Abstract
Background
Invasive-disease-free survival (IDFS) rates are excellent in breast cancer (BC) patients with hormone-receptor-positive (HR+), human-epidermal-growth-factor-receptor-2-negative (HER2−), axillary-lymph-node-negative (LN−) tumors with a 21-gene-expression assay recurrence-score (RS) of 0–10. However, outcomes among patients with a RS of 11–25 treated with endocrine therapy alone are unknown.
Methods
In this retrospective single-institution study, we described the characteristics of HR+, HER2−, LN− BC patients who underwent a 21-gene-expression assay. Additionally, among those diagnosed between 2005 and 2011 we measured IDFS, recurrence-free survival (RFS), distant-relapse-free survival (DRFS), and overall survival (OS) rates, focusing on patients with a RS of 11–25 by receipt of chemotherapy. We used the Kaplan-Meier method to estimate survival rates and multivariable Cox proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
Among 1,424 patients, the RS distribution was 0–10 in 297 patients (21%), 11–25 in 894 (63%), and >25 in 233 (16%); of these, 1.7%, 15%, and 73.4% received chemotherapy, respectively. With a median follow-up of 58 months, those with a RS of 11–25 had an IDFS rate (95% CI) at 5 years of 92.6% (89.6%–94.7%), which was comparable between those who received chemotherapy and those who did not. The HRs (95% CIs) of the effect of chemotherapy were: IDFS, 1.64 (0.73–3.71); RFS, 1.46 (0.41–5.23); DRFS, 1.25 (0.32–4.92); and OS, 2.19 (0.44–11.0).
Conclusion
Our study showed similar outcomes with or without chemotherapy in HR+, HER2−, LN− BC patients who have a RS of 11–25, but a benefit from chemotherapy in this group cannot be ruled out.
Keywords: Early-stage breast cancer, tumor genomics, gene-expression assays, hormone-receptor positive, chemotherapy
Introduction
Tumor gene expression profiling provides additional prognostic and predictive information to that obtained from traditional tumor histopathologic factors and biomarkers in patients with early-stage breast cancer (BC).1 In the United States (U.S.), the most widely used test is the 21-gene expression assay (Oncotype DX; Genomic Health Inc., Redwood City, CA), which reports a recurrence score (RS) that estimates the 10-year risk of distant recurrence in BC patients with hormone receptor-positive (HR+), human epidermal growth factor receptor-2-negative (HER2−), axillary lymph node-negative (LN−) disease.2, 3 This assay has been validated in several independent data sets4–8 and is recommended both by the American Society of Clinical Oncology and by the National Comprehensive Cancer Network guidelines in the management of HR+, HER2− early-stage BC to assess the risk of recurrence and the potential benefit of adjuvant systemic therapy.9, 10
Two prospective clinical trials reported excellent survival outcomes in HR+, HER2− early-stage BC patients with RS of <11 who were treated with endocrine therapy only. The West German Study Group Phase III Plan B trial showed a 3-year disease-free survival (DFS) rate of 98%.11 The Trial Assigning Individualized Options for Treatment (TAILORx) reported an invasive disease-free survival (IDFS) rate of 93.8% at 5 years, and the risk of distant recurrence was less than 1%, as most events were second primary breast cancers or cancers from other sites, which are not expected to be affected by the receipt of chemotherapy.12 After studies demonstrated the prognostic and predictive value of the 21-gene assay2, 7, the standard of care changed for the treatment of HR+, HER2−, LN− early-stage BC, and the use of tumor gene profiling became widespread in the U.S. The original categorization of the 21-gene-based RS was 0–17 for low risk, 18–30 for intermediate risk, and >30 for high risk.2, 7 By design, the TAILORx clinical trial investigators shifted the categorization of RS to the left of the continuum as a conservative measure to prevent the potential harm of not giving chemotherapy, and a “new” intermediate score was created, and initial results support omitting chemotherapy in patients with an RS of 0–10.3, 12. However, the results of the effects of chemotherapy in patients with a RS of 11–25 have not yet been reported.
It is unknown whether chemotherapy provides any additional benefit in outcomes in HR+, HER2−, LN− early-stage BC patients with RS of 11–25 who are treated with endocrine therapy. We therefore decided to describe the characteristics of patients who underwent a 21-gene-expression assay in our institution, and in addition, we estimated IDFS, recurrence-free survival (RFS), distant relapse-free survival (DRFS), and overall survival (OS) rates among those who had tumors with a RS of 11–25 and were diagnosed between 2005 and 2011 to allow for sufficient follow up time.
