Abstract
Oral Factor Xa (FXa) inhibitors, a growing class of direct-acting anticoagulants, are frequently used to prevent stroke and systemic embolism in patients with atrial fibrillation and to prevent and treat venous thromboembolism. These drugs reduce the risk of clotting at the expense of increasing the risk of bleeding, and currently they have no specific reversal agent. However, andexanet alfa, a recombinant modified FXa decoy molecule, is in a late-phase clinical trial in bleeding patients, and ciraparantag, a small molecule that appears to reverse many anticoagulants including the FXa inhibitors, is in development. This review summarizes the published data to date on both drugs, which have the potential to change the management approach to patients with FXa inhibitor–associated major hemorrhage.
Keywords: Andexanet alfa, Anticoagulant, Aripazine, Ciraparantag, PER977, PRT064445, PRT4445, Reversal agent
Oral factor Xa (FXa) inhibitors, a class of the direct-acting oral anticoagulants approved for prevention of stroke and systemic embolism in nonvalvular atrial fibrillation and treatment and prevention of venous thromboembolism, consistently have been shown to be noninferior to warfarin in clinical trials evaluating their safety and efficacy.1–7 As with any anticoagulant, these drugs incrementally increase the risk of bleeding to greatly reduce the risk of clotting (eg, stroke and venous thromboembolism). Before the U.S. Food and Drug Administration’s (FDA) approval of idarucizumab, a specific reversal agent for the direct thrombin inhibitor dabigatran, the lack of reversal agents was a consistent concern associated with the use of all direct-acting oral anticoagulants. Currently, there is no approved specific reversal agent for FXa inhibitors, although late-phase clinical trials of reversal agents are ongoing.8
Warfarin anticoagulation historically has been reversed with vitamin K1 (to reinitiate synthesis of affected factors II, VII, IX, and X, along with proteins C and S) and fresh-frozen plasma (to replete these factors more immediately),9 although the effectiveness of this approach has not been demonstrated in clinical trials. Despite being approved for human use in the 1950s in the United States, warfarin had no fast-acting, specific reversal agent supported by evidence from a randomized controlled trial until 2013, when a 4-Factor prothrombin complex concentrate (PCC) was approved.10–12 The first oral FXa inhibitor, rivaroxaban, was approved in the United States in 2011,13 by which time reversal agents for direct-acting oral anticoagulants were already in development. The FXa inhibitors apixaban14 and edoxaban15 have since been approved, and more are in development (note that all FXa inhibitors have the suffix “Xa-ban” [Ten-A-Ban] to denote their mechanism of action).
Preclinical and clinical data on 2 reversal agents, andexanet alfa (Portola Pharmaceuticals, South San Francisco, Calif), a recombinant modified FXa decoy molecule, and ciraparantag (PER977, Perosphere Pharmaceuticals, Danbury, Conn), a small molecule, will be covered in this review. Idarucizumab, a specific reversal agent to dabigatran, is covered in other chapters16–18 of this special issue. Off-label use of factor products, for example, PCCs or recombinant FVIIa, to treat bleeding in patients who have been anticoagulated with direct-acting oral anticoagulants is covered elsewhere19 in this special issue.
ANDEXANET ALFA
Pharmacology
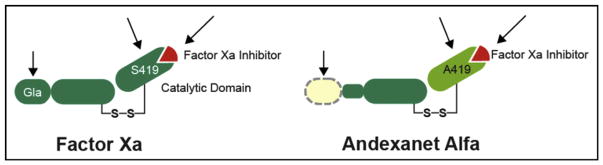
Lu et al,20 from Portola, published a proof-of-concept article on andexanet alfa in 2013, describing the molecule as a truncated form of enzymatically inactive FXa. In the coagulation cascade, FXa joins factor Va (FVa) to form the prothrombinase complex, which cleaves prothrombin (factor II [FII]) to thrombin (factor IIa [FIIa]), which in turn cleaves fibrinogen to fibrin. Two elegant modifications were made to native human FXa to create andexanet (Figure 1). First, the substitution of a serine residue with an alanine at the active site eliminates the protein’s catalytic activity, that is, its ability to cleave prothrombin to thrombin. Second, the removal of the Gla (carboxyglutamic acid) domain eliminates its ability to assemble into the prothrombinase complex, thus removing any anticoagulant effect through the prevention of binding native FVa and inhibiting prothrombin activation. The molecule retains its ability to bind direct FXa inhibitors (the Xabans), but it also binds to low-molecular-weight heparins (LMWH), pentasaccharide-activated anti-thrombin III (ATIII), and unfractionated heparin, and thereby alters the activity of these indirect FXa inhibitors (Table 1).20,21 Investigators recently reported robust double-blind, placebo-controlled safety and biomarker reversal data on nonbleeding older volunteers.24 A summary of the pre-clinical and clinical studies of andexanet is shown in Table 2 and discussed in further detail in this review.
