Abstract
Objectives
The purpose of this study was to evaluate the polymerization behavior of a model dentin adhesive with tris(trimethylsilyl)silane (TTMSS) as a co-initiator, and to investigate the polymerization kinetics and mechanical properties of copolymers in dry and wet conditions.
Methods
A co-monomer mixture based on HEMA/BisGMA (45/55, w/w) was used as a model dentin adhesive. The photoinitiator system included camphorquinone (CQ) as the photosensitizer and the co-initiator was ethyl-4-(dimethylamino) benzoate (EDMAB) or TTMSS. Iodonium salt, diphenyliodonium hexafluorophosphate (DPIHP) serving as a catalyst, was selectively added into the adhesive formulations. The control and the experimental formulations were characterized with regard to the degree of conversion (DC) and dynamic mechanical properties under dry and wet conditions.
Results
In two-component photoinitiator system (CQ/TTMSS), with an increase of TTMSS concentration, the polymerization rate and DC of C═C double bond increased, and showed a dependence on the irradiation time and curing light intensity. The copolymers that contained the three-component photoinitiator system (CQ/TTMSS/DPIHP) showed similar dynamic mechanical properties, under both dry and wet conditions, to the EDMAB-containing system.
Significance
The DC of formulations using TTMSS as co-initiator showed a strong dependence on irradiation time. With the addition of TTMSS, the maximum polymerization rate can be adjusted and the network structure became more homogenous. The results indicated that the TTMSS could be used as a substitute for amine-type co-initiator in visible-light induced free radical polymerization of methacrylate-based dentin adhesives.
Keywords: Dental adhesive, Silane, Co-initiator, Fourier transform infrared, Dynamic mechanical analysis, Polymerization kinetic
1. Introduction
Camphorquinone (CQ)/amine initiation system is the most widely employed system for visible-light curing of methacrylate-based dental restorative materials. CQ itself can photoinitiate polymerization, but at a low reaction rate. Aliphatic or aromatic amines, such as N,N-dimethylptoluidine, 2-ethyl-dimethylbenzoate, N-phenylglycine, ethyl-4-dimethylaminobenzoate (EDMAB), 2-(dimethylamino) ethyl methacrylate (DMAEMA), and many other amine-containing compounds, are widely used as co-initiators for CQ [1–9]. Among these compounds, EDMAB is a very popular co-initiator in dental restorative materials due to its low basicity and high efficiency.
The limitations of EDMAB include sensitivity to oxygen inhibition [10], unstable under acidic conditions [11] and in acidic dental resin formulations [12–14], and leached EDMAB is potentially cytotoxic [15]. Usually, an acid-base reaction occurs between electron donors and electron acceptors, which may result in a charge transfer complex (CTC). This charge transfer complex may interfere with polymerization. For example, the polymerizable co-initiator DMAEMA was ineffective for acidic monomers because of its strong basicity [6]. The search for new co-initiators remains an important issue in the development of durable dentin adhesives and composites.
Silyl radicals have widespread use in hydrosilylation and reduction reactions in organic chemistry. Tris(trimethylsilyl)silane (TTMSS) was synthesized by Gilman and co-workers in 1965 [16]. Nearly 20 years later, the Chat-gilialoglu laboratory discovered that TTMSS could serve as a radical-based agent [17,18]. Recently, the TTMSS radical has been characterized for applications in photoinitiation systems by Lalevee et al. [19–25]. Lalavee and colleagues reported that TTMSS had the following attributes: (1) a high inherent reactivity for the addition to double bonds, and (2) a low ionization potential which is associated with an oxidation process and the formation of silylium cations. Newly developed photoinitiator (PI) systems based on TTMSS exhibited a high reactivity both in free radical polymerization (FRP) [26] and free radical promoted cationic polymerization (FRPCP) [27]. The ability to efficiently consume oxygen is a particularly interesting feature of the PI systems based on TTMSS. Potentially the TTMSS-based PI systems can overcome the classical and well known oxygen inhibition of the FRP or FRPCR processes [22]. In addition, TTMSS did not exhibit a toxic response when tested in several biological test systems [28–30].
The efficacy of the PI systems depends on the H-atom donor ability of co-initiators and the compatibility of initiator components with resin. The hydrophobicity of CQ and EDMAB has limited their performance in the wet, oral environment. To address this limitation, a third component, iodonium salt, diphenyliodonium hexafluorophosphate (DPIHP), has been adopted into the two-component PI system [31,32]. DPIHP, with higher solubility in water, acts as an electron acceptor which offers dual roles, i.e., to regenerate the photosensitizer (CQ) and to generate additional active phenyl radicals [31]. Due to this unique ability, the iodonium salt is expected to promote the free radical polymerization within the CQ/TTMSS PI system.
It is well known that the structure and properties of polymeric materials are governed in part by the kinetics of the polymerization reaction. Polymerization kinetics determines the microgel structures, degree of conversion (DC), and many other characteristics [33–38]. Silanes in the presence of a PI such as benzophenone (BP), isopropylthioxanthone (ITX), or camphorquinone (CQ) are highly reactive and even better than a reference amine co-initiator such as EDMAB [22,24]. In spite of these advantages, there are no reports on the use of TTMSS as a co-initiator in methacrylate-based dental polymers.
In this work, HEMA/BisGMA (45/55, w/w) was used as a model resin. The polymerization behavior of this model resin when EDMAB or TTMSS was used as a co-initiator for photosensitizer CQ was studied in detail. The objective of this work was to evaluate the efficiency of TTMSS as co-initiator in neat methacrylate-based resin. The overall research hypotheses of this study were: (1) the polymerization behavior of neat resin formulated with TTMSS was comparable to neat resin formulated with EDMAB in the two-component PI system, (2) because of its ability to enhance radical efficacy, the addition of DPIHP will significantly increase the final degree of conversion and maximum polymerization rate, (3) the relative crosslink densities of the polymethacrylate network formulated using TTMSS was similar to EDMAB, and (4) the rate of polymerization with TTMSS will affect the mechanical properties of the neat methacrylate resin under dry and wet conditions.
2. Materials & methods
2.1. Materials
2,2-Bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]propane (BisGMA, St. Louis, MO) and 2-hydroxyethyl methacrylate (HEMA, St. Louis, MO) were used as received without further purification as monomers in dentin adhesives. Camphoroquinone (CQ), ethyl-4-(dimethylamino) benzoate (EDMAB), tris(trimethylsilyl)silane (TTMSS) and diphenyliodonium hexafluorophosphate (DPIHP) were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were reagent grade and used without further purification.
2.2. Preparation of adhesive formulations
The monomer mixtures were made with 45 wt% HEMA and 55 wt% BisGMA. The formulation containing CQ (0.5 wt%), EDMAB (0.5 wt%) and DPIHP (0.5 wt%) were used as controls [32,39]. The experimental formulations consisting of EDMAB, TTMSS and DPIHP are listed in Table 1. The mixtures of monomers/PIs are prepared in brown glass vials under amber light. The preparation of adhesive formulations and their polymer beams have been reported previously [40,41]. In brief, the solutions containing the monomers/PIs were mixed overnight at 23 ± 2 °C to promote complete dissolution and formation of a homogeneous solution. The prepared resins were injected into a glass-tubing mold (Wilmad, P 1m-1.2m-0-914m) and light-cured for 40 s at 23 ± 2 °C with a LED light curing unit (LED Curebox, 100 mW/cm2 irradiance, Proto-tech, Portland, OR). The polymerized samples were stored in the dark at 23 ± 2 °C for at least 48 h prior to testing. The resultant round beam specimens (L × D = 15 mm × 1.0 mm) were used to determine dynamic mechanical properties.
Table 1.
