Abstract
Background
Dry eye syndrome is a disorder of the tear film that is associated with symptoms of ocular discomfort. Punctal occlusion is a mechanical treatment that blocks the tear drainage system in order to aid in the preservation of natural tears on the ocular surface.
Objectives
To assess the effects of punctal plugs versus no punctal plugs, different types of punctal plugs, and other interventions for managing dry eye.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 11), MEDLINE Ovid (1946 to 8 December 2016), Embase.com (1947 to 8 December 2016), PubMed (1948 to 8 December 2016), LILACS (Latin American and Caribbean Health Sciences Literature Database) (1982 to 8 December 2016), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 18 November 2012 ‐ this resource is now archived), ClinicalTrials.gov (www.clinicaltrials.gov; searched 8 December 2016), and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en; searched 8 December 2016). We did not use any date or language restrictions in the electronic searches for trials. We also searched the Science Citation Index‐Expanded database and reference lists of included studies. The evidence was last updated on 8 December 2016
Selection criteria
We included randomized and quasi‐randomized controlled trials of collagen or silicone punctal plugs in symptomatic participants diagnosed with aqueous tear deficiency or dry eye syndrome.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We contacted study investigators for additional information when needed.
Main results
We included 18 trials (711 participants, 1249 eyes) from Austria, Canada, China, Greece, Japan, Mexico, Netherlands, Turkey, the UK, and the USA in this review. We also identified one ongoing trial. Overall we judged these trials to be at unclear risk of bias because they were poorly reported. We assessed the evidence for eight comparisons.
Five trials compared punctal plugs with no punctal plugs (control). Three of these trials employed a sham treatment and two trials observed the control group. Two trials did not report outcome data relevant to this review. There was very low‐certainty evidence on symptomatic improvement. The three trials that reported this outcome used different scales to measure symptoms. In all three trials, there was little or no improvement in symptom scores with punctal plugs compared with no punctal plugs. Low‐certainty evidence from one trial suggested less ocular surface staining in the punctal plug group compared with the no punctal plug group however this difference was small and possibly clinically unimportant (mean difference (MD) in fluorescein staining score ‐1.50 points, 95% CI ‐1.88 to ‐1.12; eyes = 61). Similarly there was a small difference in tear film stability with people in the punctal plug group having more stability (MD 1.93 seconds more, 95% CI 0.67 to 3.20; eyes = 28, low‐certainty evidence). The number of artificial tear applications was lower in the punctal plug group compared with the no punctal plugs group in one trial (MD ‐2.70 applications, 95% CI ‐3.11 to ‐2.29; eyes = 61, low‐certainty evidence). One trial with low‐certainty evidence reported little or no difference between the groups in Schirmer scores, but did not report any quantitative data on aqueous tear production. Very low‐certainty evidence on adverse events suggested that events occurred reasonably frequently in the punctal plug group and included epiphora, itching, tenderness and swelling of lids with mucous discharge, and plug displacement.
One trial compared punctal plugs with cyclosporine (20 eyes) and one trial compared punctal plugs with oral pilocarpine (55 eyes). The evidence was judged to be very low‐certainty due to a combination of risk of bias and imprecision.
Five trials compared punctal plugs with artificial tears. In one of the trials punctal plugs was combined with artificial tears and compared with artificial tears alone. There was very low‐certainty evidence on symptomatic improvement. Low‐certainty evidence of little or no improvement in ocular surface staining comparing punctal plugs with artificial tears (MD right eye 0.10 points higher, 0.56 lower to 0.76 higher, MD left eye 0.60 points higher, 0.10 to 1.10 higher) and low‐certainty evidence of little or no difference in aqueous tear production (MD 0.00 mm/5 min, 0.33 lower to 0.33 higher)
Three trials compared punctal plugs in the upper versus the lower puncta, and none of them reported the review outcomes at long‐term follow‐up. One trial with very low‐certainty evidence reported no observed complications, but it was unclear which complications were collected.
One trial compared acrylic punctal plugs with silicone punctal plugs and the trial reported outcomes at approximately 11 weeks of follow‐up (36 eyes). The evidence was judged to be very low‐certainty due to a combination of risk of bias and imprecision.
One trial compared intracanalicular punctal plugs with silicone punctal plugs at three months follow‐up (57 eyes). The evidence was judged to be very low‐certainty due to a combination of risk of bias and imprecision.
Finally, two trials with very low‐certainty evidence compared collagen punctal plugs versus silicone punctal plugs (98 eyes). The evidence was judged to be very low‐certainty due to a combination of risk of bias and imprecision.
Authors' conclusions
Although the investigators of the individual trials concluded that punctal plugs are an effective means for treating dry eye signs and symptoms, the evidence in this systematic review suggests that improvements in symptoms and commonly tested dry eye signs are inconclusive. Despite the inclusion of 11 additional trials, the findings of this updated review are consistent with the previous review published in 2010. The type of punctal plug investigated, the type and severity of dry eye being treated, and heterogeneity in trial methodology confounds our ability to make decisive statements regarding the effectiveness of punctal plug use. Although punctal plugs are believed to be relatively safe, their use is commonly associated with epiphora and, less commonly, with inflammatory conditions such as dacryocystitis.
Plain language summary
Punctal plugs for dry eye syndrome
What is the aim of this review? The aim of this Cochrane Review was to determine whether punctal plugs, which are inserted into the tear ducts to block tear drainage, can treat dry eye syndrome. Cochrane review authors searched for all relevant studies and identified 18 clinical trials.
Key messages It is unclear whether punctal plugs are effective for treating dry eye syndrome. Punctal plugs may be associated with watery eyes, though the evidence for this finding is weak.
What did we study in this review? Dry eye is a common, chronic condition that affects millions of people around the world. Dry eye sufferers frequently experience burning, foreign body sensation (something in the eye), and blurry vision, which lead them to seek medical care. The typical first‐line treatment for dry eye is over‐the‐counter artificial tears (eye drops). If these fail to relieve symptoms, persons with dry eyes may receive other treatment. Punctal plugs are one type of advanced dry eye treatment; they work by blocking the tear ducts (puncta) of the upper and lower eyelids. Punctal plugs come in several materials, shapes, and sizes.
What are the main results of the review? This review included 18 trials with 711 participants (1249 eyes), most of whom were women. The trials took place from March 1998 to May 2014 and included participants from Austria, Canada, China, Greece, Japan, Mexico, Netherlands, Turkey, the UK, and the USA. The 18 trials differed greatly in design; they compared different types of punctal plugs and reported results in different ways.
The evidence from this review suggests that punctal plugs do not conclusively improve dry eye symptoms. No type of punctal plug used in the trials we examined was significantly better than another for relieving symptoms of dry eye. It is still unclear if punctal plugs are better than oral treatment (oral pilocarpine) or eye drops such as cyclosporine or artificial tears.
The evidence from this review suggests that punctal plugs may be associated with watery eyes and sometimes with more serious conditions such as infection or swelling in the tear sac (part of the eye where tears drain).
The conclusions of this updated review are similar to the original review published in 2010, though 11 new trials were included.
How up‐to‐date is this review? Cochrane review authors searched for trials that were published up to 8 December 2016.
Summary of findings
Summary of findings for the main comparison. Summary of findings: punctal plugs versus no punctal plugs.
| Punctal plugs compared with no punctal plugs for dry eye syndrome | ||||||
|
Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: silicone or collagen punctal plugs Comparison: no punctal plugs (observation or sham treatment) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No punctal plugs | Punctal plugs | |||||
|
Symptomatic improvement Follow‐up: long‐term* A lower score favors punctal plugs |
See comments | See comments | — | 89 (2 RCT) |
⊕⊝⊝⊝ Very lowa,b |
Mansour 2007 reported little or no difference in Ocular and Oral Symptoms score, which ranged from 0 to 10 points (MD ‐0.46 points, 95% CI ‐1.24 to 0.32; eyes = 26). Nava‐Castaneda 2003 reported a slight decrease in symptom score, assumed to range from 0 to 105 points, but it does not seem to be clinically important (MD ‐2.62 points, 95% CI ‐3.32 to ‐1.93; eyes = 61). Yung 2012 reported little or no difference in dry eye symptom score, ranging from 0 to 3 points (MD ‐0.75 points, 95% CI ‐1.53 to 0.02; eyes = 28) |
|
Ocular surface staining Follow‐up: long‐term* A higher value is less advantageous Both Rose Bengal and fluorescein staining scores ranged from 0 to 4, where 0 represented no staining and 4 represented heavy staining |
The mean fluorescein staining score was 1.70 | MD 1.50 lower than observation group (1.88 to 1.12 lower (better) than observation group) |
— | 61 (1 RCT) |
⊕⊕⊝⊝ Lowc,d | — |
|
Aqueous tear production Follow‐up: long‐term* A higher value is more advantageous |
See comments | See comments | — | 28 (1 RCT) |
⊕⊕⊝⊝ Lowc,d | Yung 2012 did not provide quantitative data, but reported that "Schirmer values tended to increase in the plug group after plug insertion; however, the changes did not reach significance" |
|
Tear film stability Follow‐up: 6 months A higher value is more advantageous |
The mean tear film stability was 2.34 seconds | MD 1.93 seconds longer than observation group (0.67 to 3.20 seconds longer (better) than observation group) |
— | 28 (1 RCT) |
⊕⊕⊝⊝ Lowa,b | — |
|
Artificial tear use Follow‐up: long‐term* Fewer applications favors punctal plugs |
The mean number of applications was 3.6 applications | MD 2.70 fewer applications than observation group (3.11 to 2.29 fewer applications than observation group) |
— | 61 (1 RCT) |
⊕⊕⊝⊝ Lowe | — |
|
Adverse events Follow‐up: end of study |
See comments | See comments | — | 146 (4 RCT) | ⊕⊝⊝⊝ Very lowf,g |
Slusser 1998: all adverse events occurred in the punctal plug group, reported 23/28 participants had epiphora, 3/28 participants reported itching in area of plug placement, 1/28 participants had tenderness and swelling of lids with mucous discharge. Spontaneous plug loss occurred in 6/20 eyes with silicone punctal plugs in the Mansour 2007. One or 31 participants receiving collagen and silicone punctal plugs experienced epiphora in the Nava‐Castaneda 2003 study. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardized mean difference; RCT: randomized controlled trial. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for methodological heterogeneity. bDowngraded one level for high risk of detection and attrition bias.
cDowngraded one level for imprecision as indirectness of evidence because the confidence interval was either wide or clinically not important.
dDowngraded one level for high risk of attrition bias.
eDowngraded two levels for high risk of performance, detection, and attrition bias.
fDowngraded two levels for attrition bias.
gDowngraded one level for sparse and inconsistent data, particularly with respect to epiphora.
*We defined long‐term follow‐up as between two months and one year.
Summary of findings 2. Summary of findings: punctal plugs versus cyclosporine.
| Punctal plugs compared with cyclosporine for dry eye syndrome | |||||
|
Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs Comparison: cyclosporine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Cyclosporine | Punctal plugs | ||||
| Symptomatic improvement | Study investigators did not report this outcome at 2 weeks, 1 month, or long‐term. | ||||
|
Ocular surface staining Follow‐up: 6 months Any value greater than zero is abnormal Range: 0 to 4 points; 0 = no staining, 1 = staining of the nasal conjunctiva only, 2 = staining of both the nasal and temporal conjunctiva, 3 = peripheral corneal staining, 4 = central corneal staining |
See comments | See comments | 20 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | The study investigators of Roberts 2007 stated: "there was greater improvement in conjunctival staining with cyclosporine or the combination than with plugs alone." It was unclear whether Rose Bengal or fluorescein staining was used. Also, the study investigators did not specify the time point and so we assumed that their statement applies for 1, 3, and 6 months follow‐up. |
|
Aqueous tear production Follow‐up: 6 months A higher value is more advantageous |
The mean change in aqueous tear production was 1.5 mm/3 min lower than baseline | MD 0.80 mm/3 min higher than cyclosporine group (0.74 lower (better) to 2.34 higher (worse) than cyclosporine group) |
20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The study investigators of Roberts 2007 stated: "There was a greater increase in Schirmer score with plugs, either alone or in combination with cyclosporine." The study investigators did not specify the for which time point and so we assumed that their statement applies for 1, 3, and 6 months follow‐up. |
|
Tear film stability Follow‐up: 6 months |
Study investigators did not report this outcome at 2 weeks, 1 month, or long‐term. | ||||
|
Artificial tear use Follow‐up: 6 months range: 1‐5 applications Fewer applications favors punctal plugs |
The mean change in number of applications from baseline was 3.2 more applications than baseline | MD 1.10 applications more than baseline (0.04 fewer to 2.24 more applications than cyclosporine group) |
20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The study investigators of Roberts 2007 stated: "decreased frequency of artificial tears was greatest for combination therapy and least for punctal plugs." The study investigators did not specify the for which time point and so we assumed that their statement applies for 1, 3, and 6 months follow‐up. |
|
Adverse events Follow‐up: end of study |
See comments | See comments | 22 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Roberts 2007 reported 1/11 participants experienced plug displacement in the plug group, while 1/11 participants experienced a burning sensation in the cyclosporine group. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomized controlled trial. | |||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | |||||
aDowngraded one level for imprecision of results (wide confidence intervals).
bDowngraded two levels for high risk of detection, performance, attrition, and other bias.
*We defined long‐term follow‐up as between two months and one year.
Summary of findings 3. Summary of findings: punctal plugs versus oral pilocarpine.
| Punctal plugs compared with oral pilocarpine for dry eye syndrome | ||||||
|
Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs Comparison: oral pilocarpine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral pilocarpine | Punctal plugs | |||||
|
Symptomatic improvement Follow‐up: 3 months The study investigators defined improvements in subjective ocular symptoms as an improvement of >55 mm for responses to the eye questionnaire on a 100 mm visual analog scale. A RR less than one favors punctal plugs. |
897 per 1000 | 619 per 1000 (439 to 852) |
RR 0.69 (0.49 to 0.95) |
55 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
|
Ocular surface staining Follow‐up: 3 months A higher value is less advantageous Range: van Bijsterveld schema, which is on a scale of 0 to 9 points |
The mean change in Rose Bengal staining score was 1.00 lower (better) than baseline in the right eye | MD 0.10 higher (worse) than oral pilocarpine group (0.56 lower (better) to 0.76 higher (worse) than oral pilocarpine group) |
— | 55 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| The mean change in Rose Bengal staining score was 1.10 lower (better) than baseline in the left eye | MD 0.60 higher (worse) than oral pilocarpine group (0.10 to 1.10 higher (worse) than oral pilocarpine group) |
— | 55 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | |
|
Aqueous tear production Follow‐up: 3 months A higher value more advantageous |
The mean change in aqueous tear production was 0.30 mm/5 min higher (better) than baseline in the right eye | MD 0.10 mm/5 min lower (worse) than oral pilocarpine group (0.53 mm/5 min lower (worse) to 0.33 mm/5 min higher (better) than oral pilocarpine group) |
— | 55 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| The mean change in aqueous tear production was 1.2 mm/5 min higher (better) than baseline in the left eye | MD 0.50 mm/5 min lower (worse) than oral pilocarpine group (1.06 mm/5 min lower (worse) to 0.06 mm/5 min higher (better) than oral pilocarpine group) |
— | 55 (1 RCT) | — | ||
| Tear film stability | Study investigators did not report this outcome. | |||||
| Artificial tear use | Study investigators did not report this outcome. | |||||
|
Adverse events Follow‐up: 3 months |
See comments | See comments | — | 55 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Tsifetaki 2003 reported: "commonly reported adverse events were headache, increased sweating,nausea, and vomiting in the pilocarpine group, while 1 patient in the inferior puncta occlusion group had blepharitis and was withdrawn from the study." pg 1204 |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for high risk of performance and detection bias as the participant and outcome assessors were not masked to the treatment groups and the self‐reported symptomatic improvement might be biased. bDowngraded one level for imprecision as the confidence interval is either wide or clinically not important.
*We defined long‐term follow‐up as between two months and one year.
Summary of findings 4. Summary of findings: punctal plugs versus artificial tears.
| Punctal plugs compared with artificial tears for dry eye syndrome | ||||||
|
Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs Comparison: artificial tears | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Artificial tears | Punctal plugs | |||||
|
Symptomatic improvement Follow‐up: 3 months The study investigators defined improvements in subjective ocular symptoms as an improvement of >55 mm for responses to the eye questionnaire on a 100 mm visual analog scale. A RR greater than one favors punctal plugs. Both studies used different symptomatic improvement score; one study used the Ocular Surface Disease Index that ranged from 0 points = never to 4 points = all the time. The second study use the sum of scores for dryness, foreign body sensation, and visual fatigue; each score had a different range, but a higher score corresponded to more symptoms. |
286 per 1000 |
654 per 1000 (343 to 1000) |
RR 2.29 (1.2 to 4.38) |
54 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b,c | — |
| The mean symptomatic improvement score ranged from 15.9 to 26.92 | SMD 0.88 lower (better) than artificial tears group (1.24 to 0.51 lower (better) than artificial tears group) |
— | 130 (2 RCTs) |
— | ||
|
Ocular surface staining Follow‐up: 3 months Range: 0 = absent; 1 = trace; 2 = mild; 3 = moderate; 4 = severe A higher value is worse |
The mean change in Rose Bengal staining score was 1.0 point lower (better) than baseline in the right eye | MD 0.10 points higher (worse) than artificial tears group (0.56 lower (better) to 0.76 higher (worse) than artificial tears group) |
— | 55 (1 RCT) | ⊕⊕⊝⊝ Lowd | — |
| The mean change in Rose Bengal staining score was 1.1 lower (better) than baseline in the left eye | MD 0.60 points higher (worse) than artificial tears group (0.10 to 1.10 higher (worse) than artificial tears group) |
— | 55 (1 RCT) | — | ||
|
Aqueous tear production Follow‐up: long‐term* A higher value is more advantageous |
The mean change in aqueous tear production was 0.2 mm/5 min higher (better) than baseline in the right eye | MD 0.00 mm/5 min higher (better) than artificial tears group (0.33 mm/5 min lower (worst) to 0.33 mm/5 min higher (better) than artificial tears group) |
— | 54 (1 RCT) | ⊕⊕⊝⊝ Lowc,e | — |
| The mean change in aqueous tear production was 0.6 mm/5 min higher (better) than baseline in the left eye | MD 0.10 mm/5 min higher (better) than artificial tears group (0.35 mm/5 min lower (worst) to 0.55 mm/5 min higher (better) than artificial tears group) |
— | 54 (1 RCT) | — | ||
| The mean aqueous tear production ranged from 4.89 to 8.95 mm/ 5 min | MD 2.16 mm/ 5 min higher (better) (1.41 to 2.90 mm/ 5 min higher (better) than artificial tears group) |
— | 130 (2 RCTs) |
— | ||
|
Tear film stability Follow‐up: long‐term* A higher value is more advantageous |
The mean tear film stability ranged from 3.24 to 6 seconds | MD 1.02 seconds longer (better) than artificial tears group (0.60 to 1.44 seconds longer (better) than artificial tears group) |
— | 130 (2 RCT) | ⊕⊕⊕⊝ Moderatec | — |
| Artificial tear use | Outcome not relevant to this comparison | |||||
|
Adverse events (punctate epithelial keratopathy) Follow‐up: end of study |
375 per 1000 | 499 per 1000 (214 to 1000) |
RR 1.33 (0.57 to 3.12) | 54 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | Tsifetaki 2003 reported: "four patients had mild headache, of whom three also presented with nausea, vomiting, and sweating" (p 1205) and "one patient in the inferior puncta occlusion group had blepharitis and was withdrawn from the study" (p 1204) |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for high risk of performance and detection bias as participants and outcome assessors were unmasked to the assigned treatment group and might influence the self‐reported symptomatic improvement. bDowngraded one level for high unexplained statistical heterogeneity or inconsistent results. cDowngraded one level for imprecision of results as the confidence interval was either wide or clinically not important. dDowngraded two levels for imprecision of results as the confidence interval was wide and clinically not important. eDowngraded for inconsistent results.
*We defined long‐term follow‐up as between two months and one year.
Summary of findings 5. Summary of findings: punctal plugs in the upper versus lower puncta.
| Punctal plugs occluded in the upper puncta compared with the lower puncta for dry eye syndrome | ||||||
|
Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs occluded in the upper puncta Comparison: punctal plugs occluded in the lower puncta | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lower puncta | Upper puncta | |||||
|
Symptomatic improvement Follow‐up: long‐term* |
Study investigators did not report this outcome. | |||||
|
Ocular surface staining Follow‐up: long‐term* |
Study investigators did not report this outcome. | |||||
|
Aqueous tear production Follow‐up: long‐term* |
Study investigators did not report this outcome. | |||||
|
Tear film stability Follow‐up: long‐term* |
Study investigators did not report this outcome. | |||||
| Artificial tear use | Study investigators did not report this outcome. | |||||
| Adverse events | See comments | See comments | See comments | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Chen 2010 reported "no complication was observed in dry eye patients or control subjects during the period of this study." It is unclear which complications were collected. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level for potential bias as there was unclear risk of selection, performance, detection, attrition, and reporting bias. bDowngraded one level for imprecision of results as the confidence interval was wide.
*We defined long‐term follow‐up as between two months and one year.
Summary of findings 6. Summary of findings: acrylic versus silicone punctal plugs.
| Acrylic punctal plugs compared with silicone punctal plugs for dry eye syndrome | ||||||
|
Patient or population: mostly women with dry eye syndrome Settings: eye clinics Intervention: acrylic punctal plugs Comparison: silicone punctal plugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silicone punctal plugs | Acrylic punctal plugs | |||||
|
Symptomatic improvement Follow‐up: 11 weeks Range: 0 to 70 points A visual analog scales 10 cm in length for seven symptoms: dryness, grittiness, foreign body sensation, pain, stinging, burning, and itching was used. A lower score favors acrylic punctal plugs |
The mean symptomatic improvement score was 21.9 points | MD 0.90 points higher than silicone punctal plug group (6.94 points lower than silicone punctal plug group to 8.74 points higher than silicone punctal plug group) |
— | 36 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
|
Ocular surface staining Follow‐up: 11 weeks A higher value is less advantageous Range: 0 to 3 points |
The mean fluorescein staining score was 1.63 points | MD 0.43 points higher (worst) than silicone punctal plug group (1.61 lower (better) than silicone punctal plug group to 2.47 higher (worse) than silicone punctal plug group) |
— | 36 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| The mean Rose Bengal staining score was 0.55 points | MD 0.45 points higher (worst) than silicone punctal plug group (0.09 lower (better) than silicone punctal plug group to 0.99 higher (worst) than silicone punctal plug group) |
— | — | |||
|
Aqueous tear production Follow‐up: 11 weeks A higher value is more advantageous |
The mean aqueous tear production was 3.8 mm/5 min | MD 1.07 mm/5 min higher than silicone punctal plug group (1.62 lower than silicone punctal plug group to 3.76 higher than silicone punctal plug group) |
— | 36 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Authors did not report the time interval in which the Schirmer's test 1 without anesthesia was performed. We assumed it was done over 5 minutes. |
|
Tear film stability Follow‐up: 11 weeks A higher value is more advantageous |
The mean tear film stability was 3.5 seconds | MD 0.36 seconds longer than silicone punctal plug group (1.22 seconds shorter than silicone punctal plug group to 1.94 longer than silicone punctal plug group) |
— | 36 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
|
Artificial tear use Follow‐up: 11 weeks Range: 1‐5 applications Fewer applications favors punctal plugs |
The mean number of applications was 4.67 applications | MD 0.11 more applications than silicone punctal plug group (2.32 fewer applications than silicone punctal plug group to 2.54 more applications than silicone punctal plug group) |
— | 36 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
|
Adverse events Follow‐up: 11 weeks |
See comments | See comments | — | 36 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | 1 acrylic punctal plug participant experienced epiphora, 1 silicone punctal plug participant experienced intermittent ocular irritation, and 2 silicone and 1 acrylic punctal plug participants experienced temporary foreign body sensation. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomized controlled trial. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for imprecision of results (wide confidence intervals).
bDowngraded one level for risk of bias as selection, attrition and reporting bias were judged to be unclear.
*We defined long‐term follow‐up as between two months and one year.
