Abstract
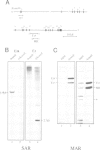
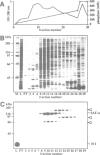
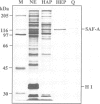
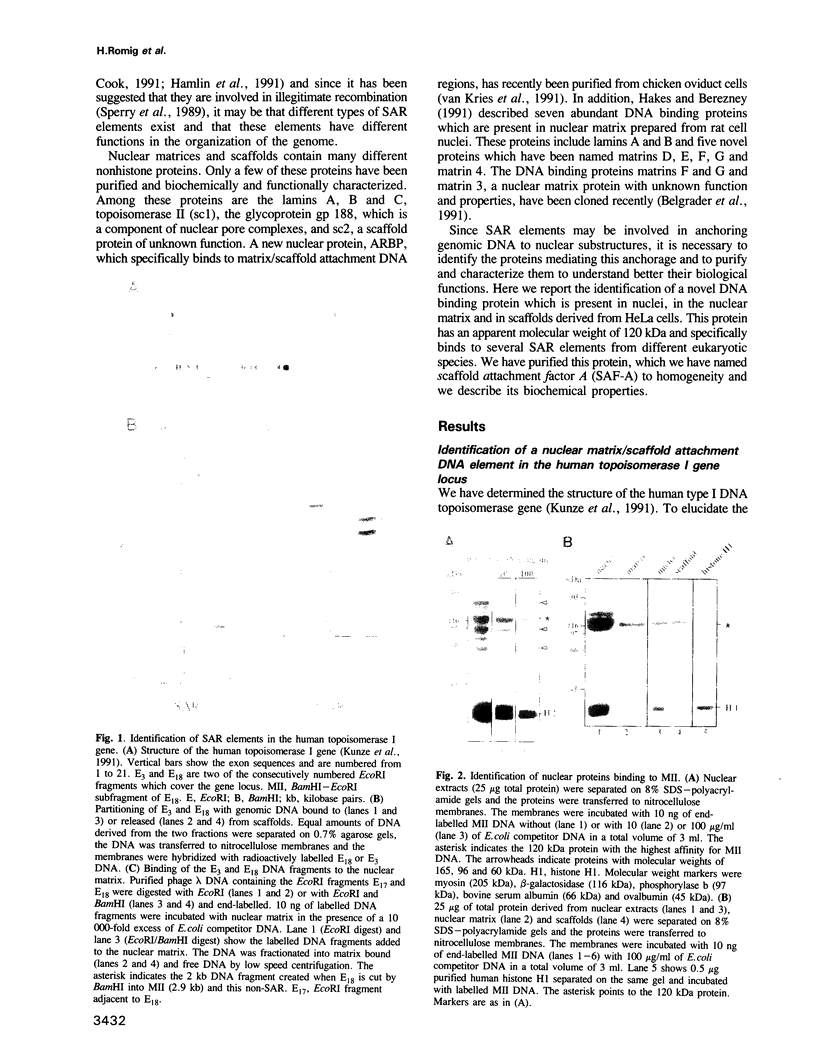
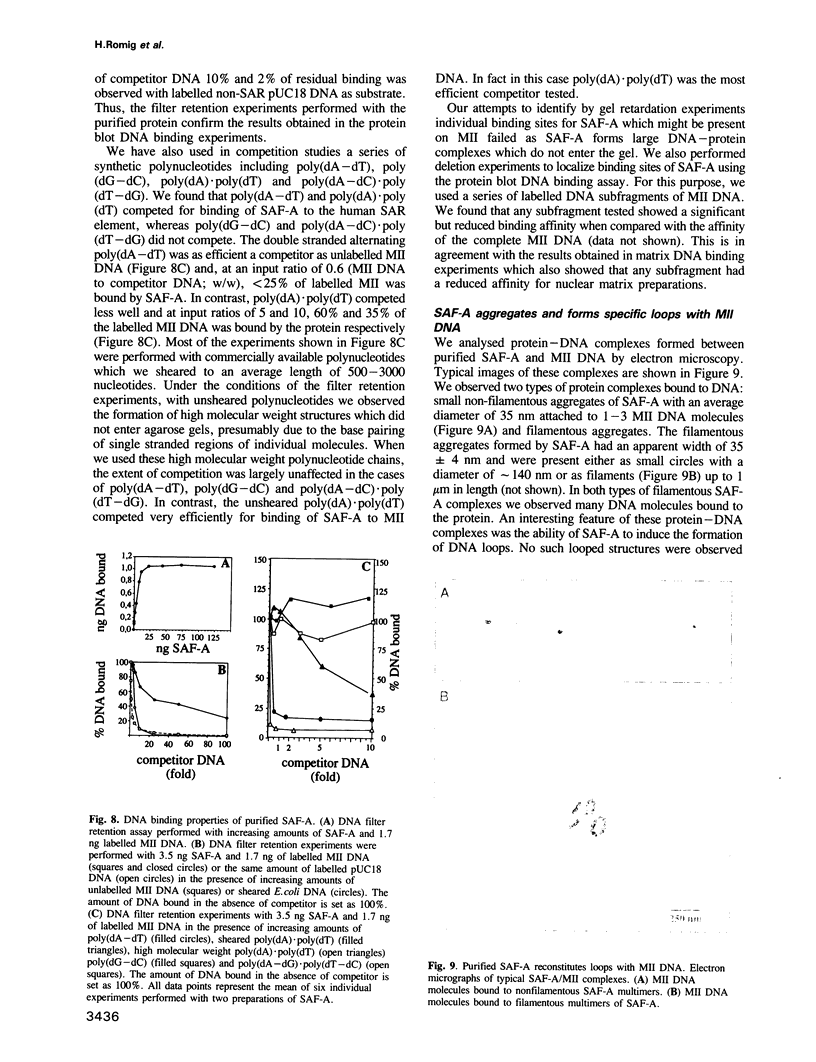
We identified four proteins in nuclear extracts from HeLa cells which specifically bind to a scaffold attachment region (SAR) element from the human genome. Of these four proteins, SAF-A (scaffold attachment factor A), shows the highest affinity for several homologous and heterologous SAR elements from vertebrate cells. SAF-A is an abundant nuclear protein and a constituent of the nuclear matrix and scaffold. The homogeneously purified protein is a novel double stranded DNA binding protein with an apparent molecular weight of 120 kDa. SAF-A binds at multiple sites to the human SAR element; competition studies with synthetic polynucleotides indicate that these sites most probably reside in the multitude of A/T-stretches which are distributed throughout this element. In addition we show by electron microscopy that the protein forms large aggregates and mediates the formation of looped DNA structures.
Full text
PDF
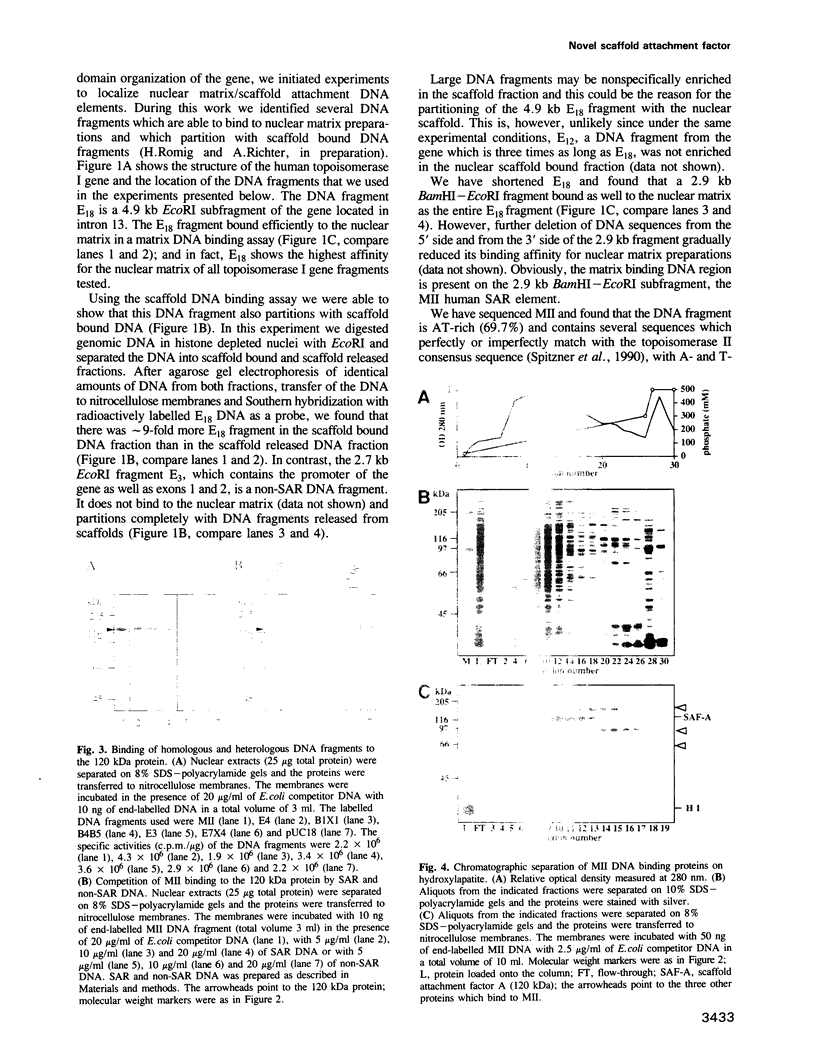




Images in this article
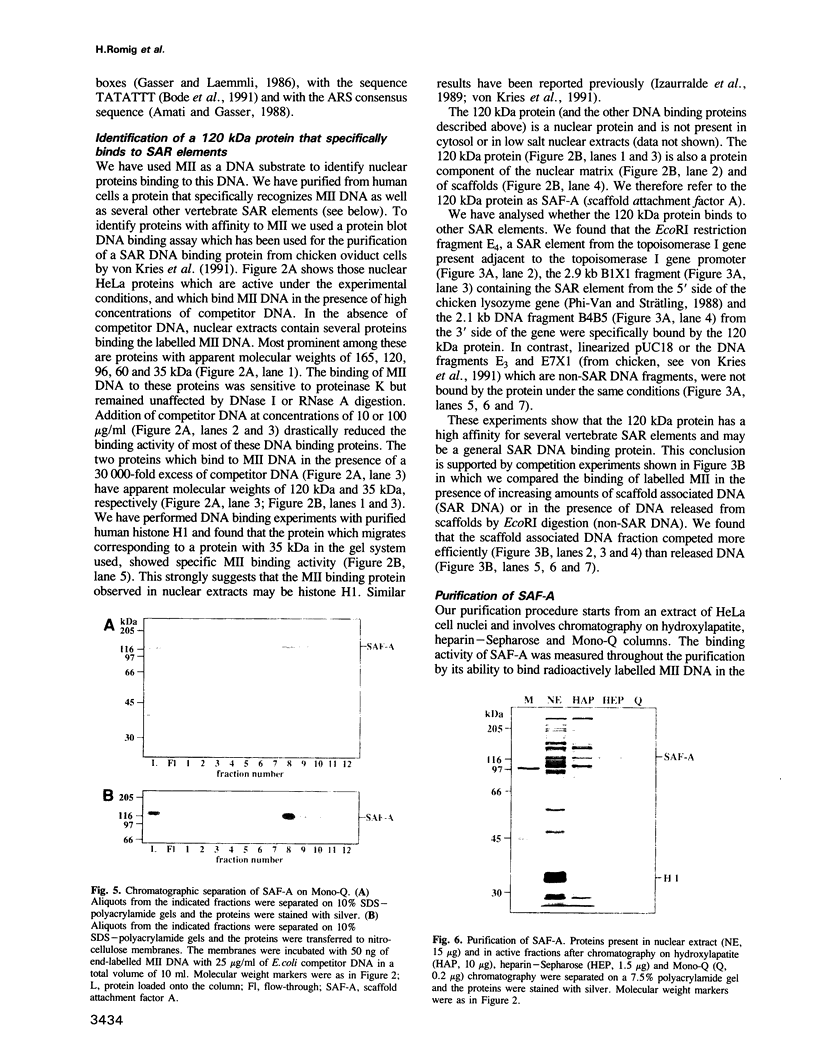
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
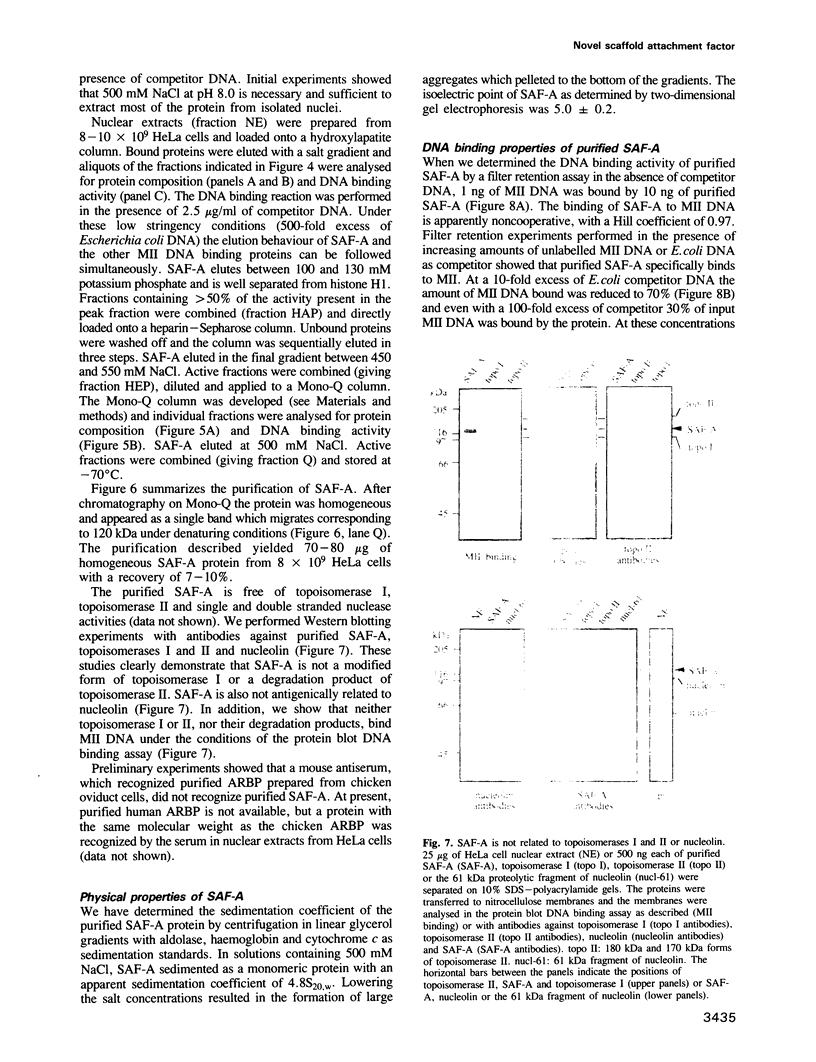
- Adachi Y., Käs E., Laemmli U. K. Preferential, cooperative binding of DNA topoisomerase II to scaffold-associated regions. EMBO J. 1989 Dec 20;8(13):3997–4006. doi: 10.1002/j.1460-2075.1989.tb08582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrader P., Siegel A. J., Berezney R. A comprehensive study on the isolation and characterization of the HeLa S3 nuclear matrix. J Cell Sci. 1991 Mar;98(Pt 3):281–291. doi: 10.1242/jcs.98.3.281. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode J., Kohwi Y., Dickinson L., Joh T., Klehr D., Mielke C., Kohwi-Shigematsu T. Biological significance of unwinding capability of nuclear matrix-associating DNAs. Science. 1992 Jan 10;255(5041):195–197. doi: 10.1126/science.1553545. [DOI] [PubMed] [Google Scholar]
- Bode J., Maass K. Chromatin domain surrounding the human interferon-beta gene as defined by scaffold-attached regions. Biochemistry. 1988 Jun 28;27(13):4706–4711. doi: 10.1021/bi00413a019. [DOI] [PubMed] [Google Scholar]
- Bodnar J. W. A domain model for eukaryotic DNA organization: a molecular basis for cell differentiation and chromosome evolution. J Theor Biol. 1988 Jun 22;132(4):479–507. doi: 10.1016/s0022-5193(88)80086-9. [DOI] [PubMed] [Google Scholar]
- Brun C., Dang Q., Miassod R. Studies of an 800-kilobase DNA stretch of the Drosophila X chromosome: comapping of a subclass of scaffold-attached regions with sequences able to replicate autonomously in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Oct;10(10):5455–5463. doi: 10.1128/mcb.10.10.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Cook P. R. The nucleoskeleton and the topology of replication. Cell. 1991 Aug 23;66(4):627–635. doi: 10.1016/0092-8674(91)90109-c. [DOI] [PubMed] [Google Scholar]
- Cook P. R. The nucleoskeleton and the topology of transcription. Eur J Biochem. 1989 Nov 20;185(3):487–501. doi: 10.1111/j.1432-1033.1989.tb15141.x. [DOI] [PubMed] [Google Scholar]