Methods
This was a single-institution retrospective descriptive analysis of a cohort of patients with a histologically confirmed diagnosis of HR+, HER2−, LN−, stage I-II BC who had received their initial treatment at The University of Texas MD Anderson Cancer Center (Houston, Texas) and had undergone a 21-gene expression assay. Patients were identified and study data were collected from the Breast Cancer Management System database and from the patients’ electronic medical charts. This study was performed with approval from the MD Anderson Institutional Review Board (protocol PA15–0387) and a waiver of consent was obtained. The exclusion criteria consisted of those who had undergone any treatment for their BC prior to their initial visit to MD Anderson, those with lymph node-positive disease, those with unknown tumor characteristics or missing treatment information, and finally we excluded patients who had enrolled and participated in the TAILORx clinical trial12 at the MD Anderson site.
We assessed patients’ demographic characteristics (age at diagnosis, race/ethnicity, sex, and menopausal status), tumor prognostic factors (size, histologic grade, histologic type, Ki-67 expression, and lymphovascular invasion [LVI]), and treatment received (type of surgery, radiation therapy, and endocrine therapy). The tumor’s estrogen receptor, progesterone receptor and HER2 status were determined by immunohistochemical analysis or fluorescence in situ hybridization using institutional laboratory thresholds and following standard current guidelines.13, 14 The tumor stage was determined using the American Joint Committee on Cancer guidelines, 6th edition.15 The last follow-up date for patients in this study was November 1, 2015.
Following the cutoff scores used in the TAILORx clinical trial12, we categorized the RS results in three groups: 0–10, 11–25 and >25. Among those diagnosed between 2005 and 2011, we estimated IDFS, RFS, DRFS, and OS rates. We used the Standardized Definitions for Efficacy Endpoints16 in adjuvant BC trial criteria to define such outcomes. We compared these outcomes of interest between patients who received chemotherapy with those who did not, focusing among those with a RS of 11–25. Patients were censored at the last day of follow-up. We determined the most recent vital status of each patient using data from the Tumor Registry at MD Anderson and via the review of the patient’s medical record.
Statistical analyses
Demographic characteristics were compared using the chi-square or Kruskal-Wallis test, as appropriate. We used the Kaplan-Meier product-limit method to estimate the survival function and the long-rank statistic to compare survival distributions by RS category. The multivariable Cox proportional hazards model was used to assess the effect of chemotherapy on IDFS, RFS, DRFS, and OS rates.17 The following pre-specified prognostic factors were considered for adjustment in the multivariable model: age at diagnosis, tumor size, grade, histologic subtype, Ki-67 expression, LVI, type of surgery, and endocrine therapy. If a pre-specified confounding variable failed to produce stable estimate of effect because of sparse distribution of events across its categories, we removed such variable from the multivariable model. The proportional hazards assumption was assessed for the continuous variable using Martingale residuals.
In an effort to minimize selection bias we also conducted a propensity score-matched analysis, obtaining the conditional probability of receiving chemotherapy given the known prognostic covariates. Propensity scores were obtained using a multivariable logistic regression model with a nearest neighborhood matching method to obtain 1:1 matched doublets.18 Two-way interactions were introduced to improve the matching. We used absolute standardized differences to assess balance in the covariates between patients who received chemotherapy vs. patients who did not, where a difference of <10% suggested a substantial reduction of bias. All statistical tests were performed using a two-sided significance level of 0.05. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute), and STATA software, version 12 (Statacorp, College Station, Texas).