Summary of findings 7. Summary of findings: intracanalicular versus silicone punctal plugs.
| Intracanalicular punctal plugs compared with silicone punctal plugs for dry eye syndrome | ||||||
|
Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: intracanalicular punctal plugs Comparison: silicone punctal plugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silicone punctal plugs | Intracanalicular punctal plugs | |||||
|
Symptomatic improvement Follow‐up: 3 months Subjective dry eye symptoms for each eye was reported; investigators measured soreness, scratching, grittiness, dryness and/or burning using a 100 mm visual analog scale (VAS; 0 mm = no symptoms, 100 mm = maximum intensity) |
The mean symptomatic improvement was 38.5 points | The mean difference in symptomatic improvement was 3.10 points lower (14.97 lower to 8.77 higher) | — | 57 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
|
Ocular surface staining Follow‐up: 3 months A higher value is less advantageous Range: 0 to 3 points; 0 = no staining and 3 = most intense staining |
The mean Rose Bengal staining score was 3.0 | MD 0.20 higher than observation group (0.71 lower to 1.11 higher than silicone punctal plugs group) |
— | 57 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| The mean fluorescein staining score was 0.7 points | MD 0.40 points higher than observation group (0.04 lower to 0.84 higher than silicone punctal plugs group) |
— | 57 (1 RCT) | — | ||
|
Aqueous tear production Follow‐up: 3 months |
Study investigators did not report this outcome. | |||||
|
Tear film stability Follow‐up: 3 months |
Study investigators did not report this outcome. | |||||
|
Artificial tear use Follow‐up: 3 months Fewer applications favors intracanalicular plugs |
The mean artificial tear use was 6.4 | MD 1.30 fewer applications (4.04 fewer to 1.44 more applications) |
— | 57 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Adverse events | Study investigators did not report on adverse events. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for imprecision of results (wide confidence intervals). bDowngraded one level for high risk of attrition bias.
*We defined long‐term follow‐up as between two months and one year.
Summary of findings 8. Summary of findings: collagen versus silicone punctal plugs.
| Collagen punctal plugs compared with silicone punctal plugs for dry eye syndrome | ||||||
|
Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: collagen punctal plugs Comparison: silicone punctal plugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silicone punctal plugs | Collagen punctal plugs | |||||
|
Symptomatic improvement Follow‐up: 3 months The Canadian Dry Eye Assessment range from 0 to 48 points; where less than 5 points was normal, 5 to 15 points was mild, 20 to 25 points was moderate, 30 to 48 points was severe. |
The mean symptomatic improvement score was 0.25 points | MD 0.81 higher than silicone punctal group (2.94 lower to 4.56 higher than silicone punctal group) |
— | 50 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | — |
|
Ocular surface staining Follow‐up: 3 months Range: 0 to 15 points A higher value is less advantageous |
The mean fluorescein stain score was 2.00 | MD 0.76 lower than silicone punctal group (18.5 lower to 17.0 higher than silicone punctal group) |
— | 50 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | — |
|
Aqueous tear production Follow‐up: 3 months A higher value is more advantageous |
The mean aqueous tear production was 16.89 mm/5 min | MD 0.67 mm/5 min higher than silicone punctal group (17.28 lower to 18.62 higher than silicone punctal group) |
— | 50 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | Authors did not report the time interval in which the Schirmer's test 1 without anesthesia was performed. We assumed it was done over 5 minutes. |
|
Tear film stability Follow‐up: 3 months A higher value is more advantageous |
The mean tear film stability was 4.67 seconds | MD 0.21 seconds higher than silicone punctal group (1.81 lower to 2.23 higher than silicone punctal group) | — | 50 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | — |
|
Artificial tear use Follow‐up: 3 months Fewer applications favors collagen punctal plugs |
The mean number of artificial tear applications was 1.34 applications | MD 0.06 fewer applications (0.23 fewer to 0.12 more applications than silicone punctal group) |
— | 50 (1 RCT) |
⊕⊕⊝⊝ Lowa | — |
|
Adverse events Follow‐up: end of study |
See comments | See comments | See comments | 98 (2 RCT) |
⊕⊝⊝⊝ Very lowa,b | Both studies reported that none of the participants developed adverse events or complications related to punctal plugs. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomized controlled trial. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for imprecision of results as the confidence interval was wide and clinically not important.
bDowngraded one level for risk of bias as risk of bias as we judged selection, performance, detection, attrition, and reporting bias to be unclear.
*We defined long‐term follow‐up as between two months and one year.
Background
Description of the condition
Dry eye syndrome, or keratoconjunctivitis sicca, is defined as "a disorder of the tear film due to tear deficiency or excessive tear evaporation which causes damage to the interpalpebral ocular surface and is associated with symptoms of ocular discomfort" (Lemp 1995; Lemp 1998; Pflugfelder 2000). Dry eye affects 10% to 20% of adults, with 1 million to 4 million affected adults aged 65 to 84 in the USA (AAO 2003; Schein 1997b). Investigators have also estimated dry eye prevalence in Sweden (15%), India (18.4%), Australia (8.6% to 16.3%), and Indonesia (27.5%) (Lee 2002; Lemp 1998; McCarty 1998; Sahai 2005). The condition has been associated with age, sex, Sjögren's syndrome, arthritis, diabetes, corneal transplants, multivitamin use, laser in situ keratomileusis (LASIK), and photorefractive keratectomy (PRK) (Dalzell 2003; De Paiva 2006; Dew 2007; Moss 2000). Symptoms may include redness, burning, itchiness, foreign body sensation, and in severe cases corneal ulceration and bacterial infection (Lemp 1998; Sheppard 2003; Wilson 2003). Assessments of ocular surface damage and measures of tear stability and hyperosmolarity also aid the diagnosis of dry eye syndrome (Lemp 1995). Due to a lack of correlation among self‐reported symptoms and clinical measures, it is difficult to diagnose dry eye precisely (Lemp 1995; Nichols 2004; Schein 1997a).
Artificial tears, the typical initial treatment for patients with dry eye, hydrate the eye and provide short‐term symptomatic relief for affected individuals (Pucker 2016). Preservative‐free artificial tears are preferable for long‐term use because preserved formulations may be toxic to the cornea and conjunctival epithelium (Lemp 1994; Pflugfelder 2000; Pucker 2016). Interventions such as bandage contact lenses (physical coverage of the ocular surface), estrogens (hormone replacement), topical corticosteroids (general immunosuppressant), cyclosporine (immunosuppressant agent that decreases T‐cell production), pilocarpine (cholinergic parasympatheticomimetic agonist), and punctal plugs (lacrimal drainage occlusion device) also have been shown to be effective treatments in selected settings (DEWS 2007a; Freeman 1975; Jehangir 2016Lemp 1994; Pflugfelder 2000; Sall 2000; Sheppard 2003; Wilson 2003).
Description of the intervention
Punctal occlusion is a non‐pharmacological intervention for dry eye when artificial tears do not ameliorate symptoms (Balaram 2001; Freeman 1975; Willis 1987). Semi‐permanent silicone or temporary collagen punctal plugs are inserted into the upper, lower, or both puncta of the affected eye(s) (Lemp 1994). Collagen plugs dissolve within four to seven days, while silicone plugs either dislodge spontaneously or are removed by a physician. Clinicians typically prescribe the silicone punctal plugs after an affected patient has found symptomatic relief with the collagen punctal plugs (Altan‐Yaycioglu 2005). Thermal cautery or argon laser achieves permanent occlusion of the puncta (AAO 2003; Dohlman 1978; Lemp 1994). Similar to punctal occlusion, intracanalicular plugs also block tear drainage, though they act by blocking the canaliculus instead of the punctum (Jehangir 2016).
How the intervention might work
Punctal plugs are believed to block tear drainage by occluding the puncta. Blockage is subsequently thought to aid in the preservation of natural tears and to improve the quality and quantity of the tear film. (Barnard 1996; Dohlman 1978; Tai 2002). The most common side effects of occlusion are epiphora (overflow of tears), inhibited tear clearance, and desensitization of the corneal surface (Lemp 1994; Sheppard 2003; Tai 2002).
Why it is important to do this review
An updated systematic review examining the efficacy of punctal occlusion, specifically punctal plugs, for managing dry eye is necessary. There was appreciable variability in the interventions and study designs of the trials included in the previous systematic review thus precluding quantitative syntheses. The effectiveness of punctal plugs for treating dry eye has not yet been established. This review summarizes the best available evidence for the use of punctal occlusion in the treatment of dry eye.
Objectives
To assess the effects of punctal plugs versus no punctal plugs, different types of punctal plugs, and other interventions for managing dry eye. This is an update to a Cochrane Review initially published in 2010 (Ervin 2010).
Methods
Criteria for considering studies for this review
Types of studies
We selected only randomized and quasi‐randomized controlled clinical trials for inclusion in this review. We considered studies to be quasi‐randomized if the investigators did not use randomization to allocate participants to treatment groups but used techniques intended to allocate patients in an unbiased fashion. Some examples include allocation based on the day of the week, year of birth, or hospital admission number.
Types of participants
We included symptomatic participants who were diagnosed with aqueous tear deficiency or keratoconjunctivitis sicca (dry eye syndrome). There were no restrictions with respect to age, sex, comorbidities, or use of adjunctive therapy.
Types of interventions
We considered clinical trials comparing occlusion of the lower or upper punctum or upper and lower puncta with collagen versus silicone punctal plugs and studies comparing these plugs to other treatments such as artificial tears, pilocarpine, cyclosporine, or diathermy (use of electrodes to heat and contract punctal tissues). We also considered clinical trials using collagen or silicone plugs in conjunction with adjunctive therapies such as artificial tears, as well as trials comparing occlusion versus no treatment, placebo, or sham treatments.
Types of outcome measures
Primary outcomes
The primary outcome was subjective report of symptomatic improvement in dry eye symptoms such as burning and grittiness and other symptoms as defined by included studies at the long‐term follow‐up visit (2‐12 months).
Secondary outcomes
We included secondary outcomes assessed post‐treatment or at other reported time points in this review.
Ocular surface staining, as defined by the mean change in total Rose Bengal score from baseline to follow‐up. We reported change in fluorescein and lissamine green scores where appropriate.
Aqueous tear production, as measured by the mean change in Schirmer I test scores (mm). We included Schirmer I tests performed with anesthesia and Schirmer II tests without anesthesia.
Tear film stability, as measured by the mean change in tear film break‐up time (seconds).
Change in the frequency of artificial tear use, as defined by included studies.
Follow‐up
We assessed the secondary outcomes at two weeks, four weeks, and at long‐term follow‐up. We considered follow‐up at 12 to 16 days to be two weeks follow‐up. We also considered follow‐up between 26 to 30 days to be four weeks follow‐up. We defined long‐term follow‐up as between two months and one year.
Adverse outcomes
We reported adverse outcomes such as epiphora, corneal ulcers, and plug extrusion (total or partial displacement of the punctal plug) where appropriate. We summarized other adverse outcomes reported in included studies.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomized controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 8 December 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 8 December 2016) (Appendix 1);
MEDLINE Ovid (1946 to 8 December 2016) (Appendix 2);
Embase.com (1947 to 8 December 2016) (Appendix 3);
PubMed (1948 to 8 December 2016) (Appendix 4);
LILACS (Latin American and Caribbean Health Science Information database (1982 to 8 December 2016) (Appendix 5);
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 18 November 2012‐ this resource is now archived) (Appendix 6);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 8 December 2016) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched 8 December 2016) (Appendix 8).
In 2016, we revised the searches of electronic databases from the 2010 publication of the original version of this review.
Searching other resources
We searched reference lists of included studies to identify any additional inclusions. We also used the Science Citation Index‐Expanded database to identify studies that may have cited trials included in this review on 8 December 2016. We did not handsearch conference proceedings or journals.
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts resulting from the literature searches according to the inclusion criteria stated above. We classified the abstracts as 'definitely exclude', 'unsure,' or 'definitely include'. We retrieved the full‐text reports corresponding to abstracts classified as 'definitely include' or 'unsure' by either review author and re‐assessed the study for inclusion. We contacted the authors of studies classified as 'unsure' for further information, as required, after examining the full report. We resolved disagreements through discussion. We excluded studies labeled as 'exclude' by both review authors from the review and documented the reasons for exclusion. We assessed studies labeled 'definitely include' for methodological quality.
Data extraction and management
Two review authors independently extracted data using a form developed by Cochrane Eyes and Vision, resolving discrepancies by discussion. One review author entered data into Review Manager 5 (RevMan 5) and a second author verified all values entered (Review Manager 5 2014). When quantitative data were not available, we abstracted data from graphs using GetData Graph Digitalizer.
Assessment of risk of bias in included studies
Two authors independently assessed the included studies for sources of systematic bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We evaluated the studies for the following criteria: sequence generation and allocation concealment before assignment (selection bias), masking of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. We reported the judgment for each criterion as 'yes (low risk of bias)', 'no (high risk of bias)' or 'unclear (information is insufficient to assess)'.
We reported the adequacy of sequence generation and allocation concealment before assignment. Methods of sequence generation considered to be at low risk of bias include references to random number tables or computer generated random numbers and coin tosses. We considered any method of allocation concealment that provided reasonable confidence that the allocation sequence was concealed from participating physicians and patients to confer low risk of bias, for example centralized randomization or use of sequentially numbered opaque envelopes.
We noted masking of outcome assessors by individual study outcomes. Masking of investigators and participants would not have been possible with the interventions being compared in some studies, but we noted it where mentioned.
For incomplete outcome data, we examined rates of follow‐up, reasons for loss to follow‐up and analysis by the principle of intention‐to‐treat (ITT). We assessed whether follow‐up rates for treatment and control arms were similar and whether data were missing for outcomes of interest. We considered studies to be at low risk of bias when there were no missing data and no participants for whom outcome data were not reported, and where all participants, including those who received some or none of the allocated treatment, were included in the analyses of outcomes. We noted the method of data imputation, when appropriate, for included studies.
We considered studies to be at low risk of bias for selective outcome reporting whenever all pre‐specified outcomes of interest in the protocol or register record were consistent with the outcomes specified in the published report.
We examined included studies for other sources of bias and considered studies at low risk of bias when there was no evidence of research misconduct or potential for bias based on study methodology, or when the study was not stopped early due to evidence of harm or benefit. When available, we used trial registration records, source(s) of funding, or conflicts of interest to identify other potential sources of bias.
We resolved disagreements through discussion. We contacted the authors of the studies for additional information on issues that were unclear from the study report. In case of failure to communicate with the primary investigators, or if no response was received within six weeks, we assessed the methodological quality on the basis of the available information.
Measures of treatment effect
We calculated a summary risk ratio for dichotomous outcomes including the primary outcome of symptomatic improvement and reports of adverse events. We verified normality of distributions of continuous outcome data and calculated mean differences for secondary outcomes as follows: mean change in Rose Bengal, fluorescein, and lissamine green staining scores, mean change in tear production as measured by the Schirmer I/Schirmer II test, and mean change in tear film break‐up time.
Unit of analysis issues
We reported the unit of analysis for the included studies in the Results section (Included studies). We did not include cross‐over trials, but if we identify eligible cross‐over trials for inclusion in a subsequent update, we will attempt to extract data from the study reports or request data from the investigators to account appropriately for the study design. If we are unable to retrieve these data, we will incorporate statistical techniques to approximate a paired analysis as outlined in Chapter 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We contacted primary authors of included studies to obtain missing or unclearly reported information such as details regarding study methods, standard deviations not reported with mean values, and data required for an ITT analysis. Whenever there was no response from the primary authors within six weeks, we imputed data where possible using available information such as P values or confidence intervals. We describe the assumptions we made during imputation where appropriate.
Assessment of heterogeneity
We assessed statistical, methodological, and clinical heterogeneity. When including additional studies to this update, we computed the I2 statistic (%) to determine statistical heterogeneity, that is, the proportion of variation due to heterogeneity among effect estimates from individual studies in a meta‐analysis. We considered an I2 value larger than 75% to suggest considerable statistical heterogeneity. We also examined the result of the Chi2 test and the degree of overlap in confidence intervals of included studies because poor overlap of estimates and confidence intervals suggests the presence of heterogeneity. We assessed clinical and methodological heterogeneity by examining variations among included studies with respect to participant characteristics, inclusion/exclusion criteria, and assessments of primary and secondary outcomes.
Assessment of reporting biases
Because fewer than 10 studies were included in any meta‐analysis, we did not assess reporting biases with funnel plots. If we identify more studies in future updates and a meta‐analysis includes data from 10 or more studies, we will assess reporting bias using funnel plots.
Data synthesis
When considering meta‐analysis, we took statistical, methodological, and clinical heterogeneity into consideration. We considered an I2 statistic greater than 75% to suggest considerable statistical heterogeneity, as defined above in 'Assessment of heterogeneity'. When the number of trials was fewer than three, we used a fixed‐effect model, which in these cases provides a more robust estimate of the treatment effect. When meta‐analysis had three or more included trials, we used a random‐effects model to estimate the overall intervention effects when appropriate. When analyzing binary (dichotomous) outcomes, we estimated the risk ratio (RR). For continuous outcomes, we estimated the mean difference (MD).
Subgroup analysis and investigation of heterogeneity
Sufficient data were not available to conduct subgroup analyses. We will use the following guidelines for future updates of this review: if there is considerable statistical heterogeneity and evidence of potential clinical or methodological heterogeneity, and if data are available, we will conduct a subgroup analysis of tear deficient classifications of dry eye as defined by the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes (Lemp 1995).
Sensitivity analysis
We did not perform sensitivity analyses due to insufficient data. We will consider sensitivity analyses during future updates of this review to determine the impact of excluding studies with lower methodological quality or industry funding as well as studies that were unpublished at the time of our review. We also will conduct sensitivity analyses to determine the impact of including quasi‐randomized trials.
Summary of findings table
We used the GRADE approach to evaluate the certainty of evidence for each outcome (GRADEpro 2014). Two review authors independently assessed each outcome as being of very low‐, low‐, moderate‐, or high‐certainty according to five criteria: risk of bias in individual trials, indirectness, heterogeneity, imprecision of estimate (wide confidence intervals), and publication bias. We resolved discrepancies by discussion. We present the main outcomes for each comparison in a 'Summary of findings' table. Since dry eye syndrome is a chronic condition, long‐term follow‐up is the most clinically relevant and patient important time point. Therefore, we included the following seven outcomes at long‐term follow‐up: symptomatic improvement, ocular surface staining with fluorescein, ocular surface staining with Rose Bengal, aqueous tear production, tear film stability, artificial tear use, and adverse events.
Results
Description of studies
We describe the full‐text studies we assessed for inclusion in the 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
In the original 2010 manuscript, the electronic search identified a total of 115 titles and abstracts (Ervin 2010). We retrieved 19 articles for full‐text assessment, and we finally included 6 and excluded 13 for the review. We identified and included one additional study from the list of references in the protocol for this systematic review and from a search of the Science Citation Index‐Expanded database.
On 8 December 2016, the electronic searches yielded an additional 211 records (Figure 1). We assessed 20 full‐text reports from 20 studies. Eleven met our eligibility criteria for inclusion, while one was ongoing. We excluded the remaining eight studies for the reasons noted in 'Characteristics of excluded studies'. Therefore, the updated version of this systematic review includes a total of 18 included studies and 1 ongoing study.
1.
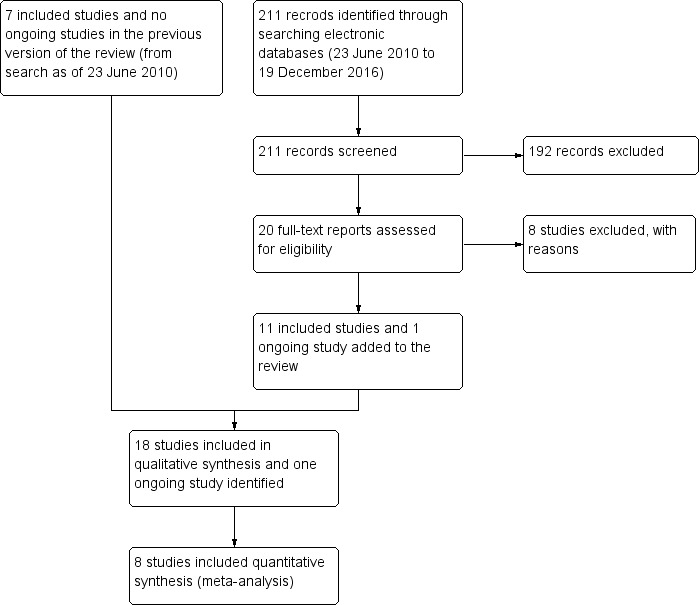
Flow diagram.
Included studies
The 18 studies included in this review enrolled 711 participants (1249 eyes); we describe the studies in the 'Characteristics of included studies' table. The intervention comparisons are summarized in Table 9. Roberts 2007 did not report the number of participants but did report the number of eyes studied.
1. Comparisons.
| 1. Punctal plugs versus observation. | ||
| Lowther 1995 | Collagen intracanalicular plugs were inserted in the upper and lower puncta | Sham treatment |
| Mansour 2007 | Silicone punctal plugs | No occlusion |
| Nava‐Castaneda 2003 | Collagen plus silicone punctal plugs | Sham treatment |
| Roberts 2007 | Bilateral collagen punctal plugs in the lower lids + cyclosporine eye drops to both eyes twice daily | Cyclosporine ophthalmic emulsion 0.05% |
| Slusser 1998 | Silicone punctal plugs in the upper and lower puncta | Sham treatment |
| Yung 2012 | Silicone punctal plugs | Observation |
| 2. Punctal plugs versus cyclosporine. | ||
| Roberts 2007 | Bilateral collagen punctal plugs in the lower lids only | Cyclosporine ophthalmic emulsion 0.05% |
| 3. Punctal plugs versus oral pilocarpine. | ||
| Tsifetaki 2003 | Collagen punctal plugs | Oral pilocarpine |
| 4. Punctal plugs versus artificial tears. | ||
| Feng 2011 | Collagen punctal plugs | Artificial tears |
| Qiu 2012 | Acrylic punctal plugs | Artificial tears |
| Qiu 2013 | Acrylic punctal plugs | Artificial tears |
| Tsifetaki 2003 | Collagen punctal plugs | Artificial tears |
| Zhou 2016 | Thermal Memory hydrophobic acrylic polymer rigid rod punctal plug | Artificial tears |
| 5. Punctal plugs in the lower puncta versus the upper puncta. | ||
| Chen 2010 | Collagen punctal plugs in the lower puncta | Collagen punctal plugs in the upper puncta |
| Farrell 2003 | Collagen punctal plugs in the lower puncta | Collagen punctal plugs in the lower and upper puncta |
| Kaido 2012 | Silcone punctal plugs in the lower puncta | Silcone punctal plugs in the upper puncta |
| 6. Acrylic punctal plugs versus silicone punctal plugs. | ||
| Burgess 2008 | Acrylic punctal plugs | Silicone punctal plugs |
| 7. Intracanalicular plugs versus Silicone punctal plugs. | ||
| Rabensteiner 2013 | Intracanicular | Silicone punctal plugs |
| 8. Collagen punctal plugs versus silicone punctal plugs. | ||
| Altan‐Yaycioglu 2005 | Collagen punctal plugs | Silicone punctal plugs |
| Brissette 2015 | Collagen punctal plugs were inserted in the lower punctum | Silicone punctal plugs were inserted in the lower punctum |
| Excluded comparisons | ||
| Tsifetaki 2003 | Artificial tears | Oral pilocarpine |
The unit of randomization for the included studies was the participant for 11 studies (Altan‐Yaycioglu 2005;Chen 2010;Farrell 2003; Kaido 2012;Nava‐Castaneda 2003; Qiu 2012; Qiu 2013;Rabensteiner 2013; Roberts 2007;Tsifetaki 2003; Yung 2012), the eye for 5 studies (Brissette 2015; Burgess 2008; Lowther 1995;Mansour 2007;Slusser 1998), and was unclear for 2 studies (Feng 2011; Zhou 2016;).
The unit of analysis was the participant for 3 studies as the average of the participant's right and left eyes was ana lysed (Rabensteiner 2013; Roberts 2007;Tsifetaki 2003). The unit of analysis was the eye for 7 studies (Brissette 2015;Feng 2011; Kaido 2012; Lowther 1995; Mansour 2007; Qiu 2013; Slusser 1998) although the study investigators did not discuss whether the analyses accounted for the correlation between eyes of a participant. The unit of analysis was unclear for the remaining 8 studies (Altan‐Yaycioglu 2005;Burgess 2008; Chen 2010; Farrell 2003;Nava‐Castaneda 2003; Qiu 2012; Yung 2012; Zhou 2016).
In this review we included the following comparisons (Table 9).
Punctal plugs versus observation.
Punctal plugs versus cyclosporine.
Punctal plugs versus oral pilocarpine.
Punctal plugs versus artificial tears.
Punctal plugs in the lower puncta versus the upper puncta.
Acrylic punctal plugs versus silicone punctal plugs.
Intracanalicular plugs versus Silicone punctal plugs.
Collagen punctal plugs versus silicone punctal plugs.
Concomitant use of artificial tears was permitted irrespective of the treatment assignment in the Brissette 2015; Burgess 2008;Rabensteiner 2013; Roberts 2007;Tsifetaki 2003; Yung 2012 studies. There was no mention of concomitant artificial tear use in the Altan‐Yaycioglu 2005; Chen 2010; Farrell 2003; Kaido 2012; Lowther 1995; Mansour 2007; Nava‐Castaneda 2003; Slusser 1998 studies. The objective in the Feng 2011, Qiu 2012, Qiu 2013, and Zhou 2016 studies was to compare artificial tears to punctal plugs so only participants assigned to the artificial tear group received this treatment.