Formulations of the control and experimental adhesive speciments with different PI.
| Type | Runa | CQ | EDMAB | TTMSS | DPIHP |
|---|---|---|---|---|---|
| Two-component PI | CE-0.5b | 0.5 | 0.5 | / | / |
| CE-1.0 | 0.5 | 1.0 | / | / | |
| CT-0.5 | 0.5 | / | 0.5 | / | |
| CT-1.0 | 0.5 | / | 1.0 | / | |
| CT-3.0 | 0.5 | / | 3.0 | / | |
| Three-component PI | CEDc | 0.5 | 0.5 | / | 0.5 |
| CTD-0.5 | 0.5 | / | 0.5 | 0.5 | |
| CTD-1.0 | 0.5 | / | 1.0 | 0.5 | |
| CTD-3.0 | 0.5 | / | 3.0 | 0.5 | |
| Binary co-initiator | CET-1.0 | 0.5 | 0.5 | 1.0 | / |
| CET-3.0 | 0.5 | 0.5 | 3.0 | / | |
| CET-0.25 | 0.5 | 0.25 | 0.25 | / |
The resin was mixed HEMA/BisGMA in the ratio of 45/55 (w/w).
The formulation was used as the control in two-component or combined co-initiator PI systems.
The formulation was used as the control in three-component PI system.
2.3. Real-time double bond conversion and maximal polymerization rate
The DC and polymerization behavior were determined by FTIR as described by our group [39,42]. Real-time in-situ monitoring of the photopolymerization behavior of the different adhesive formulations was performed using an infrared spectrometer (Spectrum 400 Fourier transform infrared spectrophotometer, Perkin-Elmer, Waltham, MA) at a resolution of 4 cm−1. One drop of adhesive solution was placed on the zinc selenide (ZnSe) crystal top plate of an attenuated total reflectance (ATR) accessory (PIKE Technologies Gladi-ATR, Madison, WI) and covered with a mylar film to prevent oxygen inhibition of polymerization. A 40 or 120-s exposure to the commercial visible-light-polymerization unit (Spectrum® 800, Dentsply, Milford, DE) at an intensity of 550 mW/cm2 was initiated after 50 infrared spectra had been recorded. Real-time IR spectra were continuously recorded for 600 s after light activation began. A time-based spectrum collector (Spectrum TimeBase, Perkin-Elmer) was used for continuous and automatic collection of spectra during polymerization. A minimum of three replicates were obtained for each adhesive formulation. The change of the band ratio profile-1637 cm−1 (C=C)/1608 cm−1 (phenyl) was monitored, and DC was calculated using the following equation based on the decrease in the absorption intensity band ratio before and after light curing.
| (1) |
The average of the last 50 values of the time-based spectra is reported as the DC value. The maximum polymerization rate (Rp(max)/[M]) was determined using the maximum slope of the linear region of the DC-time plots [32].
To determine the DC as a function of irradiation intensity, samples were irradiated with the commercial visible-light-polymerization unit (Spectrum® 800, Dentsply, Milford, DE) at intensities of 550, 300, or 50 mW/cm2. Irradiation intensity was measured at the sample surface with a visible light curing meter (Cure Rite, Model 644726, Dentsply, Milford, DE).
2.4. Dynamic mechanical analysis (DMA)
Dynamic mechanical analysis (DMA) is a thermal analysis technique that measures the properties of materials as they are deformed under periodic stress. This technique is particularly well suited for characterizing viscoelastic materials. Since the technique measures both elastic and viscous responses, it is considered a valuable tool for obtaining information regarding the crosslink density and structural heterogeneity of polymer networks [43,44].
In this study, DMA tests were performed using a TA instruments Q800 DMA (TA Instruments, New Castle, USA) with a three-point bending clamp. The dynamic mechanical properties of methacrylate-based dentin adhesives have been described by our group [41,42,45]. A sinusoidal stress is applied and the resultant strain is measured. The properties measured under this oscillating loading are storage modulus, loss modulus, and tan δ. The storage modulus (E′) represents the stiffness of a viscoelastic material and is proportional to the energy stored during a loading cycle. The loss modulus (E″) is related to the amount of energy lost due to viscous flow. The ratio of loss (E″”) to storage modulus (E′) is referred to as the mechanical damping, or tan δ. The frequency used to measure the storage modulus is 1 Hz with an amplitude of 15 μm and a preload of 0.01 N [41,45]. In the dry condition, the storage modulus is measured from 10 to 200 °C with a ramping rate of 3 °C/min. The glass transition temperature (Tg) is determined as the position of the maximum on the derivate storage modulus vs. temperature plots. Round beam specimens (1.0 mm × 15 mm) prepared as described previously are used for DMA measurements. A minimum of three specimens of each material are measured.
The inverse ratio (ζ) of the modulus in the rubbery region to the temperature was used to represent the relative crosslink density [46,47]. The full-width-at-half-maximum (FWHM) of the tan δ curves was used to represent the heterogeneity of the network.
Wet-condition DMA tests are operated using the three-point submersion clamp [46,48]. Round beam specimens (1.0 mm × 15 mm) are immersed in water at 23 °C for at least 5 days to be fully hydrated. The test temperature is varied from 10 to 80 °C with a ramping rate of 1.5 °C min−1.
2.5. Statistical analysis
The results were analyzed statistically using one-way/two-way analysis of variance (ANOVA), together with Tukey’s test at α = 0.05 (Microcal Origin Version 8.0, Microcal Software Inc., Northampton, MA) to identify significant differences in the means or interaction.
3. Results
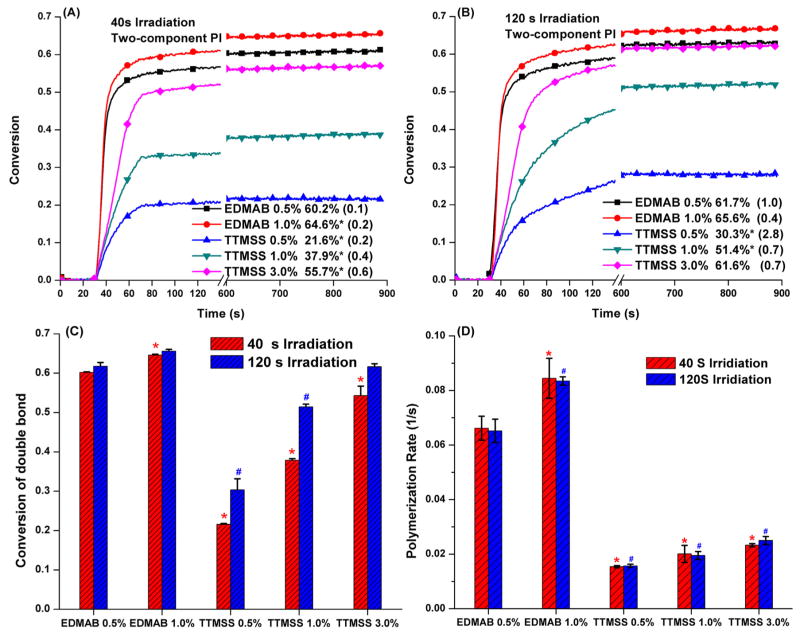
Fig. 1 shows the real-time polymerization kinetic profiles of the neat methacrylate formulation with two-component PI systems (CQ/EDMAB or CQ/TTMSS). When EDMAB was used as co-initiator, the double bond conversion and maximum polymerization rate varied slightly with an increase in EDMAB concentration from 0.5 to 1.0 wt%. These properties also showed a slight variation when the irradiation time was increased from 40 to 120s. The DC values were not significantly different (p < 0.05) after 10 min.
Fig. 1.