Results
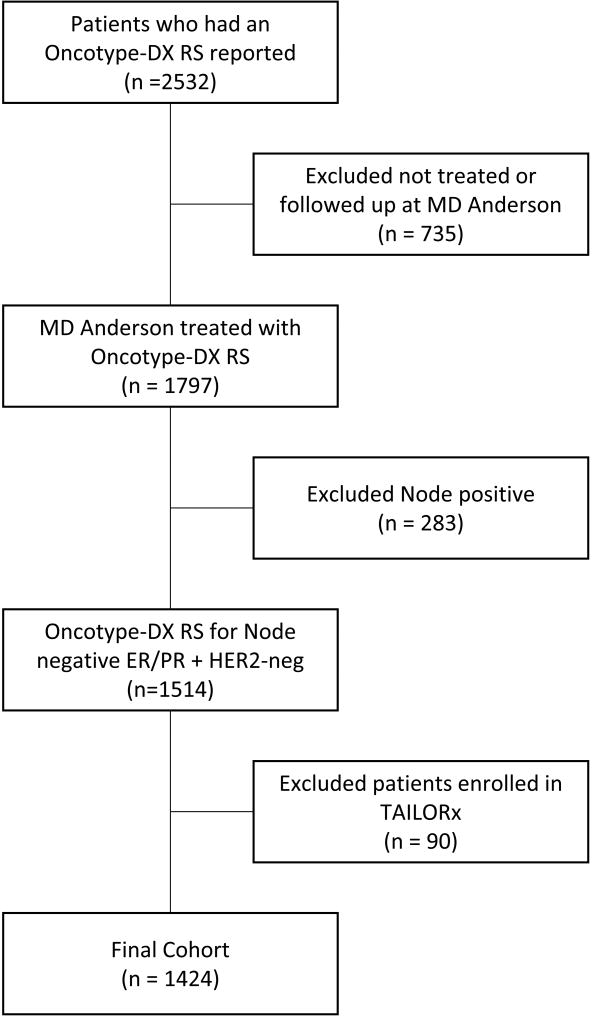
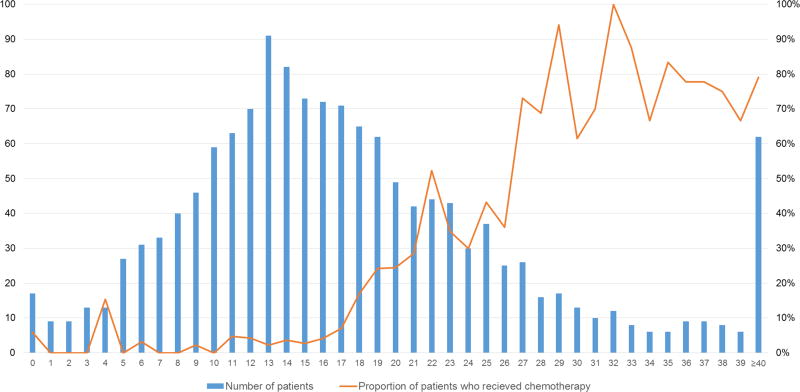
We identified 1,424 patients who met the eligibility criteria (Figure 1, Consort diagram), 99% of whom were female. Table 1 shows the characteristics of the study cohort grouped by RS category (0–10, 11–25, and >25). Age at diagnosis, menopausal status, and race/ethnicity were similarly distributed across RS categories. In the RS>25 category there were more cases of invasive ductal carcinoma, fewer cases of invasive lobular carcinoma, and more tumors with nuclear grade III and high Ki-67 expression compared to the other RS categories. However, the distribution of tumor stage was similar across the RS categories. Interestingly, in the RS 0–10 category, 11.5% of patients had tumors with nuclear grade III, 26.6% with a Ki-67 expression level of >14%, and 11.0% with LVI. The type of surgery and receipt of radiation therapy were relatively similar across the three RS categories. As expected, the rate at which patients received chemotherapy increased steadily with each category. Figure 2 depicts the distribution of patients and the proportion of patients who received chemotherapy by individual RS scores. Of note, the proportion of patients with a RS of 11–25 who received chemotherapy, which was around 15%, did not change substantially throughout the years.
Figure 1.
Consort diagram
Table 1.
Characteristics of patients by recurrence score (N= 1,424)
| ≤ 10 | 11 to 25 | > 25 | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| (N=297) | (N=894) | (N=233) | ||||||
| N | (%) | N | (%) | N | (%) | |||
| Age at diagnosis, years | ||||||||
| 20 – 40 | 27 | 9.1% | 61 | 6.8% | 17 | 7.3% | 0.650 | |
| 41 – 50 | 72 | 24.2% | 250 | 28.0% | 53 | 22.8% | ||
| 51 – 60 | 99 | 33.3% | 300 | 33.6% | 77 | 33.1% | ||
| 61 – 70 | 78 | 26.3% | 229 | 25.6% | 68 | 29.2% | ||
| 71 and above | 21 | 7.1% | 54 | 6.0% | 18 | 7.7% | ||
| Median (Q1–Q3) | 56 (48–63) | 55 (48–63) | 56 (50–64) | 0.135 | ||||
| Year of diagnosis | ||||||||
| 2005 – 2011 | 166 | 55.9% | 549 | 61.4% | 144 | 61.8% | 0.213 | |
| 2012 – 2014 | 131 | 44.1% | 345 | 38.6% | 89 | 38.2% | ||
| Race/Ethnicity | ||||||||
| Caucasian | 220 | 74.1% | 651 | 72.8% | 166 | 71.2% | 0.553 | |
| African-American | 16 | 5.4% | 58 | 6.5% | 23 | 9.9% | ||
| Hispanic | 43 | 14.5% | 125 | 14.0% | 31 | 13.3% | ||
| Other | 18 | 6.1% | 60 | 6.7% | 13 | 5.6% | ||
| Female | 294 | 99.0% | 891 | 99.7% | 230 | 98.7% | 0.119 | |
| Menopausal Status* | ||||||||
| Post | 190 | 64.6% | 598 | 67.1% | 171 | 74.4% | 0.073 | |
| Pre | 99 | 33.7% | 270 | 30.3% | 52 | 22.6% | ||
