Abstract
Objective
To determine the feasibility and acute (i.e., within 72 hrs) safety of three levels of systolic blood pressure reduction in subjects with supratentorial intracerebral hemorrhage treated within 6 hrs after symptom onset.
Design
A traditional phase I, dose-escalation, multicenter prospective study.
Settings
Emergency departments and intensive care units.
Patients
Patients with intracerebral hemorrhage with elevated systolic blood pressure ≥170 mm Hg who present to the emergency department within 6 hrs of symptom onset.
Intervention
Intravenous nicardipine to reduce systolic blood pressure to a target of: (1) 170 to 200 mm Hg in the first cohort of patients; (2) 140 to 170 mm Hg in the second cohort; and (3) 110 to 140 mm Hg in the third cohort.
Measurements and Main Results
Primary outcomes of interest were: (1) treatment feasibility (achieving and maintaining the systolic blood pressure goals for 18–24 hrs); (2) neurologic deterioration within 24 hrs; and (3) serious adverse events within 72 hrs. Safety stopping rules based on neurologic deterioration and serious adverse events were prespecified and approved by an NIH-appointed Data and Safety Monitoring Board, which provided oversight on subject safety. Each subject was followed-up for 3 months to preliminarily assess mortality and the clinical outcomes. A total of 18, 20, and 22 patients were enrolled in the respective three tiers of systolic blood pressure treatment goals. Overall, 9 of 60 patients had treatment failures (all in the last tier). A total of seven subjects with neurologic deterioration were observed: one (6%), two (10%), and four (18%) in tier one, two, and three, respectively. Serious adverse events were observed in one subject (5%) in tier two and in three subjects (14%) in tier three. However, the safety stopping rule was not activated in any of the tiers. Three (17%), two (10%), and five (23%) subjects in tiers one, two, and three, respectively, died within 3 months.
Conclusions
The observed proportions of neurologic deterioration and serious adverse events were below the prespecified safety thresholds, and the 3-month mortality rate was lower than expected in all systolic blood pressure tiers. The results form the basis of a larger randomized trial addressing the efficacy of systolic blood pressure reduction in patients with intracerebral hemorrhage.
Keywords: intracerebral hemorrhage, hypertension, nicardipine, systolic blood pressure, hematoma expansion
Acute hypertensive response (1) is observed in 46% to 75% of patients with intracerebral hemorrhage (ICH) depending on the population studied and the definition of hypertension used (2, 3). This suggests that approximately 16,650 to 35,000 ICH patients every year present with acute hypertensive response in the United States. An initial high systolic blood pressure (SBP) is associated with hematoma expansion (4, 5), increased mortality (6), and perihematoma brain edema formation (7) among patients with ICH, although the cause-and-effect relationship is not clear. Some studies have observed an inconsistent relationship between elevated SBP and hematoma expansion (8, 9). Reducing SBP may decrease the rate of hematoma expansion, although conclusive evidence is not available (10). The report from the December 2003 National Institute of Neurologic Disorders and Stroke workshop on priorities for clinical research in ICH (11) recommends clinical trials for evaluation of blood pressure management in acute ICH as a leading priority. The recommendations stated that “a prospective randomized control trial needs to be performed to determine appropriate blood pressure goals and the best strategies to achieve those goals” (10).
Recent studies suggest that reduction of SBP may be tolerated (1) because of reduced metabolism (hibernation) (12) and preserved autoregulation in the perihematoma region (13). The current American Stroke Association Stroke Council (14) and the European Stroke Initiative guidelines (15) recommend maintaining SBP <180 mm Hg in the acute period using short half-life IV antihypertensive medication. Both guidelines consider the possibility of more aggressive SBP lowering in the absence of clinical signs of elevated intracranial pressure (14) or chronic hypertension (15). Therefore, the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) study was designed to study treatment thresholds lower than those provided by current guidelines because of putative incremental benefit. The purpose of this pilot phase of the ATACH study was to primarily ascertain the feasibility and safety of aggressive SBP reduction acutely and to identify the appropriate SBP target to evaluate the clinical efficacy of this treatment in a subsequent phase III clinical trial.
MATERIALS AND METHODS
Study Overview
An open-labeled prospective study funded by National Institute of Neurologic Disorders and Stroke (R01 NS044976) was conducted to obtain an estimate of the proportion of subjects who can achieve the targeted SBP goal and to ascertain acute (i.e., within 72 hrs) safety of three levels of antihypertensive treatment goals using IV nicardipine (ESP Pharma, Edison, NJ). initiated within 6 hrs and continued for 18 to 24 hrs after onset for acute hypertensive response associated with ICH as described previously (16). The rationale for the choice of IV nicardipine, duration of treatment, and intensity of monitoring have been described in a previous publication (16) and has been based on the results of our previous studies (11, 17). The protocol was reviewed and approved by Institutional Review Boards at each participating site. Briefly, three levels of treatment goals of increasing intensity were evaluated in a step-wise fashion to ensure the tolerability and safety of a less intense SBP control range before enrolling patients in the more aggressive tiers. The goal of the first treatment tier was to reduce and maintain the SBP between 170 (inclusive) and 200 mm Hg for the initial 18 to 24 hrs. In the second and third treatment tiers, the goal was to reduce and maintain the SBP between 140 (inclusive) and 170 mm Hg and between 110 (inclusive) and 140 mm Hg, respectively.
