Abstract
Prefrontal neurons expressing D1-type dopamine receptors (D1DRs) have been implicated in a variety of cognitive processes including working memory and timing. Although D1DRs are most strongly expressed on layer V/VI projection neurons, it is unknown which brain areas are specifically targeted by these projections. Here we selectively marked D1DR neurons using cre-loxP techniques with AAV carrying mCherry fluorescent protein, and traced projection targets of D1DR+ neurons in the mouse medial frontal cortex (MFC). We found relatively strong MFC D1DR+ projections to cortical areas as well as projections to basal ganglia and thalamic nuclei. We found relatively weaker MFC D1DR+ projections to the brainstem, hypothalamus, and other subcortical nuclei. These data intimate that MFC D1DR+ projections are well-positioned to powerfully influence cortical processing and have subcortical specificity. Thus MFC D1DR+ projection neurons may play a key role in tuning cortical networks during goal-directed behavior.
Keywords: prefrontal cortex, interval timing, dopamine receptors
INTRODUCTION
Prefrontal dopamine is critical for a variety of executive functions such as working memory, attention, reasoning, and timing [1–4]. Human diseases such as Parkinson’s disease and schizophrenia can involve disruptions in prefrontal dopamine [5–8], which contributes to marked cognitive deficits. There are two broad classes of dopamine receptors, D1-type and D2-type. Of these, pharmacological agents targeting D1-type dopamine receptors have been shown to powerfully modulate cognitive processing such as working memory, flexibility, and timing [3,9–13]. Furthermore, D1-type dopamine neurons are specifically dysfunctional in human diseases such as schizophrenia [14,15]. These data indicate that prefrontal neurons expressing D1-type dopamine receptors might powerfully modulate cognitive processing and have significance for human disease.
Prefrontal neurons exert top-down control of other brain regions, and control neuronal activity in these regions to promote behavior goals [16,17]. Prefrontal neurons in layer V/VI project broadly to cortical, subcortical, and brain stem targets [18–20]. D1-type dopamine receptors are most strongly expressed in layer V [21] and this layer can be particularly dysfunctional in schizophrenia [22]. Dopamine signaling via D1DR receptors can markedly influence the firing properties of prefrontal layer V/VI neurons [23,24].
However, it is unknown if MFC D1DR+ neurons have distinct projection targets. Here, we use transgenic techniques to map the projection targets of MFC D1DR+ neurons in mice. Like all prefrontal projections, MFC D1DR+ neurons project throughout the cortex, basal ganglia, and thalamus[18,19]. We found evidence of relatively weaker projections to the brainstem. These data imply that MFC D1DR+ neurons have anatomical specificity that guides goal-directed behavior.
METHODS
We used 8 D1-dopamine receptor Cre-recombinase mice (Drd1a-cre+), strain EY262 weighing 20–30g [23,25]. Four were used for coronal sectioning, and four were used for sagittal sectioning. All animals were group housed, on a 12 h dark/light cycle with food and water available ad libitum. All animal procedures were performed in accordance with the protocol approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC).
Viral injections were performed using stereotaxic procedures. Animals were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg) and placed in a stereotaxic frame (Stoelting). Under aseptic conditions, the scalp was retracted, and the skull was leveled between bregma and lambda. After craniotomy, mice were injected unilaterally in the prefrontal cortex (AP: +1.8, ML: −0.2, DV: −2.5) with AAV2/5-EF1a-DIO-mCherry-WPRE alone or simultaneously with AAV2/5-CaMKIIa-eYFP (virus obtained from UNC vector core; Fig 1). We injected 0.5 microliter of each virus in each animal. Four animals were injected with AAV2/5-EF1a-DIO- -mCherry-WPRE alone; and four animals were co-injected with AAV2/5-EF1a-DIO -mCherry-WPRE and AAV5-CaMKIIa-eYFP. Two brains were sectioned coronally and two brains were sectioned sagittally in each group (4 with DIO-mCherry alone and 4 with DIO-mCherry and CaMKIIa; 8 brains total). The animals were given at least 2 weeks for transgene expression, viral expression, and recovery before transcardial perfusion.