Polymerization profiles of HEMA/BisGMA (45 wt%/55 wt%) under visible-light irradiation (I0 = 550 mW/cm2) in the presence of two-component PI (CQ/EDMAB or CQ/TTMSS): conversion vs. time curves with 40 s (A) and 120 s (B) irradiation, conversion (C) and maximum polymerization rate (D) vs. co-initiator concentration. ‘*’, ‘#’ significantly (p < 0.05) different from the control (CE-0.5, CQ/EDMAB = 0.5/0.5 wt%). The value in the () is the standard deviation.
When TTMSS was used as co-initiator, an increase in concentration from 0.5, 1.0, and 3.0 wt% led to an increase in final DC (10 min) from 21.6 ± 0.2, 37.9 ± 0.4, to 54.3 ± 2.4%, respectively. When the irradiation time was increased from 40 to 120 s, the final DC was 30.3 ± 2.8, 51.4 ± 0.7, and 61.6 ± 0.7%, respectively. When TTMSS was used as the co-initiator, the DC at 120 s irradiation was significantly greater (p < 0.05) than the DC at 40 s. The maximum polymerization rates were not significantly different (p < 0.05). As the irradiation time is increased, the final DC also increased. The DC is affected by several factors including the PI system and the total dose of irradiation. From Fig. 1C, when EDMAB (0.5%) was used as the co-initiator, irradiation time had limited effect on the DC and 40 s irradiation was sufficient to achieve DC of about 60%. In contrast, irradiation time had a significant effect on DC when TTMSS was used as a co-initiator.
The DC of two-component PI formulations using EDMAB or TTMSS as co-initiator is shown in Table 2. With the increase of TTMSS concentration from 0.5 to 3.0 wt%, the DC (10 min) of CT-formulations increased from 21.6 ± 0.2 to 55.7 ± 0.6%, which was significantly lower than that of the control (CE-0.5, CQ/EDMAB = 0.5 wt%/0.5 wt%). However, increasing the irradiation time from 40 to 120 s, the DC of CT-3.0 (CQ/TTMSS = 0.5/3.0 wt%) was comparable with that of the control (CE-0.5, CQ/EDMAB = 0.5//0.5 wt%). Meanwhile, the DC of two-component formulations is observed to be a function of irradiation time, and is determined by the PI system. As the irradiation time is increased, the final DC also increased. With an increase in irradiation time from 40 to 120 s, the instantaneous DC showed about 4% increase using EDMAB as co-initiator, however, the instantaneous DC increased about 10% using TTMSS as co-initiator. An irradiation time of 40 s was sufficient to achieve final DC of 60.2 ± 0.1% and 64.6 ± 0.2% at 0.5 and 1.0 wt% EDMAB, respectively.
Table 2.
Instantaneous DC of two-component PI formulations with varied irradiation time.
| Runa | CQ | EDMAB | TTMSS | DC of 40 s irradiation (%) | DC of 120 s irradiation (%) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 40 sb | 600 sc | 120 sb | 600 sc | ||||
| CE-0.5 | 0.5 | 0.5 | / | 54.6 (0.2) | 60.2 (0.1) | 58.2 (1.2) | 61.8 (1.0) |
| CE-1.0 | 0.5 | 1.0 | / | 58.7* (0.3) | 64.6* (0.2) | 62.6* (0.1) | 65.6 (0.4) |
| CT-0.5 | 0.5 | / | 0.5 | 19.5* (0.6) | 21.6* (0.2) | 29.7* (3.0) | 30.3* (2.8) |
| CT-1.0 | 0.5 | / | 1.0 | 32.7* (0.5) | 37.9* (0.4) | 46.2* (0.1) | 51.4* (0.7) |
| CT-3.0 | 0.5 | / | 3.0 | 48.1* (0.1) | 55.7* (0.6) | 57.8 (0.7) | 61.6 (0.7) |
The resin was mixture of HEMA/BisGMA (45/55, w/w) and the irradiation intensity is 550 mW/cm2.
Instantaneous DC after 40 or 120 s light irradiation turn off.
DC after light irradiation turn off for 600 s.
Significantly (p < 0.05) different from the control (CE-0.5).
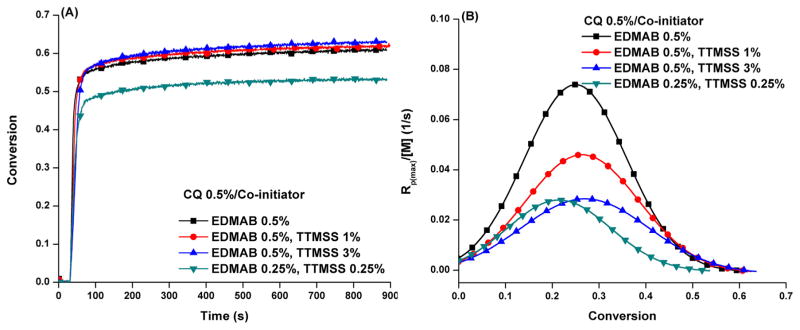
Fig. 2 shows the polymerization profiles of HEMA/BisGMA resin with binary co-initiators (combined EDMAB and TTMSS). In binary co-initiator formulations, the double bond DC was 61.0 ± 0.3 and 62.5 ± 0.6% with an increase of TTMSS concentration from 1 to 3 wt%. This DC was similar to the control (60.2 ± 0.2%). The Rp(max)/[M] was reduced from 0.043 ± 0.003 to 0.035 ± 0.003 s−1, which was lower than the control (0.066 ± 0.004 s−1). The DC decreased from 60.2 ± 0.2 to 52.4 ± 0.6% and the Rp(max)/[M] was reduced from 0.066 ± 0.004 to 0.038 ± 0.003 s−1 with a decrease in the concentration of EDMAB from 0.5 to 0.25 wt%.
Fig. 2.
Polymerization profiles of HEMA/BisGMA (45 wt%/55 wt%) under visible light (I0 = 550 mW/cm2) in the presence of binary co-initiators (EDMAB and TTMSS).
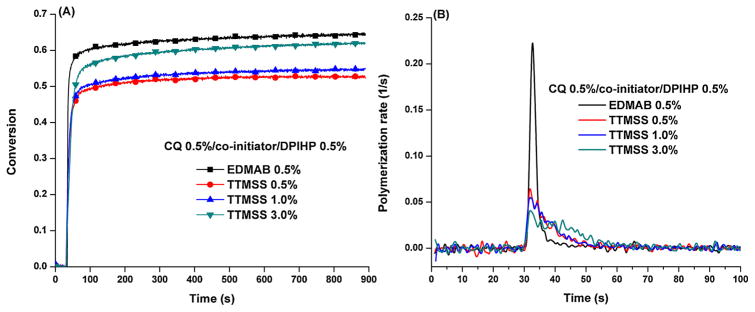
Fig. 3 shows the polymerization behavior of the neat resin with three-component PI (CQ/EDMAB/DIPHP or CQ/TTMSS/DPIHP). The DC of the control with three-component PI was about 5% greater than that of two-component PI, and with the addition of iodonium salt, the Rp(max)/[M] was increased from 0.066 to 0.23 s−1. When TTMSS was used as co-initiator and its concentration increased from 0.5 to 1.0 and even 3.0 wt%, the DC increased from 52.7 ± 0.5, 54.3 ± 0.2, and 61.5 ± 0.2%, respectively. The corresponding Rp(max)/[M] was 0.068 ± 0.002, 0.068 ± 0.011, and 0.044 ± 0.002 s−1, respectively.
Fig. 3.
Photopolymerization profiles of HEMA/BisGMA (45 wt%/55 wt%) under visible light (I0 = 550 mW/cm2) in the presence of three-component PI. (CQ 0.5 wt%, DPIHP 0.5 wt%, and EDMAB or TTMSS).