The NIH-appointed Data and Safety Monitoring Board (DSMB) was responsible for monitoring of subject recruitment and safety data. Safety stopping rules (18) were established based on the upper limit (by 95% confidence interval) for individual and cumulative rates of neurologic deterioration and serious adverse events (SAE) from previous studies in a manner similar to other phase I trials in acute stroke patents (19). However, there has been a general consensus that formal statistical methods should be used to guide decision-making rather than the hard rules by DSMB (20–23). Predefined rules can be used to trigger a hold on the study until DSMB can assess the relationship between the events and the treatment, as well as the overall risk and benefit before making its recommendations. Therefore, according to the safety stopping rule specified in the protocol, enrollment would have been suspended automatically within the treatment tier if: (1) seven or more subjects experienced neurologic deterioration or death within 24 hrs of treatment initiation; or (2) three or more subjects experienced SAE within 72 hrs of treatment initiation that necessitated termination of nicardipine treatment. In addition, if seven of any of these events occurred within a treatment tier, the safety stopping rule was to be activated. DSMB would have been notified immediately and on reviewing of the data, may consider permanent stopping or resumption of further recruitment of subjects.
Study Subjects
The specific eligibility criteria are listed in Table 1. Among patients with ICH, mortality is highest in the first 48 hrs, with approximately 20% of the patients dying within the first 24 hrs of symptom onset (24). This time-frame coincides with the main evaluation period in the ATACH pilot study. A high rate of early mortality would substantially decrease our ability to evaluate feasibility and safety outcomes in our study population. Based on the study by Broderick et al (25), patients with Glasgow Coma Scale (GCS) score >8 and hematoma volume ≤60 mL constitute the largest group of patients with ICH (59%) and are expected to have a mortality rate low enough (25%) for adequate evaluation of safety outcomes. Therefore, only patients with GCS score >8 and hematoma volume ≤60 mL were included in this ATACH study.
Table 1.
ATACH phase I trial eligibility criteria
Inclusion criteria
|
ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; IV, intravenous; CT, computed tomography; SBP, systolic blood pressure.
After a review of recruitment experience in the first tier, protocol modifications were undertaken to improve recruitment rate without compromising the scientific integrity of the original hypothesis. The eligibility criteria were modified to include patients with SBP ≥170 mm Hg (initially ≥200 mm Hg), and those with lobar hemorrhages (initially only basal ganglionic and thalamic ICH). The concern that modification of inclusion criteria may lead to inclusion of ICH not related to chronic hypertensive arterial disease was minimized by another inclusion criteria that required evidence of chronic hypertension based on previous documentation, use of antihypertensive medication, presence of hypertensive retinopathy, or electrocardiographic (EKG) findings consistent with hypertension (Table 1).
Clinical Management
All patients presenting with ICH to the emergency department at study sites were screened by one of the site investigators who reviewed the patient’s medical record and results of the diagnostic tests to confirm eligibility at presentation. Patients evaluated were divided into two groups: ineligible and eligible. Ineligible patients were entered in the screening log with the reasons for ineligibility. Each eligible patient or their relative/legal guardian (in the event that the patient was incapacitated and was unable to sign the informed consent) was approached to obtain written consent. After enrollment, blood pressure, heart rate, transcutaneous oxygen saturation, and respiratory rate were monitored continuously. A neurologic assessment by a qualified neurologist was performed before initiation of antihypertensive treatment using the National Institute of Health Stroke Scale (NIHSS) and the GCS.
IV nicardipine infusion was initiated at 5 mg/hr, and then increased by 2.5 mg/hr every 15 mins as needed, up to a maximum of 15 mg/hr. Once the target SBP was reached, the infusion rate was decreased by 1 to 3 mg/hr. If the SBP decreased to below the specified levels, infusion was reduced by 2.5 mg/hr every 15 mins until the drug was discontinued. BP was monitored using an intra-arterial catheter or automated cuff inflation device as per the discretion of the primary treating physician for up to 24 hrs after symptom onset. The frequency of measurements was as follows: every 5 mins for the first 15 mins after initiation of IV nicardipine; then, every 15 mins for the first hour, and, subsequently, every 15 to 30 mins for the duration of the study. A more frequent measurement was recommended if the prominent BP changes were observed.
Each subject was admitted in the intensive care unit for a 24-hr observation period and examined every 30 mins by the nursing staff, and a comprehensive neurologic examination was documented in the chart at 2-hr intervals. Continuous monitoring using seven-lead, three-channel EKG monitoring was performed for 18 to 24 hrs during the infusion of nicardipine. IV 0.9% sodium chloride (20 mEq/KCl added) at 1 mL/kg per hour was initiated unless contraindicated. A complete chemistry panel was performed daily for 3 days to monitor for any new renal insufficiency. Additional cardiac enzymes were drawn when clinically indicated or when EKG changes suggested myocardial ischemia. The trial mandated a noncontrast head CT scan at 24 hrs for central image analysis (16) after the initiation of treatment.
