Abstract
Mitochondria play an important role in tissue ischemia and reperfusion (IR) injury, with energetic failure and the opening of the mitochondrial permeability transition pore being the major causes of IR-induced cell death. Thus, mitochondria are an appropriate focus for strategies to protect against IR injury. Two widely studied paradigms of IR protection, particularly in the field of cardiac IR, are ischemic preconditioning (IPC) and volatile anesthetic preconditioning (APC). While the molecular mechanisms recruited by these protective paradigms are not fully elucidated, a commonality is the involvement of mitochondrial K+ channel opening. In the case of IPC, research has focused on a mitochondrial ATP-sensitive K+ channel (mitoKATP), but, despite recent progress, the molecular identity of this channel remains a subject of contention. In the case of APC, early research suggested the existence of a mitochondrial large-conductance K+ (BK, big conductance of potassium) channel encoded by the Kcnma1 gene, although more recent work has shown that the channel that underlies APC is in fact encoded by Kcnt2. In this review, we discuss both the pharmacologic and genetic evidence for the existence and identity of mitochondrial K+ channels, and the role of these channels both in IR protection and in regulating normal mitochondrial function.
Ischemia–reperfusion injury and protection
Ischemia, defined as the blockage of delivery of oxygen and nutrients to tissues, is a pathologic event that underlies some of the most prevalent causes of death in humans. Paradoxically reperfusion (i.e., the re-establishment of oxygen and nutrient delivery) is also a pathologic event. Taken together, these events comprise ischemia–reperfusion (IR) injury, the underlying cause of diverse conditions such as heart attack and stroke. The focus of this review is cardiac IR; in the US alone, there are 750 000 heart attacks a year, killing 116 000 people. In addition, over 300 000 patients undergo a ‘scheduled’ cardiac ischemic event when the heart is arrested and placed on bypass during open heart surgery [1]. Since cardiac IR injury is a major cause of mortality and morbidity, it is surprising that beyond reperfusion itself (e.g., thrombolysis or balloon angioplasty), there are virtually no drug therapies to acutely treat it [2,3].
The heart is an energetically demanding tissue, with the bulk of its ATP demand met by mitochondrial oxidative phosphorylation [4,5]. Upon ischemia, mitochondrial ATP synthesis halts, starving processes such as actin/myosin cross-bridge cycling and the maintenance of ion gradients by the Na+/K+-ATPase and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). In addition, glycolytic metabolism generates lactate, causing cellular acidosis which then activates the Na+/H+ exchanger and leads to a rise in intracellular Na+ [6,7]. Na+ export is driven by the Na+/Ca2+ exchanger, leading to a rise in cytosolic Ca2+ [8], which is compounded by the ATP-starved SERCA pump [9,10]. A cytosolic Ca2+ overload ensues, with some Ca2+ entering the mitochondrion. However, these events alone are insufficient to trigger opening of the mitochondrial permeability transition (PT) pore, since acidic pH and a reduced pyridine nucleotide pool (NADH) maintain the PT pore in a closed state [11–13]. At reperfusion, further Ca2+ overload occurs [14,15], pH rebounds [16], and a burst of reactive oxygen species (ROS) generation occurs as metabolites accumulated during ischemia are rapidly oxidized [17]. This combination of Ca2+, pH and ROS triggers opening of the PT pore, leading to cell death [18–25]. These events are summarized in Figure 1.
Figure 1. Schematic representation of pathologic events during ischemia and reperfusion.
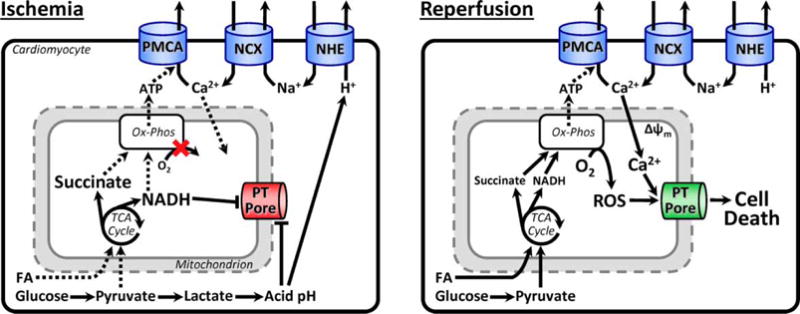
Key events are listed below each figure and described in detail in the text (Section ‘Ischemia-–Reperfusion Injury and Protection’). PMCA, plasma membrane Ca2+-ATPase; NCX, Na+/Ca2+ exchanger; NHE, Na+/H+ exchanger; FA, fatty acids; PT, permeability transition pore.
Given the universality of IR injury as a pathologic insult in biology, it should not be surprising that a diverse array of organisms [26–31] exhibit mechanisms to limit damage due to this insult. Among the best studied of such mechanisms is ischemic preconditioning (IPC), in which short periods of prior IR afford protection against subsequent IR injury. IPC is an example of hormesis (i.e., ‘what doesn’t kill you makes you stronger’) and is clinically applicable in humans [32]. IPC affords protection in two phases: the first develops in minutes, lasts 2–3 h, and involves cell signaling cascades that terminate at mitochondria [4], as will be discussed here. A second protective phase develops in ~24 h and lasts up to 72 h, requiring gene transcription and de novo protein synthesis [33], but will not be considered further. Of particular interest for this review, it is also known that halogenated volatile anesthetics (halothane, isoflurane, sevoflurane, and desflurane) can mimic the protection afforded by IPC, a process known as anesthetic preconditioning (APC) [34–36].
The centrality of mitochondria to IR pathology has driven the organelle to be a natural focus for research on IR protection. In this regard, a common mechanism believed to underlie several cardioprotective paradigms, including IPC and APC, is the opening of potassium channels in the mitochondrial inner membrane [37–42]. This review will focus on the evidence for existence and identity of these channels.
Mitochondrial K+ homeostasis and discovery of mitochondrial K+ channels
A note on nomenclature
Before discussing these channels in detail, nomenclature should be clarified. The gene encoding the mitochondrial ATP-sensitive K+ (KATP) channel is unknown, and so here we use the term ‘mitoKATP’. For other K+ channels, where possible the International Union of Basic and Clinical Pharmacology (IUPHAR) nomenclature is used [43] (see the Abbreviations list); however, there are many alternative names commonly found in the literature, described here.
The term ‘BK’ was coined in 1984 when a ‘big K+’ or large-conductance K+ channel activated by Ca2+ was recorded by patch clamp [44]. In 1986, the Drosophila slowpoke (Slo) mutation was shown to abolish a Ca2+-activated K+ current [45], and subsequently, the ‘Slo1’ gene was shown to be conserved among phyla. Hence, BK (also known as ‘maxi-K’) was the term used to describe the channel encoded by the Slo1 gene. This gene is now known as Kcnma1, and the channel is known as as KCa1.1.
A related family of Slo genes has since been identified, which now includes Slo1 (Kcnma1), Slo2.1 (Kcnt2), Slo2.2 (Kcnt1), and Slo3 (Kcnu) [46–52]. Kcnma1 and Kcnu encode Ca2+-activated K+ (KCa) channels (now known as KCa1.1 and KCa5.1, respectively). Lower organisms such as Caenorhabditis elegans have a single gene (termed ‘Slo2’) encoding a KCa channel [53], and here, we use the name SLO2 to refer to this channel in C. elegans. In contrast, this gene has diverged into two paralogs in mammals, and somewhat confusingly, the mammalian channels are, in fact, Na+-activated (KNa) channels: the gene previously known as Slo2.1 was thought to encode a channel termed KCa4.2 (also known as ‘Slick’). This gene is now known as Kcnt2 and encodes a channel known as KNa1.2. The gene previously known as Slo2.2 was thought to encode a channel termed KCa4.1 (also known as ‘Slack’). This gene is now known as Kcnt1 and encodes a channel known as KNa1.1. The umbrella term ‘KNa1.x’ is used here to refer to both mammalian KNa1.2 and KNa1.1 channels. Additional naming complexity is also imparted due to the channels encoded by the Slo family genes having alternate splice variants that can heteromultimerize [46,47,50,54,55] (see Sections ‘Mitochondrial KCa1.1 Channels and Mitochondrial KNa1.x Channels’).
The general term ‘KCa’ refers to the small-conductance (SK) channels, the intermediate-conductance (IK) channels [56], and the channel encoded by Slo1 (Kcnma1). Unfortunately, ‘KCa’ is also sometimes used to reference all channels encoded by the Slo gene family, even though it is now apparent that many of these are KNa channels (see above).
In the mitochondrial field, the terms ‘mitoBK’ and ‘mitoKCa’ (and sometimes even ‘mitoBKCa’!) have been used interchangeably. Wherever possible, we attempt to define these channels using the IUPHAR nomenclature. However, many studies have assigned channel names on the basis of pharmacology alone, prior to the advent of molecular biological identification. Therefore, in such cases, we default to the nomenclature system used by the authors of these studies.
Mitochondrial K+ channels
The mitochondrial inner membrane, while maintaining a tight barrier to proton permeability that is essential for its bioenergetic function, is also selectively permeable to numerous cations (K+, Ca2+, Mg2+, and Na+) [57–59] and anions (Cl−, PO4−, nucleotide phosphates, and di- and tri-carboxylates) [60]. This selective permeability is under the control of membrane transporters, and here we focus on those ion channels mechanistically linked to IR protection — namely, the mitoKATP channel and the K+ channels encoded by the Slo gene family. Other mitochondrial K+ transport proteins [e.g., the K+/H+ exchanger (KHE) and voltage-gated K+ channels] are reviewed extensively elsewhere [61–63].
Mitochondrial K+ permeability has been studied since the early days of bioenergetics [64–66]. K+ entry into mitochondria is accompanied by osmotically obliged water, resulting in swelling and a decreased refractive index [67], making mitochondrial volume (easily measured spectrophotometrically as the scattering of light by isolated mitochondrial suspensions) a useful surrogate measure of K+ uptake [68–70]. Energetically driven swelling in K+-containing buffers was initially attributed to an inherent K+ permeability of the mitochondrial membrane [71,72], and subsequent studies identified an electrically neutral KHE supporting the existence of a mitochondrial K+ cycle [73] (Figure 2). The fact that both K+ influx and efflux consume the transmembrane H+ gradient suggests the functional importance of the cycle, and it has been suggested that the cycle serves to regulate mitochondrial volume [74], which in turn may be an important regulator of respiratory function [75,76]. Alternatively, it has been proposed that mitochondrial K+ homeostasis serves to regulate ROS production [77,78].
Figure 2. Mitochondrial K+ cycle.
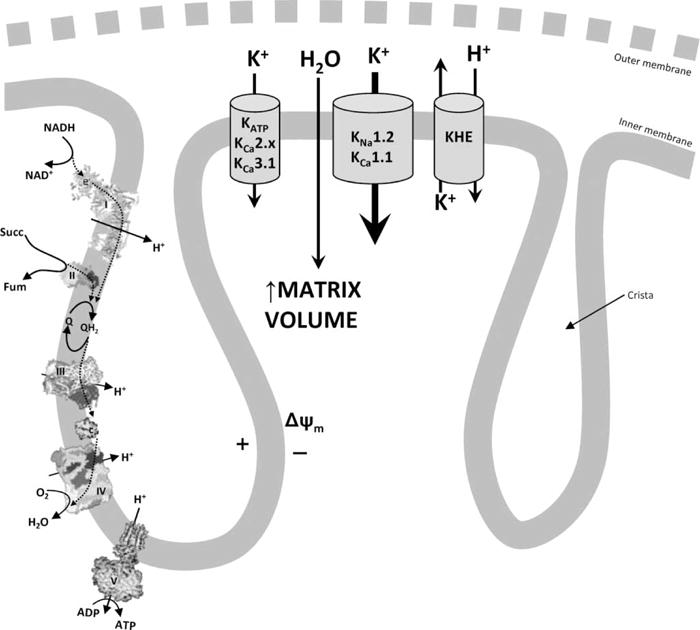
The mitochondrial inner membrane potential (Δψm), which is generated by the respiratory chain (complexes I–IV [396,397], left), drives K+ entry into the mitochondrial matrix through either small- or intermediate-conductance channels (e.g., KATP, KCa2.x, or KCa3.1) or large-conductance channels (KNa1.2 or KCa1.1). This K+ current is followed by osmotically obliged water, resulting in swelling of the matrix. K+ is removed from the matrix through the KHE that also consumes Δψm. The outer membrane is largely permeant to all solutes and hence is depicted as a dotted line.
The first report of a bona fide mitochondrial K+ channel by patch-clamp electrophysiology was in 1991 [79] and opened the way for identification of K+ currents attributable to known K+ channel families based on electrophysiological properties. Numerous K+ channels have now been reported in mitochondria, including KV1.3 in lymphocyte mitochondria [80], a KCa channel in liver [81] and fibroblast [82] mitochondria, and K+ATP in mitochondria from glioma [79] and cardiac ventricles [83]. Additional methods supporting mitochondrial K+ channel identity include the following: (i) immunologic detection such as western blot [84] and fluorescent immunocytochemistry [85]. (ii) Mitochondrial fractionation and reconstitution of channels into liposomes [86]. (iii) Indirect measurement of mitochondrial K+ uptake by fluorescent probes such as potassium binding fluorescent indicator [87] or fluorescent measurement of mitochondrial Tl+ uptake as a surrogate for K+ flux [68]. (iv) Genetic tagging of candidate K+ channel proteins and their tracking to mitochondria within cells [88]. (v) Sensitivity of these measurements to a variety of pharmacologic agents that are known to act on particular classes of K+ channel (see Sections ‘Mitochondrial KATP Channel: Composition, Pharmacology, Regulation, Role in IR Protection; Mitochondrial KCa2.x and KCa3.1 Channels: Composition, Pharmacology, Regulation, Role in IR Protection; and Mitochondrial KCa1.1 Channels and Mitochondrial KNa1.x Channels’). (vi) Generation of mice or cell lines with candidate mitochondrial K+ channel genes deleted [89–91].
While these considerable efforts support the existence of bona fide K+ channels in mitochondria, their molecular identities are still hotly debated. In particular, the mitoKATP channel is controversial [92–95]. This topic has been extensively reviewed elsewhere and so will be discussed only briefly here in the Section ‘Mitochondrial KATP Channel: Composition, Pharmacology, Regulation, Role in IR Protection’. The identity of a mitochondrial large-conductance K+ (BK) channel is also unclear [42,96–98] and is discussed in detail in Sections ‘Mitochondrial KCa2.x and KCa3.1 Channels: Composition, Pharmacology, Regulation, Role in IR Protection and Mitochondrial KCa1.1 Channels and Mitochondrial KNa1.x Channels’. For reference, a schematic of selected cardioprotective stimuli and their proposed target mitochondrial K+ channels, along with inhibitors of such cardioprotection, is shown in Figure 3.
Figure 3. Cardioprotective stimuli, molecular targets, and inhibitors.
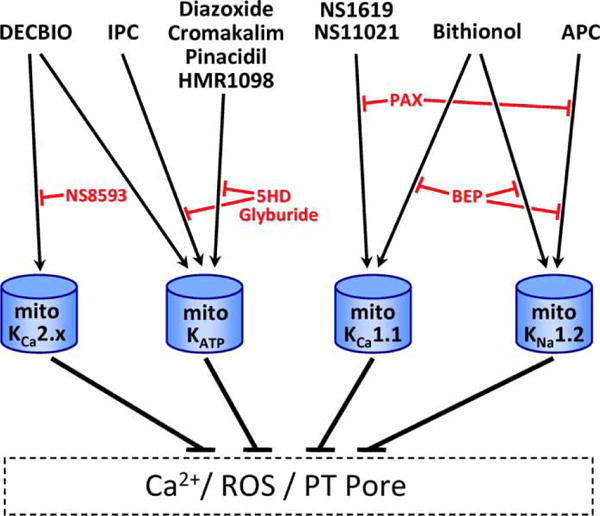
A subset of pharmacophores in Table 1 is known to confer cardioprotection. These are shown at the top of this figure, along with the cardioprotective stimuli of APC and IPC. Target mitochondrial K+ channels are depicted below (blue), with known pharmacologic inhibitors of these protective paradigms in red. As detailed in the text, a combination of genetic and pharmacologic information has demonstrated that KCa1.1 and KNa1.2 each participate in distinctly activated mechanisms of cardioprotection.
Mitochondrial KATP channel: composition, pharmacology, regulation, and role in IR protection
A study of the mitoKATP channel is incontrovertibly linked to the study of IR protection; much of the evidence for the existence of the channel comes from effects of channel-modulating drugs on cardiac IR injury, and much of the evidence for cardioprotection comes from the design and application of agents targeting putative mitoKATP channels. Thus, research into this channel’s composition or pharmacology is largely driven by its role in cardioprotection [99–101].
ATP-sensitive K+ channels (KATP) have been detected in numerous cell membranes including plasma membrane [102], sarco/endoplasmic reticulum [103], mitochondria [79], and the nuclear envelope [104]. Generally, KATP channels are octamers composed of four 2-transmembrane inward-rectifying K+ channel (KIR) subunits (KIR6.1/6.2), plus four 17-transmembrane sulfonylurea receptor (SUR) subunits (SUR1/2A/2B) [105]. The ventricular myocyte surface KATP channel comprises KIR6.2/SUR2A [106] and regulates both cell volume and action potential duration [107].
