Summary
Objective
Genome-wide association studies have identified many obesity/body mass index (BMI)-associated loci in Europeans and East Asians. Since then, a large number of studies have investigated the role of BMI-associated loci in the development of type 2 diabetes (T2D). However, the results have been inconsistent. The objective of this study was to investigate the associations of 11 obesity/BMI with T2D risk and explore how BMI influences this risk.
Methods
We retrieved published literature from PubMed and Embase. The pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated using fixed- or random- effect model.
Results
In the meta-analysis of 42 studies for 11 obesity/BMI-associated loci, we observed a statistically significant association of FTO rs9939609 polymorphism (66,425 T2D cases/239,689 normoglycemic subjects; p=1.00×10-41) and six other variants with T2D risk (17,915 T2D cases/27,531 normoglycemic individuals: n=40,629 to 130,001; all p<0.001 for SH2B1 rs7498665, FAIM2 rs7138803, TMEM18 rs7561317, GNPDA2 rs10938397, BDNF rs925946 and NEGR1 rs2568958). After adjustment for BMI, the association remained statistically significant for four of the seven variants (all p<0.05 for FTO rs9939609, SH2B1 rs7498665, FAIM2 rs7138803, GNPDA2 rs10938397). Subgroup analysis by ethnicity demonstrated similar results.
Conclusions
This meta-analysis indicates that several BMI-associated variants are significantly associated with T2D risk. Some variants increase the T2D risk independent of obesity, while others mediate this risk through obesity.
Keywords: Type 2 diabetes, Obesity, FTO, Variants, Meta-analysis
Introduction
Obesity is an established risk factor for development of type 2 diabetes (T2D). Many studies have investigated the role of obesity/ body mass index (BMI)-associated loci in predicting risk of T2D. In 2007, a variant (rs9939609) in the fat mass and obesity-associated (FTO) gene was first reported to be statistically significantly associated with obesity [odds ratio (OR)=1.32, 95% confidence interval (CI)=1.26-1.39] and with T2D in individuals of European descent (OR=1.15, 95%CI=1.09-1.23). 1 However, after adjustment for BMI, a surrogate measure of obesity, the significance of association was completely abolished (OR=1.03, 95%CI=0.96-1.10), suggesting that the effect of FTO gene polymorphism on T2D was mediated through obesity among Europeans. 1 At the same time, Scuteri et al. also reported that common variants in the FTO gene were associated with obesity related traits. 2 Since then, many studies have investigated the FTO-T2D association among different ethnic populations and obtained inconsistent results. Following the initial evidence of the BMI-independent association of FTO variant with T2D in Asians,3 a recent large meta-analysis including 96,551 East and South Asians confirmed this observation (crude OR=1.15, 95%CI=1.09-1.21; after adjustment for BMI: OR=1.10, 95%CI=1.05-1.16).4 However, the BMI-independent role of FTO in risk of T2D among Europeans still remains a matter of debate;1, 5–20 some studies indicated a significant association with T2D after correction for BMI, 5, 15, 17 while others reported a marginal or null association. 1, 5–14, 16, 18–20
Subsequently, many other loci associated with obesity or BMI have been identified 12,21 but their association with T2D is also controversial,12,13,18–20,22–24. This may be due to insufficient statistical power and/or inter-population heterogeneity. In this study, we performed a systematic meta-analysis to investigate 11 of the most commonly studied BMI-associated variants (FTO rs9939609, SH2B1 rs7498665, FAIM2 rs7138803, TMEM18 rs7561317, GNPDA2 rs10938397, BDNF rs925946, NEGR1 rs2568958, SEC16B1 rs10913469, KCTD15 rs29941, ETV5 rs7647305 and MTCH2 rs10838738) for their role in predicting risk of T2D.
Materials and methods
Literature and search strategy
To date, more than 50 BMI-associated variants or their proxies have been reportedly associated with various metabolic traits, we could use only 11 variants in the present meta-analysis due to limited data for other variants in the published studies and/or lack of response from the authors. 25 We searched the literature databases including PubMed and Embase. The search strategy was to identify all possible studies that involved the use of following key words: (FTO or SH2B1 or FAIM2 or TMEM18 or NEGR1 or GNPDA2 or SEC16B or KCTD15 or BDNF or ETV5 or MTCH2 or fat mass and obesity associated gene or Src-homology-2 (SH2) domain containing putative adaptor protein 1 or fas apoptotic inhibitory molecule 2 or transmembrane protein 18 or neuronal growth regulator 1 or glucosamine-6-phosphate deaminase 2 or SEC16 homolog B (S. cervisiae) or potassium channel tetramerization domain containing 15 or brain derived neurotrophic factor or ets variant gene 5 or mitochondrial carrier homolog 2) and (polymorphism or variant or variation) and (type 2 diabetes or T2D). The language of publication was restricted to English. The reference lists of retrieved articles were curated manually. If more than one article was published using the same case series, only the study with the largest sample size was included in the meta-analysis. The literature search was last updated on June 20, 2013.
Inclusion criteria and data extraction
We included a study in the meta-analysis if it met all the following inclusion criteria: (1) investigated the association of BMI-associated gene variant(s) with T2D; (2) used case-control or cohort design and (3) provided OR with 95% CI under an additive model or sufficient data for calculation of this estimate. Following information was extracted from each study: (1) name of the first author, (2) year of publication, (3) country of origin, (4) ethnicity of the studied population, (5) study design, (6) number of cases and controls or total subjects, (7) sex distribution and the mean ages, (8) mean BMI, and (9) studied single nucleotide polymorphisms (SNPs). All articles were independently accessed by two authors (BX and DZ) to ensure their compliance with the inclusion/exclusion criteria. Any disagreements were resolved through discussion and a consensus decision was reached. All included studies had informed consent from all the participants and were approved by the appropriate Ethics Committees.