For eight of 18 included trials, the unit of analysis was unclear, as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes (Altan‐Yaycioglu 2005; Burgess 2008; Chen 2010; Farrell 2003; Nava‐Castaneda 2003; Qiu 2012; Yung 2012; Zhou 2016). Only one paired‐eye RCT used paired t‐test (Slusser 1998), while the other four paired‐eye RCTs, but did not mention an analysis accounting for correlation between the left and right eye (Brissette 2015; Feng 2011; Lowther 1995; Mansour 2007). The remaining four of 18 trials reported averaging both eyes (Rabensteiner 2013; Roberts 2007), analyzed the right eye (Kaido 2012; Qiu 2013), or reporting the mean outcome of left and right eyes (Tsifetaki 2003).
Excluded studies
We excluded 21 studies, listing them in the 'Characteristics of excluded studies' table along with the reasons for exclusion.
Risk of bias in included studies
Figure 2 presents a summary of the risk of bias judgements for the individual included studies.
2.
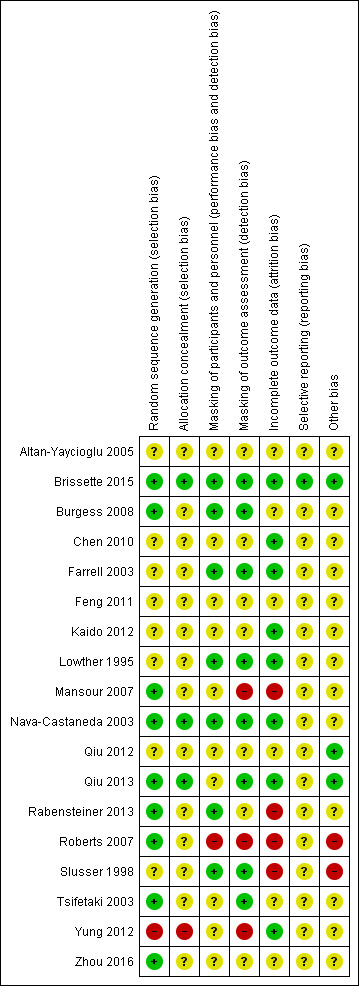
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged the methods of sequence generation to confer low risk of bias for nine studies (Brissette 2015; Burgess 2008; Mansour 2007; Nava‐Castaneda 2003; Qiu 2013; Rabensteiner 2013; Roberts 2007; Tsifetaki 2003; Zhou 2016). All these studies used some form of a computer‐generated randomization scheme. We assessed Yung 2012 as being at high risk of bias because participants were assigned based on their patient ID number; participants with an odd patient ID number were assigned to the punctal plug group, while those with even patient ID numbers were assigned to the non‐punctal plug group. The eight remaining studies did not discuss sequence generation (Altan‐Yaycioglu 2005; Chen 2010; Farrell 2003; Feng 2011; Kaido 2012; Lowther 1995; Qiu 2012; Slusser 1998).
We considered three studies at low risk of bias for allocation concealment: the investigators used opaque envelopes in Brissette 2015 and Qiu 2013, while in Nava‐Castaneda 2003 an external statistical committee prepared random assignments and placed them in sealed envelopes. We rated Yung 2012 at high risk of bias because participants were assigned to treatment groups based upon their patient ID numbers, as described above. The remaining 14 trials did not describe allocation concealment.
Masking (performance bias and detection bias)
Roberts 2007 did not mask participants and personnel, so we considered this study to be at high risk of performance bias. We judged 7 of the 18 included studies to be at low risk of both performance bias and detection bias, as they reported masking both the participants and study personnel (Brissette 2015; Burgess 2008; Farrell 2003; Lowther 1995; Nava‐Castaneda 2003; Rabensteiner 2013; Slusser 1998) although it is not possible to mask the personnel administering punctal plugs. For the remaining studies, the study investigators did not report masking of participants and personnel although given the nature of the interventions compared it was not possible to mask participants and personnel administering the punctal plugs. We judged the remaining studies as having an unclear risk of bias.
Mansour 2007 , Roberts 2007, and Yung 2012 reported that the outcome assessors were not masked, so we rated these studies as being at high risk of detection bias. Mansour 2007 reported that the same investigator performed all measurements and presumably was unmasked to treatment assignment. Roberts 2007 reported that the "medication was dispensed open‐label." In Yung 2012, the study authors informed us through email that the outcome assessors were not masked. We judged 8 of the 18 included studies to be at low risk of detection bias because they reported masking of the outcome assessors (Brissette 2015; Burgess 2008; Farrell 2003; Lowther 1995; Nava‐Castaneda 2003; Qiu 2013; Slusser 1998; Tsifetaki 2003). We judged the remaining studies to be at unclear risk of detection bias; this group included Altan‐Yaycioglu 2005, where the nuclear medicine specialist who evaluated the lacrimal scintigraphy images was masked to treatment assignment; however, lacrimal scintigraphy was not a measurement included in this review. It is unclear whether other outcome assessors were masked in Altan‐Yaycioglu 2005.
Incomplete outcome data
We judged Mansour 2007, Rabensteiner 2013, Roberts 2007, and Slusser 1998 to be at high risk of bias for failure to report complete outcome data. Mansour 2007, Roberts 2007 and Slusser 1998 excluded 20% or more participants from their analyses of outcomes. Roberts 2007 reported potential attrition bias by replacing participants who withdrew from their study (participants were not included in their analyses). In Rabensteiner 2013, the proportion of participants lost to follow‐up differed across groups.
We judged eight studies to be at low risk of bias for incomplete outcome data (Brissette 2015; Chen 2010; Farrell 2003; Kaido 2012; Lowther 1995; Nava‐Castaneda 2003; Qiu 2013; Yung 2012). These studies reported no loss to follow‐up or missing data, which we confirmed by comparing the number randomized versus the number ana lysed in the results reported.
We assessed all the remaining studies to be at unclear risk of attrition bias (Altan‐Yaycioglu 2005; Burgess 2008; Feng 2011; Qiu 2012; Tsifetaki 2003; Zhou 2016). Altan‐Yaycioglu 2005 did not report sample sizes in the Results, so it was unclear whether all participants completed follow‐up examinations. We noted that investigators assessed most outcomes immediately after insertion of the punctal plugs with the exception of ocular surface staining, which was typically assessed three days after occlusion. Burgess 2008 and Feng 2011 did not include sample sizes for all outcomes, so it was unclear whether all randomized participants completed their follow‐up examinations. In Tsifetaki 2003, one participant was lost to follow‐up and one participant discontinued due to local ocular infection, but we could not determine whether these participants were excluded from the analyses. Qiu 2012 reported that less than 10% of participants were lost to follow‐up, and Zhou 2016 did not report losses to follow‐up.
Selective reporting
We accessed study protocols and registry records and compared outcomes reported with the outcomes described in the protocol or trial registry record. We judged one study to be at low risk of bias, as we were able to confirm that all of the outcomes defined in the trial registry were reported in the full text (Brissette 2015). The reports from the remaining included studies did not include a trial registration number, and we did not have access to a study protocol, so we were unable to compare the reported outcomes in the full‐text studies with the outcomes before the study began.
Other potential sources of bias
We assessed the risk of bias for other potential sources of bias based on declared industry funding and conflict of interest. We rated two studies as being at high risk of bias because the investigators received industry funding or declared a conflict of interest (Roberts 2007; Slusser 1998). Brissette 2015, Qiu 2012, and Qiu 2013 declared no conflicts of interest and received no funding support or funding from their university, so we assigned them a low risk rating. The remaining studies did not include a source of funding or conflict of interest, and we judged them to be at unclear risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
1. Punctal plugs versus no punctal plugs
Five trials compared punctal plugs with no punctal plugs (Lowther 1995; Mansour 2007; Nava‐Castaneda 2003; Slusser 1998; Yung 2012).
When we refer to the punctal plug group, the group could include participants assigned to intracanalicular punctal plugs alone (Lowther 1995), silicone punctal plugs alone (Mansour 2007; Slusser 1998; Yung 2012), and collagen plus silicone punctal plugs (Nava‐Castaneda 2003). Similarly, the no punctal plugs group participants received sham treatment (Lowther 1995; Nava‐Castaneda 2003; Slusser 1998) or observation (Mansour 2007; Yung 2012).
Two of the five trials did not report the review outcomes at two weeks, one month, or long‐term (Lowther 1995; Mansour 2007). Mansour 2007 ascertained outcomes between 6 and 20 weeks after occlusion but did not report a more precise time point than that. Lowther 1995 reported only five days of follow‐up, so we did not report any study outcomes in this review.
Symptomatic improvement
Of the five trials, three included reports of symptomatic improvement at two weeks, one month, or long‐term (Mansour 2007; Nava‐Castaneda 2003; Yung 2012). We judged the certainty of the evidence to be very low for all follow‐up time points after downgrading for high risk of detection and attrition bias (1 level) and methodological heterogeneity (2 levels).
The three trials reported symptomatic improvement slightly differently. Nava‐Castaneda 2003 and Yung 2012 reported dry eye symptom scores that ranged from 0 to 3 points (0 = absence of the symptom, 1 = mild, 2 = moderate, and 3 = severe). However, Nava‐Castaneda 2003 also reported "a total symptoms score, which is a measure of the overall severity of the patient's conjunctival and dry eye condition, and the score was calculated by multiplying (for each individual symptom/condition) the numerical values corresponding to frequency and severity, and then adding the products for all symptoms" (p 11). For Nava‐Castaneda 2003, we assume that the score could range from 0 to 105 points based on the description in the text (minimum: 0 points for severity x 0 points for frequency x 7 symptoms; maximum: 5 points for severity x 3 points for frequency x 7 symptoms). Finally, Mansour 2007 use Ocular and Oral Symptoms score (According to the European Criteria for the Classification of Sjögren's Syndrome, and noted discomfort scores that were a summary score from 0 to 10, with higher values denoting more discomfort.
Since the three trials had high methodological heterogeneity, we report the results as a narrative synthesis. Each of the three trials used different scoring methods; Mansour 2007 used the Ocular and Oral Symptoms score, Nava‐Castaneda 2003 reported a total symptoms score, and Yung 2012 reported dry eye symptom scores. Mansour 2007 reported little or no difference in the Ocular and Oral Symptoms score, which ranged from 0 to 10 points (MD ‐0.46 points, 95% CI ‐1.24 to 0.32; eyes = 26). Nava‐Castaneda 2003 reported a slight decrease in symptom score, assumed to range from 0 to 105 points, but this finding is not clinically important (MD ‐2.07 points, 95% CI ‐2.70 to ‐1.44; eyes = 61). However, Nava‐Castaneda 2003 also noted a significant improvement in individual symptom scores at four weeks in the punctal plug group compared with the no punctal plugs group (dryness, itching, burning, foreign body sensation, fluctuating vision, light sensitivity). Yung 2012 reported little or no difference in dry eye symptom score, ranging from 0 to 3 points (MD 0.06 points, 95% CI ‐0.69 to 0.80; eyes = 28)
Two trials included long‐term results indicated slightly favorable symptomatic improvement in the punctal plug group versus the no punctal plugs group, but the confidence interval includes values that are not clinically important (Nava‐Castaneda 2003; Yung 2012). As mentioned above, different scales were used by Nava‐Castaneda 2003 and Yung 2012. Nava‐Castaneda 2003 reported a slight decrease in symptom score, although not clinically meaningful. However, Nava‐Castaneda 2003 noted there were also significant differences in the frequency of individual symptom scores (dryness, watery eyes, itching, burning, foreign body sensation, fluctuating vision, light sensitivity) with participants in the collagen/silicone group reporting lower frequency of these symptoms. Yung 2012 reported little or no difference in dry eye symptom score, ranging from 0 to 3 points (MD ‐0.75 points, 95% CI ‐1.53 to 0.02; eyes = 28)
Ocular surface staining
Three trials reported ocular surface staining as an outcome at two weeks, one month, or long‐term follow‐up (Nava‐Castaneda 2003; Slusser 1998; Yung 2012). Slusser 1998 reported Rose Bengal scores that ranged from 0 to 4, where 0 represented no staining and 4 represented heavy staining. Nava‐Castaneda 2003 and Yung 2012 reported the sodium fluorescein staining scores that ranged from 0 to 4, where 0 represented no staining and 4 represented heavy staining. We judged the certainty of the evidence to be low after downgrading for imprecision as the confidence interval was either wide or clinically not important (1 level) and there was a high risk of attrition bias (1 level).
One of the three trials included two‐week results that reported no difference between the groups (Nava‐Castaneda 2003). Nava‐Castaneda 2003 reported a slightly lower difference in sodium fluorescein staining scores in the punctal plugs group compared with the no punctal plugs group on the scale of 0 to 4, where a lower score is more advantageous (MD −0.80 points, 95% CI −1.10 to −0.50; eyes = 122; Analysis 1.3). However, we judged that the difference was not clinically significant.
1.3. Analysis.
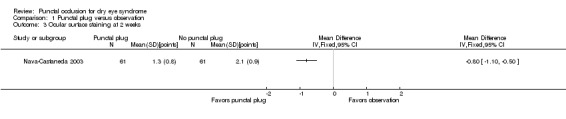
Comparison 1 Punctal plug versus observation, Outcome 3 Ocular surface staining at 2 weeks.
One of the three trials included one‐month results, which showed little or no difference between punctal plugs and no punctal plugs ( Yung 2012). Yung 2012 found little or no difference between the groups in fluorescein staining scores of 0 to 4 points (MD 0.59 points, 95% CI −0.19 to 1.37; eyes = 28; Analysis 1.4).
1.4. Analysis.
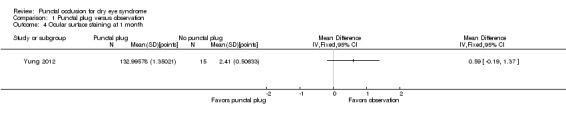
Comparison 1 Punctal plug versus observation, Outcome 4 Ocular surface staining at 1 month.
One of the three trials included long‐term results comparing punctal plugs over no punctal plugs, and punctal plugs were not consistently favored (Nava‐Castaneda 2003). Nava‐Castaneda 2003 reported that punctal plugs were somewhat better than no punctal plugs for sodium fluorescein staining score (MD ‐1.50 points, 95% CI ‐1.88 to ‐1.12; eyes = 61 Analysis 1.5).
1.5. Analysis.
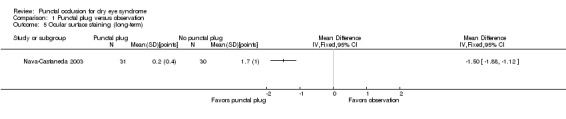
Comparison 1 Punctal plug versus observation, Outcome 5 Ocular surface staining (long‐term).
Aqueous tear production
One study reported aqueous tear production (Yung 2012) assessed using the Schirmer test with anesthesia. We judged the certainty of the evidence to be low after downgrading for imprecision as the confidence interval was either wide or clinically not important (1 level), and there was a high risk of attrition bias (1 level).
Yung 2012 reported Schirmer score results without specifying the follow‐up time point and did not provide data that could be included in a meta‐analysis. Yung 2012 reported that "Schirmer values tended to increase in the plug group after plug insertion; however, the changes did not reach significance" (p 211).
Tear film stability
Two studies reported the tear film stability outcome (Slusser 1998; Yung 2012). We judged the certainty of the evidence to be low after downgrading for inconsistency in results as TBUT did not consistently favor punctal plugs over no punctal plugs at one and three months follow‐up (1 level) and there was a high risk of attrition bias (1 level).
Slusser 1998 reported mean tear film break‐up times but did not report the respective standard deviations or the exact P value. The investigators stated: "the average prelens tear film break‐up time for all patients at the 2 baseline visits was 16.1s[econds] for the eye assigned to receive the plugs, and 16. 7s[econds] for the control eye. The difference between these values is not statistically significant (paired t‐test, p > 0.05)" (p 333).
Yung 2012 reported tear film stability at one and three months. At the one and three month follow‐up visits, Yung 2012 found little or no difference in tear film break‐up time between the groups (MD −0.41 seconds, 95% CI −1.25 to 0.43; eyes = 28 and MD 1.93 seconds, 95% CI 0.67 to 3.20; eyes = 28, Analysis 1.6 and Analysis 1.7 respectively).
1.6. Analysis.
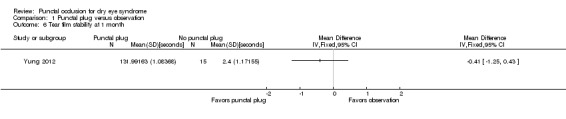
Comparison 1 Punctal plug versus observation, Outcome 6 Tear film stability at 1 month.
1.7. Analysis.
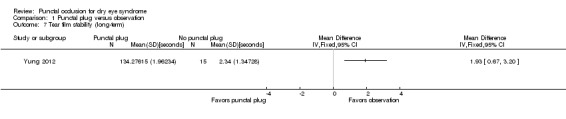
Comparison 1 Punctal plug versus observation, Outcome 7 Tear film stability (long‐term).
Artificial tear use
One trial compared the frequency of artificial tear use (Nava‐Castaneda 2003). We judged the certainty of the evidence to be very low after downgrading for high risk of performance, detection, and attrition bias (2 levels).
At two weeks follow‐up, Nava‐Castaneda 2003 reported slightly less artificial tear use in the punctal plug group compared with the no punctal plugs group (MD ‐1.40, 95% CI ‐1.86 to ‐0.94; eyes = 61; Analysis 1.8). At the one‐month follow‐up visit, there was slightly less artificial tear use in the punctal plug group compared with the no punctal plugs group (MD ‐1.80, 95% CI ‐2.09 to ‐1.51; Analysis 1.9). At the long‐term follow‐up visit Nava‐Castaneda 2003 reported slightly less artificial tear use in the punctal plug group compared with the no punctal plugs group (MD ‐2.70, 95% CI ‐3.11 to ‐2.29; eyes = 61); Analysis 1.10).
1.8. Analysis.
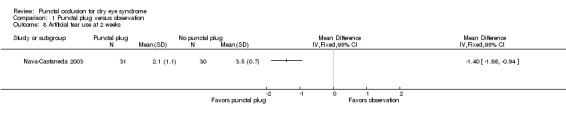
Comparison 1 Punctal plug versus observation, Outcome 8 Artificial tear use at 2 weeks.
1.9. Analysis.
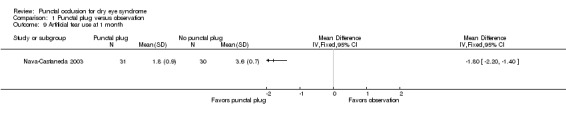
Comparison 1 Punctal plug versus observation, Outcome 9 Artificial tear use at 1 month.
1.10. Analysis.
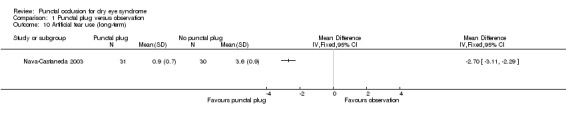
Comparison 1 Punctal plug versus observation, Outcome 10 Artificial tear use (long‐term).
Adverse outcomes
Three of the five studies reported on adverse outcomes (Slusser 1998; Mansour 2007; Nava‐Castaneda 2003). We judged the evidence to be very low‐certainty after downgrading for limitations in the design and implementation of available studies suggesting high likelihood of attrition bias (2 levels) and sparse and inconsistent data, particularly with respect to epiphora (1 level).
Slusser 1998 reported several adverse events, all in the punctal plug group (28 participants); 23 participants (82%) had epiphora, 3 (11%) reported itching in the area of plug placement, and 1 (3.5%) had tenderness and swelling of the eyelids with mucous discharge. Mansour 2007 reported spontaneous plug loss in 6 of 20 eyes (30%) with silicone punctal plugs. Nava‐Castaneda 2003 reported epiphora in one participant who had received collagen and silicone punctal plugs. The remaining studies did not report adverse events (Lowther 1995; Yung 2012).
2. Punctal plugs versus cyclosporine
One study compared punctal plugs to cyclosporine (Roberts 2007). We judged the certainty of the evidence to be very low after downgrading for imprecision of results (1 level) and high risk of detection, performance, attrition, and other bias (two levels).
Symptomatic improvement
The study investigators did not report this outcome.
Ocular surface staining
At one month, Roberts 2007 reported little or no between‐group difference in Rose Bengal staining scores on a scale of 0 to 4 points (MD 0.10 points, 95% CI −0.32 to 0.52; eyes = 20). The Rose Bengal staining score "was graded on the following scale: 0 = no staining, 1 = staining of the nasal conjunctiva only, 2 = staining of both the nasal and temporal conjunctiva, 3 = peripheral corneal staining, 4 = central corneal staining." p 806 The study investigators of Roberts 2007 stated: "there was greater improvement in conjunctival staining with cyclosporine or the combination than with plugs alone." It was unclear whether Rose Bengal or fluorescein staining was used.
Aqueous tear production
At one month, Roberts 2007 also reported little or no difference between the punctal plug and cyclosporine groups for aqueous tear production (Schirmer test without topical anesthesia: MD 6.00 mm/3 min, 95% CI 4.96 to 7.04; eyes = 20; Analysis 2.1). Likewise at long‐term follow‐up, there was little or no difference between the punctal plug and cyclosporine groups for aqueous tear production (MD 0.80 mm/3 min, 95% CI ‐0.74 to 2.34; eyes = 20; Analysis 2.2)
2.1. Analysis.
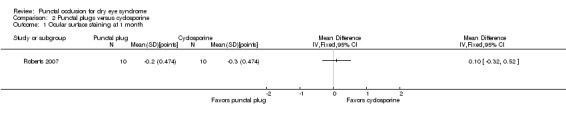
Comparison 2 Punctal plugs versus cyclosporine, Outcome 1 Ocular surface staining at 1 month.
2.2. Analysis.
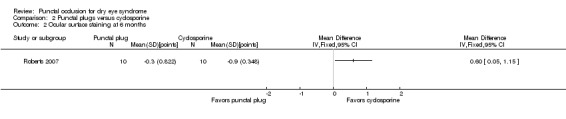
Comparison 2 Punctal plugs versus cyclosporine, Outcome 2 Ocular surface staining at 6 months.
Tear film stability
Roberts 2007 did not report this outcome.
Artificial tear use
At one month, Roberts 2007 reported less daily artificial tear use in the punctal plug group compared with the cyclosporine group (MD −1.70 applications/day, 95% CI −3.04 to −0.36; eyes = 20; Analysis 2.5). But at long‐term follow‐up, there was little or no difference between the punctal plug and cyclosporine groups for daily artificial tear use (MD 1.10 applications/day, 95% CI ‐0.04 to 2.24; eyes = 20)
2.5. Analysis.
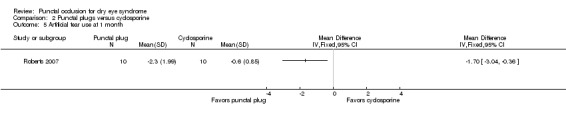
Comparison 2 Punctal plugs versus cyclosporine, Outcome 5 Artificial tear use at 1 month.
Adverse outcomes
Roberts 2007 reported that 1 of 11 participants (9%) experienced plug displacement in the punctal plug group, while 1 of 11 participants (9%) in the cyclosporine group experienced a burning sensation.
3. Punctal plugs versus oral pilocarpine
One trial compared punctal plugs to oral pilocarpine (Tsifetaki 2003).
Symptomatic improvement
Tsifetaki 2003 reported significantly greater improvements in subjective ocular symptoms in participants who received oral pilocarpine (90% improvement) versus collagen punctal plugs (60% improvement) at three months (RR 0.69, 95% CI 0.49 to 0.95; eyes = 55; Analysis 3.1). The study investigators defined improvement in subjective ocular symptoms as an improvement of >55 mm for responses to the eye questionnaire on a 100 mm VAS. We judged the certainty of the evidence to be very low after downgrading for high risk of performance and detection bias, as the participant and outcome assessors were not masked to the treatment groups, and the self‐reported symptomatic improvement could have been biased (2 levels) and imprecision as the confidence interval is either wide or clinically not important (1 level).
3.1. Analysis.
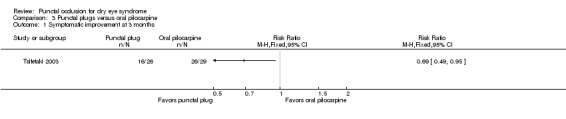
Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 1 Symptomatic improvement at 3 months.
