Abstract
Background
Recent Children’s Oncology Group (COG) trials tested the efficacy of reduced therapy in an effort to lessen late effects compared to the Intergroup Rhabdomyosarcoma Study (IRS) IV regimen with associated hematologic and hepatic toxicity, and infertility. Here, we analyze the efficacy of 45 Gray (Gy) local radiotherapy (RT) in patients with Group III orbital embryonal rhabdomyosarcoma (ERMS) enrolled on the COG low-risk study ARST0331.
Procedure
Sixty-two patients with Group III orbital ERMS were treated on ARST0331 with four cycles of vincristine (VCR), dactinomycin (DACT), and cyclophosphamide (CPM; VAC, total cumulative CPM dose 4.8 g/m2) followed by four cycles of VCR and DACT over 22 weeks. Forty-five Gray of radiation was administered in 25 fractions beginning at week 13 of therapy.
Results
Fifty-three patients were evaluable for this response analysis; seven had missing week 12 response evaluation data and two had progressive disease prior to starting RT. Median follow-up was 7.8 years. None of the 15 patients with radiographic complete response (CR) compared to 6 of the 38 patients with <CR after 12 weeks of VAC chemotherapy had local recurrences (P = 0.11). There was no difference in overall survival by response at week 12 (P = 0.52).
Conclusions
For patients with Group III orbital ERMS achieving a CR following VAC chemotherapy that includes modest dose CPM, 45 Gy may be sufficient for durable failure-free survival. However, for those with <CR treated with the ARST0331 systemic therapy, a different local therapy approach may be needed to achieve the control rate of IRS-IV without its toxicity.
Keywords: orbital rhabdomyosarcoma, radiotherapy, response based
1 INTRODUCTION
Approximately 10% of rhabdomyosarcomas (RMS) arise in the orbit.1 Orbital RMS rarely presents with regional or metastatic disease. Because of the morbidity associated with complete resection, orbital RMS is usually Group III and radiotherapy (RT) has typically been used for local control. Using this approach, the 5-year failure-free survival (FFS) rate for patients with Group III orbital embryonal RMS (ERMS) exceeds 85%, with a lower FFS rate for patients with nonembryonal histology.2–8
In Intergroup Rhabdomyosarcoma Study (IRS) III, patients with Group III orbital RMS were treated with 41.4–50.4 Gray (Gy) depending on age using conventional fractionation with vincristine (VCR) and dactinomycin (DACT; VA). The 5-year local failure rate was 16%.4 In IRS-IV, patients with Group III orbital RMS were randomized to receive either 50.4 Gy in 28 daily fractions or 59.4 Gy in twice daily hyperfractionated dosing starting after 9 weeks of chemotherapy, and they were also randomized to receive either VA plus 26.4 g/m2 of cyclophosphamide (CPM; VCR, DACT, and CPM [VAC]), VA plus ifosfamide, or VCR plus ifosfamide and etoposide. The 5-year local failure rate was 2% and 3-year FFS was 94%. Local control rates were not different between conventional fractionation and hyperfractionation.5,6
The two most recent clinical trials conducted by the Children’s Oncology Group (COG) Soft Tissue Sarcoma Committee have tested reduced-intensity chemotherapy and RT regimens for orbital ERMS. In COG D9602, patients with Group III orbital ERMS received 45 Gy in conventional fractionation after 3 weeks of chemotherapy and VA over 45 weeks. The 5-year local failure rate was 14%.7,8
Given superior IRS-IV FFS and local control rates with chemotherapy regimens that included an alkylating agent, the primary objective of COG ARST0331 was to reduce the length of therapy without compromising FFS by using VA in combination with lower dose CPM (total cumulative dose, 4.8 g/m2) plus RT for patients with subset 1 low-risk ERMS, which included those with Group III orbital ERMS.9,10 As with COG D9602, patients with Group III orbital ERMS received 45 Gy in conventional fractionation on COG ARST0331. The 3-year FFS and overall survival (OS) for eligible patients with primary orbital tumors were 87% and 97%, respectively. The overall study objective for subset 1 low-risk patients was met.11 We assessed whether initial response to chemotherapy predicted outcome for patients with unresected orbital ERMS on ARST0331 and report here local recurrence rate, FFS, and OS.