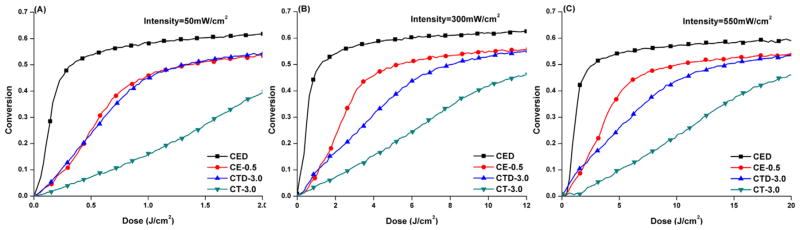
The DC values obtained from the real-time polymerization kinetics of the neat resin photocured by varying the light intensity are shown in Table 3. In two-component PI formulations (CQ/EDMAB or CQ/TTMSS), when EDMAB was used as the co-initiator, the irradiation intensity had minimal effect on the DC. When TTMSS was used to replace EDMAB, the DC was similar at about 55% except for the case when the light intensity was reduced to 50 mW/cm2. In three-component PI systems (CQ/EDMAB/DPIHP or CQ/TTMSS/DPIHP), the decrease in light intensity did not have an effect on the final DC with either EDMAB or TTMSS. When plotting the DC vs. the total dose (irradiation intensity × irradiation time), the conversion values vary significantly as a function of the dose (Fig. 4). The conversions were achieved rapidly at the lower light intensities. This result suggests that the conversion values were proportional to the light intensity at 40 s exposure.
Table 3.
DC of the two/three-component PI formulations at varied irradiation intensitya.
| Run | Irradiation intensity (mW/cm2) | DCb (%) |
|---|---|---|
| CED | 50 | 68.3 (0.2) |
| 300 | 68.0 (0.1) | |
| 550 | 63.9 (0.2) | |
| CE-0.5 | 50 | 59.5 (0.9) |
| 300 | 61.9 (0.3) | |
| 550 | 60.2 (0.2) | |
| CTD-3.0 | 50 | 59.4 (0.5) |
| 300 | 62.0 (1.7) | |
| 550 | 61.5 (0.2) | |
| CT-3.0 | 50 | 49.0 (1.0) |
| 300 | 55.6 (1.7) | |
| 550 | 54.3 (2.4) |
The irradiation time is 40 s.
DC after light irradiation turn off for 600 s.
Fig. 4.
Conversion vs. dose (irradiation intensity × 40 s) for HEMA/BisGMA (45/55, w/w) resin at irradiation intensities of (A) 50, (B) 300, and (C) 550 mW/cm2.
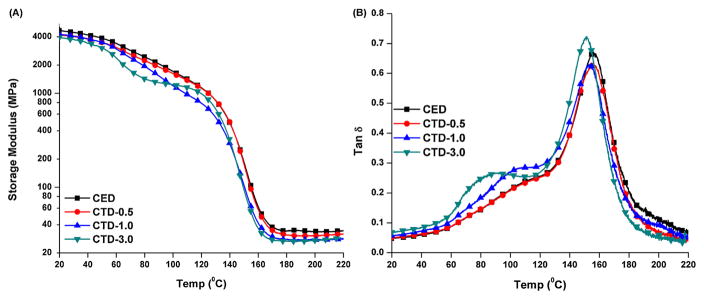
Fig. 5 shows the storage modulus (A) and tan δ (B) as a function of temperature for the control and experimental adhesives with three-component PI. At 25 °C, the storage modulus values for both polymer networks are in the range of 4000–4500 MPa. The storage moduli decrease with increasing temperature, reaching the range of 28–34 MPa in the rubbery state. The rubbery storage modulus and Tg of the experimental formulation were slightly lower than that of control, and decreased with an increase in TTMSS concentration. When the TTMSS concentration was higher than 1 wt%, an obvious shoulder appeared at lower temperatures. The DMA results acquired under both dry and wet conditions are shown in Table 4 and Table 5. The relative crosslink density, which is calculated based on the inverse ratio (ξ) of modulus in the rubbery region to the absolute temperature, showed no significant difference (p < 0.05). The value of the full-width-at-half-maximum (FWHM) of the CTD-3.0 (CQ/TTMSS/DPIHP = 0.5/3.0/0.5 wt%) was significantly lower than that of the control (p < 0.05).
Fig. 5.
The Storage modulus (A) and tan δ (B) versus temperature curves for the control and experimental adhesives with three-component PI in dry condition. (CED: EDMAB 0.5 wt%, CTD-0.5: TTMSS 0.5 wt%, CTD-1.0: TTMSS 1.0 wt%, and CTD-3.0: TTMSS 3.0 wt%. Other components in the formulation were HEMA/BisGMA = 45/55, CQ 0.5 wt%, DPIHP 0.5 wt%).
Table 4.
DMA results of the control and experimental samples containing three-component PI in dry condition.
| Run | Storage Modulus (MPa) | Tg (°C) | tan δ | ζ (K/MPa) | FWHM of tan δ (°C) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 25 °C | 37 °C | >160 °C | |||||
| CED | 4450 (159) | 4199 (124) | 33.9 (0.5) | 155.1 (1.4) | 0.66 (0.01) | 13.5 (0.2) | 37.9 (2.6) |
| CTD-0.5 | 4086a (33) | 3843a (30) | 29.8 (1.6) | 156.3 (0.2) | 0.64 (0.02) | 15.3 (0.8) | 39.4 (0.4) |
| CTD-1.0 | 4245 (111) | 3967 (123) | 29.7 (1.6) | 152.5 (2.1) | 0.63 (0.01) | 15.3 (0.9) | 37.6 (1.2) |
| CTD-3.0 | 4434 (99) | 4130 (99) | 28.9 (1.0) | 152.3 (0.5) | 0.71a (0.02) | 15.5 (0.5) | 31.6a (1.0) |
Significantly (p < 0.05) different from the control (CED, CQ/EDMAB/DPIHP = 0.5/0.5/0.5 wt%).
The value in the () is the standard deviation.
4. Discussion
In dental resins, the photopolymerization reaction is initiated by multicomponent photoinitiator systems, such as two-component CQ/amine or three-component CQ/amine/iodonium salt, which are activated by visible light. Indeed, the amine free radical, formed after the hydrogen abstraction from amine by activated photosensitizer CQ, starts and guides the polymerization of methacrylate at a high rate. It has been widely reported that the type of amine directly affected the polymerization behavior [49,50]. It has also been reported that the amine systems undergo an acid-base reaction with acidic monomers and this reaction can affect the initiation of the polymerization process [51].
Recently, the silyl radicals were reported as efficient species in FRP and FRPCP. The rate constants increase together with a decrease of the bond dissociation energy BDE(X–H). The BDE(X–H) of EDMAB and TTMSS are 92.8 and 79.8 kcal/mol, respectively [52]. Therefore, Si–H showed an efficient hydrogen transfer and high reactivity compared with C–H. It has been reported that the polymerization profiles in the presence of TTMSS for acrylate were better than that obtained in the presence of EDMAB. The profiles showed an increase of both the polymerization rates and final conversions [25]. Based on these results, TTMSS was selected as a co-initiator in the current investigation. The effect of TTMSS on the photopolymerization, and the relationship between polymerization kinetics and the mechanical performance of a model dental adhesive resin were determined.
One of the distinct clinical advantages of light-cured dental restorative materials is that light-curing offers the practitioner flexibility in terms of manipulating the material and initiating the polymerization reaction. Parameters that may negatively affect the conversion include insufficient light intensity and irradiation time that is too short. There is agreement in the literature that the source intensity should be over 400 mW/cm2, that irradiation time should be no more than 60 s [53–56], usually less than 30–40 s [1]. The parameters are based on studies that directly or indirectly determined the conversion or conversion rate at a given time after light irradiation.