End Points
The feasibility of the treatment was assessed by whether the SBP reduction and maintenance in the respective target range was achieved (treatment success) or not (failure). Initially, treatment failure was defined as the inability to reduce and maintain SBP between upper and lower limit specified for the SBP reduction tier in which a subject was enrolled. The definition of treatment failure was modified (with the DSMB approval) after completion of the first tier to exclude spontaneous decline of SBP below the lower limit of the specific tier because all such declines were asymptomatic. Treatment failure was redefined based on the observed hourly minimum SBP remaining greater than the upper limit of the target range for 2 consecutive hours after initiation of nicardipine infusion. Treatment failure was ascertained by study statistician at Medical University of South Carolina after review of hourly maximum and minimum SBP recordings.
The two primary safety end points were: (1) neurologic deterioration (defined by a decline in the GCS score ≥2 or increase in NIHSS score ≥4 points that is not explained by use of sedatives or hypnotics) within 24 hrs from treatment initiation; and (2) SAE (defined per FDA guidelines) occurring within 72 hrs of treatment initiation.
Additional measure obtained was hematoma expansion defined as an increase from baseline in the volume of intraparenchymal hemorrhage of >33% as measured by image analysis on the 24-hr CT scan. The computerized volumetric analysis for measuring hematoma volume and detecting hematoma expansion has been used in previous studies (25, 26). The cut-off for hematoma expansion was based on the definition used by Brott et al (27), which is the change in size associated with neurologic deterioration.
Postdischarge follow-up was performed at 30 ± 7 days and 90 ± 15 days to assess the modified Rankin score, NIHSS score, and Barthel Index, as well as mortality within 90 days.
Statistical Considerations
Because this was a phase I dose-escalation study to determine the treatment feasibility and safety, sample size consideration was based on the feasibility of recruiting subjects in a 3-yr study period. In oncology clinical trials, a phase I dose-escalation study typically evaluates three to six subjects per dose level with a well-defined, widely-accepted set of stopping criteria based on toxicity grading. Because no such standard guideline exists for acute ICH cases, a larger sample size of 20 was opted per treatment tier to have greater statistical confidence that the correct treatment tier is chosen and to obtain greater precision of estimates of safety parameters.
The hourly average (of maximum and minimum) SBP measurements were graphed with box-and-whiskers plot. In addition, a repeated-measures analysis of the 25 average SBP (baseline and subsequent 24 hourly measures) was conducted with a mixed effects model (assuming autoregressive covariance structure in SAS version 9.1 PROC MIXED) to determine statistically the effect of treatment intensity (tier) on the average SBP over the 24 hrs with and without adjustment for baseline NIHSS score and age. We adjusted for initial severity of deficits using baseline NIHSS score (28), instead of GCS score, to provide a higher level of discrimination among patients with GCS score >8.
For stopping criterion based on neurologic deterioration within 24 hrs, assuming a binomial distribution, the prespecified threshold value of seven (≅ 40%) is derived from the upper one-sided 95% confidence limit of 40% on the neurologic deterioration rate of 33% observed in the literature in a nontreated group of ICH patients with initial GCS >8 (16). The threshold value of seven was chosen because if seven or more subjects had neurologic deterioration, the probability of stopping at the current treatment level would be high (>0.75) if the true rate was unacceptably high (>40%), and lower (<0. 39) if the true rate was acceptable (<30%) (Table 2).
Table 2.
Probability of stopping at the current dose level if X or more number of events are observed for various true event rates and sample size of 20
| Neurologic Deterioration Criteria for Stopping: Observe ≥7 Events
|
Serious Adverse Events Criteria for Stopping: Observe ≥3 Events
|
||
|---|---|---|---|
| True Rate | Probability of Stopping | True Rate | Probability of Stopping |
| 0.25 | 0.21 | 0.05 | 0.08 |
| 0.30 | 0.39 | 0.10 | 0.32 |
| 0.35 | 0.58 | 0.15 | 0.60 |
| 0.40 | 0.75 | 0.20 | 0.79 |
| 0.45 | 0.87 | 0.25 | 0.91 |
The lower and upper 95% confidence limits on the rates observed in the literature (bolded and italicized are presented). The probability is based on safety stopping rule activation and does not take into account resumption of further recruitment of subjects based on Data and Safety Monitoring Board recommendation after data review.
Similar to the stopping rule for neurologic deterioration, the expected rate of SAE in subjects treated with IV nicardipine was ≤10% from previous studies in subjects with postoperative hypertension, subarachnoid hemorrhage, and ICH (29–33). The threshold of three or more subjects in each treatment tier (≅ 20%) is derived from the upper one-sided confidence limit on the 10% observed in Flamm et al (39). This threshold was chosen because if three or more subjects experienced one or more SAE, the probability of stopping at the current treatment level would be high (>0.79) if the true rate was unacceptably high (>20%), and lower (<0.32) if the true rate was acceptable (<10%; Table 2).
Because the primary aim of the study was to identify a treatment tier that was safe, the chosen threshold levels for treatment failure, neurologic deterioration, and SAE remained consistent for all three tiers.