- Devlin C., Tice-Baldwin K., Shore D., Arndt K. T. RAP1 is required for BAS1/BAS2- and GCN4-dependent transcription of the yeast HIS4 gene. Mol Cell Biol. 1991 Jul;11(7):3642–3651. doi: 10.1128/mcb.11.7.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson P., Cook P. R., Jackson D. A. Active RNA polymerase I is fixed within the nucleus of HeLa cells. EMBO J. 1990 Jul;9(7):2207–2214. doi: 10.1002/j.1460-2075.1990.tb07390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Heck M. M. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. doi: 10.1083/jcb.100.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Getzenberg R. H., Coffey D. S. Tissue specificity of the hormonal response in sex accessory tissues is associated with nuclear matrix protein patterns. Mol Endocrinol. 1990 Sep;4(9):1336–1342. doi: 10.1210/mend-4-9-1336. [DOI] [PubMed] [Google Scholar]
- Hakes D. J., Berezney R. DNA binding properties of the nuclear matrix and individual nuclear matrix proteins. Evidence for salt-resistant DNA binding sites. J Biol Chem. 1991 Jun 15;266(17):11131–11140. [PubMed] [Google Scholar]
- Hakes D. J., Berezney R. Molecular cloning of matrin F/G: A DNA binding protein of the nuclear matrix that contains putative zinc finger motifs. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6186–6190. doi: 10.1073/pnas.88.14.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Vaughn J. P., Dijkwel P. A., Leu T. H., Ma C. Origins of replication: timing and chromosomal position. Curr Opin Cell Biol. 1991 Jun;3(3):414–421. doi: 10.1016/0955-0674(91)90068-a. [DOI] [PubMed] [Google Scholar]