Figure 1.
Design of andexanet alfa. Serine, the active site of FXa, was substituted with alanine, rendering the molecule unable to cleave and activate prothrombin. The Gla domain of FXa was removed to prevent its assembly into the prothrombinase complex, thus removing any anticoagulant effects. Gla = gamma-carboxyglutamic acid-rich. Reproduced with permission from Portola Pharmaceuticals, Inc (South San Francisco, Calif).
Table 1.
FXa and “Universal” Reversal Agent Drug Targets
| Anticoagulant | Andexanet | Ciraparantag |
|---|---|---|
| FXa | ||
| Apixaban | ✔ | ✔ |
| Edoxaban | ✔ | ✔ |
| Rivaroxaban | ✔ | ✔ |
| FIIa | ||
| Dabigatran | — | ✔ |
| Heparin | ||
| UFH | ✔ | ✔ |
| LMWH | ✔ | ✔ |
| ATIII-FXa | ||
| Fondaparinux | Unknown | ✔ |
| VKA | ||
| Warfarin | — | — |
Table 2.
Summary of Preclinical and Clinical Studies of Andexanet
| Enoxaparin | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|---|
| Animal in vivo | |||||
| Rat tail transection | Decreased bleeding by >50%25 | – | – | – | – |
| Mouse tail transection | – | – | Decreased bleeding by >80% (+ ASA)26 | – | |
| Rabbit liver laceration | – | – | Decreased bleeding by >80%27 | – | |
| Human in vitro | |||||
| – | – | Dose-dependent reversal of anti-FXa activity in a buffer system of purified human FXa; reversal of direct and ATIII-dependent FXa inhibitors in human plasma28 Anti-FXa activity of exogenous rivaroxaban was reduced in human plasma from healthy subjects pre-treated with andexanet29 |
Dose-dependent reversal of anti-FXa activity in a buffer system of purified human FXa; reversal of direct and ATIII-dependent FXa inhibitors in human plasma28 | Dose-dependent reversal of anti-FXa activity in a buffer system of purified human FXa; reversal of direct and ATIII-dependent FXa inhibitors in human plasma28 | |
| Human in vivo | |||||
| Reversal of anti-FXa activity in healthy subjects22 | – | Reversal of anti-FXa activity in healthy subjects22 Dose-dependent reversal of anti-FXa activity in healthy subjects and partial restoration of thrombin generation and partial reversal of prolongation of clotting time30 Sustained reversal of anti-FXa activity with bolus plus infusion in healthy older subjects24 |
Reversal of anti-FXa activity in healthy subjects22 Sustained reversal of anti-FXa activity with bolus plus infusion in healthy subjects31 Sustained reversal of anti-FXa activity with bolus plus infusion in healthy older subjects24 |
Dose-dependent reversal of anti-FXa activity in healthy subjects and restoration of thrombin generation and reversal of prolongation of clotting time.32 | |
ASA = acetylsalicylic acid; ATIII = antithrombin III; FXa = factor Xa.
Preclinical Animal Studies and Human Blood in Vitro Studies
The seminal article by Lu et al20 demonstrated a rapid effect of andexanet, which appeared to reverse direct FXa inhibitors completely in human and rat plasma and to restore hemostasis and reduce bleeding in mouse tail transection and rabbit liver laceration models. In humans, andexanet has an effective half-life of approximately 1 hour, which depends on the dose of andexanet and the particular FXa inhibitor with which it is used.24 The FDA granted Portola breakthrough therapy designation, intended to expedite the development and review of drugs for serious or life-threatening conditions, for andexanet in 2013.33
Beyond the primary effect of reestablishing hemostatic efficacy, there are 2 major safety concerns, one general to all anticoagulant “reversal” agents and one specific to the andexanet approach, neither of which has been demonstrated despite ongoing surveillance. Generally, reversal agents must effectively restore hemostasis without creating excessive rebound hypercoagulable states and risk for clinically significant thromboembolic events. Specific to andexanet alfa, a modified clotting factor must not lead to an antibody response against its native cousin, potentially causing an iatrogenic clotting factor deficiency.
