Abstract
The impact of light intensity on the degree of conversion (DC), rate of polymerization and network structure was investigated for hydrophobic and hydrophilic dental adhesive resins. Two and three component photoinitiating (PI) systems were used in this study. Low light intensities had a negative impact on the polymerization efficiency for the hydrophilic resin with 2 component PI system. Incorporation of iodonium salt in the hydrophilic resin significantly improved the polymerization efficiency of the HEMA/BisGMA system and led to a substantial DC, even at low light intensities. The results suggested that shorter polymer chains were formed in the presence of iodonium salt. It appears that there is little or no impact of light intensity on the polymer structure of the 2 component PI system. Light intensity has subtle impact on the polymer structure of the 3 component PI system. In the case of the hydrophobic resin, the polymer is so highly cross-linked that the presence of shorter chains for the 3 component PI system does not cause a decrease in the glass transition temperature (Tg) when compared to the 2 component PI system. For the hydrophilic resin, the presence of shorter polymer chains in the 3 component PI system reduces the Tg when compared with the corresponding 2 component PI system.
Keywords: hydrophobic phase, hydrophilic phase, light intensity, dental adhesive, photopolymerization, crosslink structure
INTRODUCTION
Secondary decay is a primary factor in the premature failure and limited clinical lifetime of moderate to large class II dental composite restorations.1 The gingival margin of the class II dental composite restoration is particularly vulnerable to early failure and at this margin, the dentin/adhesive bond is the primary barrier to oral fluids, cariogenic bacteria, and other noxious agents that will undermine the composite restoration.2 Failure of the dentin/adhesive bond has been associated with incomplete polymerization of the adhesive monomers, incomplete infiltration, adhesive phase separation, and hydrolytic/enzymatic degradation.3–6
The adhesive resin separates into hydrophobic- and hydrophilic-rich phases as it infiltrates the wet, demineralized dentin collagen.6,7 As the resin penetrates deeper into the wet collagen matrix, the concentration of the cross-linker and the widely used photoinitiator (camphoquinone and EDMAB) decreases. The major components within the hydrophilic-rich phase are water and the monomethacrylate, 2-hydroxyethyl methacrylate (HEMA).8,9 The lack of photoinitiators (PI) within the hydrophilic-rich phase could compromise the degree of conversion (DC) of this phase.10 The limited DC could result in early failure and leaching of the unreacted species. Since visible light is commonly used for polymerization of adhesives and composites, it is important to understand the impact of light intensity on the polymerization of the phases that develop as a result of adhesive phase separation.
Previous investigations regarding the impact of light intensity on polymerization have focused primarily on the dental composite. The impact of light intensity on volumetric shrinkage and hardness of composite restorations has been investigated by several authors.11,12 Discacciati et al. demonstrated that irradiation time and light intensity, for example, 200 and 400 mW/cm2, had a significant effect on Vickers hardness, but these parameters had no effect on volumetric polymerization shrinkage of dental composite.11 Kaban et al. showed that higher light intensity, for example, 700 mW/cm2, increases shrinkage which could lead to marginal gap formation.12 The effect of irradiation time, at low and high light intensities, as well as ramp-curing on the DC and polymerization shrinkage of composite restorations has been investigated.13–15 The impact of different light curing units (LCU) on the polymerization of commercially available composite resins has been studied, but the efficiency of the LCU is dependent on the spectral absorbance of the photosensitizer in the resins.14,16–18 In general, higher light intensity has been proposed to increase the mechanical properties and depth of cure of the composite resin.19 Higher light intensity is expected to reduce the curing time without compromising the material properties.
Emami et al. showed that total energy (light intensity × exposure time) is more important than light intensity.15 The work by Emami et al. showed that irrespective of light intensity, composites possessing a similar DC, Young’s modulus, and volumetric shrinkage could be produced as long as the total energy is the same, that is, the formation of reactive species depends on the number of useful photons.15 Lovell et al. investigated the copolymerization of 2,2 bis[4-(2-hydoxy-3-methacryloxypropoxy)phenyl] propane (BisGMA) and triethylene glycol dimethacrylate (TEGDMA) under various temperatures and UV light intensities.20 Lovell and his colleagues showed that both the DC and rate of polymerization were enhanced with an increase in light intensity; the rate for 50/50 BisGMA/TEGDMA at 25°C was proportional to the light intensity raised to the power of 0.6.20 For the copolymerization of BisGMA and TEGDMA, a change in the light intensity did not have a significant impact on the network formation.21 Lovell et al. investigated the conversion and flexural strength of the viscous dimethacrylate system for two LCUs (QTH and PAC) at 200 and 2000 mW/cm2.22 The researchers concluded that the final flexural strength was independent of light intensity for similar final conversion.22
With a few exceptions, the work regarding the effect of light intensity on polymerization was conducted using dental composite resin. Ye et al. investigated the effect of light intensity on three commercially available dentin adhesives (Single Bond, One-up Bond F, and Adper Prompt) and showed that for the more hydrophilic adhesive, that is, Adper Prompt, the DC depended on the light intensity (60 s curing time).23 Yamamoto et al. investigated commercially available dentin adhesive systems and reported lower shear bond strength values for low light intensity, that is, 150 mW/cm2.24 These resins were rich in cross-linker concentration and are comparable to the hydrophobic-rich phase in the phase-separated dentin adhesives.
There has been limited investigation involving the effect of light intensity on the polymerization behavior of the hydrophilic-rich phase of dentin adhesives. Methacrylate hydrogels are similar in composition to the hydrophilic-rich phase and the effect of light intensity on the polymerization of these hydrogels has been studied.25,26 He et al. showed that for the methacrylic acid (MAA)/TEGDMA system, excessively high UV light intensity had a negative impact on the photopolymerization. Light intensity had a significant effect on the onset of macrogelation.25 He et al. proposed structure formation involving initiation, microgel, cluster formation, macrogelation, and post gelation for the MAA/TEGDMA system.25 Abedin et al. proposed a reaction mechanism involving polymerization- and solvent-induced phase separation for dilute BisGMA/HEMA system containing deuterium oxide (D2O).27 Previous investigation on the dilute HEMA/diethylene glycol dimethacrylate hydrogel showed that the reaction profiles varied as a function of the light intensity and increased light intensity delayed macrogelation.26
Studies have shown that light intensity varies as a function of distance from the tip of the LCU and the decrease in light intensity with distance had an adverse effect on the polymerization of composite resin.28,29 Under clinical conditions, there will be variable light intensity along the depth and breadth of the hybrid layer and this variable light intensity may impact the polymerization of the hydrophilic-rich phase. The objective of this study was to investigate the influence of visible light intensity on the DC, rate of polymerization and polymer structure of simplified model dentin adhesive resin representing the hydrophobic- and hydrophilic-rich phases. To eliminate the complications associated with water, that is, evaporation leading to variable water concentration, water was not included in the formulations for this investigation. The simplified model dentin adhesive resin representing the hydrophilic-rich phase was rich in the monomethacrylate component HEMA, 95 wt % and poor in the dimethacrylate component BisGMA, 5 wt %. Two types of photoinitiating (PI) systems, 2 components (2PI,) and 3 components (3PI), were used in this study. The results were compared with simplified model dentin adhesive resin representing the hydrophobic-rich phase. The latter consisted of 45 wt % HEMA and 55 wt % BisGMA; this composition is similar to a variety of commercially available dentin adhesives.30 These simplified formulations provide a straightforward means of studying the impact of light intensity on the polymerization of phases that develop as a result of phase separation in dentin adhesive resin.