- Hofmann J. F., Laroche T., Brand A. H., Gasser S. M. RAP-1 factor is necessary for DNA loop formation in vitro at the silent mating type locus HML. Cell. 1989 Jun 2;57(5):725–737. doi: 10.1016/0092-8674(89)90788-5. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Käs E., Laemmli U. K. Highly preferential nucleation of histone H1 assembly on scaffold-associated regions. J Mol Biol. 1989 Dec 5;210(3):573–585. doi: 10.1016/0022-2836(89)90133-2. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Transcription occurs at a nucleoskeleton. EMBO J. 1985 Apr;4(4):919–925. doi: 10.1002/j.1460-2075.1985.tb03719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klehr D., Maass K., Bode J. Scaffold-attached regions from the human interferon beta domain can be used to enhance the stable expression of genes under the control of various promoters. Biochemistry. 1991 Feb 5;30(5):1264–1270. doi: 10.1021/bi00219a015. [DOI] [PubMed] [Google Scholar]
- Kunze N., Yang G. C., Dölberg M., Sundarp R., Knippers R., Richter A. Structure of the human type I DNA topoisomerase gene. J Biol Chem. 1991 May 25;266(15):9610–9616. [PubMed] [Google Scholar]
- Käs E., Chasin L. A. Anchorage of the Chinese hamster dihydrofolate reductase gene to the nuclear scaffold occurs in an intragenic region. J Mol Biol. 1987 Dec 20;198(4):677–692. doi: 10.1016/0022-2836(87)90209-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loc P. V., Strätling W. H. The matrix attachment regions of the chicken lysozyme gene co-map with the boundaries of the chromatin domain. EMBO J. 1988 Mar;7(3):655–664. doi: 10.1002/j.1460-2075.1988.tb02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Richter A., Otto B., Knippers R. Replication of SV40 chromatin in extracts from eggs of Xenopus laevis. Nucleic Acids Res. 1981 Aug 11;9(15):3793–3807. doi: 10.1093/nar/9.15.