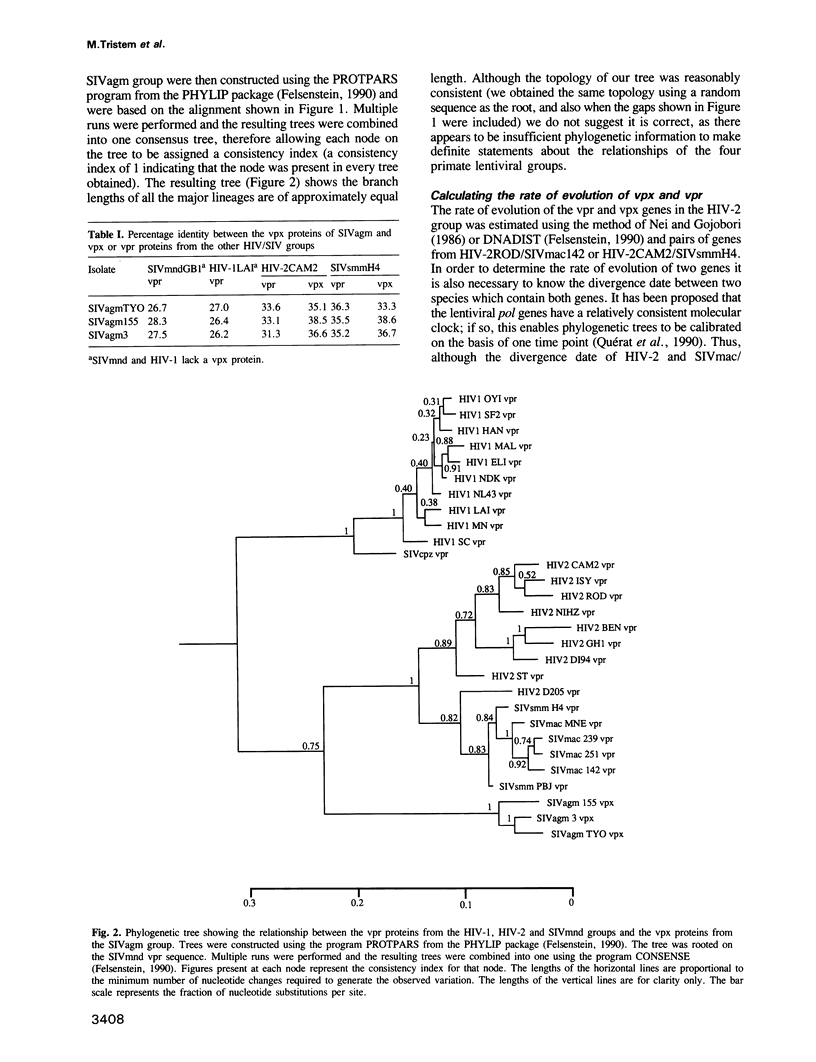
Abstract
The genomes of the four primate lentiviral groups are complex and contain several regulatory or accessory genes. Two of these genes, vpr and vpx, are found in various combinations within the four groups and encode proteins whose functions have yet to be elucidated. Comparison of the encoded protein sequences suggests that the vpx gene within the HIV-2 group arose by the duplication of an ancestral vpr gene within this group. Evolutionary distance analysis showed that both genes were well conserved when compared with viral regulatory genes, and indicated that the duplication occurred at approximately the same time as the HIV-2 group and the other primate lentivirus groups diverged from a common ancestor. Furthermore, although the SIVagm vpx proteins are homologous to the HIV-2 group vpx proteins, there are insufficient grounds from sequence analysis for classifying them as vpx proteins. Because of their similarity to the vpr proteins of other groups, we suggest reclassifying the SIVagm vpx gene as a vpr gene. This creates a simpler and more uniform picture of the genomic organization of the primate lentiviruses and allows the genomic organization of their common precursor to be defined; it probably contained five accessory genes: tat, rev, vif, nef and vpr.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Baier M., Werner A., Cichutek K., Garber C., Müller C., Kraus G., Ferdinand F. J., Hartung S., Papas T. S., Kurth R. Molecularly cloned simian immunodeficiency virus SIVagm3 is highly divergent from other SIVagm isolates and is biologically active in vitro and in vivo. J Virol. 1989 Dec;63(12):5119–5123. doi: 10.1128/jvi.63.12.5119-5123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Barton G. J., Sternberg M. J. A strategy for the rapid multiple alignment of protein sequences. Confidence levels from tertiary structure comparisons. J Mol Biol. 1987 Nov 20;198(2):327–337. doi: 10.1016/0022-2836(87)90316-0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guyader M., Guétard D., Sallé M., Montagnier L., Alizon M. Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature. 1986 Dec 18;324(6098):691–695. doi: 10.1038/324691a0. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Dehni G., Sodroski J. G., Haseltine W. A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990 Jun;64(6):3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Dedera D., Hu W., Vander Heyden N., Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol. 1989 Jul;63(7):3205–3208. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. HIV-1 origins. A finger on the missing link. Nature. 1990 May 24;345(6273):288–289. doi: 10.1038/345288a0. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Embretson J. E., Anderson D. C., Mullins J. I., Fultz P. N. Sequence analysis and acute pathogenicity of molecularly cloned SIVSMM-PBj14. Nature. 1990 Jun 14;345(6276):636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- Dietrich U., Adamski M., Kreutz R., Seipp A., Kühnel H., Rübsamen-Waigmann H. A highly divergent HIV-2-related isolate. Nature. 1989 Dec 21;342(6252):948–950. doi: 10.1038/342948a0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Emau P., McClure H. M., Isahakia M., Else J. G., Fultz P. N. Isolation from African Sykes' monkeys (Cercopithecus mitis) of a lentivirus related to human and simian immunodeficiency viruses. J Virol. 