Clinical Studies
In a phase 2 dose-ranging, proof-of-concept study in healthy, nonbleeding volunteers treated with apixaban, rivaroxaban, the LMWH enoxaparin,22 or edoxaban,32 andexanet demonstrated rapid (within 2 minutes)34,35 reversal of anticoagulation activity, as measured by anti-FXa activity assays; anti-FXa activity returned to placebo levels in approximately 2 hours after treatment in the study of reversal of edoxaban-induced anticoagulation.32 The reversal could be extended with a continuous infusion.22 There were no observed thrombotic events, serious adverse events, discontinuation because of adverse events, or antibodies to endogenous human FXa or FX.22,32 Andexanet has since been studied in phase 3, double-blind, placebo-controlled studies as a single bolus or a bolus plus 2-hour infusion in nonbleeding healthy older adults (50–75 years) treated with apixaban or rivaroxaban.24 Andexanet was administered as a bolus plus 2-hour infusion because the half-life of the drug is approximately 1 hour (Figure 2). The apixaban and rivaroxaban groups were administered 400 or 800 mg of andexanet, respectively; doses were selected on the basis of the stoichiometric ratio needed for the reversal of each anticoagulant. In the apixaban group, anti-FXa activity was reduced by 94% in subjects (n = 24) who received an andexanet bolus versus 21% in participants (n = 9) who received placebo (P < .001), and the concentration of unbound apixaban was reduced by 9.3 ng/mL versus 1.9 ng/mL (P < .001); thrombin generation was fully restored within 2 to 5 minutes in 100% versus 11% of subjects (P < .001) (Figure 2A).24 In the rivaroxaban group, anti-FXa activity was reduced by 92% in subjects (n = 27) who received an andexanet bolus versus 18% in subjects (n = 14) who received placebo (P < .001), and the concentration of unbound rivaroxaban was reduced by 23.4 ng/mL versus 4.2 ng/mL (P < .001); thrombin generation was fully restored in 96% versus 7% of subjects (P < .001) (Figure 2B).24 When andexanet was administered as a bolus plus 2-hour infusion, these effects were sustained in subjects treated with apixaban and rivaroxaban (Figure 2C and D). Transient increases in levels of D-dimer and prothrombin fragments 1 and 2 (F1.2) occurred in a subgroup of subjects, raising a concern for prothrombotic effect, but this normalized within 24 to 72 hours.24 Mild infusion reactions were reported in some subjects, and 1 subject developed hives.24 Subjects were followed for 6 weeks, and there were no serious adverse events, thromboembolic events, or neutralizing antibody development.24
Figure 2.
Time courses of plasma concentrations of unbound apixaban or rivaroxaban before and after administration of andexanet. Concentrations of unbound apixaban or rivaroxaban in plasma were measured before and after administration of andexanet or placebo on study day 4. (A) Data from participants in the apixaban study who received andexanet, as a 400-mg intravenous bolus, or placebo. (B) Participants in the rivaroxaban study who received andexanet, as an 800-mg intravenous bolus, or placebo. (C) Participants in the apixaban study who received andexanet, as a 400-mg intravenous bolus plus a 4-mg-per minute infusion for 120 minutes, or placebo. (D) Participants in the rivaroxaban study who received andexanet, an 800-mg intravenous bolus plus an 8-mg-per minute infusion for 120 minutes, or placebo. The dashed horizontal line represents the calculated no-effect level for anticoagulant activity (3.5 ng/mL of apixaban and 4.0 ng/mL of rivaroxaban). The points on the graph represent the mean unbound inhibitor plasma concentrations, and I bars indicate the standard error. There was a significant difference (P < .05) between andexanet and placebo until 2 hours after the end of the bolus and 1 hour after the end of the infusion in the apixaban study and until 3 hours after the end of the bolus and 3 hours after the end of the infusion in the rivaroxaban study. Reprinted with permission from Massachusetts Medical Society.24
The pivotal Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa Inhibitors (ANNEXA)-4 trial (NCT02329327)36 is now under way, with a goal of enrolling 270 bleeding patients. It is the first to study andexanet in bleeding humans and shares some similarities with the trials of the 4-Factor PCC for warfarin reversal.10,11 One major difference is the lack of a control group (eg, plasma in the 4-Factor PCC trials). Because there is no standard of care for reversing these drugs, and thus no reasonable active control, it would not be ethical or feasible to give placebos to patients with major hemorrhages. The same reasoning accounts for the single-arm design in the idarucizumab study, REVERSal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD).37 One of the ANNEXA-4 study’s primary outcomes is clinical hemostatic efficacy36 similar to a scale developed for the 4-Factor PCC trials,10 and safety outcomes also are being gathered. The trial may take several years to enroll its cohort, but given its FDA breakthrough designation, the drug could be approved before completion, as was the case with idarucizumab.