Initial observations suggested a role for surface KATP channels in IPC [108–110], with depressed contractility thought to be the mechanism of cardioprotection [107,111,112]. However, the discovery that KATP activators [diazoxide (DZX) [113], cromakalim [114], and aprikalim [115]] were capable of protecting noncontracting myocytes, in a manner blocked by KATP inhibitors [5-hydroxydecanoate (5-HD), glyburide, and HMR1098 [116–118]], suggested a protective mechanism independent of depressed contractility. The discovery of a mitochondrial KATP channel with sensitivity to DZX and cromakalim [79,83,119] provided a candidate mechanism, with further support provided by evidence that cardiac surface KATP channels are insensitive to DZX [120–123]. Subsequently, a channel with pharmacologic properties ascribed to mitoKATP was recognized as a key player in IPC signaling, despite an ongoing debate regarding the molecular identity of this channel. Weak evidence also exists for a potential role of a mitochondrial KCa channel in IPC [124,125] and will be discussed in Section ‘Mitochondrial KCa1.1 Channels and Mitochondrial KNa1.x Channels’.
Although global knockout mice exist for KATP channel subunits (i.e., KIR6.1/6.2 and SUR1/2), several complications preclude their use to study IPC. For example, Kir6.1−/− mice [126] exhibit a form of angina, and it is known that patients with unstable angina exhibit a preconditioned phenotype [127,128]. While Kir6.2−/− mice [91,121] exhibit blunting of protection by IPC, these mice also have impaired insulin secretion and mild glucose intolerance [91], and it is known that diabetes abrogates protection by IPC [129]. Similarly, both Sur1−/− and Sur2−/− mice exhibit glycemic disturbances and are endogenously protected against cardiac IR injury [130–132], precluding their use to study cardioprotective signaling. Furthermore, the Sur1−/− mouse was demonstrated to still express alternate splice variants [133], again confounding studies attempting to assign functions to the SUR1 protein. Owing to these confounds, assignment of a particular combination of KIR/SUR subunits as underlying IPC has not been possible to date. In addition, evidence favoring a mitoKATP composition of the canonical KIR6/SUR proteins needs to be balanced against the discovery that the original antibodies used to identify these proteins in purified mitochondria recognize off-target proteins unrelated to K+ channel function [134]. Furthermore, although a smaller 55 kDa splice variant of SUR2A has been reported in mitochondria [135], this was detected using custom antibodies which failed to detect the same 55 kDa band in similar samples from the same laboratory a year earlier [133]. A subsequent study [136] also suggested rather equivocal evidence for the existence of this 55 kDa band, and overall caution should be used in interpreting any immunologic evidence for a mitoKATP channel.
The pharmacology of mitoKATP is conserved in humans [137], rats [79], plants [138], amoeba [139], trypanosomes [140], and C. elegans [141], and remains the default method to assign a role for mitoKATP in IR protection. A catalog of K+ channel pharmacophores applicable to mitochondrial research is given in Table 1. While many channel modulators are available, the two most commonly linked to mitoKATP are the channel-activating benzothiadiazine derivative DZX and the antagonist 5-HD [142]. We see that 10 μM DZX is a relatively specific mitoKATP agonist and mimics the protective effects of IPC [113,116], whereas 5-HD prevents both IPC- and DZX-mediated IR protection [143,144]. At concentrations >40 μM, DZX has several other mitochondrial effects (e.g., complex II inhibition and protonophoric activity [145,146]). The specificity of 5-HD has also been questioned, since it undergoes β-oxidation to yield 5-HD-CoA and other derivatives [147,148]. However, this compound is also effective within 1 s in mitoKATP assays, suggesting that such metabolism is irrelevant for its acute effects on the mitoKATP [149]. Additional KATP channel activators including cromakalim and pinacidil [150–153] (Table 1) have also been studied in the context of cardiac IR protection and are thought to elicit protective effects via the mitoKATP.
Table 1.
Commonly used pharmacologic agents in the fields of mitochondrial K+ channel research and cardioprotection, for KATP, KCa2.x, KCa1.1, and KNa1.x channels
| Channel | Actions | Drugs | EC50/IC50 | Refs |
|---|---|---|---|---|
| KATP | Activators | Atpenin A5 | 10 nM | [39] |
| Cromakalim | 1 μM | [113,114,398] | ||
| Diazoxide | 10 μM | [113] | ||
| Pinacidil | 50 μM | [115,399,400] | ||
| Inhibitors | Fluoxetine | 2.4 μm | [68] | |
| Glyburide | 50 μm | [113,119] | ||
| 5-HD | 100 μM | [116] | ||
| Quinine | 100 μM | [119] | ||
| HMR1098 | 100 μM | [119] | ||
| KCa2.x | Activator | DECBIO | 3 μm | [180] |
| Inhibitors | ChTx | 50 nM | [191] | |
| Apamin | 1 μM | [191] | ||
| Fluoxetine | 9 μM | [192] | ||
| NS8593 | 10 μM | [180] | ||
| KCa1.1 | Activators | Emodepside | 14 nM | [258] |
| Rottlerin | 500 nM | [238] | ||
| NS11021 | 500 nM | [242] | ||
| NS004 | 10 μM | [401] | ||
| NS1619 | 10 μM | [242] | ||
| 17-β Estradiol | 30 μM | [313] | ||
| Niflumic acid | 33 μM | [309] | ||
| Ethanol | 20 mM | [315] | ||
| Inhibitors | SloTx | 1.5 nm | [402] | |
| IbTx | 50 nM | [232] | ||
| Charybdotoxin | 200 nM | [403] | ||
| Paxilline | 1 μm | [42] | ||
| KNa1.x | Activators | Niclosamide | 2.9 μM | [317] |
| loxapine | 4.4 μM | [317] | ||
| 17-β Estradiol | 10 μM | [314] | ||
| Bithionol | 10 μM | [310] | ||
| Isoflurane | 300 μM | [307] | ||
| Niflumic acid | 2.1 mM | [294] | ||
| Inhibitors | Bepridil | 500 nM | [310] | |
| Pax | 1 μM | [97] | ||
| Verapimil | 100 μM | [291] | ||
| Quinidine | 100 μM | [308] | ||
| Clofilium | 109–331 μM | [290] |
A common feature that has arisen in the field of mitoKATP pharmacology is complex II of the mitochondrial respiratory chain [39,145,149,154–157]. In short, several compounds that open the mitoKATP channel are known to be complex II inhibitors, and in turn many complex II inhibitors have been discovered to open the channel. Among these, the most potent is the complex II inhibitor atpenin A5 (AA5), which is an effective mitoKATP activator at low nM concentrations and is cardioprotective in a manner blocked by 5-HD [39,157,158]. Additionally, a mitochondrial membrane fraction enriched in complex II, mitochondrial ATP-binding cassette protein 1, phosphate carrier, adenine nucleotide translocator, and ATP synthase was shown to have mitoKATP-like activity when reconstituted in lipid bilayers [86]. The exact nature of the relationship between complex II and the mitoKATP is reviewed extensively elsewhere [159]. Similarly, an interaction between complex IV and a paxilline-sensitive K+ channel has been reported in membranes isolated from brain mitochondria [160], although the identity of this channel is currently unclear.
Despite the assignment of IPC protection to a mitochondrial channel with KATP-like properties, the molecular identity of the channel remains unclear. Specifically, none of the KIR6.1/6.2 or SUR1/2A/2B proteins are known to contain mitochondrial targeting sequences [134,161–165]. Furthermore, although there are 14 KIR channel isoforms in mammals [105,166], the genetic model organism C. elegans contains only three such proteins (encoded by the irk-1,2,3 genes) [167]. In C. elegans with ablation of all three irk genes, no alteration in protection by hypoxic preconditioning or baseline sensitivity to hypoxic injury was seen [88]. Furthermore, mitochondria from these worms exhibited K+ channel activity with all of the pharmacologic properties of a KATP channel [88]. These data suggest that mitoKATP might not be a canonical KIR6/SUR channel.
Alternatively, another member of the Kir gene family, Kir1.1 (also known as renal outer medullary K+ channel, ROMK), has recently been proposed to encode a mitoKATP channel [95]. Specifically, ROMK variant 2 (ROMK2) contains an N-terminal mitochondrial localization sequence, and although its endogenous expression could only be detected by reverse-transcriptase polymerase chain reaction (RT-PCR), overexpression of recombinant ROMK2 fused to an epitope tag allowed immunodetection of its co-localization with mitochondrial markers. Recently, specific ROMK2 activators have been reported [168–170], but it is yet to be determined if these molecules can elicit protection against IR injury or activate a mitochondrial K+ flux. In addition, although a whole-body ROMK knockout exists [171,172], renal insufficiency and hypertension render this model unsuitable for cardiovascular studies such as IR injury, and as of the submission of the present study, a cardiac-specific ROMK knockout mouse has not been reported. Finally, the pharmacologic properties of the mitoKATP (e.g., sensitivity to DZX ATP, phosphadityl inositol bisphosphate (PIP2), and fluoxetine) do not match those reported for ROMK (see ref. [159]). It is also intriguing that a recent abstract [173] claims to have identified the mitoKATP channel as a previously unknown protein (i.e., not ROMK). Hence, it seems prudent to keep an open mind as to whether ROMK2 is the bona fide mitoKATP channel.
Mitochondrial KCa2.x and KCa3.1 channels: composition, pharmacology, regulation, and role in IR protection
There are four genes in the Kcnn family. Kcnn1, Kcnn2, and Kcnn3, respectively, code for the SK potassium channels with IUPHAR names KCa2.1, KCa2.2, and KCa2.3 [174], while the Kcnn4 gene encodes an IK potassium (IK) channel termed KCa3.1 [43]. The KCa3.1 channel has not been implicated in IR protection; however, it does have a role in post-ischemic cardiac remodeling [175,176] and Kcnn4−/− mice exhibit more damage in ischemic stroke [177]. The KCa2.x channels are expressed in atrial cells but not in ventricular tissue [178]. Their activation by DECBIO is protective against cardiac IR injury [179,180], and this protection is blocked by the KCa2.x antagonist NS8593. More recently, it has been claimed, on the basis of immunologic evidence, that the KCa2.x channels responsible for this cardioprotection are mitochondrial, and of the KCa2.2 and KCa2.3 variety [180], although similarity between these SK proteins precludes identification of the exact subtype.
KCa2.x channels are nominally activated by sub-micromolar Ca2+, co-ordinated by a calmodulin (CaM)-binding domain. Additional regulation of channel activity is also afforded by phosphorylation at the N- and C-termini [181–183]. These channels are also known to be activated by stimuli implicated in cardioprotection, such as 11,12-epoxyeicosatrienoic acid (EET) and NO· [184,185]. However, both of these species can elicit protection via pleiotropic mechanisms [186,187], including other KCa channels [188–190]. The KCa2.x channels are selectively blocked by apamin, and are also nonselectively blocked by charybdotoxin (ChTx) [191] and fluoxetine, which also target BK and KATP channels, respectively [157,192]. This overlapping pharmacology with BK and KATP channels should be taken into consideration when interpreting pharmacologic evidence for a mitochondrial or cardioprotective role of KCa2.x channels. Ultimately, the use of Kcnn1–4−/− mice [177,193,194] may be informative regarding the contribution of these channels to IR protection.
Mitochondrial KCa1.1 channels
KCa1.1 channels — composition
The KCa1.1 channel consists of a tetramer of Kcnma1 encoded pore-forming α subunits, each of which can be accompanied by a β subunit, with the entire complex also binding γ subunits. Each α subunit has seven trans-membrane helices, a voltage sensor, β/γ interaction domains, and a large cytosolic region containing two RCK (regulation of conductance of K+) domains which house the Ca2+ sensing ‘bowl’. The exon 19–23 region of Kcnma1 (between the RCK domains) can be alternatively spliced (Figure 4) [48,195–197], yielding isoforms termed ‘zero’ (no exons 19–23), ‘e20’ (IYF insert between 19 and 20) [198], ‘e21/STREX’ (59 AA insert in exon 21) [198–201], ‘e22’ (inclusion of exon 22) [198], ‘Δe23’ (loss of exon 23) [202,203], and ‘DEC’ (C-terminal splice variant) [204]. Notably, the DEC variant has been suggested to impart mitochondrial localization [204]; however, this moiety has also been observed to increase surface KCa1.1 expression in combination with a β4 subunit [205,206].
Figure 4. Venn diagram representing the various K+ channel channels, isoforms, and their subgroupings.
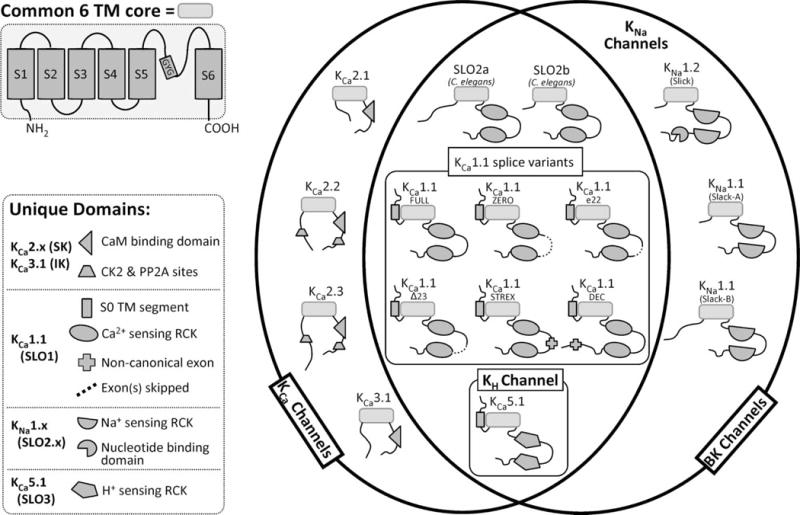
The 6-transmembrane (6TM) pore-forming core is represented as a rounded rectangle with the individual N- and C-termini and their corresponding unique exons/domains illustrated as darker shaded shapes (see key). As only gross structural motifs are depicted in this figure, specific differences in amino acid sequence (i.e., differences in charged residues in S4 between KCa1.1 and KNa1.x or unique sequences in KNa1.1 and KNa1.2 RCK domains) are not represented.
There are four genes encoding KCa1.1 β subunits (Kcnmb1–4) [207,208] and their expression is tissue-specific [208,209]. These proteins interact with KCa1.1-α, altering activity and drug sensitivity [209–214]. β1 and β2 both increase Ca2+ sensitivity [213–216], and β1 also slows activation/inactivation kinetics [213,217] and affects cellular localization [215]. β2 and some splice variants of β3 also contain globular N-terminal domains, which confer rapid ‘N-type inactivation’ to KCa1.1 currents [211,214,218–222], whereas β4 down-regulates channel activity [223]. Mitochondrial localization of the β subunits, particularly β1 [189], β3 [82], and β4 [189], has been reported; however, these reports are largely informed by co-immunoprecipitation of the β subunits with the α subunit from mitochondrial-enriched tissue preparations. Currently, there are no data demonstrating that the β subunits complex with the α subunits in mitochondria and affect the channels’ pharmacologic or electro-physiologic properties. KCa1.1 γ subunits are single transmembrane leucine-rich repeat proteins encoded by the Lrrc26 (γ1), Lrr52 (γ2), Lrr55 (γ3), and Lrr38 (γ4) genes. The γ subunit interaction with the extracellular face of KCa1.1-α lowers channel voltage sensitivity [224,225]. Although γ subunits are diversely expressed, to date none have been detected in the heart [226].
Knockout mice exist for the genes encoding several components of the KCa1.1 channel, including the Kcmna1 [90,227], Kcnmb1 [220], and Kcnmb4 [228]. These mice exhibit a variety of phenotypes including spontaneous death, motor dysfunction, circadian rhythm disruption, and vasoconstriction, although this has not precluded their use in studying the role of KCa1.1 in cardioprotection (see below). Functional insight has also been afforded by KCa1.1 channel crystal structures [229,230].
KCa1.1 channels — pharmacology and regulation
There are no drugs that distinguish KCa1.1-α splice variants, although β/γ subunits have been shown to affect KCa1.1 pharmacology. The peptide toxins ChTx, iberotoxin (IbTx), and slotoxin (SloTx) all occlude the pore on the outer face of the channel [231–233] and are useful for measuring surface KCa1.1 function, but their membrane impermeability renders them unsuitable for probing intracellular KCa1.1 activity. Sensitivity to ChTx is increased 20-fold by β1 [231], whereas sensitivity to SloTx is decreased by β1 or β4 [234], and inhibition by ChTx or IbTx is lost in the KCa1.1-α/β4 composition [235,236]. Although the molecule rottlerin (also known as mallotoxin, historically thought to be a PKC inhibitor [237]) is known to activate KCa1.1 [238], γ1 subunit-containing channels are resistant to such activation [239,240]. The small-molecule paxilline is reportedly a membrane-permeable KCa1.1 blocker, but its specificity has been questioned by the reported efficacy in Kcnma1−/− mice [97]. The neurosearch (NS) class of compounds (NS004, NS1619, and NS11021) was developed as KCa1.1-specific activators [241,242] and is cardioprotective (Figure 2 and Table 1) [96,243–245], but also exhibits multiple KCa1.1-independent effects [246–253]. This includes inhibition of SERCA [254], L-type Ca2+ channels [246], Ca2+-activated Cl− and Na+ channels [247], and CaV channels [248,255,256]. In addition, these drugs activate KV7.4 and nAchR α7, and are known to have multiple effects on mitochondrial function [98,252,257]. Finally, emodepside has been reported to activate KCa1.1 at nM concentrations [258,259]. Overall consensus is that peptide-based KCa1.1 modulators are specific but of limited in situ utility, whereas current small-molecule KCa1.1 modulators are less specific but more useful in a variety of cell, organ, or in vivo settings.