2 PROCEDURES
2.1 Patients
Eighty-two eligible patients with ERMS of the orbit or eyelid were treated on ARST0331. Of those patients, 62 had orbital Group III disease, all of whom had orbital primary disease. The eligibility requirements for this study have been previously published.11
2.2 Treatment
Patients with Stage 1, Group III orbital RMS were assigned to subset 1 of ARST0331 and received four cycles of VAC followed by four cycles of VA over 22 weeks and RT starting after 12 weeks of chemotherapy. DACT was omitted during RT. Chemotherapy dosing was based on age. For children >3 years of age, total cumulative doses of VCR, DACT, and CPM were 27 mg/m2, 0.36 mg/kg, and 4.8 g/m2, respectively.
The RT gross tumor volume (GTV) was the prechemotherapy extent of disease. The clinical target volume (CTV) was the GTV plus 1 cm, confined to the orbit, providing there was no orbital bone erosion. The entire orbit was not irradiated, only the CTV with a 0.3–0.5 cm margin for the planning target volume (PTV). The lens, cornea, and lacrimal gland were shielded as possible. The prescription dose was 45 Gy in 1.8 Gy daily fractions, with five fractions per week.
Radiation oncology plans were centrally reviewed by the Quality Assurance Review Center. Study RT guidelines permitted deviations from study guidelines for patients aged ≤ 24 months.
2.3 Statistical analysis
Records were reviewed for response following four cycles of VAC chemotherapy over 12 weeks, local recurrences, FFS, and OS. Response to four cycles of VAC was determined by imaging. Complete response (CR) was defined as complete disappearance of the tumor by exam and imaging. Partial response (PR) was defined as at least 64% decrease in tumor volume compared to the measurement obtained at study enrollment. Progressive disease (PD) was defined as at least 40% increase in tumor volume compared to the smallest volume obtained after beginning therapy. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as a reference the smallest disease volume after starting treatment. Patients with chemotherapy-response data who received RT on study were included in a subset analysis.
FFS was defined as the time from the start of treatment to disease progression, recurrence, or death as a first event. OS was defined as the time from the start of treatment to death from any cause. The Kaplan–Meier method was used to estimate FFS and OS distributions.12 Confidence intervals (CIs) were calculated using Greenwood’s formula.13 Recurrence was defined as local if the tumor recurred at the site of primary disease, regional if regional lymph nodes were involved, and distant if metastatic disease was present at the time of recurrence. Only first recurrences were considered for this analysis. Differences between curves were analyzed by the log-rank test. Data regarding infertility or ocular late effects were not collected on ARST0331. Analyses were based on data available by July 2016.
3 RESULTS
3.1 Patient characteristics and outcomes
ARST0331 accrued 304 subset 1 patients from September 17, 2004 to August 13, 2010. Characteristics of the 62 patients with Group III ERMS of the orbit are shown in Table 1. There was one infant (aged <1 year) enrolled on the study who did not receive radiation therapy. The median follow-up was 7.8 years. The 5-year FFS and OS for the entire cohort of 62 patients was 87% (95% CI, 78–96%), and 97% (95% CI, 92–100%), respectively.
TABLE 1.
Patient and tumor characteristics
| N (%) | Median | Range | |
|---|---|---|---|
| Age (in years) | 6.2 | 0.6–23.4 | |
| <6 | 29 (46.8%) | ||
| ≥6 | 33 (53.2%) | ||
| Gender | |||
| Male | 38 (61.3%) | ||
| Female | 24 (38.7%) | ||
| Tumor size (cm) | 62 | 2.9 | 0.2–5.2 |
| ≤5 cm | 60 (96.8%) | ||
| >5 cm | 1 (1.6%) | ||
| Missing | 1 (1.6%) | ||
| RT dose (Gy) | 45 | 36a–47.4 | |
| No RTb | 5 (8.1%) | ||
| 45 | 51 (82.2%) | ||
| <45 | 1 (1.6%) | ||
| >45 | 2 (3.2%) | ||
| Missing | 3 (4.8%) | ||
| Follow-up (in years) | 62 | 7.8 | 0.5–10.7 |
| Disease status at week 12 | |||
| CR | 15 (24.2%) | ||
| PR | 31 (50.0%) | ||
| SD | 7 (11.3%) | ||
| PD | 2 (3.2%) | ||
| Unknown | 7 (11.3%) | ||
One patient was enrolled as Group III but was subsequently determined to have microscopic residual disease, so a dose of 36 Gy was assessed as appropriate. The patient’s response was classified as CR.