| Perimenopausal /Unknown | 5 | 1.7% | 23 | 2.6% | 7 | 3.0% | ||
| Histology | ||||||||
| Ductal | 236 | 79.5% | 654 | 73.2% | 209 | 89.7% | p<0.001 | |
| Lobular | 33 | 11.1% | 126 | 14.1% | 13 | 5.6% | ||
| Mixed | 15 | 5.1% | 68 | 7.6% | 6 | 2.6% | ||
| Other | 13 | 4.4% | 46 | 5.2% | 5 | 2.2% | ||
| Nuclear Grade | ||||||||
| I | 45 | 15.2% | 122 | 13.7% | 4 | 1.7% | p<0.001 | |
| II | 212 | 71.4% | 618 | 69.1% | 68 | 29.2% | ||
| III | 34 | 11.5% | 144 | 16.1% | 151 | 64.8% | ||
| Unknown | 6 | 2.0% | 10 | 1.1% | 10 | 4.3% | ||
| Ki-67 expression† | ||||||||
| Median (range) | 10 (1 – 60) | 10 (0 – 88) | 32.5 (4 – 95) | p<0.001 | ||||
| >14% | 46 | 26.6% | 212 | 41.3% | 109 | 83.9% | ||
| Lymphovascular invasion‡ | 32 | 11.0% | 110 | 12.4% | 35 | 15.2% | 0.326 | |
| Tumor Stage | ||||||||
| I | 220 | 74.1% | 674 | 75.4% | 164 | 70.4% | 0.296 | |
| II | 77 | 25.9% | 220 | 24.6% | 69 | 29.6% | ||
| Tumor size (cm) | ||||||||
| < 1.0 | 61 | 20.5% | 178 | 19.9% | 27 | 11.6% | 0.002 | |
| 1.1 –1.9 | 150 | 50.5% | 444 | 49.7% | 118 | 50.6% | ||
| 2.0 – 2.9 | 54 | 18.2% | 171 | 19.1% | 57 | 24.5% | ||
| 3.0 – 3.9 | 19 | 6.4% | 47 | 5.3% | 25 | 10.7% | ||
| ≥ 4.0 | 13 | 4.4% | 54 | 6.0% | 6 | 2.6% | ||
| Median (Q1–Q3) | 1.4 (1.0–2.1) | 1.5 (1.0–2.0) | 1.7 (1.2–2.3) | 0.002 | ||||
| Surgery type | ||||||||
| Partial mastectomy | 161 | 54.2% | 544 | 60.9% | 148 | 63.5% | 0.060 | |
| Mastectomy | 136 | 45.8% | 350 | 39.2% | 85 | 36.5% | ||
| Chemotherapy | 5 | 1.7% | 134 | 15.0% | 171 | 73.4% | p<0.001 | |
| Radiation therapy | 161 | 54.2% | 511 | 57.2% | 128 | 54.9% | 0.618 | |
| Endocrine therapy | 273 | 91.9% | 835 | 93.4% | 194 | 83.3% | p<0.001 | |
In female patients
Available for 816 patients
Available for 1,417 patients
Figure 2.
Number of patients and percentage of patients who received chemotherapy per individual RS
Table 2 shows the characteristics of 549 patients, all females, diagnosed between 2005 and 2011, who had tumors with a RS of 11–25, categorized by receipt of chemotherapy. Patients who received chemotherapy were younger, had larger tumors, and had more stage II tumors than did those who did not receive chemotherapy, with a tendency for these patients of also having higher Ki-67scores and more LVI. However, the distribution of the other demographic and tumor characteristics, and treatments received were comparable. In these patients, after a median follow-up time of 58 months (interquartile range 46–73 months), there were 41 outcome events (overall rate 7.5%): 10 distant recurrences, 3 local recurrences, 7 deaths, 11 second primary BCs, and 10 primary cancers at other sites.
Table 2.