It is worth noting that there are some key differences between the ANNEXA-4 study36 and the study of idarucizumab for the reversal of dabigatran in the RE-VERSE AD trial.37 The ANNEXA-4 study strictly requires that subjects meet major hemorrhage criteria for inclusion, and it excludes participation in the efficacy analysis if patients do not meet these criteria.36 Therefore, a strength of the ANNEXA-4 study is that it includes patients with well-characterized major hemorrhage who are in need of immediate reversal.36 In contrast, RE-VERSE AD was more permissive, allowing physician discretion on who required immediate reversal therapy and thus was designed to mimic a “real-world” population.37 In addition, the primary end point of the RE-VERSE AD study was the percentage reversal of the anticoagulant effect of dabigatran. This calculation included the measurements of dilute thrombin time or ecarin clotting time, both of which correlate consistently with the concentration of unbound dabigatran across a wide range of dabigatran concentrations. The ANNEXA-4 study uses dual primary outcomes of a biomarker (anti-FXa levels) plus a clinical outcome of hemostatic efficacy.36,38
CIRAPARANTAG
Pharmacology
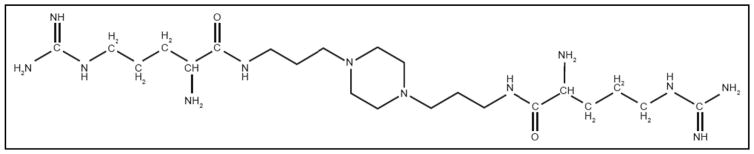
Perosphere is developing ciraparantag (di-arginine piperazine; formerly known as “aripazine” or “PER977”). Ciraparantag is a small (512 Da) synthetic molecule that reportedly binds to unfractionated heparin, LMWH fondaparinux, and the direct-acting oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban23,39,40 (Table 1). It inactivates anticoagulants via noncovalent hydrogen binding, which blocks the binding to the target sites of FIIa and FXa. It does not bind to coagulation factors or other plasma proteins23,41,42 and has no measurable prothrombotic effect as measured by D-dimer, F1.2, or tissue factor pathway inhibitor.43 The chemical structure of ciraparantag is shown in Figure 3. A summary of the preclinical and clinical studies of ciraparantag is shown in Table 3 and discussed in further detail in this review.
Figure 3.
Molecular structure of ciraparantag. Reprinted with permission from Ansell et al.44
Table 3.
Summary of Preclinical and Clinical Studies of Ciraparantag
| Enoxaparin | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|---|
| Animal in vivo | |||||
| Rat tail transection | – | Decreased bleeding by >90%39 | Decreased bleeding by >90%39 | Decreased bleeding by >90%39 | Decreased bleeding45,48 |
| Rat liver laceration | – | – | – | – | Decreased bleeding, reversed change in WBCT, and normalized clot fibrin diameter45,46 |
| Rabbit liver laceration | – | – | – | – | Decreased bleeding by up to 76%47 |
| Human in vitro | |||||
| – | – | Dose-dependent reversal of anti-FXa levels in spiked plasma39,48 | Dose-dependent reversal of anti-FXa levels in spiked plasma39,48 | – | |
| Human in vivo | |||||
| Increase in WBCT reversed within 5 min43 No procoagulation detected by D-dimer, F1.2, or TFPI43 |
– | – | – | Dose-dependent reversal of increase in WBCT44 | |
F1.2 = prothrombin fragments 1 and 2; FXa = factor Xa; TFPI = tissue factor pathway inhibitor; WBCT = whole blood clotting time.
Preclinical Animal Studies
Laboratory rats were dosed with edoxaban and followed by ciraparantag or saline in 3 models: tail transection, liver laceration, and measurement of whole blood clotting time. Ciraparantag decreased bleeding within 7.5 minutes in the tail transection model, within 10 minutes in the liver laceration model,45 and within 30 minutes in the whole blood clotting time model.46 Ciraparantag also has been shown to reduce bleeding from tail transection by more than 90% in rats overdosed with dabigatran, rivaroxaban, or apixaban.39
Andexanet manufacturer Portola synthesized ciraparantag and compared the reversal effects in a rabbit liver laceration model with prior studies with andexanet.47 High-dose ciraparantag reversed blood loss to a similar extent as andexanet. In nonanticoagulated rabbits, there was a reduction in blood loss suggesting ciraparantag may have procoagulant effects, although this trend was not statistically significant. Ciraparantag required a 30:1 molar ratio (compared with 1:1 with andexanet), consistent with the difference in mechanism of action.