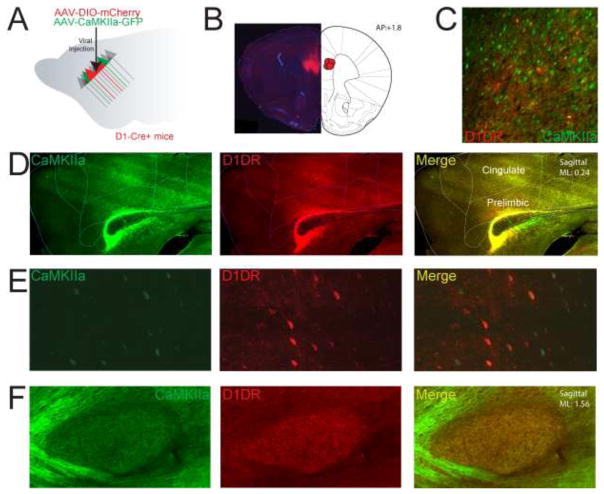
Figure 1. Tracing projections of medial frontal D1DR+ neurons.
A) In D1DR-Cre+ mice, we simultaneously injected AAV2/5-EF1a-DIO-mCherry-WPRE to visualize projections of MFC D1DR+ neurons, and AAV2/5-CaMKIIa-eYFP to non-specifically tag projections of prefrontal excitatory neurons. B) Injection target in the mouse MFC targeting dorsal prelimbic cortex. C) In Layer V, we observe robust transfection of MFC D1DR+ cells and CamKIIα+ neurons. D) A sagittal section of the prelimbic cortex contralateral to injection site reveals bright axonal labeling of CamKIIα+ and D1DR+ axons coursing from the prelimbic injection site. E) Labeling of prefrontal neurons expressing CaMKIIα+ and D1DR+ neurons. F) Example of CaMKIIα+ and D1DR+ fiber tracts in the internal capsule and synaptic projection fields in the subthalamic nucleus.
The animals were anesthetized and transcardially perfused using a cold PBS (phosphate buffer solution) followed by 4% paraformaldehyde to fix the tissue. The top of the skull of removed using a bone rongeur and brain of the animal was extracted using fine forceps. The brain was post-fixed in 4% paraformaldehyde solution. After post-fixation for 24 hours at 4°C, the brain tissue was transferred to a 30% sucrose solution at 4°C for 1–2 days before being cryoprotected in tissue freezing medium.
All 8 brains were either cut coronally or sagitally using cryostat (Leica, CM 1510) at −22°C with section thickness of 40 micrometers. The sliced brain sections were collected into two 12 well plate containing free-floating PBS with no more than 10 sections per well. The sections from each brain were placed into the wells in order of cuts such that the first cut section was placed in well 1 then second cut in well 2 until well 24 then starting back at well 1 until all of the brain was cut.
Brain sections were stained free floating with DAPI at 100ng/ml concentration as fluorescence marker for cell nuclei. Sections were mounted on Fisher-brand superfrost plus microscope slides using Prolong Diamond Antifade Mountant (Life Technology).
A fluorescent microscope, Zeiss Axio.M2 equipped with ApoTome (Carl Zeiss, Oberkochen, Germany), a digital camera and calibrated motorized stage controller that allows precise control of y-, x and z axes was used to collect images for each brain section. Using the virtual tissue program in StereoInvestigator software (MBF Bioscience, Colchester, Vermont), composite images at 10x magnification of every brain sections with mCherry were taken. MFC D1DR+ neuron and MFC CamKIIα+ projection targets were mapped and compared to the Allen mouse brain atlas.
Projections’ fluorescence levels were quantified using imageJ. Mean fluorescence levels were taken from 4 areas without projections to be used as background and also from the projection target area. The average of the background levels were removed from the target area levels to normalize signal. Projection target areas were determined by prior literature; areas without any identifiable mCherry were not analyzed.
RESULTS
AAV2/5-EF1a-DIO-mCherry and AAV2/5-CaMKIIa-eYFP were simultaneously injected into the mouse MFC of four animals (Fig 1A). Our projections targeted the dorsal prelimbic cortex; our prior work has shown that D1DR+ neurons in this region are critical for interval timing and feeding [1,16,26]. 2 weeks later, MFC cell bodies were brightly fluorescent for eYFP, mCherry, or both (Fig 1B). eYFP+ and mCherry+ synaptic projection fields were visible in the contralateral MFC for eYFP and mCherry; these were densest in layer V/VI (Fig 1C–E) and sent synaptic projections to downstream areas (Fig 1F).