Characteristics of 549 female patients diagnosed between 2005 and 2011 with a RS of 11–25 by receipt of chemotherapy
| Chemotherapy | No chemotherapy | p-value | |||
|---|---|---|---|---|---|
| (N= 92) | (N= 457) | ||||
| N | (%) | N | (%) | ||
| Age at diagnosis, years | |||||
| 20 – 40 | 15 | 16.3% | 24 | 5.3% | p<0.001 |
| 41 – 50 | 37 | 40.2% | 113 | 24.7% | |
| 51 – 60 | 23 | 25% | 158 | 34.6% | |
| 61 – 70 | 13 | 14.1% | 136 | 29.8% | |
| 71 and above | 4 | 4.4% | 26 | 5.7% | |
| Median (Q1–Q3) | 50 (44–57) | 57 (49–63) | p<0.001 | ||
| Race/Ethnicity | |||||
| Caucasian | 70 | 76.1% | 346 | 75.7% | 0.652 |
| African-American | 7 | 7.6% | 23 | 5.0% | |
| Hispanic | 9 | 9.8% | 60 | 13.1% | |
| Other | 6 | 6.5% | 28 | 6.1% | |
| Menopausal Status | |||||
| Post | 52 | 56.5% | 329 | 72.0% | 0.010 |
| Pre | 36 | 39.1% | 119 | 26.0% | |
| Perimenopausal/Unknown | 4 | 4.4% | 9 | 2.0% | |
| Histology | |||||
| Ductal | 69 | 75.0% | 331 | 72.4% | 0.571 |
| Lobular | 10 | 10.9% | 65 | 14.2% | |
| Mixed | 10 | 10.9% | 37 | 8.1% | |
| Other | 3 | 3.3% | 24 | 5.3% | |
| Nuclear Grade | |||||
| I | 11 | 12.0% | 65 | 14.2% | 0.727 |
| II | 64 | 69.6% | 320 | 70.0% | |
| III | 17 | 18.5% | 72 | 15.8% | |
| Ki-67 expression† | |||||
| Median (range) | 10 (1 – 60) | 10 (0 – 88) | 0.689 | ||
| >14% | 23 | 41.1% | 99 | 39.4% | 0.822 |
| Lymphovascular invasion‡ | 14 | 15.2% | 50 | 11.1% | 0.250 |
| Tumor Stage | |||||
| I | 64 | 69.6% | 367 | 80.3% | 0.02 |
| II | 28 | 30.4% | 90 | 19.7% | |
| Tumor size (cm) | |||||
| < 1.0 | 12 | 13.0% | 103 | 22.5% | 0.050 |
| 1.1 – 1.9 | 45 | 48.9% | 234 | 51.2% | |
| 2.0 – 2.9 | 22 | 23.9% | 78 | 17.1% | |
| 3.0 – 3.9 | 8 | 8.7% | 17 | 3.7% | |
| ≥ 4.0 | 5 | 5.4% | 25 | 5.5% | |
| Median (Q1–Q3) | 1.5 (1.2–2.2) | 1.4 (1.0–2.0) | 0.052 | ||
| Surgery type | |||||
| Partial mastectomy | 56 | 60.9% | 280 | 61.3% | 0.943 |
| Mastectomy | 36 | 39.1% | 177 | 38.7% | |
| Radiation therapy | 53 | 57.6% | 274 | 60.0% | 0.676 |
| Endocrine therapy | 90 | 97.8% | 436 | 95.4% | 0.290 |
Available for 307 patients
Available for 547 patients