In addition to direct pharmacology, many signaling pathways are known to endogenously regulate KCa1.1 channels in a cellular context. These include arachidonic acid metabolism (i.e., EETs) [189,260], NO· metabolism [261,262], pH [263], Zn2+ homeostasis [264], phosphorylation by PKA, PKG, PKC, and CaMKII [265–268], and palmitoylation and myristoylation [269–271]. Unfortunately, given the enormous scope of this subject, full discussion of these signaling pathways and their context in IR injury and protection is not possible in the current review. Finally, given that KCa1.1 is a KCa channel, it should be noted that modulation of intracellular Ca2+ by other Ca2+ channels (e.g., ryanodine and IP3 receptors) can also affect its activity [272–275].
Mitochondrial KCa1.1 channels — role in IR protection
The first report of a mitochondrial large-conductance (295pS) K+ channel employing patch clamp of glial cell mitochondrial inner membranes [81] showed that the channel was activated by Ca2+ (0.1–1 μM) and voltage, and blocked by ChTx. A large-conductance K+ (BK) channel opened by NS1619 was also found in isolated liver mitochondria, and this compound was protective in a rabbit heart model of IR injury, in a manner blocked by paxilline [42]. Further work confirmed the cardioprotective nature of the NS compounds and also reported on a potential role for a mitochondrial BK channel in cardioprotection by volatile APC [74,97,242]. Specifically, the KCa1.1 antagonist IbTx was reported to block volatile anesthetic or NS protection in a model of ischemic postconditioning [276]. These studies assigned a variety of names to the mitochondrial BK channel including BK, KCa, and BKCa (see Section ‘Mitochondrial K+ Homeostasis and Discovery of Mitochondrial K+ Channels’), although a specific gene or protein name was conspicuously absent. Notably, cardioprotection induced by the KCa1.1-activating NS compounds was not blocked by the mitoKATP antagonist 5-HD [125]. In addition, the mitochondrial BK channel itself was found to be insensitive to DZX and 5-HD [277], suggesting that this channel is distinct from mitoKATP. Later, immunologic studies documented KCa1.1 expression in the heart [196,198,207], and it was found that KCa1.1 channels are not expressed at the myocyte plasma membrane or sarcoplasmic reticulum membrane [42,277]. Furthermore, KCa1.1 was found specifically in mitochondrial membranes [42,81,204,278,279], including the presence of the β1 subunit [41,42,278,280,281]. Together, these studies suggested the existence of a bona fide KCa1.1 channel in the mitochondrial inner membrane, with a proposed role in mediating the cardioprotective effects of APC.
Despite these early studies, the case for a role of KCa1.1 in cardioprotection by APC has previously been challenged by our finding that APC protection was intact in Kcnma1−/− hearts [97]. Thus, KCa1.1 is dispensable for APC. Furthermore, as detailed above, the specificity of the NS compounds for KCa1.1 has been repeatedly questioned [119,246–253,257]. For example, the PKA inhibitor H-89 blocks APC protection [282], but does not block protection afforded by NS1619 [41], thus suggesting that NS compounds and APC protect via different mechanisms. In general, the NS compounds are unsuitable for drawing conclusions about the molecular identity of channels that underlie APC.
This concept is further illustrated by the finding that while IR protection by NS compounds was absent from Kcnma1−/− hearts, it was still present in Kcnma1−/− cardiomyocytes. This suggests that a KCa1.1-independent side effect of the NS compounds was responsible for their protective effects in the isolated cell system [98], but for poorly understood reasons this KCa1.1-independent side effect was not able to be recruited for protective benefit in the intact heart. Further experimentation revealed a role for KCa1.1 channels within intrinsic cardiac neurons (where KCa1.1 is known to be expressed [283,284]) in mediating the protective effects of NS compounds in the intact heart [98]. Overall, it is suggested that APC cardioprotection does not require KCa1.1, whereas NS cardioprotection requires KCa1.1 in a noncardiomyocyte cell (probably cardiac neurons). It should be noted that our data do not preclude the possibility that a mitochondrially localized KCa1.1 channel exists and may indeed be a viable drug target to induce cardioprotection. However, such a channel has no role in APC and cannot be inferred from the use of NS compounds.
Finally, although the use of nonspecific KCa channel inhibitors has led to suggestions of a potential role for mitochondrial KCa1.1 channel in IPC [124,125], such a case is refuted by our finding that protection induced by IPC was completely intact in Kcnma1−/− hearts [98]. Thus, without a defined role in IPC or APC, any potential cardioprotective effects of a mitochondrial KCa1.1 channel are limited to specific agonists that are yet to be discovered.
Mitochondrial KNa1.x channels
KNa1.x channels — composition
The KNa1.x channels consist of tetramers of pore-forming α subunits encoded by either Kcnt1 or Kcnt2. These genes are classified as being in the Slo gene family, despite having only 7% homology to Kcnma1. KNa1.x channels lack the S0 domain of KCa1.1 and are therefore unable to interact with β subunits [50]. They also lack the voltage-sensing positive residues in transmembrane S4. KNa1.x and KCa1.1 also differ in their cytosolic domains, with KNa1.x RCK domains activated by Na+ and Cl− and inhibited by Ca2+ (Figure 4) [285]. The KNa1.1 paralog has five splice variants (termed Slack-A, Slack Ax2, Slack B, Slack Bx2, and Slack M [286]) in the cytosolic N-terminal region, of which two have been characterized: Slack-B is the canonical KNa1.1 channel, whereas Slack-A produces a channel with properties similar to KNa1.2 [49,55,286]. While no splice variant of KNa1.2 has been discovered, KNa1.2 can heteromultimerize with Slack-B [55] or with KCa1.1, to produce channels with novel characteristics [50]. The KNa1.x channels have been mainly studied in the brain where they are highly expressed, and currently, only KNa1.2 has been detected in the heart [51,89,286,287]. This is consistent with early observations of surface KNa channels in the heart [288].
The KNa1.2 channel is unique among channels encoded by the Slo gene family, in that it has a nucleotide-binding domain on the C-terminus (Figure 4). ATP binding to this domain inhibits the channel in rodent cells [49], but activates in human and frog cells [287,289–297]. Meanwhile, NAD+ and NADP+ both activate KNa1.2 presumably via this nucleotide-binding domain [298], but it should be noted that pyridine nucleotides can also act on KATP [299] and KCa channels [300,301], so this property cannot be used to distinguish these channels. Interestingly, in neurons of both humans and rats, KNa1.2 transcription is regulated by nuclear factor kappa B (NF-κB) activation [302], which itself is activated by IPC and APC [303–306]. Knockout mice for Kcnt1, Kcnt2, and a double knockout are available and have been used to determine the role of these channels in cardioprotection (see below) [89,307].
KNa1.x channels — pharmacology and regulation
KNa1.x channel pharmacology is largely informed by KNa1.1, although several of these drugs are also known to affect the lone C. elegans SLO2 channel [97], and thus, not surprisingly, most KNa1.x drugs exhibit similar effects on both mammalian paralogs. There are numerous drugs that nonspecifically inhibit both KCa1.1 and KNa1.x channels, including paxilline, verapamil, and bepridil [308,309]. Similarly, several drugs can nonspecifically activate both KCa1.1 and KNa1.x channels: bithionol [310–312], 17β-estradiol [313,314], and nonsteroidal anti-inflammatory fenmates such as niflumic acid [294]. In addition, several drugs that target other K+ channels do not affect KNa1.x channels, including (target) dendrotoxin (KV), apamin (KCa2.x), glibenclamide (KATP), or DZX (KATP). This can help in ruling out a role for KNa channels in any particular phenomenon [49,50].
In terms of distinguishing KCa1.1 from KNa1.x, ChTx and IbTx have already been described above as KCa1.1-specific inhibitors, and NS1619 and EtOH as KCa1.1 activators; none of these agents affect KNa1.x [315]. There are also inhibitors of KNa1.x including clofilium and quinidine [290,308,309,316] and activators of KNa1.x including loxapine and niclosamide (see Table 1) [317]. None of these agents have an impact on KCa1.1. Unfortunately, many of these drugs also affect other channels, including KCa5.1 (also known as SLO3, encoded by Kcnu) [318], KV11 (human ether-a-go-go related gene) [319], KV10 (human ether-a-go-go potassium channel) [320], KV1.5 [321], KV7 (Kcnq) [322], and KV4 (Kcne) [323] channels. As such, the use of pharmacologic agents to assign distinct functions to KNa1.x channels is susceptible to a multitude of off-target effects.
In addition to pharmacologic regulation, several signaling pathways are known to regulate KNa1.x channels either directly or at the transcriptional level. NAD+ regulates both KNa1.2 [298] and KNa1.1, resulting in activation and a lower EC50 for Na+. In the brain, KNa1.2 channels are inhibited by PKC, Gα, Gq, M1, and mGluR1 receptor signaling pathways, whereas KNa1.1 channels are activated by these same pathways [55].
KNa1.x channels — role in mitochondria and cardioprotection
In isolated cardiac mitochondria, functional assays (Tl+ flux) have demonstrated a channel with BK-like pharmacologic sensitivity (i.e., activation by bithionol or isoflurane and inhibition by bepridil) that is absent from mitochondria from Kcnt2−/− mice [307]. This channel was still present in Kcnt1−/− mice and absent from Kcnt1/Kcnt2 double knockouts, supporting the existence of a KNa1.2 channel in heart mitochondria [307]. To date, immunologic and other approaches have not yielded solid evidence for the mitochondrial KNa1.2 channel, primarily due to issues of antibody specificity (unpublished observations), although this is a problem not unique to KNa1.2 (see discussion above on mitoKATP and KCa1.1 channels). Electrophysiology (patch clamp) studies on mitoplasts from wild-type and Kcnt2−/− mice are currently underway in our laboratory.
Both Kcnt2−/− and Kcnt1−/− mice retain cardioprotection by IPC and DZX, consistent with the action of a mitoKATP channel and not a KNa1.x channel in such protection. In addition, the Kcnt1−/− heart was protected by APC (via isoflurane). However, in Kcnt2−/− and in Kcnt1/Kcnt2 double knockout hearts, no protection by APC was observed [307]. These data suggest an absolute requirement of KNa1.2 for the protective effects of APC. Furthermore, the KNa1.x activator bithionol was found to be protective when delivered exogenously, supporting that opening of KNa1.x alone is sufficient to confer IR protection.
The single C. elegans SLO2 channel is also required for hypoxic protection. Specifically, protection against anoxia–reoxygenation injury by the volatile anesthetic isoflurane was lost in Slo2−/− worms. In addition, mitochondria from Slo2−/− worms lacked a BK-like channel activity seen in wild-type mitochondria (i.e., activated by bithionol or isoflurane, blocked by bepridil, and insensitive to IbTx) [97]. Taken together, these knockout organism experiments demonstrate that a channel with BK-like activity in the mitochondrion (SLO2 in worms and KNa1.2 in mammals) is a conserved mechanism for protection against IR injury triggered by APC.
In the context of ion homeostasis in IR injury and cardioprotection, there are clear reasons for hypothesizing that both Na+-activated and Ca2+-activated mitochondrial channels (i.e., KNa1.2 and KCa1.1, respectively) may be opened by the high prevailing concentrations of Na+ or Ca2+ during ischemia (see Section ‘Ischemia–Reperfusion Injury and Protection’) [51,324–326]. However, evidence for the opening of these channels in baseline IR injury alone is lacking, and blockers of these channels do not exacerbate IR injury [92,307]. In addition, how the levels of Na+ and Ca2+ in the heart differ in ischemia following either IPC or APC is poorly understood. There is currently no rationale for the hypothesis that these channels would open in response to their natural ligands (Na+ or Ca2+) under cardioprotective stimulus conditions. In the case of KNa1.2, elevated Ca2+ would be predicted to inhibit the channel despite the raise in Na+ [50,285]. The likely activators of these channels in IPC are upstream protein kinase signaling pathways (see above), while in the case of APC it is likely that volatile anesthetics are direct channel ligands.
While the use of volatile anesthetics is positively linked with reduced mortality in cardiac surgery [327], there is also evidence that repeated exposures can result in acute hepatitis [328]. Therefore, the identification of the channel that underlies the clinically important phenomenon of APC potentially paves the way for the development of novel KNa1.2-targeted cardioprotective therapeutics [329]. However, an important caveat to these results is that we are the only laboratory that has to date investigated or provided any evidence for mitochondrial KNa1.2. As such, validation of these findings by other laboratories will be necessary before moving toward any potential clinical applications.
Downstream mechanisms of protection due to mitochondrial K+ channel opening
The mitochondrial PT pore is a fundamental arbiter of cell survival in IR injury (Figure 1) [71,330–334]. As such, numerous events at the mitochondrial level that are known to regulate the PT pore (e.g., mitochondrial Ca2+ overload, ROS generation, energetics, and pH) have been shown to interface with upstream signaling pathways implicated in cardioprotection (GSK-3B, NO·, signaling ROS, PKA, PKC, and others) [335–339]. However, despite a proposed central role for mitochondrial K+ channels in IR protection (see Section ‘Mitochondrial K+ Homeostasis and Discovery of Mitochondrial K+ Channels’), how such channels elicit downstream protective mitochondrial events is poorly understood.
There are numerous attractive hypotheses linking mitochondrial K+ channels to the PT pore, and these can be roughly broken down into those dependent on membrane potential, and those that are not. Owing to the mitochondrial K+ cycle (Figure 2), it is apparent that opening of a mitochondrial K+ channel may serve (coupled with a KHE) to uncouple mitochondrial oxidative phosphorylation. Mild uncoupling of Ox-Phos alone is known to be cardioprotective [22,340–342] and may have many salutary effects on IR injury, such as those described in the following paragraphs.
ROS generation
Tissue reperfusion following ischemia is known to trigger a burst of ROS, and it is also known that mitochondrial ROS generation is exquisitely sensitive to membrane potential [77,78]. As such, it has been proposed that mild uncoupling may serve to depress ROS generation in early reperfusion [343–345]. This may be achievable via opening of a mitochondrial K+ channel [244,245,346–348]. In addition, APC protection may also decrease ROS at reperfusion via mild uncoupling [349–351]. These findings would appear to position mitochondrial K+ channel opening upstream of a decrease in pathologic ROS.
However, the interplay between ROS and mitochondrial K+ channels in the setting of IR injury is much more complicated. Specifically, it is well known that low levels of ROS (termed ‘signaling ROS’) are in fact required for the cardioprotective effects of IPC [352–356] and APC [357]. This is consistent with the notion that ROS is hormetic, and indeed, low levels of ROS alone are known to confer IR protection [353,358,359]. In addition, the mitoKATP channel is redox-sensitive and opens in response to a variety of ROS [360]. These findings position mitochondrial K+ channel opening downstream from signaling ROS.
Still further complication arises from the claim that opening of a mitochondrial K+ channel itself can trigger ROS generation by complex I [361], which would position channel opening upstream of signaling ROS. Overall, it appears that the relationship between mitochondrial K+ channels and ROS generation may be bi-directional, with signaling ROS and mitochondrial K+ channel opening perhaps exhibiting an amplification loop behavior during the early trigger phase of cardioprotection, leading to an overall decrease in pathologic ROS at reperfusion. Unfortunately, beyond the brute-force application of antioxidants, the evidence for a role of ROS in transmitting a protective signal as part of an IPC or mitochondrial K+ channel signaling cascade is somewhat limited. Clearly, much remains to be done, in elucidating the order of events relating mitochondrial K+ channels and ROS in IR protection. Recent progress in identifying the molecular constituents of mitochondrial K+ channels (see Sections ‘Mitochondrial KATP Channel: Composition, Pharmacology, Regulation, Role in IR Protection; Mitochondrial KCa2.x and KCa3.1 Channels: Composition, Pharmacology, Regulation, Role in IR Protection; Mitochondrial KCa1.1 Channels and Mitochondrial KNa1.x Channels; and Mitochondrial KNa1.x Channels’) may also permit the identification of redox-sensitive residues (e.g., cysteine and methionine) within these proteins that are responsible for the interplay of these channels with ROS.
Mitochondrial Ca2+ and autophagy
Another potential benefit of mild uncoupling via opening of a mitochondrial K+ channel would be the prevention of mitochondrial Ca2+ uptake, which is driven by the membrane potential. As such, opening of a mitochondrial K+ channel may prevent mitochondrial Ca2+ overload [362,363], thus serving to prevent PT pore opening. A third potential benefit of mild uncoupling downstream from mitochondrial K+ channel opening could be the triggering of mitophagy [364], which is itself known to be cardioprotective [342,365]. The ability of mitochondrial K+ channel opening to regulate mitophagy has not been rigorously investigated, although it was shown that the mitoKATP opener DZX induces mitophagy in murine hearts [366], and it is also known that isoflurane induces cardiac mitophagy [367].