Ocular surface staining
Tsifetaki 2003 noted small differences in Rose Bengal ocular surface staining scores, with participants who received oral pilocarpine showing more improvement at three months than participants randomized to collagen punctal plugs. The Rose Bengal ocular surface staining scores was graded using the van Bijsterveld schema, which is on a scale of 0 to 9 points (right eyes: MD 0.10 points, 95% CI −0.56 to 0.76; eyes = 55; Analysis 3.2.1; left eyes: MD 0.60 points, 95% CI 0.10 to 1.10; eyes = 55; Analysis 3.2.1). Mean differences ± standard deviations at follow‐up were: −1.0 point ± 1.3 (right eye) and −1.1 points ± 1.0 (left eye) for oral pilocarpine; and −0.9 points ± 1.2 (right eye) and −0.5 points ± 0.9 (left eye) for collagen punctal plugs.
3.2. Analysis.
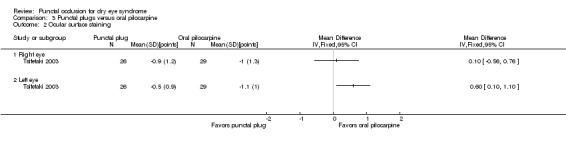
Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 2 Ocular surface staining.
Aqueous tear production
Tsifetaki 2003 noted no difference in the Schirmer test (without topical anesthesia) scores when comparing oral pilocarpine versus collagen punctal plugs (right eyes: MD −0.10 mm/5 min, 95% CI −0.53 to 0.33; eyes = 55; Analysis 3.3.1; left eyes: MD −0.50 mm/5 min, 95% CI −1.06 to 0.06; eyes = 55; Analysis 3.3.2). Mean differences ± standard deviations at follow‐up were: 0.3 ± 1.1 mm (right eyes) and 1.2 ± 1.3 mm (left eyes) for oral pilocarpine; and 0.2 ± 0.4 mm (right eyes) and 0.7 ± 0.8 mm (left eyes) for collagen punctal plugs.
3.3. Analysis.
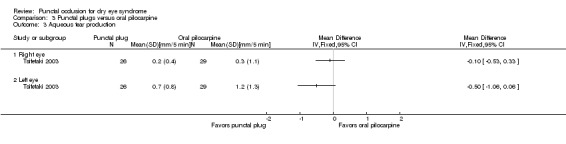
Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 3 Aqueous tear production.
Tear film stability
The study investigators did not report this outcome.
Artificial tear use
The study investigators did not report this outcome.
Adverse outcomes
Tsifetaki 2003 reported that "four patients had mild headache, of whom three also presented with nausea, vomiting, and sweating" (p 1205), and "one patient in the inferior puncta occlusion group had blepharitis and was withdrawn from the study" (p 1204).
4. Punctal plugs versus artificial tears
Five trials compared punctal plugs with artifical tears (Feng 2011; Qiu 2012; Qiu 2013; Tsifetaki 2003; Zhou 2016). Tsifetaki 2003 compared punctal plugs plus artificial tears versus artificial tears alone, while the rest of the trials compared punctal plugs versus artificial tears. Also Tsifetaki 2003 randomized both eyes of each participants to the same treatment group, hence they reported the mean and SD for the left and right eyes individually. All participants included in Feng 2011 had undergone LASIK surgery.
Symptomatic improvement
Four of the five trials reported symptomatic improvement (Qiu 2012; Qiu 2013; Tsifetaki 2003; Zhou 2016). We judged the certainty of the evidence to be very low after downgrading for there was a high risk of performance and detection bias, as the lack of masking in participants and outcome assessors could have influenced self‐reported symptomatic improvement (2 levels), and there was unexplained statistical heterogeneity or inconsistent results (1 level), and imprecision of results as the confidence interval was either wide or clinically not important (1 level).
At two weeks, Qiu 2012 reported eight symptom outcomes: dryness, foreign body sensation, visual fatigue, burning, red eye, stinging, secretion on eyelash, and difficulty opening eyes in the morning due to dryness. Each symptom was scored from "1 to 10; 1 being the absence of the type of discomfort and 10 being the most severe level that one could bear." There was little or no difference in symptomatic improvement score (MD −0.30 points, 95% CI −3.87 to 3.27; eyes = 28; Analysis 4.1).
4.1. Analysis.
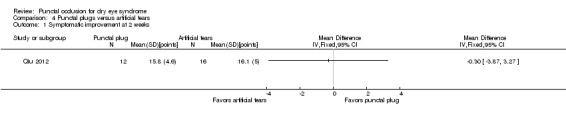
Comparison 4 Punctal plugs versus artificial tears, Outcome 1 Symptomatic improvement at 2 weeks.
At three months, Tsifetaki 2003 reported more improvements in ocular symptoms in the group receiving punctal plugs plus artificial tears than in the group receiving artifical tears alone (RR 2.29, 95% CI 1.20 to 4.38; eyes = 54; Analysis 4.2). As mentioned above, improvements in subjective ocular symptoms was defined as an improvement of >55 mm for responses to the eye questionnaire on a 100 mm VAS.
4.2. Analysis.
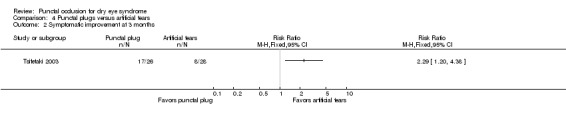
Comparison 4 Punctal plugs versus artificial tears, Outcome 2 Symptomatic improvement at 3 months.
At three months, Zhou 2016 and Qiu 2013 reported mean symptomatic improvement scores. Qiu 2013 reported scores on the 12‐item Ocular Surface Disease Index (OSDI). Each item on the questionnaire is graded from 0 to 4 points, where 0 = never, 1 = some of the time, 2 = half of the time, 3 = most of the time, and 4 = all the time. Zhou 2016 reported a symptoms score, defined as the sum of scores for dryness, foreign body sensation, and visual fatigue. The dryness score was graded from 0 to 6 points, where 0 = none, 2 = occasional, 4 = my eyes are often dry and uncomfortable, 6 = my eyes are always unbearably dry. Foreign body sensation was scored from 0 to 3 points, where 0 = none; 1 = occasional; 2 = often, I often want to blink; and 3 = often, I have to blink frequently, and want to rub my eyes with my hands. The visual fatigue score ranged from 0 to 6 points, where 0 = none, 2 = quick to fatigue, 4 = the duration that I can read or see has shortened, 6 = my eyelids feel like falling off, so I cannot read or see. Punctal plugs showed slightly better symptomatic improvement than artificial tears group at three months (SMD −0.88, 95% CI −1.24 to −0.51; eyes = 130; I2 = 86%; Analysis 4.3)
4.3. Analysis.
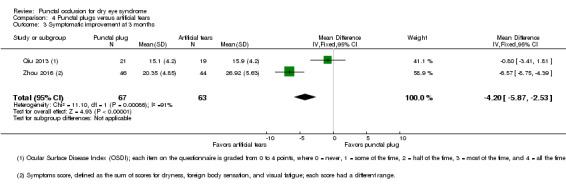
Comparison 4 Punctal plugs versus artificial tears, Outcome 3 Symptomatic improvement at 3 months.
Ocular surface staining
We judged the certainty of the evidence to be low after downgrading for imprecision in results as the confidence interval is wide and clinically not important (2 levels). At two‐week follow‐up, Feng 2011 reported little or no difference in Rose Bengal scores between groups (MD 0.81 points, 95% CI −0.09 to 1.71; eyes = 54; Analysis 4.4)
4.4. Analysis.
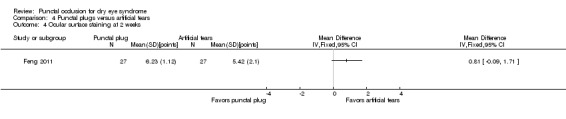
Comparison 4 Punctal plugs versus artificial tears, Outcome 4 Ocular surface staining at 2 weeks.
Both Tsifetaki 2003 and Qiu 2013 reported ocular surface staining at three months follow‐up but assessed it differently. Because Tsifetaki 2003 randomized both eyes of each participant to the same treatment group, they reported the mean and SD for the left and right eye respectively. Further more, both treatment groups received artificial tears, and noted small differences in Rose Bengal ocular surface staining scores on a scale of 0 to 3 (right eyes: MD 0.10 points, 95% CI −0.56 to 0.76; Analysis 4.5.1; left eyes: MD 0.60 points, 95% CI 0.10 to 1.10; Analysis 4.5.2). Qiu 2013 reported little or no difference in fluorescein staining scores on a scale of 0 to 3 (MD −0.21 points, 95% CI −0.49 to 0.07; eyes = 40; Analysis 4.6)
4.5. Analysis.
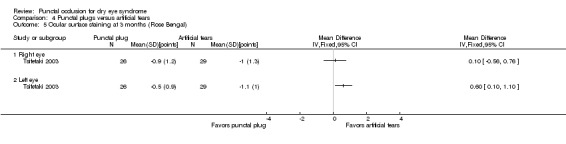
Comparison 4 Punctal plugs versus artificial tears, Outcome 5 Ocular surface staining at 3 months (Rose Bengal).
4.6. Analysis.
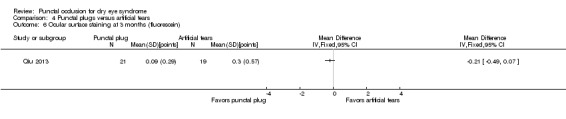
Comparison 4 Punctal plugs versus artificial tears, Outcome 6 Ocular surface staining at 3 months (fluorescein).
Aqueous tear production
We judged the certainty of the evidence to be low after downgrading for imprecision as the confidence interval are wide or clinically not important (1 level) and results were inconsistent (1 level). At two weeks, Feng 2011 noted little or no difference in Schirmer scores (without topical anesthesia) when comparing collagen punctal plugs versus artificial tears (MD 0.83 mm/5 min, 95% CI ‐1.05 to 2.71; eyes = 82; Analysis 4.7).
4.7. Analysis.
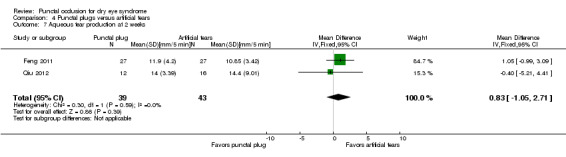
Comparison 4 Punctal plugs versus artificial tears, Outcome 7 Aqueous tear production at 2 weeks.
At three months, Tsifetaki 2003 noted small differences in Schirmer scores (without topical anesthesia) when both treatment groups received artificial tears and both eyes of each participant was in the same treatment group (right eye: MD 0.00 mm/5 min, 95% CI ‐0.33 to 0.33; Analysis 4.8.1; left eye: MD 0.10 mm/5 min, 95% CI ‐0.35 to 0.55; Analysis 4.8.2). The meta‐analysis of Qiu 2013 and Zhou 2016 favored punctal plugs over artificial tears (MD 2.16 mm/5 min, 95% CI 1.41 to 2.90; eyes = 130; I2 = 0%; Analysis 4.9)
4.8. Analysis.
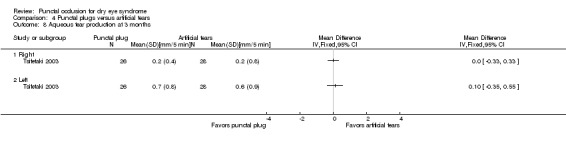
Comparison 4 Punctal plugs versus artificial tears, Outcome 8 Aqueous tear production at 3 months.
4.9. Analysis.
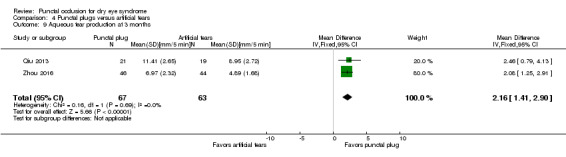
Comparison 4 Punctal plugs versus artificial tears, Outcome 9 Aqueous tear production at 3 months.
Tear film stability
We judged the certainty of the evidence to be moderate after downgrading for imprecision of results (1 level).
At two weeks, the pooled analysis of Feng 2011 and Qiu 2012 showed little or no difference in tear film stability when comparing collagen punctal plugs versus artificial tears (MD 0.26 seconds, 95% CI −0.57 to 1.09; eyes = 82; I2 = 0%; Analysis 4.10).
4.10. Analysis.
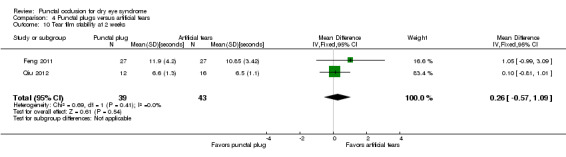
Comparison 4 Punctal plugs versus artificial tears, Outcome 10 Tear film stability at 2 weeks.
At three months, the pooled analysis of Qiu 2013 and Zhou 2016 showed little or no difference in tear film stability when comparing collagen punctal plugs versus artificial tears (MD 1.02 seconds, 95% CI 0.60 to 1.44; eyes = 130; I2 = 55%; Analysis 4.11).
4.11. Analysis.
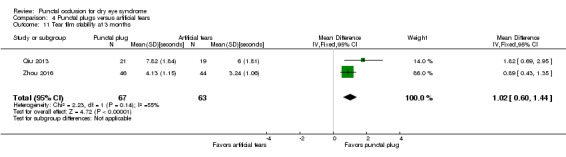
Comparison 4 Punctal plugs versus artificial tears, Outcome 11 Tear film stability at 3 months.
Artificial tear use
The investigators of these studies did not report this outcome, as one of the interventions consisted of artificial tears.
Adverse outcomes
We judged the certainty of the evidence to be low after downgrading for imprecision because of wide confidence intervals (2 levels). Tsifetaki 2003 reported that "four patients had mild headache, of whom three also presented with nausea, vomiting, and sweating" (p 1205) and that "one patient in the inferior puncta occlusion group had blepharitis and was withdrawn from the study" (p 1204).
Qiu 2012 reported little or no difference in punctate epithelial keratopathy between the intervention groups (RR 1.33, 95% CI 0.57 to 3.12; eyes = 28; Analysis 4.12).
4.12. Analysis.
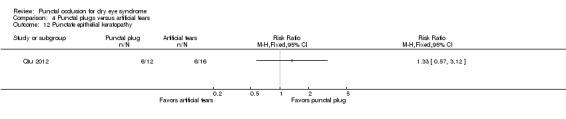
Comparison 4 Punctal plugs versus artificial tears, Outcome 12 Punctate epithelial keratopathy.
Feng 2011, Qiu 2013, and Zhou 2016 did not report adverse outcomes.
5. Punctal plugs in the upper versus lower puncta
Three trials compared upper versus lower punctal plug placement (Chen 2010; Farrell 2003; Kaido 2012). Farrell 2003 compared participants with lower puncta occlusion only, with participants receiving a combination of upper and lower puncta occlusion. Chen 2010 and Farrell 2003 investigated collagen punctal plugs while Kaido 2012 included silicone punctal plugs. Chen 2010 reported only 10 days of follow‐up, so we present no quantitative data for this comparison.
Symptomatic improvement
We judged the certainty of the evidence to be low after downgrading for imprecision of results as the confidence interval was wide (1 level) and potential bias as there was unclear risk of selection, performance, detection, attrition, and reporting bias (1 level). None of the studies reported this outcome beyond one month of follow‐up. Chen 2010 did not report this outcome.
Farrell 2003 reported median McMonnies symptom scores for participants receiving collagen punctal plugs in the lower puncta versus the upper and lower puncta for the right and left eyes separately. While study investigators of Farrell 2003 noted using a modified McMonnies questionnaire, they did not publish the modified questionnaire, but did note that higher scores denote increased symptom reports. The median (range) values for the right eye at baseline, 5 days, and 12 days post‐occlusion were 7 points (3 to 12), 3 points (0 to 8), and 3 points (0 to 10), respectively, for participants with collagen plugs in their lower puncta and 7 points (6 to 13), 3 points (0 to 11) and 3 points (0 to 7), respectively, for participants with collagen plugs in their lower and upper puncta. The authors stated that "the median symptom score reduced significantly from 7 to 3 between baseline and day 5," but "there was no significant shift in symptom score between days 5 and 12 (Wilcoxon signed‐rank test)" (p 3).
At one month, Kaido 2012 noted little or no difference in symptomatic improvement when comparing punctal plugs in the upper versus lower puncta (RR 1.07, 95% CI 0.85 to 1.36; eyes = 43; Analysis 5.1). Kaido 2012 reported the number of participants who expressed satisfaction with resolution of symptoms or satisfaction with resolution of symptoms despite epiphora.
5.1. Analysis.
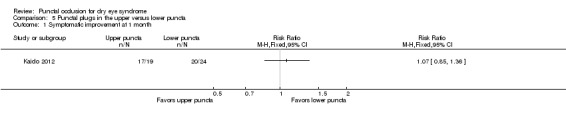
Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 1 Symptomatic improvement at 1 month.
Ocular surface staining
We judged the certainty of the evidence to be low after downgrading for imprecision of results as the confidence interval was wide (1 level) and potential bias as there was unclear risk of selection, performance, detection, attrition, and reporting bias (1 level). Chen 2010 and Kaido 2012 measured ocular surface staining using a sodium fluorescein strip graded from 0 to 3 points, with 0 indicating no staining and 3 indicating the most intense staining.
At one month, Kaido 2012 reported the means ± standard deviation of the upper and lower puncta group to be 0.4 ± 0.9 points and 0.0 ± 0.0 points; therefore, we were not able to estimate the mean difference. None of the trials reported this outcome at two weeks or for long‐term follow‐up. Farrell 2003 did not report this outcome at any follow‐up time.
Aqueous tear production
We judged the certainty of the evidence to be low after downgrading for imprecision of results as the confidence interval was wide (1 level) and potential bias as there was unclear risk of selection, performance, detection, attrition, and reporting bias (1 level). Chen 2010 measured aqueous tear production using a Schirmer test with anesthesia, but Kaido 2012 used a Schirmer test without anesthesia (mm/5 min).
At one month, Kaido 2012 reported slightly less aqueous tear production in the peripheral cornea when comparing punctal plugs in the upper puncta to punctal plugs in the lower puncta (MD −4.50 mm/5 min, 95% CI −8.63 to −0.37; eyes = 43; Analysis 5.2). None of the trials reported this outcome at two weeks or for long‐term follow‐up. Farrell 2003 did not report this outcome.
5.2. Analysis.
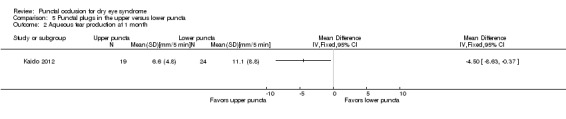
Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 2 Aqueous tear production at 1 month.
Tear film stability
We judged the certainty of the evidence to be low after downgrading for imprecision of results as the confidence interval was wide (1 level) and potential bias as there was unclear risk of selection, performance, detection, attrition, and reporting bias (1 level). Both Chen 2010 and Kaido 2012 reported tear film stability via assessment with sodium fluorescein.
At one month, Kaido 2012 noted little or no difference in tear film break‐up time between punctal plugs in the upper versus lower puncta (MD −0.10 seconds, 95% CI −1.73 to 1.53; eyes = 43; Analysis 5.3). None of the trials reported this outcome at two weeks or at long‐term follow‐up. Farrell 2003 did not report this outcome.
5.3. Analysis.
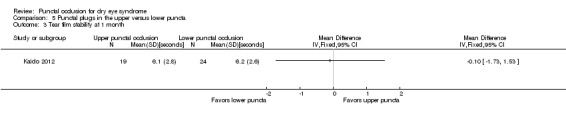
Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 3 Tear film stability at 1 month.
Artificial tear use
None of the studies reported this outcome.
Adverse outcomes
We judged the certainty of the evidence to be low after downgrading for imprecision of results as the confidence interval was wide (1 level) and potential bias as there was unclear risk of selection, performance, detection, attrition, and reporting bias (1 level). Chen 2010 reported that "no complication was observed in dry eye patients or control subjects during the period of this study." It is unclear which complications were ana lysed. Kaido 2012 and Farrell 2003 did not report this outcome.
6. Acrylic punctal plugs versus silicone punctal plugs
Burgess 2008 was the only trial that compared acrylic punctal plugs with silicone punctal plugs; the investigators reported outcomes at approximately 11 weeks of follow‐up. We judged the certainty of the evidence for all outcomes to be low after downgrading for imprecision as the confidence interval was wide (2 levels) and risk of bias as selection, attrition and reporting bias were judged to be unclear (1 level). The study investigators did not report this outcome at two weeks or four weeks.
Symptomatic improvement
Burgess 2008 reported no difference in mean subjective symptom score when comparing silicone and acrylic punctal plugs. The study investigators used a "subjective symptom scoring was assessed for each eye by using standard, vertical, nonanchored visual analog scales 10 cm in length for symptoms of dryness, grittiness, foreign body sensation, pain, stinging, burning, and itching. After rating symptoms individually, we summed scores to give the total symptom score." We assumed the score ranged from 0 to 70 points, where a lower score was more advantageous. At baseline and approximately 11 weeks follow‐up, mean scores ± standard deviations were 29.6 points ± 16.9 and 21.0 points ± 14.5, respectively, among participants with silicone plugs, and 38.9 points ± 16.7 and 21.9 points ± 18.8 among participants with acrylic plugs group (MD 0.90 points, 95% CI −6.94 to 8.74; eyes = 36; Analysis 6.1).
6.1. Analysis.
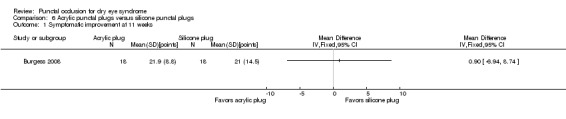
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 1 Symptomatic improvement at 11 weeks.
Ocular surface staining
Burgess 2008 found no differences in Rose Bengal and sodium fluorescein ocular surface staining when comparing silicone versus acrylic punctal plugs (Rose Bengal baseline versus approximately 11 weeks follow‐up on a scale of 0 to 3 points: MD 0.45 points, 95% CI −0.09 to 0.99; eyes = 36; Analysis 6.2.1; sodium fluorescein baseline versus approximately 11 weeks follow‐up on a scale of 0 to 3 points: MD 0.43 points, 95% CI −1.61 to 2.47; eyes = 36; Analysis 6.2.2).
6.2. Analysis.
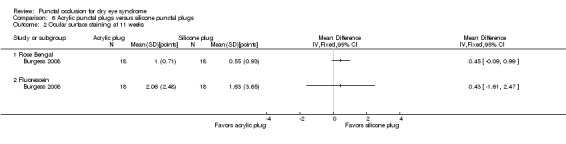
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 2 Ocular surface staining at 11 weeks.
Aqueous tear production
Similarly, Burgess 2008 found no difference in mean Schirmer test results (without topical anesthesia) when comparing silicone versus acrylic punctal plugs (MD 1.07 mm, 95% CI −1.62 to 3.76; eyes = 36; Analysis 6.3). However, Burgess 2008 did not report the duration of the Schirmer test.
6.3. Analysis.
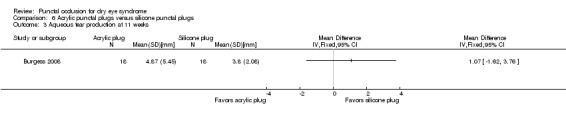
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 3 Aqueous tear production at 11 weeks.
Tear film stability
Burgess 2008 reported no difference in tear film break‐up time when comparing silicone versus acrylic punctal plugs (MD 0.36 seconds, 95% CI −1.22 to 1.94; eyes = 36; Analysis 6.4).
6.4. Analysis.
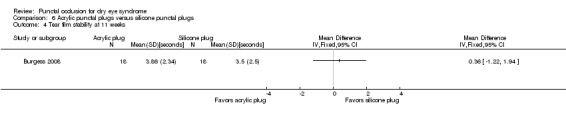
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 4 Tear film stability at 11 weeks.
Artificial tear use
In the Burgess 2008 study, silicone and acrylic punctal plugs reduced the use of artificial tears in 10 (55.6%) and 11 (61.1%) participants, respectively, but the difference in mean reduction in artificial tear use (as measured by the symptom score) was not statistically significant (mean 1.55 applications, 95% CI −1.50 to 4.60; P = 0.27, t test).
Adverse outcomes
Burgess 2008 reported that 1 of 18 eyes receiving an acrylic punctal plug experienced epiphora, 1 of 18 eyes receiving a silicone punctal plug eye experienced intermittent ocular irritation, and 2 eyes in the silicone punctal plug group and 1 in the acrylic punctal plug experienced temporary foreign body sensation.
7. Intracanalicular punctal plugs versus silicone punctal plugs
Rabensteiner 2013 was the only trial to compare intracanalicular punctal plugs with silicone punctal plugs. For all outcomes, we downgraded the evidence two levels to very low‐certainty because of wide confidence intervals indicating imprecision of effect estimates (2 levels) and high risk of attrition bias (1 level).
Symptomatic improvement
Rabensteiner 2013 reported subjective dry eye symptoms for each eye. Investigators measured soreness, scratching, grittiness, dryness and/or burning using a 100 mm visual analog scale (VAS; 0 mm = no symptoms, 100 mm = maximum intensity). At three months follow‐up, moderate‐certainty evidence showed little or no difference in symptomatic improvement score (MD −3.10 mm, 95% CI −14.97 to 8.77; eyes = 57; Analysis 7.1).