For the TTMSS silyl radical, a high reactivity with addition rate constant (kadd~2.2 × 107 M−1 s−1) to methyl acrylate was noted [22]. For the EDMAB generated radical, the value is 5 × 105 M−1 s−1 [57]. These results demonstrate the high potential of the silyl radicals to act as a photoinitiating species. In the present study, the generation of silyl radical involves an interaction between excited CQ and TTMSS that leads to hydrogen abstraction. When TTMSS replaces EDMAB as co-initiator, a lower reactivity is clearly noted in Fig. 1. In the two-component PI system, the DC of the TTMSS-containing formulation was significantly lower (p < 0.05) than that of the control (CE-0.5, CQ/EDMAB = 0.5/0.5 wt%). The maximum polymerization rate with TTMSS is slower than that with EDMAB. It is reported that the hydrogen abstraction rate constants of PI/silane (kH ~106 – 108 M−1 s−1) are much lower than that of PI/amines ( ) [22]. This has been attributed primarily to a different mechanism, i.e., electron/proton transfer for amine vs. hydrogen atom transfer for silane [10]. The lower CQ/TTMSS interaction could limit the concentration of produced silyl free radical, which is the crucial step to determine the polymerization rate and the final conversion of methacrylate. It is observed that the DC of TTMSS-containing formulations increased significantly with an increase in TTMSS concentration from 0.5 to 3.0 wt% or irradiation time from 40 to 120 s (Table 2). Meanwhile, the maximum polymerization rates remained similar, which indicated that the maximum polymerization rate was not determined by the TTMSS concentration or irradiation time. Thus, based on these results the first hypothesis, which state that the polymerization behavior of neat resins formulated with TTMSS or EDMAB is comparable in the two-component PI system, is rejected.
Assuming that the termination of free radical photopolymerization occurs primarily through a bimolecular process, the rate of free radical photopolymerization Rp is expressed by Eq. (2) [58,59], where kp is the propagation rate constant, kt the termination rate constant, and Ri the initiation rate as defined in Eq. (3) [58]. In Eq. (3), ϕi represents the initiation quantum yield that corresponds to the number of starting polymer chains per photon absorbed, I0 is the intensity of incident light, ε is the molar extinction coefficient of the PI, [I] is the concentration of the PI, and d is the thickness of the sample.
| (2) |
| (3) |
With an increase in irradiation time from 40 to 120 s, the Iabs was enhanced and the Ri increased accordingly with both EDMAB and TTMSS. All of the Rp are proportional to the irradiation time or the total dose. However, the hydrogen abstraction rates between CQ/EDMAB and CQ/TTMSS were different, which led to different ϕi values. Due to the fast hydrogen abstraction rate of CQ/EDMAB, the DC increased slightly with an increase in irradiation time. When TTMSS was used as co-initiator, with an increase in irradiation time, the DC increased significantly (see Fig. 1B and C). It must be noted that the 120 s irradiation time, utilized in this work, is not necessarily applicable or appropriate for the clinical setting. The clinical recommendation is generally less than 60 s [53].
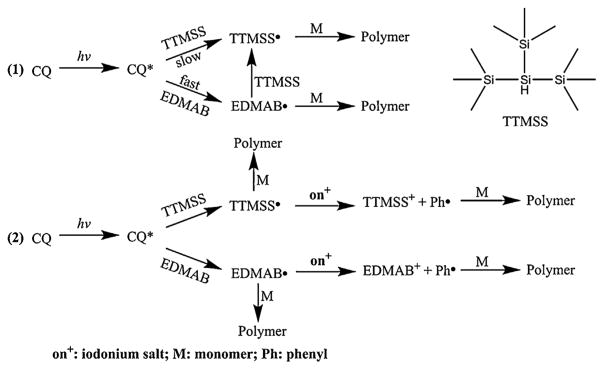
In the binary co-initiator formulations (mixture of EDMAB and TTMSS as co-initiator), with the increase of TTMSS concentration from 1.0 to 3.0 wt%, the concentration of the generated free radical was increased and the final DC was increased slightly. However, the maximum Rp was decreased accordingly. This result was attributed to a competition reaction between the generated amine radical with monomer or TTMSS (See Scheme 1). It was reported that some carbon-centered radicals can abstract a hydrogen atom from TTMSS, and the rate constants were about (1.4–3.8) × 105 M−1 s−1 [60]. Because the addition rate constant is very close to the hydrogen abstraction rate constant, part of the amine radicals can react with TTMSS and generate silyl radicals (Scheme 1), which then react with monomers and produce the macroradicals. At the same time, due to the lower bond dissociation energy of Si–H, the macroradical can react with TTMSS via “chain transfer” reaction. The polymer chain is terminated and generates a TTMSS radical, which can then begin a new polymer chain. Since the initiation stage of CQ/TTMSS is slower than CQ/EDMAB, it can be assumed that the EDMAB radicals will be formed first during the irradiation and initiate the poly-merization. Following the initiation, two reactions may occur: (1) EDMAB radical reacts with TTMSS, and (2) macroradicals react with TTMSS, which reduced the maximum polymerization rate.
Scheme 1.
The illustration for the generation of free radicals in two-component and three-component PI systems.
It is well known that the reasonable intensity of the irradiation light has a dramatic effect on the polymerization rate [61]. When EDMAB was used as co-initiator, whether in the two-component or three-component PI system, the DC and the polymerization rate were similar even with a decrease in the light intensity from 550 to 50 mW/cm2. The results indicated that EDMAB was an efficient co-initiator at these light intensities. When TTMSS was used as a co-initiator, without the addition of iodoium salt (DPIHP), the DC and polymerization rates increased with an increase in the light intensity. With the increase in irradiation intensity, the amount of light absorbed (Iabs) increased and the Ri was promoted, which showed a positive effect on the Rp. At the same time, with the addition of DIPHP, there was no significant difference in DC or polymerization rate with an increase in irradiation intensity. Although, the reaction mechanism using a three-component PI system is more complex, it was postulated that these results can be attributed to the generation of higher activity phenyl free radical [62] (see Scheme 1).
It has been reported that the polymerization rate and final conversion could be dramatically improved with the addition of iodonium salt, DPIHP, into the two-component initiator system [32]. The improvement in these properties was due to generation of the higher reactive phenyl radical between the amine or silyl radical and the DPIHP. Our results showed the same trend whether in CQ/EDMAB/DPIHP or CQ/TTMSS/DPIHP system. Meanwhile, the maximum polymerization rates of TTMSS-containing formulations were still significantly lower than that of the control (CED, CQ/EDMAB/DPIHP = 0.5/0.5/0.5 wt%). Therefore, in the three-component PI system, with the addition of TTMSS, the maximum polymerization rate was reduced due to the slower initiation stage. However, the conversion (after irradiated 10 min) showed no significant difference (p < 0.05) when compared to the system with EDMAB as co-initiator. Thus, the results support acceptance of the second hypothesis, i.e., the addition of DPIHP will significantly increase the final conversion and maximum polymerization rate.
The copolymer specimens prepared with CQ/TTMSS system were relatively soft and could not be retrieved for dynamic mechanical analysis. Therefore, only the specimens prepared with three-component PI system were used to determine the mechanical properties shown in Fig. 5. At low temperature, both the control and experimental specimens showed a gradual decrease in storage moduli with increasing temperature. With the increase of TTMSS concentration from 0.5 to 3 wt%, the slope became more distinct. Near the glass transition temperature, storage moduli decreased drastically. As heating continues the storage moduli reached the rubbery plateau. During the visible-light irradiation, microgel formation at initiation sites and cyclization reactions have created a heterogeneous cross-linked network structure [63]. The loosely cross-linked regions, highly cross-linked regions and the unreacted monomers or initiators could give a network with a broad distribution of mobility [63]. When the TTMSS content was 0.5 wt%, the tan δ curve was similar with the control. With an increase of the TTMSS content to 3 wt%, rubbery moduli were slightly lower than that of the control and the shoulder peak of tan δ curve became more obvious. The shoulder peak was attributed to the side chains and unreacted ends in the polymer network [45].
