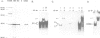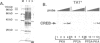Abstract
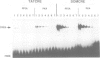
Cyclic AMP treatment of hepatoma cells leads to increased protein binding at the cyclic AMP response element (CRE) of the tyrosine aminotransferase (TAT) gene in vivo, as revealed by genomic footprinting, whereas no increase is observed at the CRE of the phosphoenolpyruvate carboxykinase (PEPCK) gene. Several criteria establish that the 43 kDa CREB protein is interacting with both of these sites. Two classes of CRE with different affinity for CREB are described. One class, including the TATCRE, is characterized by asymmetric and weak binding sites (CGTCA), whereas the second class containing symmetrical TGACGTCA sites shows a much higher binding affinity for CREB. Both classes show an increase in binding after phosphorylation of CREB by protein kinase A (PKA). An in vivo phosphorylation-dependent change in binding of CREB increases the occupancy of weak binding sites used for transactivation, such as the TATCRE, while high affinity sites may have constitutive binding of transcriptionally active and inactive CREB dimers, as demonstrated by in vivo footprinting at the PEPCK CRE. Thus, lower basal level and higher relative stimulation of transcription by cyclic AMP through low affinity CREs should result, allowing finely tuned control of gene activation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D. M., Jones N. C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990 Mar;5(3):295–302. [PubMed] [Google Scholar]
- Bohmann D. Transcription factor phosphorylation: a link between signal transduction and the regulation of gene expression. Cancer Cells. 1990 Nov;2(11):337–344. [PubMed] [Google Scholar]
- Boshart M., Weih F., Nichols M., Schütz G. The tissue-specific extinguisher locus TSE1 encodes a regulatory subunit of cAMP-dependent protein kinase. Cell. 1991 Sep 6;66(5):849–859. doi: 10.1016/0092-8674(91)90432-x. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weih F., Schmidt A., Fournier R. E., Schütz G. A cyclic AMP response element mediates repression of tyrosine aminotransferase gene transcription by the tissue-specific extinguisher locus Tse-1. Cell. 1990 Jun 1;61(5):905–916. doi: 10.1016/0092-8674(90)90201-o. [DOI] [PubMed] [Google Scholar]
- Comb M., Mermod N., Hyman S. E., Pearlberg J., Ross M. E., Goodman H. M. Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. EMBO J. 1988 Dec 1;7(12):3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P. K., Karl K. A., Colicos M. A., Prywes R., Kandel E. R. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., Blakeley M. S., Newby R. F., Ghazal P., Hennighausen L., Bourgeois S. Forskolin inducibility and tissue-specific expression of the fibronectin promoter. Mol Cell Biol. 1989 Apr;9(4):1498–1506. doi: 10.1128/mcb.9.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Habener J. F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwarki V. J., Montminy M., Verma I. M. Both the basic region and the 'leucine zipper' domain of the cyclic AMP response element binding (CREB) protein are essential for transcriptional activation. EMBO J. 1990 Jan;9(1):225–232. doi: 10.1002/j.1460-2075.1990.tb08099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fink J. S., Verhave M., Kasper S., Tsukada T., Mandel G., Goodman R. H. The CGTCA sequence motif is essential for biological activity of the vasoactive intestinal peptide gene cAMP-regulated enhancer. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6662–6666. doi: 10.1073/pnas.85.18.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Mellström B., Benusiglio E., Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992 Jan 2;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Gaire M., Chatton B., Kedinger C. Isolation and characterization of two novel, closely related ATF cDNA clones from HeLa cells. Nucleic Acids Res. 1990 Jun 25;18(12):3467–3473. doi: 10.1093/nar/18.12.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Menzel P., Leonard J., Fischer W. H., Montminy M. R. Characterization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991 Mar;11(3):1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Shenk T. Adenoviral control regions activated by E1A and the cAMP response element bind to the same factor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4171–4175. doi: 10.1073/pnas.85.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H. C., Masson N., Jones N. C., Lee K. A. The cellular transcription factor CREB corresponds to activating transcription factor 47 (ATF-47) and forms complexes with a group of polypeptides related to ATF-43. Mol Cell Biol. 1990 Dec;10(12):6192–6203. doi: 10.1128/mcb.10.12.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H. C., Totty N. F., Jones N. C. Identification and functional characterisation of the cellular activating transcription factor 43 (ATF-43) protein. Nucleic Acids Res. 1991 Sep 11;19(17):4601–4609. doi: 10.1093/nar/19.17.