Abstract
Rheumatoid arthritis (RA) is an autoimmune joint disease maintained by aberrant immune responses involving CD4+ T helper (Th)1 and Th17 cells. In this study, we tested the therapeutic efficacy of Ligand Epitope Antigen Presentation System (LEAPS™) vaccines in two Th1 cell-driven mouse models of RA, cartilage proteoglycan (PG)-induced arthritis (PGIA) and PG G1-domain-induced arthritis (GIA). The immunodominant PG peptide PG70 was attached to a DerG or J immune cell binding peptide, and the DerG-PG70 and J-PG70 LEAPS vaccines were administered to the mice after the onset of PGIA or GIA symptoms. As indicated by significant decreases in visual and histopathological scores of arthritis, the DerG-PG70 vaccine inhibited disease progression in both PGIA and GIA, while the J-PG70 vaccine was ineffective. Splenic CD4+ cells from DerG-PG70-treated mice were diminished in Th1 and Th17 populations but enriched in Th2 and regulatory T (Treg) cells. In vitro spleen cell-secreted and serum cytokines from DerG-PG70-treated mice demonstrated a shift from a pro-inflammatory to an anti-inflammatory/regulatory profile. DerG-PG70 peptide tetramers preferentially bound to CD4+ T-cells of GIA spleen cells. We conclude that the DerG-PG70 vaccine (now designated CEL-4000) exerts its therapeutic effect by interacting with CD4+ cells, which results in an antigen-specific down-modulation of pathogenic T-cell responses in both the PGIA and GIA models of RA. Future studies will need to determine the potential of LEAPS vaccination to provide disease suppression in patients with RA.
Keywords: Therapeutic vaccine, Peptide vaccine, Rheumatoid arthritis, Murine models, Autoimmunity, Immunotherapy, Cytokines
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by chronic inflammation and systemic destruction of the peripheral joints [1–6]. RA is a very heterogeneous disease that may have different initiators and be driven by different types of inflammatory responses for each individual. Although the initiating events of RA are unknown, the disease is maintained by pro-inflammatory mediators produced during T helper (primarily Th1 and Th17) cell-driven autoimmune responses [1].
Current treatment of RA largely focuses on alleviation of symptoms and delaying disease progression [7]. The two primary treatment modalities are synthetic, small molecule disease-modifying anti-rheumatic drugs (sDMARDs), and biologic large molecule DMARDs (bDMARDs) [8]. Both sDMARDs and bDMARDS suppress elements of the entire immune system in order to curtail the inflammatory process.
The pro-inflammatory cytokine interleukin (IL)17 has become a popular target for treating the inflammation associated with RA. IL17-targeting therapies still only treat the symptoms, do not act on the source of the inflammatory pathways, are not disease- or antigen-specific, and may ablate important anti-microbial immune responses [6,9].
Vaccination as an immunotherapy is an alternative to treatment with either sDMARDs or bDMARDs and has the potential to modulate the different cells and cytokines involved in the ongoing autoimmune and inflammatory responses. In contrast to other therapies for RA, therapeutic vaccines focus on the antigen specific disease-driving cells that are upstream of disease presentation to modulate the pro-inflammatory Th1 or Th17 responses or enhance regulatory T-cell (Treg) responses in a beneficial manner [10]. A key advantage of therapeutic vaccination is antigen specificity, which is focused on the disease initiators, but the vaccine must also modulate the aberrant ongoing immune reactions of the patient.
LEAPS3 (Ligand Epitope Antigen Presentation System) vaccines offer the opportunity to immunize with a disease-related antigen and concurrently modulate the subsequent immune response. LEAPS vaccines are heteroconjugate peptides composed of a disease- specific antigenic epitope and an immune cell binding ligand (ICBL)[10]. The J peptide ICBL is from human β2 microglobulin and acts first on dendritic cells (DC) [11–13] and the DerG peptide from domain 2 of the β chain of the human MHC class II (HLA-DR) molecule acts on CD4+ T cells [14–16].
The LEAPS vaccine CEL-2000 (a conjugate composed of a J ICBL and an immunodominant type II collagen (CII) epitope) was shown to be therapeutic when administered after the onset of disease symptoms in collagen induced arthritis (CIA), a Th17-driven mouse model of RA [17]. The therapeutic effect was accompanied by reduced serum levels of pro-inflammatory cytokines. Other J-LEAPS heteroconjugate vaccines were effective in treating experimental autoimmune myocarditis [18] or viral infections in animal models [11,12,19,20].
Proteoglycan (PG)-induced arthritis (PGIA) and G1 domain (of PG) induced arthritis (GIA) are autoimmune animal models of RA,3 induced by intraperitoneal (i.p.) immunization of BALB/c female mice with PG of human articular cartilage [21] or the recombinant G1 domain (rhG1) of PG [22]. Both the PGIA and GIA models are predominantly driven by Th1 interferon-gamma (IFNγ) responses [22–25], and generate Th17 as well as other pro- and anti-inflammatory cytokines responses. The PGIA [21,26] and GIA [22] models resemble human RA better than other animal models.
We report here the results of testing J-LEAPS (J-PG70) and DerG-LEAPS (DerG-PG70, also called CEL-4000) vaccines, heteroconjugates with the PG70 peptide,3 in the PGIA and GIA models of RA. The effects of vaccines on the disease process, joint histopathology, and serum levels of anti-inflammatory and pro-inflammatory cytokines were evaluated. T-cell phenotypes and production of cytokines were also examined in vitro using spleen cells from vaccinated and control diseased animals. Binding of vaccine component peptides to different types of immune cells was examined to identify cells likely involved in the response to the vaccines.
2. Materials and methods3
2.1. Peptides
The sequences of the peptides used for the in vivo and in vitro studies were as follows: PG70 (ATEGRVRVNSAYQDK), DerG (DGQEE KAGVVSTGLIGGG), J (DLLKNGERIEKVEGGG), and conjugates DerG-PG70 (DGQEEKAGWSTGLIGGGATEGRVRVNSAYQDK) and J-PG70 (DLLKNGERIEKVEGGGATEGRVRVNSAYQDK). Peptides were purchased from 21st Century Biochemicals (Marlborough, MA) or Ambiopharm (North Augusta, SC) at ≥95% purity with amino acid sequences and mass confirmed by mass spectrometry. Biotinylated peptides were purchased from Biomatik, Inc. (Wilmington, DE).
2.2. Antigens used for inducing arthritis
Human cartilage PG and rhG1 protein were prepared as described.3
2.3. Mice, immunization, and visual assessment of arthritis
Adult (retired breeder) female BALB/c mice were obtained from the National Cancer Institute (NCI; Frederick, MD) or from the former NCI facility acquired by Charles River (Wilmington, MA).