RESULTS
A total of 774 patients were screened for recruitment in the study; of these, 714 were excluded. The most common reasons for exclusion were ICH related to trauma (n = 83); presentation after 6 hrs (n = 80); initial SBP <170 mm Hg (n = 72); GCS score <8 (n = 68); unknown reasons (n = 61); and history of bleeding diathesis or coagulopathy (n = 50). Of those screened, 60 patients were enrolled, with 18, 20, and 22 patients recruited in tier one (SBP ≥170 mm Hg and <200 mm Hg), tier two (SBP ≥140 mm Hg and <170 mm Hg), and tier three (SBP ≥110 mm Hg and <140 mm), respectively. The total number of patients per tier was adjusted in consultation with the DSMB. Recruitment in the first tier was discontinued after 18 patients because the rate of safety events would be below the rate specified by safety stopping rule even if the two additional patients experienced safety events. The last tier continued recruitment to meet the total recruitment goal of 60 patients. The demographic and clinical characteristics of the patients are summarized in Table 3.
Table 3.
Demographic and clinical and treatment characteristics of subjects by SBP target tier
| Characteristics | First Tier, SBP 170–200 mm Hg, n = 18 |
Second Tier, (SBP 140–170 mm Hg, n = 20 |
Third Tier, SBP 110–140 mm Hg, n = 22 |
|---|---|---|---|
| Mean age (±SD) | 62.0 (17.7) | 58.5 (13.0) | 65.1 (14.6) |
| Men | 9 (50%) | 12 (60%) | 13 (59%) |
| Race/ethnicity | |||
| White | 5 (28%) | 13 (65%) | 13 (59%) |
| Black | 11 (61%) | 7 (35%) | 7 (32%) |
| Others | 2 (11%) | 0 | 2 (9%) |
| Initial SBP in mm Hg, median | 209 | 212 | 201 |
| Initial NIHSS score, median | 11 | 9 | 8 |
| Initial GCS score, median | 14 | 15 | 15 |
| Mean time from symptom onset to emergency department arrival (±SD) | 1.72 hrs (1.27) | 1.70 hrs (1.13) | 1.86 hrs (1.78) |
| Mean time from symptom onset to initiating treatment (±SD) | 3.94 hrs (1.45) | 4.13 hrs (1.50) | 4.44 hrs (2.08) |
| N (%) treated within 3 hrs of symptom onset | 7 (39%) | 5 (25%) | 6 (27.3%) |
| N (%) with previous use of oral antihypertensive medications | 6 (33.3%) | 11 (55%) | 14 (63.6%) |
| N (%) compliant with oral antihypertensive medications | 0 | 3 (15%) | 8 (36.4%) |
| N (%) noncompliant or unknown compliance with oral antihypertensive medications | 6 (33%) | 8 (40%) | 6 (27%) |
| N (%) with diabetes mellitus | 2 (11.1%) | 4 (20%) | 4 (18.2%) |
| N (%) who currently smoke cigarettes | 4 (22.2%) | 6 (30%) | 4 (18.2%) |
| N (%) with hyperlipidemia | 2 (11.1%) | 4 (20%) | 5 (22.7%) |
| Initial hematoma volume (±SD) | 15.45 mL (14.60) | 14.84 mL (17.15) | 10.94 mL (10.87) |
| Duration of nicardipine infusion (±SD) | 12.93 hrs (13.5) | 30.06 hrs (23.8) | 45.82 hrs (37.3) |
| Maximum dose of nicardipine used (±SD) | 8.47 (5.75) | 8.90 (4.48) | 12.52 (6.76) |
NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Scale; SBP, systolic blood pressure.
Figure 1 presents the box-and-whisker plots of the hourly average SBP within each treatment tier. The repeated measure analysis of the hourly average SBP showed that the SBP were significantly different among the tiers both with (p < 0.0028) and without (p < 0.0001) adjusting for baseline NIHSS score and age. Primary treatment failure was observed in 9 of 60 subjects, all in the last tier. Tables 4 and 5 provide respective summaries of neurologic deteriorations and SAE observed in the study. No significant difference (p = 0.4682 from Wilcoxon rank sum test) was observed in average SBP change (as a surrogate to evaluate the relationship between early SBP reduction and safety events) at 2 hrs after treatment initiation between subjects who did (mean decrease = 48.1 mm Hg; SE = 9.7) or did not (mean decrease = 56.7 mm Hg; SE= 4.3) have neurologic deterioration within 24 hrs For subjects who experienced neurologic deterioration within 24 hrs, graphs of their individual hourly average SBP and nicardipine doses with a notation on when the event occurred are presented in Appendix B. None of the neurologic deteriorations were preceded by an acute SBP reduction or IV nicardipine initiation or dose adjustment. Such plots are not provided for subjects who had SAE because they occurred either before nicardipine infusion or after 24 hrs.
Figure 1.