Statistical analysis
We calculated the summary estimate under an additive genetic model in this meta-analysis because majority of the included studies only provided OR with 95%CI under this model.4 We analyzed the associations of 11 BMI-associated gene variants with T2D by calculating pooled ORs and 95% CIs. The significance of the OR was determined by a Z test (p<0.05 was considered statistically significant) and Cochrane’s Q test was performed to test the between-study heterogeneity using a cut-off of p<0.10 as statistically significant. We used a random- (DerSimonian-Laird method) or fixed- (Mantel-Haenszel method) effects model to calculate pooled OR in the presence (p<=0.10) or absence (p>0.10) of heterogeneity, respectively. We used Begg’s test and Egger’s test (p<0.05 was considered statistically significant) to examine any publication bias. To evaluate the stability of the results, we performed sensitivity analysis by removing one study at a time. Statistical analyses for meta-analyses were performed using STATA version 11.0 (StataCorp LP, College Station, TX, USA). The associations were not corrected for multiple testing sine the used loci for association testing have strong priors.
Results
Characteristics of the studies on FTO variant and 10 other BMI associated loci
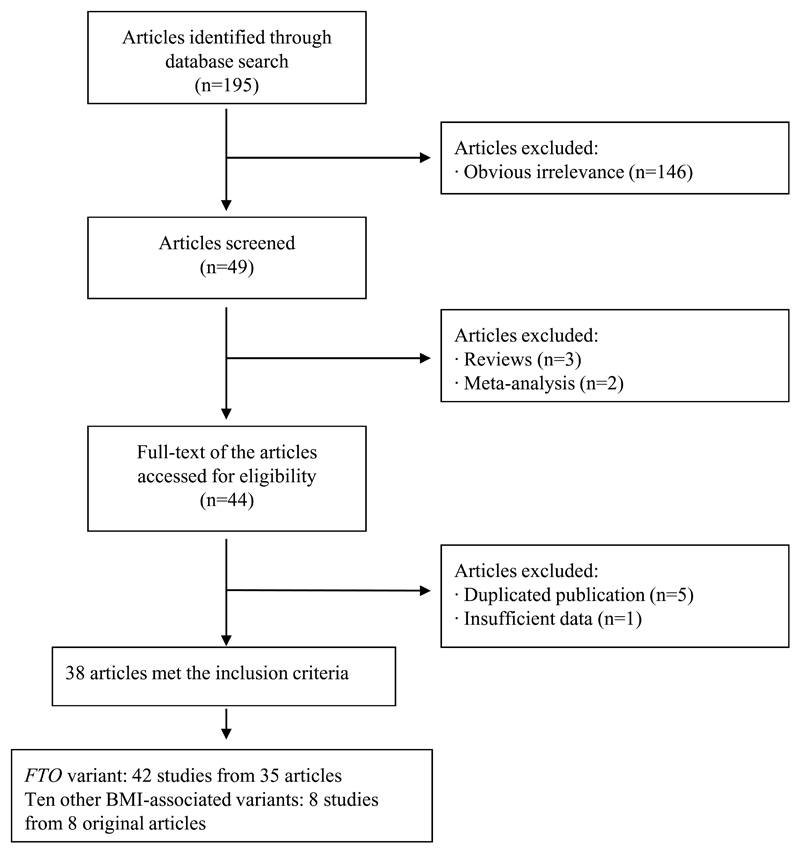
Details of the process of inclusion/exclusion of various studies in the meta-analysis are described in Figure 1. We identified a total of 195 potential relevant articles from the literature search. Of these, 146 were excluded at the outset because of obvious irrelevance as observed from the title or the abstract (e.g. those articles that evaluated the association of FTO gene variant with obesity only, metabolic syndrome, type 1 diabetes, gestational diabetes, cardiovascular disease, polycystic ovary syndrome or cancer). In addition, three review articles and two meta-analyses were also excluded. Six articles were further excluded on account of duplicated publications 22, 26–29 or unavailability of OR with 95% CI values.25 Therefore, a total of 38 articles met the inclusion criteria 1,3–21, 23,24, 30–45.
Figure 1.