MATERIALS AND METHODS
The monomers used in the formulations were HEMA from Acros Organics and Bisphenol A glycerolate dimethacrylate (BisGMA) from Polysciences, Washington, PA. The photoinitiators were camphorquinone (CQ) and ethyl 4-(dimethylamino) benzoate (EDMAB). The photoinitiators were obtained from Aldrich, Milwaukee, WI. The composition of the 2PI system is CQ and EDMAB. The accelerator, Diphenyliodonium hexafluorophosphate (DPIHP), which acts as the third component in the 3PI system, was also from Aldrich, Milwaukee, WI.
The hydrophilic formulation (HB95NR) was prepared by adding photoinitiators, CQ and EDMAB, to the desired quantity of HEMA. BisGMA was added to the solution to achieve a mass ratio of HEMA to BisGMA of 95/5. The mixture was agitated overnight until a homogeneous solution was obtained. In the case of the 2PI system, the photoinitiators were added such that there was 0.5 wt % of CQ and EDMAB each in the solution; the concentrations were based on the total mass of the solution. For the 3PI system, in addition to the CQ and EDMAB, 0.5 wt % DPIHP was also added.
The hydrophobic resin (HB45NR) was prepared similar to the approach described above but the mass ratio for HEMA to BisGMA was 45/55. There were four types of formulations, two hydrophilic and two hydrophobic. The compositions are given in Table I. Three samples were prepared for each formulation.
TABLE I.
Composition of Monomethacrylate and Dimethacrylate Monomers in the Formulations
| PI component | Name | % wt HEMA | % wt BisGMA |
|---|---|---|---|
| 2PIa | HB45NR2PI | 44.96 ± 0.03 | 55.04 ± 0.03 |
| HB95NR2PI | 94.96 ± 0.06 | 5.04 ± 0.06 | |
| 3PIb | HB45NR3PI | 45.00 ± 0.01 | 55.00 ± 0.01 |
| HB95NR3PI | 94.98 ± 0.01 | 5.02 ± 0.01 |
Two component PI system contains 0.5 wt % camphoquinone (CQ) and EDMAB each.
Three component PI system contains 0.5 wt % camphoquinone (CQ), EDMAB and iodonium salt (DPIHP) each.
The formulation name HBxNRyPI means that the formulation contains x wt % of HEMA and (100 − x) wt % of BisGMA, HB stands for HEMA/BisGMA, NR means neat resin, y = 2 or 3 based on the type of PI system.
Polymerization kinetics study
The polymerization kinetics study was carried out using Perkin-Elmer Spectrum 400 Fourier transform infrared spectrophotometer (FTIR) with a resolution of 4 cm−1 in the ATR sampling mode.23,30 A time resolved spectrum collector allowed in-situ monitoring of the photopolymerization reaction. For each formulation, 30 μL was placed on the ATR crystal and covered with a plastic coverslip. The edges of the coverslip were sealed to prevent diffusion of oxygen from the environment; oxygen diffusion could interfere with the polymerization reaction. The sample was cured for 40 s using a dental curing light unit (Spectrum® 800, Dentsply, Milford, DE). The kinetic study for each formulation was carried out by curing the samples at different light intensities, that is, 25, 50, 100, 229, 455, and 679 mW/cm2.
The halogen LCU, used in this study, has a broad emission spectrum with peak wavelength at 488 nm.31 It has a built in system that allows the operator to vary the light intensity from 300 to 800 mW/cm2. To achieve light intensities of 25, 50, and 100 mW/cm2 the distance between the tip of the LCU and sample was varied. The first step was to determine the distance between the tip of the LCU and the visible light intensity meter that led to a reading of 25, 50, or 100 mW/cm2. The distance between the tip of the LCU and the meter was recorded. The tip of the LCU was then set at the appropriate distance from the sample on the ATR crystal to obtain intensities of 25, 50, or 100 mW/cm2. For the remaining intensities, the LCU was set at 300, 550, or 800 mW/cm2 and the corresponding intensities were read by using the visible light intensity meter. The readings of the intensity meter are given in Table II. The polymerization kinetics of the hydrophobic resin was monitored in-situ for 1 h and for the hydrophilic resin it was 2 h. The ratio of the absorption of C═C at 1637 cm−1 to C═O at 1716 cm−1 was monitored and Eq. (1) was used to calculate the DC. For each formulation, the polymerization kinetics at each light intensity was monitored in triplicate.
TABLE II.
Light Intensity Setting on the LCU and Corresponding Intensity Reading on the Visible Light Intensity Meter
| Light Intensity on the LCU (mW/cm2) | Light Intensity Read by the Visible Light Intensity Meter (mW/cm2) |
|---|---|
| 300 | 229 |
| 550 | 455 |
| 800 | 679 |
| (1) |
In the case of the hydrophilic resins, the final DC was measured using pan samples. The neat resins were placed into low-mass aluminum DSC pans and the pan was covered with a coverslip following transfer of the sample into the pan. The sample was cured at the desired light intensity for 40 s. For each formulation, three samples per light intensity were prepared. The pan samples were stored in the dark at room temperature for 24 h before removing the coverslip. The ratio of the absorption of C═C at 1637 cm−1 to C═O at 1716 cm−1 was measured for the top and bottom surfaces of each pan sample using FTIR-ATR. The DC was calculated using Eq. (1). The sample size for the polymerization kinetics study and final DC using pan samples was 3 for each intensity.
Differential Scanning Calorimetry (DSC) Study
The variation of polymer structure when cured at different light intensities was investigated for both hydrophobic and hydrophilic resins using DSC. Pan samples were prepared similar to the approach described above. The samples were kept in the dark for 24 h at room temperature before removing the coverslip. After 24 h, the samples were stored in a vacuum chamber at 37°C. The mass of the samples was measured at specific time intervals until the difference in the consecutive mass was < 0.0003 g. The hydrophobic resin samples were cured at intensities of 100, 229, 455, and 679 mW/cm2. For the hydrophilic resins, the samples were cured at all six intensities (Table IV). Storing the samples in a vacuum chamber at higher pressure ensured that there was minimum unreacted monomer in the samples before the DSC test.
TABLE IV.