To induce PGIA, mice were immunized i.p. with human PG (100 μg protein in 100 μl sterile phosphate-buffered saline (PBS, pH 7.2)) [21,26] emulsified in dimethyl-dioctadecyl ammonium bromide adjuvant (DDA; Sigma-Aldrich, St Louis, MO) three times, three weeks apart [25]. Mice were inspected for signs of arthritis (swelling and redness) twice a week after the second PG immunization. Upon disease onset, the degree of arthritis for each paw was visually scored every other day on a scale of 0 to 4 for each limb, summing the individual paw scores to a maximum visual arthritis (VA) score of 16 per animal3 [25,27].
Similarly, groups of mice were immunized with rhG1 (40 μg per injection in DDA) i.p. three times to induce GIA [22]. The limbs of animals with GIA were visually scored for the degree of arthritis 3 times a week as described for PGIA.3
2.4. Vaccine treatment of mice with PGIA or GIA
Vaccine treatment was initiated after the mice developed arthritis (PGIA or GIA). Briefly, mice with a similar average VA score in each group (ranging from 2 to 4 in the different studies) were sorted into treatment groups. Doses (100 nmol) of LEAPS conjugates or individual peptides were prepared in 100 μl sterile PBS (pH 7.2) and emulsified at a 1:1 ratio with Montanide ISA-51VG adjuvant (Seppic, Paris, France). The vaccines (or control PBS in adjuvant) were administered s.c. at the nape of the neck into anesthetized mice. The mice were treated first on the day of initial grouping (day 0) and the same dose was administered s.c. on day 14. Monitoring of the VA scores continued three weeks after the second vaccination.3
2.5. Collection of blood and tissues from mice
At the end of the experiments, mice were anesthetized, and bled. Serum samples were stored at −70 °C until use. After blood draw, the anesthetized mice were euthanized via CO2 inhalation. Spleens were harvested under aseptic conditions for in vitro studies, and hind limbs excised and fixed in formalin. All animal procedures3 were approved by the Institutional Animal Care and Use Committee (IACUC) of Rush University Medical Center (IACUC permit number: 14-032).
2.6. Histology
Hind limbs tissue sections were prepared, processed, examined, photographed and scored as described3 [21,25–27].
2.7. Spleen cell cultures
Single cell suspensions from the spleens of mice with PGIA or GIA were processed as described.3 The spleen cells were seeded into 48-well culture plates (3 × 106 viable cells per well) in the absence or presence of PG (50 μg/ml) or rhG1 (7.5 μg/ml) for cells from PGIA and GIA mice, respectively, and cultured for 4 days.
2.8. Determination of T-cell phenotypes and cytokine profiles by flow cytometry
The percentages of Th (CD4+) spleen cells containing intracellular cytokines or Foxp3 in cultures of antigen-stimulated spleen cells were determined by flow cytometry [28] on a BD FACS Canto II flow cytometer and analyzed using FACS Diva software (BD Flow Cytometry Systems, San Jose, CA).3
2.9. Assays of in vitro cytokine secretion
For cytokine secretion studies, spleen cells were either left untreated (native cultures) or treated with human PG or rhG1 (for PGIA and GIA cells, respectively) as specified above. On the fourth day of culture, the plates were centrifuged, supernatants were collected and frozen at −70 °C. For the PGIA study, the concentrations of cytokines were determined by ELISA (IFNγ, IL17, IL4, IL10 kits (Peprotech, Rocky Hill, NJ) and transforming growth factor (TGFβ1; R & D Systems, Minneapolis, MN), according to the manufacturers’ instructions. For the GIA studies, multiple cytokines were measured using the multi-plex mouse Th17 kit (IL1β, IL2, IL4, IL6, IL10, IL12p70, IL17A, IL17F, IFNγ, TNFα) or a single-plex kit for TGFβ1 (both from EMD Millipore, Billerica, MA), according to the manufacturer’s instructions and analyzed using a MagPix® reader with MilliPlex Analyst software (both from EMD Millipore).3
2.10. Serum cytokine assays
Cytokine levels in the serum samples of GIA mice were measured using the MagPix® technology as described above for cell culture supernatants.
2.11. Generation of bone marrow-derived dendritic cells (BM-DCs)
BM was obtained from naïve mice and was enriched in DCs as described previously [28,29]. DCs (MHC class II+ CD11c+ cells) constituted over 60% of the collected cell population, as determined by flow cytometry [29].3
2.12. Assay of peptide tetramer binding to cells
The avidity of peptide binding was increased by tetramerization of biotinylated (B)-peptides with streptavidin-phycoerythrin (SA-PE) according to a protein tetramerization protocol provided by the National Institutes of Health (NIH) Tetramer Core Facility (Emory University, Atlanta, GA). Peptide binding to cells was detected by flow cytometry as described.3
2.13. Statistical analysis
The GraphPad Prism 6 software (GraphPad, La Jolla, CA) was used for both statistical analysis and plotting of data. Results are presented as mean ± SEM. The statistical tests used as well as other details are provided in the Figure Captions and in the footnote to Table 1. P values less than 0.05 were accepted as statistically significant (Material handling and data collection details as described).3
Table 1.
Histopathology scores of the hind limbs of mice with GIA following in vivo treatment with LEAPS peptide vaccines or control agents.1
| GIA (Study 2)2
|
GIA (Study 3)2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBS + adj. | J-PG70 + adj. | DerG-PG70 + adj. | PBS + adj. | J-PG70 + adj. | DerG-PG70 + adj. | PG70 + adj. | DerG + adj. | PBS | ||
| Inflammation | 4.70 ± 0.1 | 4.00 ± 0.9 | 3.45 ± 0.6 | 4.56 ± 0.2 | 5.00 ± 0.0 | 2.97 ± 0.6*,# | 3.72 ± 0.6 | 4.88 ± 0.1 | 4.50 ± 0.2 | |
| Pannus formation | 1.00 ± 0.0 | 0.80 ± 0.2 | 0.65 ± 0.1* | 1.38 ± 0.2 | 2.13 ± 0.2 | 0.81 ± 0.2 | 1.13 ± 0.3 | 1.56 ± 0.1 | 1.38 ± 0.2 | |
| Cartilage damage | 4.60 ± 0.2 | 3.95 ± 0.9 | 3.45 ± 0.6 | 3.84 ± 0.4 | 4.94 ± 0.1 | 2.34 ± 0.5# | 3.41 ± 0.7 | 4.88 ± 0.1 | 4.13 ± 0.4 | |
| Bone resorption | 1.00 ± 0.0 | 0.80 ± 0.2 | 0.65 ± 0.1* | 1.38 ± 0.2 | 2.06 ± 0.2 | 0.78 ± 0.2 | 1.13 ± 0.3 | 1.56 ± 0.1 | 1.38 ± 0.2 | |
| Periosteal bone formation | 2.10 ± 0.0 | 2.10 ± 0.4 | 1.70 ± 0.3 | 1.94 ± 0.2 | 2.06 ± 0.1 | 1.19 ± 0.3 | 1.88 ± 0.4 | 2.06 ± 0.1 | 2.19 ± 0.2 | |
| Cumulative scores | 13.40 ± 0.2 | 11.65 ± 2.6 | 9.90 ± 1.6 | 13.09 ± 1.0 | 16.13 ± 0.5 | 8.09 ± 1.8# | 11.25 ± 2.1 | 14.94 ± 0.2 | 13.09 ± 1.0 | |
Hind limbs of mice with rhG1-induced arthritis (GIA), treated with either LEAPS peptide vaccines in adjuvant (+adj.) or control agents, were subjected to joint histopathology evaluation as described in the Materials and Methods. Scores of individual histopathology parameters (inflammation, pannus formation, etc.) and cumulative scores identified as italized values are listed.