7.1. Analysis.
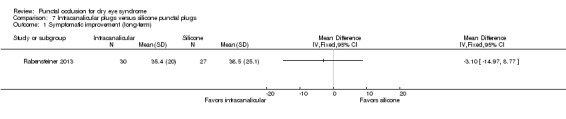
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 1 Symptomatic improvement (long‐term).
Ocular surface staining
At three months follow‐up, moderate‐certainty evidence showed little or no difference in ocular surface staining for Rose Bengal on the van Bijsterveld scale of 0 to 9 points (MD 0.20 points, 95% CI −0.71 to 1.11; Analysis 7.2.1) and fluorescein of a scale from 0 to 4 points (MD 0.40 points, 95% CI −0.04 to 0.84; Analysis 7.2.2). Fluorescein was graded using zero points indicates no staining, one point less than one‐third, two points less than two thirds, and three points more than two‐thirds staining of the cornea.
7.2. Analysis.
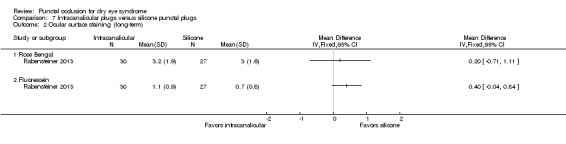
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 2 Ocular surface staining (long‐term).
Aqueous tear production
At three months follow‐up, moderate‐certainty evidence showed little or no difference in aqueous tear production (MD −0.70 mm/5 min, 95% CI −3.46 to 2.06; Analysis 7.3).
7.3. Analysis.
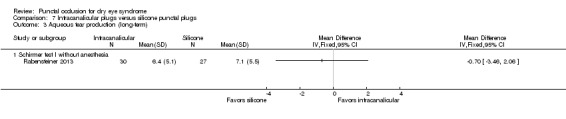
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 3 Aqueous tear production (long‐term).
Tear film stability
At three months follow‐up, moderate‐certainty evidence showed little or no difference in tear film stability (MD 0.80 seconds, 95% CI −0.00 to 1.60; Analysis 7.4).
7.4. Analysis.
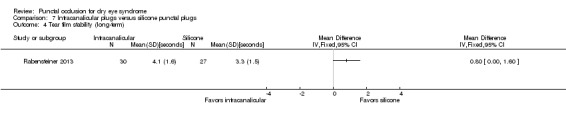
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 4 Tear film stability (long‐term).
Artificial tear use
At three months follow‐up, moderate‐certainty evidence showed little or no difference in daily artificial tear use (MD −1.30 applications, 95% CI −4.04 to 1.44; Analysis 7.5).
7.5. Analysis.
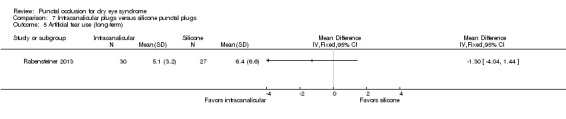
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 5 Artificial tear use (long‐term).
Adverse outcomes
No studies reported adverse events.
8. Collagen punctal plugs versus silicone punctal plugs
Altan‐Yaycioglu 2005 and Brissette 2015 compared collagen punctal plugs with silicone punctal plugs. Altan‐Yaycioglu 2005 did not report any review outcomes at two weeks, one month, or at long‐term follow‐up. Brissette 2015 reported symptomatic improvement (measured using the Canadian Dry Eye Assessment), aqueous tear production (Schirmer I without anesthesia), and tear film stability at one month and at long‐term follow‐up (six months). The Canadian Dry Eye Assessment range from 0 to 48 points; where less than 5 points was normal, 5 to 15 points was mild, 20 to 25 points was moderate, 30 to 48 points was severe. However, the study investigators did not report standard deviations or the exact P value at the one month follow‐up. At the long‐term follow‐up, we used the reported group means and exact P values to compute the MD and respective 95% CI.
We judged the certainty of the evidence for all outcomes to be very low after downgrading for imprecision of estimates as the confidence interval were wide or clinically not important (2 levels) and risk of bias as we judged selection, performance, detection, attrition, and reporting bias to be unclear (1 level).
Symptomatic improvement
At long‐term follow‐up, Brissette 2015 reported little or no difference between the groups for Canadian Dry Eye Assessment scores on a scale of 0 to 48 points (MD 0.81 points, 95% CI −2.94 to 4.56; eyes = 50).
Ocular surface staining
At long‐term follow‐up, Brissette 2015 reported both sodium fluorescein staining scores and lissamine green staining scores. There were few or no between‐group differences for fluorescein staining score on a scale of 0 to 4 points (MD −0.76 points, 95% CI −18.5 to 17.0) and for lissamine green score on a scale of 0 to 3 points (MD 0.03 points, 95% CI −0.15 to 0.21; eyes = 50).
Aqueous tear production
At long‐term follow‐up, Brissette 2015 reported little or no difference between groups for the Schirmer test I without anesthesia (MD 0.67 mm, 95% CI −17.28 to 18.62; eyes = 50).
Tear film stability
At long‐term follow‐up, Brissette 2015 reported little or no between‐group differences for tear break‐up time (MD 0.21 seconds, 95% CI −1.81 to 2.23; eyes = 50).
Artificial tear use
At long‐term follow‐up, Brissette 2015 reported little or no difference between groups in daily frequency of artificial tear applications (MD −0.06 applications/day, 95% CI −0.23 to 0.12; eyes = 50).
Adverse outcomes
Altan‐Yaycioglu 2005 stated that "none of the patients developed adverse events related to the procedure" (p 88.e3). Likewise, Brissette 2015 reported that "there were no additional significant differences between groups and no plug complications reported" (p 238).
Discussion
Summary of main results
Despite the inclusion of 18 trials, only a maximum of two studies contributed outcome data at any given follow‐up time point across eight comparisons due to significant study heterogeneity. Punctal plugs did not show consistent symptomatic improvement over the observation group at 2 weeks, 1 month, or at 2 to 12 months follow‐up. When comparing symptomatic improvement in different types of punctal plugs at long‐term follow‐up, there was little or no difference between silicone and collagen or acrylic punctal plugs. Punctal plugs may be more effective than oral pilocarpine, but artificial tears may be more effective than punctal plugs for treating dry eye signs and symptoms. The location of the occlusion (lower versus upper puncta) resulted in little or no difference in symptomatic improvement. Overall, this review update includes new evidence, though we cannot draw strong conclusions from the current body of evidence.
Adverse outcomes
Tsifetaki 2003 was the only study to report on adverse outcomes with collagen plugs only; 1 participant of 28 discontinued the study due to a local infection. The most common adverse outcomes in silicone plug studies were: spontaneous plug loss (6 of 28 in Mansour 2007, 6 of 20 in Burgess 2008); epiphora (1 case each in Burgess 2008 and Nava‐Castaneda 2003, 4 in Farrell 2003); ocular irritation or foreign body sensation (3 cases in Burgess 2008); and local inflammatory reaction to silicone (1 case in Mansour 2007). In addition, Mansour 2007 reported one case of corneal melting and perforation, and Nava‐Castaneda 2003 reported one instance of corneal ulceration. The trial investigators believed that neither of these serious conditions was related to the punctal plugs.
Overall completeness and applicability of evidence
We included 18 trials, but no more than two studies reported the same review outcome at any review follow‐up time points across eight comparisons of interventions. Meta‐analysis was not possible for the primary review outcome thus the evidence regarding punctal occlusion for dry eye‐related symptoms is incomplete.
The certainty of the evidence ranged from moderate to very low, as we frequently downgraded the evidence for the high risk of bias or imprecision in effect estimates.
Potential biases in the review process
We used standard Cochrane methods to conduct this review and are unaware of any potential bias.
Agreements and disagreements with other studies or reviews
Many investigators have published reviews of punctal plug use for dry eye syndrome (Hamano 2005; Marcet 2015; Yellepeddi 2015; Jehangir 2016). Nevertheless, these reviews do not include meta‐analyses of randomized controlled trials or other comparative studies. Hamano 2005 and Yellepeddi 2015 was a general review of punctal plug types and the ways in which punctal plugs are used for treating dry eye. Marcet 2015 was a narrative review of 27 studies, which included both case series and randomized trials. Jehangir 2016 provided an extensive review of the available punctal plug materials, designs, and uses, as well as the complications associated with punctal plugs treatment. Overall, the authors concluded that punctal plugs were an effective and relatively safe means for treating dry eye.
Authors' conclusions
Implications for practice.
The effectiveness of punctal plugs for treating dry eye syndrome cannot be assessed based on the evidence in this updated systematic review. The investigators of the included studies suggest that punctal plugs are efficacious, but the strength of the evidence is limited due to high methodological and clinical heterogeneity. Effectiveness may vary by the characteristics of the punctal plug, i.e., placement, materials used, shape, and size. It is also possible that punctal plugs may not be an effective treatment for every patient based upon the classification, etiology, and severity of dry eye. Thus, additional randomized trials are necessary to assess the effectiveness of punctal plugs based on design, classification of disease, and against the appropriate gold standard treatment.
Overall, punctal plugs are believed to be a relatively safe treatment, yet their use is not without potential complications, including epiphora, punctal plug loss, or rarely, a more serious complication such as dacryocystitis.
Implications for research.
The current evidence suggests that punctal plugs are a modestly effective means of treating dry eye, though we cannot draw strong conclusions about the effectiveness of punctal plugs based on the findings of this systematic review. Heterogeneity in methodology among the included studies, such as varied participant characteristics, testing protocols, follow‐up periods, punctal plug designs and comparison interventions were limiting factors. The limitations highlighted in this review should be addressed in future trials to maximize study comparability and enable more comprehensive and generalizable conclusions about the utility of punctal plugs for treating dry eyes.
Investigators should recruit a sufficient number of participants utilizing statistical techniques to determine the appropriate sample size based on the anticipated treatment effect. Trial investigators should also avoid quasi randomization and should employ appropriate methods for allocation concealment and masking of participants and personnel assessing outcomes. When masking of personnel administering the intervention is not possible, excluding these persons from the assessment of treatment outcomes through use of masked examiners and objective outcomes minimizes information bias. Assessment of outcomes at two months or longer should be considered to provide evidence of the long term effect of punctal occlusion. Standardized patient oriented outcome measures would allow comparisons across individual studies. As well, investigators should report the unit of analysis and appropriately account for the correlation among fellow eyes in analyses.
Future randomized controlled trials on dry eye should examine the efficacy of punctal plugs for treating the two primary types of dry eye, aqueous deficient and evaporative dry eye, or more specifically the utility of using punctal plugs for treating conditions like Sjögren's syndrome (DEWS 2007a). Comparisons of punctal plugs and other dry eye treatments such as topical cyclosporine and serum should be conducted to guide treatment decisions for each classification of dry eye (DEWS 2007b).
What's new
| Date | Event | Description |
|---|---|---|
| 16 June 2017 | New search has been performed | Issue 6, 2017: electronic searches updated on 8 December 2016. |
| 16 June 2017 | New citation required and conclusions have changed | Issue 6, 2017: 11 new trials added to the review; one ongoing trial identified. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 26 June 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank Iris Gordon and Lori Rosman, Informationists for Cochrane Eyes and Vision (CEV), for designing the search strategy and conducting the electronic searches. We also acknowledge the CEV editorial team's support during the preparation of this review and thank Oliver Schein and Robert Wojciechowski for their contributions to the prior review published in 2010. We would like to also acknowledge Ke Chen, Xinyi Chen, and Shi‐ming Li for their help in screening full‐text reports, abstracting data, and translating Mandarin publications. Finally, we would like to thank our peer reviewers Jod Mehta and Barbara Hawkins.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dry Eye Syndromes] explode all trees #2 (dry near/2 eye*) #3 (ocular near/2 dry*) #4 MeSH descriptor: [Tears] explode all trees #5 tear* #6 MeSH descriptor: [Xerophthalmia] explode all trees #7 xerophthalmi* #8 MeSH descriptor: [Vitamin A Deficiency] explode all trees #9 ("vitamin A" near/3 deficien*) #10 ("avitaminosis a" or retinol deficien* or "hypovitaminosis A") #11 MeSH descriptor: [Keratoconjunctivitis Sicca] explode all trees #12 (Keratoconjunctiv* or kerato conjunctivitis) #13 MeSH descriptor: [Sjogren's Syndrome] explode all trees #14 ((Sjogren* or Sjoegren*) near/2 (syndrom* or disease*)) #15 sicca syndrom* #16 MeSH descriptor: [Stevens‐Johnson Syndrome] explode all trees #17 (Steven* and Johnson and (syndrom* or disease*)) #18 MeSH descriptor: [Pemphigoid, Benign Mucous Membrane] explode all trees #19 Benign Muco* Pemphigoid* #20 (Cicatricial near/2 Pemphigoid*) #21 blepharoconjunctiviti* #22 MeSH descriptor: [Meibomian Glands] explode all trees #23 (meibomian or tarsal) #24 MeSH descriptor: [Lacrimal Apparatus Diseases] explode all trees #25 (lacrima* or epiphora) #26 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27 (occlu* or plug* or cauter*) near/4 (puncta* or punctum* or canalicula* or Intracanalicula* or lacrima*) #28 (Silicone near/2 plug*) or (Collagen near/2 plug*) #29 #27 or #28 #30 #26 and #29
Appendix 2. MEDLINE (Ovid) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp dry eye syndromes/ 13. (dry adj2 eye*).tw. 14. (ocular adj2 dry*).tw. 15. exp tears/ 16. tear*.tw. 17. exp xerophthalmia/ 18. xerophthalmi*.tw. 19. exp vitamin A deficiency/ 20. (vitamin A adj3 deficien*).tw. 21. (avitaminosis a or retinol deficien* or hypovitaminosis A).tw. 22. exp keratoconjunctivitis sicca/ 23. (Keratoconjunctiv* or kerato conjunctivitis).tw. 24. exp Keratoconjunctivitis/ 25. limit 24 to yr="1966 ‐ 1985" 26. exp Sjogren's syndrome/ 27. ((Sjogren* or Sjoegren*) adj2 (syndrom* or disease*)).tw. 28. sicca syndrom*.tw. 29. exp Stevens Johnson syndrome/ 30. (Steven* and Johnson and (syndrom* or disease*)).tw. 31. exp Pemphigoid, Benign Mucous Membrane/ 32. Benign Muco* Pemphigoid*.tw. 33. (Cicatricial adj2 Pemphigoid*).tw. 34. blepharoconjunctiviti*.tw. 35. exp meibomian glands/ 36. (meibomian or tarsal).tw. 37. exp lacrimal apparatus diseases/ 38. (lacrima* or epiphora).tw. 39. or/12‐23,25‐38 40. ((occlu* or plug* or cauter*) adj4 (puncta* or punctum* or canalicula* or Intracanalicula* or lacrima*)).tw. 41. ((Silicone adj2 plug*) or (Collagen adj2 plug*)).tw. 42. 40 or 41 43. 11 and 39 and 42
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'dry eye'/exp #34 (dry NEAR/2 eye*):ab,ti #35 (ocular NEAR/2 dry*):ab,ti #36 'lacrimal fluid'/exp #37 tear*:ab,ti #38 'xerophthalmia'/exp #39 xerophthalmi*:ab,ti #40 'retinol deficiency'/exp #41 ('vitamin a' NEAR/3 deficien*):ab,ti #42 'avitaminosis a':ab,ti OR (retinol NEAR/1 deficien*):ab,ti OR 'hypovitaminosis a':ab,ti #43 'keratoconjunctivitis sicca'/exp #44 keratoconjunctiv*:ab,ti OR 'kerato conjunctivitis':ab,ti #45 'sjoegren syndrome'/exp #46 ((sjogren* OR sjoegren*) NEAR/2 (syndrom* OR disease*)):ab,ti #47 (sicca NEXT/1 syndrom*):ab,ti #48 'stevens johnson syndrome'/exp #49 steven*:ab,ti AND johnson:ab,ti AND (syndrom*:ab,ti OR disease*:ab,ti) #50 'mucous membrane pemphigoid'/exp #51 benign AND muco* AND pemphigoid*:ab,ti #52 (cicatricial NEAR/2 pemphigoid*):ab,ti #53 blepharoconjunctiviti*:ab,ti #54 'meibomian gland'/exp #55 meibomian:ab,ti OR tarsal:ab,ti #56 'lacrimal gland disease'/exp #57 lacrima*:ab,ti OR epiphora:ab,ti #58 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 #59 ((occlu* OR plug* OR cauter*) NEAR/4 (puncta* OR punctum* OR canalicula* OR intracanalicula* OR lacrima*)):ab,ti #60 (silicone NEAR/2 plug*):ab,ti OR (collagen NEAR/2 plug*):ab,ti #61 #59 OR #60 #62 #32 AND #58 AND #61
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. (dry[tw] AND eye*[tw]) NOT Medline[sb] 3. (ocular[tw] AND dry*[tw]) NOT Medline[sb] 4. tear*[tw] NOT Medline[sb] 5. xerophthalmi*[tw] NOT Medline[sb] 6. ("vitamin A"[tw] AND deficien*[tw]) NOT Medline[sb] 7. ("avitaminosis a"[tw] OR retinol deficien*[tw] OR "hypovitaminosis A"[tw]) NOT Medline[sb] 8. (Keratoconjunctiv*[tw] OR "kerato conjunctivitis"[tw]) NOT Medline[sb] 9. ((Sjogren*[tw] OR Sjoegren*[tw]) AND (syndrom*[tw] OR disease*[tw])) NOT Medline[sb] 10. sicca syndrom*[tw] NOT Medline[sb] 11. (Steven*[tw] AND Johnson[tw] AND (syndrom*[tw] OR disease*[tw])) NOT Medline[sb] 12. Benign Muco* Pemphigoid*[tw] NOT Medline[sb] 13. (Cicatricial[tw] AND Pemphigoid*[tw]) NOT Medline[sb] 14. blepharoconjunctiviti*[tw] NOT Medline[sb] 15. (Meibomian[tw] OR tarsal[tw]) NOT Medline[sb] 16. (lacrima*[tw] OR epiphora[tw]) NOT Medline[sb] 17. #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 18. ((occlu*[tw] OR plug*[tw] OR cauter*[tw]) AND (puncta*[tw] OR punctum*[tw] OR canalicula*[tw] OR Intracanalicula*[tw] OR lacrima*[tw])) NOT Medline[sb] 19. ((Silicone[tw] AND plug*[tw]) OR (Collagen[tw] AND plug*[tw])) NOT Medline[sb] 20. #18 OR #19 21. #1 AND #17 AND #20
Appendix 5. LILACS search strategy
((occlu$ OR plug$ OR cauter$) AND (puncta$ OR punctum$ OR canalicula$ OR Intracanalicula$ OR lacrima$)) OR (silicone plug$) OR (collagen plug$)
Appendix 6. metaRegister of Controlled Trials search strategy
punctal or punctum
Appendix 7. ClinicalTrials. gov search strategy
(punctal OR punctum OR canalicular OR Intracanalicular OR lacrimal) AND (occlusion OR plug OR cautery)
Appendix 8. ICTRP search strategy
Punctal OR punctum OR canalicular OR intracanalicular OR lacrimal
Data and analyses
Comparison 1. Punctal plug versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic improvement at 1 month | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Symptomatic improvement (long‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Ocular surface staining at 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Ocular surface staining at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Ocular surface staining (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Tear film stability at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Tear film stability (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Artificial tear use at 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Artificial tear use at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Artificial tear use (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
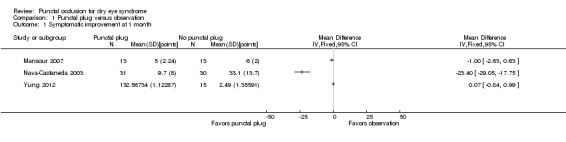
Comparison 1 Punctal plug versus observation, Outcome 1 Symptomatic improvement at 1 month.
1.2. Analysis.
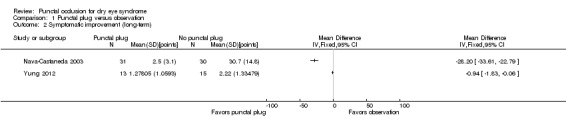
Comparison 1 Punctal plug versus observation, Outcome 2 Symptomatic improvement (long‐term).
Comparison 2. Punctal plugs versus cyclosporine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ocular surface staining at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Aqueous tear production at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Aqueous tear production at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Artificial tear use at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Artificial tear use at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.3. Analysis.
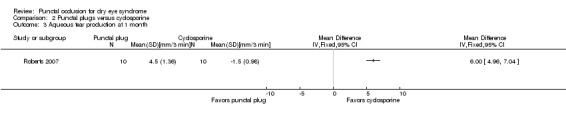
Comparison 2 Punctal plugs versus cyclosporine, Outcome 3 Aqueous tear production at 1 month.
2.4. Analysis.
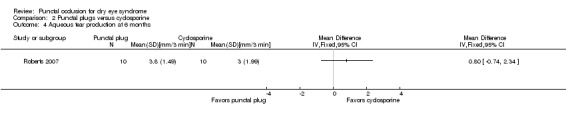
Comparison 2 Punctal plugs versus cyclosporine, Outcome 4 Aqueous tear production at 6 months.
2.6. Analysis.
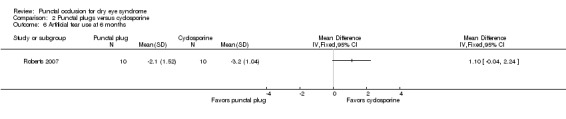
Comparison 2 Punctal plugs versus cyclosporine, Outcome 6 Artificial tear use at 6 months.
Comparison 3. Punctal plugs versus oral pilocarpine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic improvement at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Punctal plugs versus artificial tears.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic improvement at 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Symptomatic improvement at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic improvement at 3 months | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐5.87, ‐2.53] |
| 4 Ocular surface staining at 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Ocular surface staining at 3 months (Rose Bengal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Ocular surface staining at 3 months (fluorescein) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Aqueous tear production at 2 weeks | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐1.05, 2.71] |
| 8 Aqueous tear production at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Right | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Left | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Aqueous tear production at 3 months | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 2.16 [1.41, 2.90] |
| 10 Tear film stability at 2 weeks | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.57, 1.09] |
| 11 Tear film stability at 3 months | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [0.60, 1.44] |
| 12 Punctate epithelial keratopathy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Punctal plugs in the upper versus lower puncta.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic improvement at 1 month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Aqueous tear production at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Tear film stability at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Acrylic punctal plugs versus silicone punctal plugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic improvement at 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining at 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Rose Bengal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fluorescein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production at 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Tear film stability at 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Artificial tear use at 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
6.5. Analysis.
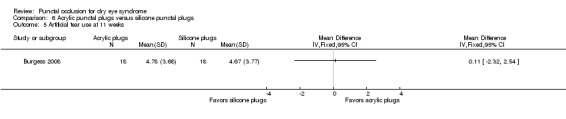
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 5 Artificial tear use at 11 weeks.