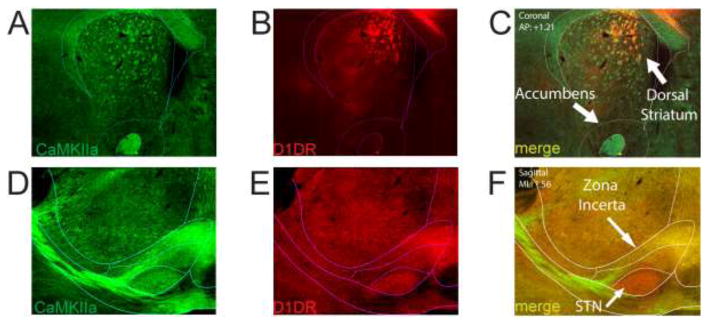
Next, we examined projection targets of MFC D1DR+ neurons in the thalamus. We observed bright mCherry corresponding to synaptic projection fields of several groups of thalamic nuclei, including dorsal and central nuclei (Fig 2A–B). However, we observed comparatively weaker MFC D1DR+ projections to the lateral habenula; despite the fact that this nuclei received robust non-specific MFC input (Fig 2A). These data imply that MFC D1DR+ neurons do not project equally to all prefrontal projection sites, and that MFC D1DR+ neurons have some specificity (Fig 2C). Consistent with prior work, MFC D1DR+ projections sent strong projections to the dorsal and ventral striatum (Fig 3A–C)[18,19]. We also observed projections to the subthalamic nucleus and zona incerta (Fig 3D–F). We observed very few MFC D1DR+ projections to brainstem nuclei in the midbrain and periaqueductal gray.
Figure 2. MFC D1DR+ can be specific.
A) Coronal section of projection targets of MFC CamKIIα+ neurons labeling the lateral habenula and mediodorsal thalamus. B) By contrast, we observed comparatively less MFC D1DR+ signal in the lateral habenula with comparatively stronger signal in the mediodorsal nuclei of thalamus. C) Co-labeling of MFC CamKIIα+ and MFC D1DR+ projections indicated that these projections were not entirely overlapping.
Figure 3. MFC D1DR+ projections to the basal ganglia.
A–C) Coronal sections of projections of MFC neurons to the basal ganglia; note that intense projections are white matter tracts (internal capsule/anterior commissure) with prefrontal labeling. D–F) Sagittal sections of MFC projections to the subthalamic nucleus and zona incerta.
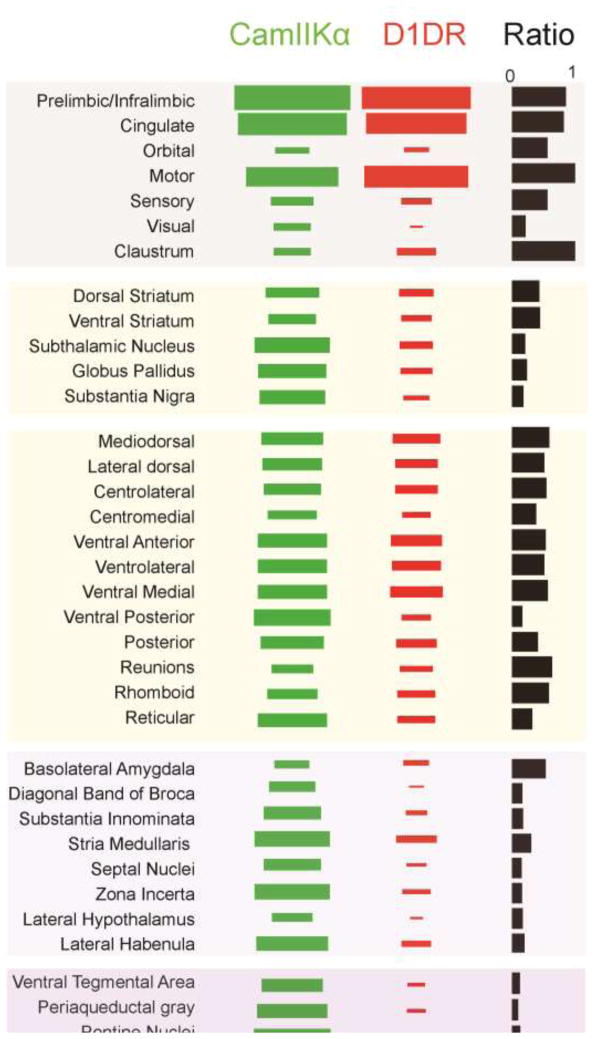
To visualize the connectivity pattern of MFC D1DR+ projections, we sectioned the entire brain of 4 animals co-injected with AAV2/5-EF1a-DIO-mCherry and AAV2/5-CaMKIIa-eYFP in the MFC. In this brains, MFC D1DR+ projections were labeled with mCherry and CaMKII α+ projections were labeled with eYFP. We quantified the synaptic projection fields of known MFC projection targets using imageJ. This data was compared with MFC CamKIIα+ projections in the same brain, which largely matches past work using retrograde/anterograde tracers [18,19,27,28]. MFC D1DR+ projections were densely observed in cortical areas at similar strengths to MFC CamKIIα+ projections, with the exception of visual cortex which had MFC CamKIIα+ projections that were ~4x as prominent as MFC D1DR+ projections. In the basal ganglia, projection strengths were consistently stronger to striatum, pallidum, and the subthalamic nucleus for MFC CamKIIα+ projections than for MFC D1DR+ projections. MFC D1DR+ projections were notably weaker to the substantia nigra pars reticulata. In the thalamus, projections were more evenly matched except for lateral dorsal thalamic nucleus, posterior thalamus, and the reticular nucleus. Figure 4 shows while MFC D1DR+ projections to the amygdala were similar to MFC CamKIIα+ projections[16], projections to hypothalamus and brainstem nuclei were much weaker than MFC CamKIIα+ projections. For example, D1DR+ projections to the visual cortex was ~0.4 times the strength of the CamKIIα+ projections. These data provide some insight into the specificity of MFC D1DR+ projections.