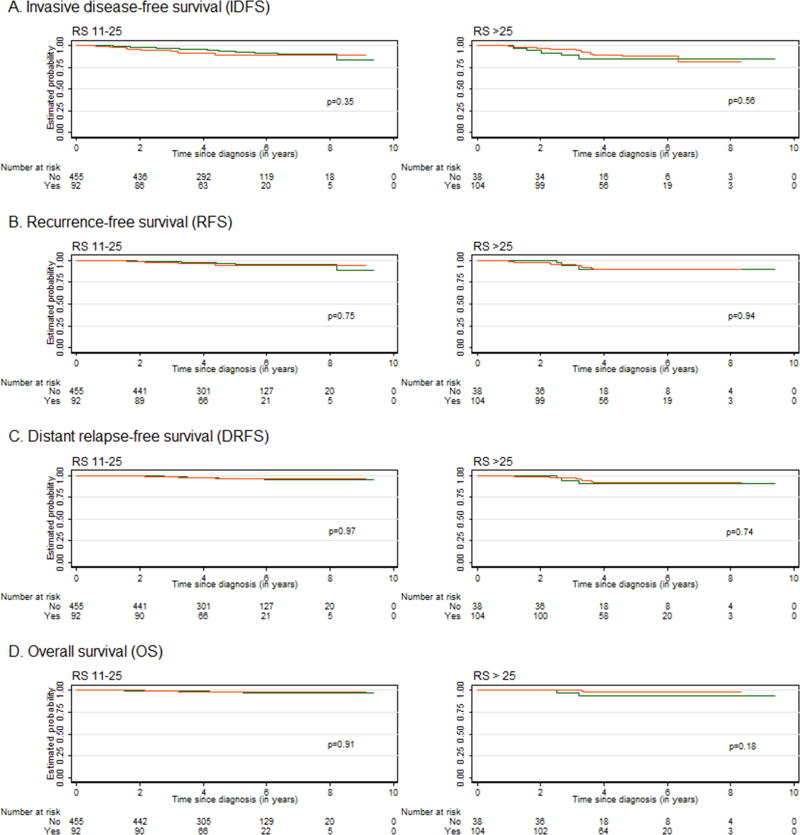
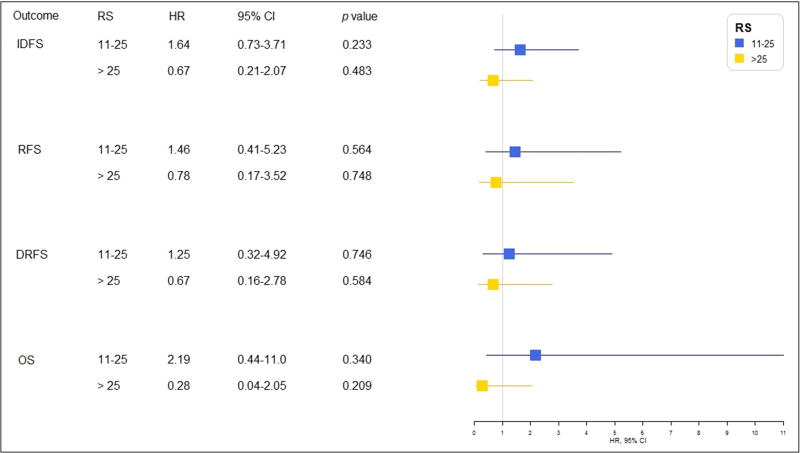
Figure 3 depicts the Kaplan-Meier curves for each outcome by receipt of chemotherapy for the RS categories of 11–25 and >25. There was no significant separation of the curves in any of the RS categories for any of the four outcomes. Figure 4 shows the results of the multivariable analysis of the effect of chemotherapy on each outcome in patients with an RS of 11–25 diagnosed between 2005 and 2011. In the multivariable model, we excluded Ki-67 due to a high frequency of missing values. All HRs were above 1.0, but none reached statistical significance. The estimated 5-year IDFS rates (95% CI) were 89% (80–94%) and 93% (90–95%) among those who received chemotherapy and those who did not, respectively. Similarly, the 5-year RFS rates were 95% (86–98%) and 96% (94–98%), the 5-year DRFS rates were 96% (87–99%) and 96% (94–98%), and the 5-year OS rates were 98% (91–99%) and 98% (96–99%), respectively. Among patients who had tumors with an RS >25, all HRs were <1.0, suggesting a benefit from chemotherapy; however, statistical significance was not reached. Among patients with a RS 11–25 diagnosed between 2005–2001, 92 received chemotherapy. The logistic regression-based propensity score model selected a matched cohort of 178 patients, where 89 patients received chemotherapy and 89 did not. The balance statistics of the distribution of the confounding variables in patients who received chemotherapy and who did not (Supplementary Table 1) showed that patients were well matched in their prognostic factors. Findings in the survival differences (Supplementary Figure 1) in this matched cohort were consistent with that in our primary analysis.
Figure 3.
Kaplan-Meier curves for (A) invasive disease-free survival (IDFS), (B) recurrence-free survival (RFS), (C) distant relapse-free survival (DRFS), and (D) overall survival (OS) in patients who did or did not receive chemotherapy, by recurrence score (RS) category; orange line represents patients who received chemotherapy, and green line those who did not.
Figure 4.