Comparison 7. Intracanalicular plugs versus silicone punctal plugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic improvement (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Rose Bengal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fluorescein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Schirmer test I without anesthesia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Tear film stability (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Artificial tear use (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altan‐Yaycioglu 2005.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? No missing data reported Reported power calculation: NR Unusual study design: 22 eyes of age‐ and gender‐matched healthy volunteers were included (tear production and film stability, lacrimal scintigraphy measurements only; no punctal plugs inserted) |
|
| Participants |
Country: Turkey Number randomized: 24 participants (48 eyes) in total 11 participants (22 eyes) in collagen punctal plugs group 13 participants (26 eyes) in silicone punctal plugs group Exclusions after randomization: none reported Number analyzed: 24 participants (48 eyes) in total 11 participants (22 eyes) in collagen punctal plugs group 13 participants (26 eyes) in silicone punctal plugs group Losses to follow‐up: none reported Overall mean age (SD): NR Age range: NR Sex (%): 21 women (88%) and 3 men (12%) in total; by group not reported Inclusion criteria: participants were diagnosed with aqueous tear deficiency; no previous history of punctal plug insertion. All using artificial tears with no subjective or objective improvement in symptoms Exclusion criteria: NR |
|
| Interventions | No mention of artificial tears use Intervention 1: collagen punctal plugs were inserted in the lower punctum Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Intervention 2: silicone punctal plugs were inserted in the lower punctum Punctal plug model: Odyssey‐Parasol Punctal Occluder A14 to 203 Manufacturer of punctal plug: Oasis Medical Location of manufacturer: Memphis, TN Length of follow‐up: Planned: protocol not available Actual: 3 days |
|
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: tear production (Schirmer I test at 5 minutes performed before and immediately after occlusion); tear film stability (tear break‐up time (TBUT) measured in seconds before and immediately after occlusion); ocular surface staining (Rose Bengal strip before and 3 days after occlusion) Adverse events reported: authors reported "no adverse events," but did not define which adverse events were collected Intervals at which outcomes assessed: before treatment, after treatment, and 3 days post‐treatment |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported Investigators did not discuss how they accounted for the correlation between eyes of the same person |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants and people administering interventions. |
| Masking of outcome assessment (detection bias) | Unclear risk | Nuclear medicine specialist (outcome assessor) evaluating lacrimal scintigraphy images was masked to treatment assignment (but not an outcome of this review) Unclear whether other outcome assessors masked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No sample size information included in Results section, so it is unclear whether all participants completed follow‐up examinations. Most outcomes assessed immediately after insertion of the punctal plugs (ocular surface staining assessed 3 days after occlusion) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine. Trial registry information and protocol were not available for comparison |
| Other bias | Unclear risk | Insufficient information to determine. Source of funding and conflict of interest not reported |
Brissette 2015.
| Methods |
Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: each eye of each participant was randomized to a different intervention How were missing data handled? Excluded from analysis Reported power calculation: sample size = 50 participants (25 participants per group); power = 80.7% Unusual study design: none |
|
| Participants |
Country: Canada Number randomized: 26 participants (52 eyes) in total 26 participants (26 eyes) in collagen punctal plugs group 26 participants (26 eyes) in silicone punctal plugs group Exclusions after randomization: none reported Number analyzed: 25 participants (50 eyes) in total 25 participants (25 eyes) in collagen punctal plugs group 25 participants (25 eyes) in silicone punctal plugs group Losses to follow up: 1 participant (2 eyes) in total 1 participants (1 eyes) in collagen punctal plugs group 1 participants (1 eyes) in silicone punctal plugs group Mean age (SD): 60.05 years (NR) overall 61.7 (17.67) years in the collagen punctal plug group 58.4 (16.18) years in the silicone punctal plug group Age range: NR Sex (%): 21 women (81%) and 5 men (19%) in total 10 women (77%) and 3 men (23%) in the collagen punctal plug group 11 women (85%) and 2 men (15%) in the silicone punctal plug group Inclusion criteria: "moderate to severe subjective dry eye symptoms as per the Canadian Dry Eye Assessment (CDEA), a validated dry eye symptoms questionnaire based on the Ocular Surface Disease Index (OSDI)" (p 239) Exclusion criteria: "dry eye secondary to systemic inflammatory conditions, punctal cautery, punctal stenosis, silicone allergy, and inability to attend multiple follow‐up visits for 6 months" (p 239) |
|
| Interventions | Both groups were allowed to use artificial tears throughout the follow up period Intervention 1: collagen punctal plugs were inserted in the lower punctum Punctal plug model: Parasol Manufacturer of punctal plug: Odyssey Medical Location of manufacturer: Memphis, TN, USA Intervention 2: silicone punctal plugs were inserted in the lower punctum Punctal plug model: Super Flex Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN, USA Length of follow‐up: Planned: 6 months Actual: 6 months |
|
| Outcomes |
Primary outcomes reported: punctal plug retention at 6 months "Retention was characterized by last examined date with the punctal plug in place. For example, if a patient returned for his or her 4‐month visit, and the plug was gone, the plug was recorded as 3 months of retention." (p 239) Secondary outcomes reported: Schirmer I (mm), tear meniscus height as measured at the slit lamp (mm), TBUT (in seconds), inferior fluorescein corneal staining (National Eye Institute (NEI) scale), and average lissamine green conjunctival staining (NEI scale), artificial tear drop frequency Adverse events reported: none reported Intervals at which outcomes assessed: monthly for primary outcome up to 6 months; months 1, 3, and 6 for secondary outcomes |
|
| Notes |
Trial registry: NCT01947517 (clinicaltrials.gov) Type of study: published full‐text Funding sources: "The authors indicate no funding support" (p 242) Disclosures of interest: "none were reported" (p 242) Study period: September 2013 to May 2014 (from clinicaltrials.gov) *We contacted the authors via email and have received additional information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was achieved using a mathematical computer‐generated allocation schema based on permuted blocks with blocks of random sizes" (p 239). "Each eye was assigned randomly with equal probability to receive either Super Flex or Parasol brand punctal plugs" (p 239). |
| Allocation concealment (selection bias) | Low risk | From email correspondence with authors: "we had opaque envelopes with assignment once deemed eligible for inclusion." |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | "Participants and all study staff, except an unmasked investigator (A.B.) who inserted punctal plugs, were masked to treatment arms" (p 239). |
| Masking of outcome assessment (detection bias) | Low risk | "Plug retention and all other secondary outcomes were evaluated by 1 examiner masked to the treatment arms (Z.M.)" (p 239). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/50 eyes (4%) were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Trial was registered at clinicaltrials.gov and all pre‐specified outcomes in the registry were reported in the full‐text publications. |
| Other bias | Low risk | "All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest and none were reported. The authors indicate no funding support" (p 242). |
Burgess 2008.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: unclear as both eyes of 15 participants could have receive the same or different intervention Unit of randomization: “each eye was treated as independent for the purposes of the study” (p 391) How were missing data handled? NA, no missing data reported Reported power calculation: NR Unusual study design: authors did not perform the appropriate pair‐wise analysis and each eye treated as being independent |
|
| Participants |
Country: UK (assumed from author's origin) Number randomized: 21 participants (36 eyes) in total NR participant (18 eyes) in silicone punctal plug group NR participants (18 eyes) in acrylic punctal plug group Exclusions after randomization: none reported Number analyzed: 21 participants (36 eyes) in total NR participant (18 eyes) in silicone punctal plug group NR participants (18 eyes) in acrylic punctal plug group Losses to follow‐up: none reported Overall mean age (SD): 60.0 (NR) years in total; by group not reported Age range: 33‐78 years; by group not reported Sex (%): 20 women (95%) and 1 man (5%) in total; by group not reported Inclusion criteria: participants with subjective symptoms consistent with dry eye, tear film break‐up time of < 5 seconds, and ocular surface abnormalities as demonstrated by fluorescein or Rose Bengal staining. All used artificial tears for more than 6 months with no subjective or objective improvement in symptoms. Exclusion criteria: use of punctal plugs within previous 6 months or contact lens use |
|
| Interventions | Both groups were allowed to use artificial tears throughout the follow up period Intervention 1: silicone punctal plugs were inserted in the lower punctum Punctal plug model: Soft Plug Manufacturer of punctal plug: Oasis Medical Location of manufacturer: Glendora, CA Intervention 2: acrylic punctal plugs were inserted in the lower punctum Punctal plug model: SmartPlugs Manufacturer of punctal plug: Medennium Location of manufacturer: Irvine, CA Length of follow‐up: Planned: NR Actual: mean follow‐up 11.2 weeks (range, 8‐18 weeks) in total 11.27 ± 2.54 weeks in silicone punctal plug group 11.11 ± 2.56 weeks in acrylic punctal plug group |
|
| Outcomes | Primary and secondary outcome were not distinguished Outcomes reported: Subjective symptoms: dryness, foreign body sensation, grittiness, stinging, pain, itching and burning; 10 cm visual analog scale; scores added to derive a summary score Tear film stability: TBUT measured in seconds Tear meniscus height: measured using calibrated slit‐lamp in mm, midway between canthi along the lower lid Average of 3 measurements Tear production: Schirmer I test without anesthesia Ocular surface staining: Rose Bengal staining in nasal and temporal cornea and conjunctiva; graded on 0‐3 scale; fluorescein staining in 5 areas of cornea; graded on 0‐3 scale Topical artificial tears used Adverse events reported: NR Intervals at which outcomes assessed: all above outcomes were assessed before and at approximately 11 weeks after occlusion (mean 11.2 weeks; range 8 to 18 weeks) |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: National Health Service Lothian, Edinburgh, UK Disclosures of interest: "the authors state that they have no proprietary interest in the products named in this article" Study period: NR Reported subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomization; computer generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were also masked to treatment assignment |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessments performed by investigators not involved in the treatment administration and not informed of treatment status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size information is not included for all outcomes, so it is unclear whether all randomized participants completed follow‐up examinations |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make comparison. All outcomes reported in the methods section were reported |
| Other bias | Unclear risk | Received government funding (National Health Service Lothian, Edinburgh, UK) |
Chen 2010.
| Methods |
Study design: parallel randomized control trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? NA Reported power calculation: no Unusual study design: the study included 40 participants, 20 of whom were healthy controls. For this review, we will only refer to 20 dry eye participants. Healthy controls were also randomly assigned: "The 40 eyes of the normal subjects (group II) were similarly assigned to group IIA (upper punctal occlusion group, n = 20 eyes) or group IIB (lower punctal occlusion group, n = 20 eyes)." |
|
| Participants |
Country: China Number randomized: Total: 20 participants (40 eyes) Per group: 10 participants (20 eyes) Exclusions after randomization: none Number analyzed: Total: 20 participants (40 eyes) Per group: 10 participants (20 eyes) Losses to follow‐up: none Mean age ± SD (years): 22.5 ± 2.4 total; by group not reported Sex (%): 16 women (80%) and 4 men (20%); by group not reported Inclusion criteria: subjective symptoms of dry eye, a Schirmer I test result < 5 mm or TBUT < 5 seconds, and evidence of corneal surface damage on fluorescein staining Exclusion criteria: history of atopy; allergic diseases; Stevens‐Johnson syndrome; chemical, thermal, or radiation injury; or any other ocular or systemic disorder or had undergone any ocular surgery or contact lens use that would create an ocular surface problem or dry eye; lacrimal dysfunction, as determined by slit lamp examination and irrigation Equivalence of baseline characteristics: yes |
|
| Interventions | No mention of artificial tear use Intervention 1: collagen punctal plugs in the lower puncta (1A) Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Intervention 2: collagen punctal plugs in the upper puncta (1B) Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Length of follow‐up: Planned: protocol not available Actual: 10 days |
|
| Outcomes |
Primary and secondary outcome not differentiated, as defined in study reports: symptom scoring, upper and lower tear menisci, tear breakup time, corneal fluorescein staining, and Schirmer I test Adverse events reported: "no complication was observed in dry eye patients or control subjects during the period of this study" Intervals at which outcomes assessed: day 1, 4, 7, and 10 |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: Chinese National Science and Technology Development Supporting Program and Zhejiang Provincial Program for the Cultivation of High‐level Innovative Health Talents Disclosures of interest: none Study period: NR Reported subgroup analyses: yes, healthy controls and dry eye groups Did trial investigators need to be contacted? yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make comparison. All outcomes reported in the Methods section were reported |
| Other bias | Unclear risk | Received government funding (Chinese National Science and Technology Development Supporting Program and Zhejiang Provincial Program for the Cultivation of High‐level Innovative Health Talents) |
Farrell 2003.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? NA Reported power calculation: NR Unusual study design: 45 age‐ and gender‐ matched healthy volunteers were included (for comparison with dry eye participant baseline measurements only; no punctal plugs inserted) |
|
| Participants |
Country: UK Number randomized: 62 participants (121 eyes) in total NR participants (71 eyes) in lower punctum group NR participants (50 eyes) in lower and upper punctum group Exclusions after randomization: none reported Number analyzed: 62 participants (121 eyes) in total NR participants (71 eyes) in lower punctum group NR participants (50 eyes) in lower and upper punctum group Losses to follow‐up: none reported Overall mean age (SD): NR Age range: 24‐87 years Sex (%): 52 women (84%) and 10 men (16%) in total; by group not reported Inclusion criteria: (at least 3 of the 4 had to be met to be eligible): Schirmer score < 10 mm at 5 minutes; TBUT < 10 seconds; Mucin filaments and tear meniscus discontinuity; Rose Bengal score of ≥ 3.5 (corneal epithelium and bulbar conjunctiva) Exclusion criteria: NR |
|
| Interventions | No mention of artificial tears use Intervention 1: collagen punctal plugs were inserted in the lower punctum of 71 eyes Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics, Inc Location of manufacturer: NR Intervention 2: collagen punctal plugs were inserted in the lower and upper puncta of 50 eyes Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics, Inc Location of manufacturer: NR Length of follow‐up: Actual: 12 days |
|
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported:
Adverse events reported: Intervals at which outcomes assessed: 5 and 12 days after occlusion |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: Lacrimedics, Inc donated collagen plugs Disclosures of interest: "none of the authors have any other vested interest in Lacrimedics Inc. or their products" Study period: NR Reported subgroup analyses: none reported Each eye treated as independent |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were also masked to treatment assignment |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessors measuring tear parameters were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sample sizes reported in the results were consistent with the number randomized, so no loss to follow‐up or missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine |
| Other bias | Unclear risk | Insufficient information to determine |
Feng 2011.
| Methods |
Study design: unclear if it was a parallel or paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: unclear if each eye of each participant were randomized to the same or different interventions How were missing data handled? NR Reported power calculation: none reported Unusual study design: no |
|
| Participants |
Country: China Number randomized: Total: 54 participants (108 eyes) Per group: 27 participants (54 eyes) Exclusions after randomization: none Number analyzed: Total: 54 participants (108 eyes) Per group: 27 participants (54 eyes) Losses to follow‐up: NR Mean age ± SD: 20 ± 6 years; by group not reported Age range: 18‐34 years in total; by group not reported Sex (%): 19 men (35%) and 35 women (65%) in total; by group not reported Inclusion criteria: "patients treated with LASIK in our hospital" (p 1666) Exclusion criteria: NR |
|
| Interventions |
Intervention 1: collagen punctual occlusion Punctal plug model: A12‐103 Manufacturer of punctal plug: Odyssey Location of manufacturer: NR Intervention 2: artificial tear (dextran and hypromellose eye drops) by Alcon, 1 drop 3 times/day. Length of follow‐up: Planned: protocol not available Actual: 2 weeks after punctual occlusion surgery |
|
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported:
Adverse events reported: NR Intervals at which outcomes assessed: NA |
|
| Notes |
Type of study: published Funding sources: NR Disclosures of interest: NR Study period: June 2009 to September 2009 Reported subgroup analyses: no Do trial investigators need to be contacted? Yes, the investigators need to be contacted for randomization method |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The investigator stated "we randomly picked 54 patients (108 eyes) receiving LASIK surgery in our hospital from June to September in 2009 . . . The patients were randomly assigned to 2 groups, 27 individuals (54 eyes) in each group (p 1666)" The trial investigator did not describe how the random sequence were generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions. |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Due to the nature of the treatments, participants and personnel cannot be masked for this study, and the results are likely to be influenced by the lack of masking. |
| Masking of outcome assessment (detection bias) | Unclear risk | The investigator stated that "all the tests and surgeries were conducted by the same physician", thus the outcome assessor (i.e., the physician) were aware of the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available and all of the outcomes were reported as prespecified in the Methods section |
| Other bias | Unclear risk | Funding sources and disclosures of interest were not reported. |
Kaido 2012.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: right eye of each participant Unit of randomization: participant; both eyes received the same treatment but only the right eye was analyzed How were missing data handled? NA Reported power calculation: none reported Unusual study design: unclear whether both of eyes had similar values of TBUT at baseline |
|
| Participants |
Country: Japan Number randomized: 43 participants (43 eyes) in total 19 participants (19 eyes) in upper occlusion group 24 participants (24 eyes) in lower occlusion group Exclusions after randomization: none Number analyzed: 43 participants (43 eyes) in total 19 participants (19 eyes) in upper occlusion group 24 participants (24 eyes) in lower occlusion group Losses to follow‐up: none Mean age ± SD: 55.8 ± 16.1 years; by group not reported Age range: 22‐82 years in total; by group not reported Sex (%): 9 men (21%) and 34 women (79%) in total; by group not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | No mention of artificial tears Intervention 1: silicone punctal plugs in the lower puncta Punctal plug model: Super Flex plug Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN Intervention 2: silicone punctal plugs in the upper puncta Punctal plug model: Super Flex plug Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN Length of follow‐up: Planned: protocol not available Actual: 1 month |
|
| Outcomes |
Primary and secondary outcomes not differentiated, as defined in study reports:
Adverse events reported: no Intervals at which outcomes assessed: 1 month |
|
| Notes |
Type of study: published full‐text Trial registry: NR Funding sources: NR Disclosures of interest: "The authors have no financial or conflicts of interest to disclose (p 1009)." Study period: NR Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine |
| Other bias | Unclear risk | Source of funding was not reported and authors explicitly stated no conflicts of interest |
Lowther 1995.
| Methods |
Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: eyes; each eye of each participant were randomized to a different intervention How were missing data handled? NA Reported power calculation: yes, sample size = 32 (64 eyes); P value = 0.05; 50% of the patients indicated that the eye with the implants was better, 25% said the opposite eye was better, and 25% said no difference Unusual study design: authors did not perform the appropriate pair‐wise analysis and each eye treated as independent |
|
| Participants |
Country: USA (assumed from author's origin) Number randomized: Total: 32 participants (64 eyes) Per group: 32 eyes Exclusions after randomization: none reported Number analyzed: 32 participants (64 eyes); 32 eyes in each group Losses to follow‐up: none reported Overall mean age (SD): participant age was collected but not reported Age range: participant age was collected but not reported Sex (%): NR Inclusion criteria: participants reporting bilateral dry eye symptoms (based on responses to a modified McMonnies' questionnaire) and wearing hydrogel contact lenses were included Exclusion criteria: none reported |
|
| Interventions | No mention of artificial tears Intervention 1: collagen intracanalicular plugs were inserted in the upper and lower puncta Punctal plug model: 0.3 mm diameter Manufacturer of punctal plug: Lacrimedics, Inc. Location of manufacturer: Rosemead, CA Intervention 2: sham plug insertion in participants contralateral eye Length of follow‐up: Planned: protocol not available Actual: 5 days |
|
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: Subjective symptoms: McMonnies symptom questionnaire (modified) Tear meniscus height: measured using video slit‐lamp in mm Tear film stability: TBUT measured in seconds Ocular surface staining: Rose Bengal and fluorescein staining scored 0 (no staining) to 4 (heavy, coalesced staining) Tear lactoferrin immunoassay test: lactoplate tear lactoferrin immunoassay test; left in cul‐de‐sac for 2‐4 minutes; precipitation ring diameter measured in mm 3 days later Adverse events reported: Intervals at which outcomes assessed: before and at 5 days after occlusion |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: funded by Bausch and Lomb inVision Institute; Eagle Vision, Inc. supplied implants and Lactoplate tests Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript. "The patients were randomly assigned to have the implants put into either the right or the left eye but were lead to believe that implants were put in both eyes" (p 238). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript. "The eye to receive the implants was determined just before insertion by randomization" (p 239). |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were also masked to treatment assignment. "All of the steps of inserting the implants were performed on both eyes, but an implant was inserted in the upper and lower punctum of one eye only. Therefore, the patients did not know that only one eye received the implants. Because the patients did not know that the puncta of only one eye received the implants and the investigator making the measurements did not know which eye of the patient received the implant, the study was double masked" (p 239). |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessors were masked. "Because the patients did not know that the puncta of only one eye received the implants and the investigator making the measurements did not know which eye of the patient received the implant, the study was double masked" (p 239). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sample sizes reported in the results were consistent with the number randomized, so no loss to follow‐up or missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine. Trial registry information and protocol not available for comparison |
| Other bias | Unclear risk | Insufficient information to determine. The source of funding of the study (Bausch and Lomb inVision Institute) was different from the manufacturer (Lacrimedics, Inc.) |
Mansour 2007.