Preclinical Human Blood In Vitro Studies
Rivaroxaban or apixaban was added to plasma from healthy human volunteers at 1 and 2 times the therapeutic maximum concentration.39 The effect of ciraparantag on anti-FXa activity was measured by a chromogenic assay, and rivaroxaban and apixaban anti-FXa activity were completely reversed in a dose-dependent manner.
Clinical Studies
Forty healthy subjects were given enoxaparin 1.5 mg/kg, and 4 hours later they received placebo or 100 mg, 200 mg, or 300 mg of ciraparantag, and whole blood clotting times were measured.43 Of note, enoxaparin increased whole blood clotting time by 28.5% ± 3.3%. Ciraparantag completely reversed the increase in whole blood clotting time within 20 minutes in subjects receiving 100 mg and within 5 minutes with the 200-mg dose, and the effect was sustained for 24 hours. There was no signal for hypercoagulability as measured by D-dimer, F1.2, or tissue factor pathway inhibitor.
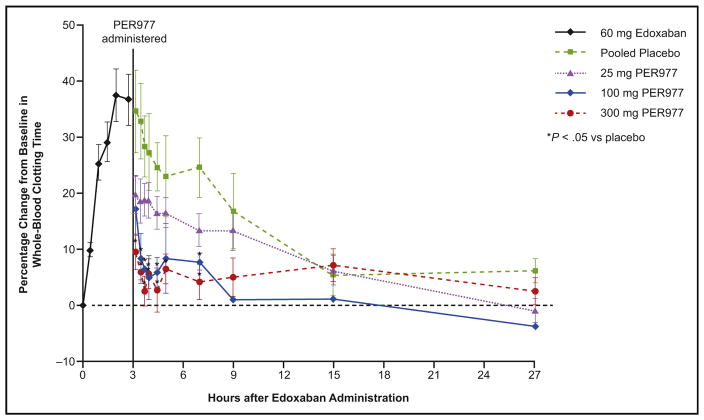
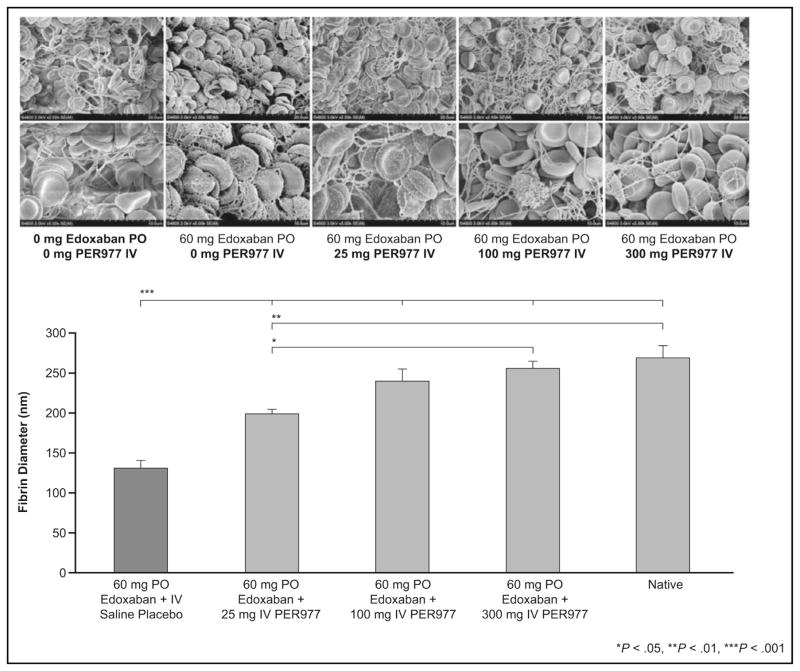
In a dose-escalation study, 80 healthy volunteers were divided into 8 cohorts, each with a different dose (5, 15, 25 [2 cohorts], 50, 100, 200, or 300 mg of ciraparantag).44 All patients received 60 mg of edoxaban and were randomized in an 8:2 ratio to ciraparantag or placebo 3 hours after edoxaban administration. Edoxaban increased whole blood clotting time by 37% over baseline, and ciraparantag 100 mg and 300 mg reduced this to within 10% of baseline within 10 minutes, and the effect persisted for 24 hours (Figure 4). Subjects receiving placebo required approximately 12 to 15 hours to decrease whole blood clotting times to within 10% of baseline. Mean fibrin–fiber diameter was determined from analyzing scanning electron micrographs of clots using a computer algorithm (Figure 5). Edoxaban decreased the mean fibrin–fiber diameter from approximately 250 nm to 125 nm, and the diameter was restored within 30 minutes of ciraparantag doses that had been shown to reverse elevation in whole blood clotting times. There was no evidence of hypercoagulability as measured by changes in D-dimer, F1.2, tissue factor pathway inhibitor, or whole blood clotting time. Potentially ciraparantag-related adverse reactions included transient mild perioral and facial flushing, dysgeusia, and headache. One subject had moderate muscle cramping and elevated post-treatment creatinine phosphokinase levels, but these events were not considered to be related to the study drug.