Multivariable analysis of patients who received chemotherapy versus those who did not, by recurrence score (RS) category. Abbreviations: IDFS, invasive disease-free survival; RFS, recurrence-free survival; DRFS, distant relapse-free survival; OS, overall survival; RS, recurrence score (RS); HR, hazard ratio; CI, confidence interval
Discussion
In this large single-institution, unselected cohort of early-stage BC patients with HR+, HER2−, LN− disease, among patients with tumors with a RS of 11–25, we found similar outcomes among those who received chemotherapy compared to those who did not. These results were limited by the low number of events and by the relatively short median follow-up time. Among those patients who did not receive chemotherapy, the estimated rates of freedom from outcomes at 5 years ranged from 93% IDFS to 98% OS, which are very good and comparable to those who did receive chemotherapy. Interestingly, the 5-year IDFS rate was very close to the 93.8% reported by the TAILORx trial for the subgroup with a RS of 0–11, and also close to the 92% predefined lower boundary of the 95% CI of the rate of 5-year survival without distant metastasis in the Microarray in Node-Negative and 1 to 3 Positive Lymph Node Disease May Avoid Chemotherapy (MINDACT) study.12, 19
The benefits of chemotherapy must always be balanced against its short- and long-term side effects; therefore, estimates of risks are essential for making clinical decisions. BC represents a variety of different diseases and currently we have prognostic and predictive biomarkers of treatment effectiveness.20 The standard of care for the treatment of HR+, HER2−, LN− early-stage BC using a tumor gene profiling assay has become widespread in the U.S. After the original classification of the 21-gene-based RS was suggested to be 0–17 for low risk, 18–30 for intermediate risk, and >30 for high risk2, 7 the benefit of chemotherapy for patients with tumors who landed in the intermediate risk has since remained unclear.
A report from the Clalit Health Services of 1,594 early-stage BC patients with HR+, HER2−, and LN− or LN−microscopic disease showed that among those with an intermediate RS of 18–30, there were no differences in distant recurrence between those who underwent chemotherapy and those who did not.21, 22 The report from the MINDACT investigators showed that among 1,550 female patients with early-stage BC (26% with node-positive disease) who were classified as having high clinical risk for recurrence using a modified version of Adjuvant! Online23 and low genomic risk using a 70-gene signature, those who received chemotherapy had a non-significant 1.5% improvement in the 5-year rate of survival without distant metastasis.19 Interestingly, if we were to apply the same criteria used by the MINDACT study, 72.5% (398 of 549) of the patients from our study with a RS of 11–25 would have been classified as low clinical risk and would have not benefited from chemotherapy.
As a planned conservative measure, the TAILORx clinical trial investigators decided to shift the categorization of RS to the left of the continuum, and a “new” intermediate score was then created.3, 12 Initial results showed a very low event rate at 5 years among patients with tumors with a “very low” RS of 0–10. Comparable results were seen in the West German Plan B trial.11 These results clearly justify omitting chemotherapy in patients with an RS of 0–10. The benefits of chemotherapy in patients with a RS of 11–25 will remain unclear until we learn the outcome results of the 6,897 patients in the TAILORx trial who were randomly assigned to chemotherapy versus no chemotherapy. Although our study showed similar outcomes between those who received chemotherapy compared to those who did not in the group of patients with a RS of 11–25, we clearly do not promote a change of current clinical practice as our study did not have the statistical power to show a difference. It was interesting to observe that known prognostic tumor variables do not correlate entirely with the RS. As seen by others11, 12, there is a small but considerable proportion of patients who have tumors with a RS of <11 but have grade 3 disease, high Ki-67 expression levels, and high LVI, which are traditionally considered as tumor high risk factors. Therefore, determining the RS of tumors with these characteristics is justifiable.
Recent U.S. population-based studies have reported on trends of the use of the 21-gene RS assay associated with variables of interest. Using the National Cancer Database, investigators identified 143,032 early-stage BC patients of whom 54% had the RS assay performed, which significantly influenced clinicians’ recommendation of chemotherapy, with significant differences in its use by race, type of insurance, and facility.24 A Surveillance, Epidemiology, and End Results (SEER) population-based study of 38,568 BC patients showed unadjusted 5-year BC-specific mortality rates of 0.4%, 1.4%, and 4.4% in patients with RS of <18, 18–30, and >30, respectively; however, due to under-reporting of chemotherapy in SEER and a possible selection bias, the investigators did not report outcomes by treatment choice.25 Using the Pennsylvania Cancer Registry, investigators identified a retrospective cohort of 7,287 early-stage BC patients among whom the use of the RS assay was associated with a decrease in the use of chemotherapy and lower costs in women younger than 55 years and a modest increase in chemotherapy use and costs among patients 75 years and older.26 The Carolina Breast Cancer Study of 1,468 women reported no racial disparities in RS testing for LN− patients, although black women had tumors with worse known prognostic markers, such as high tumor grade and tumor size.27
Our study has several limitations, of which the main one is its relatively short follow-up time, which may explain the low number of events. Additionally, a relatively low number of patients with a RS 11–25 received chemotherapy. However, the Kaplan-Meier curves in the RS 11–25 category did not show any separation, while there was a minuscule separation in the RS>25 category. In addition, the estimated 5-year rates of freedom of all outcomes were 93% and above. We therefore decided to run an exploratory analysis using the original RS cut-off points of <18, 18–30, and >30, and this showed a separation of the curves (p-value 0.12) among the subset of patients with an RS >30; the results of the multivariable analysis suggested that chemotherapy was beneficial in patients with a RS >30 (Supplementary Table 2). The other limitations of our study are inherent to retrospective studies, such as unbalanced characteristics among comparison groups and selection bias, reason why we performed a propensity score matched analysis.