In the present work, TTMSS may act as plasticizer, which facilitates the movement of the side chains. The rubbery modulus has been related to the crosslink density of the polymeric materials. The inverse ratio (ζ) of the modulus in rubbery region to the temperature has been used to represent the relative crosslink density [46]. The value of ζ suggested comparable relative crosslink density when TTMSS was used to replace EDMAB. The maximum polymerization rates were depressed with the addition of TTMSS, but the crosslink densities were not significantly different. Therefore, based on these results, the third hypothesis is accepted.
In Table 4 and Fig. 5, it is noted that the full-width-at-half-maximum (FWHM) values for the tan δ curves decreased with an increase in the TTMSS concentration. With the exception of the experimental specimen that contained 3 wt% TTMSS, the heights of tan δ peak for the experimental specimens were not significantly different from the control (p < 0.05). Previous investigators have reported decreased polymerization rate and more homogenous network structure with the addition of TTMSS [64]. The current investigation shows similar results, i.e., the heterogeneity of the copolymer was decreased by the slower polymerization rate associated with an increase in the TTMSS concentration.
Traditionally, HEMA/BisGMA dental resins initiated with three-component PI system form polymers with a heterogeneous network structure, because of the unequal reactivity of the functional groups in the co-monomers. The formation of microgels can be determined by many factors, such as the monomer structure, initiation rate, viscosity, and so forth [63,65]. It has been shown that the propagating radicals can react with pendant double bond by primary cyclization, or secondary cyclization. The crosslinking mechanism associated with the primary cyclization causes reduction in the effective crosslink density [34]. In the present study, the slower Rp with the addition of TTMSS promoted the primary cyclization; meanwhile, due to the lower bond dissociation energy of Si–H, the macroradical can react with TTMSS via “chain transfer” reaction. Therefore, the effective crosslink density could be decreased. In this manner, the copolymer of HEMA/BisGMA was expected to increase the homogeneity. Although the heterogeneity of copolymer was decreased by the slower polymerization rate, under dry conditions, the mean storage modulus for the experimental specimens (Table 4) was not significantly different from the control (p < 0.05).
When adhesive formulations are photopolymerized in the mouth, the copolymer can become saturated with water and this can lead to plasticization of the polymer. The three-point bending water-submersion clamp method is used to study the polymer under conditions that simulate the wet, oral environment. The moduli of the control and experimental specimens were significantly lower than that of the dry samples. These differences could be due to the plasticizing effect of water. When TTMSS was 1 wt%, the storage modulus at 25 or 37 °C was not significantly different from that of the control (Table 5). However, the moduli of the experimental specimens at 70 °C were significantly lower than that of the control (p < 0.05). These findings suggest that the effective crosslink density of network structure of the HEMA/BisGMA copolymer system was affected by changes in polymerization behavior and the structure was affected negatively by the wet environment. Two-away ANOVA has been used to analyze the effect of environment conditions (dry vs. wet). Based on the results from the mechanical property measurements under dry and wet conditions, the fourth hypothesis is accepted. The fourth hypothesis states that the polymerization rates will affect the mechanical properties of the polymethacrylate in dry and wet conditions.
In the literature, the TTMSS-based radicals were reported to possess outstanding reactivity and new photoinitiating systems have been proposed based on TTMSS [19–25]. In the present work, the maximum polymerization rates of methacrylate-based dentin adhesive were reduced dramatically by TTMSS. Compared with EDMAB, a decrease of both the polymerization rates and the final conversion were contradictory with Lalevee’s result [66]. These differences are attributed primarily to the use of acrylate monomers in Lalevee’s work while methacrylate monomers were used in the current study. In the present study, with the exception of the slow polymerization rates, comparable DC of C=C bond and mechanical properties, under dry condition, were observed with the addition of TTMSS. Placement of resin composite over the uncured adhesive surface layer will potentially produce further polymerization of the bonding agent by diffusion of reactive components from the composite into the adhesive. It has also been theorized that the placement of the resin composite will displace the uncured adhesive and generate a mixed layer of the two compounds at the interface. The diffusion of unfilled resin into the overlying resin composite was reported by Rueggeberg and Margeson [67], and they concluded that this diffusion allowed more opportunities for the migration of free radicals into the composite material. It is unclear whether the uncured adhesive layer is required for the coupling of a resin composite. Some studies have suggested a positive correlation between the oxygen-inhibition layer, which characteristically exhibits low conversion, and composite bonding [68–70]. Investigation of the oxygen-inhibited layer and its impact on the interfacial composite/adhesive bond are ongoing.
5. Conclusions
The polymerization behavior and the mechanical properties of methacrylate formulations using TTMSS as a co-initiator have been determined. The DC varied as a function of the total irradiation dose and the PI system. In the two (CQ/TTMSS) or three (CQ/TTMSS/DPIHP)-component PI system, the Rp and DC were depressed compared with the control. Without the addition of iodonium salt, the Rp and DC showed an obvious dependence on the irradiation time and light intensity. Our results indicated that the two-component PI system based on the TTMSS exhibited a low reactivity in free radical polymerization, which was attributed to the decrease of the hydrogen abstraction yield between TTMSS and excited CQ. In three-component PI systems, when the co-initiator TTMSS was used at a concentration of 3 wt%, the degree of conversion was close to that of EDMAB at 0.5 wt%. In dry conditions, the storage modulus was insensitive to the polymerization rate. The relative crosslink densities of the experimental formulations showed no significant difference (p < 0.05) when compared to the control. The decrease of FWHM value with an increase of TTMSS concentration indicated that a more homogeneous structure can be obtained by slowing the polymerization rate. Further study is needed to provide a thorough understanding of the behavior of TTMSS when used under conditions representative of the wet, oral environment.