Two had early progression (at Day 78 and 81); the other three did not progress but went off protocol therapy before week 13 for reasons of “physician determination,” “refusal by patient/parent,” and “Intolerable toxicity.”
3.2 Response to 12 weeks of VAC chemotherapy and associated outcomes
Fifteen patients (24%) had a CR to VAC chemotherapy and 38 patients (61%) had <CR either a PR (n =31) or SD (n =7). Two patients (4%) had PD before week 12 evaluation, and seven patients (11%) had insufficient or missing week 12 evaluation data.
There were eight events, all of which were local disease progression either before RT (n = 2) or following RT (n = 6). There were six local failures among the 31 patients with PR. There were no events among the patients with CR or SD following induction chemotherapy. Six of the eight patients are alive following local recurrence. The salvage regimens were not collected by the study. The two patients died of PD, including the infant in the study who did not receive RT.
Figure 1 shows the FFS for patients with CR versus patients with PR/SD at week 12 There was a nonsignificant trend for improved 5-year FFS for patients with CR (100%) at week 12 compared with patients with PR/SD (84%; 95% CI, 71–96%) at week 12 (P = 0.11). There was no difference in 5-year OS between patients with CR (100%) and PR/SD (97%; 95% CI, 91–100%) at week 12 (P = 0.52).
FIGURE 1.
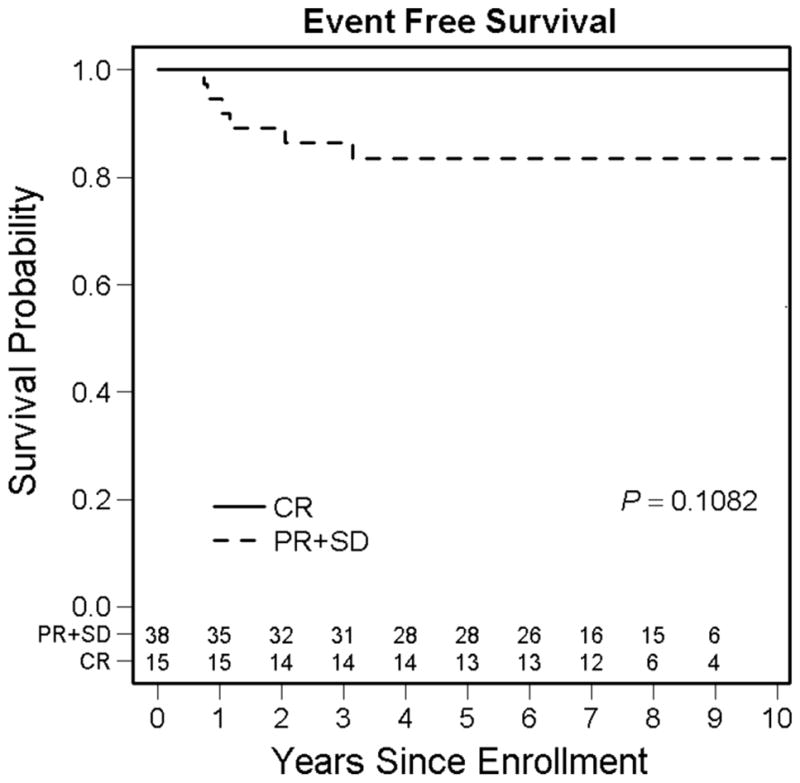
Failure-free survival for patients with Group III orbital embryonal rhabdomyosarcoma by response to induction chemotherapy
Protocol RT deviations did not differ between patients with recurrence (one minor deviation among six patients) and patients without recurrence (10 minor deviations among 47 patients). Eight deviations (including one in a patient with a recurrence) were related to CTV or PTV delineation, and three deviations were related to target volume coverage. There were no deviations for total radiation dose.