Flow chart of exclusion/inclusion of individual articles (or studies) for meta-analysis
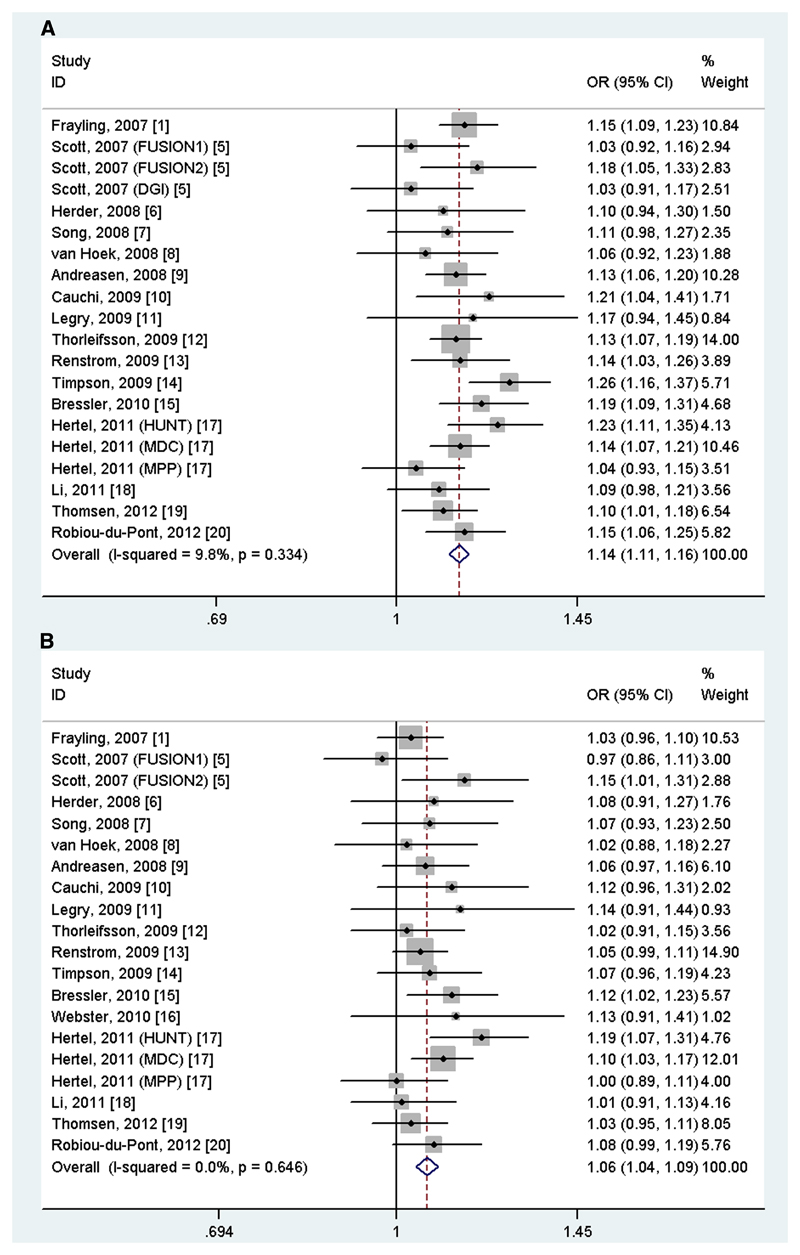
Since more than one study on FTO variant was included in each of the articles by Scott et al. 5 and by Hertel et al., 17 they were considered as separate studies in the meta-analysis. Thus, the final meta-analysis included a total of 42 studies (21 studies for Europeans, 15 studies for East Asians and 6 studies for South Asians) from 35 articles 1, 3–21,23,24,30–45 that had data on rs9939609 (or proxy [r2>0.85]) variant in FTO gene. Out of 21 studies in Europeans, 12 had data on rs9939609, 5 on rs8050136, 2 on rs1121980 and 2 on rs1421085. Only one variant was selected if any study analyzed more than one variant, and we used the FTO variant rs9939609 to represent other polymorphisms because they are in strong linkage disequilibrium (LD) with each other (r2>0.85). All studies provided the crude (except the study by Webster et al 16) and BMI-adjusted (except the study by Scott et al 5) ORs with 95% CIs for FTO-T2D association. Characteristics of the included studies for FTO variant(s) in Europeans are listed in Supplementary Table 1.
For 10 other BMI-associated gene variants included in the meta-analysis, the data on specific SNPs (or proxies) in specific genes was available in variable number of studies. All the variants and their proxies were in strong LD with each other (all r2>0.85 in Hapmap-CEU, CHB and JPT for each SNP). All studies provided the crude and BMI-adjusted ORs with 95%CIs for the association of specific variants with T2D. Characteristics of the included studies for these ten loci are listed in Supplementary Table 2. The genotypes of all 11 BMI-associated variants were in Hardy-Weinberg equilibrium in controls of all included studies (all p>0.05).
Meta-analysis results for FTO variant
A total of 66,425 T2D cases and 239,689 normoglycemic controls for FTO rs9939609 (or proxy) were identified from all the included studies. We observed a statistically significant association of rs9939609 variant with the risk of T2D [(OR=1.14, 95%CI=1.12-1.16, p(z-test)=1.00×10-41), I2=0.0%, p for heterogeneity=0.386, Table 1]. Interestingly, the association remained statistically significant after adjustment for BMI [(OR=1.07, 95%CI=1.05-1.09, p(z-test)=6.42×10-41, I2=0.0%, p for heterogeneity=0.576)]. In the subgroup analysis by ethnicity, similar results were found in Europeans (Table 1 and Figure 2A and 2B), East Asians and South Asians without or with adjustment for BMI (Table 1).
Table 1. Meta-analysis of BMI-associated gene variants and type 2 diabetes risk based on ethnicity.