Values are the means ± SEM; n = 5 mice/group (Study 2) and n = 8 mice/group (Study 3). Data were transformed (Y = Y2) before statistical analysis using one-way ANOVA followed by Fisher’s LSD test (Study 2) or by Dunnett’s test (Study 3) for multiple comparisons. The lowest individual and cumulative scores were noted in mice treated with DerG-PG70 LEAPS vaccine in both studies. Statistically significant differences are indicated as:
p < 0.05 (DerG-PG70 + adj. vs. PBS + adj. control).
p < 0.05 (DerG-PG70 + adj. vs. PBS control).
3. Results
3.1. DerG-PG70 LEAPS vaccine limits arthritis progression in vivo
3.1.1. Visual arthritis (VA) scores
The therapeutic activities of LEAPS vaccines containing Der-G-PG70 or J-PG70 peptide conjugates (emulsified with Seppic ISA-51VG adjuvant) were compared to controls containing vehicle (PBS with or without adjuvant), in the PGIA (Fig. 1A: Study 1) and GIA (Fig. 1B: Study 2, and Fig. 1C: Study 3) models of RA. In Study 3 (Fig. 1C), additional controls of peptides PG70 and DerG in adjuvant as well as PBS without adjuvant were tested. As indicated by the VA scores, treatment with the DerG-PG70 vaccine significantly (*p < 0.05) limited disease progression within 3 weeks in all three studies whereas J-PG70 or individual peptides had no significant effects as compared with the PBS/adjuvant or PBS control (Fig. 1). Importantly, the DerG-PG70 vaccine effectively curtailed arthritis symptoms in GIA (Fig. 1B and 1C) despite its more aggressive disease course compared to PGIA (Fig. 1A).
Fig. 1.

Visual arthritis (VA) scores of mice with PGIA or GIA treated with LEAPS conjugate vaccines or control agents. VA scores of mice in the (A) initial PGIA study (Study 1), (B) initial GIA study(Study 2) and (C) confirmatory GIA study (Study 3)with additional control groups (dotted lines). VA Scoring: sum of value for each paw: 0 = no evidence of inflammation, 1 = slight swelling of the paw or at least 3 finger joints, 2 = moderate swelling of the paw and fingers, 3 = moderate swelling of the paw, fingers, and ankle/wrist joints, 4 = severe swelling and redness of paw, fingers, and ankle/wrist joints, summing the individual paw scores to a maximum VA score of 16 per animal [34–38 ]. Groups of mice (n = 8–9 mice per group) with VA scores averaging ~3 in each group were injected s.c. on day 0 (black arrows with squares) and day 14 (black arrows with diamonds) with the following: (A) and (B) PBS emulsified with Seppic ISA-51VG adjuvant (solid green lines, vehicle control), J-PG70 in PBS + adjuvant (solid orange lines), or DerG-PG70 in PBS + adjuvant (solid blue lines). (C) Groups treated as shown in (A) and (B) and the three additional control groups were treated with PBS (no adjuvant) (dotted gray line), PG70in PBS + adjuvant (dotted brown line) or DerG in PBS + adjuvant (dotted blue line). Data are expressed as the mean ± SEM. Asterisks depict statistically significant (*p < 0.05) differences between the DerG-PG70-treated and PBS + adjuvant) control groups. Data were analyzed using (two-way repeated measures analysis of variance ANOVA with post hoc Fisher’s least significant differences (LSD) test). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1.2. Histopathological changes
Histopathological changes in the joints, induced by autoimmune inflammatory damage, or as modulated by vaccine treatment, were evaluated in comparison with normal joints of naïve mice (see Supplementary Figs. 1 and 2).3
Table 1 shows the individual and cumulative histopathology scores of the limbs of GIA mice (Study 2 and Study 3) after the animals underwent the in vivo treatments. In Study 2, mice treated with DerG-PG70 in adjuvant had significantly (*p < 0.05) reduced pannus formation and bone resorption as well as lower cumulative histopathology scores as compared to controls treated with PBS in adjuvant. In Study 3, DerG-PG70-treated animals demonstrated significantly reduced degree of inflammation, cartilage damage, and cumulative histopathology score, while J-PG70-treated mice generally had greater joint damage than PBS/adjuvant-treated controls. While the effect of DerG-PG70 on reduction of inflammation, pannus formation, cartilage damage, bone resorption, periosteal bone formation, or summed scores did not always reach the level of statistical significance in Studies 2 and 3, a protective effect was indicated by strong reductions in these parameters in both studies (Table 1). The in vivo VA scores (Fig. 1) and joint histopathology (Table 1 and Supplementary Figs. 1 and 2) indicate that DerG-PG70 treatment of PGIA and GIA mice had a consistent and reproducible therapeutic effect.
Fig. 2.
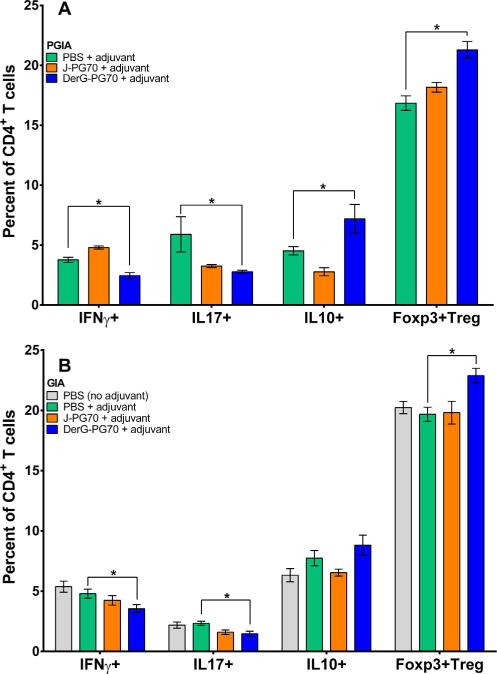
Effects of LEAPS vaccines on the phenotype of cultured CD4+ T-cell populations from the spleens of mice with (A) PGIA or (B) GIA. Percentages of Th1 (IFNγ+), Th17 (IL17+), Th2/Treg (IL10+), and CD25+ Tregs (Foxp3+) in PG-stimulated cultures of spleen cells from control or LEAPS-vaccine treated PGIA or GIA mice as determined by intracellular cytokine or Foxp3 staining and flow cytometry. For culture conditions see Materials and Methods. Data shown are the mean ± SEM of the percent of cytokine-expressing CD4+ T-cell populations in the cultures from the 3 groups of PGIA mice (spleen cells from n = 4 per group) and GIA mice (n = 8 per group). Data were analyzed in both (A) and (B) using one-way ANOVA with Fisher’s LSD test. Asterisks depict statistically significant differences (*p < 0.05).