Tg at for Hydrophobic and Hydrophilic Resins Cured at Various Light Intensities
| Sample Name |
Tg (°C)
|
|||||
|---|---|---|---|---|---|---|
| 25 (mW/cm2) | 50 (mW/cm2) | 100 (mW/cm2) | 229 (mW/cm2) | 455 (mW/cm2) | 679 (mW/cm2) | |
| HB45NR2PI | N/A | N/A | 126.7 ± 1.1 | 127.7 ± 1.7 | 127.3 ± 1.3 | 123.4 ± 3.8 |
| HB45NR3PI | N/A | N/A | 131.0 ± 1.1 | 130.9 ± 1.5 | 135.1 ± 2.9 | 135.9 ± 2.4 |
| HB95NR2PI | 103.6 ± 0.4 | 102.5 ± 1.2 | 103.9 ± 1.0 | 101.0 ± 1.8 | 102.1 ± 2.2 | 101.4 ± 3.7 |
| HB95NR3PI | 96.3 ± 2.8 | 96.7 ± 3.3 | 93.7 ± 1.3 | 90.4 ± 3.4 | 94.2 ± 1.2 | 98.3 ± 1.7 |
Modulated Temperature DSC was carried out using TA instruments Q200 DSC.27,30 Temperature was modulated sinusoidally with an amplitude of 2°C every 60 s in the presence of purged nitrogen gas at a flow rate of 40 mL/min. The temperature was varied from −10 to 200°C. Samples were heated and cooled at 3°C/min. Two heating/cooling cycles were carried out for each sample. The results were analyzed using Universal Analysis software (TA Instruments, New Castle, DE). The glass transition temperature (Tg) for the second cycle was analyzed. This analysis provided insight into the overall average characteristics of the structure of the polymer. The sample size for DSC analysis was 3 per intensity for the hydrophobic resin polymers and 6 per intensity for the hydrophilic resin polymers.
Statistical Analysis
The differences in DC, rates of polymerization and Tg at various light intensities for each formulation were evaluated using one-way analysis of variance (ANOVA) and two sample t test at α =0.05. The statistical analysis was carried out using Microcal Origin (Version 6.0, Microcal Software, Northampoton, MA).
RESULTS
Polymerization kinetics
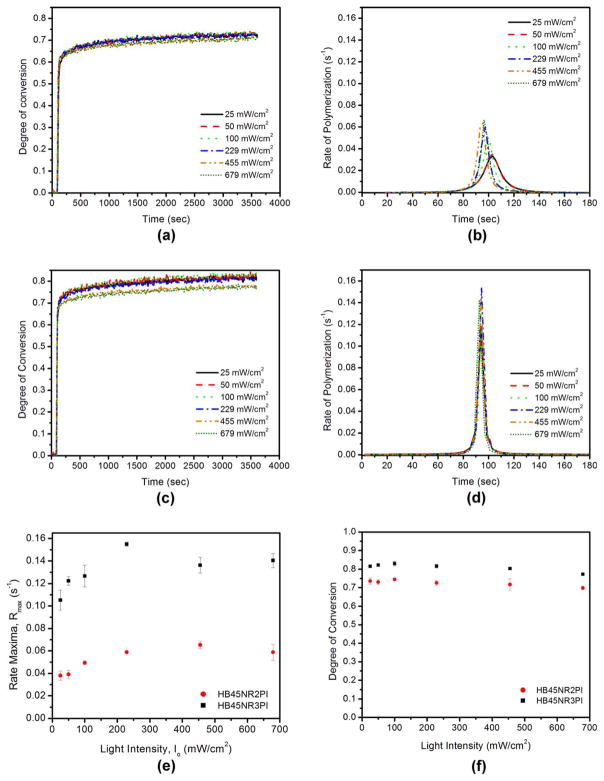
The DC was substantial for the hydrophobic-rich phase at all of the light intensities. In the case of HB45NR2PI, there was no statistically significant difference in DC at 1 h for samples cured at different light intensities. The rate maxima varied significantly with light intensity (one-way ANOVA, p < 0.0001) for both HB45NR2PI and HB45NR3PI. Significant differences in DC at 1 h were observed for HB45NR3PI samples (one-way ANOVA, p < 0.0001) cured at different light intensities. Figure 1 shows the representative results from the kinetic study for the hydrophobic resins. The rate maxima (Rmax) showed a trend of increasing with increasing light intensity for HB45NR2PI until 455 mW/cm2 and beyond this the rate maxima reached a plateau.
FIGURE 1.
Representative polymerization kinetics result for the hydrophobic resin. (a, b) 2PI system. (c, d) 3PI system. (e) Variation of rate maxima, Rmax with light intensity for the hydrophobic resin 2PI system and 3PI system. (f) Impact of light intensity on DC at 1 h for hydrophobic resins.
For HB45NR3PI, the rate maxima showed a relative increase with light intensity until 229 mW/cm2. For light intensities >229 mW/cm2, the rate maxima decreased and reached a plateau [Figure 1(e)]. There was no significant difference between rate maxima at 455 and 679 mW/cm2 (Two sample t test, p < 0.05). There was a relative decrease in DC at 1 h when the light intensity exceeded 229 mW/cm2.
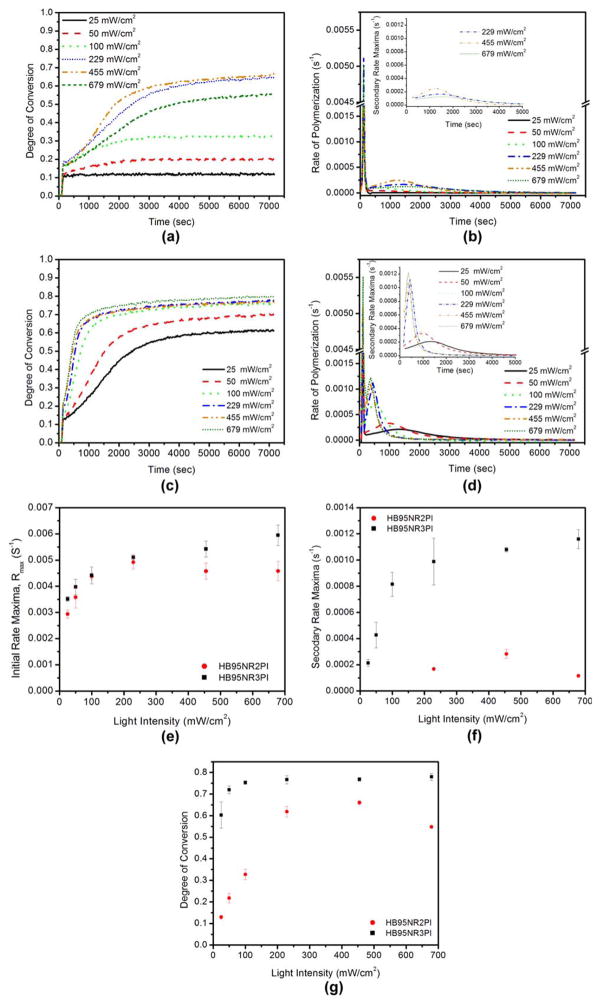
A statistically significant difference in the DC and initial rate maxima for various light intensities was seen for the hydrophilic resin with both the 2PI and 3PI systems (one way ANOVA, p < 0.0001). Figure 2 shows representative results from the kinetic study for both HB95NR2PI and HB95NR3PI. For HB95NR2PI, there was a relative increase in DC at 2 h post curing until 455 mW/cm2. For the light intensity 679 mW/cm2 there was a relative decrease in DC at 2 h post curing. At lower light intensities, that is, 25, 50, and 100 mW/cm2, the DC at 2 h was suboptimal for HB95NR2PI. The initial rate of polymerization exhibited an increasing trend until 229 mW/cm2, beyond this the initial rate maxima reached a plateau. Post polymerization was observed for the hydrophilic resins with both the 2PI and 3PI systems. For HB95NR2PI, secondary rate maxima was observed only at higher light intensities, that is, 229, 455, and 679 mW/cm2 [Figure 2(b)]. A statistically significant difference in secondary rate maxima with light intensity (one-way ANOVA, p < 0.0002) was observed for HB95NR2PI. An increasing trend in the secondary rate maxima was seen until 455 mW/cm2 and for intensities higher than 455 mW/cm2, the secondary rate maxima decreased [Figure 2(f)].