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamph W. W., Dwarki V. J., Ofir R., Montminy M., Verma I. M. Negative and positive regulation by transcription factor cAMP response element-binding protein is modulated by phosphorylation. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4320–4324. doi: 10.1073/pnas.87.11.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lee C. Q., Yun Y. D., Hoeffler J. P., Habener J. F. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990 Dec;9(13):4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Gonzalez G. A., Yamamoto K. K. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990 May;13(5):184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Müller B., Restle T., Weiss S., Gautel M., Sczakiel G., Goody R. S. Co-expression of the subunits of the heterodimer of HIV-1 reverse transcriptase in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):13975–13978. [PubMed] [Google Scholar]
- Patel L., Abate C., Curran T. Altered protein conformation on DNA binding by Fos and Jun. Nature. 1990 Oct 11;347(6293):572–575. doi: 10.1038/347572a0. [DOI] [PubMed] [Google Scholar]
- Quinn P. G., Wong T. W., Magnuson M. A., Shabb J. B., Granner D. K. Identification of basal and cyclic AMP regulatory elements in the promoter of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1988 Aug;8(8):3467–3475. doi: 10.1128/mcb.8.8.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik A., Schütz G., Stewart A. F. Glucocorticoids are required for establishment and maintenance of an alteration in chromatin structure: induction leads to a reversible disruption of nucleosomes over an enhancer. EMBO J. 1991 Sep;10(9):2569–2576. doi: 10.1002/j.1460-2075.1991.tb07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Boshart M., Bosch F. X., Schmid W., Fournier R. E., Schütz G. Two genetically defined trans-acting loci coordinately regulate overlapping sets of liver-specific genes. Cell. 1990 Jun 1;61(5):895–904. doi: 10.1016/0092-8674(90)90200-x. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Cole T. J., Boshart M., Schmid E., Schütz G. Multiple mRNA isoforms of the transcription activator protein CREB: generation by alternative splicing and specific expression in primary spermatocytes. EMBO J. 1992 Apr;11(4):1503–1512. doi: 10.1002/j.1460-2075.1992.tb05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Granner D. K. Regulation of phosphoenolpyruvate carboxykinase gene transcription by insulin and cAMP: reciprocal actions on initiation and elongation. Proc Natl Acad Sci U S A. 1988 May;85(9):2954–2958. doi: 10.1073/pnas.85.9.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Thompson M. A., Greenberg M. E. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991 Jun 7;252(5011):1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Schütz G. Camptothecin-induced in vivo topoisomerase I cleavages in the transcriptionally active tyrosine aminotransferase gene. Cell. 1987 Sep 25;50(7):1109–1117. doi: 10.1016/0092-8674(87)90177-2. [DOI] [PubMed] [Google Scholar]
- Tan S., Richmond T. J. DNA binding-induced conformational change of the yeast transcriptional activator PRTF. Cell. 1990 Jul 27;62(2):367–377. doi: 10.1016/0092-8674(90)90373-m. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Weih F., Stewart A. F., Boshart M., Nitsch D., Schütz G. In vivo monitoring of a cAMP-stimulated DNA-binding activity. Genes Dev. 1990 Aug;4(8):1437–1449. doi: 10.1101/gad.4.8.1437. [DOI] [PubMed] [Google Scholar]
- Weih F., Stewart A. F., Schütz G. A novel and rapid method to generate single stranded DNA probes for genomic footprinting. Nucleic Acids Res. 1988 Feb 25;16(4):1628–1628. doi: 10.1093/nar/16.4.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. A., Ellenberger T., Wobbe C. R., Lee J. P., Harrison S. C., Struhl K. Folding transition in the DNA-binding domain of GCN4 on specific binding to DNA. Nature. 1990 Oct 11;347(6293):575–578. doi: 10.1038/347575a0. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Lugo T. G., Short J. M., Fournier R. E., Hanson R. W. Identification of a cAMP regulatory region in the gene for rat cytosolic phosphoenolpyruvate carboxykinase (GTP). Use of chimeric genes transfected into hepatoma cells. J Biol Chem. 1984 Oct 10;259(19):12161–12169. [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Menzel P., Rivier J., Montminy M. R. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990 Feb 23;60(4):611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Andrisani O. M., Pot D. A., Dixon J. E. Purification and characterization of a 43-kDa transcription factor required for rat somatostatin gene expression. J Biol Chem. 1989 Apr 15;264(11):6550–6556. [PubMed] [Google Scholar]
- von der Ahe D., Pearson D., Nagamine Y. Macromolecular interaction on a cAMP responsive region in the urokinase-type plasminogen activator gene: a role of protein phosphorylation. Nucleic Acids Res. 1990 Apr 25;18(8):1991–1999. doi: 10.1093/nar/18.8.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]