1991 Apr;65(4):2135–2140. doi: 10.1128/jvi.65.4.2135-2140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Fargnoli K. A., Giombini F., Jagodzinski L., De Rossi A., Bosch M., Biberfeld G., Fenyo E. M., Albert J., Gallo R. C. Molecular and biological characterization of a replication competent human immunodeficiency type 2 (HIV-2) proviral clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2433–2437. doi: 10.1073/pnas.86.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Franchini G., Rusche J. R., O'Keeffe T. J., Wong-Staal F. The human immunodeficiency virus type 2 (HIV-2) contains a novel gene encoding a 16 kD protein associated with mature virions. AIDS Res Hum Retroviruses. 1988 Aug;4(4):243–250. doi: 10.1089/aid.1988.4.243. [DOI] [PubMed] [Google Scholar]
- Fukasawa M., Miura T., Hasegawa A., Morikawa S., Tsujimoto H., Miki K., Kitamura T., Hayami M. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature. 1988 Jun 2;333(6172):457–461. doi: 10.1038/333457a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey K. J., Oberste M. S., Elser J. E., Braun M. J., Gonda M. A. Nucleotide sequence and genome organization of biologically active proviruses of the bovine immunodeficiency-like virus. Virology. 1990 Apr;175(2):391–409. doi: 10.1016/0042-6822(90)90424-p. [DOI] [PubMed] [Google Scholar]
- Gojobori T., Moriyama E. N., Ina Y., Ikeo K., Miura T., Tsujimoto H., Hayami M., Yokoyama S. Evolutionary origin of human and simian immunodeficiency viruses. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4108–4111. doi: 10.1073/pnas.87.11.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., McLachlan A. D., Eisenberg D. Profile analysis: detection of distantly related proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4355–4358. doi: 10.1073/pnas.84.13.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgo C., Guo H. G., Franchini G., Aldovini A., Collalti E., Farrell K., Wong-Staal F., Gallo R. C., Reitz M. S., Jr Envelope sequences of two new United States HIV-1 isolates. Virology. 1988 Jun;164(2):531–536. doi: 10.1016/0042-6822(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Montagnier L., Peden K. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 1989 Apr;8(4):1169–1175. doi: 10.1002/j.1460-2075.1989.tb03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa A., Tsujimoto H., Maki N., Ishikawa K., Miura T., Fukasawa M., Miki K., Hayami M. Genomic divergence of HIV-2 from Ghana. AIDS Res Hum Retroviruses. 1989 Dec;5(6):593–604. doi: 10.1089/aid.1989.5.593. [DOI] [PubMed] [Google Scholar]
- Hattori N., Michaels F., Fargnoli K., Marcon L., Gallo R. C., Franchini G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8080–8084. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Benveniste R. E., Sowder R., Copeland T. D., Schultz A. M., Oroszlan S. Molecular characterization of gag proteins from simian immunodeficiency virus (SIVMne). J Virol. 1988 Aug;62(8):2587–2595. doi: 10.1128/jvi.62.8.2587-2595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R. C., Copeland T. D., Benveniste R. E., Oroszlan S. Isolation and characterization of a novel protein (X-ORF product) from SIV and HIV-2. Science. 1988 Jul 8;241(4862):199–201. doi: 10.1126/science.3388031. [DOI] [PubMed] [Google Scholar]