FIGURE 2.
Representative polymerization kinetics result for the hydrophilic resin. (a, b) 2PI system. (c, d) 3PI system. (e) Variation of initial rate maxima, Rmax with light intensity for the hydrophilic resin 2PI system and 3PI system. (f) Variation of secondary rate maxima with light intensity for the hydrophilic resin. (g) Impact of light intensity on DC at 2 h for hydrophilic resins.
For the hydrophilic resin with 3PI, the DC was substantial at all light intensities. At 2 h, there was a trend of increasing DC with an increase in light intensity until 100 mW/cm2. At higher light intensities, there was no statistically significant difference in DC (Two sample t test, p < 0.05). The initial rate maxima showed a trend of increasing with an increase in light intensity [Figure 2(e)].
Secondary rate maxima were observed for HB95NR3PI at all the intensities studied here. The secondary rate maxima appears earlier with increasing light intensity in the case of the 3PI hydrophilic resin. There was a statistically significant difference in the secondary rate maxima with light intensity (one-way ANOVA, p < 0.0001). An increasing trend in the secondary rate maxima with light intensity was seen until 229 mW/cm2 and at higher intensities, the rate reached a plateau [Figure 2(f)]. No significant difference was observed in the secondary rate maxima at intensities higher than 100 mW/cm2 (Two sample t test, p < 0.05).
The DC and rate maxima for resins containing 3PI were much higher when compared to the corresponding resins with 2PI (Table III). The impact of the 3PI system on the hydrophilic resin was significant. Substantial DC (at 2 h) and secondary rate maxima were observed at low intensities 25, 50, and 100 mW/cm2 for the 3PI hydrophilic resin. The initial rate maxima for the 2PI and 3PI hydrophilic resins were of the same order but the secondary rate maxima was much higher for the 3PI.
TABLE III.
DC, Initial Rate Maxima (Rmax) and Secondary Rate Maxima for Hydrophobic and Hydrophilic Resins
| Light Intensity (mW/cm2) | DC
|
Initial Rate Maxima (s−1) × 104
|
Secondary Rate Maxima (s−1) × 104
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HB45NR2PI | HB45NR3PI | HB95NR 2PI | HB95NR 3PI | HB45NR 2PI | HB45NR 3PI | HB95NR 2PI | HB95NR 3PI | HB95NR 2PI | HB95NR 3PI | |
| 25 | 0.736 ± 0.018 | 0.816 ± 0.003 | 0.129 ± 0.008 | 0.603 ± 0.061 | 379.0 ± 41.4 | 1052.1 ± 89.9 | 29.4 ± 1.6 | 35.1 ± 0.9 | N/A | 2.14 ± 0.27 |
| 50 | 0.729 ± 0.013 | 0.822 ± 0.003 | 0.217 ± 0.022 | 0.720 ± 0.018 | 391.4 ± 35.7 | 1222.8 ± 39.2 | 35.8 ± 4.1 | 39.8 ± 2.9 | N/A | 4.27 ± 0.98 |
| 100 | 0.744 ± 0.007 | 0.829 ± 0.012 | 0.327 ± 0.024 | 0.753 ± 0.008 | 493.3 ± 15.7 | 1266.3 ± 96.9 | 43.8 ± 1.3 | 44.2 ± 3.2 | N//A | 8.15 ± 0.93 |
| 229 | 0.726 ± 0.013 | 0.816 ± 0.009 | 0.618 ± 0.024 | 0.767 ± 0.019 | 587.9 ± 14.3 | 1548.9 ± 19.5 | 49.2 ± 2.5 | 51.1 ± 0.9 | 1.67 ± 0.02 | 9.88 ± 1.79 |
| 455 | 0.717 +0.030 | 0.803 ± 0.005 | 0.660 ± 0.009 | 0.768 ± 0.009 | 652.8 ± 33.8 | 1362.7 ± 70.6 | 45.8 ± 3.1 | 54.2 ± 3.0 | 2.82 ± 0.35 | 10.80 ± 0.17 |
| 679 | 0.698 ± 0.009 | 0.773 ± 0.004 | 0.548 ± 0.007 | 0.780 ± 0.017 | 587.3 ± 71.5 | 1404.3 ± 63.6 | 45.8 ± 3.7 | 59.5 ± 3.9 | 1.15 ± 0.08 | 11.6 ± 0.72 |
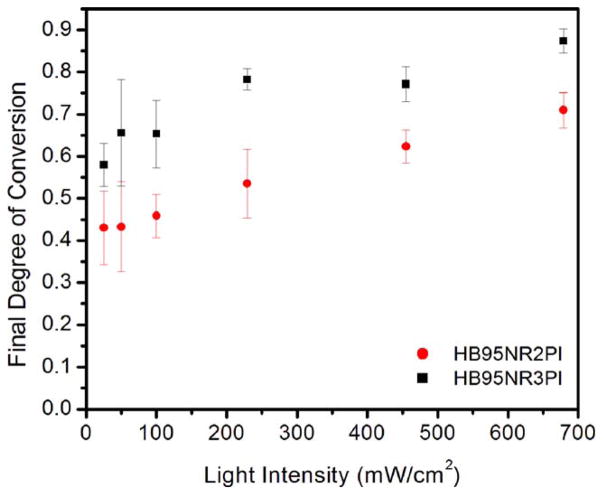
Figure 3 shows the final DC (at 24 h) at various light intensities for the 2PI and 3PI hydrophilic resin pan samples. There was a statistically significant difference (one-way ANOVA, p < 0.05) in final DC for pan samples cured at various light intensities for the 2PI hydrophilic resin pan samples. An increasing trend in the final DC was noted with an increase in light intensity for the hydrophilic 2PI resin. At low light intensities (25, 50, and 100 mW/cm2) the DC (at 24 h) of the pan samples was higher than the corresponding samples from the kinetic study; the kinetic study was conducted for a period of 2 h vs. measurement carried out at 24 h post curing for the pan samples. The DC for the pan samples cured at low intensities (25, 50, and 100 mW/cm2) was still lower than the corresponding samples cured at higher intensities. These results indicate that light intensity has an impact on the final DC for the hydrophilic 2PI resin. For light intensities 229 and 455 mW/cm2, the DC for the samples in the kinetic study and pan samples were similar in the case of the 2PI hydrophilic resin. At very high light intensity, for example, 679 mW/cm2, the DC at 2 h from the kinetic study was observed to be lower compared to the DC of pan samples at 24 h.