In considering the above phenomena, a caveat should be rendered regarding any link between mitochondrial K+ channel opening and mitochondrial uncoupling, in terms of the size of the K+ conductance involved. Specifically, it is known that opening of the mitoKATP channel only drops membrane potential by 1–2 mV in isolated cardiac mitochondria (from its baseline value of ~180 mV) [368]. Thus, any such uncoupling mediated by a mitoKATP channel is likely to be insufficient to affect mitochondrial function (i.e., ATP production), although it could be sufficient to affect the driving force for mitochondrial ion fluxes. In contrast, the larger conductance of mitochondrial BK channels renders them more attractive candidates for inducing uncoupling. In this regard, we have found that the KNa1.x opener biothionol (which is cardioprotective, [307]) is also capable of inducing mitochondrial uncoupling in cardiomyocytes (unpublished data). In addition, the KNa1.x opener niclosamide was recently reported to uncouple mitochondria [369]. It is not yet known if the uncoupling effect of niclosamide is mediated by a mitochondrial K+ channel or is capable of conferring cardioprotection.
Membrane potential-independent effects and volume
Beyond effects that depend on membrane potential, mitochondrial K+ channels are thought to play a role in regulating mitochondrial volume [74] (see Section ‘Mitochondrial K+ Homeostasis and Discovery of Mitochondrial K+ Channels’ and Figure 2). Thus, it is possible that mild swelling associated with mitochondrial K+ channel opening [370] may be part of a protective signaling cascade. Mitochondrial volume has been historically linked to respiratory function, with the transition between classical respiratory state 4 (quiescent) and state 3 (phosphorylating) being associated with a contraction of the mitochondrial matrix. [75,76,371]. As such, mild swelling would be expected to coincide with a lower overall mitochondrial respiratory function. How this would lead to protection against IR injury is not clear.
Alternatively, mitochondrial swelling could confer protection by many other mechanisms as follows: (i) by improving efficiency of the creatine kinase energy shuttle, for example, by changing the distance between inner and outer mitochondrial membranes. (ii) By regulating the supra-molecular assembly of respiratory chain complexes and super-complexes [372]. For example, it has been shown that mitochondria from hearts protected by APC had improved ATP synthase function [335]. Furthermore, it has recently been proposed that the cardio-protective drug SS-31 (Bendavia) [373] may confer protection via the stabilization of super-complexes involving cardiolipin [374–376]. (iii) Mild swelling could interfere with PT pore assembly [332,377]. (iv) Mild swelling would also be expected to dilute the contents of the mitochondrial matrix, which may directly affect the activity of enzymes in the tricarboxylic acid cycle by lowering substrate concentrations, or may affect concentrations of important mitochondrial enzyme allosteric regulators such as Ca2+, NADH, acetyl-CoA, and phosphate. (v) By physiologic coupling to other mitochondrial channels or transporters that can sense volume or osmolarity.
From a perspective of long-term protective benefits of mitochondrial KATP opening, it has been shown that the treatment of cells with DZX causes mild in situ mitochondrial swelling, which can trigger a signaling cascade involving cyclic AMP responsive binding element (CREB) and NF-κB, leading to resistance to apoptosis [378]. Thus, there are clearly cell signaling mechanisms triggered by mitochondrial volume changes, which may play an important role in IR protection and remain to be determined.
In summary, the events linking mitochondrial K+ channel opening to protection from IR injury are currently poorly understood, both at the mechanistic and molecular levels. It is hoped that the future availability of specific mitochondrial K+ channel ligands (facilitated by the molecular identification of these channels) will permit the independent interrogation of mitochondrial K+ channel opening and swelling as a signaling trigger mechanism, to elucidate these downstream pathways.
Physiologic role of mitochondrial K+ channels beyond cardioprotection
Given the importance of K+ as a cytosolic solute and the conserved nature of mitochondrial K+ channels, it is important to consider the endogenous physiologic role(s) of these channels in the cell, beyond protection from IR injury. Such considerations could also provide insights into novel mechanisms of regulating mitochondrial function.
At the organism level, as already discussed in Section ‘Mitochondrial KCa2.x and KCa3.1 Channels: Composition, Pharmacology, Regulation, Role in IR Protection’, mammalian KATP channels play important roles in glucose-stimulated insulin secretion in pancreatic β-cells [379], and these channels are the pharmacologic target of the widely used antidiabetic sulfonylurea class of drugs. In addition, mammalian BK channels are broadly recognized to play roles in regulating vascular smooth muscle tone [380,381], in muscle relaxation [382], in regulating circadian rhythms [383,384], and in the function of neurons in the dorsal root ganglion [89]. Both KNa1.x paralogs are highly expressed in the brain, and the majority of research on endogenous KNa1. x channels has been conducted in neurons, focused mainly on KNa1.1 [287]. Whether any of these functions attributed to KNa1.x channels are in fact due to such channels located in mitochondria is not known.
Evidence for a direct physiologic function of mitochondrially localized K+ channels beyond their role on protection against IR injury is very sparse. Historically, uncoupling has been viewed as an important contributor to basal metabolic rate, perhaps best envisioned in brown adipose tissue (BAT), which burns fat to generate heat via mitochondrial uncoupling. The discovery of homologs of the BAT uncoupling protein (now called UCP1, [385]) in other tissues has led to a consensus that these proteins (UCP2–5, [386–389]) may serve a role in regulating whole-organism energy expenditure [390–392]. An alternative uncoupling mechanism has also recently been proposed, involving a nonspecific pore formed by the c-subunit of the ATP synthase in mitochondria [393]. Whether uncoupling by opening of a mitochondrial K+ channel (presumably of the KNa1.x variety — see Section ‘Downstream Mechanisms of Protection by Mitochondrial K+ Channel Opening’) is capable of having a similar effect on whole-organism energy balance remains to be seen, although it is exciting that the KNa1.x activator niclosamide is reported to have an antiobesity effect similar to the uncoupler 2,4-dinitrophenol [369]. This result potentially positions mitochondrial KNa1.2 as a candidate antiobesity drug target.
Outlook
In the roughly three decades, since the discovery of IPC [394] and APC [395], there has been a plethora of research devoted to understanding the molecular underpinnings of these phenomena. Although mitochondrial K+ channels were identified as candidate players early in this research arc, only in the past 4 years have viable molecular identities been assigned to these channels: KNa1.2 [307], KCa2.2/KCa2.3 [180], and KIR1.1 (with caveats as outlined in Section ‘Mitochondrial KATP Channel: Composition, Pharmacology, Regulation, Role in IR Protection’) [95]. These identities can now be used to develop novel molecules to afford protection of organs such as the heart and brain from ischemic injury in a clinical setting.
Finally, it is noteworthy that the field of mitochondrial K+ channel research has used model organisms at multiple stages, including genetically engineered mice, C. elegans, plants [138], amoeba [139], and trypanosomes. In addition, the field exists as a clear demonstration of the importance of basic biomedical research toward understanding a clinically relevant phenomenon in humans. The discoveries made regarding mitochondrial K+ channels in the past 4 years provide a rich resource for future development of clinical therapies.
Acknowledgments
We thank all past and present members of the mitochondrial research group in Rochester who have contributed to our work in this area over the past decade, primarily Andrew Wojtovich and Jolanta Skalska. In addition, Chris Lingle (WUSTL), Liz Jonas (Yale), and Kathleen Kinally (NYU) have been invaluable and generous collaborators for our efforts in this field.
Funding
Work in the laboratories of P.S.B. and K.N. is funded by grants from the US National Institutes of Health [R01-HL071158, R01-GM087483, and R01-HL127891] and by a grant from the US National Science Foundation [NSF-1352836].
Abbreviations
- 5-HD
5-hydroxydecanoate
- APC
anesthetic preconditioning
- BAT
brown adipose tissue
- BK
big conductance of potassium
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- ChTx
charybdotoxin
- DZX
diazoxide
- EET
eicosaepoxytrienoic acid
- IbTx
iberotoxin
- IK
intermediate conductance
- IPC
ischemic preconditioning
- IR
ischemia–reperfusion
- IUPHAR
International Union of Basic and Clinical Pharmacology
- KCa1.1
channel encoded by Kcnma1, also known as SLO1
- KCa2.1
channel encoded by Kcnn1, also known as SK1
- KCa2.2
channel encoded by Kcnn2, also known as SK2
- KCa2.3
channel encoded by Kcnn3, also known as SK3
- KCa3.1
channel encoded by Kcnn4, also known as IK, SK4
- KCa5.1
channel encoded by Kcnu, also known as SLO3
- KHE
K+/H+ exchanger
- KIR
inwardly rectifying potassium channel
- KNa1.1
channel encoded by Kcnt1 (formerly Slo2.2), also known as Slack, KCa4.1, SLO2.2
- KNa1.2
channel encoded by Kcnt2 (formerly Slo2.1), also known as Slick, KCa4.2, SLO2.1
- mitoKATP
ATP-sensitive mitochondrial potassium channel
- NCX
sodium/calcium exchanger
- NHE
sodium/proton exchanger
- NO·
nitric oxide
- PKA
cAMP-dependent protein kinase
- PKC
Ca2+/diacylglycerol-dependent protein kinase
- PKG
cGMP-dependent protein kinase
- PT
permeability transition
- RCK
regulation of conductance of K+
- ROMK
renal outer medullary potassium channel
- ROS
reactive oxygen species
- SERCA
sarco/endoplasmic reticulum calcium-ATPase
- SK
small conductance
- SLO
slowpoke
- Slo2
C. elegans gene encoding the single isotype SLO2 KCa channel
- SloTx
slotoxin
- SUR
sulfonylurea receptor
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics — 2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.CJ-09-0338. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury — the search continues. N Engl J Med. 2015;373:1073–1075. doi: 10.1056/NEJMe1509718. [DOI] [PubMed] [Google Scholar]
- 4.Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW, Kitsis RN, et al. Mitochondrial function, biology, and role in disease. Circ Res. 2016;118:1960–1991. doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, et al. Assessing cardiac metabolism: a scientific statement from the American Heart Association. Circ Res. 2016;118:1659–1701. doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tani M, Neely JR. Vascular washout reduces Ca2+ overload and improves function of reperfused ischemic hearts. Am J Physiol. 1990;258:H354–H361. doi: 10.1152/ajpheart.1990.258.2.H354. [DOI] [PubMed] [Google Scholar]
- 7.Tani M, Neely JR. Na+ accumulation increases Ca2+ overload and impairs function in anoxic rat heart. J Mol Cell Cardiol. 1990;22:57–72. doi: 10.1016/0022-2828(90)90972-5. [DOI] [PubMed] [Google Scholar]
- 8.Tani M, Neely JR. Intermittent perfusion of ischemic myocardium. Possible mechanisms of protective effects on mechanical function in isolated rat heart Circulation. 1990;82:536–548. doi: 10.1161/01.CIR.82.2.536. [DOI] [PubMed] [Google Scholar]
- 9.Talukder MAH, Zweier JL, Periasamy M. Targeting calcium transport in ischaemic heart disease. Cardiovasc Res. 2009;84:345–352. doi: 10.1093/cvr/cvp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KS, Ladinsky H, Stuckey JH. Decreased Ca2+ uptake by sarcoplasmic reticulum after coronary artery occlusion for 60 and 90 minutes. Circ Res. 1967;21:439–444. doi: 10.1161/01.RES.21.4.439. [DOI] [PubMed] [Google Scholar]
- 11.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 13.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 14.Khandoudi N, Bernard M, Cozzone P, Feuvray D. Intracellular pH and role of Na+/H+ exchange during ischaemia and reperfusion of normal and diabetic rat hearts. Cardiovasc Res. 1990;24:873–878. doi: 10.1093/cvr/24.11.873. [DOI] [PubMed] [Google Scholar]
- 15.Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther. 2001;89:29–46. doi: 10.1016/S0163-7258(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 16.Baysal K, Jung DW, Gunter KK, Gunter TE, Brierley GP. Na(+)-dependent Ca2+ efflux mechanism of heart mitochondria is not a passive Ca2+/2Na+ exchanger. Am J Physiol. 1994;266:C800–C808. doi: 10.1152/ajpcell.1994.266.3.C800. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 18.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 19.Asimakis GK, Conti VR. Myocardial ischemia: correlation of mitochondrial adenine nucleotide and respiratory function. J Mol Cell Cardiol. 1984;16:439–447. doi: 10.1016/S0022-2828(84)80615-X. [DOI] [PubMed] [Google Scholar]
- 20.Hardy DL, Clark JB, Darley-Usmar VM, Smith DR. Reoxygenation of the hypoxic myocardium causes a mitochondrial complex I defect. Biochem Soc Trans. 1990;18:549. doi: 10.1042/bst0180549. [DOI] [PubMed] [Google Scholar]
- 21.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS Lett. 1998;424:155–158. doi: 10.1016/S0014-5793(98)00161-6. [DOI] [PubMed] [Google Scholar]
- 22.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395:611–618. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turrens JF, Beconi M, Barilla J, Chavez UB, McCord JM. Mitochondrial generation of oxygen radicals during reoxygenation of ischemic tissues. Free Radic Res Commun. 1991;13(Pt 2):681–689. doi: 10.3109/10715769109145847. [DOI] [PubMed] [Google Scholar]
- 24.García-Rivas GJ, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac postischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. doi: 10.1042/bj3410233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloner RA, Muller J, Davis V. Effects of previous angina pectoris in patients with first acute myocardial infarction not receiving thrombolytics. Am J Cardiol. 1995;75:615–617. doi: 10.1016/S0002-9149(99)80628-6. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Miyazaki S, Miyao Y, Morii I, Matsumoto T, Daikoku S, et al. Effect on survival of previous angina pectoris after acute myocardial infarction. Am J Cardiol. 1997;79:1534–1538. doi: 10.1016/S0002-9149(97)00188-4. [DOI] [PubMed] [Google Scholar]
- 28.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.CIR.84.1.350. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Downey JM. Ischemic preconditioning protects against infarction in rat heart. Am J Physiol. 1992;263:H1107–H1112. doi: 10.1152/ajpheart.1992.263.4.H1107. [DOI] [PubMed] [Google Scholar]
- 30.Schott RJ, Rohmann S, Braun ER, Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res. 1990;66:1133–1142. doi: 10.1161/01.RES.66.4.1133. [DOI] [PubMed] [Google Scholar]
- 31.Jia B, Crowder CM. Volatile anesthetic preconditioning present in the invertebrate Caenorhabditis elegans. Anesthesiology. 2008;108:426–433. doi: 10.1097/ALN.0b013e318164d013. [DOI] [PubMed] [Google Scholar]
- 32.Otani H. Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:207–248. doi: 10.1089/ars.2007.1679. [DOI] [PubMed] [Google Scholar]
- 33.Pagliaro P, Gattullo D, Rastaldo R, Losano G. Ischemic preconditioning: from the first to the second window of protection. Life Sci. 2001;69:1–15. doi: 10.1016/S0024-3205(01)01113-4. [DOI] [PubMed] [Google Scholar]
- 34.Toller WG, Kersten JR, Pagel PS, Hettrick DA, Warltier DC. Sevoflurane reduces myocardial infarct size and decreases the time threshold for ischemic preconditioning in dogs. Anesthesiology. 1999;91:1437–1446. doi: 10.1097/00000542-199911000-00037. [DOI] [PubMed] [Google Scholar]
- 35.Novalija E, Fujita S, Kampine JP, Stowe DF. Sevoflurane mimics ischemic preconditioning effects on coronary flow and nitric oxide release in isolated hearts. Anesthesiology. 1999;91:701–712. doi: 10.1097/00000542-199909000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 37.O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa ADT, Garlid KD. MitoKATP activity in healthy and ischemic hearts. J Bioenerg Biomembr. 2009;41:123–126. doi: 10.1007/s10863-009-9213-y. [DOI] [PubMed] [Google Scholar]
- 39.Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol. 2009;104:121–129. doi: 10.1007/s00395-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riess ML, Eells JT, Kevin LG, Camara AKS, Henry MM, Stowe DF. Attenuation of mitochondrial respiration by sevoflurane in isolated cardiac mitochondria is mediated in part by reactive oxygen species. Anesthesiology. 2004;100:498–505. doi: 10.1097/00000542-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Redel A, Lange M, Jazbutyte V, Lotz C, Smul TM, Roewer N, et al. Activation of mitochondrial large-conductance calcium-activated K+ channels via protein kinase A mediates desflurane-induced preconditioning. Anesth Analg. 2008;106:384–391. doi: 10.1213/ane.0b013e318160650f. table of contents. [DOI] [PubMed] [Google Scholar]