4 DISCUSSION
As shown in Table 2, among IRS Group (IRSG) and COG studies preceding ARST0331, the highest local control rate for patients with Group III orbital ERMS was seen in IRS-IV, which achieved a 5-year local control rate of 98% and 3-year FFS rate of 94% using a high cumulative CPM dose with the risk of substantial hematologic and hepatic toxicity, and infertility. IRS-IV also included 50.4 Gy with daily fractionation or 59.4 Gy with hyperfractionation for local therapy. The preceding study and subsequent study (IRS-III and COG D9602, respectively) had somewhat lower local control rates in patients with orbital RMS.13 ARST0331 included a modest CPM dose in an effort to improve outcome compared to IRS-III and D9602 and decrease toxicity compared to IRS-IV; however, the local control rate for patients with orbital ERMS on ARST0331 was approximately the same as IRS-III and D9602.
TABLE 2.
Treatment and outcome for Group III orbital ERMS
| Protocol | Dose (Gy) | Chemotherapya | Timing of RT (week) | 5-year local failure rate (%) | 5-year failure-free survival (%) |
|---|---|---|---|---|---|
| IRS-III (n = 71) | 41.4–50.4 | VA | 2 or 6b | 16 | 79 |
| IRS-IV (n = 49) | 50.4–59.4 | VAC (26.4 g/m2)/VAI/VIE | 9 | 2 | 94c |
| D9602 (n = 77) | 45 | VA | 3 | 14 | 86 |
| ARST0331d | 45 | VAC (4.8 g/m2) | 13 | ||
| Overall (n = 62e) | 13 | 87 | |||
| Week 12 CR (n = 15) | 0 | 100 | |||
| Week 12 PR/SD (n = 38) | 16 | 84 |
ERMS, embryonal rhabdomyosarcoma; IRS, Intergroup Rhabdomyosarcoma Study; VA, vincristine and dactinomycin; VAC, vincristine, dactinomycin, and cyclophosphamide; VAI, vincristine, dactinomycin, and ifosfamide; VIE, vincristine, ifosfamide, and etoposide.
Cumulative dose of cyclophosphamide shown in parentheses.
Patients less than 6 years of age with tumors less than 5 cm received radiation at week 2, others received radiation at week 6.
Only ARST0331 assessed response to induction chemotherapy.
3-year failure free survival.
Two patients with progressive disease at the end of induction chemotherapy and seven patients with unknown disease status at week 13 or incomplete information.
Two of 62 patients had PD at week 11 of ARST0331. The timing of RT on ARST0331 was later than in previous trials; however, earlier RT on D9602 (after 3 weeks of chemotherapy) resulted in nearly identical outcomes, and RT was administered after 9 weeks of chemotherapy on IRS-IV. In patients with intermediate-risk RMS, PD at less than 120 days is rare, occurring in only 2% of patients.14 Further, intermediate-risk patients with parameningeal tumors with cranial nerve palsy or cranial base bony erosion have equivalent clinical outcomes whether they are treated with RT at week 0 or week 12.15 RT timing during the first 13 weeks may not impact outcome.
In addition to showing the local control and FFS for the aforementioned studies, Table 2 also shows response-based local control and FFS for ARST0331. These were suboptimal for patients achieving <CR after neoadjuvant 12 weeks of VAC chemotherapy on ARST0331. We observed a nonsignificant trend toward improved outcome based on response to neoadjuvant chemotherapy, with 100% local control and FFS in patients achieving a CR.