FIGURE 3.
Final DC for hydrophilic resin 2PI and 3PI obtained from pan samples stored in the dark for 24 h.
For the 3PI hydrophilic resin, the DC was similar for corresponding samples in the kinetic study and pan samples. A statistically significant difference in final DC of pan samples for various light intensities was observed in the case of HB95NR3PI (one-way ANOVA, p < 0.05). A relative increase in final DC was noted with an increase in light intensity for the 3PI hydrophilic resin.
Determination of Tg
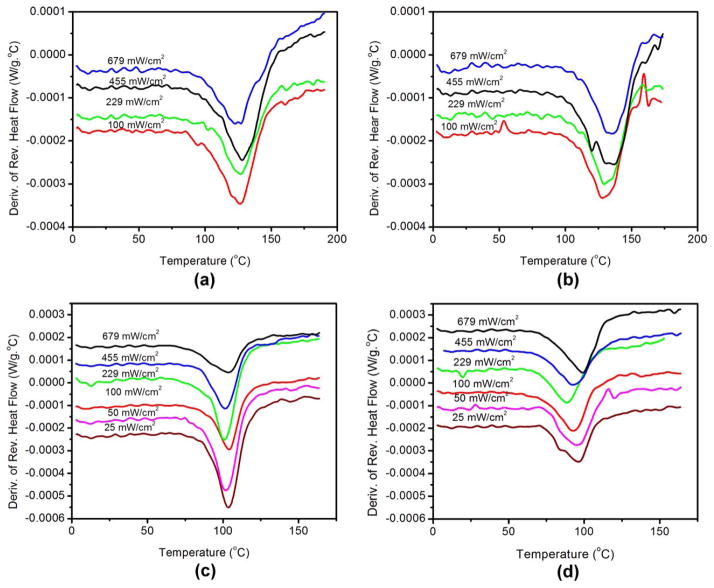
The polymer structure of the hydrophobic and hydrophilic resins was studied using DSC. Figure 4 exhibits the variation of Tg with light intensity for the resins studied here. Figure 4 exhibits an average of all the samples per intensity for each formulation. Table IV, summarizes the Tg of HB45NR2PI, HB45NR3PI, HB95NR2PI, and HB95NR3PI for the second heating/cooling cycle.
FIGURE 4.
Variation of Tg with light intensity for (a) HB45NR2PI, (b) HB45NR3PI, (c) HB95NR2PI, and (d) HB95NR3PI.
There was no statistically significant difference in Tg with light intensity for the 2PI hydrophobic resins (one-way ANOVA, p < 0.05). There was also no statistically significant difference in the Tg with light intensity for the 2PI hydrophilic resins (one-way ANOVA, p < 0.05). These results suggest that light intensity has little impact on the Tg for the 2PI resins if the samples have had sufficient time for postpolymerization.
There was a statistically significant difference in the Tg for the 3PI hydrophobic resins cured at various light intensities (one-way ANOVA, p < 0.05). For the hydrophobic 3PI resin, the Tg showed a slight increase with light intensity. There was a significant difference (one-way ANOVA, p < 0.0001) in the Tg of HB95NR3PI samples cured at various light intensities.
Comparing the corresponding Tg of the 2PI and 3PI samples for the hydrophobic resins, it can be seen that there is a trend of Tg being slightly higher for the 3PI than that for 2PI. Comparing the corresponding Tg of the 2PI and 3PI samples for the hydrophilic resins, there is a trend of Tg being slightly lower for the 3PI as compared with the 2PI system.
DISCUSSION
The radical generation rate (Ri) increases with light intensity as given by the following Eq. (2).22
| (2) |
where ϕ is the initiator efficiency, [A] is the initiator concentration, b is the thickness of the sample, ε and I0 are the extinction coefficient of the initiator and light intensity in light quanta per second, respectively. In this study, for each PI system, [A], b, and ε remained the same but the light intensity was varied. The initiator efficiency, ϕ, was much higher for the 3PI system compared with the 2PI system.
The rate of propagation for polymerization is proportional to the monomer radicals and the formation of monomer radicals is dependent on the rate of generation of the initiator radicals. Lovell et al. showed a power law relation between the rate of polymerization and light intensity for the BisGMA/TEGDMA system.20 Increasing light intensity enhances radical generation, which enhances the rate of polymerization. The kinetic study for the hydrophobic resin was conducted for 1 h because it was observed previously that 1 h was sufficient for the hydrophobic resins to reach the final DC.32 Results from an earlier study showed that the hydrophilic resin exhibited lower polymerization efficiency compared with the hydrophobic resin,10 therefore the kinetic study for the hydrophilic resin was conducted for 2 h instead of 1 h. Since some of the hydrophilic samples continued to polymerize after 2 h, additional study was carried out with pan samples for the hydrophilic resins to determine the DC at 24 h.
The radical generation rate is expected to be much higher for the 3PI system than the 2PI system since the terminating ketyl radical, after abstraction of hydrogen from tertiary amine, is replaced by the phenyl radical following oxidation of the ketyl radical by DPIHP.33 The oxidation regenerates CQ which can again absorb photon and the generated phenyl radical is active in initiation.33 The aminoalkyl radical also undergoes oxidation to generate phenyl radicals. The consumption of both aminoalkyl and ketyl radicals reduces back electron transfer within the CQ/EDMAB exciplex.33 These events may lead to generation of radicals in higher concentration in the 3PI system as compared to the 2PI system. Therefore, ϕ in Eq. (2) will be much higher for 3PI system.
An increasing trend in rate maxima with increasing light intensity until 455 mW/cm2 had no impact on DC at 1 hr for HB45NR2PI. For HB45NR3PI, it is possible that at higher light intensities, the generation of radicals becomes too high and the viscous system may cause the radicals to become trapped in close proximity. This could cause the radicals to terminate each other reducing the concentration of effective radicals at higher light intensities. The decrease in rate maxima after 229 mW/cm2 for HB45NR3PI could be attributed to the termination of radicals by each other. It is possible that the termination could result in a constant concentration of the effective radicals for intensities 455 and 679 mW/cm2, causing the rate maxima to plateau. The same phenomenon could explain the rate maxima reaching plateau beyond 455 mW/cm2 for HB45NR2PI. The decreasing trend of DC at 2 h for light intensity beyond 229 mW/cm2 in case of HB45NR3PI could be due to the termination of radicals.
HB95NR2PI is a low viscous system allowing movement of radicals during and after autoacceleration. Therefore, HB95NR2PI does not experience the restricted movement of the reactive species as in HB45NR2PI. As a result, the termination rate is higher in HB95NR2PI compared with HB45NR2PI. In HB45NR2PI, the radicals experience restricted movement due to higher viscosity and crosslinking reducing the termination rate. This accounts for the high initial polymerization rate in HB45NR2PI compared to HB95NR2PI although both formulations contain the same concentration of photoinitiators.
