Abstract
Many embryonic species are initially transcriptionally quiescent after fertilization. In this issue of Developmental Cell, Blythe et al. reveal that β-catenin acts very early in Xenopus development to specifically modify the chromatin of Organizer genes, poising them for rapid activation when transcription begins.
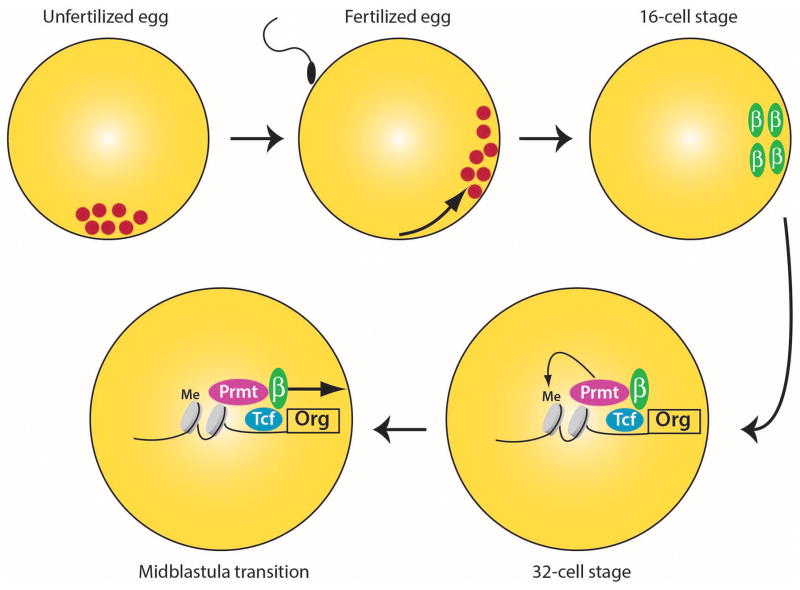
The midblastula transition (MBT) represents a critical stage in the early development of many embryos when zygotic transcription first begins. By keeping transcription quiescent for a period of time after fertilization, the embryo first becomes multicellular without new gene expression. Then when transcription begins at the MBT, many genes can be activated in discrete regions of the embryo because their transcriptional activators are restricted to a limited subset of cells. A classic case of this involves the regulation of the organizer genes in Xenopus embryos. During oogenesis a set of determinants are localized to the vegetal pole (Figure 1). Upon fertilization, the determinants translocate to what will become the future dorsal side of the embryo, where the determinants stabilize the transcription factor β-catenin (reviewed in Weaver and Kimelman, 2004). Thus, with the onset of transcription at the MBT, β-catenin dependent genes are activated in only one region of the embryo, which will become Spemann’s Organizer. The organizer in turn then secretes a panoply of intercellular signaling factors to regulate formation of the embryonic axes.
Figure 1.
Regulation of Organizer gene expression in the early Xenopus embryo
Earlier evidence revealed that β-catenin could be stabilized as early as the 16–32 cell stage (Larabell et al., 1997) whereas the MBT does not occur until the 4000-cell stage in Xenopus, leaving several hours in which the β-catenin was presumed to be bound to DNA via its DNA binding co-factor Tcf3 but unable to initiate transcription. A challenge to this view came from previous work of the Klein group, which demonstrated that two organizer genes, the nodal genes Xnr5 and Xnr6, were transcribed at relatively low levels on the future dorsal side as early as the 256-cell stage in a β-catenin dependent process (Yang et al., 2002). While this could be viewed just as leakiness of the pre-MBT transcriptional inhibition system, the authors presented compelling evidence indicating that the pre-MBT transcription mediated by β-catenin is important for formation of the embryonic axes.
In their current work, Blythe et al. (2010) present a somewhat different view of the role of β-catenin. The essential function of the early β-catenin is to regulate the chromatin in the pre-MBT embryo such that when transcription begins, the organizer genes are immediately ready to be transcribed at a high level. This is very important because a large body of work has shown that the organizer region is essentially fighting a battle with the rest of the embryo, which is working to suppress organizer gene expression (reviewed in De Robertis, 2009). By gaining a head-start on the competition, the organizer thus ensures its own survival.