Data regarding acute and late morbidity from RT administered in the era prior to three-dimensional conformal RT, such as orbital hypoplasia, keratoconjunctivitis, corneal injury, uveitis, retinal injury, eye dryness, cataract, pituitary dysfunction, and secondary malignancy are well recognized by clinicians today.2,16 Raney et al. investigated, by questionnaire, the morbidity among 103 surviving patients treated on IRS-III between 1984 and 1991 who received between 41.4 and 50.4 Gy of RT. The eye preservation rate was 86% but impaired visual acuity occurred in 70% of the remaining patients.17 The marginal increase in toxicity between 45 and 50.4 Gy is unknown, and none of the most recent trials collected data regarding ocular function. With modern radiation techniques including three-dimensional conformal, intensity modulated, volumetric modulated arc, and proton RT, treatments have become increasingly more conformal, thus decreasing the likelihood of similar ocular findings.18,19
The overall prognosis remained favorable for patients with Group III orbital ERMS on ARST0331. Indeed, for patients with Group III orbital ERMS with a CR at week 12 to neoadjuvant chemotherapy on ARST0331, there were no local recurrences using 45 Gy, suggesting this combination of chemotherapy and RT may be adequate for these patients. Trials assessing further reduction in RT are unlikely for this rare subset of patients.
However, a higher local failure rate among patients on ARST0331 with <CR suggests that these patients may benefit from alternative approaches. Local control, FFS, OS acute toxicity, and long-term morbidity should be taken into account to determine the optimal treatment approach for patients with Group III orbital ERMS. IRS-IV therapy had the most favorable local control and FFS rates, although with more toxicity. Dose escalation to 50.4 Gy for patients with Group III orbital ERMS on a backbone of ARST0331 chemotherapy has not been studied prospectively for efficacy, but should have an acceptable toxicity profile and was previously used for many patients on IRS-III and IRS-IV. Positron emission tomography response20 or gene expression signatures such as MG521 could be investigated in the future to identify candidates for augmented or reduced therapy. Finally, one could hypothesize that earlier identification of patients with <CR could allow intensified therapy to those patients; however, doing so could subject some patients to more intensive therapy who would have achieved a CR to induction chemotherapy by week 13.
5 CONCLUSIONS
For patients with low-risk Group III orbital ERMS achieving a CR following induction chemotherapy on ARST0331 that includes modest dose CPM, 45 Gy may be sufficient for durable FFS. However, for patients with Group III orbital ERMS with <CR after induction chemotherapy, the ARST0331 treatment algorithm is associated with lower poorer local control than was achieved on the more intensive IRS-IV therapy and warrants consideration of different local treatment algorithms in future RMS clinical trials.
Acknowledgments
Grant sponsor: National Cancer Institute; Grant numbers: CA-98543 and CA-98413.
The research in this study was supported in part by grant nos. CA-98543 and CA-98413 from the National Cancer Institute, Bethesda, MD.
Abbreviations
- CI
confidence interval
- COG
Children’s Oncology Group
- CPM
cyclophosphamide
- CR
complete response
- CTV
clinical target volume
- DACT
dactinomycin
- ERMS
embryonal rhabdomyosarcoma
- FFS
failure-free survival
- GTV
gross tumor volume
- Gy
Gray
- IRS
Intergroup Rhabdomyosarcoma Study
- OS
overall survival
- PD
progressive disease
- PR
partial response
- PTV
planning target volume
- RT
radiotherapy
- SD
stable disease
- VA
vincristine and DACT
- VCR
vincristine
Footnotes
CONFLICT OF INTEREST
Dr. Anderson previously served on independent Data Monitoring Committees for Amgen, Merck, and SFJ Parma sponsored studies. There is no other conflict of interest.