| Gene/SNP | No. of studies (cases/controls) | OR (95%CI) without BMI adjustment | Pza | Effect model | I2 (%) | PH b | OR (95%CI) with BMI adjustment | Pz a | Effect model | I2 (%) | PHb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | |||||||||||
| FTO rs9939609 | 42 (66,425/239,689) | 1.14 (1.12-1.16) | 1.00×10-41 | Fixed | 5.7 | 0.386 | 1.07 (1.05-1.09) | 6.42×10-41 | Fixed | 0.0 | 0.576 |
| SH2B1 rs7498665 | 7 (24,063/68,660) | 1.08 (1.05-1.12) | 2.28×10-7 | Fixed | 43.5 | 0.101 | 1.06 (1.02-1.09) | 8.71×10-4 | Fixed | 24.1 | 0.245 |
| FAIM2 rs7138803 | 6 (23,025/64,775) | 1.08 (1.05-1.11) | 1.35×10-7 | Fixed | 0.0 | 0.723 | 1.05 (1.02-1.08) | 0.001 | Fixed | 0.0 | 0.728 |
| TMEM18 rs7561317 | 8 (27,531/130,001) | 1.13 (1.06-1.21) | 4.47×10-4 | Random | 62.4 | 0.009 | 1.08 (1.00-1.17) | 0.051 | Random | 68.2 | 0.003 |
| GNPDA2 rs10938397 | 6 (20,187/40,629) | 1.07 (1.03-1.10) | 5.86×10-5 | Fixed | 5.8 | 0.379 | 1.04 (1.01-1.08) | 0.021 | Fixed | 40.5 | 0.136 |
| BDNF rs925946 | 6 (23,025/64,775) | 1.06 (1.03-1.10) | 1.08×10-4 | Fixed | 28.1 | 0.224 | 1.02 (0.97-1.07) | 0.525 | Random | 50.7 | 0.071 |
| NEGR1 rs2568958 | 7 (18,953/67,646) | 1.04 (1.01-1.08) | 0.015 | Fixed | 43.3 | 0.102 | 1.03 (0.94-1.06) | 0.158 | Fixed | 2.1 | 0.409 |
| SEC16B rs10913469 | 6 (23,025/64,775) | 1.00 (0.97-1.04) | 0.938 | Fixed | 0.0 | 0.468 | 0.97 (0.93-1.00) | 0.062 | Fixed | 38.7 | 0.147 |
| KCTD15 rs29941 | 7 (24,063/68,660) | 1.02 (0.99-1.05) | 0.245 | Fixed | 0.0 | 0.754 | 1.01 (0.97-1.04) | 0.738 | Fixed | 0.0 | 0.988 |
| ETV5 rs7647305 | 6 (17,915/63,761) | 1.05 (0.98-1.12) | 0.202 | Random | 56.5 | 0.042 | 1.02 (0.95-1.10) | 0.544 | Random | 55.8 | 0.046 |
| MTCH2 rs10838738 | 6 (20,187/40,629) | 1.00 (0.97-1.16) | 0.999 | Fixed | 19.3 | 0.288 | 1.00 (0.95-1.05) | 0.883 | Random | 46.5 | 0.096 |
| Europeans | |||||||||||
| FTO rs9939609 | 21 (32,681/196,140) | 1.14 (1.11-1.16) | 1.36×10-36 | Fixed | 9.8 | 0.334 | 1.06 (1.04-1.09) | 3.51×10-8 | Fixed | 0.0 | 0.646 |
| SH2B1 rs7498665 | 5 (11,269/59,661) | 1.09 (1.05-1.13) | 2.45×10-6 | Fixed | 38.9 | 0.162 | 1.06 (1.02-1.10) | 0.003 | Fixed | 32.1 | 0.207 |
| FAIM2 rs7138803 | 4 (10,231/55,776) | 1.08 (1.05-1.13) | 1.69×10-5 | Fixed | 0.0 | 0.557 | 1.05 (1.01-1.10) | 0.008 | Fixed | 0.0 | 0.452 |
| TMEM18 rs7561317 | 6 (14,737/121,002) | 1.14 (1.05-1.24) | 0.003 | Random | 66.8 | 0.010 | 1.08 (0.98-1.18) | 0.133 | Random | 69.2 | 0.006 |
| GNPDA2 rs10938397 | 4 (7,393/31,630) | 1.06 (1.01-1.11) | 0.020 | Fixed | 35.4 | 0.200 | 1.03 (0.95-1.12) | 0.414 | Random | 56.6 | 0.075 |
| BDNF rs925946 | 4 (10,231/55,776) | 1.04 (1.00-1.09) | 0.065 | Fixed | 37.7 | 0.186 | 1.00 (0.96-1.04) | 0.903 | Fixed | 43.2 | 0.152 |
| NEGR1 rs2568958 | 5 (11,269/59,661) | 1.06 (1.02-1.10) | 0.002 | Fixed | 0.0 | 0.787 | 1.03 (1.00-1.07) | 0.072 | Fixed | 0.0 | 0.740 |
| SEC16B rs10913469 | 4 (10,231/55,776) | 0.97 (0.93-1.02) | 0.225 | Fixed | 0.0 | 0.807 | 0.93 (0.88-0.97) | 0.002 | Fixed | 0.0 | 0.566 |
| KCTD15 rs29941 | 5 (11,269/59,661) | 1.01 (0.97-1.05) | 0.597 | Fixed | 0.0 | 0.866 | 1.00 (0.96-1.04) | 0.998 | Fixed | 0.0 | 0.954 |
| ETV5 rs7647305 | 4 (10,231/55,776) | 1.03 (0.94-1.13) | 0.483 | Random | 69.6 | 0.020 | 1.00 (0.92-1.08) | 0.910 | Random | 54.7 | 0.085 |
| MTCH2 rs10838738 | 4 (7,393/31,630) | 1.03 (0.99-1.08) | 0.174 | Fixed | 0.0 | 0.845 | 1.02 (0.96-1.07) | 0.557 | Fixed | 12.1 | 0.332 |
| East Asians | |||||||||||
| FTO rs9939609 | 15 (27,401/31,708) | 1.15 (1.09-1.22) | 2.43×10−7 | Fixed | 40.0 | NA | 1.11 (1.05-1.17) | 3.0×10-4 | Fixed | 35.9 | NA |
| SH2B1 rs7498665 | 2 (12,794/8,999) | 1.11 (0.95-1.30) | 0.179 | Random | 74.6 | 0.049 | 1.05 (0.99-1.12) | 0.130 | Fixed | 50.0 | 0.157 |
| FAIM2 rs7138803 | 2 (12,794/8,999) | 1.07 (1.02-1.11) | 0.002 | Fixed | 0.0 | 0.479 | 1.05 (1.00-1.09) | 0.058 | Fixed | 0.0 | 0.733 |
| TMEM18 rs7561317 | 2 (12,794/8,999) | 1.11 (0.95-1.29) | 0.175 | Random | 66.1 | 0.086 | 1.10 (0.97-1.26) | 0.133 | Random | 52.5 | 0.147 |
| GNPDA2 rs10938397 | 2 (12,794/8,999) | 1.08 (1.03-1.13) | 0.001 | Fixed | 0.0 | 0.589 | 1.06 (1.01-1.12) | 0.015 | Fixed | 0.0 | 0.617 |
| BDNF rs925946 | 2 (12,794/8,999) | 1.09 (1.04-1.14) | 0.001 | Fixed | 0.0 | 0.633 | 1.07 (1.01-1.12) | 0.006 | Fixed | 0.0 | 0.481 |