| Methods |
Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: each eye of each participant was randomized to a different intervention How were missing data handled? Participants lost to follow‐up were excluded from analysis Reported power calculation: NR Unusual study design: authors did not perform the appropriate pair‐wise analysis and each eye treated as independent |
|
| Participants |
Country: Netherlands (assumed from location of ethics committee) Number randomized: Total: 20 participants (40 eyes) Per group: 20 eyes in each group Exclusions after randomization: none reported Number analyzed: 13 participants (26 eyes); 13 eyes in each group Losses to follow‐up: 7 participants (14 eyes); 7 eyes in each group Overall mean age (SD): NR Age range: NR Sex (%): 17 women (85%) and 3 men (15%) Inclusion criteria: European criteria for the diagnosis of Sjögren's syndrome were used to identify eligible participants: subjective symptom report (ocular and oral symptoms of dryness). Schirmer test, Rose Bengal staining, and TBUT; xerostomia Exclusion criteria: none reported |
|
| Interventions | No mention of artificial tears Intervention 1: silicone punctal plugs inserted in the upper and lower puncta Punctal plug model: tapered‐shaft silicone punctal plugs, 0.7 mm diameter Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN Duration of plug occlusion was 6 to 20 weeks If plugs extruded during course of study, larger plug inserted and follow‐up deferred for period of at least 6 weeks Intervention 2: other eye of participant remained unoccluded Length of follow‐up: Planned: protocol not available Actual: between 6 to 20 weeks |
|
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: Subjective discomfort (at least 1 of the following complaints): foreign body sensation, photophobia, stinging, pain, burning, and ocular fatigue Subjective symptoms: abovementioned symptom discomfort complaints scored and scores added to derive a summary score (0 to 10) Tear production: Schirmer test without anesthesia Ocular surface staining: Rose Bengal staining scored according to Van Bijsterveld classification Mucus debris: measured on scale of 0 (no mucus debris) to 3 (mucus threads and filaments) Adverse events reported: NR Intervals at which outcomes assessed: before and at least 6 weeks after occlusion |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization scheme. "The eye to be occluded was chosen at random using a computer‐generated randomization scheme" (p 148). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions |
| Masking of outcome assessment (detection bias) | High risk | The same investigator performed all measurements and was presumably unmasked to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/20 participants (30%) excluded from the analysis because of spontaneous plug loss and 1 excluded after an inflammatory reaction to plug material |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine. Trial registry information and protocol were not available for comparison |
| Other bias | Unclear risk | Insufficient information to determine. Funding source and disclosure of interest not reported |
Nava‐Castaneda 2003.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? NA, no missing data reported Reported power calculation: yes, sample size = 30 participants in each group; "based on the assumption that 80% of the patients subjected to canalicular occlusion would have a successful outcome (reduction in dry eye/conjunctivitis symptoms), in contrast to 30% of those receiving medical treatment alone (artificial tears). It was further assumed that 74 patients would have to be randomized to the 2 treatment groups to allow for dropouts and ensure that 60 patients would complete the study" (p 11) |
|
| Participants |
Country: Mexico Number randomized: 61 participants (122 eyes) in total 31 participants (31 eyes) in Collagen/silicone plug group 30 participants (30 eyes) in sham group Exclusions after randomization: 1 participant (2 eyes) Number analyzed: 60 participants (120 eyes); 30 participants (60 eyes) in each group Losses to follow‐up: none reported Overall mean age (SD): 49.8 (NR) years in total; by group not reported Age range: 23‐80 years Sex (%): 50 women (82%) and 11 men (18%) in total; by group not reported Inclusion criteria: 18‐80 years old, 2 subjective symptoms of dry eye, ocular surface abnormalities as demonstrated by fluorescein score > 1, conjunctivitis of ≥ 1 month Exclusion criteria: participants were excluded if dry eye attributed to other ocular conditions (see Nava‐Castaneda 2003) or if diagnosed with asthma |
|
| Interventions | No mention of artificial tears use Intervention 1: collagen/silicone plug group (experimental): Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Collagen punctal plugs inserted in the upper and lower canaliculi of both eyes. 2 weeks after initial insertion, 1 silicone punctal plug inserted in the upper and 2 collagen plugs inserted in lower canaliculi of both eyes. 4 weeks after initial insertion, permanent plug inserted in lower canaliculi Control: sham plug insertion at same intervals as collagen/silicone plug group. Same procedures as collagen/silicone group, but eyes were not occluded Length of follow‐up: Planned: protocol not available Actual: 8 weeks after initial occlusion |
|
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: Subjective symptoms: visual performance and comfort; assessed using a 10 cm visual analogue scale, very poor vision/very uncomfortable and very good vision/very comfortable at the boundaries of the scale Frequency and severity of dry eye (watery eyes, itching, burning, dryness, fluctuating vision, sandy/foreign body sensation, light sensitivity) and conjunctival (discharge and redness) symptoms; frequency scored 0 (never) to 5 (continually, every hour of the day) and severity 0 (no symptom) to 3 (severe); frequency and severity score for each symptom multiplied and scores summed to derive summary score. Ocular surface staining: fluorescein staining scored 0 (absent) to 4 (severe) Topical artificial tears used: frequency scored 0 (never) to 5 (continually, every hour of the day) Visual acuity Adverse events reported: yes, corneal ulcer (1 participant; not related to treatment) and epiphora (1 participant; related to treatment) Intervals at which outcomes assessed: before and at 1 hour and 2, 4, 8 weeks after initial occlusion |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: Lacrimedics, Inc Disclosures of interest: none reported Study period: NR Reported subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme. |
| Allocation concealment (selection bias) | Low risk | Random assignments prepared by Statistical Committee and placed in sealed envelopes numbered 1‐74; randomization list maintained by Statistical Committee |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants masked to treatment assignment. "Patients subjected to the sham procedure were treated identically to the collagen/silicone plug implantation group (i.e., the punctum was dilated and the canaliculus probed), except that a plug was not actually inserted" (p 11). |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessors masked to treatment assignment. "Subsequent patient evaluations were performed by one of the initial evaluators who were kept uninformed of the patient's treatment status" (p 11). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 randomized participant was found to be ineligible, but was not excluded from the analysis. 1/61 participants (2%) discontinued treatment and was excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine although the investigators list the following protocol deviations in the publication: 61 patients instead of 60 enrolled, 1 patient was ineligible, and covariate analyses were not performed |
| Other bias | Unclear risk | Device manufacturer is the funding source |
Qiu 2012.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? Excluded from analysis Reported power calculation: NR Unusual study design: no |
|
| Participants |
Country: China Number randomized: 28 participants (56 eyes) in total 12 participants (24 eyes) in punctal plug group 16 participants (32 eyes) in artificial tears group Exclusions after randomization: none reported Number analyzed: 28 participants (56 eyes) in total 12 participants (24 eyes) in punctal plug group 16 participants (32 eyes) in artificial tears group Losses to follow‐up: none reported Mean age (SD): 31.75 (NR) years 31.4 (15.1) years in punctal plug group 32.1 (12.8) years in artificial tears group Age range: 22‐67 Sex (%): 18 women (64%) and 10 men (36%) in total; by group not reported Inclusion criteria: "The entry criteria for the patients were that they were diagnosed with dry eyes at our ophthalmology clinic and had no evidence of ocular diseases other than those associated with dry eye changes, such as superficial punctate keratopathy (SPK)" (p 20) Exclusion criteria: none listed |
|
| Interventions |
Intervention 1: acrylic punctal plug Punctal plug model: SmartPLUG500 Manufacturer of punctal plug: Medenium Location of manufacturer: Irvine, CA Control: artificial tear solution (Zhuhai Yisheng, Guangdong, China) containing a carbomer gel and basic fibroblast growth factor Length of follow‐up: Planned: 2 weeks Actual: 2 weeks, extended to 4 weeks for corneal fluorescein staining |
|
| Outcomes | Primary and secondary outcomes not differentiated Outcomes, as defined by study:
Adverse events reported: punctate epithelial keratopathy Intervals at which outcomes assessed: baseline, 2 weeks |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: "seed fund (no. 79495‐01) and Linhu fund (no. 79495‐02) of Peking University Hospital" Disclosures of interest: reported no conflicts of interest Enrollement period: May 2009 to October 2009 Reported subgroup analyses: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not report how the random sequence was generated. "They were randomly assigned into artificial tears group and punctual plugs group" (p 20) |
| Allocation concealment (selection bias) | Unclear risk | Authors did not report how allocation was concealed. "They were randomly assigned into artificial tears group and punctual plugs group" (p 20) |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Due to the nature of the treatments, participants and personnel cannot be masked for this study, and the results are likely to be influenced by the lack of masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 2/42 lost to follow‐up, even across groups. However, reason for lost to follow‐up was not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and trial registry number not reported |
| Other bias | Low risk | Non‐industry funding and reported no conflict of interest |
Qiu 2013.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: right eye of each participant Unit of randomization: participant as only the right eye of each participant was randomized to intervention or control How were missing data handled? Excluded from analysis Reported power calculation: NR Unusual study design: no |
|
| Participants |
Country: China Number randomized: 42 participants (42 eyes) in total 22 participants (22 eyes) in punctal plug group 20 participants (20 eyes) in artificial tears group Exclusions after randomization: none reported Number analyzed: 40 participants (40 eyes) in total 21 participants (21 eyes) in punctal plug group 19 participants (19 eyes) in artificial tears group Losses to follow‐up: 2 participants (2 eyes) in total 1 participant (1 eye) in punctal plug group 1 participant (1 eye) in artificial tears group Mean age (SD): overall NR 35.2 (16.5) years in punctal plug group 34.6 (1.3) years in artificial tears group Age range: 22‐67 years Sex (%): 36 women (90%) and 4 men (10%) in total 19 women (90.5%) and 2 men (9.5%) in the intervention group 17 women (89.5%) and 2 men (10.5%) in the observation group Inclusion criteria: "patients with dry eye who sought for treatment in our ophthalmology clinic from March to October in 2010, diagnosed with primary Sjögren's syndrome …The entry criteria for the patients were that they were diagnosed with dry eyes at our ophthalmology clinic and had no evidence of ocular diseases other than those associated with dry eye changes, such as superficial punctuate keratopathy (SPK)" (p 2544). Exclusion criteria: none specified |
|
| Interventions |
Intervention 1: acrylic punctal plug punctal plug model: SmartPLUG500 Manufacturer of punctal plug: Medenium Location of manufacturer: Irvine, CA Control: artificial tears (Zhuhai Yisheng, Guangdong, China) containing a carbomer gel and bFGF Length of follow‐up:3 months Planned: 3 months Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes not differentiated Outcomes, defined by the study:
Adverse events reported: NR Intervals at which outcomes assessed: baseline, 3 months |
|
| Notes |
Trial registry: not reported Type of study: published full‐text Funding sources: Seed Fund (No. 79495‐01) and Linhu fund (no. 79495) of Peking University Third Hospital Disclosures of interest: no conflicts of interest to report Enrollement period: March 2010 to October 2010 Reported subgroup analyses: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned into artificial tears group and punctual plugs group using a computer‐generated random number table" (p 2544). |
| Allocation concealment (selection bias) | Low risk | "Written allocation assignments were sealed in individual opaque envelopes marked only with study identification numbers" (p 2544). |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | "All para‐clinical examinations and analyses were performed by the same experienced technician and the same clinical staff who both were masked to the type of treatment" (p 2544). However given the nature of the interventions, participants and personnel could not be masked. |
| Masking of outcome assessment (detection bias) | Low risk | "All para‐clinical examinations and analyses were performed by the same experienced technician and the same clinical staff who both were masked to the type of treatment" (p 2544). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients did not complete the follow‐up period and were excluded from the analysis" (p 2545). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and trial registry number not reported |
| Other bias | Low risk | Non‐industry funding and reported no conflict of interest |
Rabensteiner 2013.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: participant; trial investigators reported the average of both eyes of each participant Unit of randomization: participant; both eyes received the same intervention How were missing data handled? Excluded from analysis Reported power calculation: NR Unusual study design: no |
|
| Participants |
Country: Austria Number randomized: 30 participants (60 eyes) in total 15 participants (30 eyes) in silicone punctual plugs group 15 participants (30 eyes) in intracanalicular SmartPlugs group Exclusions after randomization: none reported Number analyzed: 30 participants (57 eyes) in total 15 participants (27 eyes) in silicone punctual plugs group 15 participants (30 eyes) in intracanalicular SmartPlugs group Losses to follow‐up: none reported Mean age (SD): overall NR; by group not reported Age range: NR Sex (%): NR Inclusion criteria: "moderate to severe dry eye syndrome as described by the DEWS report 2007… typical dry eye symptoms, reduced tear break–up time of less than 5 seconds with either a Schirmer test without local anesthesia below 5 mm/ 5 min, or a vital staining score of the cornea (fluorescein) and conjunctiva (Rose Bengal) according to van Bijsterveld (> 3)" (p 522) Exclusion criteria: "Sjogren's syndrome, eyelid or blinking problems, contact lens use and previous punctal plug use" (p 522) |
|
| Interventions | Both groups used artificial tears as needed Intervention 1: silicone punctual plugs Punctal plug model: silicone punctual plugs Manufacturer of punctal plug: FCI Opthalmics Location of manufacturer: Issy‐les‐Moulineaux Cedex, France Intervention 2: intracanalicular SmartPlugs Punctal plug model: punctual plugs Manufacturer of punctal plug: Medenium Location of manufacturer: Irvine, CA Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes not differentiated Outcomes, as defined by study:
Intervals at which outcomes assessed: baseline, 3 months |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: "no funding source to declare" (p 524) Disclosures of interest: no conflicts of interest Enrollement period: unclear Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 30 patients were randomized into two groups … using the next available number from a set of block randomized computer numbers" (p 522). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were masked to treatment group. "Patients were not informed into which arm of the trial they had been allocated" (p 522). |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was differential across groups. "All 30 patients completed the study, but in three eyes of group I (collared silicone plugs) spontaneous loss of the plug was noticed at the follow‐up visit" (p 522) "Eyes with a spontaneous lost punctual plug at follow up visit were excluded" (p 522). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and trial registry number not reported |
| Other bias | Unclear risk | Non‐industry funding and reported no conflict of interest |
Roberts 2007.
| Methods |
Study design: parallel group randomized controlled trial Unit of analysis: participant; trial investigators reported the average of both eyes of each participant Unit of randomization: participant, both eyes received the same intervention How were missing data handled? Excluded from analyses Reported power calculation: no Unusual study design: "for Schirmer testing and rose bengal staining, data were collected from both eyes and averaged before statistical analysis" |
|
| Participants |
Country: USA Number randomized: 32 participants (64 eyes) in total 11 participants (22 eyes) in punctal plug group 11 participants (22 eyes) in cyclosporine group 10 participants (20 eyes) in cyclosporine + punctal plugs Exclusions after randomization: none Number analyzed: 30 participants (60 eyes) in total 10 participants (20 eyes) in each group Losses to follow‐up: 2 participants Mean age: 52.1 years in total; by group not reported Age range: 38‐63 years; by group not reported Sex (%): 25 women (83.3%) and 5 men (16.7%); by group not reported Inclusion criteria: "(1) chronic symptoms of burning, sandy, or scratchiness in both eyes; (2) daily need for multiple applications of artificial tears; and (3) rose bengal staining of grade 2 or higher (scale described below)." (p 391) Exclusion criteria:
|
|
| Interventions | All groups were allowed to use artificial tears throughout the follow up period Intervention 1: cyclosporine ophthalmic emulsion 0.05% (RESTASIS; Allergan, Irvine, CA) eye drops to both eyes twice daily Intervention 2: bilateral collagen punctal plugs in the lower lids only Punctal plug model: PARASOL (Punctal Occluder) Manufacturer of punctal plug: Odyssey Medical Location of manufacturer: Memphis, TN Intervention 3: bilateral collagen punctal plugs in the lower lids + cyclosporine eye drops to both eyes twice daily Length of follow‐up: Planned: protocol not available Actual: 6 months |
|
| Outcomes |
Primary and secondary outcome not differentiated, as defined in study reports: "Schirmer scores without anaesthesia, corneal and conjunctival rose bengal staining, and artificial tear use" Adverse events reported: 2 participants withdrew: 1 due to discomfort of plugs and 1 due to burning caused by cyclosporine Intervals at which outcomes assessed: 1, 3, and 6 months after occlusion |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: Allergan Disclosures of interest: "Dr. Roberts is a consultant for Allergan. The authors state that they have no proprietary interest in the products named in this article" (p 391) Study period: October 2003 to January 2005 Reported subgroup analyses: none reported Do trial investigators need to be contacted? Yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[A] computer‐generated randomization schedule" (p 391). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias and detection bias) | High risk | "Medication was dispensed open‐label" (p 391). |
| Masking of outcome assessment (detection bias) | High risk | "Medication was dispensed open‐label" (p 391). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who with drew were replaced and data from withdrawals not included in analyses |
| Selective reporting (reporting bias) | Unclear risk | Trial registry record not reported; we were not able to compare the reported outcomes with the trial registry record |
| Other bias | High risk | Industry funded study |
Slusser 1998.
| Methods |
Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; used paired t‐tests to account for correlation between left and right eyes of each participant Unit of randomization: each eye of each participant was randomized to a different intervention How were missing data handled? Excluded from analysis Reported power calculation: no Unusual study design: used correct matched analysis |
|
| Participants |
Country: USA Number randomized: Total: 35 participants (70 eyes) Per group: 35 eyes per group Exclusions after randomization: 7 participants (14 eyes) Number analyzed: Total: 28 participants (56 eyes) Per group: 28 eyes Losses to follow‐up: 1 participant (2 eyes) Mean age (SD): NR Age range: 21‐69 years in total; by group not reported Sex (%): 26 women (74%) and 9 men (26%) in total; by group not reported Inclusion criteria: "1. Dry eye based on the response of at least 'sometimes' on at least 2 of the 3 questions concerning dryness, lens awareness, and cloudy vision on the recruitment questionnaire (see Table 2). 2. At least one of the following objective signs: grade 1 or greater vital staining; prelens tear film break‐up time of less than 15 seconds; tear meniscus height of less than 0.5 mm on slitlamp examination; grade 2 or more tear debris on a 0 to 4 scale. 3. Bilateral involvement of the above criteria. 4. Ability to understand and complete subjective scales daily. 5. Wearing contact lenses that are of equal age, type, and material. 6. Must be able to wear both contact lenses at least 20 hours/week." Exclusion criteria: "1. Dry eye attributed to poor lid apposition or blinking mechanism. 2. Contact lens surface abnormalities (deposits), which cause distortion of a reflected grid pattern. 3. Clinically apparent nasolacrimal occlusion. 4. Patients who take certain hormones (birth control pills, menopausal replacement therapy) who cannot maintain current dosage throughout the study duration. 5. Ocular infection, including active corneal ulcers, keratitis, or conjunctivitis. 6. Use of any topical agents in the eye other than artificial tears, saline, or rewetting drops at the time of study entry. 7. Under 18 yr of age. 8. Known pregnancy." |
|
| Interventions |
Intervention 1: silicone punctal plugs in the lower and upper puncta Punctal plug model: Herrick Lacrimal Plugs Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Rialto, CA (upper and lower) – 1 eye 1st 4 weeks; plugs + re‐wetting drops 5th week Intervention 2: sham treatment No plugs (sham) ‐ fellow eye – 1st 4 weeks; re‐wetting drops only 5th week Length of follow‐up: Planned: protocol not available Actual: 5 weeks |
|
| Outcomes |
Primary and secondary outcome not differentiated, and were defined in study reports: tear film break‐up time, lens water content, vital staining, bulbar conjunctiva with fluorescein, Rose Bengal, patient questionnaires Adverse events reported: yes, 3 participants reported epiphora and plugs removed Intervals at which outcomes assessed: day 0, 7, 28, 35 |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: Vistaken; Dr. Slusser. "Lacrimedics, Inc. (Rialto, CA) donated the lacrimal plugs and supportive equipment used in this study. Allergan, Inc. (Irvine, CA) provided the non‐preserved rewetting drops (p 337)." Disclosures of interest: NR Study period: March to June; year of study period not reported Reported subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | "To provide a placebo control, the fellow eye was carefully manipulated in a similar fashion without actual insertion of any plugs. The patient was masked as much as possible to avoid visualizing the plugs during the insertion procedure." |
| Masking of outcome assessment (detection bias) | Low risk | "Outcome assessors masked as independent investigator inserted the plugs and performed sham insertion for those receiving no plugs" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/35 participants (20%) were excluded after randomization |
| Selective reporting (reporting bias) | Unclear risk | Trial registry or protocol was not reported, hence we were not able to check if all outcomes in the protocol were reported in the full‐text publication |
| Other bias | High risk | Received industry funding |
Tsifetaki 2003.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: participant; (both eyes of each participant; mean outcome of left and right eyes reported respectively) Unit of randomization: participant; both eyes received the same intervention How were missing data handled? NR Reported power calculation: power = 90%; sample size = 25 participants; assuming 90% response rate in the group receiving pilocarpine and a 30% response rate in the group receiving artificial tears Unusual study design: participants with dry eye due to Sjögren's syndrome |
|
| Participants |
Country: Greece (assumed from author's origin) Number randomized: 85 participants (170 eyes) in total 28 participants (NR eyes) in collagen plugs group 29 participants (NR eyes) in oral pilocarpine group 28 participants in artificial tears only group Exclusions after randomization: 1 participant (2 eyes) in collagen plugs group Number analyzed: NR Losses to follow‐up: 1 participant (2 eyes) in collagen plugs group Mean age (SD): 57.8 (12.9) years in collagen plugs group 59.9 (9.9) years in oral pilocarpine group 57.0 (11.5) years in artificial tears only group Age range: NR Sex (%): 85 women (100%) and 0 men in total Inclusion criteria: European criteria for the diagnosis of Sjögren's syndrome were used to identify eligible participants Exclusion criteria: none reported |
|
| Interventions | All groups used artificial tears Intervention 1: collagen plugs inserted in the lower puncta of both eyes for 7 days then permanent collagen plugs for the duration of the trial + artificial tears Punctal plug model: Collagen Plugs Manufacturer of punctal plug: Lacrimedics Inc. Location of manufacturer: NR Intervention 2: oral pilocarpine (5 mg twice a day) + artificial tears Intervention 3: artificial tears only Length of follow‐up: Planned: protocol not available Actual: 12 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: assessed with a dry eye questionnaire
Secondary outcomes, as defined in study reports: Subjective symptoms: 100 mm visual analogue scale; score defined as improvement of > 55 mm in symptoms over course of study Tear production: Schirmer I test without anesthesia Ocular surface staining: Rose Bengal staining Tear film stability: TBUT Fluorophotometer method Imprint test (conjunctival impression cytology): improvement defined as increase in cytoplasm/nucleus ratio (epithelial cells) and goblet cells Adverse events reported: yes, "four patients had mild headache, of whom three also presented with nausea, vomiting, and sweating" (p 1205). Intervals at which outcomes assessed: every week for the first month, then every month after up to 12 weeks |
|
| Notes |
Trial registry: Not reported (NR) Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme. "Patients were randomized according to a computer generated schedule" (p 1204) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | It is difficult to mask participants and personnel as the interventions may be visible during examination. Eye examination, ocular surface staining, aqueous tear production, and imprint test performed by masked investigators |
| Masking of outcome assessment (detection bias) | Low risk | Eye examination, ocular surface staining, aqueous tear production, and imprint test performed by masked investigators. "Eye examination, Schirmer‐I test, and rose bengal staining were all performed by another investigator (GK), who was unaware of the treatment allocation. Furthermore, the imprint test was completed by another investigator (CAP) who was also unaware of the treatment arms and the results of Schirmer‐I and rose bengal tests" (p 1204) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1/85 participants (1%) lost to follow‐up and 1/85 participants (1%) discontinued due to local infection; not clear if these participants were excluded from the analyses |
| Selective reporting (reporting bias) | Unclear risk | Trial registry information and protocol were not available for comparison |
| Other bias | Unclear risk | Source of funding and conflict of interest not reported |
Yung 2012.
| Methods |
Study design: quasi‐randomized trial – divided into 2 groups on the basis of their ID numbers; the plug (even numbers) and non‐plug (odd numbers) groups Unit of analysis: unclear as both eyes of 10 participants receive the same intervention, but the trial investigators did not report how they analyzed them Unit of randomization: participant; assuming ID numbers were assigned to each participant, both eyes of 10 participants receive the same intervention How were missing data handled? Excluded from analysis Reported power calculation: none Unusual study design: participants' patient ID number as used to assigned them to each group |
|
| Participants |
Country: Japan Number randomized: 18 participants (28 eyes) in total 9 participants (13 eyes) in silicone punctal plugs group 9 participants (15 eyes) in non‐plug group Exclusions after randomization: 2 participants (NR eyes) in total 2 participants (NR eyes) in silicone punctal plugs group 0 participants (0 eyes) in non‐plug group Number analyzed: 18 participants (28 eyes) in total 9 participants (13 eyes) in silicone punctal plugs group 9 participants (15 eyes) in non‐plug group Losses to follow‐up: none Mean age (SD): 32.32 (7.69) years in total* 35.67 (10.74) years in silicone punctal plugs group 30.89 (3.89) years in non‐plug group Age range: (20‐56) years overall Sex (%): 16 women (89%) and 2 men (11%) in total 8 women (89%) and 1 men (11%) in silicone punctal plugs group 8 women (89%) and 1 men (11%) in non‐plug group Inclusion criteria: "patients who underwent LASIK. All eyes fulfilled the Japanese dry‐eye criteria and had not responded to conventional treatment with artificial tears by 1 month postsurgery (p 208)." Exclusion criteria: none reported |
|
| Interventions | Both groups used artificial tears Intervention 1: silicone punctal plugs in upper and lower puncta + artificial tears Punctal plug model: Eagle plug Manufacturer of punctal plug: EagleVison Location of manufacturer: Memphis, TN Intervention 2: observation + artificial tears Length of follow‐up: Planned: protocol not available Actual: 3 months |
|
| Outcomes | Outcomes not identified as primary or secondary. As defined in study reports: subjective symptoms and satisfaction, corneal sensitivity, tear function and ocular surface (Schimer value, TBUT, and fluorescein score), and visual performance (uncorrected and best‐corrected visual acuity (UCVA and BCVA), manifest refraction, and functional visual acuity (FVA)) Adverse events reported: yes, excessive lacrimation and plugs that were lost were reinserted Intervals at which outcomes assessed: 1 and 3 months |
|
| Notes |
Trial registry: NR Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: January 2008 to March 2009 Reported subgroup analyses: none reported *We contacted the authors via email and have received additional information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi randomized study – assigned based on patient ID number (odd/even) |
| Allocation concealment (selection bias) | High risk | "These candidates were divided into two groups on the basis of their ID numbers; the plug (even numbers) and non‐plug (odd numbers) groups" (p 209) "They were randomly divided into a plug and a non‐plug group" (p 208) |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Given the treatment groups it is not possible to mask participants or people administering interventions. From email correspondence with authors: "The participant and those assessing the outcome were not blinded." |
| Masking of outcome assessment (detection bias) | High risk | From email correspondence with authors: "The participant and the those assessing the outcome were not blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "2 patients lost plug(s) from either eye during the follow‐up and had new plug(s) re‐inserted but were excluded from the study" (p 208) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison |
| Other bias | Unclear risk | From email correspondence with authors: "Source of funding and conflict of interest not reported. There was no financial support for the trial. We did not use a clinical trial registry for this study.The protocol is only in the patients and methods in the manuscript. I will attach the paragraphs bellow (quoted from full‐text)." |
Zhou 2016.
| Methods |
Study design: parallel randomized controlled trial Unit of analysis: unclear as both eyes of 10 participants could have receive the same or different intervention Unit of randomization: unclear How were missing data handled? NA, no missing data reported Reported power calculation: NR Unusual study design: for 10 participants, both eyes were included, but for the remaining 70 participants only 1 eye was included |
|
| Participants |
Country: China (assumed from author's affiliations) Number randomized: 80 participants (90 eyes) in total 40 participants (46 eyes) in punctal plug group 40 participants (44 eyes) in artificial tears group Exclusions after randomization: none reported Number analyzed: 80 participants (90 eyes) in total 40 participants (46 eyes) in punctal plug group 40 participants (44 eyes) in artificial tears group Losses to follow‐up: none reported Mean age (SD): 35.21 (NR) overall 35.28 (5.58) years in the punctal plug group 35.13 (6.25) years in the artificial tears group Age range: 22 to 46 years in the punctal plug group 21 to 48 years in the artificial tears group Sex (%): 31 women (39%) and 49 men (61%)in total 15 women (38%) and 25 men (62%) in the punctal plug group 16 women (40%) and 24 men (60%) in the artificial tears group Inclusion criteria: time on viewing a monitor ≥ 5 hours per day; itching, foreign body sensation, tearing, redness, and photophobia; aqueous tear production < 10 mm/5 min, TBUT< 10 s; artificial tears use > 3 times/d Exclusion criteria: not meeting inclusion criteria; corneal, conjunctiva, iris disorder; pregnant; diabetes |
|
| Interventions |
Intervention 1: Thermal Memory hydrophobic acrylic polymer rigid rod punctal plug Punctal plug model: NR Manufacturer of punctal plug: NR Location of manufacturer: NR Control: 1g/L sodium hyaluronate eye drops; 1 drop/time; 4‐6 times /day Length of follow‐up: Planned: NR Actual: 3 months |
|
| Outcomes |
Primary and secondary outcomes not differentiated: Outcomes reported: tear secretion test, tear film break‐up time, scores of dry eye symptoms Adverse events reported: inflammation: 12 in control group and 10 in intervention group. Plug prolapses: 1 in control group and 2 in intervention group Intervals at which outcomes assessed: baseline and 3 months |
|
| Notes |
Trial registry: none reported Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Enrollement period: March 2013 to March 2015 Reported subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated using SAS 9.2 program |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants were reported to have been lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Published protocol or trial registry number were not available for comparison |
| Other bias | Unclear risk | Source of funding and conflict of interest were not reported |
NA: not applicable; NR: not reported; SD: standard deviation; TBUT: tear break‐up time.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bukhari 2015 | Wrong intervention; botulinum neurotoxin type A |
| Capita 2015 | Wrong intervention; 0.05 mL of hypromellose |
| Charnetski‐Sites 2001 | No diagnosis of dry eye syndrome |
| Geldis 2008 | Synthetic punctal plugs |
| Giovagnoli 1992 | Not a randomized or quasi‐randomized controlled trial |
| Goto 2003 | Not a randomized or quasi‐randomized controlled trial |
| Guzey 2001 | Not a randomized or quasi‐randomized controlled trial |
| Hamano 2002 | No relevant comparisons |
| Kojima 2014 | Wrong comparison. Trial registration number: JPRN‐UMIN000011574 |
| Li 2012 | No relevant comparisons |
| Malet 1997 | Not a randomized or quasi‐randomized controlled trial |
| Murube del Castillo 1995 | Not a randomized or quasi‐randomized controlled trial |
| Nishii 2004 | No relevant outcome data |
| Ozkan 2001 | Not a randomized or quasi‐randomized controlled trial |
| Patten 1976 | No relevant comparisons |
| Sainz 2000 | Not a randomized or quasi‐randomized controlled trial |
| Schultze 2004 | Not a randomized or quasi‐randomized controlled trial |
| Sharpe 2001 | Conference proceeding abstract; study met inclusion criteria, but data were not presented by relevant treatment groups; investigators were unable to provide further details |
| Virtanen 1996 | Not a randomized or quasi‐randomized controlled trial |
| Ward 2001 | Conference proceeding abstract; study met inclusion criteria, but no data presented in abstract; unable to locate contact information for sole investigator |
| Zhou 2001 | Not a randomized or quasi‐randomized controlled trial. Authors could not be contacted |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IPR‐16007760.