Figure 4.
Effect of PER977 (ciraparantag) on whole-blood clotting time. Shown are the mean whole blood clotting times after administration of a single oral 60-mg dose of edoxaban, followed 3 hours later by a single intravenous dose of 25 mg, 100 mg, or 300 mg PER977 (ciraparantag) or placebo. Reprinted with permission from Massachusetts Medical Society.44
Figure 5.
Dose-dependent normalization of fibrin diameter with ciraparantag (PER977) in human volunteers treated with edoxaban. Top: Clot fibrin structure pre- and post-ciraparantag (upper and lower photographs represent lower and higher magnification). Bottom: Computer algorithm-based quantification of clot fibrin diameter. IV = intravenous; PO = oral. Reprinted with permission from Massachusetts Medical Society.44
Ciraparantag is being developed as a “universal” anticoagulant reversal agent to block the anticoagulant effect of unfractionated heparin, LMWH, fondaparinux, and the new direct oral anticoagulants (Table 1), and the FDA has granted fast-track designation to its development.49
CONCLUSIONS
At the present time, there are no specific reversal agents available for the direct oral FXa inhibitors. Emerging data from early-phase studies of andexanet alfa and ciraparantag are encouraging, and phase 3 studies in patients who require reversal for significant bleeding or urgent invasive procedure/surgery are eagerly anticipated. Approximately 50 years elapsed before there were randomized controlled trial data demonstrating the efficacy and safety of a rapid onset reversal agent for warfarin. Current developments are encouraging that patients taking oral anti-FXa anticoagulants will not have to wait as long.
Acknowledgments
Funding: This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). Editorial support was provided by Daniella Babu, PhD, of Envision Scientific Solutions, which was contracted and funded by BIPI. The authors received no direct compensation related to the development of the manuscript.
Footnotes
Conflict of Interest: TJM is a member of the executive committee for the ANNEXA-4 trial, for which he receives consulting income from the Public Health Research Institute at McMaster University; has served as a consultant for CSL Behring; and has participated in the Speakers’ Bureau for CSL Behring, Boehringer Ingelheim, and Janssen. SK reports personal fees from Janssen, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, CSL Behring, Portola, and Daiichi Sankyo.
Authorship: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors were responsible for all content and editorial decisions, were involved at all stages of manuscript development, and approved the final version. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
References
- 1.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 2.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 3.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 4.Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 6.Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 7.Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 8.Sarich TC, Seltzer JH, Berkowitz SD, et al. Novel oral anticoagulants and reversal agents: considerations for clinical development. Am Heart J. 2015;169:751–757. doi: 10.1016/j.ahj.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Bristol-Myers Squibb. [Accessed February 23, 2016];Coumadin (warfarin sodium) prescribing information. 2015 Available at: http://packageinserts.bms.com/pi/pi_coumadin.pdf.
- 10.Sarode R, Milling TJ, Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–1243. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein JN, Refaai MA, Milling TJ, Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385:2077–2087. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CSL Behring GmbH. [Accessed February 23, 2016];Kcentra (prothrombin complex concentrate [human]) 2014 Available at: http://labeling.cslbehring.com/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf.
- 13.Janssen Pharmaceuticals, Inc. [Accessed February 23, 2016];Xarelto (rivaroxaban) prescribing information. 2015 Available at: https://www.xarelto-us.com/shared/product/xarelto/prescribing-information.pdf.