Figure 4. MFC D1DR+ projectome.
Hinton plots of relative projection strength in 4 mouse for MFC-CamKIIα+ (green) and MFC-D1DR+ (red). The third column indicates the ratio of MFC-D1DR+ to CamKIIα+ projections. For instance, there was the same amount of D1DR+/CamKIIα+ projections medial frontal, motor and sensory cortices, but D1DR+ projections to visual cortex was 0.4 times as strong as CaMKIIα+, and D1DR+ projections to the periaqueductal gray were 0.1 times as strong as CaMKIIα+.
DISCUSSION
We used transgenic mice to map the projections of MFC D1DR+ neurons that are intimately involved in cognitive processing and dysfunctional in human diseases that impair cognitive function. We found that these neurons projected to similar regions as other MFC projection neurons in the cortex, basal ganglia, and thalamus. By contrast, MFC D1DR+ neurons had relatively weak projections to the hypothalamus and brainstem. These data imply that MFC D1DR+ projections may guide behavior in part by shaping ongoing cortical activity to promote goal-directed behavior.
Here, we used viral methods to express the fluorescent protein mCherry with a cre-dependent EF1a promoter within infected D1DR+ neurons, or YFP with a CamKIIα promoter –which is non-selectively expressed in excitatory cortical cells. In this technique, areas with synaptic projection fields have the fluorescent signal corresponding to mCherry or eYFP. Viral techniques are quite distinct from classic anterograde/retrograde tracers, which are axonally transported by individual neurons [18–20,27,29]; however, they revealed a similar pattern of prefrontal projections to these studies. Also of note, whereas previous studies were chiefly in rat, our report is exclusively in mouse [18,19].
Many of these areas that received MFC input do not get strong prefrontal input from D1DR+ projections. For instance, there was sparse MFC D1DR+ innervation to the VTA. Similarly, although there are robust MFC projections to subcortical nuclei, we saw few strong MFC D1DR+ inputs with the exception of the amygdala. The former connection has been demonstrated to play a specific and powerful rule in top-down control of feeding behaviors [16]. To our knowledge, this study is one of the few demonstrations of MFC D1DR+ projections with a specific behavioral effect, although other work demonstrates that stimulation of prefrontal afferents to specific nuclei can also selectively modulate depressive behavior [30].