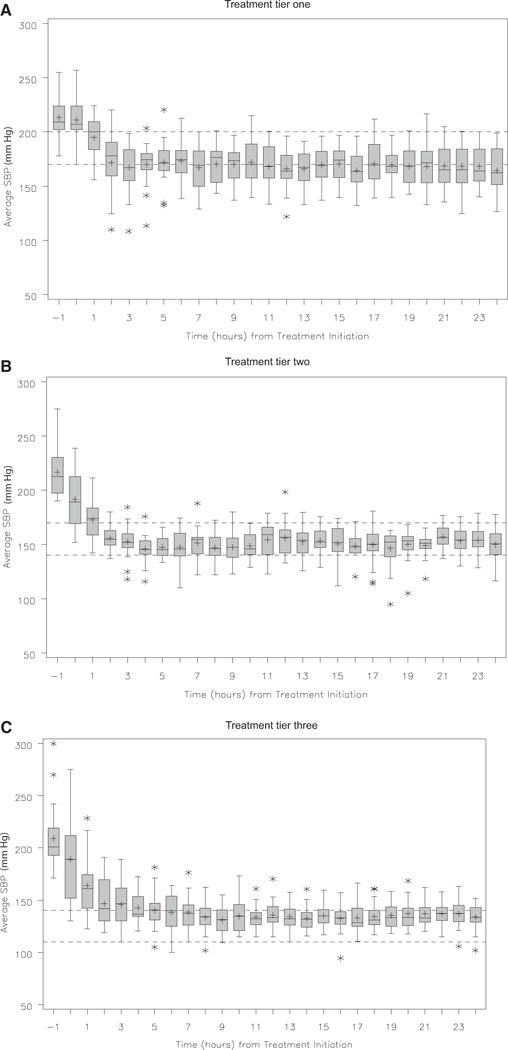
Box plot of systolic blood pressure (SBP) recordings over a 24-hr period in each treatment group.
Table 4.
Demographic and clinical characteristics of subjects who had neurologic deterioration within 24 hrs of treatment initiation
| Age/Gender | Tier | Initial SBP, mm Hg | Initial NIHSS Score | Initial GCS Score | Time Interval Between Symptom Onset and Treatment | Cause of Neurologic Deterioration | Time Interval Between Initiation of Treatment and Deterioration | 1-mo and 3-mo mRS |
|---|---|---|---|---|---|---|---|---|
| 55/F | 1 | 250 | 14 | 10 | 2.67 hrs | Hydrocephalus | 12 hrs | Lost to follow-up |
| 45/M | 2 | 192 | 8 | 15 | 2.75 hrs | Hematoma expansion | 5 mins | 1-mo mRS: 4 3-mo mRS: 3 |
| 59/F | 2 | 212 | 10 | 8 | 5.25 hrs | Hematoma expansion | 15 mins | 1-mo mRS: 5 3-mo mRS: 5 |
| 74/Ma | 3 | 219 | 5 | 15 | 5.23hrs | Hematoma expansion | 14.7 hrs | 1-mo mRS: 5 3-mo mRS: 6 |
| 43/M | 3 | 181 | 6 | 15 | 2.33 hrs | Hematoma expansion | Before infusion | 1-mo mRS: 4 3-mo mRS: 4 |
| 82/M | 3 | 183 | 34 | 13 | 3.92 hrs | Hematoma expansion | 18.75 hrs | 1-mo mRS: 6 |
| 46/M | 3 | 228 | 13 | 12 | 5.25 hrs | Hematoma expansion | 13.25 hrs | 1-mo mRS: 5 3-mo mRS: 4 |
M, male; F, female; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Scale.
Also had severe adverse events.
Table 5.
Demographic and clinical characteristics of subjects with SAE within 72 hrs of treatment initiation
| Age/Gender | Tier | Initial SBP, mm Hg | Initial NIHSS Score | Initial GCS Score | Time Interval Between Symptom Onset and Recruitment | Description of SAE | Time Interval Between Initiation of Treatment and SAE | 1-mo and 3-mo mRS |
|---|---|---|---|---|---|---|---|---|
| 64/M | 2 | 247 | 0 | 15 | 4.67 hrs | Respiratory distress | 65 hrs | 1 mo mRS: 6 |
| 74/M | 3 | 219 | 5 | 15 | 5.23 hrs | Acute respiratory failure | 40 hrs | 1 mo mRS: 5 3 mo mRS: 6 |
| 69/F | 3 | 200 | 6 | 15 | 4.25 hrs | Hyperglycemia and hypokalemia | Before infusion | 1 mo mRS: 2 3 mo mRS: 2 |
| 67/F | 3 | 203 | 27 | 9 | 3.92 hrs | Acute respiratory distress syndrome | 60 hrs | 1 mo mRS: 5 3 mo mRS: 4 |
SAE, serious adverse event; SBP, sytolic blood pressure; NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Scale; M, male; F, famale; mRS, modified Rankin scale.
Deaths within 3 months were observed in three (17%), two (10%), and five (23%) subjects in tiers one, two, and three, respectively. The 3-month functional outcome was available in 52 of 60 patients recruited in the study. The 1- and 3-month functional outcomes are provided in Table 6.
Table 6.