| NEGR1 rs2568958 | 2 (7,684/7,985) | 0.92 (0.83-1.02) | 0.106 | Fixed | 54.8 | 0.137 | 0.95 (0.85-1.06) | 0.351 | Fixed | 51.1 | 0.153 |
| SEC16B rs10913469 | 2 (12,794/8,999) | 1.04 (0.99-1.09) | 0.154 | Fixed | 0.0 | 0.733 | 1.01 (0.96-1.07) | 0.610 | Fixed | 0.0 | 0.791 |
| KCTD15 rs29941 | 2 (12,794/8,999) | 1.03 (0.98-1.08) | 0.223 | Fixed | 42.6 | 0.187 | 1.01 (0.96-1.07) | 0.593 | Fixed | 0.0 | 0.761 |
| ETV5 rs7647305 | 2 (7,684/7,985) | 1.10 (0.98-1.24) | 0.116 | Fixed | 4.0 | 0.307 | 1.15 (1.01-1.30) | 0.039 | Fixed | 20.5 | 0.262 |
| MTCH2 rs10838738 | 2 (12,794/8,999) | 0.97 (0.93-1.02) | 0.196 | Fixed | 45.9 | 0.174 | 0.98 (0.88-1.08) | 0.627 | Random | 64.1 | 0.095 |
| South Asians | |||||||||||
| FTO rs9939609 | 6 (6,271/11,841) | 1.13 (1.03-1.24) | 0.01 | Random | 54.6 | NA | 1.10 (1.00-1.21) | 0.05 | Random | 55.6 | NA |
Notes : OR, odds ratio; CI, confidence interval; NA, not available
P value for Z test
P value for x2-based Q test (If P<=0.10, the random effect model was used; otherwise, the fixed effect model was applied)
Figure 2.
Meta-analysis of the association between FTO rs9939609 variant and type 2 diabetes (A) without and (B) with adjustment for body mass index
Meta-analysis results for 10 other BMI-associated loci
We had a variable number of subjects for association analysis of each BMI-associated gene variant with T2D. The sample size in T2D cases ranged from 17,915 to 27,531, and from 40,629 to 130,001 for normoglycemic controls. We observed a statistically significant association of six BMI-associated gene variants with the risk of T2D (SH2B1 rs7498665: OR=1.08, 95%CI=1.05-1.12, p(z-test)=2.28×10-7; FAIM2 rs7138803: OR=1.08, 95%CI=1.05-1.11, p(z-test)=1.35×10-7; TMEM18 rs7561317: OR=1.13, 95%CI=1.06-1.21, p(z-test)=4.47×10-4; GNPDA2 rs10938397: OR=1.07, 95%CI=1.03-1.10, p(z-test)= 5.86×10-5; BDNF rs925946: OR=1.06, 95%CI=1.03-1.10, p(z-test)=1.08×10-4, NEGR1 rs2568958: OR=1.04, 95%CI=1.01-1.08, p(z-test)=0.015 (Table 1). After adjustment for BMI, the associations remained statistically significant for three variants (SH2B1 rs7498665: OR=1.06, 95%CI=1.02-1.09, p(z-test)=8.71×10-4; FAIM2 rs7138803: OR=1.05, 95%CI=1.02-1.08, p(z-test)=0.001; GNPDA2 rs10938397: OR=1.04, 95%CI=1.01-1.08, p(z-test)=0.021) but was abolished for three other variants (TMEM18 rs7561317, BDNF rs925946 and NEGR1 rs2568958) (Table 1). However, we did not observe statistically significant associations of four other BMI-associated gene variants (SEC16B rs10913469, KCTD15 rs29941, ETV5 rs7647305, MTCH2 rs10838738) with T2D without or with adjustment for BMI (Table 1).
In the Europeans, five BMI-associated gene variants (SH2B1 rs7498665, FAIM2 rs7138803, TMEM18 rs7561317, GNPDA2 rs10938397 and NEGR1 rs2568958) were significantly associated with the risk of T2D. However, the associations remained statistically significant for only two variants (SH2B1 rs7498665 and FAIM2 rs7138803) after adjustment for BMI (Table 1). In the East Asians, three BMI-associated gene variants (FAIM2 rs7138803, GNPDA2 rs10938397 and BDNF rs925946) were significantly associated with the risk of T2D without or with adjustment for BMI (Table 1). The overall sample size was adequately powered (>90%) to detect the association.
Sensitivity analysis and publication bias
We performed sensitivity analysis by excluding one study at a time. The results confirmed the statistically significant association between 11 BMI-associated variants and the risk of T2D without or with adjustment for BMI (data not shown). There was no evidence of any publication bias for all the variants (p>0.05 for Begg’s test and Egger’s test).
Discussion
In this study, we performed an extensive review and meta-analysis to investigate the role of BMI-associated gene variants in predicting risk of T2D. Our study indicates that in addition to FTO, polymorphisms in six other BMI associated genes (SH2B1, FAIM2, TMEM18 GNPDA2, BDNF and NEGR1) were statistically significantly associated with an increased risk of T2D in Europeans and East Asians. Associations of variants in four genes including FTO, SH2B1, FAIM2 and GNPDA2 with T2D may not be entirely mediated via obesity (BMI).