- 42.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, et al. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 43.Kaczmarek LK, Aldrich RW, Chandy KG, Grissmer S, Wei AD, Wulff H. International union of basic and clinical pharmacology. C. Nomenclature and properties of calcium-activated and sodium-activated potassium channels. Pharmacol Rev. 2017;69:1–11. doi: 10.1124/pr.116.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trautmann A, Marty A. Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci USA. 1984;81:611–615. doi: 10.1073/pnas.81.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elkins T, Ganetzky B, Wu CF. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci USA. 1986;83:8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- 47.Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, et al. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-F. [DOI] [PubMed] [Google Scholar]
- 48.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding ‘maxi’ calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joiner WJ, Tang MD, Wang LY, Dworetzky SI, Boissard CG, Gan L, et al. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat Neurosci. 1998;1:462–469. doi: 10.1038/2176. [DOI] [PubMed] [Google Scholar]
- 51.Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, et al. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37:765–773. doi: 10.1016/S0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 52.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–3516. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- 53.Salkoff L, Yuan A, Dourado M, Butler A, Walton N, Wei A, et al. SLO-2, a K+ channel with an unusual Cl− dependence. Nat Neurosci. 2000;3:771–779. doi: 10.1038/77670. [DOI] [PubMed] [Google Scholar]
- 54.Lagrutta A, Shen KZ, North RA, Adelman JP. Functional differences among alternatively spliced variants of Slowpoke, a Drosophila calcium-activated potassium channel. J Biol Chem. 1994;269:20347–20351. [PubMed] [Google Scholar]
- 55.Chen H, Kronengold J, Yan Y, Gazula VR, Brown MR, Ma L, et al. The N-terminal domain of Slack determines the formation and trafficking of Slick/Slack heteromeric sodium-activated potassium channels. J Neurosci. 2009;29:5654–5665. doi: 10.1523/JNEUROSCI.5978-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International union of pharmacology. LII Nomenclature and molecular relationships of calcium-activated potassium channels Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 57.Szabo I, Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol Rev. 2014;94:519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 58.Xu H, Martinoia E, Szabo I. Organellar channels and transporters. Cell Calcium. 2015;58:1–10. doi: 10.1016/j.ceca.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Checchetto V, Teardo E, Carraretto L, Leanza L, Szabo I. Physiology of intracellular potassium channels: a unifying role as mediators of counterion fluxes? Biochim Biophys Acta, Bioenerg. 2016;1857:1258–1266. doi: 10.1016/j.bbabio.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Ponnalagu D, Singh H. Anion channels of mitochondria. In: Barrett James E., editor. Handbook of Experimental Pharmacology. Springer; NY: 2016. p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laskowski M, Augustynek B, Kulawiak B, Koprowski P, Bednarczyk P, Jarmuszkiewicz W, et al. What do we not know about mitochondrial potassium channels? Biochim Biophys Acta. 2016;1857:1247–1257. doi: 10.1016/j.bbabio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Szabò I, Leanza L, Gulbins E, Zoratti M. Physiology of potassium channels in the inner membrane of mitochondria. Pflugers Arch Eur J Physiol. 2012;463:231–246. doi: 10.1007/s00424-011-1058-7. [DOI] [PubMed] [Google Scholar]
- 64.Krishnamoorthy G, Hinkle PC. Non-ohmic proton conductance of mitochondria and liposomes. Biochemistry. 1984;23:1640–1645. doi: 10.1021/bi00303a009. [DOI] [PubMed] [Google Scholar]
- 65.O’Shea PS, Chappell JB. The relationship between the rate of respiration and the protonmotive force. The role of proton conductivity. Biochem J. 1984;219:401–404. doi: 10.1042/bj2190401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Racker E. Resolution and reconstitution of a Mammalian membrane. J Gen Physiol. 1969;54:38–49. doi: 10.1085/jgp.54.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 68.Wojtovich AP, Williams DM, Karcz MK, Lopes CMB, Gray DA, Nehrke KW, et al. A novel mitochondrial KATP channel assay. Circ Res. 2010;106:1190–1196. doi: 10.1161/CIRCRESAHA.109.215400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/S0014-5793(01)02316-X. [DOI] [PubMed] [Google Scholar]
- 70.Garlid KD, Paucek P. Mitochondrial potassium transport: the K+ cycle. Biochim Biophys Acta, Bioenerg. 2003;1606:23–41. doi: 10.1016/S0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 71.Garlid KD. Cation transport in mitochondria — the potassium cycle. Biochim Biophys Acta, Bioenerg. 1996;1275:123–126. doi: 10.1016/0005-2728(96)00061-8. [DOI] [PubMed] [Google Scholar]
- 72.Shi GY, Jung DW, Garlid KD, Brierley GP. Induction of respiration-dependent net efflux of K+ from heart mitochondria by depletion of endogenous divalent cations. J Biol Chem. 1980;255:10306–10311. [PubMed] [Google Scholar]
- 73.Halestrap AP. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta, Bioenerg. 1989;973:355–382. doi: 10.1016/S0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- 74.Riess ML, Costa AD, Carlson R, Garlid KD, Heinen A, Stowe DF. Differential increase of mitochondrial matrix volume by sevoflurane in isolated cardiac mitochondria. Anesth Analg. 2008;106:1049–1055. doi: 10.1213/ane.0b013e318167875e. [DOI] [PubMed] [Google Scholar]
- 75.Hackenbrock CR. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. J Cell Biol. 1966;30:269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scalettar BA, Abney JR, Hackenbrock CR. Dynamics, structure, and function are coupled in the mitochondrial matrix. Proc Natl Acad Sci USA. 1991;88:8057–8061. doi: 10.1073/pnas.88.18.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 78.Brookes PS. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 79.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 80.Szabò I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, et al. A novel potassium channel in lymphocyte mitochondria. J Biol Chem. 2005;280:12790–12798. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- 81.Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- 82.Kicinska A, Augustynek B, Kulawiak B, Jarmuszkiewicz W, Szewczyk A, Bednarczyk P. A large-conductance calcium-regulated K+ channel in human dermal fibroblast mitochondria. Biochem J. 2016;473:4457–4471. doi: 10.1042/BCJ20160732. [DOI] [PubMed] [Google Scholar]
- 83.Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- 84.Lacza Z, Snipes JA, Kis B, Szabó C, Grover G, Busija DW. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res. 2003;994:27–36. doi: 10.1016/j.brainres.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 85.Singh H, Lu R, Rodríguez PFG, Wu Y, Bopassa JC, Stefani E, et al. Visualization and quantification of cardiac mitochondrial protein clusters with STED microscopy. Mitochondrion. 2012;12:230–236. doi: 10.1016/j.mito.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jezek P, Mahdi F, Garlid KD. Reconstitution of the beef heart and rat liver mitochondrial K+/H+ (Na+/H+) antiporter. Quantitation of K+ transport with the novel fluorescent probe, PBFI. J Biol Chem. 1990;265:10522–10526. [PubMed] [Google Scholar]
- 88.Wojtovich AP, DiStefano P, Sherman T, Brookes PS, Nehrke K. Mitochondrial ATP-sensitive potassium channel activity and hypoxic preconditioning are independent of an inwardly rectifying potassium channel subunit in Caenorhabditis elegans. FEBS Lett. 2012;586:428–434. doi: 10.1016/j.febslet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinez-Espinosa PL, Wu J, Yang C, Gonzalez-Perez V, Zhou H, Liang H, et al. Knockout of Slo2.2 enhances itch, abolishes KNa current, and increases action potential firing frequency in DRG neurons. eLife. 2015;4:e10013. doi: 10.7554/eLife.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 91.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wojtovich AP, Urciuoli WR, Chatterjee S, Fisher AB, Nehrke K, Brookes PS. Kir6.2 is not the mitochondrial KATP channel but is required for cardioprotection by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2013;304:H1439–H1445. doi: 10.1152/ajpheart.00972.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garlid KD, Halestrap AP. The mitochondrial KATP channel — fact or fiction? J Mol Cell Cardiol. 2012;52:578–583. doi: 10.1016/j.yjmcc.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gross GJ. Selective ATP-sensitive potassium channel openers: fact or fiction. J Mol Cell Cardiol. 2003;35:1005–1007. doi: 10.1016/S0022-2828(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 95.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, et al. Mitochondrial ROMK channel is a molecular component of MitoKATP. Circ Res. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. Activation of big conductance Ca2+-activated K+ channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch Eur J Physiol. 2009;457:979–988. doi: 10.1007/s00424-008-0583-5. [DOI] [PubMed] [Google Scholar]
- 97.Wojtovich AP, Sherman TA, Nadtochiy SM, Urciuoli WR, Brookes PS, Nehrke K. Slo-2 is cytoprotective and contributes to mitochondrial potassium transport. PLoS ONE. 2011;6:e28287. doi: 10.1371/journal.pone.0028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wojtovich AP, Nadtochiy SM, Urciuoli WR, Smith CO, Grunnet M, Nehrke K, et al. A non-cardiomyocyte autonomous mechanism of cardioprotection involving the SLO1 BK channel. PeerJ. 2013;1:e48. doi: 10.7717/peerj.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 101.Lim S, Davidson S, Hausenloy D, Yellon D. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 103.Salari S, Ghasemi M, Fahanik-Babaei J, Saghiri R, Sauve R, Eliassi A. Evidence for a KATP channel in rough endoplasmic reticulum (rerKATP channel) of rat hepatocytes. PLoS ONE. 2015;10:e0125798. doi: 10.1371/journal.pone.0125798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quesada I, Rovira JM, Martin F, Roche E, Nadal A, Soria B. Nuclear KATP channels trigger nuclear Ca2+ transients that modulate nuclear function. Proc Natl Acad Sci USA. 2002;99:9544–9549. doi: 10.1073/pnas.142039299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 106.Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- 107.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the Guinea pig ventricular action potential and contraction. Circ Res. 1991;68:280–287. doi: 10.1161/01.RES.68.1.280. [DOI] [PubMed] [Google Scholar]
- 108.Armstrong SC, Liu GS, Downey JM, Ganote CE. Potassium channels and preconditioning of isolated rabbit cardiomyocytes: effects of glyburide and pinacidil. J Mol Cell Cardiol. 1995;27:1765–1774. doi: 10.1016/S0022-2828(95)90986-9. [DOI] [PubMed] [Google Scholar]
- 109.Gross GJ, Auchampach JA. Role of ATP dependent potassium channels in myocardial ischaemia. Cardiovasc Res. 1992;26:1011–1016. doi: 10.1093/cvr/26.11.1011. [DOI] [PubMed] [Google Scholar]
- 110.Mizumura T, Nithipatikom K, Gross GJ. Bimakalim, an ATP-sensitive potassium channel opener, mimics the effects of ischemic preconditioning to reduce infarct size, adenosine release, and neutrophil function in dogs. Circulation. 1995;92:1236–1245. doi: 10.1161/01.CIR.92.5.1236. [DOI] [PubMed] [Google Scholar]
- 111.Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I Signalling and cytoprotective mechanisms. Br J Anaesth. 2003;91:551–565. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- 112.Zaugg M, Lucchinetti E, Garcia C, Pasch T, Spahn DR, Schaub MC. Anaesthetics and cardiac preconditioning. Part II Clinical implications. Br J Anaesth. 2003;91:566–576. doi: 10.1093/bja/aeg206. [DOI] [PubMed] [Google Scholar]
- 113.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels: possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.RES.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 114.Das B, Sarkar C. Mitochondrial KATP channel activation is important in the antiarrhythmic and cardioprotective effects of non-hypotensive doses of nicorandil and cromakalim during ischemia/reperfusion: a study in an intact anesthetized rabbit model. Pharmacol Res. 2003;47:447–461. doi: 10.1016/S1043-6618(02)00335-3. [DOI] [PubMed] [Google Scholar]
- 115.Pignac J, Lacaille C, Dumont L. Protective effects of the K+ATP channel opener, aprikalim, against free radicals in isolated rabbit hearts. Free Radic Biol Med. 1996;20:383–389. doi: 10.1016/0891-5849(96)02091-6. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.CIR.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 117.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.RES.84.9.973. [DOI] [PubMed] [Google Scholar]
- 118.Lee HL, Lu CH, Chang PC, Chou CC, Wo HT, Wen MS. Contrasting effects of HMR1098 on arrhythmogenicity in a langendorff-perfused phase-2 myocardial infarction rabbit model. Pacing Clin Electrophysiol. 2014;37:1058–1066. doi: 10.1111/pace.12381. [DOI] [PubMed] [Google Scholar]
- 119.Bednarczyk P, Kicińska A, Kominkova V, Ondrias K, Dolowy K, Szewczyk A. Quinine inhibits mitochondrial ATP-regulated potassium channel from bovine heart. J Membr Biol. 2004;199:63–72. doi: 10.1007/s00232-004-0676-9. [DOI] [PubMed] [Google Scholar]
- 120.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, et al. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 121.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI0214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochim Biophys Acta, Biomembr. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 123.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 124.Shintani Y, Node K, Asanuma H, Sanada S, Takashima S, Asano Y, et al. Opening of Ca2+-activated K+ channels is involved in ischemic preconditioning in canine hearts. J Mol Cell Cardiol. 2004;37:1213–1218. doi: 10.1016/j.yjmcc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 125.Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther. 2005;312:644–650. doi: 10.1124/jpet.104.074476. [DOI] [PubMed] [Google Scholar]
- 126.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, et al. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 127.Kloner RA, Shook T, Antman EM, Cannon CP, Przyklenk K, Yoo K, et al. Prospective temporal analysis of the onset of preinfarction angina versus outcome: an ancillary study in TIMI-9B. Circulation. 1998;97:1042–1045. doi: 10.1161/01.CIR.97.11.1042. [DOI] [PubMed] [Google Scholar]
- 128.Ottani F, Galvani M, Ferrini D, Sorbello F, Limonetti P, Pantoli D, et al. Prodromal angina limits infarct size. A role for ischemic preconditioning. Circulation. 1995;91:291–297. doi: 10.1161/01.CIR.91.2.291. [DOI] [PubMed] [Google Scholar]
- 129.Kersten JR, Toller WG, Gross ER, Pagel PS, Warltier DC. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol. 2000;278:H1218–H1224. doi: 10.1152/ajpheart.2000.278.4.H1218. [DOI] [PubMed] [Google Scholar]
- 130.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for KATP channel-independent regulation of insulin secretion. J Biol Chem. 2000;275:9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 131.Elrod JW, Harrell M, Flagg TP, Gundewar S, Magnuson MA, Nichols CG, et al. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008;117:1405–1413. doi: 10.1161/CIRCULATIONAHA.107.745539. [DOI] [PubMed] [Google Scholar]
- 132.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, et al. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci USA. 2001;98:11760–11764. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J Mol Cell Cardiol. 2008;44:188–200. doi: 10.1016/j.yjmcc.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brian Foster D, Rucker JJ, Marbán E. Is Kir6.1 a subunit of mitoKATP? Biochem Biophys Res Commun. 2008;366:649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, et al. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fahrenbach JP, Stoller D, Kim G, Aggarwal N, Yerokun B, Earley JU, et al. Abcc9 is required for the transition to oxidative metabolism in the newborn heart. FASEB J. 2014;28:2804–2815. doi: 10.1096/fj.13-244459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang MT, Ljubkovic M, Nakae Y, Shi Y, Kwok WM, Stowe DF, et al. Characterization of human cardiac mitochondrial ATP-sensitive potassium channel and its regulation by phorbol ester in vitro. Am J Physiol Heart Circ Physiol. 2006;290:H1770–H1776. doi: 10.1152/ajpheart.01084.2005. [DOI] [PubMed] [Google Scholar]
- 138.Pastore D, Stoppelli MC, Di Fonzo N, Passarella S. The existence of the K+ channel in plant mitochondria. J Biol Chem. 1999;274:26683–26690. doi: 10.1074/jbc.274.38.26683. [DOI] [PubMed] [Google Scholar]
- 139.Kicinska A, Swida A, Bednarczyk P, Koszela-Piotrowska I, Choma K, Dolowy K, et al. ATP-sensitive potassium channel in mitochondria of the eukaryotic microorganism Acanthamoeba castellanii. J Biol Chem. 2007;282:17433–17441. doi: 10.1074/jbc.M701496200. [DOI] [PubMed] [Google Scholar]
- 140.Costa ADT, Krieger MA. Evidence for an ATP-sensitive K+ channel in mitoplasts isolated from Trypanosoma cruzi and Crithidia fasciculata. Int J Parasitol. 2009;39:955–961. doi: 10.1016/j.ijpara.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wojtovich AP, Burwell LS, Sherman TA, Nehrke KW, Brookes PS. The C. elegans mitochondrial K+ATP channel: a potential target for preconditioning. Biochem Biophys Res Commun. 2008;376:625–628. doi: 10.1016/j.bbrc.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grover GJ, Garlid KD. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- 143.Auchampach JA, Grover GJ, Gross GJ. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res. 1992;26:1054–1062. doi: 10.1093/cvr/26.11.1054. [DOI] [PubMed] [Google Scholar]
- 144.Hide EJ, Thiemermann C. Limitation of myocardial infarct size in the rabbit by ischaemic preconditioning is abolished by sodium 5-hydroxydecanoate. Cardiovasc Res. 1996;31:941–946. doi: 10.1016/S0008-6363(96)00041-7. [DOI] [PubMed] [Google Scholar]