3.2. T-cell phenotypes of splenocytes from vaccine treated PGIA and GIA mice
The phenotypes of CD4+ T-cells within the spleen cell cultures of the treatment groups of PGIA and GIA mice were determined by flow cytometric evaluation of intracellular cytokines IFNγ (Th1), IL17 (Th17), or IL10, or the Treg cell marker, Foxp3, following in vitro stimulation with antigen (PG or rhG1). Treatment of PGIA (Fig. 2A) or GIA (Fig. 2B) mice with the DerG-PG70 vaccine led to a reduction of pro-inflammatory Th1 and Th17 cells (even after antigen stimulation) and an increase in the frequency of anti-inflammatory IL10-producing as well as protective Treg cells in both models.
3.3. In vitro secretion of cytokines by splenocytes from vaccine-treated PGIA and GIA mice
Cytokine production from cell cultures prepared from the spleens of vaccinated mice were measured to evaluate ongoing or antigen-induced responses. Cytokine production by spleen cells receiving no additional in vitro antigen stimulation represent cellular responses resulting from the combination of the effects of disease and vaccine treatments on the mice, whereas cytokine production following in vitro antigen stimulation represents additional antigen-specific responses resulting from the combined effects of disease, vaccination, and in vitro antigen treatment. We specifically focused on the ratios of anti-inflammatory (IL4 or IL10) to pro-inflammatory (IFNγ or IL17) T-cell cytokines in the spleen cell cultures of PGIA and GIA mice (Fig. 3) as calculated from the concentrations of secreted cytokines (Supplementary Fig. 3).3 In the PGIA study, these ratios were significantly increased in favor of anti-inflammatory cytokine release in both the non-stimulated (Fig. 3A) and PG-stimulated (Fig. 3B) spleen cell cultures of DerG-PG70-treated mice as compared to PBS/adjuvant-treated controls. In the GIA study, the IL4:IL17(A+F) ratio was significantly increased in the in vitro non-stimulated (Fig. 3C), but not in the rhG1-stimulated cultures (Fig. 3C). These ratios reflect the reductions in pro-inflammatory cytokines (IFNγ and IL17(A+F)) and increases in anti-inflammatory cytokines (IL4, TGFβ, and IL10) due to DerG-PG70 vaccine treatment, as compared to control treatment in all the PGIA cultures, although the differences did not always reach significance (Supplementary Figs. 3A and 3B).3 In the cell cultures from GIA mice, DerG-PG70 vaccination resulted in significant reductions in the production of IL17(A + F) and IFNγ, but other cytokines were affected to a lesser degree (Supplementary Fig. 3C and 3D).3
Fig. 3.
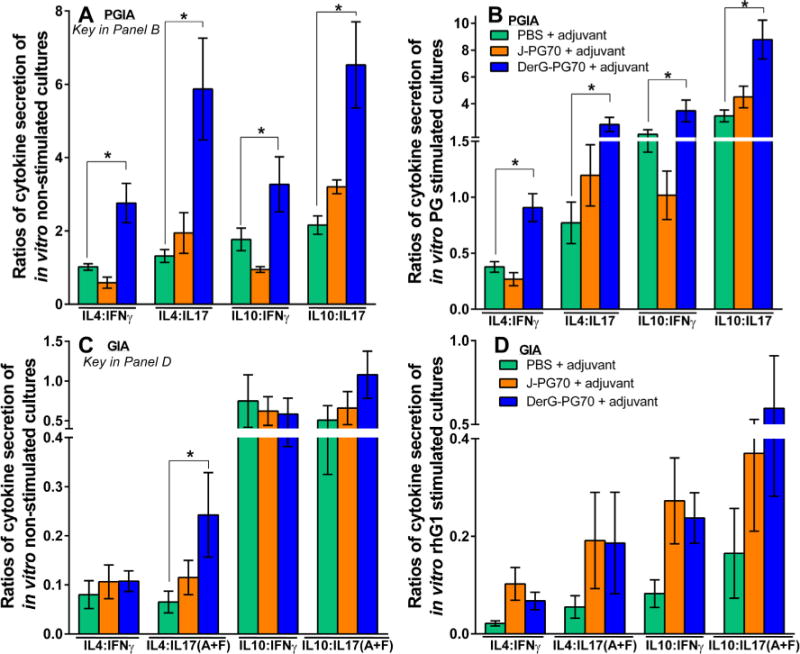
Ratios of key anti-inflammatory to pro-inflammatory T-cell cytokines in in vitro cultures of spleen cells from control and LEAPS vaccine-treated PGIA (A and B) and GIA (C and D) mice. Ratios of secreted anti-inflammatory (IL4 or IL10) to pro-inflammatory (IL17 or IFNγ) cytokines detected in the supernatants of spleen cells from mice with PGIA cultured in the absence of antigen (A) (in vitro non-stimulated cultures), or (B) in the presence of PG antigen (in vitro PG-stimulated cultures). The ratios of the same cytokines in the supernatants of spleen cells from GIA mice cultured in the absence of antigen (C) (in vitro non-stimulated cultures) or (D) in the presence of rhG1 antigen (in vitro rhG1-stimulated cultures) (see Supplementary Fig. 31 for cytokine concentrations). Data were analyzed with one-wayANOVA and pairwise comparisons between the control and LEAPS-treated groups were made using Fisher’s LSD test. Asterisks depict statistically significant differences (*p < 0.05).
Differences in the results between the PGIA and GIA spleen cell cytokine secretion studies may reflect differences in the detection methods (i.e., ELISA versus MagPix® with the latter being more sensitive than the former), but it is more likely that they reflect differences between the two animal models [22]. The relative inability of the DerG-PG70 vaccine to suppress IFNγ secretion by native or rhG1-stimulated spleen cells from GIA mice as opposed to a suppressive effect on IFNγ production by PGIA spleen cells might be due to the overproduction of IFNγ in the GIA model compared to the PGIA model, as demonstrated here and as reported previously [22]. Ultimately, the pro-inflammatory response was reduced by DerG-PG70 treatment in both models.