Acknowledgments
This investigation was supported by Research Grant: R01 DE022054 and 3R01DE022054-04S1 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
References
- 1.Jakubiak J, Allonas X, Fouassier JP, Sionkowska A, Andrzejewska E, Linden LA, et al. Camphorquinone-amines photoinitating systems for the initiation of free radical polymerization. Polymer. 2003;44:5219–26. [Google Scholar]
- 2.Bi YB, Neckers DC. A visible-light initiating system for free-radical promoted cationic polymerization. Macromolecules. 1994;27:3683–93. [Google Scholar]
- 3.Cook WD. Photopolymerization kinetics of dimethacrylates using the camphorquinone amine initiator system. Polymer. 1992;33:600–9. [Google Scholar]
- 4.Nie J, Bowman CN. Synthesis and photopolymerization of N,N′-dimethyl,-N,N′-di(methacryloxy ethyl)-1,6-hexanediamine as a polymerizable amine coinitiator for dental restorations. Biomaterials. 2002;23:1221–6. doi: 10.1016/s0142-9612(01)00241-1. [DOI] [PubMed] [Google Scholar]
- 5.Popielarz R, Vogt O. Effect of coinitiator type on initiation efficiency of two-component photoinitiator systems based on Eosin. J Polym Sci, A: Polym Chem. 2008;46:3519–32. [Google Scholar]
- 6.Guo XL, Peng ZH, Spencer P, Wang Y. Effect of initiator on photopolymerization of acidic, aqueous dental model adhesives. J Biomed Mater Res, A. 2009;90A:1120–7. doi: 10.1002/jbm.a.32185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei J, Lu R, Liu F. Novel, highly efficient polymeric benzophenone photoinitiator containing coinitiator moieties for photopolymerization. Polym Adv Technol. 2010;21:656–62. [Google Scholar]
- 8.Wei J, Liu F. Novel highly efficient macrophotoinitiator comprising benzophenone, coinitiator amine, and thio moieties for photopolymerization. Macromolecules. 2009;42:5486–91. [Google Scholar]
- 9.Wang Y, Spencer P, Yao X, Ye Q. Effect of coinitiator and water on the photoreactivity and photopolymerization of HEMA/camphoquinone-based reactant mixtures. J Biomed Mater Res, A. 2006;78A:721–8. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tehfe MA, El-Roz M, Lalevee J, Morlet-Savary F, Graff B, Fouassier JP. Bifunctional co-initiators: a new strategy for the design of efficient systems in radical photopolymerization reactions under air. Eur Polym J. 2012;48:956–62. [Google Scholar]
- 11.Munchow EA, Valente LL, Peralta SL, Fernandez MR, Lima GD, Petzhold CL, et al. 1,3-Diethyl-2-thiobarbituric acid as an alternative coinitiator for acidic photopolymerizable dental materials. J Biomed Mater Res, B: Appl Biomater. 2013;101:1217–21. doi: 10.1002/jbm.b.32933. [DOI] [PubMed] [Google Scholar]
- 12.Bowen RL, Cobb EN, Rapson JE. Adhesive bonding of various materials to hard tooth tissues—improvement in bond strength to Dentin. J Dent Res. 1982;61:1070–6. doi: 10.1177/00220345820610090901. [DOI] [PubMed] [Google Scholar]
- 13.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 14.Ikemura K, Endo T. A review of our development of dental adhesives—effects of radical polymerization initiators and adhesive monomers on adhesion. Dent Mater J. 2010;29:109–21. doi: 10.4012/dmj.2009-057. [DOI] [PubMed] [Google Scholar]
- 15.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–16. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilman H, Atwell WH, Sen PK, Smith CL. Branched-chain organic polysilanes containning silicon-hydrogen group. J Organomet Chem. 1965;4:163–7. [Google Scholar]
- 17.Kanabuskaminska JM, Hawari JA, Griller D, Chatgilialoglu C. Reducation of silicon-hydrogen bond strengths. J Am Chem Soc. 1987;109:5267–8. [Google Scholar]
- 18.Chatgilialoglu C, Griller D, Lesage M. Tris(trimethylsilyl)silane—a new reducing agent. J Org Chem. 1988;53:3641–2. [Google Scholar]
- 19.Lalevee J, Allonas X, Fouassier JP. Tris(trimethylsilyl)silane (TTMSS)-derived radical reactivity toward alkenes: a combined quantum mechanical and laser flash photolysis study. J Org Chem. 2007;72:6434–9. doi: 10.1021/jo0706473. [DOI] [PubMed] [Google Scholar]
- 20.Lalevee J, Blanchard N, El-Roz M, Graff B, Allonas X, Fouassier JP. New photoinitiators based on the silyl radical chemistry: polymerization ability, ESR spin trapping, and laser flash photolysis investigation. Macromolecules. 2008;41:4180–6. [Google Scholar]
- 21.Lalevee J, Blanchard N, Graff B, Allonas X, Fouassier JP. Tris(trimethylsilyl)silyl versus tris(trimethylsilyl)germyl: radical reactivity and oxidation ability. J Organomet Chem. 2008;693:3643–9. [Google Scholar]
- 22.Lalevee J, Dirani A, El-Roz M, Allonas X, Fouassier JP. Silanes as new highly efficient co-initiators for radical polymerization in aerated media. Macromolecules. 2008;41:2003–10. [Google Scholar]
- 23.Lalevee J, El-Roz M, Allonas X, Fouassier JP. Free-radical-promoted cationic photopolymerization under visible light in aerated media: new and highly efficient silane-containing initiating systems. J Polym Sci, A: Polym Chem. 2008;46:2008–14. [Google Scholar]
- 24.Lalevee J, Blanchard N, Chany AC, El-Roz M, Souane R, Graff B, et al. Silyl radical chemistry and conventional photoinitiators: a route for the design of efficient systems. Macromolecules. 2009;42:6031–7. [Google Scholar]
- 25.Chatgilialoglu C, Lalevee J. Recent applications of the (TMS)(3)SiH radical-based reagent. Molecules. 2012;17:527–55. doi: 10.3390/molecules17010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalevee J, Tehfe MA, Morlet-Savary F, Graff B, Allonas X, Fouassier JP. Radical photopolymerization reactions under air upon lamp and diode laser exposure: the input of the organosilane radical chemistry. Prog Org Coat. 2011;70:83–90. [Google Scholar]
- 27.Lalevee J, Tehfe MA, Morlet-Savary F, Graff B, Allonas X, Fouassier JP. Oxygen mediated and wavelength tunable cationic photopolymerization reactions under air and low intensity: a new concept. Prog Org Coat. 2011;70:23–31. [Google Scholar]
- 28.Schummer D, Hofle G. Tris(trimethylsilyl)silane as reagent for the radical deoxygenation of alcohols. Synlett. 1990:705–6. [Google Scholar]
- 29.Giese B, Dickhaut J, Chatgilialoglu C. Tris(trimethylsilyl)silane, e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley and Sons, Inc; 2007. http://www.carborad.com/Volume%20I/volumeI.html. [Google Scholar]
- 30.Chatgilialoglu C. Organosilanes as radical-based reducing agents in synthesis. Acc Chem Res. 1992;25:188–94. [Google Scholar]
- 31.Padon KS, Scranton AB. A mechanistic investigation of a three-component radical photoinitiator system comprising methylene blue, N-methyldiethanolamine, and diphenyliodonium chloride. J Polym Sci, A: Polym Chem. 2000;38:2057–66. [Google Scholar]
- 32.Guo X, Wang Y, Spencer P, Ye Q, Yao X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent Mater. 2008;24:824–31. doi: 10.1016/j.dental.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anseth KS, Goodner MD, Reill MA, Kannurpatti AR, Newman SM, Bowman CN. The influence of comonomer composition on dimethacrylate resin properties for dental composites. J Dent Res. 1996;75:1607–12. doi: 10.1177/00220345960750081301. [DOI] [PubMed] [Google Scholar]
- 34.Elliott JE, Bowman CN. Kinetics of primary cyclization reactions in cross-linked polymers: an analytical and numerical approach to heterogeneity in network formation. Macromolecules. 1999;32:8621–8. [Google Scholar]
- 35.Lovell LG, Newman SM, Bowman CN. The effects of light intensity, temperature, and comonomer composition on the polymerization behavior of dimethacrylate dental resins. J Dent Res. 1999;78:1469–76. doi: 10.1177/00220345990780081301. [DOI] [PubMed] [Google Scholar]
- 36.Lovell LG, Stansbury JW, Syrpes DC, Bowman CN. Effects of composition and reactivity on the reaction kinetics of dimethacrylate dimethacrylate copolymerizations. Macromolecules. 1999;32:3913–21. [Google Scholar]