- 14.Bristol-Myers Squibb Company. [Accessed February 23, 2016];Eliquis (apixaban) prescribing information. 2015 Available at: http://packageinserts.bms.com/pi/pi_eliquis.pdf.
- 15.Daiichi Sankyo, Inc. [Accessed February 23, 2016];Savaysa (edoxaban) prescribing information. 2015 Available at: http://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true.
- 16.Huisman M, Fanikos J. Idarucizumab and factor xa reversal agents: role in hospital guidelines and protocols. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Levy JH. Discontinuation and management of direct-acting anticoagulants for emerging procedures. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Reilly P, van Ryn J, Grottke O, Glund S, Stangier J. Idarucizumab, a specific reversal agent for dabigatran: mode of action, pharmacokinetics and pharmacodynamics, and safety and efficacy in phase 1 subjects. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Eikelboom J, Merli G. Bleeding with direct oral anticoagulants versus warfarin: clinical experience. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–451. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 21.Lu G, Lin J, Curnutte JT, Conley PB. Reversal of heparin-induced anticoagulation by andexanet alfa, a universal antidote for factor Xa inhibitors. Proceedings of the 57th Annual Meeting & Exposition of the American Society of Hematology; December 5–8, 2015; Orlando, FL. 2015. [Accessed February 23, 2016]. Available at: https://ash.confex.com/ash/2015/webprogram/Paper81760.html. [Google Scholar]
- 22.Crowther M, Lu G, Conley PB, et al. Reversal of factor Xa inhibitors-induced anticoagulation in healthy subjects by andexanet alfa. Crit Care Med. 2014;42(Suppl):A1469. Abstract 455. [Google Scholar]
- 23.Sullivan DW, Jr, Gad SC, Laulicht B, Bakhru S, Steiner S. Nonclinical safety assessment of PER977: a small molecule reversal agent for new oral anticoagulants and heparins. Int J Toxicol. 2015;34:308–317. doi: 10.1177/1091581815590667. [DOI] [PubMed] [Google Scholar]
- 24.Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–2424. doi: 10.1056/NEJMoa1510991. [DOI] [PubMed] [Google Scholar]
- 25.Hollenbach SJ, Lu G, Deguzman F, Curnutte J, Conley PB, Sinha U. Bolus administration of PRT064445, a recombinant factor Xa inhibitor antidote, reverses blood loss and PD markers in a rat model following enoxaparin-induced anticoagulation. Eur Heart J. 2012;33(Suppl 1) Abstract P1857. [Google Scholar]
- 26.Lu G, Deguzman FR, Karbarz MJ, et al. Reversal of rivaroxaban mediated anticoagulation in animal models by a recombinant antidote protein (r-Antidote, PRT064445) Eur Heart J. 2011;32(Suppl 1):640–641. Abstract 3715. [Google Scholar]
- 27.Pine P, Hollenbach SJ, DeGuzman F, et al. Andexanet alfa but not four-factor prothrombin complex concentrate reverses rivaroxaban-induced anticoagulation as measured by reduction in blood loss in a rabbit liver laceration model. J Thromb Haemost. 2015;13(Suppl 2):216–217. Abstract A18218. [Google Scholar]
- 28.Lu G, Kotha J, Cardenas JM, et al. In vitro characterization of andexanet alfa (PRT064445), a specific fXa inhibitor antidote versus aripazine (PER977), a nonspecific reversal agent. Circulation. 2014;130 Abstract A18218. [Google Scholar]
- 29.Crowther M, Kitt M, McClure M, et al. Randomized, double-blind, placebo-controlled single ascending dose pharmacokinetic and pharmacodynamic study of PRT064445, a universal antidote for factor Xa inhibitors. Arterioscler Thromb Vasc Biol. 2013;33 Abstract 10. [Google Scholar]
- 30.Crowther M, Vandana M, Michael K, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of rivaroxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), an antidote for FXa inhibitors. Blood. 2013;122 Abstract 3636. [Google Scholar]
- 31.Crowther M, Lu G, Conley P, et al. Sustained reversal of apixaban anticoagulation with andexanet alfa using a bolus plus infusion regimen in a phase 2 placebo controlled trial. Eur Heart J. 2014;35 Abstract P738. [Google Scholar]
- 32.Crowther M, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Blood. 2014;124 Abstract 4269. [Google Scholar]
- 33.Portola Pharmaceuticals, Inc. [Accessed November 20, 2015];Portola Pharmaceuticals receives breakthrough therapy designation from FDA for andexanet alfa (PRT4445*), investigational factor Xa inhibitor antidote: only agent that has demonstrated clinical reversal of anti-Xa activity of factor Xa inhibitors. 2013 Available at: http://investors.portola.com/phoenix.zhtml?c=198136&p=irol-newsroomArticle&ID=1879666.