3.4. Serum levels of cytokines
Concentrations of 10 different cytokines (IL4, TNFa, IFNγ, IL2, IL1β, IL6, IL10, IL12p70, IL17A, and IL17F) in serum samples from LEAPS-vaccinated and control GIA mice (Studies 2 and 3) were evaluated as an additional indication of the action of DerG-PG70 on the immune and inflammatory processes. In both GIA studies, IL17F serum levels were significantly reduced in DerG-PG70-treated mice as compared to the PBS/adjuvant-treated controls, but the concentrations of other cytokines were comparable among the treatment groups (Supplementary Fig. 4). Ratios of anti-inflammatory T-cell cytokines (IL4 and IL10) to pro-inflammatory T-cell cytokines (IL17(A + F) and IFNγ) in serum from DerG-PG70 treated mice were significantly increased in favor of anti-inflammatory responses (Fig. 4A and B).
Fig. 4.
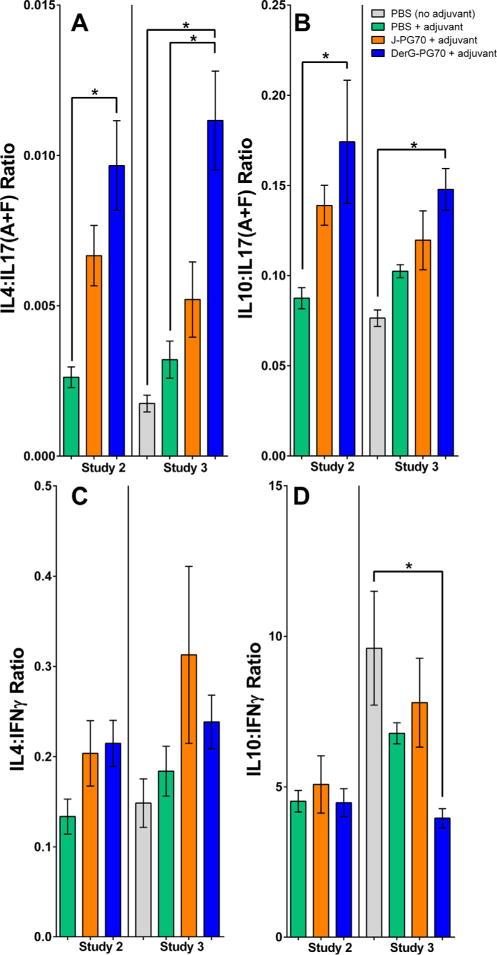
Ratios of key anti-inflammatory to pro-inflammatory cytokines in serum samples collected from control and LEAPS-treated GIA mice. Ratio of anti-inflammatory IL4 (A) or IL10 (B) to pro-inflammatory IL17(A + F) in the sera of GIA mice at the end of studies. Ratio of anti-inflammatory IL4 (C) or IL10 (D) to pro-inflammatory IFNγ in the sera of the same mice. Cytokine concentrations were determined using a multiplex Luminex MagPix® kit (serum concentrations of 10 different cytokines are shown in Supplementary Fig. 4).3 Results are expressed as the mean ± SEM of cytokine ratios (n = 8 mice in Study 2 and n = 9 mice in Study 3 per group). Data analysis was performed using one-way ANOVA followed by Fisher’s LSD test. Asterisks indicate statistically significant differences (*p < 0.05).
3.5. Binding of LEAPS conjugates and related individual peptides to immune cells in vitro
The binding of tetramers of DerG-PG70, J-PG70, and their component peptides to spleen cells from GIA mice and BM-derived DCs from normal mice was examined in vitro to identify the cell types that can be affected by treatment. SA-PE-B-Peptide tetramers were prepared to increase the avidity and add fluorescence to the peptide ligands to facilitate analysis of their binding to cells. Tetramers were formed with SA-PE using a method developed for detecting the binding of MHC-peptide complexes to T-cells by flow cytometry [30]. However, as the LEAPS heteroconjugate peptides contained a cell binding ligand (either DerG or J), addition of MHC was not necessary. Although the DerG-PG70 tetramer bound to CD4+ T-cells (Fig. 5A, top) better than the other ligands, binding specificity to the other cell types was relatively difficult to discern. Specifically, the binding patterns to DCs were obscured by the high background fluorescence (e.g., SA-PE alone), possibly due to phagocytic activity of DCs [31] (Fig. 5A, bottom). Therefore, the net mean fluorescence intensity levels (above the SA-PE background) were calculated as a measure of specific peptide binding to cells (Fig. 5B). By comparing the binding of DerG-PG70 and J-PG70, two similar-sized peptides differing in the ICBL, the binding preference of the DerG-PG70 tetramer for CD4+ T-cells was evident. Similarly, the binding preference of J-PG70 for MHC II-bearing cells (APCs) and CD11c+ BM-DCs became evident. Tetramers containing the individual peptides (PG70, DerG or J) bound very weakly to all of the cell types examined (Fig. 5B).
Fig. 5.
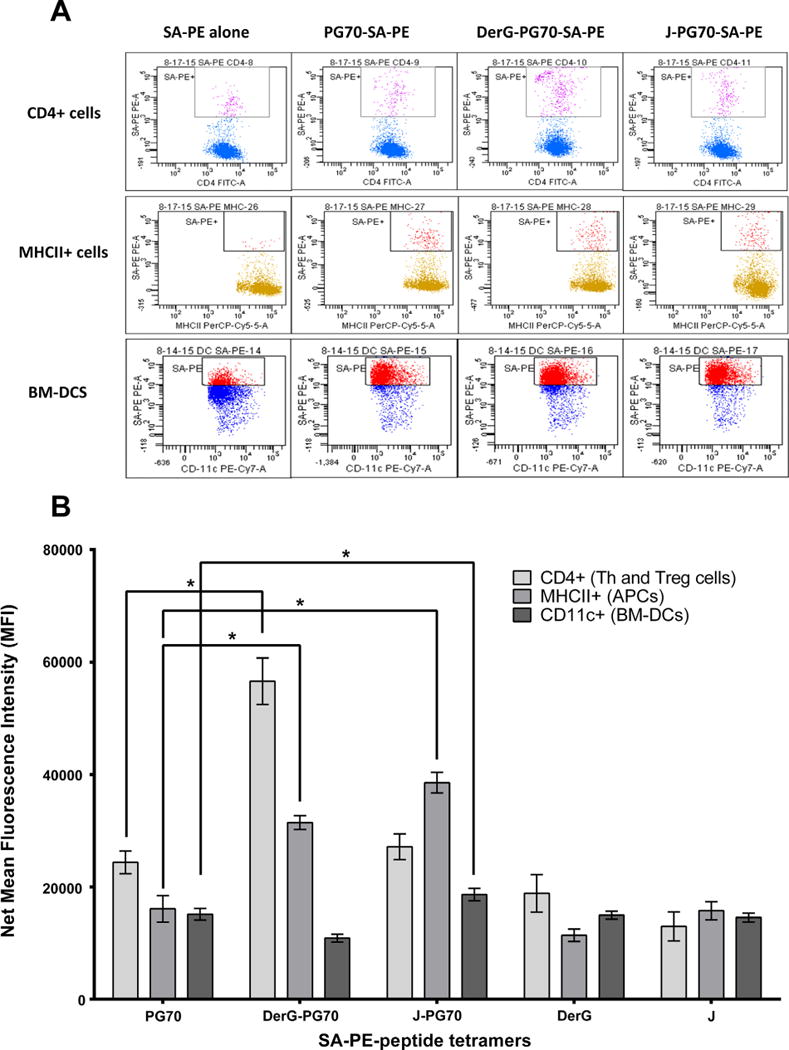
Binding of LEAPS conjugates or individual peptides to cells in vitro as determined by flow cytometry. (A) Representative flow cytometry panels of streptavidin-phycoerythrin (SA-PE)-biotinylated (B)-peptide tetramer binding to splenic CD4+ T-cells or APCs from GIA mice or to BM-DCs from naïve mice. Cells binding (SA-PE)-B-peptide tetramer are shown as dots in the box within each of the flow panel frames. (B) Summary of (SA-PE)-B-peptide tetramer binding flow cytometry data using GIA spleen cells (n = 8 mice) or BM-DCs from naïve mice (n = 6). Results are expressed as the means ± SEM of net mean fluorescence intensity (net MFI: total MFI minus SA-PE alone background MFI). For statistical analysis, data were transformed (Y = Y2) and then analyzed using one-way ANOVA followed by Dunnett’s test for multiple comparisons. Asterisks indicate statistically significant differences (*p < 0.05).
