design
To identify a ZEBOV genomic target for amplification, we analyzed an alignment of 143 whole genome sequences, including isolates from 1976 to the present. From this alignment, a highly conserved region in the L protein coding region was identified, corresponding to reference NC_002549 positions 13135–13599. This region was synthesized and cloned, and the resulting plasmid was used as a template for in vitro transcription of the sense strand, giving rise to a small negative-sense RNA that was used for assay development and optimization.
We next designed LAMP primers capable of recognizing a wide breadth of ZEBOV sequences. By targeting a highly-conserved genomic region and substituting degenerate bases at highly polymorphic sites, we designed 3 primer sets with perfect identity to >99% of the ZEBOV isolates in the LANL alignment (Table 1). This approach generated primers with better ZEBOV genome homology than previously reported primers (Fig. 1) (Kurosaki et al., 2007). While all 3 primers were capable of RNA template amplification, primer set 29 demonstrated the best reaction kinetics during a 90-min experiment based on time-to-threshold at less than 30 min compared to over 30 min for the other primer sets (Supplemental Fig. 1). Primer set 29 was used for the remainder of the experiments. BLAST analysis of primer set 29 was performed and found no significant cross reaction. These in silico findings were confirmed by RT-LAMP reaction with our EBOV primer mix using RNA generated in vitro from a homologous region of MARV and EBOV L-gene (Fig. 2).
Fig. 2. RT-LAMP specificity. Gel electrophoresis of EBOV vs MARV T3 and T7.
RT-LAMP with EBOV primer mix demonstrated only amplification of EBOV-L without amplification of either the T3 or T7 transcribed MARV-L RNA, confirming in silico findings of primer specificity.
3.2. Visual detection of ZEBOV RNA using RT-LAMP
Prior studies have taken advantage of the large amount of pyrophosphate generated during the reaction to detect positive reactions by turbidity (Mori et al., 2001) or by dequenching of manganese-bound calcein dye (Tomita et al., 2008). Unfortunately, the visual effect is not always clear with these approaches, requiring considerable judgement on the part of the observer. The use of the SYBR Green I intercalating dye has been reported (Tao et al., 2011; Zhou et al., 2014), but the dye inhibits the LAMP reaction and thus must be added after the reaction is complete, risking contamination of the work area. To overcome this limitation, we explored the use of other DNA intercalating dyes. Interestingly, we found that the cyanine dye SYBR Safe did not inhibit the reaction, and when added during reaction setup produced a high signal-to-noise ratio. Visualization of SYBR Safe-containing reactions using a handheld UV lamp allowed for easy, unambiguous determination of positive reactions, with equivalent sensitivity as gel electrophoresis (Fig. 3A and B middle panel). Visualization turbidity and with a hand-held blue LED light source was also possible, but with inferior ease of detection (Fig. 3B top and bottom panel). SybrSafe with hand-held UV lamp, turbidity visualization with handheld blue LED light source and gel electrophoresis were qualitatively similar methods for detection.
Fig. 3. Determination of positive reactions by DNA Gel electrophoresis and SYBR safe-containing LAMP reactions.
A. DNA Gel Electrophoresis of LAMP reactions with varying concentrations of ZEBOV nucleic acid. B. Visualization of SYBR Safe-containing reactions by handheld UV lamp determination, with equivalent sensitivity to gel electrophoresis. top panel turbidity, middle panel hand-held UV light, bottom panel blue-spectrum LED.