- 34.Portola Pharmaceuticals, Inc. [Accessed February 23, 2016];Portola Pharmaceuticals announces first phase 2 results demonstrating extended duration infusion with andexanet alfa (PRT4445*) provides prolonged reversal of anticoagulation activity of factor Xa inhibitor Eliquis(R): rapid and nearly complete reversal of anticoagulation effect of Eliquis(R) (apixaban) sustained for duration of infusion. 2013 Available at: http://investors.portola.com/phoenix.zhtml?c=198136&p=irol-newsArticle&ID=1864273.
- 35.Crowther M, Kitt M, Lorenz T, et al. A phase 2 randomized, double-blind, placebo-controlled trial of PRT064445, a novel, universal antidote for direct and indirect factor Xa inhibitors. Thromb Haemost. 2013;11 Abstract AS 20.21. [Google Scholar]
- 36.U.S. National Institutes of Health-ClinicalTrials.gov. [Accessed February 23, 2016];A study in patients with acute major bleeding to evaluate the ability of andexanet alfa to reverse the anticoagulation effect of direct and indirect oral anticoagulants ( NCT02329327) 2014 Available at: https://clinicaltrials.gov/ct2/show/NCT02329327.
- 37.Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 38.Portola Pharmaceuticals, Inc. [Accessed February 23, 2016];Corporate update. 2016 Available at: http://investors.portola.com/phoenix.zhtml?c=198136&p=irol-eventDetails&EventId=5214445.
- 39.Laulicht B, Bakhru S, Lee C, et al. Small molecule antidote for anticoagulants. Circulation. 2012;126 Abstract A11395. [Google Scholar]
- 40.Laulicht B, Bakhru S, Jiang X, et al. Antidote for new oral anticoagulants: mechanism of action and binding specificity of PER977. Thromb Haemost. 2013;11 Abstract AS 47.41. [Google Scholar]
- 41.Costin J, Ansell J, Laulicht B, Bakhru S, Steiner S. Reversal agents in development for the new oral anticoagulants. Postgrad Med. 2014;126:19–24. doi: 10.3810/pgm.2014.11.2829. [DOI] [PubMed] [Google Scholar]
- 42.Bakhru S, Laulicht B, Noveck R, et al. A synthetic small molecule which reverses overdosage and bleeding by the new oral anticoagulants. Circulation. 2013;128 Abstract 18809. [Google Scholar]
- 43.Costin J, Laulicht B, Bakhru S, Steiner S. PER977 reverses low molecular weight heparin in addition to IIa and Xa new oral anticoagulants. J Am Coll Cardiol. 2015;65:A2056. Abstract. [Google Scholar]
- 44.Ansell JE, Bakhru SH, Laulicht BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014;371:2141–2142. doi: 10.1056/NEJMc1411800. [DOI] [PubMed] [Google Scholar]
- 45.Bakhru S, Laulicht B, Jiang X, et al. Reversal of anticoagulant-induced bleeding in external and internal bleeding models by PER977, a small molecule anticoagulant antidote. Circulation. 2014;130 Abstract 19361. [Google Scholar]
- 46.Bakhru S, Laulicht B, Jiang X, et al. Reversal of anticoagulant-induced bleeding in external and internal bleeding models by PER977, a small molecule anticoagulant antidote. [Accessed November 9, 2015];2014 Poster 19361. Available at: http://perosphere.com/content/media/documents/PerosphereAHAPosterNovember2014.pdf.
- 47.Hollenbach S, Lu G, DeGuzman F, et al. Andexanet-alfa and PER977 (Arapazine) correct blood loss in a rabbit liver laceration model - only andexanet reverses markers of fXa-mediated anticoagulation. Circulation. 2014;130:A14657. :Abstract. [Google Scholar]
- 48.Bakhru S, Laulicht B, Jiang X, et al. A synthetic small molecule antidote for anticoagulants. Eur Heart J. 2013;34(Suppl):188–189. Abstract 1078. [Google Scholar]
- 49.Perosphere, Inc. [Accessed November 9, 2015];Perosphere receives FDA fast track designation for investigational anticoagulant reversal agent PER977. 2015 Available at: http://perosphere.com/documents/PerosphereFDAFastTrack.pdf.