- 145.Schäfer G, Wegener C, Portenhauser R, Bojanovski D. Diazoxide, an inhibitor of succinate oxidation. Biochem Pharmacol. 1969;18:2678–2681. doi: 10.1016/0006-2952(69)90200-7. [DOI] [PubMed] [Google Scholar]
- 146.Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–170. doi: 10.1016/j.febslet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 147.Hanley PJ, Dröse S, Brandt U, Lareau RA, Banerjee AL, Srivastava DK, et al. 5-Hydroxydecanoate is metabolised in mitochondria and creates a rate-limiting bottleneck for β-oxidation of fatty acids. J Physiol. 2005;562:307–318. doi: 10.1113/jphysiol.2004.073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hanley PJ, Mickel M, Löffler M, Brandt U, Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: Implications for ischemic preconditioning. Biochim Biophys Acta, Bioenerg. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Critz SD, Liu GS, Chujo M, Downey JM. Pinacidil but not nicorandil opens ATP-sensitive K+ channels and protects against simulated ischemia in rabbit myocytes. J Mol Cell Cardiol. 1997;29:1123–1130. doi: 10.1006/jmcc.1996.0335. [DOI] [PubMed] [Google Scholar]
- 151.Richer C, Pratz J, Mulder P, Mondot S, Giudicelli JF, Cavero I. Cardiovascular and biological effects of K+ channel openers, a class of drugs with vasorelaxant and cardioprotective properties. Life Sci. 1990;47:1693–1705. doi: 10.1016/0024-3205(90)90342-O. [DOI] [PubMed] [Google Scholar]
- 152.Cecchetti V, Tabarrini O, Sabatini S. From cromakalim to different structural classes of KATP channel openers. Curr Top Med Chem. 2006;6:1049–1068. doi: 10.2174/156802606777323683. [DOI] [PubMed] [Google Scholar]
- 153.Englert HC, Gerlach U, Goegelein H, Hartung J, Heitsch H, Mania D, et al. Cardioselective KATP channel blockers derived from a new series of m-anisamidoethylbenzenesulfonylthioureas. J Med Chem. 2001;44:1085–1098. doi: 10.1021/jm000985v. [DOI] [PubMed] [Google Scholar]
- 154.Ockaili RA, Bhargava P, Kukreja RC. Chemical preconditioning with 3-nitropropionic acid in hearts: role of mitochondrial K(ATP) channel. Am J Physiol Heart Circ Physiol. 2001;280:H2406–H2411. doi: 10.1152/ajpheart.2001.280.5.H2406. [DOI] [PubMed] [Google Scholar]
- 155.Shiva S, Crawford JH, Ramachandran A, Ceaser EK, Hillson T, Brookes PS, et al. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J. 2004;379:359–366. doi: 10.1042/bj20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia–reperfusion injury. Antioxid Redox Signal. 2008;10:579–600. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 157.Wojtovich AP, Nehrke KW, Brookes PS. The mitochondrial complex II and ATP-sensitive potassium channel interaction: quantitation of the channel in heart mitochondria. Acta Biochim Pol. 2010;57:431–434. [PMC free article] [PubMed] [Google Scholar]
- 158.Drose S, Bleier L, Brandt U. A common mechanism links differently acting complex II inhibitors to cardioprotection: modulation of mitochondrial reactive oxygen species production. Mol Pharmacol. 2011;79:814–822. doi: 10.1124/mol.110.070342. [DOI] [PubMed] [Google Scholar]
- 159.Wojtovich AP, Smith CO, Haynes CM, Nehrke KW, Brookes PS. Physiological consequences of complex II inhibition for aging, disease, and the mKATP channel. Biochim Biophys Acta, Bioenerg. 2013;1827:598–611. doi: 10.1016/j.bbabio.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bednarczyk P, Wieckowski MR, Broszkiewicz M, Skowronek K, Siemen D, Szewczyk A, et al. Putative structural and functional coupling of the mitochondrial BKCa channel to the respiratory chain. PLoS ONE. 2013;8:e68125. doi: 10.1371/journal.pone.0068125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guda C, Fahy E, Subramaniam S. MITOPRED: a genome-scale method for prediction of nucleus-encoded mitochondrial proteins. Bioinformatics. 2004;20:1785–1794. doi: 10.1093/bioinformatics/bth171. [DOI] [PubMed] [Google Scholar]
- 163.Cotter D, Guda P, Fahy E, Subramaniam S. MitoProteome: mitochondrial protein sequence database and annotation system. Nucleic Acids Res. 2004;32:D463–D467. doi: 10.1093/nar/gkh048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumar M, Verma R, Raghava GPS. Prediction of mitochondrial proteins using support vector machine and hidden markov model. J Biol Chem. 2005;281:5357–5363. doi: 10.1074/jbc.M511061200. [DOI] [PubMed] [Google Scholar]
- 165.Foster DB, O’Rourke B, Van Eyk JE. What can mitochondrial proteomics tell us about cardioprotection afforded by preconditioning? Expert Rev Proteomics. 2008;5:633–636. doi: 10.1586/14789450.5.5.633. [DOI] [PubMed] [Google Scholar]
- 166.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 167.Salkoff L, Wei AD, Baban B, Butler A, Fawcett G, Ferreira G, et al. Potassium channels in C elegans. WormBook; 2006. pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Denton JS, Weaver CD, Lewis LM, Chauder BA, Lindsley CW. Probe Reports from NIH Mol Libr Progr. National Center for Biotechnology Information; Bethesda, MD: 2010. Discovery of a small molecule inhibitor of ROMK and Kir7.1. https://www.ncbi.nlm.nih.gov/books/NBK50685/ [PubMed] [Google Scholar]
- 169.Lewis LM, Bhave G, Chauder BA, Banerjee S, Lornsen KA, Redha R, et al. High-throughput screening reveals a small-molecule inhibitor of the renal outer medullary potassium channel and Kir7.1. Mol Pharmacol. 2009;76:1094–1103. doi: 10.1124/mol.109.059840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kharade SV, Flores D, Lindsley CW, Satlin LM, Denton JS. ROMK inhibitor actions in the nephron probed with diuretics. Am J Physiol Ren Physiol. 2015;310:F732–F737. doi: 10.1152/ajprenal.00423.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou X, Zhang Z, Shin MK, Horwitz SB, Levorse JM, Zhu L, et al. Heterozygous disruption of renal outer medullary potassium channel in rats is associated with reduced blood pressure. Hypertension. 2013;62:288–294. doi: 10.1161/HYPERTENSIONAHA.111.01051. [DOI] [PubMed] [Google Scholar]
- 172.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, et al. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter’s syndrome. J Biol Chem. 2002;277:37871–37880. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]
- 173.Paggio A, Checchetto V, Szabò I, Rizzutto R, De Stefani D. Molecular identification of the mitochondrial ATP sensitive potassium channel (mitoKATP) Biochim Biophys Acta, Bioenerg. 2016;1857:e70. doi: 10.1016/j.bbabio.2016.04.366. [DOI] [Google Scholar]
- 174.Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 175.Saito T, Fujiwara Y, Fujiwara R, Hasegawa H, Kibira S, Miura H, et al. Role of augmented expression of intermediate-conductance Ca2+-activated K+ channels in postischaemic heart. Clin Exp Pharmacol Physiol. 2002;29:324–329. doi: 10.1046/j.1440-1681.2002.03652.x. [DOI] [PubMed] [Google Scholar]
- 176.Ju CH, Wang XP, Gao CY, Zhang SX, Ma XH, Liu C. Blockade of KCa3.1 attenuates left ventricular remodeling after experimental myocardial infarction. Cell Physiol Biochem. 2015;36:1305–1315. doi: 10.1159/000430298. [DOI] [PubMed] [Google Scholar]
- 177.Chen YJ, Nguyen HM, Maezawa I, Grössinger EM, Garing AL, Köhler R, et al. The potassium channel KCa3.1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. J Cereb Blood Flow Metab. 2016;36:2146–2161. doi: 10.1177/0271678X15611434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chang PC, Chen PS. SK channels and ventricular arrhythmias in heart failure. Trends Cardiovasc Med. 2015;25:508–514. doi: 10.1016/j.tcm.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Skibsbye L, Poulet C, Diness JG, Bentzen BH, Yuan L, Kappert U, et al. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc Res. 2014;103:156–167. doi: 10.1093/cvr/cvu121. [DOI] [PubMed] [Google Scholar]
- 180.Stowe DF, Gadicherla AK, Zhou Y, Aldakkak M, Cheng Q, Kwok WM, et al. Protection against cardiac injury by small Ca2+-sensitive K+ channels identified in guinea pig cardiac inner mitochondrial membrane. Biochim Biophys Acta, Biomembr. 2013;1828:427–442. doi: 10.1016/j.bbamem.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, et al. Protein kinase CK2 is coassembled with small conductance Ca2+-Activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 182.Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci. 2007;27:2369–2376. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Luján R, Maylie J, Adelman JP. New sites of action for GIRK and SK channels. Nat Rev Neurosci. 2009;10:475–480. doi: 10.1038/nrn2668. [DOI] [PubMed] [Google Scholar]
- 184.Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol. 2007;42:687–691. doi: 10.1016/j.yjmcc.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Burnham MP, Bychkov R, Félétou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, et al. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol. 2009;46:960–968. doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nadtochiy SM, Zhu Q, Urciuoli W, Rafikov R, Black SM, Brookes PS, et al. Nitroalkenes confer acute cardioprotection via adenine nucleotide translocase 1. J Biol Chem. 2012;287:3573–3580. doi: 10.1074/jbc.M111.298406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 189.Loot AE, Moneke I, Keserü B, Oelze M, Syzonenko T, Daiber A, et al. 11,12-EET stimulates the association of BK channel α and β1 subunits in mitochondria to induce pulmonary vasoconstriction. PLoS ONE. 2012;7:e46065. doi: 10.1371/journal.pone.0046065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.RES.80.6.877. [DOI] [PubMed] [Google Scholar]
- 191.Sollini M, Frieden M, Bény JL. Charybdotoxin-sensitive small conductance KCa channel activated by bradykinin and substance P in endothelial cells. Br J Pharmacol. 2002;136:1201–1209. doi: 10.1038/sj.bjp.0704819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Terstappen GC, Pellacani A, Aldegheri L, Graziani F, Carignani C, Pula G, et al. The antidepressant fluoxetine blocks the human small conductance calcium-activated potassium channels SK1, SK2 and SK3. Neurosci Lett. 2003;346:85–88. doi: 10.1016/S0304-3940(03)00574-3. [DOI] [PubMed] [Google Scholar]
- 193.Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, et al. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 195.Pallanck L, Ganetzky B. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum Mol Genet. 1994;3:1239–1243. doi: 10.1093/hmg/3.8.1239. [DOI] [PubMed] [Google Scholar]
- 196.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, et al. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 197.McCobb DP, Fowler NL, Featherstone T, Lingle CJ, Saito M, Krause JE, et al. A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 1995;269(3 Pt 2):H767–H777. doi: 10.1152/ajpheart.1995.269.3.H767. [DOI] [PubMed] [Google Scholar]
- 198.Chen L, Tian L, MacDonald SHF, McClafferty H, Hammond MSL, Huibant JM, et al. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α-subunits generated from a single site of splicing. J Biol Chem. 2005;280:33599–33609. doi: 10.1074/jbc.M505383200. [DOI] [PubMed] [Google Scholar]
- 199.Saito M, Nelson C, Salkoff L, Lingle CJ. A cysteine-rich domain defined by a novel exon in aSlo variant in rat adrenal chromaffin cells and PC12 cells. J Biol Chem. 1997;272:11710–11717. doi: 10.1074/jbc.272.18.11710. [DOI] [PubMed] [Google Scholar]
- 200.Tian L, Duncan RR, Hammond MSL, Coghill LS, Wen H, Rusinova R, et al. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- 201.McCartney CE, McClafferty H, Huibant JM, Rowan EG, Shipston MJ, Rowe ICM. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel α-subunits. Proc Natl Acad Sci USA. 2005;102:17870–17876. doi: 10.1073/pnas.0505270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem. 2001;276:16232–16239. doi: 10.1074/jbc.M008852200. [DOI] [PubMed] [Google Scholar]
- 203.Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, Stefani E, et al. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci USA. 2004;101:10072–10077. doi: 10.1073/pnas.0302919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. mitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci USA. 2013;110:10836–10841. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang SX, Ikeda M, Guggino WB. The cytoplasmic tail of large conductance, voltage- and Ca2+-activated K+ (MaxiK) channel is necessary for its cell surface expression. J Biol Chem. 2003;278:2713–2722. doi: 10.1074/jbc.M208411200. [DOI] [PubMed] [Google Scholar]
- 206.Kwon SH, Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci USA. 2004;101:15237–15242. doi: 10.1073/pnas.0404877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel β-subunit genes: cloning and characterization. Genomics. 1999;55:57–67. doi: 10.1006/geno.1998.5627. [DOI] [PubMed] [Google Scholar]
- 208.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, et al. Primary sequence and immunological characterization of beta-subunit of high conductance Ca(2+)-activated K+ channel from smooth muscle. J Biol Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- 209.Torres YP, Granados ST, Latorre R. Pharmacological consequences of the coexpression of BK channel α and auxiliary β subunits. Front Physiol. 2014;5:383. doi: 10.3389/fphys.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 211.Orio P, Latorre R. Differential effects of β1 and β2 subunits on BK channel activity. J Gen Physiol. 2005;125:395–411. doi: 10.1085/jgp.200409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: β-subunits and voltage-dependent K+ channels. J Biol Chem. 2007;282:24485–24489. doi: 10.1074/jbc.R700022200. [DOI] [PubMed] [Google Scholar]
- 213.McManus OB, Helms LMH, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 214.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc Natl Acad Sci USA. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Toro B, Cox N, Wilson RJJ, Garrido-Sanabria E, Stefani E, Toro L, et al. KCNMB1 Regulates surface expression of a voltage and Ca2+-activated K+ Channel via endocytic trafficking signals. Neuroscience. 2006;142:661–669. doi: 10.1016/j.neuroscience.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 216.Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bao L, Cox DH. Gating and ionic currents reveal how the BKCa channel’s Ca2+ sensitivity is enhanced by its β1 subunit. J Gen Physiol. 2005;126:393–412. doi: 10.1085/jgp.200509346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Restituito S, Cens T, Barrere C, Geib S, Galas S, De Waard M, et al. The [beta]2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J Neurosci. 2000;20:9046–9052. doi: 10.1523/JNEUROSCI.20-24-09046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brenner R, Yu JY, Srinivasan K, Brewer L, Larimer JL, Wilbur JL, et al. Complementation of physiological and behavioral defects by a slowpoke Ca2+-activated K+ channel transgene. J Neurochem. 2000;75:1310–1319. doi: 10.1046/j.1471-4159.2000.751310.x. [DOI] [PubMed] [Google Scholar]
- 220.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 221.Xia XM, Ding JP, Lingle CJ. Inactivation of BK channels by the NH2 terminus of the β2 auxiliary subunit: an essential role of a terminal peptide segment of three hydrophobic residues. J Gen Physiol. 2003;121:125–148. doi: 10.1085/jgp.20028667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Orio P, Torres Y, Rojas P, Carvacho I, Garcia ML, Toro L, et al. Structural determinants for functional coupling between the β and α subunits in the Ca2+-activated K+ (BK) channel. J Gen Physiol. 2006;127:191–204. doi: 10.1085/jgp.200509370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cox N, Toro B, Pacheco-Otalora LF, Garrido-Sanabria ER, Zarei MM. An endoplasmic reticulum trafficking signal regulates surface expression of β4 subunit of a voltage- and Ca2+-activated K+ channel. Brain Res. 2014;1553:12–23. doi: 10.1016/j.brainres.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 224.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466:513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 225.Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc Natl Acad Sci USA. 2012;109:7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang J, Yan J. Regulation of BK channels by auxiliary γ subunits. Front Physiol. 2014;5:401. doi: 10.3389/fphys.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol. 2008;294:C810–C819. doi: 10.1152/ajpcell.00511.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 229.Contreras GF, Castillo K, Enrique N, Carrasquel-Ursulaez W, Castillo JP, Milesi V, et al. A BK (Slo1) channel journey from molecule to physiology. Channels. 2013;7:442–458. doi: 10.4161/chan.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Braun AP. Structural insights into the cytoplasmic domain of a human BK channel. Channels. 5:1–3. doi: 10.4161/chan.5.1.14818. [DOI] [PubMed] [Google Scholar]
- 231.Giangiacomo KM, Fremont V, Mullmann TJ, Hanner M, Cox RH, Garcia ML. Interaction of charybdotoxin S10A with single maxi-K channels: kinetics of blockade depend on the presence of the β1 subunit. Biochemistry. 2000;39:6115–6122. doi: 10.1021/bi992865z. [DOI] [PubMed] [Google Scholar]
- 232.Giangiacomo KM, Garcia ML, McManus OB. Mechanism of iberiotoxin block of the large-conductance calcium-activated potassium channel from bovine aortic smooth muscle. Biochemistry. 1992;31:6719–6727. doi: 10.1021/bi00144a011. [DOI] [PubMed] [Google Scholar]
- 233.Garcia-Valdes J, Zamudio FZ, Toro L, Possani LD, Possan LD. Slotoxin, αKTx1.11, a new scorpion peptide blocker of MaxiK channels that differentiates between α and α+β (β1 or β4) complexes. FEBS Lett. 2001;505:369–373. doi: 10.1016/S0014-5793(01)02791-0. [DOI] [PubMed] [Google Scholar]