4. Discussion
Since Th1-dominated diseases might benefit from a shift away from the pro-inflammatory Th1 to a more balanced Th2 or Treg response, our hypothesis was that treatment with a Th2-promoting DerG LEAPS heteroconjugate incorporating PG70 would initiate an antigen specific response to ameliorate the arthritis symptoms, while a Th1-promoting J-LEAPS heteroconjugate with PG70 would either have no effect or exacerbate disease. Indeed, the DerG-PG70 vaccine reproducibly curtailed the progression of disease in PGIA and two independent GIA studies as indicated by the VA scores of in vivo monitored animals and joint histopathology. As expected, treatment with the J-PG70 vaccine was either ineffective or slightly exacerbated disease in these models. Although the outcomes in the two disease systems were similar, there were differences in the kinetics of both disease development and vaccine effects between PGIA and GIA.
As in RA, the inflammatory disease in both the PGIA and GIA models is driven and sustained primarily by Th1 but also by Th17 cells and cytokines produced by them. DerG-PG70 treatment promoted more balanced, less inflammatory cytokine responses by increasing Th2 (IL4+ and IL10+) and Treg (Foxp3+ and TGFβ1+) cells and reducing Th1 and Th17 cells in PGIA, and to a somewhat lesser extent in the GIA system, as represented by the T-cell phenotypes from the treated animals. The switch from a pro-inflammatory T-cell population to one favoring regulation and modulation by DerG-PG70 treatment in the spleen is likely to reflect the systemic changes seen in the curtailed progression of disease.
Although showing similar trends, the immune responses driving disease and subsequently, the responses to DerG-PG70 and J-PG70 for the PGIA and GIA models were subtly different. There is more robust production of IFNγ in the GIA model compared to the PGIA model, as seen herein and an earlier study [22]. In both models, the therapeutic effect of DerG-PG70 treatment was reflected in the reduction in IL17 production and the proportion of Th17 cells, critical players in RA pathogenesis. The reduction in these pro-inflammatory responses was likely due to increases in Th2 and/or Treg activity.
The ICBL component of a LEAPS vaccine attached to the disease related antigenic peptide promotes interaction with immune cells and hence directs the subsequent immune response. Using the SA-PE-peptide tetramers it was possible to demonstrate that the DerG-PG70 conjugate preferentially bound to splenic CD4+ Th (or Treg) cells, whereas J-PG70 showed preference for binding to MHC II-bearing APCs and CD11c+ DCs, known to be involved in PGIA [32]. Preference for CD4+ T-cells is in keeping with previous studies for the DerG ICBL peptide [10,13–16,33,34], and preference for APCs for the J ICBL, is consistent with its ability to promote the maturation of DCs [11,12,20]. The binding studies suggest that the DerG-PG70 conjugate modulates T-cell responses via direct binding to CD4+ T-cells in the PGIA and GIA mice.
The ability of DerG-PG70 to steer T-cells away from pro-inflammatory responses and promote anti-inflammatory/regulatory activities explains the therapeutic success of DerG-PG70 in restricting the progression of disease in the treated PGIA and GIA mice. Unlike cytokine ablation [23,35], which also suppresses PGIA, DerG-PG70 appears to act directly on the T-cell drivers of the immune response rather than the subsequently elicited arthritis related cytokines to ameliorate disease progression. Focusing the therapy on the inflammatory disease-promoting immune responses in an antigen-specific manner with LEAPS vaccines appears to bring the system to an appropriate balance, presumably without loss of important immune protections.
RA is different for different patients and failure to respond to certain therapies may reflect differences in the initiators and responses driving their disease. In this study, the DerG-PG70 vaccine was effective in curtailing progression of arthritis driven by Th1 responses whereas a collagen peptide-containing J-LEAPS vaccine, CEL-2000, was effective in blocking the progression of arthritis in the Th17-driven CIA model [17]. As such, knowledge of signature T-cell cytokine phenotypes that drive the patient’s disease can facilitate the choice of the appropriate LEAPS vaccine therapy.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) of USA. Research reported in this publication was supported by the NIAMS under award numbers R43AR063504 for DH Zimmerman, R01AR064206 for K Mikecz, and R01AR062991 for TT Glant as well as by CEL-SCI Corporation.
The authors would also like to acknowledge the expert contributions by Alison Bendele of Bolder BioPATH, Boulder, CO, and by Alison Finnegan and Yanxia Cao of Rush University Medical Center, Chicago, IL.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Rush University Medical Center, CEL-SCI Corporation or Roseman University of Health Sciences College of Medicine.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.05.009.
Footnotes
For related information see Supplementary Information.
5. Conflicts of interest
Authors A.M., J.K., declare no conflict of interest.
The following authors declare conflict of interest as specified: K.M. and T.T.G. are inventors on patent applications related to this study.
K.S.R. is inventor on some LEAPS-related patents or patent applications.
R.E.C., S.M.C. and H.L.S. are inventors on patent applications related to this study, and they are employees and stockholders or option holders of CEL-SCI Corporation.
D.H.Z. is a discoverer of LEAPS technology, inventor on LEAPS patents and patent applications related to this study, and he is also a Company officer, employee, stockholder and option holder of CEL-SCI Corporation.