| Trial name or title | Treatment of dry eye with absorbable punctual plug (VisiPlug) in a randomized, observer‐blind and parallel study |
| Methods | Randomized controlled trial |
| Participants | Inclusion criteria: "1. Voluntarily participated in this clinical study and signed an informed consent 2. Male or female, aged 18 to 70 years old 3. Patients with dry eye signs and symptoms, consistent with either criterion as follows:
4. Willing to follow the requirements of this study; 5. Subjects did not participate in other clinical trials during the last 4 weeks; 6. Subjects did not use any topical medications other than artifical tears, or have used these medications, but withdrew them more than 2 weeks before; 7. The daily life vision of included subjects eye should be equal to or more than 0.1." Exclusion criteria: "1. Subjects with inflammation and infection in lacrimal system; 2. Subjects with nasolacrimal duct blocking or stenosis; 3. Subjects with severe conjunctivochalasis; 4. Allergy to any ingredient of the test materials; 5. Clinically diagnosed as fungal, bacterial or viral keratitis/conjunctivitis in active stage; 6. Co‐existence with other conjunctiva, cornea and iris lesions; 7. Patients with severe primary disease such as severe heart, brain and blood vessels, liver, kidney and hematopoietic systems disease; 8. Patients who received intraocular surgery or with intraocular trauma in the last 6 months; 9. Postmenopausal women with hormone replacement therapy; 10. Patients received permanent punctal occlusion or absorbable punctal occlusion in the last 6 months; 11. Patients who cannot stop wearing contact lenses during the trial; 12. Patients who cannot obey the required treatments and follow‐ups during the trial." |
| Interventions |
Intervention 1: both upper and lower punctal occlusion Intervention 2: lower punctal occlusion Intervention 3: upper punctal occlusion |
| Outcomes | Primary outcomes*:
Secondary outcomes**:
* Primary outcomes will be measured at week 4 after treatment ** Secondary outcomes will be measured at week 1, week 4 or week 12 after treatment |
| Starting date | NR |
| Contact information | Lan Gong (13501798683@139.com) Eye & ENT Hospital of Fudan University, 83 Fenyang Road, Shanghai 200031 |
| Notes |
Trial registration number: ChiCTR‐IPR‐16007760 (registered at Chinese Clinical Trial Registry) Source of funding: Eye & ENT Hospital of Fudan University Accessed on 9 January 2017 |
Differences between protocol and review
Cochrane methodology regarding assessments of the risk of bias in included studies has changed, and the review authors updated the 'Assessment of risk of bias in included studies' section of the Methods to reflect these updated methodological. We also added methods for assessing the certainty of evidence using the GRADE approach and preparing 'Summary of findings' tables.
Data synthesis: We did not solely base our decision to perform meta‐analysis on the I2 statistic; we took statistical, methodological, and clinical heterogeneity into consideration.
We modified the follow‐up time points for our primary and secondary outcomes to two and four weeks. Two and four weeks were considered the clinically relevant time points for this review because this is when clinicians tend to schedule follow‐up visits for dry eye patients. We also chose these time points because two other systematic reviews (treatment of dry eye with over the counter artifical tears and treatment of dry eye with autologous serum) on dry eye used these time points when comparing similar dry eye outcomes (Pan 2013; Pucker 2016).
Contributions of authors
Review co‐ordination: AE, AL
Data collection for the review update
Designing search strategies: AE, RW, OS, CEV Trials Search Co‐ordinator
Undertaking searches: CEV Trials Search Co‐ordinator
Screening search results: AE, AL
Organizing retrieval of papers: AL
Screening retrieved papers against inclusion criteria: AE, AL
Appraising quality of papers: AE, AL
Extracting data from papers: AE, AL
Writing to authors of papers for additional information: AE
Providing additional data about papers: AE
Obtaining and screening data on unpublished studies: AE, AL
Data management for the review
Entering data into RevMan: AE, AL
Analyzing data: AE, AL
Interpretation of data
Providing a methodological perspective: AE, AL
Providing a clinical perspective: AP
Providing a policy perspective: AE, AP
Providing a consumer perspective: AE
Writing the review: AE, AL, AP Providing general advice on the review: AE, AL, AP Securing funding for the review: NA Performing previous work that was the foundation of the current study: NA
Guarantor for review: AE
Sources of support
Internal sources
-
National Human Genome Research Institute Intramural funds, National Institutes of Health, USA.
This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health
External sources
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
AE: none known. AL: none known. AP: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Altan‐Yaycioglu 2005 {published data only}
- Altan‐Yaycioglu R, Gencoglu EA, Akova YA, Dursun D, Cengiz F, Akman A. Silicone versus collagen plugs for treating dry eye: results of a prospective randomized trial including lacrimal scintigraphy. American Journal of Ophthalmology 2005;140(1):88‐93. [DOI] [PubMed] [Google Scholar]
Brissette 2015 {published data only}
- Brissette 2015. Query regarding your publication in AJO: Punctal plug retention rates for the treatment of moderate to severe dry eye (for inclusion in a systematic review). Email to: Dr. Baxter 19 December 2016.
- Brissette AR, Mednick ZD, Schweitzer KD, Bona MD, Baxter SA. Punctal plug retention rates for the treatment of moderate to severe dry eye: a randomized, double‐masked, controlled clinical trial. American Journal of Ophthalmology 2015; Vol. 160, issue 2:238‐42. [DOI] [PubMed]
Burgess 2008 {published data only}
- Burgess PI, Koay P, Clark P. SmartPlug versus silicone punctal plug therapy for dry eye: a prospective randomized trial. Cornea 2008;27(4):391‐4. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen F, Wang J, Chen W, Shen M, Xu S, Lu F. Upper punctal occlusion versus lower punctal occlusion in dry eye. Investigative Ophthalmology and Visual Science 2010;51(11):5571‐7. [DOI] [PubMed] [Google Scholar]
Farrell 2003 {published data only}
- Farrell J, Patel S, Grierson DG, Sturrock RD. A clinical procedure to predict the value of temporary occlusion therapy in keratoconjunctivitis sicca. Ophthalmic and Physiological Optics 2003;23(1):1‐8. [DOI] [PubMed] [Google Scholar]
Feng 2011 {published data only}
- Feng YN, Fang XJ, Su Y, Mo JB, Shi TY. Using punctum plugs at the early stage after LASIK [泪道塞在LASIK 术后的早期应用]. International Journal of Ophthalmology 2011;11(9):1666‐7. [Google Scholar]
Kaido 2012 {published data only}
- Kaido M, Ishida R, Dogru M, Tsubota K. Visual function changes after punctal occlusion with the treatment of short BUT type of dry eye. Cornea 2012;31(9):1009‐13. [DOI] [PubMed] [Google Scholar]
Lowther 1995 {published data only}
- Lowther GE, Semes L. Effect of absorbable intracanalicular collagen implants in hydrogel contact lens patients with symptoms of dryness. International Contact Lens Clinic 1995;22:238‐43. [Google Scholar]
- Lowther GE, Semes L. Effect of intracanalicular collagen implants on drying symptoms and the tear film of hydrogel contact lens patients. American Academy of Optometry; 1991 Dec; Anaheim (CA). 1991:Abstract number 75.
Mansour 2007 {published data only}
- Mansour K, Leonhardt CJ, Kalk WW, Bootsma H, Bruin KJ, Blanksma LJ. Lacrimal punctum occlusion in the treatment of severe keratoconjunctivitis Sicca caused by Sjogren syndrome: a uniocular evaluation. Cornea 2007;26(2):147‐50. [DOI] [PubMed] [Google Scholar]
Nava‐Castaneda 2003 {published data only}
- Nava‐Castaneda A, Tovilla‐Canales JL, Rodriguez L, Tovilla Y, Pomar JL, Jones CE. Effects of lacrimal occlusion with collagen and silicone plugs on patients with conjunctivitis associated with dry eye. Cornea 2003;22(1):10‐4. [DOI] [PubMed] [Google Scholar]
Qiu 2012 {published data only}
- Qiu W, Liu Z, Zhang Z, Ao M, Li X, Wang W. Punctal plugs versus artificial tears for treating dry eye: a comparative observation of their effects on contrast sensitivity. Journal of Ocular Biology, Diseases, and Informatics 2012;5(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiu 2013 {published data only}
- Qiu W, Liu Z, Ao M, Li X, Wang W. Punctal plugs versus artificial tears for treating primary Sjögren's syndrome with keratoconjunctivitis SICCA: a comparative observation of their effects on visual function. Rheumatology International 2013;33(10):2543‐8. [DOI] [PubMed] [Google Scholar]
Rabensteiner 2013 {published data only}
- Rabensteiner DF, Boldin I, Klein A, Horwath‐Winter J. Collared silicone punctal plugs compared to intracanalicular plugs for the treatment of dry eye. Current Eye Research 2013;38(5):521‐5. [DOI] [PubMed] [Google Scholar]
Roberts 2007 {published data only}
- Carniglia P, Roberts C. Cyclosporine with and without punctal plugs in the relief of symptoms and reduction of signs of dry eye. Optometry 2005;76(6):391. [ID Number: CN‐00772991] [Google Scholar]
- Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea 2007;26(7):805‐9. [DOI] [PubMed] [Google Scholar]
Slusser 1998 {published data only}
- Slusser 1998. Questions for Dr. Slusser regarding a publication in OVS for inclusion in asystematic review. Email to Dr. Todd Slusser 20th December 2016.
- Slusser TG, Lowther GE. Effects of lacrimal drainage occlusion with non‐dissolvable intracanalicular plugs on hydrogel contact lens related dry eye. American Academy of Optometry. 1994; Vol. 15. [DOI] [PubMed]
- Slusser TG, Lowther GE. Effects of lacrimal drainage occlusion with nondissolvable intracanalicular plugs on hydrogel contact lens wear. Optometry and Vision Science 1998;75(5):330‐8. [DOI] [PubMed] [Google Scholar]
Tsifetaki 2003 {published data only}
- Tsifetaki N, Kitsos G, Paschides CA, Alamanos Y, Eftaxias V, Voulgari PV, et al. Oral pilocarpine for the treatment of ocular symptoms in patients with Sjogren's syndrome: a randomised 12 week controlled study. Annals of the Rheumatic Diseases 2003;62(12):1204‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yung 2012 {published data only}
- Yung 2012. Query regarding your publication in the Japanese Journal of Ophthalmology (for inclusion in a Cochrane systematic review). Email to: Dr. Toda 12th December 2016.
- Yung YH, Toda I, Sakai C, Yoshida A, Tsubota K. Punctal plugs for treatment of post‐LASIK dry eye. Japanese Journal of Ophthalmology 2012;56(3):208‐13. [DOI] [PubMed] [Google Scholar]
Zhou 2016 {published data only}
- Zhou ZX, Yi SP. Effect of lacrimal plug treated refractory dry eye video terminal. International Eye Science 2016;16(12):2285‐7. [Google Scholar]
References to studies excluded from this review
Bukhari 2015 {published data only}
- Bukhari AA. Botulinum neurotoxin type A versus punctal plug insertion in the management of dry eye disease. Oman Journal of Ophthalmology 2014;7(2):61‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capita 2015 {published data only}
Charnetski‐Sites 2001 {published data only}
- Charnetski‐Sites C, Bucci FA Jr. Do silicone punctal plugs impact tear lactoferrin levels following LASIK. American Academy of Optometry; 2001 Dec; Philadelphia (PA). 2001:Abstract 203.
Geldis 2008 {published data only}
- Geldis JR, Nichols JJ. The impact of punctal occlusion on soft contact lens wearing comfort and the tear film. Eye and Contact Lens 2008;34(5):261‐5. [DOI] [PubMed] [Google Scholar]
Giovagnoli 1992 {published data only}
- Giovagnoli D, Graham SJ. Inferior punctal occlusion with removable silicone punctal plugs in the treatment of dry‐eye related contact lens discomfort. Journal of the American Optometric Association 1992;63(7):481‐5. [PubMed] [Google Scholar]
Goto 2003 {published data only}
- Goto E, Yagi Y, Kaido M, Matsumoto Y, Konomi K, Tsubota K. Improved functional visual acuity after punctal occlusion in dry eye patients. American Journal of Ophthalmology 2003;135(5):704‐5. [DOI] [PubMed] [Google Scholar]
Guzey 2001 {published data only}
- Guzey M, Ozardali I, Kilic A, Basar E, Dogan Z, Satici A, et al. The treatment of severe trachomatous dry eye with canalicular silicone plugs. Eye 2001;15(Pt 3):297‐303. [DOI] [PubMed] [Google Scholar]
Hamano 2002 {published data only}
- Hamano T. Atelocollagen punctal occlusion for the treatment of the dry eye. Advances in Experimental Medicine and Biology 2002;506(Pt B):1283‐4. [DOI] [PubMed] [Google Scholar]
Kojima 2014 {published data only}
- Kojima T, Matsumoto Y, Ibrahim OM, Wakamatsu TH, Dogru M, Tsubota K. Evaluation of a thermosensitive atelocollagen punctal plug treatment for dry eye disease. American Journal of Ophthalmology 2014;157(2):311‐7. [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li M, Wang J, Shen M, Cui L, Tao A, Chen Z, et al. Effect of punctal occlusion on tear menisci in symptomatic contact lens wearer. Cornea 2012;31(9):1014‐22. [DOI] [PubMed] [Google Scholar]
Malet 1997 {published data only}
- Malet T, David N, Maalouf T, Angioi K, Bertr A, George JL. Scintigraphic evaluation of the degree of lacrimal duct occlusion after implantation of an intracanalicular plug. Orbit 1997;16(4):241‐6. [Google Scholar]
Murube del Castillo 1995 {published data only}
- Murube del Castillo J, Olivares C, Murube E. Treatment of dry eye by punctum patch. Orbit 1995;14(1):1‐7. [Google Scholar]
Nishii 2004 {published data only}
- Nishii M, Yokoi N, Komuro A, Kinoshita S. Clinical investigation of extrusion of a new punctal plug (Flex Plug). Nippon Ganka Gakkai Zasshi 2004;108(3):139‐43. [PubMed] [Google Scholar]
Ozkan 2001 {published data only}
- Ozkan Y, Bozkurt B, Gedik S, Irkec M, Orhan M. Corneal topographical study of the effect of lacrimal punctum occlusion on corneal surface regularity in dry eye patients. European Journal of Ophthalmology 2001;11(2):116‐9. [DOI] [PubMed] [Google Scholar]
Patten 1976 {published data only}
- Patten JT. Punctal occlusion with n‐butyl cyanoacrylate tissue adhesive. Ophthalmic Surgery 1976;7(2):24‐6. [PubMed] [Google Scholar]
Sainz 2000 {published data only}
- Sainz M, Simon Castellvi C, KABBANI 0. Nonpreserved topical steroids and punctal occlusion for severe keratoconjunctivitis sicca [Esteroides tópicos sin conservantes y oclusión de los puntos lagrimales para la queraconjuntivitis sicca grave]. Archivos de la Sociedad Española de Oftalmología 2000;75:751‐6. [PubMed] [Google Scholar]
Schultze 2004 {published data only}
- Schultze RL, Michra G. Comparison of topical cyclosporine (Restasis) and punctal occlusion in the treatment of dry eye syndrome. American Academy of Optometry; 2004 Oct; New Orleans (LA). 2004.
Sharpe 2001 {published data only}
- Sharpe J, Connor C. The effect of site selection for punctal occlusion on patient symptom relief. American Academy of Optometry; 2001 Dec; Philadelphia (PA). 2001:Abstract number 299.
Virtanen 1996 {published data only}
- Virtanen T, Huotari K, Harkonen M, Tervo T. Lacrimal plugs as a therapy for contact lens intolerance. Eye 1996;10(Pt 6):727‐31. [DOI] [PubMed] [Google Scholar]
Ward 2001 {published data only}
- Ward HJ. A preliminary comparison of superior to inferior silicone punctal occlusion in hydrogel contact lens wearers with dry eye signs and symptoms. American Academy of Optometry; 2001 Dec; Philadelphia (PA). 2001:Abstract number 300.
Zhou 2001 {published data only}
- Zhou S, Wilcox C, Liau C. SmartPlugTM ‐ one size fits all punctal plug for dry eyes. Investigative Ophthalmology and Visual Science 2001;42:ARVO E‐abstract 206. [Google Scholar]
References to ongoing studies
ChiCTR‐IPR‐16007760 {published data only}
- ChiCTR‐IPR‐16007760. Treatment of dry eye with absorbable punctual plug (VisiPlug) in a randomized, observer‐blind and parallel study. www.chictr.org.cn/hvshowproject.aspx?id=5168 (accessed 28 March 2016).
Additional references
AAO 2003
- American Academy of Ophthalmology (AAO) Cornea/External Disease Panel. Preferred Practice Pattern for Dry Eye Syndrome. 2003. one.aao.org/CE/PracticeGuidelines/default.aspx (accessed 27 April 2010).
Balaram 2001
- Balaram M, Schaumberg DA, Dana MR. Efficacy and tolerability outcomes after punctal occlusion with silicone plugs in dry eye syndrome. American Journal of Ophthalmology 2001;131(1):30‐6. [DOI] [PubMed] [Google Scholar]
Barnard 1996
- Barnard NA. Punctal and intracanalicular occlusion ‐ a guide for the practitioner. The College of Optometrists. Ophthalmic and Physiological Optics 1996;16 Suppl 1:S15‐22. [PubMed] [Google Scholar]
Dalzell 2003
- Dalzell MD. Dry eye: prevalence, utilization, and economic implications. Managed Care 2003;12(12 Suppl):9‐13. [PubMed] [Google Scholar]
De Paiva 2006
- Paiva CS, Chen Z, Koch DD, Hamill MB, Manuel FK, Hassan SS, et al. The incidence and risk factors for developing dry eye after myopic LASIK. American Journal of Ophthalmology 2006;141(3):438‐45. [DOI] [PubMed] [Google Scholar]
Dew 2007
- Dew K. A health researcher's guide to qualitative methodologies. Australian and New Zealand Journal of Public Health 2007;31:1753‐6405. [DOI] [PubMed] [Google Scholar]
DEWS 2007a
- Definition and Classification Subcommittee of the International Dry Eye WorkShop. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). The Ocular Surface 2007;5(2):75‐92. [DOI] [PubMed] [Google Scholar]
DEWS 2007b
- Management and Therapy Subcommittee of the International Dry Eye WorkShop. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). The Ocular Surface 2007;5(2):163‐78. [DOI] [PubMed] [Google Scholar]
Dohlman 1978
- Dohlman CH. Punctal occlusion in keratoconjunctivitis sicca. Ophthalmology 1978;85(12):1277‐81. [DOI] [PubMed] [Google Scholar]
Freeman 1975
- Freeman JM. The punctum plug: evaluation of a new treatment for the dry eye.. Transactions. Section on Ophthalmology. American Academy of Ophthalmology and Otolaryngology 1975;79(6):874‐9. [PubMed] [Google Scholar]
GetData Graph Digitalizer [Computer program]
- www.getdata‐graph‐digitizer.com. GetData Graph Digitalizer. Version 2.26. www.getdata‐graph‐digitizer.com, 2013.
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 14 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hamano 2005
- Hamano T. Lacrimal duct occlusion for the treatment of dry eye. Seminars Ophthalmology 2005;20:71‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jehangir 2016
- Jehangir N, Bever G, Mahmood SM, Moshirfar M. Comprehensive review of the literature on existing punctal plugs for the management of dry eye disease. Journal of Ophthalmology 2016;2016:9312340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2002
- Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. British Journal of Ophthalmology 2002;86(12):1347‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemp 1994
- Lemp MA. Management of the dry‐eye patient. International Ophthalmology Clinics 1994;34(1):101‐13. [DOI] [PubMed] [Google Scholar]
Lemp 1995
- Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. The Contact Lens Association of Ophthalmologists Journal 1995;21(4):221‐32. [PubMed] [Google Scholar]
Lemp 1998
- Lemp MA. Epidemiology and classification of dry eye. Advances in Experimental Medicines and Biology 1998;438:791‐803. [DOI] [PubMed] [Google Scholar]
Marcet 2015
- Marcet MM, Shtein RM, Bradley EA, Deng SX, Meyer DR, Bilyk JR, et al. Safety and efficacy of lacrimal drainage system plugs for dry eye syndrome. Ophthalmology 2015;122(8):1681‐7. [DOI] [PubMed] [Google Scholar]
McCarty 1998
- McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology 1998;105(6):1114‐9. [DOI] [PubMed] [Google Scholar]
Moss 2000
- Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Archives of Ophthalmology 2000;118(9):1264‐8. [DOI] [PubMed] [Google Scholar]
Nichols 2004
- Nichols K, Nichols J, Lynn MG. The lack of association between signs and symptoms in patients with dry eye disease. Journal of Cornea and External Disease 2004;23(8):762‐70. [DOI] [PubMed] [Google Scholar]
Pan 2013
- Pan Q, Angelina A, Zambrano A, Marrone M, Stark WJ, Heflin T, et al. Autologous serum eye drops for dry eye. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009327.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pflugfelder 2000
- Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty‐five‐year review. Cornea 2000;19(5):644‐9. [DOI] [PubMed] [Google Scholar]
Pucker 2016
- Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD009729.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sahai 2005
- Sahai A, Malik P. Dry eye: prevalence and attributable risk factors in a hospital‐based population. Indian Journal of Ophthalmology 2005;53(2):87‐91. [DOI] [PubMed] [Google Scholar]
Sall 2000
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000;107(4):631‐9. [DOI] [PubMed] [Google Scholar]
Schein 1997a
- Schein OD, Tielsch JM, Munoz B, Bandeen‐Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population‐based perspective. Ophthalmology 1997;104(9):1395‐401. [DOI] [PubMed] [Google Scholar]
Schein 1997b
- Schein OD, Munoz B, Tielsch JM, Bandeen‐Roche K, West S. Prevalence of dry eye among the elderly. American Journal of Ophthalmology 1997;124(6):723‐8. [DOI] [PubMed] [Google Scholar]
Sheppard 2003
- Sheppard JD. Dry eye moves beyond palliative therapy. Managed Care 2003;12(12 Suppl):6‐8. [PubMed] [Google Scholar]
Tai 2002
- Tai MC, Cosar CB, Cohen EJ, Rapuano CJ, Laibson PR. The clinical efficacy of silicone punctal plug therapy. Cornea 2002;21(2):135‐9. [DOI] [PubMed] [Google Scholar]
Willis 1987
- Willis RM, Folberg R, Krachmer JH, Holland EJ. The treatment of aqueous‐deficient dry eye with removable punctal plugs. A clinical and impression‐cytologic study. Ophthalmology 1987;94(5):514‐8. [DOI] [PubMed] [Google Scholar]
Wilson 2003
- Wilson SE. Inflammation: a unifying theory for the origin of dry eye syndrome. Managed Care 2003;12(12 Suppl):14‐9. [PubMed] [Google Scholar]
Yellepeddi 2015
- Yellepeddi VK, Sheshala R, McMillan H, Gujral C, Jones D, Raghu Raj Singh T. Punctal plug: a medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discovery Today 2015;20(7):884‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ervin 2007
- Ervin AM, Wojciechowski R, Schein O. Punctal occlusion for dry eye syndrome. Cochrane Database of Systematic Reviews 2007, Issue 10. [DOI: 10.1002/14651858.CD006775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ervin 2010
- Ervin AM, Wojciechowski R, Schein O. Punctal occlusion for dry eye syndrome. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD006775.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