- Hirsch V. M., Olmsted R. A., Murphey-Corb M., Purcell R. H., Johnson P. R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989 Jun 1;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet T., Cheynier R., Meyerhans A., Roelants G., Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990 May 24;345(6273):356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- Huet T., Dazza M. C., Brun-Vézinet F., Roelants G. E., Wain-Hobson S. A highly defective HIV-1 strain isolated from a healthy Gabonese individual presenting an atypical western blot. AIDS. 1989 Nov;3(11):707–715. doi: 10.1097/00002030-198911000-00004. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Fomsgaard A., Allan J., Gravell M., London W. T., Olmsted R. A., Hirsch V. M. Simian immunodeficiency viruses from African green monkeys display unusual genetic diversity. J Virol. 1990 Mar;64(3):1086–1092. doi: 10.1128/jvi.64.3.1086-1092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappes J. C., Conway J. A., Lee S. W., Shaw G. M., Hahn B. H. Human immunodeficiency virus type 2 vpx protein augments viral infectivity. Virology. 1991 Sep;184(1):197–209. doi: 10.1016/0042-6822(91)90836-z. [DOI] [PubMed] [Google Scholar]
- Kappes J. C., Morrow C. D., Lee S. W., Jameson B. A., Kent S. B., Hood L. E., Shaw G. M., Hahn B. H. Identification of a novel retroviral gene unique to human immunodeficiency virus type 2 and simian immunodeficiency virus SIVMAC. J Virol. 1988 Sep;62(9):3501–3505. doi: 10.1128/jvi.62.9.3501-3505.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Sherman L., Dahlberg J., Gazit A., Yaniv A., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of equine infectious anemia virus proviral DNA. Virology. 1987 Jun;158(2):300–312. doi: 10.1016/0042-6822(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Klemm E., Schneweis K. E., Horn R., Tackmann W., Schulze G., Schneider J. HIV-II infection with initial neurological manifestation. J Neurol. 1988 May;235(5):304–307. doi: 10.1007/BF00314179. [DOI] [PubMed] [Google Scholar]
- Kumar P., Hui H. X., Kappes J. C., Haggarty B. S., Hoxie J. A., Arya S. K., Shaw G. M., Hahn B. H. Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J Virol. 1990 Feb;64(2):890–901. doi: 10.1128/jvi.64.2.890-901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Kühnel H., von Briesen H., Dietrich U., Adamski M., Mix D., Biesert L., Kreutz R., Immelmann A., Henco K., Meichsner C. Molecular cloning of two west African human immunodeficiency virus type 2 isolates that replicate well in macrophages: a Gambian isolate, from a patient with neurologic acquired immunodeficiency syndrome, and a highly divergent Ghanian isolate. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2383–2387. doi: 10.1073/pnas.86.7.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. A rate-independent technique for analysis of nucleic acid sequences: evolutionary parsimony. Mol Biol Evol. 1987 Mar;4(2):167–191. doi: 10.1093/oxfordjournals.molbev.a040433. [DOI] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Shibata R., Kiyomasu T., Higuchi I., Kishida Y., Ishimoto A., Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989 Sep;63(9):4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Hirsch V. M., Purcell R. H., Johnson P. R. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querat G., Audoly G., Sonigo P., Vigne R. Nucleotide sequence analysis of SA-OMVV, a visna-related ovine lentivirus: phylogenetic history of lentiviruses. Virology. 1990 Apr;175(2):434–447. doi: 10.1016/0042-6822(90)90428-t. [DOI] [PubMed] [Google Scholar]
- Regier D. A., Desrosiers R. C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Saltarelli M., Querat G., Konings D. A., Vigne R., Clements J. E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990 Nov;179(1):347–364. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Sauermann U., Schneider J., Mous J., Brunckhorst U., Schedel I., Jentsch K. D., Hunsmann G. Molecular cloning and characterization of a German HIV-1 isolate. AIDS Res Hum Retroviruses. 1990 Jun;6(6):813–823. doi: 10.1089/aid.1990.6.813. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Understanding the origins of AIDS viruses. Nature. 1988 Nov 24;336(6197):315–315. doi: 10.1038/336315a0. [DOI] [PubMed] [Google Scholar]