End points observed within subjects according to SBP target tier
| Characteristics | First Tier, SBP 170–200 mm Hg, n = 18 |
Second Tier, SBP 140–170 mm Hg, n = 20 |
Third Tier, SBP 110–140 mm Hg, n = 22 |
|---|---|---|---|
| Treatment failure | 0 | 0 | 9 (41%) |
| N (%) with SAE within 72 hrs | 0 | 1 (5%) | 3 (14%) |
| N (%) with neurologic deterioration within 24 hrs | 1 (6%) | 2 (10%) | 4 (18%) |
| N (%) with symptomatic hematoma expansion | 0 | 1 (5%) | 4 (18%) |
| N (%) with asymptomatic hematoma expansion | 6 (33%) | 2 (10%) | 3 (14%) |
| N (%) with in-hospital mortality | 2 (11%) | 1 (5%) | 1 (4 %) |
| N (%) with 3-mo mortality | 3 (17%) | 2 (10%) | 5 (23%) |
| 1-mo favorable outcome, mRS 0–2 | 4 (3 missing) | 6 (3 missing) | 4 (2 missing) |
| 3-mo favorable outcome, mRS 0–2 | 8 (3 missing) | 9 (4 missing) | 7 (2 missing) |
SBP, systolic blood pressue; SAE, serious adverse event; mRS, modified Rankin score.
DISCUSSION
The presented ATACH phase I trial provided the data on treatment failure, neurologic deteriorations within 24 hrs, and SAE within 72 hrs for three different SBP treatment targets. The highest number of treatment failures was observed in the tier with the most intense SBP goal. However, the average SBP values were significantly lower in the last tier (110–140 mm Hg) compared with the other two tiers, suggesting that current treatment protocol can achieve groups with discernibly different SBP to test the effect of various intensities of SBP reduction. The proportion of subjects with neurologic deterioration and SAE were below the prespecified safety thresholds in each tier. However, cautious interpretation is recommended because of the higher rate of treatment failures, neurologic deteriorations, and SAE in the third tier. Of the four neurologic deteriorations in the last tier, one deterioration event occurred before initiation of infusion. Three occurred after the first 12 hrs of treatment, suggesting no clear temporal correlation between acute exposure to either SBP reduction or IV nicardipine treatment. Three SAE occurred in the last tier, but all occurred either before initiation of treatment or after the termination of active SBP reduction period (first 24 hrs). No clear relationship was demonstrable between the dose of nicardipine, initial SBP change after nicardipine initiation, or SBP before deterioration and neurologic deterioration. However, the possibility that neurologic deteriorations were related to either SBP reduction or IV nicardipine cannot be completely ruled out. The overall rate of SAE was below the threshold set to activate a safety stopping rule, and an independent review by the DSMB concluded that the rate of such events were not disproportionately higher than expected in any tier. We acknowledge that the study was not designed to provide comparative rates of events between the tiers. Because of small sample size and likelihood of imbalances in subject characteristics among the three tiers, we have avoided providing comparisons among groups on the safety and effectiveness of study treatment. We also acknowledge that clinical measures, such as neurologic deteriorations, may be insensitive to small regions of focal ischemic injury precipitated by SBP reduction that may impact overall clinical outcome.
The Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage (INTERACT) trial (35) completed recruitment, randomizing 404 patients with ICH and elevated SBP (≥150 and <220 mm Hg) within 6 hrs of symptom onset (35) to either intensive blood pressure management (SBP <140 mm Hg) or American Stroke Association guideline based blood pressure management (SBP <180 mm Hg) using available antihypertensive agents. The primary efficacy end point was proportional change (expansion) in hematoma volume at 24 hrs. The results suggested that early intensive blood pressure reduction appears to attenuate hematoma expansion in patients with ICH. In subgroup analyses, patients recruited within 3 hours and patients with an initial SBP ≥181 mm Hg appeared to have the greatest benefit with intensive blood pressure reduction. The rate of SAE or poor clinical outcome at 90 days was not different between the two groups. No significant difference in the 3-month mortality was observed between the groups, although the study was not powered to demonstrate a difference in rates of clinical outcomes.
There are some differences between the ATACH and INTERACT study. The initial SBP ≥180 mm Hg was observed in 47% of the patients recruited in INTERACT, but in all patients recruited in ATACH. The antihypertensive agents used were different; either IV uradipil or furosemide in INTERACT and IV nicardipine in ATACH. The location of the ICH was supratentorial in 90% and 100% of the patients recruited in INTERACT and ATACH study, respectively. The SBP goals were achieved in 42% and 66% of the subjects at 1 and 6 hrs after treatment initiation in INTERACT. In ATACH, the SBP goals were achieved in 90% of the patients by 2 hours. The patients recruited in INTERACT were Chinese (95%), but in ATACH, the patents were predominantly whites (52%) and blacks (42%). The impact of these differences on the results is unknown. It is reasonable to expect that faster and more effective SBP reduction in a patient population with higher initial SBP as seen in the ATACH study will result in a greater therapeutic benefit.
Both the ATACH and INTERACT studies provide the necessary background data for a randomized trial comparing intensive SBP reduction (SBP <140 mm Hg) with standard SBP reduction (SBP <180 mm Hg) using IV nicardipine with treatment initiated within 3 hrs after onset of ICH and continued for the next 24 hrs. Although the threshold of SBP reduction that will provide the greatest benefit for reducing the rate of hematoma expansion is not known, it is reasonable to consider that the most aggressive reduction in SBP may provide the greatest benefit, provided it can be well-tolerated. The target for the intensive treatment group to reduce and maintain SBP ≤140 mm Hg is supported by the results of the INTERACT study and by two studies demonstrating very low rates of hematoma expansion when SBP was maintained at < 140mm Hg (34–36). Another study (37) assessed 76 consecutive patients with hypertensive ICH and attempted to lower SBP to <140, <150, or <160 mm Hg. Lowest rates of hematoma expansion were observed in subjects with the lowest SBP. These studies suggest that the <140-mm Hg tier maybe the most efficacious SBP range for reducing hematoma expansion.