Prefrontal D1DRs are necessary for cognitive operations such as working memory and timing [31–33]. MFC D1DRs are expressed throughout all cortical layers but most strongly on layer V projection neurons [21,23]. D1DRs are expressed on dendritic spines, shafts, and soma of these neurons, which facilitate powerful modulation by dopamine signaling, particularly by recurrent cortical projections [8,34]. In support of this idea, a recent study by our lab showed that during a timing task, MFC D1DR+ neurons did not have particularly strong temporal processing, but stimulating these neurons strongly increased temporal processing among all MFC networks and could compensate for behavioral deficits caused by depleting dopamine [1]. This effect, combined with the data in Figure 4 revealing a strong cortico-cortical role of MFC D1DR+ projections, suggest that MFC D1DRs may play a role in tuning and optimizing cognitive processing.
Our technique has several limitations. First, viral techniques only transfect a minority of cells in a target brain area; hence it is possible that the absence of the signal in a target brain area is related to the viral expression of mCherry rather than a lack of MFC D1DR+ input. However, we observe similar projection patterns to well-validated tracer-based work in some key brain areas. Furthermore, because AAV-mediated expression is most compatible with genetically-encoded fluorescent proteins, we are further constrained by background issues related to immunofluorescence and cannot use chromogenic (i.e., peroxidase) or radioassays. Viral injections typically affect a larger volume than tracers, which can be iontophoresed at very small volumes. For these reasons, it is important to compare signal from MFC-D1DR+ mCherry to AAV- CamKIIα+ YFP, which is also virally based, and appears to project to the same brain areas as more traditional tracers. Finally, we did not screen MFC D1DR+ projections for their effect on target nuclei or behavior. This effort is likely to further elucidate the role that the MFC D1DR+ projectome plays in goal-directed behavior.
HIGHLIGHTS.
We studied projections of prefrontal neurons expressing D1-type dopamine receptors
Prefrontal D1DR+ neurons projected strongly to cortical areas
Prefrontal D1DR+ neurons did not project strongly to the brainstem
These data provide information about the prefrontal D1DR+ projectome
Acknowledgments
This project was supported by R01 NS089470 to NSN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim YC, Han SW, Alberico SL, Ruggiero RN, De Corte B, Chen KH, Narayanan NS. Optogenetic Stimulation of Frontal D1 Neurons Compensates for Impaired Temporal Control of Action in Dopamine-Depleted Mice. Curr Biol CB. 2017;27:39–47. doi: 10.1016/j.cub.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci. 2013;24:267–278. doi: 10.1515/revneuro-2013-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker KL, Ruggiero RN, Narayanan NS. Infusion of D1 Dopamine Receptor Agonist into Medial Frontal Cortex Disrupts Neural Correlates of Interval Timing. Front Behav Neurosci. 2015;9:294. doi: 10.3389/fnbeh.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Parker KL, Kim YC, Kelley RM, Nessler AJ, Chen KH, Muller-Ewald VA, Andreasen NC, Narayanan NS. Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Naryanan NS. Medial frontal ~4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol. 2015 doi: 10.1152/jn.00412.2015. jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain J Neurol. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- 8.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 9.Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Narayanan NS. D1-Dependent 4 Hz Oscillations and Ramping Activity in Rodent Medial Frontal Cortex during Interval Timing. J Neurosci. 2014;34:16774–16783. doi: 10.1523/JNEUROSCI.2772-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puig MV, Miller EK. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron. 2012;74:874–886. doi: 10.1016/j.neuron.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 12.Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- 14.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci Off J Soc Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. 20026302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 16.Land B, Narayanan N, Liu R, Gianessi C, Brayton C, Grimadli D, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. n.d doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 19.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 20.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 21.Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 22.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 23.Seong HJ, Carter AG. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J Neurosci Off J Soc Neurosci. 2012;32:10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci Off J Soc Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 28.Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- 29.Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–87. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- 30.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y-C, Alberico S, Emmons E, Narayanan N. New therapeutic strategies targeting D1-type dopamine receptors for neuropsychiatric disease. Front Biol. 2015:1–9. doi: 10.1007/s11515-015-1360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laubach M, Caetano MS, Narayanan NS. Mistakes were made: Neural mechanisms for the adaptive control of action initiation by the medial prefrontal cortex. J Physiol-Paris. n.d doi: 10.1016/j.jphysparis.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci Off J Soc Neurosci. 2005;25:1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]