The FTO gene on chromosome 16q12.2 was first identified as a susceptibility locus for T2D in Europeans by genome-wide association study (GWAS). 1 However, based on complete abolition of the FTO variant-T2D association on adjustment for BMI, the study concluded that the effect of the FTO variant on T2D was fully mediated through adiposity. 1 Subsequently, many individual studies have reported inconsistent results. 1, 5–20 A recent meta-analysis of the association between FTO variant and incident T2D in three cohorts showed influence of the FTO variant on the risk of T2D independent of BMI. 17 In the present study, we also found that BMI had no substantial impact on the association between FTO rs9939609 variant and risk of T2D and the association was observed irrespective of ethnicity. Our meta-analysis includes probably the largest sample size to date investigating this association and hence the results are highly convincing. In addition, we observed a similar effect size among Europeans and Asians with or without adjustment for BMI, suggesting a global role for FTO variants in predicting an independent risk for T2D.
Two recent GWAS studies initially designed to identify obesity susceptibility loci reported 10 other BMI-associated gene variants. 12, 21 Although several studies have investigated the associations of these reported variants with T2D, results have not been replicated. Since obesity is one of the main risk factors for T2D, exploration of obesity-associated genes in the development of T2D has important implications. We have recently established the association of BMI-associated variant in MC4R with risk of T2D and demonstrated no influence of BMI on the strength of association. 46 Our present observations provide evidence of possible existence of two types of obesity-associated genetic variants that could explain the link between obesity and T2D; most increase risk of T2D through obesity, while some have independent association with T2D. To our knowledge, no meta-analysis on this aspect has been performed and our results might shed light on the underlying mechanism on how obesity increases the risk of T2D.
It is still not clear how variants in the BMI associated genes could independently influence the risk of T2D. As is known, the FTO protein is highly expressed in the central nervous system (CNS) and regulates energy metabolism. 21 Many studies have also indicated that variants in FTO influences energy-dense food intake rather than regulation of energy expenditure. 47 In addition, FTO variants are reported to be associated with diabetes-related metabolic traits (including higher fasting insulin, glucose and triglycerides, and lower HDL cholesterol), although the associations disappeared after adjustment for BMI.48 Furthermore, the FTO is also highly expressed in muscle, and a recent study supported an important role of FTO in oxidative metabolism, lipogenesis and oxidative stress in muscle, 49 which suggests its potential involvement in the muscle defects that characterize T2D. Similar to FTO, SH2B1, FAIM2, TMEM18, NEGR1, GNPDA2 and BDNF are also highly expressed in the CNS and thus may play influence the above mentioned traits.21 SH2B1 is specifically implicated in the insulin signaling pathway and Sh2b1-null mice tend to have high-fat diet-induced hyperglycemia, hyperinsulinemia, and glucose intolerance.50 NEGR1 plays an important role in neuronal outgrowth.51 BDNF is suggested to regulate blood glucose homeostasis and insulin sensitivity peripherally.52 The potential role of FAIM2, TMEM18, and GNPDA2 proteins in T2D associated pathophysiological processes needs to be investigated further.
Our data shows that although the association of several of BMI-associated variants with type 2 diabetes is statistically significant, their effect size is small. 53 This suggests that globally BMI-associated SNPs may play relatively small role in the pathophysiology of T2D. However, majority of the T2D-associated variants identified to date influence beta-cell function rather than insulin resistance and hence it may not be unreasonable to assume that BMI-associated variants might have more of an effect on insulin resistance than on beta-cell dysfunction.54 This may also explain why majority of these variants do not predict strong risk for T2D.
Our meta-analysis is subject to several limitations. First, BMI is not the ideal measure of obesity and adjustment for BMI may not fully consider the effect of obesity on variant-T2D association. Other measures of obesity such as waist circumference and waist-hip ratio should be taken into account in future studies. Second, the diabetic status and subsequent anti-diabetic treatment or life-style intervention may influence adiposity and BMI. Third, we cannot rule out the well-known reporting biases that have been identified after the onset of diseases like T2D, and thus prospective studies will be superior to cross-sectional designs for answering such questions with reasonable confidence. Fourth, since most of the studies included in this meta-analysis did not provide data on diet, physical activity and other metabolic variables, we could not address their influence on the effect of FTO variant on obesity and obesity comorbidity. Fifth, to date only three studies 13, 18, 20 have examined the cumulative risk of several obesity-associated loci (some in the form of genetic risk score) on T2D, which impeded our attempts of pooled analysis. Finally, in this meta-analysis, we only included published studies, thus, the exclusion of unpublished data may bias the results. To overcome these limitations, a nested T2D case-control (matched for ethnicity, sex, age, adiposity) recruited from a multi-ethnic prospective study seems to be an optimal design to properly assess the association of obesity-related variants with T2D risk.
In conclusion, our meta-analysis with sufficient statistical power has confirmed the statistically significant associations of seven BMI-associated genes (FTO, SH2B1, FAIM2, TMEM18, BDNF, GNPDA2, NEGR1) with risk of T2D in Europeans or East Asians, and several variants seem to predict risk of T2D, independent of BMI. However, observations on several other BMI-associated genes in the development of T2D could not be replicated. The findings suggest that it will be important to dissect the pathways that separate the roles of these variants in the risk of T2D.
Supplementary Material
Acknowledgements
This study was partially supported by Council of Scientific and Industrial Research (CSIR), and Ministry of Science of Technology, Govt. of India, India through their XII FYP titled “CARDIOMED”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr Mark I McCarthy (Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, UK), Dr Michael Boehnke and Dr Heather M. Stringham (Department of Biostatistics and Center for Statistical Genetics, University of Michigan, Ann Arbor, USA) for providing data.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FTO
fat mass and obesity-associated
- OR
odds ratio
- T2D
type 2 diabetes
Footnotes
Declaration of interest
None of the authors has any potential conflicts of interest to declare.