- 37.Lovell LG, Lu H, Elliott JE, Stansbury JW, Bowman CN. The effect of cure rate on the mechanical properties of dental resins. Dent Mater. 2001;17:504–11. doi: 10.1016/s0109-5641(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 38.Lovell LG, Newman SM, Donaldson MM, Bowman CN. The effect of light intensity on double bond conversion and flexural strength of a model, unfilled dental resin. Dent Mater. 2003;19:458–65. doi: 10.1016/s0109-5641(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 39.Ye Q, Park J, Topp E, Spencer P. Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent Mater. 2009;25:452–8. doi: 10.1016/j.dental.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J, Ye Q, Singh V, Kieweg SL, Misra A, Spencer P. Synthesis and evaluation of novel dental monomer with branched aromatic carboxylic acid group. J Biomed Mater Res, B: Appl Biomater. 2012;100B:569–76. doi: 10.1002/jbm.b.31987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parthasarathy R, Misra A, Park J, Ye Q, Spencer P. Diffusion coefficients of water and leachables in methacrylate-based crosslinked polymers using absorption experiments. J Mater Sci: Mater Med. 2012;23:1157–72. doi: 10.1007/s10856-012-4595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J, Ye Q, Topp EM, Misra A, Kieweg SL, Spencer P. Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. J Biomed Mater Res, A. 2010;93A:1245–51. doi: 10.1002/jbm.a.32617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mesquita RV, Geis-Gerstorfer J. Influence of temperature on the visco-elastic properties of direct and indirect dental composite resins. Dent Mater. 2008;24:623–32. doi: 10.1016/j.dental.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Kannurpatti AR, Anseth JW, Bowman CN. A study of the evolution of mechanical properties and structural heterogeneity of polymer networks formed by photopolymerizations of multifunctional (meth)acrylates. Polymer. 1998;39:2507–13. [Google Scholar]
- 45.Park JG, Ye Q, Topp EM, Lee CH, Kostoryz EL, Misra A, et al. Dynamic mechanical analysis and esterase degradation of dentin adhesives containing a branched methacrylate. J Biomed Mater Res, B: Appl Biomater. 2009;91B:61–70. doi: 10.1002/jbm.b.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JG, Ye Q, Topp EM, Misra A, Spencer P. Water sorption and dynamic mechanical properties of dentin adhesives with a urethane-based multifunctional methacrylate monomer. Dent Mater. 2009;25:1569–75. doi: 10.1016/j.dental.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podgorski M, Matynia T. Network structure/mechanical property relationship in multimethacrylates-derivatives of nadic anhydride. J Appl Polym Sci. 2008;109:2624–35. [Google Scholar]
- 48.Song LY, Ye Q, Ge XP, Misra A, Laurence JS, Berrie CL, et al. Synthesis and evaluation of novel dental monomer with branched carboxyl acid group. J Biomed Mater Res, B: Appl Biomater. 2014;102:1473–84. doi: 10.1002/jbm.b.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn KD, Han DK, Lee SH, Lee CW. New aromatic tert-amines for application as photoinitiator components in photocurable dental materials. Macromol Chem Phys. 2003;204:1628–35. [Google Scholar]
- 50.Schneider LFJ, Pfeifer CSC, Consani S, Prahl SA, Ferracane JL. Influence of photoinitiator type on the rate of polymerization, degree of conversion, hardness and yellowing of dental resin composites. Dent Mater. 2008;24:1169–77. doi: 10.1016/j.dental.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Ilie N, Hickel R. Can CQ be completely replaced by alternative initiators in dental adhesives? Dent Mater J. 2008;27:221–8. doi: 10.4012/dmj.27.221. [DOI] [PubMed] [Google Scholar]
- 52.El-Roz M, Lalevee J, Allonas X, Fouassier JP. Mechanistic investigation of the silane, germane, and stannane behavior when incorporated in Type I and Type II photoinitiators of polymerization in aerated media. Macromolecules. 2009;42:8725–32. [Google Scholar]
- 53.Davidson-Kaban SS, Davidson CL, Feilzer AJ, de Gee AJ, Erdilek N. The effect of curing light variations on bulk curing and wall-to-wall quality of two types and various shades of resin composites. Dent Mater. 1997;13:344–52. doi: 10.1016/s0109-5641(97)80105-4. [DOI] [PubMed] [Google Scholar]
- 54.Ye QA, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dentin adhesives as a function of light source and irradiance. J Biomed Mater Res, B: Appl Biomater. 2007;80B:440–6. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JS, Choi YH, Cho BH, Son HH, Lee IB, Um CM, et al. Effect of light-cure time of adhesive resin on the thickness of the oxygen-inhibited layer and the microtensile bond strength to dentin. J Biomed Mater Res, B: Appl Biomater. 2006;78B:115–23. doi: 10.1002/jbm.b.30463. [DOI] [PubMed] [Google Scholar]
- 56.Uhl A, Mills RW, Vowles RW, Jandt KD. Knoop hardness depth profiles and compressive strength of selected dental composites polymerized with halogen and LED light curing technologies. J Biomed Mater Res. 2002;63:729–38. doi: 10.1002/jbm.10390. [DOI] [PubMed] [Google Scholar]
- 57.Lalevee J, Graff B, Allonas X, Fouassier JP. Aminoalkyl radicals: direct observation and reactivity toward oxygen, 2,2,6,6-tetramethylpiperidine-N-oxyl, and methyl acrylate. J Phys Chem A. 2007;111:6991–8. doi: 10.1021/jp071720w. [DOI] [PubMed] [Google Scholar]
- 58.Odian G. Principles of Polymerization. 4. Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 59.Broer DJ, Mol GN, Challa G. Temperature effects on the kinetics of photoinitiated polymerization of dimethacrylates. Polymer. 1991;32:690–5. [Google Scholar]
- 60.Binkley RW, Binkley ER. Radical Reactions of Carbohydrates. Volume I: Structure and Reactivity of Carbohydrate Radicals. 2013 http://www.carborad.com/Volume%20I/volumeI.html.
- 61.Wydra JW, Cramer NB, Stansbury JW, Bowman CN. The reciprocity law concerning light dose relationships applied to BisGMA/TEGDMA photopolymers: theoretical analysis and experimental characterization. Dent Mater. 2014;30:605–12. doi: 10.1016/j.dental.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogliari FA, Ely C, Petzhold CL, Demarco FF, Piva E. Onium salt improves the polymerization kinetics in an experimental dental adhesive resin. J Dent. 2007;35:583–7. doi: 10.1016/j.jdent.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Elliott JE, Lovell LG, Bowman CN. Primary cyclization in the polymerization of bis-GMA and TEGDMA: a modeling approach to understanding the cure of dental resins. Dent Mater. 2001;17:221–9. doi: 10.1016/s0109-5641(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 64.Kilambi H, Reddy SK, Schneidewind L, Lee TY, Stansbury JW, Bowman CN. Design, development, and evaluation of monovinyl acrylates characterized by secondary functionalities as reactive diluents to diacrylates. Macromolecules. 2007;40:6112–8. doi: 10.1021/ma062708p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lovell LG, Bowman CN. The effect of kinetic chain length on the mechanical relaxation of crosslinked photopolymers. Polymer. 2003;44:39–47. [Google Scholar]
- 66.Tehfe MA, Lalevee J, Morlet-Savary F, Graff B, Fouassier JP. A breakthrough toward long wavelength cationic photopolymerization: initiating systems based on violanthrone derivatives and silyl radicals. Macromolecules. 2011;44:8374–9. [Google Scholar]
- 67.Rueggeberg FA, Margeson DH. The effect of oxygen inhibition on an unfilled/filled composite system. J Dent Res. 1990;69:1652–8. doi: 10.1177/00220345900690100501. [DOI] [PubMed] [Google Scholar]
- 68.Oyama K, Tsujimoto A, Otsuka E, Shimizu Y, Shiratsuchi K, Tsubota K, et al. Influence of oxygen inhibition on the surface free energy and enamel bond strength of self-etch adhesives. Dent Mater J. 2012;31:26–31. doi: 10.4012/dmj.2011-162. [DOI] [PubMed] [Google Scholar]
- 69.El-Askary FS, Fawzy AS, Elmohsen HMA. Tensile bond strength of immediately repaired anterior microfine hybrid restorative composite using nontrimmed hourglass specimens. J Adhes Dent. 2009;11:41–7. [PubMed] [Google Scholar]
- 70.Truffier-Boutry D, Place E, Devaux J, Leloup G. Interfacial layer characterization in dental composite. J Oral Rehabil. 2003;30:74–7. doi: 10.1046/j.1365-2842.2003.01008.x. [DOI] [PubMed] [Google Scholar]