- Shibata R., Miura T., Hayami M., Ogawa K., Sakai H., Kiyomasu T., Ishimoto A., Adachi A. Mutational analysis of the human immunodeficiency virus type 2 (HIV-2) genome in relation to HIV-1 and simian immunodeficiency virus SIV (AGM). J Virol. 1990 Feb;64(2):742–747. doi: 10.1128/jvi.64.2.742-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Spire B., Sire J., Zachar V., Rey F., Barré-Sinoussi F., Galibert F., Hampe A., Chermann J. C. Nucleotide sequence of HIV1-NDK: a highly cytopathic strain of the human immunodeficiency virus. Gene. 1989 Sep 30;81(2):275–284. doi: 10.1016/0378-1119(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Staden R. An improved sequence handling package that runs on the Apple Macintosh. Comput Appl Biosci. 1990 Oct;6(4):387–393. doi: 10.1093/bioinformatics/6.4.387. [DOI] [PubMed] [Google Scholar]
- Stockwell P. A., Petersen G. B. HOMED: a homologous sequence editor. Comput Appl Biosci. 1987 Mar;3(1):37–43. doi: 10.1093/bioinformatics/3.1.37. [DOI] [PubMed] [Google Scholar]
- Tristem M., Hill F., Karpas A. Nucleotide sequence of a Guinea-Bissau-derived human immunodeficiency virus type 2 proviral clone (HIV-2CAM2). J Gen Virol. 1991 Mar;72(Pt 3):721–724. doi: 10.1099/0022-1317-72-3-721. [DOI] [PubMed] [Google Scholar]
- Tristem M., Marshall C., Karpas A., Petrik J., Hill F. Origin of vpx in lentiviruses. Nature. 1990 Sep 27;347(6291):341–342. doi: 10.1038/347341b0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H., Hasegawa A., Maki N., Fukasawa M., Miura T., Speidel S., Cooper R. W., Moriyama E. N., Gojobori T., Hayami M. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature. 1989 Oct 12;341(6242):539–541. doi: 10.1038/341539a0. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Chanda P. K., Ghrayeb J. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987 Spring;3(1):33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Chung L., Gojobori T. Molecular evolution of the human immunodeficiency and related viruses. Mol Biol Evol. 1988 May;5(3):237–251. doi: 10.1093/oxfordjournals.molbev.a040495. [DOI] [PubMed] [Google Scholar]
- Yu X. F., Ito S., Essex M., Lee T. H. A naturally immunogenic virion-associated protein specific for HIV-2 and SIV. Nature. 1988 Sep 15;335(6187):262–265. doi: 10.1038/335262a0. [DOI] [PubMed] [Google Scholar]
- Yu X. F., Matsuda M., Essex M., Lee T. H. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J Virol. 1990 Nov;64(11):5688–5693. doi: 10.1128/jvi.64.11.5688-5693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. F., Yu Q. C., Essex M., Lee T. H. The vpx gene of simian immunodeficiency virus facilitates efficient viral replication in fresh lymphocytes and macrophage. J Virol. 1991 Sep;65(9):5088–5091. doi: 10.1128/jvi.65.9.5088-5091.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Matsuda Z., Matsuda M., Essex M., Lee T. H. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]
- Zagury J. F., Franchini G., Reitz M., Collalti E., Starcich B., Hall L., Fargnoli K., Jagodzinski L., Guo H. G., Laure F. Genetic variability between isolates of human immunodeficiency virus (HIV) type 2 is comparable to the variability among HIV type 1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5941–5945. doi: 10.1073/pnas.85.16.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]