3.3 Real time detection of ZEBOV RNA by RT-LAMP
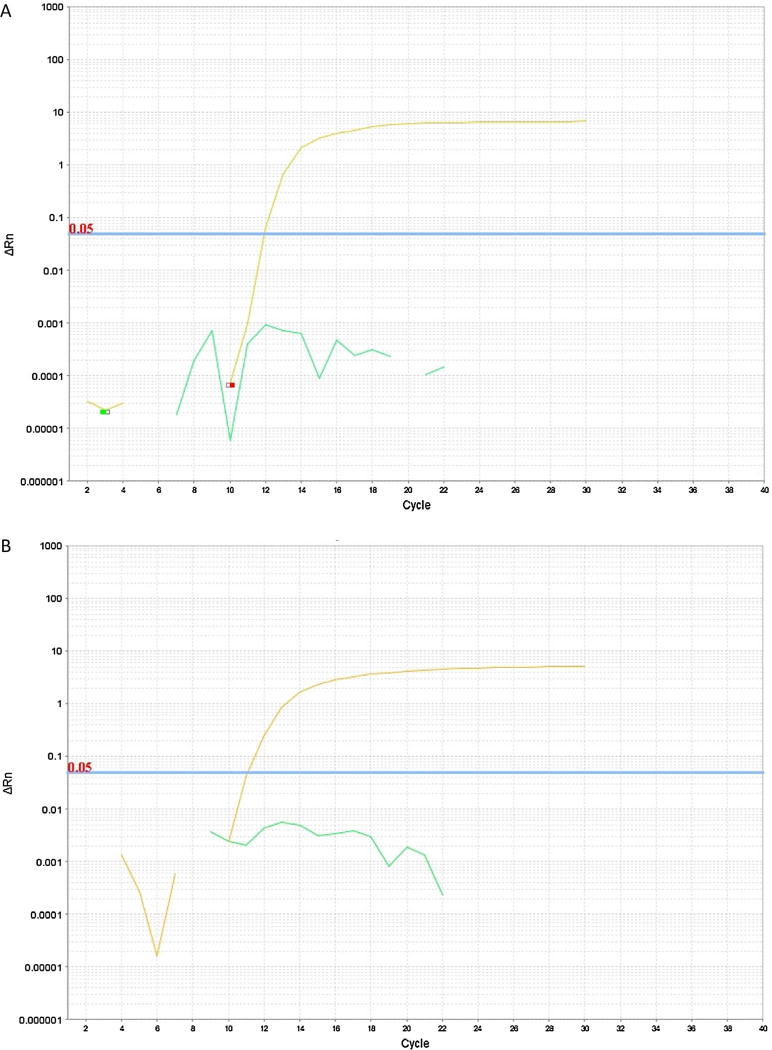
In order to optimize reaction conditions and to determine the kinetics of the ZEBOV RT-LAMP assay, we sought to develop a real-time detection approach. Given the success of SYBR-Safe dye for reaction visualization, we applied the same approach using a real-time thermocycler, and observed exponential amplification with an end-point fluorescence 102–103 times brighter than baseline. This allowed optimization of reaction conditions, including primer set, temperature, magnesium concentration, and enzyme concentration. With optimized parameters, we observed amplification of RNA template with rapid kinetics, with detection of product as fast as 15 min, and completion of exponential-phase amplification within 30 min (Fig. 4A). Under these conditions, we reliably detected 10–100 copies of RNA template (Fig. 4B). In probit analysis, the limit of detection with a 95% probability was 208 viral copies on 9 runs of the plasmid standard (Supplemental Fig. 2). To evaluate the impact of human sera on RT-LAMP, 10% volume heat inactivated serum was added to reactions with no difference in time to threshold observed (Fig. 5).
Fig. 4. Amplification kinetics of ZEBOV RT-LAMP.
RT-LAMP reaction in triplicate using decreasing template copy numbers as indicated. Amplification curves are shown in panel A, and time-to-positive is shown in panel B. One out of three triplicate reactions using 10 template copies was positive (denoted by solid dot in panel A and asterisk in panel B).
Fig 5. Impact of Human Sera on RT-LAMP Reaction.
A) No serum. B) 2.5% heat-inactivated serum. Addition of up to 10% human sera to the RT-LAMP reaction did not significantly impact the RT-LAMP reaction.