At low light intensity, the generation of radicals is much lower and because termination is higher for HB95NR2PI compared with HB45NR2PI, the concentration of effective radicals taking part in polymerization decreases. The low concentration of effective radicals could lead to suboptimal DC at 2 h for light intensities of 25, 50, and 100 mW/cm2. For HB95NR2PI, the effective number of radicals increase with increasing light intensity, leading to an increasing trend in the initial rate maxima until 229 mW/cm2. Hence, the DC at 2 h post curing from the kinetic study also exhibited an increasing trend until 455 mW/cm2 for HB95NR2PI.
Secondary rate maxima in the hydrophilic system is associated with the formation of microgels.10,27,34 The absence of secondary rate peaks for HB95NR2PI at lower light intensities could be due to the low concentration of effective radicals. There may not be a sufficient quantity of radicals to initiate a secondary gel effect within the microgels at low light intensities for HB95NR2PI. At higher light intensities, the increasing trend in the secondary peak with light intensity could be due to an increase in the concentration of effective radicals. For HB95NR2PI, it is possible that at very high light intensity, for example, 679 mW/cm2, there is an excessive number of radicals trapped within the microgels following initial rate maxima. This phenomenon would enhance termination and reduce the availability of effective radicals within the microgels. The reduced concentration of effective radicals within microgels could lead to a decrease in the secondary gel effect and hence the secondary rate maxima would be reduced at 679 mW/cm2. These events could account for the lower DC for this light intensity at 2 h.
The final DC, which was measured at 24 h post curing, was higher for the pan samples as compared with the samples from the kinetic study. This difference was noted especially at low light intensities. Unreacted radicals remain trapped within the polymer system. These unreacted radicals are responsible for the post polymerization reaction that occurs in the dark and hence the final DC (at 24 h) is higher.35,36 Despite post polymerization, the DC for HB95NR2PI at lower light intensities was still much lower than that at higher light intensities. These results suggest that lower light intensity exhibits an adverse impact on the polymerization efficiency of hydrophilic 2PI resin. For HB95NR2PI, the final DC showed a trend of increasing with an increase in light intensity; these results suggest that there is an increase in the radicals available to participate in the postpolymerization with increasing light intensity. The results from the kinetic study and pan samples indicate that for HB95NR2PI, very high light intensity, for example, 679 mW/cm2 slows the secondary gel effect and a lower DC is observed at 2 h post curing but the remaining radicals are sufficient to continue the reaction causing a higher final DC at 24 h. A similar result of slower secondary peak at high intensity was also observed for the MAA/TEGDMA system.25
In the case of the HB95NR3PI, the generation of radicals was much higher than the corresponding samples prepared with the 2PI system. This could account for the higher initial and secondary rate maxima for HB95NR3PI and the presence of secondary rate maxima at lower light intensities, for example, 25, 50, and 100 mW/cm2. Despite the possibility of enhanced termination when excess radicals are generated at higher light intensity, it appears that the concentration of effective radicals increases with increasing curing light intensity for HB95NR3PI. Hence, there is an increase in initial and secondary rate maxima with increasing light intensity for HB95NR3PI. For HB95NR3PI, the DC at 2 h from the kinetic study increased until 100 mW/cm2 and there was minimal difference in the DC at 2 h post curing for light intensities >100 mW/cm2. In contrast, the final DC from the pan samples for this formulation showed a relative increase with light intensity. Although the final DC increases with light intensity, the DC for corresponding samples in the kinetic study and pan samples were similar, indicating that the 3PI hydrophilic resin reaches almost complete conversion in 2 h. The results suggest that the effective radicals remain within the polymer network for further postpolymerization and the concentration of these remaining radicals could be higher at increased light intensities. This could contribute to an increase in the final DC for the HB95NR3PI pan samples with an increase in the light intensity. The extensive difference between the secondary rate maxima of 2PI and 3PI hydrophilic resins indicated that the excess radicals, which were generated due to the presence of iodonium salt, mostly contributed to the secondary gel effect within the microgels.
For the 2PI hydrophobic and hydrophilic resins, increasing light intensity did not have a significant effect on the Tg. These results suggest that increasing light intensity had little or no effect on the cross-linking density for these formulations. Similar results were suggested for the BisGMA/TEGDMA system.21 For HB45NR3PI, although increasing light intensity showed a significant effect on Tg, the differences in Tg at various light intensities was minimal, indicating that there were slight differences in crosslinking density among the samples. The corresponding Tg was higher for HB45NR3PI compared to HB45NR2PI.
Lovell et al. reported that an increase in initiation rate led to shorter kinetic chain length and this may impact the network structure.21 Since the 3PI system generates a higher concentration of radicals which leads to a higher initiation/secondary rate, it is possible that the kinetic chains are shorter for this system as compared to the corresponding 2PI system. However, HB45NR3PI polymerizes to an intensely cross-linked structure and thus, the results suggest that the short chains did not impact the network significantly. The higher Tg of the HB45NR3PI may be attributable to differences in the crosslinking density of HB45NR3PI as compared with HB45NR2PI. For HB45NR3PI shorter chains do not impact the Tg adversely since the formulation contains a high concentration of the multifunctional monomer, BisGMA. The high concentration of BisGMA potentially leads to a highly crosslinked structure. For the viscous hydrophobic resins, Tg could be dominated by the cross-linking density. This phenomenon was also reported by Lovell et al. for BisGMA/TEGDMA.21 For HB45NR3PI, slight increase in Tg with light intensity suggests slightly enhanced cross-linking density.
There is an overall trend of decreasing Tg for HB95NR3PI as compared with the corresponding samples for HB95NR2PI. It is possible that for a system with low viscosity and a high concentration of the monofunctional monomer, the chain length could impact the network structure. For such a system, the Tg could be dominated by the chain length. The initiation/secondary rate of the hydrophilic 3PI system is higher than the 2PI hydrophilic system; the difference in the rate could lead to shorter chain lengths for the hydrophilic 3PI system. The presence of shorter chains in a system, which contains mostly linear chains with limited cross-linking could reduce the Tg. Moreover, it is possible that the high rate of polymerization in the HB95NR3PI system could promote intramolecular cyclizations, which could account for its higher DC compared with HB95NR2PI. Intramolecular cyclizations do not contribute to an increase in the cross-linking density and could cause the overall average cross-linking density to be lower for HB45NR3PI compared with HB95NR2PI. He et al. also found that faster polymerization rate enhanced intramolecular cyclizations for low viscous MAA/TEGDMA with 50 wt % solvent.25 An increase in the light intensity may have very little effect on the chain length or cross-linking for HB95NR2PI. Therefore, for HB95NR2PI system with mostly linear polymer chains, the Tg remains almost the same irrespective of the light intensity. For HB95NR3PI, the Tg was similar among samples cured at various light intensities indicating subtle differences in the polymer structures.