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge M., Dahmus M. E., Bradbury E. M. Chromosomal loop/nuclear matrix organization of transcriptionally active and inactive RNA polymerases in HeLa nuclei. J Mol Biol. 1988 Jun 5;201(3):545–555. doi: 10.1016/0022-2836(88)90636-5. [DOI] [PubMed] [Google Scholar]
- Sapp M., Knippers R., Richter A. DNA binding properties of a 110 kDa nucleolar protein. Nucleic Acids Res. 1986 Sep 11;14(17):6803–6820. doi: 10.1093/nar/14.17.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp M., König H., Riedel H. D., Richter A., Knippers R. A newly detected class of mammalian single strand-specific DNA-binding proteins. Effects on DNA polymerase alpha-catalyzed DNA synthesis. J Biol Chem. 1985 Feb 10;260(3):1550–1556. [PubMed] [Google Scholar]
- Schiedner G., Wessel R., Scheffner M., Stahl H. Renaturation and DNA looping promoted by the SV40 large tumour antigen. EMBO J. 1990 Sep;9(9):2937–2943. doi: 10.1002/j.1460-2075.1990.tb07485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Nasmyth K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell. 1987 Dec 4;51(5):721–732. doi: 10.1016/0092-8674(87)90095-x. [DOI] [PubMed] [Google Scholar]
- Smith H. C., Berezney R. DNA polymerase alpha is tightly bound to the nuclear matrix of actively replicating liver. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1541–1547. doi: 10.1016/s0006-291x(80)80041-6. [DOI] [PubMed] [Google Scholar]