3.4 Development of stabilized, freeze-dried RT-LAMP
One limitation of the LAMP assay in resource-limited settings is the need for cold storage of reaction components. To create a stabilized form of the reaction, we attempted lyophilization of a RT-LAMP reaction mixture, which contained all reaction components except Sybr Safe. We found that addition of the non-reducing sugar D-trehalose stabilized the reagents during lyophilization; however, the lyophilized reaction mixture rapidly lost activity during room-temperature storage (Fig. 6, open circles). Hypothesizing that glycerol present in the enzyme preparations might prevent adequate water removal, we dialyzed the mixtures prior to lyophilization. This resulted in a dehydrated reagent mixture that remained stable at room temperature with little loss of activity (Fig. 6, closed circles).
Fig 6. Stability of lyophilized ZEBOV RT-LAMP reaction mixtures.
Lyophilized reaction mixtures prepared using standard enzyme preparation (open circles) and dialyzed enzyme preparations (closed circles) were stored at room temperature for the indicated amount of time before being reconstituted and run using 1E6 copies of RNA template.
4. Discussion
RT-LAMP represents an attractive approach for rapidly deployable emerging pathogen detection. In the present work, we report a RT-LAMP system for detection of ZEBOV that addresses some of the major limitations of the assay which have hindered deployment in resource limited settings.
Simple readout of positive reactions represents one limitation of RT-LAMP technology. Prior studies have taken advantage of turbidity created by calcium pyrophosphate precipitation to detect positive reactions, but the presence of turbidity is not always obvious, and requires considerable subjective interpretation. One attractive approach is to use DNA intercalating fluorescent dyes, however these have been demonstrated to inhibit the LAMP reaction. Thus the use of these dyes requires introduction following completion of the reaction, which increases workflow complexity and risks contamination of the work area. We have tested several DNA intercalating dyes, and interestingly found that the cyanine dye SYBR Safe does not inhibit the reaction when added at set-up, and allows for unambiguous detection of positive reactions, as well as real time detection. The reason for this is unclear, but we hypothesize that higher DNA affinity dyes such as SYBR green may block efficient strand displacement by the BST polymerase.
Another significant barrier to the use of RT-LAMP in resource limited settings is the need for cold storage of reaction components. In the present work, we found that RT-LAMP reaction mixes can be lyophilized and maintain excellent activity with room temperature storage. We found that two key modifications of the reaction components were necessary for stability of the lyophilized reagents. Firstly, addition of the non-reducing sugar trehalose was needed, an approach that has been previously employed for PCR reaction lyophilization (Chua et al., 2011). Secondly, dialysis of the enzyme preparations used in lyophilized reaction mixtures was found to be crucial for stabilization of the reaction. We hypothesize that dialysis removed the highly hygroscopic glycerol from the enzyme preparations, allowing effective water removal during lyophilization process. Success of this lyophilization technique depends on multiple environmental conditions, like temperature, humidity, storage conditions and will require real world field testing in subsequent studies.
Finally, we report a novel amplification target in the ZEBOV genome, which is highly conserved among isolates from major outbreaks from 1979 to the present, and represents a significant improvement over previously reported LAMP targets. Our approach highlights how rapid whole genome sequencing and public sequence databases could allow an approach such as RT-LAMP to be rapidly modified and deployed in an outbreak setting.
5. Conclusions
In summary, this study sought to develop methods that were better suited to detect viral infection in resource limited settings. We have detailed the development and evaluation procedures for ZEBOV LAMP including primer sequences and assay methods, new visualization and real time detection procedures, and development of stabilized, freeze-dried RT-LAMP reagents. We hypothesize methods such as these could be used to detect ZEBOV and other emerging pathogens such as Zika virus with deployment in settings and at scales unattainable by current methodologies.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Department of Veterans Affairs and the National Institutes of Health [grant numbers AI100665, EB015365, AI036214, AI007384]; the James B. Pendleton Charitable Trust. This publication resulted [in part] from research supported by the University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program [P30 AI036214], which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jviromet.2017.02.013.