6. Authors’ contributions
Conceived and designed the experiments: D.H.Z., K.M., T.T.G. Performed the experiments: K.M., A.M., J.K., H.L.S., R.E.C., T.T.G. Analyzed the data: K.M., S.M.C., R.E.C., H.L.S., A.M., J.K., D.H.Z., T.T.G., K.S.R. Contributed reagents/materials/analysis tools: D.H.Z., T.T.G., K.M. Wrote the paper: D.H.Z., K.S.R., K.M., T.T.G.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. http://dx.doi.org/10.7748/phc2011.11.21.9.29.c8797. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, Jacobsson L, Bergström U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatol (Oxford) 1999;38:668–74. doi: 10.1093/rheumatology/38.7.668. [DOI] [PubMed] [Google Scholar]
- 3.Moreland L. Unmet needs in rheumatoid arthritis. Arthritis Res Ther. 2005;7(Suppl 3):S2–8. doi: 10.1186/ar1736. http://dx.doi.org/10.1186/ar1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1316–22. doi: 10.1136/annrheumdis-2013-204627. http://dx.doi.org/10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 5.Sacks JJ, Luo Y-H, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res (Hoboken) 2010;62:460–4. doi: 10.1002/acr.20041. http://dx.doi.org/10.1002/acr.20041. [DOI] [PubMed] [Google Scholar]
- 6.Kugyelka R, Kohl Z, Olasz K, Mikecz K, Rauch TA, Glant TT, et al. Enigma of IL-17 and Th17 Cells in Rheumatoid Arthritis and in Autoimmune Animal Models of Arthritis. Mediators Inflamm. 2016;2016:1–11. doi: 10.1155/2016/6145810. http://dx.doi.org/10.1155/2016/6145810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colmegna I, Ohata BR, Menard HA. Current understanding of rheumatoid arthritis therapy. Clin Pharmacol Ther. 2012;91:607–20. doi: 10.1038/clpt.2011.325. http://dx.doi.org/10.1038/clpt.2011.325. [DOI] [PubMed] [Google Scholar]
- 8.Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewé R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis. 2014;73:3–5. doi: 10.1136/annrheumdis-2013-204317. http://dx.doi.org/10.1136/annrheumdis-2013-204317. [DOI] [PubMed] [Google Scholar]
- 9.Chang MR, Lyda B, Kamenecka TM, Griffin PR. Pharmacologic repression of retinoic acid receptor-related orphan nuclear receptor γ is therapeutic in the collagen-induced arthritis experimental model. Arthritis Rheumatol. 2014;66:579–88. doi: 10.1002/art.38272. http://dx.doi.org/10.1002/art.38272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal KS, Mikecz K, Steiner HL, Glant TT, Finnegan A, Carambula RE, et al. Rheumatoid arthritis vaccine therapies: perspectives and lessons from therapeutic ligand epitope antigen presentation system vaccines for models of rheumatoid arthritis. Expert Rev Vaccines. 2015:1–18. doi: 10.1586/14760584.2015.1026330. http://dx.doi.org/10.1586/14760584.2015.1026330. [DOI] [PMC free article] [PubMed]
- 11.Taylor PR, Koski GK, Paustian CC, Bailey E, Cohen Pa, Moore FB-G, et al. J-LEAPS vaccines initiate murine Th1 responses by activating dendritic cells. Vaccine. 2010;28:5533–42. doi: 10.1016/j.vaccine.2010.06.043. http://dx.doi.org/10.1016/j.vaccine.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PR, Paustian CC, Koski GK, Zimmerman DH, Rosenthal KS. Maturation of dendritic cell precursors into IL12-producing DCs by J-LEAPS immunogens. Cell Immunol. 2010;262:1–5. doi: 10.1016/j.cellimm.2010.01.003. http://dx.doi.org/10.1016/j.cellimm.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthal KS, Taylor P, Zimmerman DH. J-LEAPS peptide and LEAPS dendritic cell vaccines. Microb Biotechnol. 2012;5:203–13. doi: 10.1111/j.1751-7915.2011.00278.x. http://dx.doi.org/10.1111/j.1751-7915.2011.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.König R, Huang LY, Germain RNN. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature. 1992;356:796–8. doi: 10.1038/356796a0. http://dx.doi.org/10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- 15.König R, Shen X, Germain RN. Involvement of both major histocompatibility complex class II alpha and beta chains in CD4 function indicates a role for ordered oligomerization in T cell activation. J Exp Med. 1995;182:779–87. doi: 10.1084/jem.182.3.779. http://dx.doi.org/10.1084/jem.182.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen X, Hu B, McPhie P, Wu X, Fox A, Germain RN, et al. Peptides corresponding to CD4-interacting regions of murine MHC class II molecules modulate immune responses of CD4+ T lymphocytes in vitro and in vivo. J Immunol. 1996;157:87–100. [PubMed] [Google Scholar]
- 17.Zimmerman DH, Taylor P, Bendele A, Carambula R, Duzant Y, Lowe V, et al. CEL-2000: A therapeutic vaccine for rheumatoid arthritis arrests disease development and alters serum cytokine/chemokine patterns in the bovine collagen type II induced arthritis in the DBA mouse model. Int Immunopharmacol. 2010;10:412–21. doi: 10.1016/j.intimp.2009.12.016. http://dx.doi.org/10.1016/j.intimp.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Cihakova D, Barin JG, Baldeviano GC, Kimura M, Talor MV, Zimmerman DH, et al. L.E.A.P.S heteroconjugate is able to prevent and treat experimental autoimmune myocarditis by altering trafficking of autoaggressive cells to the heart. Int Immunopharmacol. 2008;8:624–33. doi: 10.1016/j.intimp.2008.01.004. http://dx.doi.org/10.1016/j.intimp.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal KS, Mao H, Horne WI, Wright C, Zimmerman D. Immunization with a LEAPS heteroconjugate containing a CTL epitope and a peptide from beta-2-microglobulin elicits a protective and DTH response to herpes simplex virus type 1. Vaccine. 1999;17:535–42. doi: 10.1016/s0264-410x(98)00231-x. [DOI] [PubMed] [Google Scholar]
- 20.Boonnak K, Vogel L, Orandle M, Zimmerman D, Talor E, Subbarao K. Antigen-activated dendritic cells ameliorate influenza A infections. J Clin Invest. 2013;123:2850–61. doi: 10.1172/JCI67550. http://dx.doi.org/10.1172/JCI67550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–12. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 22.Glant TT, Radacs M, Nagyeri G, Olasz K, Laszlo A, Boldizsar F, et al. Proteoglycan-induced arthritis and recombinant human proteoglycan aggrecan G1 domain-induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis Rheum. 2011;63:1312–21. doi: 10.1002/art.30261. http://dx.doi.org/10.1002/art.30261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doodes PD, Cao Y, Hamel KM, Wang Y, Farkas B, Iwakura Y, et al. Development of proteoglycan-induced arthritis is independent of IL-17. J Immunol. 2008;181:329–37. doi: 10.4049/jimmunol.181.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–90. [PubMed] [Google Scholar]
- 25.Hanyecz A, Berlo SE, Szántó S, Broeren CPM, Mikecz K, Glant TT. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis Rheum. 2004;50:1665–76. doi: 10.1002/art.20180. http://dx.doi.org/10.1002/art.20180. [DOI] [PubMed] [Google Scholar]