The presence of two Tgs during the first heating/cooling cycle of the DSC study of the hydrophilic and hydrophobic resins (unpublished data) indicate the presence of two different phases (higher cross-linked and lower cross-linked regions). The presence of two phases (microgel and matrix) for the hydrophilic resin system was also proposed by Abedin et al.27
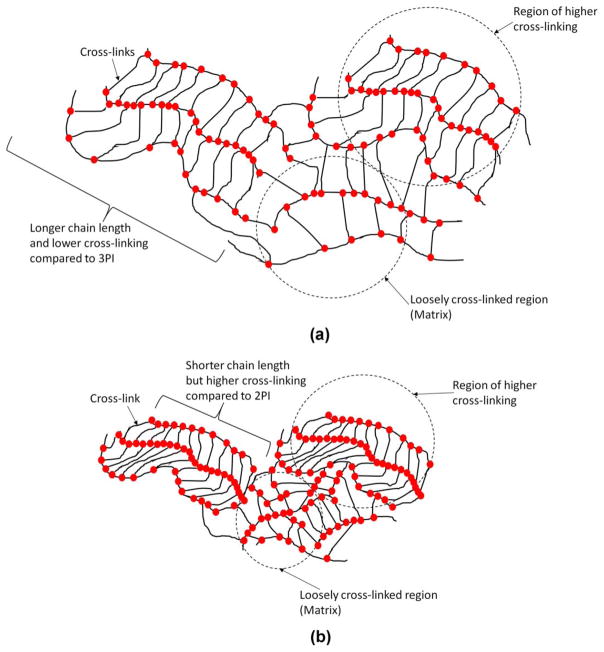
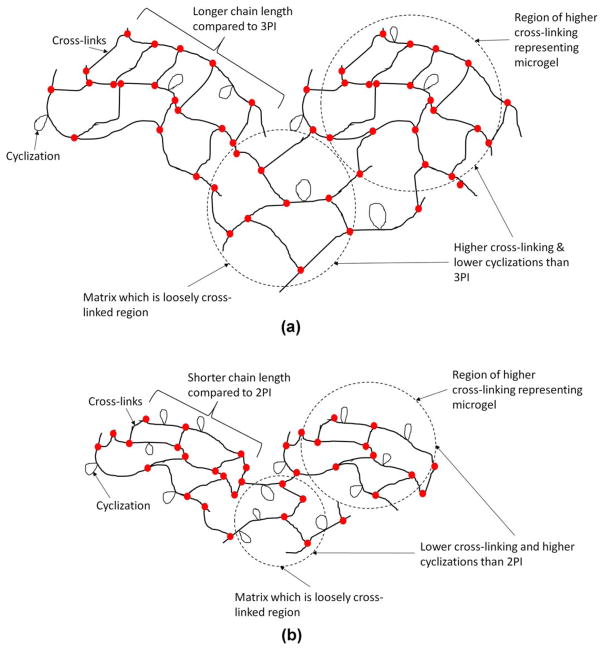
The DSC results support formation of shorter polymer chains for the 3PI samples as compared to the corresponding 2PI samples. The polymer from the hydrophobic 3PI resin could possess shorter chains but higher cross-linking density as compared with the corresponding polymer from the 2PI hydrophobic resin. According to the DSC study, the polymer from the 3PI hydrophilic resin could have shorter chains, more cyclizations, slightly lower cross-linking density compared to the corresponding polymer from the 2PI hydrophilic resin. Based on the DSC results schematics of comparative network structure for the four different polymers have been proposed in Figures 5 and 6.
FIGURE 5.
Schematic showing possible network structure for (a) HB45NR2PI (b) HB45NR3PI. The 3PI hydrophobic system could have shorter chain lengths compared with their corresponding 2PI hydrophobic system. The polymer from the hydrophobic 3PI resin (HB45NR3PI) could have higher cross-linking density than that from the HB45NR2PI.
FIGURE 6.
Schematic showing possible network structure for (a) HB95NR2PI and (b) HB95NR3PI. The 3PI system could have shorter chain lengths compared with their corresponding 2PI system. The possibility of formation of cyclization is higher for HB95NR3PI compared with HB95NR2PI.
Since the samples for kinetic study represent a thin film, dissolved oxygen, especially within the hydrophilic resin, could interfere with the polymerization. This study indicates that the viscous hydrophobic resin, rich in multifunctional monomer, undergoes a substantial DC within a short period of time (in a few minutes) irrespective of the light intensities. For the hydrophilic 2PI samples, the suboptimal DC at 2 h post curing at lower light intensities, indicate that the light intensity could significantly impact the polymerization efficiency. Although previous investigations have shown that the efficiency of polymerization of dentin adhesive hydrophilic 2PI system is lower than the hydrophobic 2PI system,10 this study shows that decreased light intensity significantly lowers the polymerization efficiency of the dentin adhesive hydrophilic 2PI system. Incorporation of iodonium salt to the BisGMA/HEMA based dentin adhesive hydrophilic resin significantly improved the polymerization efficiency, leading to a substantial DC at 2 h post curing even at lower light intensities.
This study provides important information related to the polymerization of the hydrophilic-rich phase and shows how the variable light intensity could impact the hydrophilic-rich phase. The light intensity will vary along the length and breadth of the hybrid layer. Although iodonium salt could improve the DC of BisGMA/HEMA based hydrophilic resin at low light intensities, the resultant polymer will be susceptible to degradation due to shorter chain lengths and lower cross-linking density. Incorporation of hydrophilic multifunctional monomers to dentin adhesive formulations could overcome these limitations by enhancing the crosslinking density. It should be noted that the impact of the iodonium salt on the polymerization reaction depends on the type of monomers present in the system. It was found that the iodonium salt failed to enhance the photopolymerization in the presence of methacrylate phosphonic acid monomers.37 From the range of intensities studied here, the appropriate light intensity to obtain substantial DC with good polymerization efficiency for hydrophilic 2PI resin is in the range 229–679 mW/cm2. Both the hydrophobic resins and hydrophilic 3PI resin yield substantial DC with good efficiency at all of the light intensities studied here.