In this single-institution, retrospective descriptive study of a cohort of early-stage HR+, HER2−, LN− BC patients, after a median follow-up time of 58 months, high RFS and IDFS rates were found among those who had a RS of 11–25, and there were similar outcomes among those who received chemotherapy compared to those who did not. A benefit from chemotherapy in this group cannot be ruled out as longer follow-up time is required to observe future events.
Supplementary Material
Supplementary Figure 1. Propensity score analysis Kaplan-Meier curves for invasive disease-free survival (IDFS), recurrence-free survival (RFS), distant relapse-free survival (DRFS), and overall survival (OS) in patients who did or did not receive chemotherapy, by recurrence score (RS) category
Acknowledgments
Funding: None
Footnotes
Potential conflict of interest:
Co-author Dr. Gabriel N Hortobagyi is currently chair of the Scientific Advisory Board of Agendia, which is the sponsor of Mammaprint
References
- 1.Adaniel C, Jhaveri K, Heguy A, Esteva FJ. Genome-based risk prediction for early stage breast cancer. Oncologist. 2014;19:1019–1027. doi: 10.1634/theoncologist.2014-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 3.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 4.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. The Lancet Oncology. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 8.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris LN, Ismaila N, McShane LM, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:324–354. doi: 10.6004/jnccn.2016.0037. [DOI] [PubMed] [Google Scholar]
- 11.Gluz O, Nitz UA, Christgen M, et al. West German Study Group Phase III PlanB Trial: First Prospective Outcome Data for the 21-Gene Recurrence Score Assay and Concordance of Prognostic Markers by Central and Local Pathology Assessment. J Clin Oncol. 2016;34:2341–2349. doi: 10.1200/JCO.2015.63.5383. [DOI] [PubMed] [Google Scholar]
- 12.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 15.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 17.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. Springer Science & Business Media; 2005. [Google Scholar]
- 18.Ho D, Imai K, King G, Stuart E. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis. 2007;15:199–236. [Google Scholar]
- 19.Cardoso F, van't Veer LJ, Bogaerts J, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 20.Ballman KV. Biomarker: Predictive or Prognostic? J Clin Oncol. 2015;33:3968–3971. doi: 10.1200/JCO.2015.63.3651. [DOI] [PubMed] [Google Scholar]
- 21.Stemmer SM, Steiner M, Rizel S, et al. ASCO Annual Meeting. Chicago: 2016. Real-lif analysis evaluating 1594 N0/Nmic breast cancer pateints for whom treatment decisions incorporated the 21-gene recurrence score result 5-year KM estimate for breast cancer specific survival with recurrence score results <30 is 98% [Google Scholar]
- 22.Stemmer SM, Klang SH, Ben-Baruch N, et al. The impact of the 21-gene Recurrence Score assay on clinical decision-making in node-positive (up to 3 positive nodes) estrogen receptor-positive breast cancer patients. Breast Cancer Res Treat. 2013;140:83–92. doi: 10.1007/s10549-013-2603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 24.Jasem J, Amini A, Rabinovitch R, et al. 21-Gene Recurrence Score Assay As a Predictor of Adjuvant Chemotherapy Administration for Early-Stage Breast Cancer: An Analysis of Use, Therapeutic Implications, and Disparity Profile. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petkov VI, Miller DP, Howlader N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. Npj Breast Cancer. 2016;2:16017. doi: 10.1038/npjbcancer.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein AJ, Wong YN, Mitra N, et al. Adjuvant Chemotherapy Use and Health Care Costs After Introduction of Genomic Testing in Breast Cancer. J Clin Oncol. 2015;33:4259–4267. doi: 10.1200/JCO.2015.61.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts MC, Weinberger M, Dusetzina SB, et al. Racial Variation in the Uptake of Oncotype DX Testing for Early-Stage Breast Cancer. J Clin Oncol. 2016;34:130–138. doi: 10.1200/JCO.2015.63.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Propensity score analysis Kaplan-Meier curves for invasive disease-free survival (IDFS), recurrence-free survival (RFS), distant relapse-free survival (DRFS), and overall survival (OS) in patients who did or did not receive chemotherapy, by recurrence score (RS) category