Financial disclosure
None of the authors have received any financial benefit for their participation in the research nor the writing of this article.
Author Contributions
Conceived and designed the experiments: BX, DZ, GRC
Performed the experiments: FT, AM, APM, WZ, HP
Analyzed the data: BX, FT, AM, APM, WZ, HP
Contributed reagents/materials/analysis tools: FT, AM, APM, NK, JCC, YSC, WZ, KLM, JSK, XOS, HP, EST, HP, JYW
Contributed to the writing of the manuscript: BX, DZ, GRC
ICMJE criteria for authorship read and met: BX, DZ, FT, AM, APM, NK, JCC, YSC, WZ, KLM, JSK, XOS, HP, EST, HP, JYW, GRC
Agree with manuscript results and conclusions: BX, DZ, FT, AM, APM, NK, JCC, YSC, WZ, KLM, JSK, XOS, HP, EST, HP, JYW, GRC
All authors have read and approved the final manuscript.
References
- 1.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omori S, Tanaka Y, Takahashi A, et al. Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes. 2008;57:791–795. doi: 10.2337/db07-0979. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Kilpeläinen TO, Liu C, et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia. 2012;55:981–995. doi: 10.1007/s00125-011-2370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herder C, Rathmann W, Strassburger K, et al. Variants of the PPARG, IGF2BP2, CDKAL1, HHEX, and TCF7L2 genes confer risk of type 2 diabetes independently of BMI in the German KORA studies. Horm Metab Res. 2008;40:722–726. doi: 10.1055/s-2008-1078730. [DOI] [PubMed] [Google Scholar]
- 7.Song Y, You NC, Hsu YH, et al. FTO polymorphisms are associated with obesity but not diabetes risk in postmenopausal women. Obesity (Silver Spring) 2008;16:2472–2480. doi: 10.1038/oby.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hoek M, Dehghan A, Witteman JC, et al. Predicting type 2 diabetes based on polymorphisms from genome-wide association studies: a population-based study. Diabetes. 2008;57:3122–3128. doi: 10.2337/db08-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 10.Cauchi S, Stutzmann F, Cavalcanti-Proença C, et al. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med (Berl) 2009;87:537–546. doi: 10.1007/s00109-009-0451-6. [DOI] [PubMed] [Google Scholar]
- 11.Legry V, Cottel D, Ferrières J, et al. Effect of an FTO polymorphism on fat mass, obesity, and type 2 diabetes mellitus in the French MONICA Study. Metabolism. 2009;58:971–975. doi: 10.1016/j.metabol.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 13.Renström F, Payne F, Nordström A, et al. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009;18:1489–1496. doi: 10.1093/hmg/ddp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timpson NJ, Lindgren CM, Weedon MN, et al. Adiposity-related heterogeneity in patterns of type 2 diabetes susceptibility observed in genome-wide association data. Diabetes. 2009;58:505–510. doi: 10.2337/db08-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bressler J, Kao WH, Pankow JS, et al. Risk of type 2 diabetes and obesity is differentially associated with variation in FTO in whites and African-Americans in the ARIC study. PLoS One. 2010;5:e10521. doi: 10.1371/journal.pone.0010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster RJ, Warrington NM, Beilby JP, et al. The longitudinal association of common susceptibility variants for type 2 diabetes and obesity with fasting glucose level and BMI. BMC Med Genet. 2010;11:140. doi: 10.1186/1471-2350-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hertel JK, Johansson S, Sonestedt E, et al. FTO, type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes. 2011;60:1637–1644. doi: 10.2337/db10-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Zhao JH, Luan J, et al. Genetic predisposition to obesity leads to increased risk of type 2 diabetes. Diabetologia. 2011;54:776–782. doi: 10.1007/s00125-011-2044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomsen M, Dahl M, Tybjærg-Hansen A, et al. β2-Adrenergic Receptor Thr164Ile Polymorphism, Obesity, and Diabetes: Comparison with FTO, MC4R, and TMEM18 Polymorphisms in More Than 64,000 Individuals. J Clin Endocrinol Metab. 2012;97:E1074–1079. doi: 10.1210/jc.2011-3282. [DOI] [PubMed] [Google Scholar]
- 20.Robiou-du-Pont S, Bonnefond A, Yengo L, et al. Contribution of 24 obesity-associated genetic variants to insulin resistance, pancreatic beta-cell function and type 2 diabetes risk in the French population. Int J Obes (Lond) 2013;37:980–985. doi: 10.1038/ijo.2012.175. [DOI] [PubMed] [Google Scholar]
- 21.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandholt CH, Vestmar MA, Bille DS, et al. Studies of metabolic phenotypic correlates of 15 obesity associated gene variants. PLoS One. 2011;6:e23531. doi: 10.1371/journal.pone.0023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng MC, Tam CH, So WY, et al. Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J Clin Endocrinol Metab. 2010;95:2418–2425. doi: 10.1210/jc.2009-2077. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi F, Yamamoto K, Katsuya T, et al. Association of genetic variants for susceptibility to obesity with type 2 diabetes in Japanese individuals. Diabetologia. 2011;54:1350–1359. doi: 10.1007/s00125-011-2086-8. [DOI] [PubMed] [Google Scholar]
- 25.Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertel JK, Johansson S, Raeder H, et al. Genetic analysis of recently identified type 2 diabetes loci in 1,638 unselected patients with type 2 diabetes and 1,858 control participants from a Norwegian population-based cohort (the HUNT study) Diabetologia. 2008;51:971–977. doi: 10.1007/s00125-008-0982-3. [DOI] [PubMed] [Google Scholar]