References
- 1.Crist W, Gehan EA, Ragab AH, et al. The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 2.Oberlin O, Rey A, Anderson J, et al. Treatment of orbital rhabdomyosarcoma: survival and late effects of treatment–results of an international workshop. J Clin Oncol. 2001;19:197–204. doi: 10.1200/JCO.2001.19.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Kodet R, Newton WA, Jr, Hamoudi AB, Asmar L, Wharam MD, Maurer HM. Orbital rhabdomyosarcomas and related tumors in childhood: relationship of morphology to prognosis—an Intergroup Rhabdomyosarcoma Study. Med Pediatr Oncol. 1997;29:51–60. doi: 10.1002/(sici)1096-911x(199707)29:1<51::aid-mpo10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Wharam MD, Meza J, Anderson J, et al. Failure pattern and factors predictive of local failure in rhabdomyosarcoma: a report of group III patients on the third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 2004;22:1902–1908. doi: 10.1200/JCO.2004.08.124. [DOI] [PubMed] [Google Scholar]
- 5.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. 200. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma—a report from the IRSG. Children’s Oncology Group Soft Tissue Sarcoma Committee (formerly Intergroup Rhabdomyosarcoma Group) representing the Children’s Oncology Group and the quality assurance review center. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 7.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2011;29:1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breneman J, Meza J, Donaldson SS, et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: a report from the Children’s Oncology Group D9602 study. Int J Radiat Oncol Biol Phys. 2012;83:720–726. doi: 10.1016/j.ijrobp.2011.06.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega JA, Donaldson SS, Ivy SP, Pappo A, Maurer HM. Venoocclusive disease of the liver after chemotherapy with vincristine, actinomycin D, and cyclophosphamide for the treatment of rhabdomyosarcoma. A report of the Intergroup Rhabdomyosarcoma Study Group. Children’s Cancer Group, the Pediatric Oncology Group, and the Pediatric Intergroup Statistical Center. Cancer. 1997;79:2435–2439. doi: 10.1002/(sici)1097-0142(19970615)79:12<2435::aid-cncr21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Kenney LB, Laufer MR, Grant FD, Grier H, Diller L. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91:613–621. doi: 10.1002/1097-0142(20010201)91:3<613::aid-cncr1042>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Walterhouse DO, Pappo AS, Meza JL, et al. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2014;32:3547–3552. doi: 10.1200/JCO.2014.55.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Simon R, Wittes RE. Methodologic guidelines for reports of clinical trials. Cancer Treat Rep. 1985;69:1–3. [PubMed] [Google Scholar]
- 14.Minn AY, Lyden E, Anderson JR, et al. Early treatment failure in intermediate-risk rhabdomyosarcoma: results from IRS-IV and D9803—a report from the Children’s Oncology Group. J Clin Oncol. 2010;28:4228–4232. doi: 10.1200/JCO.2010.29.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spalding AC, Hawkins DS, Donaldson SS, et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: an analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys. 2013;87(3):512–516. doi: 10.1016/j.ijrobp.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyn R, Ragab A, Raney RB, Jr, et al. Late effects of therapy in orbital rhabdomyosarcoma in children. A report from the Intergroup Rhabdomyosarcoma Study. Cancer. 1986;57:1738–1743. doi: 10.1002/1097-0142(19860501)57:9<1738::aid-cncr2820570905>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Raney RB, Anderson JR, Kollath J, et al. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: report from the Intergroup Rhabdomyosarcoma Study (IRS)-III, 1984–1991. Med Pediatr Oncol. 2000;34:413–420. doi: 10.1002/(sici)1096-911x(200006)34:6<413::aid-mpo6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Wolden SL, Wexler LH, Kraus DH, Laquaglia MP, Lis E, Meyers PA. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2005;61(5):1432–1438. doi: 10.1016/j.ijrobp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Combs SE, Kessel KA, Herfarth K, et al. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): establishment of workflow and initial clinical data. Radiat Oncol. 2012;17(7):170. doi: 10.1186/1748-717X-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey DL, Wexler LH, Fox JJ, et al. Predicting outcome in patients with rhabdomyosarcoma: role of [(18)f]fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2014;90(5):1136–1142. doi: 10.1016/j.ijrobp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Hingorani P, Missiaglia E, Shipley J, et al. Clinical application of prognostic gene expression signature in fusion gene-negative Rhabdomyosarcoma: a report from the Children’s Oncology Group. Clin Cancer Res. 2015;21(20):4733–4739. doi: 10.1158/1078-0432.CCR-14-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]