References
- Cenciarelli O, Pietropaoli S, Malizia A, Carestia M, D’Amico F, Sassolini A, DiGiovanni D, Rea S, Gabbarini V, Tamburrini A, Palombi L, Bellecci C, Gaudio P. Ebola virus disease 2013–2014 outbreak in west Africa: an analysis of the epidemic spread and response. Int. J. Microbiol. 2015;2015:769121. doi: 10.1155/2015/769121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AL, Elina HT, Lim BH, Yean CY, Ravichandran M, Lalitha P. Development of a dry reagent-based triplex PCR for the detection of toxigenic and non-toxigenic Vibrio cholerae. J. Med. Microbiol. 2011;60(Pt. 4):481–485. doi: 10.1099/jmm.0.027433-0. [DOI] [PubMed] [Google Scholar]
- Curtis KA, Rudolph DL, Owen SM. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP) J. Virol. Methods. 2008;151:264–270. doi: 10.1016/j.jviromet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Evenson WE, Boden LM, Muzikar KA, O’Leary DJ. 1H and 13C NMR assignments for the cyanine dyes SYBR Safe and thiazole orange. J. Org. Chem. 2012;77(23):10967–10971. doi: 10.1021/jo3021659. [DOI] [PubMed] [Google Scholar]
- Fakruddin M, Mannan KS, Chowdhury A, Mazumdar RM, Hossain MN, Islam S, Chowdhury MA. Nucleic acid amplification: alternative methods of polymerase chain reaction. J. Pharm. Bioallied Sci. 2013;5:245–252. doi: 10.4103/0975-7406.120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P, Ghaemi A. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids. 2008;27:224–243. doi: 10.1080/15257770701845204. [DOI] [PubMed] [Google Scholar]
- Hu D, Hao L, Zhang J, Yao P, Zhang Q, Lv H, Gong X, Pan X, Cao M, Zhu J, Zhang Y, Feng Y, Wang C. Development of reverse transcription loop-mediated isothermal amplification assays to detect Hantaan virus and Seoul virus. J. Virol. Methods. 2015;221:68–73. doi: 10.1016/j.jviromet.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Kurosaki Y, Takada A, Ebihara H, Grolla A, Kamo N, Feldmann H, Kawaoka Y, Yasuda J. Rapid and simple detection of Ebola virus by reverse transcription-loop-mediated isothermal amplification. J. Virol. Methods. 2007;141:78–83. doi: 10.1016/j.jviromet.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Liu J, Nian QG, Li J, Hu Y, Li XF, Zhang Y, Deng YQ, Zhu SY, Zhu QY, Qin ED, Jiang T, Qin CF. Development of reverse-transcription loop-mediated isothermal amplification assay for rapid detection of novel avian influenza A (H7N9) virus. BMC Microbiol. 2014;14:271. doi: 10.1186/s12866-014-0271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001;289:150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- Njiru ZK. Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl. Trop. Dis. 2012;6:e1572. doi: 10.1371/journal.pntd.0001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao ZY, Zhou HY, Xia H, Xu S, Zhu HW, Culleton RL, Han ET, Lu F, Fang Q, Gu YP, Liu YB, Zhu GD, Wang WM, Li JL, Cao J, Gao Q. Adaptation of a visualized loop-mediated isothermal amplification technique for field detection of Plasmodium vivax infection. Parasit Vectors. 2011;4:115. doi: 10.1186/1756-3305-4-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh BT, Sam SS, Tan KK, Johari J, Danlami MB, Hooi PS, Md-Esa R, AbuBakar S. Detection of dengue viruses using reverse transcription-loop-mediated isothermal amplification. BMC Infect. Dis. 2013;13:387. doi: 10.1186/1471-2334-13-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- Zhou D, Guo J, Xu L, Gao S, Lin Q, Wu Q, Wu L, Que Y. Establishment and application of a loop-mediated isothermal amplification (LAMP) system for detection of cry1Ac transgenic sugarcane. Sci. Rep. 2014;4:4912. doi: 10.1038/srep04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.