- 27.Pettersen E, Skorpen F, Kvaløy K, et al. Genetic heterogeneity in latent autoimmune diabetes is linked to various degrees of autoimmune activity: results from the Nord-Trøndelag Health Study. Diabetes. 2010;59:302–310. doi: 10.2337/db09-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220–2232. doi: 10.1056/NEJMoa0801869. [DOI] [PubMed] [Google Scholar]
- 29.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Song F, Jiang H, et al. A genetic variation in the fat mass- and obesity-associated gene is associated with obesity and newly diagnosed type 2 diabetes in a Chinese population. Diabetes Metab Res Rev. 2010;26:128–132. doi: 10.1002/dmrr.1066. [DOI] [PubMed] [Google Scholar]
- 31.Tan JT, Dorajoo R, Seielstad M, et al. FTO variants are associated with obesity in the Chinese and Malay populations in Singapore. Diabetes. 2008;57:2851–2857. doi: 10.2337/db08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Wu Y, Loos RJ, et al. Variants in the fat mass- and obesity-associated (FTO) gene are not associated with obesity in a Chinese Han population. Diabetes. 2008;57:264–268. doi: 10.2337/db07-1130. [DOI] [PubMed] [Google Scholar]
- 33.Ng MC, Tam CH, So WY, et al. Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J Clin Endocrinol Metab. 2010;95:2418–2425. doi: 10.1210/jc.2009-2077. [DOI] [PubMed] [Google Scholar]
- 34.Wen J, Ronn T, Olsson A, et al. Investigation of type 2 diabetes risk alleles support CDKN2A/B, CDKAL1, and TCF7L2 as susceptibility genes in a Han Chinese cohort. PLoS One. 2010;5:e9153. doi: 10.1371/journal.pone.0009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang YC, Liu PH, Lee WJ, et al. Common variation in the fat mass and obesity-associated (FTO) gene confers risk of obesity and modulates BMI in the Chinese population. Diabetes. 2008;57:2245–2252. doi: 10.2337/db08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu XO, Long J, Cai Q, et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 2010;6:e1001127. doi: 10.1371/journal.pgen.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Liu Z, Song Y, et al. Meta-analysis added power to identify variants in FTO associated with type 2 diabetes and obesity in the Asian population. Obesity (Silver Spring) 2010;18:1619–1624. doi: 10.1038/oby.2009.469. [DOI] [PubMed] [Google Scholar]
- 38.Hu C, Zhang R, Wang C, et al. PPARG, KCNJ11, CDKAL1, CDKN2A-CDKN2B, IDE-KIF11-HHEX, IGF2BP2 and SLC30A8 are associated with type 2 diabetes in a Chinese population. PLoS One. 2009;4:e7643. doi: 10.1371/journal.pone.0007643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karasawa S, Daimon M, Sasaki S, et al. Association of the common fat mass and obesity associated (FTO) gene polymorphism with obesity in a Japanese population. Endocr J. 2010;57:293–301. doi: 10.1507/endocrj.k09e-305. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi F, Serizawa M, Yamamoto K, et al. Confirmation of multiple risk loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes. 2009;58:1690–1699. doi: 10.2337/db08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng MC, Park KS, Oh B, et al. Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes. 2008;57:2226–2233. doi: 10.2337/db07-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marvelle AF, Lange LA, Qin L, et al. Association of FTO with obesity-related traits in the Cebu Longitudinal Health and Nutrition Survey (CLHNS) Cohort. Diabetes. 2008;57:1987–1991. doi: 10.2337/db07-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yajnik CS, Janipalli CS, Bhaskar S, et al. FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia. 2009;52:247–252. doi: 10.1007/s00125-008-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanghera DK, Ortega L, Han S, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet. 2008;9:59. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chambers JC, Elliott P, Zabaneh D, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 46.Xi B, Takeuchi F, Chandak GR, et al. Common polymorphism near the MC4R gene is associated with type 2 diabetes: data from a meta-analysis of 123,373 individuals. Diabetologia. 2012;55:2660–2666. doi: 10.1007/s00125-012-2655-5. [DOI] [PubMed] [Google Scholar]
- 47.Razquin C, Marti A, Martinez JA. Evidences on three relevant obesogenes: MC4R, FTO and PPARγ. Approaches for personalized nutrition. Mol Nutr Food Res. 2011;55:136–149. doi: 10.1002/mnfr.201000445. [DOI] [PubMed] [Google Scholar]
- 48.Freathy RM, Timpson NJ, Lawlor DA, et al. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes. 2008;57:1419–1426. doi: 10.2337/db07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bravard A, Lefai E, Meugnier E, et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes. 2011;60:258–268. doi: 10.2337/db10-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris DL, Cho KW, Zhou Y, et al. SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes. 2009;58:2039–2047. doi: 10.2337/db08-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schafer M, Brauer AU, Savaskan NE, et al. Neurotractin/kilon promotes neurite outgrowth and is expressed on reactive astrocytes after entorhinal cortex lesion. Mol Cell Neurosci. 2005;29:580–590. doi: 10.1016/j.mcn.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 53.Xi B, Mi J. Genome-wide Association Studies of Common Obesity: Now and Future. Biomed Environ Sci. 2013;26:787–791. doi: 10.3967/bes2013.001. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsson JA, Klovins J, Kapa I, et al. Novel genetic variant in FTO influences insulin levels and insulin resistance in severely obese children and adolescents. Int J Obes (Lond) 2008;32:1730–1735. doi: 10.1038/ijo.2008.168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.