Mechanistically, the authors show that β-catenin recruits the protein arginine methyltransferase Prmt2, which asymmetrically dimethylates histone 3 arginine 8 (Figure 1). Together with additional chromatin modifying activities and Pol2, this creates what the authors call a “fully poised” state in which the β-catenin target genes of the organizer are ready for transcription when the general transcription block is released at the MBT. Importantly, partial knockdown of maternal prmt2 mRNA, leads to a loss of organizer gene expression and a ventralized (i.e. loss of organizer) phenotype. Curiously, inhibition of Prmt2 translation with a morpholino oligonucleotide not only inhibits organizer gene expression but it often precludes survival of the embryo until the MBT stage, demonstrating that Prmt2 is likely to do considerably more in the embryo than just regulate the β-catenin pathway.
In a clever experiment, the authors attach Prmt2 to the Tcf-related factor Lef1, which had the β-catenin binding site removed. This fusion protein can rescue organizer formation when expressed in β-catenin depleted embryos, demonstrating that β-catenin is dispensable as long as Prmt2 is recruited to the promoter of organizer genes. This result is surprising since β-catenin is known to bind several transcriptional regulators such as Pygopus and Bcl9 (reviewed in Mosimann et al., 2009), which have been shown to be required for axis formation in Xenopus (Belenkaya et al., 2002; Kennedy et al., 2010). Whether these factors are solely required to recruit Prmt2, or whether they can be recruited to the promoter independently of β-catenin once Prmt2 modifies the chromatin, remains for future analysis (see also Mosimann et al., 2009).
This new work conflicts with the previous claim from this group that β-catenin is only needed to initiate a very early (pre-MBT) transcription event in order to establish the organizer (Yang et al., 2002) whereas here the authors argue that β-catenin is needed to poise organizer genes so that they can be transcribed at the MBT. The likely reason for this discrepancy is that while the earlier work used pharmacological inhibitors of Pol2 to temporally block transcription at the early cleavage stages, here the authors β-catenin dependent histone modification. Thus, addition of Pol2 inhibitors in the very early embryo inhibits the establishment of the poised state, which leads to alterations in the transcription of β-catenin target genes at the MBT (Blythe et al., 2010).
As a result of this finding, however, the new work opens afresh the question of whether the pre-MBT transcription of the β-catenin target genes Xnr5 and Xnr6 is important, or if it just represents minor leakiness in the poised state. In addition, this work raises the question of why organizer genes respond to maternal β-catenin to establish a poised state, whereas other genes are only activated after the MBT by β-catenin stabilized in response to zygotic Wnt signals. Finally, is the β-catenin/Prmt poising a unique mechanism to deal with the special requirements of the MBT, or is this a more general phenomenon in which β-catenin establishes a poised state that is activated at later times? Despite all the new questions, the work of Blythe et al. (2010) provides a clear and important mechanistic insight into how maternal β-catenin poises organizer genes during the pre-MBT period so that they can get off to a fast start when zygotic transcription begins. Using similar approaches, it will be interesting to see if other genes are similarly poised in the pre-MBT embryo, allowing them to hit the fast-track at this major embryonic transition.
During oogenesis, determinants (red dots) are localized to the vegetal pole. Upon sperm entry, the determinants localize to one side of the embryo. During the early cleavage stages, the determinants stabilize β-catenin (green), providing a localized region of high β-catenin. β-catenin binds the DNA binding factor Tcf (blue) and the methyl transferase Prmt2 (red), which methylates histone H3 in the promoters of organizer genes (Org). Together with additional chromatin modifications and Pol2 (not shown) this establishes the fully poised state. With the activation of transcription at the midblastula transition, the poised organizer genes are immediately ready to begin transcription at a high level.
References
- 1.Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- 2.De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 2009;126:925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy MW, Cha SW, Tadjuidje E, Andrews PG, Heasman J, Kao KR. A co-dependent requirement of xBcl9 and Pygopus for embryonic body axis development in Xenopus. Dev Dyn. 2010;239:271–283. doi: 10.1002/dvdy.22133. [DOI] [PubMed] [Google Scholar]
- 4.Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosimann C, Hausmann G, Basler K. B-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 6.Weaver C, Kimelman D. Move it or lose it: axis specification in Xenopus. Development. 2004;131:3491–3499. doi: 10.1242/dev.01284. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Tan C, Darken RS, Wilson PA, Klein PS. B-catenin/Tcf-regulated transcription prior to the midblastula transition. Development. 2002;129:5743–5752. doi: 10.1242/dev.00150. [DOI] [PubMed] [Google Scholar]