The goal for standard treatment (SBP <180 mm Hg) was defined by existing recommendations from professional organizations. The European Stroke Initiative in 2006 (15) and the Writing Group of the American Heart Association Stroke Council in 2007 (14) recommended that antihypertensive treatment should be initiated in patients with ICH under specific conditions if SBP is ≥180 mm Hg. Although different from the two SBP tiers in our study (designed before the guidelines), investigators and planning committee felt the need to have the comparative group of standard treatment consistent with existing recent guidelines. In 1999 before design of ATACH, the Americal Heart Association guidelines (38) had recommended that blood pressure levels be maintained below a mean arterial pressure of 130 mm Hg in persons with a history of hypertension (level of evidence V data from anecdotal case series) and acknowledged the possibility of perihematomal ischemia worsening. In 2007, American Heart Association guidelines (14) indicated that possibility of perihematomal ischemia worsening was mitigated based on new data demonstrating absence of regional ischemia with modest blood pressure reduction in clinical (by positron emission tomography [13]) or experimental (by microspheres [39]) studies, and low neurologic deterioration rates in studies (11, 17) reporting on patients in whom SBP was pharmacologically reduced. The current guidelines recommended maintaining SBP <180 mm Hg in the acute period with the possibility of more aggressive SBP lowering in selected circumstances (class IIb—usefulness/efficacy is less well-established and based on consensus opinion of experts).
Although a longer time window was considered adequate for a feasibility and safety study, a shorter time window (<3 hrs) was proposed for efficacy trial on the basis of two observations. First, the initial 3 hrs represent the time interval for maximum rates of hematoma expansion (the proposed mechanism for beneficial effect of SBP reduction) after symptom onset. In one study, the mean time from symptom onset to arrival was 1.3 hrs in patients who underwent hematoma expansion within the subsequent hour. Second, significant improvement in the beneficial effect of recombinant factor VII was observed in the FAST trial if the analysis was only limited to patients recruited within 2.5 hrs after symptom onset (40). Therefore, recruiting patients with symptom onset after 3 hrs will result in inclusion of patients who already either have hematoma expansion or are at very low risk for hematoma expansion.
Even the best-designed, randomized, controlled trials can suffer when subjects are lost to follow-up (41). Incomplete follow-up biases the results of a trial when patients who drop out are different from those who complete follow-up. Based on the relatively high rate of dropout (8 out of 60 patients), the phase III trial sample size planning will take into account the potential dilution effect on the efficacy outcome as a result of treatment failure, cross-over, and those lost to follow-up. We have also planned to implement a central approach (http://omnitrace.com/lost-to-follow-up.html) to monitor all subject follow-up and to aid in finding lost patients if the rate of those lost to follow-up exceeds 10%.
CONCLUSIONS
Blood pressure treatment is a strategy that can be made widely available without specialized equipment or personnel and may make a major impact on outcome in ICH patients. Both the ATACH and INTERACT studies provide additional data from prospective multicenter studies with independent oversight for designing a randomized trial comparing intensive SBP reduction with standard SBP reduction in ICH patients.
Acknowledgments
Dr. Qureshi has received funding from National Institutes of Health RO-1-NS44976-01A2 (medication provided by ESP Pharma), American Heart Association Established Investigator Award 0840053N, and Minnesota Medical Foundation, Minneapolis, MN.
Dr. Palesch is funded by the NIH U01 NS054630, U01 NS059041, R01 NS057127, and R01 NS062778, and by Boehringer-Ingelheim.