- 26.Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987;30:306–18. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- 27.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms, and genetics. Crit Rev Immunol. 2003;23:199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 28.Kurkó J, Vida A, Ocskó T, Tryniszewska B, Rauch TA, Glant TT, et al. Suppression of proteoglycan-induced autoimmune arthritis by myeloid-derived suppressor cells generated in vitro from murine bone marrow. PLoS One. 2014;9:e111815. doi: 10.1371/journal.pone.0111815. http://dx.doi.org/10.1371/joumal.pone.0111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egelston C, Kurkó J, Besenyei T, Tryniszewska B, Rauch TA, Glant TT, et al. Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 2012;64:3179–88. doi: 10.1002/art.34494. http://dx.doi.org/10.1002/art.34494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobezda T, Ghassemi-Nejad S, Glant TT, Mikecz K. In vivo two-photon imaging of T cell motility in joint-draining lymph nodes in a mouse model of rheumatoid arthritis. Cell Immunol. 2012;278:158–65. doi: 10.1016/j.cellimm.2012.08.003. http://dx.doi.org/10.1016/j.cellimm.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Roche PA. Macropinocytosis in phagocytes: regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front Physiol. 2015;6 doi: 10.3389/fphys.2015.00001. http://dx.doi.org/10.3389/fphys.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174:3781–8. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 33.Cammarota G, Scheirle A, Takacs B, Doran DM, Knorr R, Bannwarth W, et al. Identification of a CD4 binding site on the beta 2 domain of HLA-DR molecules. Nature. 1992;356:799–801. doi: 10.1038/356799a0. http://dx.doi.org/10.1038/356799a0. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman DH, Steiner H, Carmabula R, Talor E, Rosenthal KS. LEAPS therapeutic vaccines as antigen specific suppressors of inflammation in infectious and autoimmune diseases. J Vaccines Vaccin. 2012;3:1000149. doi: 10.4172/2157-7560.1000149. http://dx.doi.org/10.4172/2157-7560.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doodes PD, Cao Y, Hamel KM, Wang Y, Rodeghero RL, Mikecz K, et al. IFN-gamma regulates the requirement for IL-17 in proteoglycan-induced arthritis. J Immunol. 2010;184:1552–9. doi: 10.4049/jimmunol.0902907. http://dx.doi.org/10.4049/iimmunol.0902907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markovics A, Ocskó T, Katz RS, Buzás EI, Glant TT, Mikecz K. Immune Recognition of Citrullinated Proteoglycan Aggrecan Epitopes in Mice with Proteoglycan-Induced Arthritis and in Patients with Rheumatoid Arthritis. PLoS One. 2016;11:e0160284. doi: 10.1371/journal.pone.0160284. http://dx.doi.org/10.1371/joumal.pone.0160284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman DH, Morris S, Rouse D, Worthington K, Elliott D, Rosenthal K. Immunization with peptide heterconjugates primes a t helper cell type 1-associated antibody (IgG2a) response that recognizes the native epitope on the 38-kDA protein of Mycobacterium tuberculosis. Vaccine Res. 1996;5:103–18. [Google Scholar]
- 38.Goel N, Rong Q, Zimmerman D, Rosenthal KS. A, L.E.A.P.S heteroconjugate vaccine containing a T cell epitope from HSV-1 glycoprotein D elicits Th1 responses and protection. Vaccine. 2003;21:4410–20. doi: 10.1016/s0264-410x(03)00429-8. http://dx.doi.org/10.1016/S0264-410X(03)00429-8. [DOI] [PubMed] [Google Scholar]
- 39.Buzás EI, Végvári A, Murad YM, Finnegan A, Mikecz K, Glant TT. T-cell recognition of differentially tolerated epitopes of cartilage proteoglycan aggrecan in arthritis. Cell Immunol. 2005;235:98–108. doi: 10.1016/j.cellimm.2004.08.006. http://dx.doi.org/10.1016/j.cellimm.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Glant TT, Buzás EI, Finnegan A, Negroiu G, Cs-Szabó G, Mikecz K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 1998;160:3812–9. [PubMed] [Google Scholar]
- 41.Szántó S, Bárdos T, Szabó Z, David CS, Buzás EI, Mikecz K, et al. Induction of arthritis in HLA-DR4-humanized and HLA-DQ8-humanized mice by human cartilage proteoglycan aggrecan but only in the presence of an appropriate (non-MHC) genetic background. Arthritis Rheum. 2004;50:1984–95. doi: 10.1002/art.20285. http://dx.doi.org/10.1002/art.20285. [DOI] [PubMed] [Google Scholar]
- 42.Guerassimov A, Zhang Y, Banerjee S, Cartman A, Leroux JY, Rosenberg LC, et al. Cellular immunity to the G1 domain of cartilage proteoglycan aggrecan is enhanced in patients with rheumatoid arthritis but only after removal of keratan sulfate. Arthritis Rheum. 1998;41:1019–25. doi: 10.1002/1529-0131(199806)41:6<1019::AID-ART8>3.0.CO;2-X. http://dx.doi.org/10.1002/1529-0131(199806)41:6<1019::AID-ART8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 43.de Jong H, Berlo SE, Hombrink P, Otten HG, van Eden W, Lafeber FP, et al. Cartilage proteoglycan aggrecan epitopes induce proinflammatory autoreactive T-cell responses in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2010;69:255–62. doi: 10.1136/ard.2008.103978. http://dx.doi.org/10.1136/ard.2008.103978. [DOI] [PubMed] [Google Scholar]
- 44.von Delwig A, Locke J, Robinson JH, Ng W-F. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:143–9. doi: 10.1002/art.25064. http://dx.doi.org/10.1002/art.25064. [DOI] [PubMed] [Google Scholar]
- 45.Law SC, Street S, Yu C-HA, Capini C, Ramnoruth S, Nel HJ, et al. T cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. http://dx.doi.org/10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutterotti A, Yousef S, Sputtek A, Stürner KH, Stellmann J-P, Breiden P, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5:188ra75. doi: 10.1126/scitranslmed.3006168. http://dx.doi.org/10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marek-Trzonkowska N, Mysliwiec M, Iwaszkiewicz-Grzes D, Gliwmski M, Derkowska I, Zalmska M, et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med. 2016;14:332. doi: 10.1186/s12967-016-1090-7. http://dx.doi.org/10.1186/s12967-016-1090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer DC, Balasubramaniam S, Hanada K, Wrzesinski C, Yu Z, Farid S, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–16. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatum AM, Mylin LM, Bender SJ, Fischer Ma, Vigliotti Ba, Tevethia MJ, et al. CD8+ T cells targeting a single immunodominant epitope are sufficient for elimination of established SV40 T antigen-induced brain tumors. J Immunol. 2008;181:4406–17. doi: 10.4049/jimmunol.181.6.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