- 234.Garcia ML, Gao YD, McManus OB, Kaczorowski GJ. Potassium channels: from scorpion venoms to high-resolution structure. Toxicon. 2001;39:739–748. doi: 10.1016/S0041-0101(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 235.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang B, Jaffe DB, Brenner R. Current understanding of iberiotoxin-resistant BK channels in the nervous system. Front Physiol. 2014;5:382. doi: 10.3389/fphys.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gschwendt M, Müller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 238.Zakharov SI, Morrow JP, Liu G, Yang L, Marx SO. Activation of the BK (SLO1) potassium channel by mallotoxin. J Biol Chem. 2005;280:30882–30887. doi: 10.1074/jbc.M505302200. [DOI] [PubMed] [Google Scholar]
- 239.Clements RT, Cordeiro B, Feng J, Bianchi C, Sellke FW. Rottlerin increases cardiac contractile performance and coronary perfusion through BKCa++ channel activation after cold cardioplegic arrest in isolated hearts. Circulation. 2011;124:S55–S61. doi: 10.1161/CIRCULATIONAHA.110.012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Almassy J, Begenisich T. The LRRC26 protein selectively alters the efficacy of BK channel activators. Mol Pharmacol. 2012;81:21–30. doi: 10.1124/mol.111.075234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gessner G, Cui YM, Otani Y, Ohwada T, Soom M, Hoshi T, et al. Molecular mechanism of pharmacological activation of BK channels. Proc Natl Acad Sci USA. 2012;109:3552–3557. doi: 10.1073/pnas.1114321109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bentzen BH, Nardi A, Calloe K, Madsen LS, Olesen SP, Grunnet M. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol Pharmacol. 2007;72:1033–1044. doi: 10.1124/mol.107.038331. [DOI] [PubMed] [Google Scholar]
- 243.Sargent CA, Grover GJ, Antonaccio MJ, McCullough JR. The cardioprotective, vasorelaxant and electrophysiological profile of the large conductance calcium-activated potassium channel opener NS-004. J Pharmacol Exp Ther. 1993;266:1422–1429. [PubMed] [Google Scholar]
- 244.Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2070–H2077. doi: 10.1152/ajpheart.00431.2004. [DOI] [PubMed] [Google Scholar]
- 245.Jin C, Wu J, Watanabe M, Okada T, Iesaki T. Mitochondrial K+ channels are involved in ischemic postconditioning in rat hearts. J Physiol Sci. 2012;62:325–332. doi: 10.1007/s12576-012-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Park WS, Kang SH, Son YK, Kim N, Ko JH, Kim HK, et al. The mitochondrial Ca2+-activated K+ channel activator, NS 1619 inhibits L-type Ca2+ channels in rat ventricular myocytes. Biochem Biophys Res Commun. 2007;362:31–36. doi: 10.1016/j.bbrc.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 247.Saleh SN, Angermann JE, Sones WR, Leblanc N, Greenwood IA. Stimulation of Ca2+-gated Cl− currents by the calcium-dependent K+ channel modulators NS1619 [1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one] and isopimaric acid. J Pharmacol Exp Ther. 2007;321:1075–1084. doi: 10.1124/jpet.106.118786. [DOI] [PubMed] [Google Scholar]
- 248.Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Aldakkak M, Stowe DF, Cheng Q, Kwok WM, Camara AKS. Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening. Am J Physiol Cell Physiol. 2010;298:C530–C541. doi: 10.1152/ajpcell.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Aon MA, Cortassa S, Wei AC, Grunnet M, O’Rourke B. Energetic performance is improved by specific activation of K+ fluxes through KCa channels in heart mitochondria. Biochim Biophys Acta, Bioenerg. 2010;1797:71–80. doi: 10.1016/j.bbabio.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cancherini DV, Queliconi BB, Kowaltowski AJ. Pharmacological and physiological stimuli do not promote Ca2+-sensitive K+ channel activity in isolated heart mitochondria. Cardiovasc Res. 2007;73:720–728. doi: 10.1016/j.cardiores.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 252.Debska G, Kicinska A, Dobrucki J, Dworakowska B, Nurowska E, Skalska J, et al. Large-conductance K+ channel openers NS1619 and NS004 as inhibitors of mitochondrial function in glioma cells. Biochem Pharmacol. 2003;65:1827–1834. doi: 10.1016/S0006-2952(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 253.Kicinska A, Szewczyk A. Large-conductance potassium cation channel opener NS1619 inhibits cardiac mitochondria respiratory chain. Toxicol Mech Methods. 2004;14:59–61. doi: 10.1080/15376520490257482. [DOI] [PubMed] [Google Scholar]
- 254.Wrzosek A. The potassium channel opener NS1619 modulates calcium homeostasis in muscle cells by inhibiting SERCA. Cell Calcium. 2014;56:14–24. doi: 10.1016/j.ceca.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 255.Edwards G, Niederste-Hollenberg A, Schneider J, Noack Th, Weston AH. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol. 1994;113:1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Olesen SP, Munch E, Wätjen F, Drejer J. NS 004 — an activator of Ca2+-dependent K+ channels in cerebellar granule cells. Neuroreport. 1994;5:1001–1004. doi: 10.1097/00001756-199404000-00037. [DOI] [PubMed] [Google Scholar]
- 257.Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, et al. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2007;293:H1400–H1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 258.Guest M, Bull K, Walker RJ, Amliwala K, O’Connor V, Harder A, et al. The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int J Parasitol. 2007;37:1577–1588. doi: 10.1016/j.ijpara.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 259.Holden-Dye L, Crisford A, Welz C, von Samson-Himmelstjerna G, Walker RJ, O’Connor V. Worms take to the slo lane: a perspective on the mode of action of emodepside. Invert Neurosci. 2012;12:29–36. doi: 10.1007/s10158-012-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, et al. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation. 2003;107:769–776. doi: 10.1161/01.CIR.0000047278.28407.C2. [DOI] [PubMed] [Google Scholar]
- 261.Climent B, Schubert R, Stankevicius E, García-Sacristán A, Simonsen U, Rivera L. Large conductance Ca2+-activated K+ channels modulate endothelial cell outward currents and nitric oxide release in the intact rat superior mesenteric artery. Biochem Biophys Res Commun. 2012;417:1007–1013. doi: 10.1016/j.bbrc.2011.12.076. [DOI] [PubMed] [Google Scholar]
- 262.L’Heureux MC, Muinuddin A, Gaisano HY, Diamant NE. Nitric oxide activation of a potassium channel (BKCa) in feline lower esophageal sphincter. World J Gastroenterol. 2010;16:5852–5860. doi: 10.3748/wjg.v16.i46.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Avdonin V, Tang XD, Hoshi T. Stimulatory action of internal protons on Slo1 BK channels. Biophys J. 2003;84:2969–2980. doi: 10.1016/S0006-3495(03)70023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hou S, Vigeland LE, Zhang G, Xu R, Li M, Heinemann SH, et al. Zn2+ activates large conductance Ca2+-activated K+ channel via an intracellular domain. J Biol Chem. 2010;285:6434–6442. doi: 10.1074/jbc.M109.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tian L, McClafferty H, Chen L, Shipston MJ. Reversible tyrosine protein phosphorylation regulates large conductance voltage- and calcium-activated potassium channels via cortactin. J Biol Chem. 2008;283:3067–3076. doi: 10.1074/jbc.M706826200. [DOI] [PubMed] [Google Scholar]
- 266.Hosseinzadeh Z, Almilaji A, Honisch S, Pakladok T, Liu G, Bhavsar SK, et al. Upregulation of the large conductance voltage- and Ca2+-activated K+ channels by Janus kinase 2. Am J Physiol Cell Physiol. 2014;306:C1041–C1049. doi: 10.1152/ajpcell.00209.2013. [DOI] [PubMed] [Google Scholar]
- 267.Fezai M, Ahmed M, Hosseinzadeh Z, Lang F. Up-regulation of the large-conductance Ca2+-activated K+ channel by glycogen synthase kinase GSK3β. Cell Physiol Biochem. 2016;39:1031–1039. doi: 10.1159/000447810. [DOI] [PubMed] [Google Scholar]
- 268.Toro L, Li M, Zhang Z, Singh H, Wu Y, Stefani E. MaxiK channel and cell signalling. Pflugers Arch Eur J Physiol. 2014;466:875–886. doi: 10.1007/s00424-013-1359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tian L, Jeffries O, McClafferty H, Molyvdas A, Rowe ICM, Saleem F, et al. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc Natl Acad Sci USA. 2008;105:21006–21011. doi: 10.1073/pnas.0806700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhou X, Wulfsen I, Korth M, McClafferty H, Lukowski R, Shipston MJ, et al. Palmitoylation and membrane association of the stress axis regulated insert (STREX) controls BK channel regulation by protein kinase C. J Biol Chem. 2012;287:32161–32171. doi: 10.1074/jbc.M112.386359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shipston MJ. Regulation of large conductance calcium- and voltage-activated potassium (BK) channels by S-palmitoylation. Biochem Soc Trans. 2013;41:67–71. doi: 10.1042/BST20120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Grunnet M, Kaufmann WA. Coassembly of big conductance Ca2+-activated K+ channels and L-type voltage-gated Ca2+ channels in rat brain. J Biol Chem. 2004;279:36445–36453. doi: 10.1074/jbc.M402254200. [DOI] [PubMed] [Google Scholar]
- 273.Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, et al. Profiling the phospho-status of the BKCa channel α subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics. 2008;7:2188–2198. doi: 10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 275.Weaver AK, Olsen ML, McFerrin MB, Sontheimer H. BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: a novel mechanism for coupling [Ca2+]i to ion channel activation. J Biol Chem. 2007;282:31558–31568. doi: 10.1074/jbc.M702866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Stumpner J, Lange M, Beck A, Smul TM, Lotz CA, Kehl F, et al. Desflurane-induced post-conditioning against myocardial infarction is mediated by calcium-activated potassium channels: role of the mitochondrial permeability transition pore. Br J Anaesth. 2012;108:594–601. doi: 10.1093/bja/aer496. [DOI] [PubMed] [Google Scholar]
- 277.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 278.Zhang XY, Wang S, Yan Z, Wan Y, Wang W, Cui GB, et al. Molecular cloning, tissue distribution and bioinformatics analyses of the rabbit BK channel β1 subunit gene. Mol Biol Rep. 2008;35:649–655. doi: 10.1007/s11033-007-9135-x. [DOI] [PubMed] [Google Scholar]
- 279.Cheng Y, Debska-Vielhaber G, Siemen D. Interaction of mitochondrial potassium channels with the permeability transition pore. FEBS Lett. 2010;584:2005–2012. doi: 10.1016/j.febslet.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 280.Skalska J, Piwońska M, Wyroba E, Surmacz L, Wieczorek R, Koszela-Piotrowska I, et al. A novel potassium channel in skeletal muscle mitochondria. Biochim Biophys Acta, Bioenerg. 2008;1777:651–659. doi: 10.1016/j.bbabio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 281.Zoratti M, De Marchi U, Gulbins E, Szabò I. Novel channels of the inner mitochondrial membrane. Biochim Biophys Acta, Bioenerg. 2009;1787:351–363. doi: 10.1016/j.bbabio.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 282.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/bj3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Franciolini F, Hogg R, Catacuzzeno L, Petris A, Trequattrini C, Adams DJ. Large-conductance calcium-activated potassium channels in neonatal rat intracardiac ganglion neurons. Pflugers Arch Eur J Physiol. 2001;441:629–638. doi: 10.1007/s004240000471. [DOI] [PubMed] [Google Scholar]
- 284.Callewaert G, Vereecke J, Carmeliet E. Existence of a calcium-dependent potassium channel in the membrane of cow cardiac Purkinje cells. Pflugers Arch Eur J Physiol. 1986;406:424–426. doi: 10.1007/BF00590947. [DOI] [PubMed] [Google Scholar]
- 285.Budelli G, Sun Q, Ferreira J, Butler A, Santi CM, Salkoff L. SLO2 channels are inhibited by all divalent cations that activate SLO1 K+ channels. 2016;291:7347–7356. doi: 10.1074/jbc.M115.709436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Brown MR, Kronengold J, Gazula VR, Spilianakis CG, Flavell RA, Von Hehn CAA, et al. Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J Physiol. 2008;586:5161–5179. doi: 10.1113/jphysiol.2008.160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kaczmarek LK. Slack, slick and sodium-activated potassium channels. ISRN Neurosci. 2013;2013:1–14. doi: 10.1155/2013/354262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984;309:354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- 289.Bhattacharjee A, Kaczmarek L. For K channels, Na is the new Ca. Trends Neurosci. 2005;28:422–428. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 290.Tejada MdLA, Stolpe K, Meinild AK, Klaerke DA. Clofilium inhibits Slick and Slack potassium channels. Biologics Targets Therapy. 2012;6:465–470. doi: 10.2147/BTT.S33827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Garg P, Gardner A, Garg V, Sanguinetti MC. Structural basis of ion permeation gating in Slo2.1K+ channels. J Gen Physiol. 2013;142:523–542. doi: 10.1085/jgp.201311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Paulais M, Lachheb S, Teulon J. A Na+- and Cl−-activated K+ channel in the thick ascending limb of mouse kidney. J Gen Physiol. 2006;127:205–215. doi: 10.1085/jgp.200509360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Garg P, Sanguinetti MC. Intracellular ATP does not inhibit Slo2.1 K+ channels. Physiol Rep. 2014;2:e12118. doi: 10.14814/phy2.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dai L, Garg V, Sanguinetti MC. Activation of Slo2.1 channels by niflumic acid. J Gen Physiol. 2010;135:275–295. doi: 10.1085/jgp.200910316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Garg P, Sanguinetti MC. Structure-activity relationship of fenamates as Slo2.1 channel activators. Mol Pharmacol. 2012;82:795–802. doi: 10.1124/mol.112.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gribble FM, Ashcroft FM. Sulfonylurea sensitivity of adenosine triphosphate-sensitive potassium channels from β cells and extrapancreatic tissues. Metabolism. 2000;49:3–6. doi: 10.1053/meta.2000.17822. [DOI] [PubMed] [Google Scholar]
- 297.Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol. 1997;504:35–45. doi: 10.1111/j.1469-7793.1997.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tamsett TJ, Picchione KE, Bhattacharjee A. NAD+ activates KNa channels in dorsal root ganglion neurons. J Neurosci. 2009;29:5127–5134. doi: 10.1523/JNEUROSCI.0859-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Dabrowski M, Trapp S, Ashcroft FM. Pyridine nucleotide regulation of the KATP channel Kir6.2/SUR1 expressed in Xenopus oocytes. J Physiol. 2003;550:357–363. doi: 10.1113/jphysiol.2003.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lee S, Park M, So I, Earm YE. NADH and NAD modulates Ca2+-activated K+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflugers Arch Eur J Physiol. 1994;427:378–380. doi: 10.1007/BF00374548. [DOI] [PubMed] [Google Scholar]
- 301.Tipparaju SM, Saxena N, Liu SQ, Kumar R, Bhatnagar A. Differential regulation of voltage-gated K+ channels by oxidized and reduced pyridine nucleotide coenzymes. Am J Physiol Cell Physiol. 2005;288:C366–C376. doi: 10.1152/ajpcell.00354.2004. [DOI] [PubMed] [Google Scholar]
- 302.Tomasello DL, Gancarz-Kausch AM, Dietz DM, Bhattacharjee A. Transcriptional regulation of the sodium-activated potassium channel SLICK (KCNT2) promoter by nuclear factor-κB. J Biol Chem. 2015;290:18575–18583. doi: 10.1074/jbc.M115.643536303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lee J-C, Tae H-J, Kim IH, Cho JH, Lee T-K, Park JH, et al. Roles of HIF-1α, VEGF, and NF-κB in ischemic preconditioning-mediated neuroprotection of hippocampal CA1 pyramidal neurons against a subsequent transient cerebral ischemia. Mol Neurobiol. 2016:15. doi: 10.1007/s12035-016-0219-2. [DOI] [PubMed] [Google Scholar]
- 304.Qiao S, Xie H, Wang C, Wu X, Liu H, Liu C. Delayed anesthetic preconditioning protects against myocardial infarction via activation of nuclear factor-κB and upregulation of autophagy. J Anesth. 2013;27:251–260. doi: 10.1007/s00540-012-1494-3. [DOI] [PubMed] [Google Scholar]
- 305.Shi S, Yang W, Tu X, Chen C, Wang C. Ischemic preconditioning reduces ischemic brain injury by suppressing nuclear factor kappa B expression and neuronal apoptosis. Neural Regen Res. 2013;8:633–638. doi: 10.3969/j.issn.1673-5374.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wilhide ME, Tranter M, Ren X, Chen J, Sartor MA, Medvedovic M, et al. Identification of a NF-κB cardioprotective gene program: NF-κB regulation of Hsp70.1 contributes to cardioprotection after permanent coronary occlusion. J Mol Cell Cardiol. 2011;51:82–89. doi: 10.1016/j.yjmcc.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wojtovich AP, Smith CO, Urciuoli WR, Wang YT, Xia XM, Brookes PS, et al. Cardiac Slo2.1 is required for volatile anesthetic stimulation of K+ transport and anesthetic preconditioning. Anesthesiology. 2016;124:1065–1076. doi: 10.1097/ALN.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mori K, Kobayashi S, Saito T, Masuda Y, Nakaya H. Inhibitory effects of class I and IV antiarrhythmic drugs on the Na+-activated K+ channel current in guinea pig ventricular cells. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:641–648. doi: 10.1007/PL00005306. [DOI] [PubMed] [Google Scholar]
- 309.Li L, Vaali K, Vapaatalo H, Kankaanranta H. Effects of K+ channel inhibitors on relaxation induced by flufenamic and tolfenamic acids in guinea-pig trachea. Eur J Pharmacol. 1999;383:169–176. doi: 10.1016/S0014-2999(99)00634-2. [DOI] [PubMed] [Google Scholar]
- 310.Yang B, Gribkoff V, Pan J, Damagnez V, Dworetzky S, Boissard C, et al. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology. 2006;51:896–906. doi: 10.1016/j.neuropharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 311.Enzie FD, Colglazier ML. Preliminary trials with bithionol against tapeworm infections in cats, dogs, sheep, and chickens. Am J Vet Res. 1960;21:628–630. [PubMed] [Google Scholar]