- Sperry A. O., Blasquez V. C., Garrard W. T. Dysfunction of chromosomal loop attachment sites: illegitimate recombination linked to matrix association regions and topoisomerase II. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5497–5501. doi: 10.1073/pnas.86.14.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzner J. R., Chung I. K., Muller M. T. Eukaryotic topoisomerase II preferentially cleaves alternating purine-pyrimidine repeats. Nucleic Acids Res. 1990 Jan 11;18(1):1–11. doi: 10.1093/nar/18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stief A., Winter D. M., Strätling W. H., Sippel A. E. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989 Sep 28;341(6240):343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Strausfeld U., Richter A. Simultaneous purification of DNA topoisomerase I and II from eukaryotic cells. Prep Biochem. 1989;19(1):37–48. doi: 10.1080/10826068908544895. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais M. L., Sentenac A. Asymmetric DNA bending induced by the yeast multifunctional factor TUF. J Biol Chem. 1989 May 25;264(15):8463–8466. [PubMed] [Google Scholar]
- White M. A., Wierdl M., Detloff P., Petes T. D. DNA-binding protein RAP1 stimulates meiotic recombination at the HIS4 locus in yeast. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9755–9759. doi: 10.1073/pnas.88.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- van Driel R., Humbel B., de Jong L. The nucleus: a black box being opened. J Cell Biochem. 1991 Dec;47(4):311–316. doi: 10.1002/jcb.240470405. [DOI] [PubMed] [Google Scholar]
- von Kries J. P., Buhrmester H., Strätling W. H. A matrix/scaffold attachment region binding protein: identification, purification, and mode of binding. Cell. 1991 Jan 11;64(1):123–135. doi: 10.1016/0092-8674(91)90214-j. [DOI] [PubMed] [Google Scholar]