Acknowledgments
Contract grant sponsor: National Institute of Dental and Craniofacial Research; contract grant number: R01 DE022054 and 3R01DE022054-04S1
Contract grant sponsor: National Institutes of Health, Bethesda, MD
References
- 1.Opdam NJM, Bronkhorst EM, Roeters JM, Loomans BAC. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent Mater. 2007;23:2–8. doi: 10.1016/j.dental.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Spencer P, Ye Q, Park J, Topp E, Misra A, Marangos O, Wang Y, Bohaty B, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann Biomed Eng. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin–dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–3803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 4.Kostoryz EL, Dharmala K, Ye Q, Wang Y, Huber J, Park J-G, Snider G, Katz JL, Spencer P. Enzymatic biodegradation of HEMA/bisGMA adhesives formulated with different water content. J Biomed Mater Res B Appl Biomater. 2009;88B:394–401. doi: 10.1002/jbm.b.31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santini A, Miletic V. Quantitative micro-Raman assessment of dentine demineralization, adhesive penetration, and degree of conversion of three dentine bonding systems. Eur J Oral Sci. 2008;116:177–183. doi: 10.1111/j.1600-0722.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- 6.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 7.Toledano M, Yamauti M, Osorio E, Monticelli F, Osorio R. Characterization of micro- and nanophase separation of dentin bonding agents by stereoscopy and atomic force microscopy. Microsc Microanal. 2012;18:279–288. doi: 10.1017/S1431927611012621. [DOI] [PubMed] [Google Scholar]
- 8.Ye Q, Park J, Parthasarathy R, Pamatmat F, Misra A, Laurence JS, Marangos O, Spencer P. Quantitative analysis of aqueous phase composition of model dentin adhesives experiencing phase separation. J Biomed Mater Res B Appl Biomater. 2012;100B:1086–1092. doi: 10.1002/jbm.b.32675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J Dent Res. 2003;82:141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 10.Abedin F, Ye Q, Parthasarathy R, Misra A, Spencer P. Polymerization behavior of hydrophilic-rich phase of dentin adhesive. J Dent Res. 2015;94:500–507. doi: 10.1177/0022034514565646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Discacciati JAC, Neves AD, Oréfice RL, Pimenta FJGS, Sander HH. Effect of light intensity and irradiation time on the polymerization process of a dental composite resin. Mater Res. 2004;7:313–318. [Google Scholar]
- 12.Davidson-Kaban SS, Davidson CL, Feilzer AJ, de Gee AJ, Erdilek N. The effect of curing light variations on bulk curing and wall-to-wall quality of two types and various shades of resin composites. Dent Mater. 1997;13:344–352. doi: 10.1016/s0109-5641(97)80105-4. [DOI] [PubMed] [Google Scholar]
- 13.Silikas N, Eliades G, Watts DC. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dent Mater. 2000;16:292–296. doi: 10.1016/s0109-5641(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann N, Hugo B, Klaiber B. Effect of irradiation type (LED or QTH) on photo-activated composite shrinkage strain kinetics, temperature rise, and hardness. Eur J Oral Sci. 2002;110:471–479. doi: 10.1034/j.1600-0722.2002.21359.x. [DOI] [PubMed] [Google Scholar]
- 15.Emami N, Soderholm K-JM, Berglund LA. Effect of light power density variations on bulk curing properties of dental composites. J Dent. 2003;31:189–196. doi: 10.1016/s0300-5712(03)00015-0. [DOI] [PubMed] [Google Scholar]
- 16.Lohbauer U, Rahiotis C, Kramer N, Petschelt A, Eliades G. The effect of different light-curing units on fatigue behavior and degree of conversion of a resin composite. Dent Mater. 2005;21:608–615. doi: 10.1016/j.dental.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Lee S-K, Kim T-W, Son S-A, Park J-K, Kim J-H, Kim H-I, Kwon YH. Influence of light-curing units on the polymerization of low-shrinkage composite resins. Dent Mater J. 2013;32:688–694. doi: 10.4012/dmj.2013-027. [DOI] [PubMed] [Google Scholar]
- 18.Franco EB, Santos PAd, Mondelli RFL. The effect of different light-curing units on tensile strength and microhardness of a composite resin. J Appl Oral Sci. 2007;15:470–474. doi: 10.1590/S1678-77572007000600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennison JB, Yaman P, Seir R, Hamilton JC. Effect of variable light intensity on composite shrinkage. J Prosthet Dent. 2000;84:499–505. doi: 10.1067/mpr.2000.110494. [DOI] [PubMed] [Google Scholar]
- 20.Lovelh LG, Newman SM, Bowman CN. The effects of light intensity, temperature, and comonomer composition on the polymerization behavior of dimethacrylate dental resins. J Dent Res. 1999;78:1469–1476. doi: 10.1177/00220345990780081301. [DOI] [PubMed] [Google Scholar]
- 21.Lovell LG, Lu H, Elliott JE, Stansbury JW, Bowman CN. The effect of cure rate on the mechanical properties of dental resins. Dent Mater. 2001;17:504–511. doi: 10.1016/s0109-5641(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 22.Lovell LG, Newman SM, Donaldson MM, Bowman CN. The effect of light intensity on double bond conversion and flexural strength of a model, unfilled dental resin. Dent Mater. 2003;19:458–465. doi: 10.1016/s0109-5641(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 23.Ye Q, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dentin adhesives as a function of light source and irradiance. J Biomed Mater Res B Appl Biomater. 2007;80B:440–446. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto A, Tsubota K, Takamizawa T, Kurokawa H, Rikuta A, Ando S, Takigawa T, Kuroda T, Miyazaki M. Influence of light intensity on dentin bond strength of self-etch systems. J Oral Sci. 2006;48:21–26. doi: 10.2334/josnusd.48.21. [DOI] [PubMed] [Google Scholar]
- 25.He H, Li L, Lee LJ. Photopolymerization and structure formation of methacrylic acid based hydrogels: The effect of light intensity. React Funct Polym. 2008;68:103–113. [Google Scholar]
- 26.Li L, Lee LJ. Photopolymerization of HEMA/DEGDMA hydrogels in solution. Polymer. 2005;46:11540–11547. [Google Scholar]
- 27.Abedin F, Ye Q, Good HJ, Parthasarathy R, Spencer P. Polymerization- and solvent-induced phase separation in hydrophilic-rich dentin adhesive mimic. Acta Biomater. 2014;10:3038–3047. doi: 10.1016/j.actbio.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price RB, DÉRand T, Sedarous M, Andreou P, Loney RW. Effect of distance on the power density from two light guides. J Esthet Dent. 2000;12:320–327. doi: 10.1111/j.1708-8240.2000.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 29.Rueggeberg FA, Jordan DM. Effect of light-tip distance on polymerization of resin composite. Int J Prosthodont. 1993;6:364–370. [PubMed] [Google Scholar]
- 30.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res A. 2007;80A:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Perez MM, Perez-Ocon F, Lucena-Martin C, Pulgar R. Stability and reproducibility of radiometric properties of light curing units (LCUs) Part I: QTH LCUs Dental Mater J. 2008;27:284–291. [PubMed] [Google Scholar]
- 32.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nanoheterogeneous dentin adhesive. J Dent Res. 2008;87:829–833. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook WD, Chen F. Enhanced photopolymerization of dimethacrylates with ketones, amines, and iodonium salts: The CQ system. J Polym Sci Part A: Polym Chem. 2011;49:5030–5041. [Google Scholar]
- 34.Horie K, Otagawa A, Muraoka M, Mita I. Calorimetric investigation of polymerization reactions. V. Crosslinked copolymerization of methyl methacrylate with ethylene dimethacrylate. J Polym Sci: Polym Chem Ed. 1975;13:445–454. [Google Scholar]
- 35.Lovell LG, Berchtold KA, Elliott JE, Lu H, Bowman CN. Understanding the kinetics and network formation of dimethacrylate dental resins. Polym Adv Technol. 2001;12:335–345. [Google Scholar]
- 36.Gao X, Nie J. Low-temperature photopolymerization and post-cure characteristics of acrylates. Polym Int. 2007;56:707–710. [Google Scholar]
- 37.Besse V, Le Pluart L, Cook WD, Pham T-N, Madec P-J. Polymerization kinetics of phosphonic acids and esters using an iodonium initiator. J Polym Sci Part A: Polym Chem. 2013;51:5046–5055. [Google Scholar]