- 312.Barr FS, Collins GF, Wyatt LG. Potentiation of the antimicrobial activity of bithionol. J Pharm Sci. 1965;54:801–802. doi: 10.1002/jps.2600540534. [DOI] [PubMed] [Google Scholar]
- 313.Ohya S, Kuwata Y, Sakamoto K, Muraki K, Imaizumi Y. Cardioprotective effects of estradiol include the activation of large-conductance Ca2+-activated K+ channels in cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2005;289:H1635–H1642. doi: 10.1152/ajpheart.00016.2005. [DOI] [PubMed] [Google Scholar]
- 314.Zhang L, Sukhareva M, Barker JL, Maric D, Hao Y, Chang YH, et al. Direct binding of estradiol enhances slack (sequence like a calcium-activated potassium channel) channels’ activity. Neuroscience. 2005;131:275–282. doi: 10.1016/j.neuroscience.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 315.Liu J, Bukiya AN, Kuntamallappanavar G, Singh AK, Dopico AM. Distinct sensitivity of Slo1 channel proteins to ethanol. Mol Pharmacol. 2013;83:235–244. doi: 10.1124/mol.112.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lee KS, Tsien RW. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983;302:790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- 317.Biton B, Sethuramanujam S, Picchione KE, Bhattacharjee A, Khessibi N, Chesney F, et al. The antipsychotic drug loxapine is an opener of the sodium-activated potassium channel slack (Slo2.2) J Pharmac Exp Ther. 2012;340:706–715. doi: 10.1124/jpet.111.184622. [DOI] [PubMed] [Google Scholar]
- 318.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci USA. 2011;108:5879–5884. doi: 10.1073/pnas.1100240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Perry M, de Groot MJ, Helliwell R, Leishman D, Tristani-Firouzi M, Sanguinetti MC, et al. Structural determinants of HERG channel block by clofilium and ibutilide. Mol Pharmacol. 2004;66:240–249. doi: 10.1124/mol.104.000117. [DOI] [PubMed] [Google Scholar]
- 320.Gessner G, Heinemann SH. Inhibition of hEAG1 and hERG1 potassium channels by clofilium and its tertiary analogue LY97241. Br J Pharmacol. 2003;138:161–171. doi: 10.1038/sj.bjp.0705025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Malayev AA, Nelson DJ, Philipson LH. Mechanism of clofilium block of the human Kv1.5 delayed rectifier potassium channel. Mol Pharmacol. 1995;47:198–205. [PubMed] [Google Scholar]
- 322.Honoré E, Attali B, Romey G, Heurteaux C, Ricard P, Lesage F, et al. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991;10:2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yang WP, Levesque PC, Little WA, Conder ML, Shalaby FY, Blanar MA. KvLQT1, a voltage-gated potassium channel responsible for human cardiac arrhythmias. Proc Natl Acad Sci USA. 1997;94:4017–4021. doi: 10.1073/pnas.94.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res. 2009;104:292–303. doi: 10.1161/CIRCRESAHA.108.189050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Santi CM, Ferreira G, Yang B, Gazula VR, Butler A, Wei A, et al. Opposite regulation of Slick and Slack K+ channels by neuromodulators. J Neurosci. 2006;26:5059–5068. doi: 10.1523/JNEUROSCI.3372-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yang B, Desai R, Kaczmarek LK. Slack and Slick KNa channels regulate the accuracy of timing of auditory neurons. J Neurosci. 2007;27:2617–2627. doi: 10.1523/JNEUROSCI.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Uhlig C, Bluth T, Schwarz K, Deckert S, Heinrich L, De Hert S, et al. Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: a systematic review and meta-analysis. Anesthesiology. 2016;124:1230–1245. doi: 10.1097/ALN.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 328.Nicoll A, Moore D, Njoku D, Hockey B. Repeated exposure to modern volatile anaesthetics may cause chronic hepatitis as well as acute liver injury. Case Reports. 2012 doi: 10.1136/bcr-2012-006543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wagner NM, Gross ER, Patel HH. A slick way volatile anesthetics reduce myocardial injury. Anesthesiology. 2016;124:986–988. doi: 10.1097/ALN.0000000000001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Garlid KD, Costa ADT, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46:858–866. doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Costa ADT, Quinlan CL, Andrukhiv A, West IC, Jabůrek M, Garlid KD. The direct physiological effects of mitoKATP opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–H415. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 332.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–H2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 333.Murphy MP. Understanding and preventing mitochondrial oxidative damage. Biochem Soc Trans. 2016;44:1219–1226. doi: 10.1042/BST20160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2004;94:7–16. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- 335.Novalija E, Kevin LG, Eells JT, Henry MM, Stowe DF. Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology. 2003;98:1155–1163. doi: 10.1097/00000542-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 336.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Jang S, Lewis TS, Powers C, Khuchua Z, Baines CP, Wipf P, et al. Elucidating mitochondrial electron transport chain supercomplexes in the heart during ischemia–reperfusion. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KHH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 340.Minners J, van den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res. 2000;47:68–73. doi: 10.1016/S0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 341.Minners J, Lacerda L, McCarthy J, Meiring JJ, Yellon DM, Sack MN. Ischemic and pharmacological preconditioning in Girardi cells and C2C12 myotubes induce mitochondrial uncoupling. Circ Res. 2001;89:787–792. doi: 10.1161/hh2101.098372. [DOI] [PubMed] [Google Scholar]
- 342.Zhang J, Nadtochiy SM, Urciuoli WR, Brookes PS. The cardioprotective compound cloxyquin uncouples mitochondria and induces autophagy. Am J Physiol Heart Circ Physiol. 2016;310:H29–H38. doi: 10.1152/ajpheart.00926.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3:186. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Chen Q, Camara AKS, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 345.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Penna C, Perrelli MG, Pagliaro P. Mitochondrial pathways, permeability transition pore, and redox signaling in cardioprotection: therapeutic implications. Antioxid Redox Signal. 2013;18:556–599. doi: 10.1089/ars.2011.4459. [DOI] [PubMed] [Google Scholar]
- 347.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–466. doi: 10.1161/01.RES.87.6.460. [DOI] [PubMed] [Google Scholar]
- 348.Fretwell L, Dickenson JM. Role of large-conductance Ca2+-activated potassium channels in adenosine A1 receptor-mediated pharmacological preconditioning in H9c2 cells. Eur J Pharmacol. 2009;618:37–44. doi: 10.1016/j.ejphar.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 349.Ge ZD, Pravdic D, Bienengraeber M, Pratt PF, Auchampach JA, Gross GJ, et al. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology. 2010;112:73–85. doi: 10.1097/ALN.0b013e3181c4a607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Tessier-Vetzel D, Tissier R, Waintraub X, Ghaleh B, Berdeaux A. Isoflurane inhaled at the onset of reperfusion potentiates the cardioprotective effect of ischemic postconditioning through a NO-dependent mechanism. J Cardiovasc Pharmacol. 2006;47:487–492. doi: 10.1097/01.fjc.0000211731.69045.fe. [DOI] [PubMed] [Google Scholar]
- 351.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 352.Altugˇ S, Demiryürek AT, Kane KA, Kanzik I. Evidence for the involvement of peroxynitrite in ischaemic preconditioning in rat isolated hearts. Br J Pharmacol. 2000;130:125–131. doi: 10.1038/sj.bjp.0703280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 354.Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–429. doi: 10.1161/01.RES.77.2.424. [DOI] [PubMed] [Google Scholar]
- 355.Das DK, Maulik N, Sato M, Ray PS. Reactive oxygen species function as second messenger during ischemic preconditioning of heart. Mol Cell Biochem. 1999;196:59–67. doi: 10.1023/A:1006966128795. [DOI] [PubMed] [Google Scholar]
- 356.Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol. 1997;29:2441–2450. doi: 10.1006/jmcc.1997.0481. [DOI] [PubMed] [Google Scholar]
- 357.Müllenheim J, Ebel D, Frädorf J, Preckel B, Thämer V, Schlack W, et al. Isoflurane preconditions myocardium against infarction via release of free radicals. Anesthesiology. 2002;96:934–940. doi: 10.1097/00000542-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 358.Valen G, Starkopf J, Takeshima S, Kullisaar T, Vihalemm T, Kengsepp AT, et al. Preconditioning with hydrogen peroxide (H2O2) or ischemia in H2O2-induced cardiac dysfunction. Free Radic Res. 1998;29:235–245. doi: 10.1080/10715769800300271. [DOI] [PubMed] [Google Scholar]
- 359.Zhou T, Chuang CC, Zuo L. Molecular characterization of reactive oxygen species in myocardial ischemia-reperfusion injury. Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/864946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Queliconi BB, Wojtovich AP, Nadtochiy SM, Kowaltowski AJ, Brookes PS. Redox regulation of the mitochondrial KATP channel in cardioprotection. Biochim Biophys Acta, Mol Cell Res. 2011;1813:1309–1315. doi: 10.1016/j.bbamcr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Costa ADT, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKC, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295:H874–H882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Stamm C, Friehs I, Choi YH, Zurakowski D, McGowan FX, del Nido PJ. Cytosolic calcium in the ischemic rabbit heart: assessment by pH- and temperature-adjusted rhod-2 spectrofluorometry. Cardiovasc Res. 2003;59:695–704. doi: 10.1016/S0008-6363(03)00467-X. [DOI] [PubMed] [Google Scholar]
- 363.Seppet EK, Kallikorm AP, Dzhavadov SA, Preobrazhenskiĭ AN, Lakomkin VA. [Energy-related disorders of myocardial contractility in calcium overload of the cardiomyocytes] Kardiologiia. 1987;27:72–76. [PubMed] [Google Scholar]
- 364.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Sin J, Andres AM, Taylor DJR, Weston T, Hiraumi Y, Stotland A, et al. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2016;12:369–380. doi: 10.1080/15548627.2015.1115172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM, et al. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res. 2010;3:365–373. doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ma L, Zhu J, Gao Q, Rebecchi MJ, Wang Q, Liu L. Restoring pharmacologic preconditioning in the aging heart: role of mitophagy/autophagy. J Gerontol A Biol Sci Med Sci. 2016:glw168. doi: 10.1093/gerona/glw168. [DOI] [PubMed] [Google Scholar]
- 368.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 369.Tao H, Zhang Y, Zeng X, Shulman GI, Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med. 2014;20:1263–1269. doi: 10.1038/nm.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Miyadera H, Shiomi K, Ui H, Yamaguchi Y, Masuma R, Tomoda H, et al. Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase) Proc Natl Acad Sci USA. 2003;100:473–477. doi: 10.1073/pnas.0237315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Cortese JD, Voglino AL, Hackenbrock CR. The ionic strength of the intermembrane space of intact mitochondria is not affected by the pH or volume of the intermembrane space. Biochim Biophys Acta, Bioenerg. 1992;1100:189–197. doi: 10.1016/0005-2728(92)90081-C. [DOI] [PubMed] [Google Scholar]
- 372.Wittig I, Carrozzo R, Santorelli FM, Schägger H. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim Biophys Acta, Bioenerg. 1757:1066–1072. doi: 10.1016/j.bbabio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 373.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective peptide. J Am Heart Assoc. 2012;1:e001644. doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Shi J, Dai W, Hale SL, Brown DA, Wang M, Han X, et al. Bendavia restores mitochondrial energy metabolism gene expression and suppresses cardiac fibrosis in the border zone of the infarcted heart. Life Sci. 2015;141:170–178. doi: 10.1016/j.lfs.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Brown DA, Sabbah HN, Shaikh SR. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther. 2013;140:258–266. doi: 10.1016/j.pharmthera.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 376.Shaikh SR, Sullivan EM, Alleman RJ, Brown DA, Zeczycki TN. Increasing mitochondrial membrane phospholipid content lowers the enzymatic activity of electron transport complexes. Biochemistry. 2014;53:5589–5591. doi: 10.1021/bi500868g. [DOI] [PubMed] [Google Scholar]
- 377.da Silva MM, Sartori A, Belisle E, Kowaltowski AJ. Ischemic preconditioning inhibits mitochondrial respiration, increases H2O2 release, and enhances K+ transport. Am J Physiol Heart Circ Physiol. 2003;285:H154–H162. doi: 10.1152/ajpheart.00955.2002. [DOI] [PubMed] [Google Scholar]
- 378.Eliseev RA, Vanwinkle B, Rosier RN, Gunter TE. Diazoxide-mediated preconditioning against apoptosis involves activation of cAMP-response element-binding protein (CREB) and NFκB. J Biol Chem. 2004;279:46748–46754. doi: 10.1074/jbc.M406217200. [DOI] [PubMed] [Google Scholar]
- 379.Bennett K, James C, Hussain K. Pancreatic β-cell KATP channels: hypoglycaemia and hyperglycaemia. Rev Endocr Metab Disord. 2010;11:157–163. doi: 10.1007/s11154-010-9144-2. [DOI] [PubMed] [Google Scholar]
- 380.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 381.Calderone V. Large-conductance, Ca2+-activated K+ channels: function, pharmacology and drugs. Curr Med Chem. 2002;9:1385–1395. doi: 10.2174/0929867023369871. [DOI] [PubMed] [Google Scholar]
- 382.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 383.Whitt JP, Montgomery JR, Meredith AL. BK channel inactivation gates daytime excitability in the circadian clock. Nat Commun. 2016;7:10837. doi: 10.1038/ncomms10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Nicholls DG. A history of UCPI. Biochem Soc Trans. 2001;29:751–755. doi: 10.1042/bst0290751. [DOI] [PubMed] [Google Scholar]
- 386.Brand MD, Brindle KM, Buckingham JA, Harper JA, Rolfe DFS, Stuart JA. The significance and mechanism of mitochondrial proton conductance. Int J Obes Relat Metab Disord. 1999;23(Suppl 6):S4–S11. doi: 10.1038/sj.ijo.0800936. [DOI] [PubMed] [Google Scholar]
- 387.Alán L, Smolková K, Kronusová E, Šantorová J, Ježek P. Absolute levels of transcripts for mitochondrial uncoupling proteins UCP2, UCP3, UCP4, and UCP5 show different patterns in rat and mice tissues. J Bioenerg Biomembr. 2009;41:71–78. doi: 10.1007/s10863-009-9201-2. [DOI] [PubMed] [Google Scholar]
- 388.Hoang T, Smith MD, Jelokhani-Niaraki M. Toward understanding the mechanism of ion transport activity of neuronal uncoupling proteins UCP2, UCP4, and UCP5. Biochemistry. 2012;51:4004–4014. doi: 10.1021/bi3003378. [DOI] [PubMed] [Google Scholar]
- 389.Jia JJ, Zhang X, Ge CR, Jois M. The polymorphisms of UCP2 and UCP3 genes associated with fat metabolism, obesity and diabetes. Obes Rev. 2009;10:519–526. doi: 10.1111/j.1467-789X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 390.Nedergaard J, Cannon B. Sulfonates are low-affinity ligands for the GDP-binding site of brown-fat mitochondria. Biochim Biophys Acta, Bioenerg. 1994;1185:311–317. doi: 10.1016/0005-2728(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 391.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 392.MacLellan JD, Gerrits MF, Gowing A, Smith PJS, Wheeler MB, Harper ME. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005;54:2343–2350. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- 393.Mnatsakanyan N, Beutner G, Porter GA, Alavian KN, Jonas EA. Physiological roles of the mitochondrial permeability transition pore. J Bioenerg Biomembr. 2016:1–13. doi: 10.1007/s10863-016-9652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 395.Warltier DC, Al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988;69:552–565. doi: 10.1097/00000542-198810000-00016. [DOI] [PubMed] [Google Scholar]
- 396.Chaban Y, Boekema EJ, Dudkina NV. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim Biophys Acta, Bioenerg. 2014;1837:418–426. doi: 10.1016/j.bbabio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 397.Vinothkumar KR, Zhu J, Hirst J. Architecture of mammalian respiratory complex I. Nature. 2014;515:80–84. doi: 10.1038/nature13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- 399.Yu T, Fu XY, Liu XK, Yu ZH. Protective effects of pinacidil hyperpolarizing cardioplegia on myocardial ischemia reperfusion injury by mitochondrial KATP channels. Chin Med J. 2011;124:4205–4210. [PubMed] [Google Scholar]
- 400.Kopustinskiene D, Liobikas J, Skemiene K, Malinauskas F, Toleikis A. Direct effects of KATP channel openers pinacidil and diazoxide on oxidative phosphorylation of mitochondria in situ. Cell Physiol Biochem. 2010;25:181–186. doi: 10.1159/000276552. [DOI] [PubMed] [Google Scholar]
- 401.Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE, et al. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol Pharmacol. 1996;50:206–217. [PubMed] [Google Scholar]
- 402.Garcia-Valdes J, Zamudio FZ, Toro L, Possan LD. Slotoxin, K KTx111, a new scorpion peptide blocker of MaxiK channels that di ¡ erentiates between K and K + L (L 1 or L 4) complexes. FEBS Lett. 2001;505:369–373. doi: 10.1016/S0014-5793(01)02791. [DOI] [PubMed] [Google Scholar]
- 403.MacKinnon R, Miller C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science. 1989;245:1382–1385. doi: 10.1126/science.2476850. [DOI] [PubMed] [Google Scholar]