APPENDIX A
List of centers/hospitals, investigators, and recruitment by center
| Coordinating Centers | Principal Investigators and Coordinators |
|---|---|
| Clinical coordinating center | |
| University of Minnesota, Minneapolis, MN (n = 2) | Adnan I. Qureshi, MD–Principal Investigator Nauman Tariq, MD, Afskin A. Divani, MD–Imaging Analysis Jill Novitzke, RN Haitham H. Hussein MD–Study Coordinator |
| Statistical coordinating center | |
| Medical University of South Carolina, Charleston, SC | Yuko Y. Palesch, PhD–Statistician Renee’ Martin, PhD–Statistician Catherine Dillon–Project Manager |
|
| |
| Hospital or Center, City, State | Principal Investigators and Coordinators |
|
| |
| University of Medicine and Dentistry of New Jersey, Newark, NJ (n = 33) | Adnan I Qureshi, MD–Neurologist/Neurointensivist Jawad F. Kirmani, MD–Neurologist/Neurointensivist Mustapha A. Ezzeddine, MD–Neurologist/Neurointensivist Ibrahim Mohammad, MD–Neurologist M. Fareed K. Suri, MD–Neurologist Pansy Harris-Lane, RN–Coordinator |
| Case Western Reserve University, Cleveland, OH (n = 2) | Jose I. Suarez, MD–Neurologist/Neurointensivist Eliahu Feen, MD–Neurologist Warren Selman, MD–Neurosurgeon Christopher Murphy, RN–Coordinator |
| Columbia University Medical Center, New York, NY (n = 0) | Stephan A. Mayer, MD–Neurologist/Neurointensivist Augusto Parra, MD–Neurologist Kiwon Lee, MD–Neurologist/Neurointensivist Noeleen Ostapkovich, RN–Coordinator |
| JFK Medical Center, Edison, NJ (n =1) | Nikolaos I. H. Papamitsakis, MD–Neurologist Spozhmy Panezai, MD–Neurologist Chinekwu Anyanwu, MD–Coordinator |
| Kansas University Medical Center, Kansas City, KS (n = 3) | John Terry, MD–Neurologist/Neurointensivist Kelly Dickerson, RN–Coordinator |
| Massachusetts General Hospital, Boston, MA (n = 3) | Joshua Goldstein, MD, PhD–Emergency Medicine Lauren Wendell–Coordinator |
| Ohio State University, Columbus, OH (n = 1) | Yousef M. Mohammad, MD–Neurologist Hoda Jradi–Coordinator |
| Saint Louis University, Saint Louis, MO (n = 7) | Salvador Cruz-Flores, MD–Neurologist/Neurointensivist Eve Holzemer, RN–Coordinator |
| University of Southern CA, Los Angeles, CA (n = 1) | Gene Sung, MD–Neurologist/Neurointensivist Vangie Thomson, RN–Coordinator |
| Via Christi Regional Medical Center, Wichita, KS (n = 7) | As’ad Ehtisham, MD–Neurologist/Neurointensivist Betty Brown, RN–Coordinator |
Data and Safety Monitoring Board: William R. Clarke, PhD; Steven Greenberg, MD, PhD; Leslie Ain McClure, PhD; Emmy R. Miller, PhD, RN; J. Paul Muizelaar, MD, PhD; Howard Yonas, MD.
Funding Organization: National Institute of Neurologic Diseases and Stroke (NINDS).
APPENDIX B
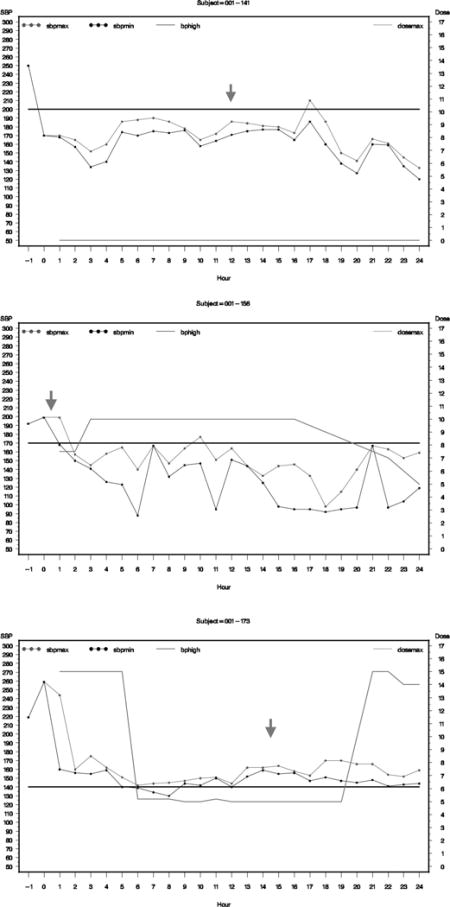
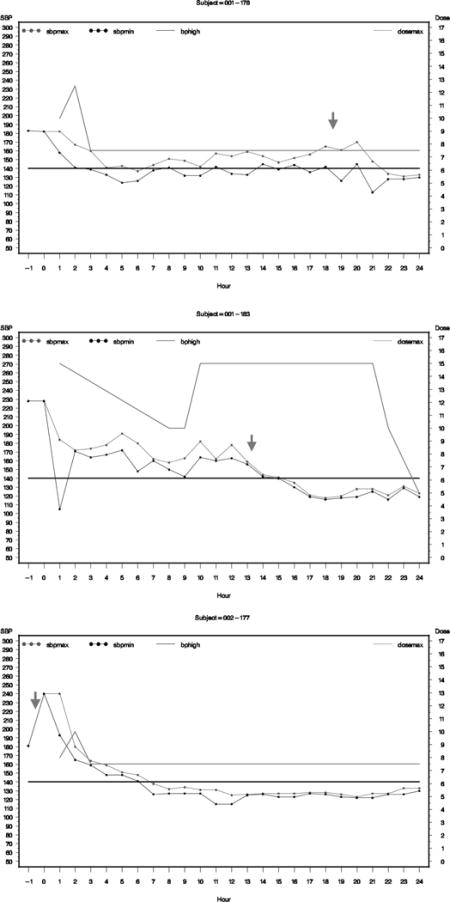
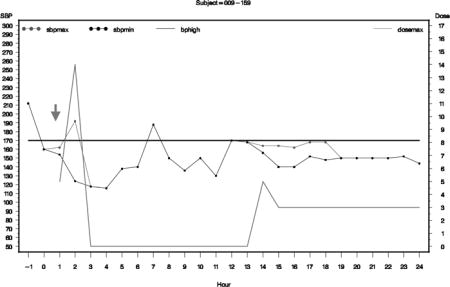
Graphs of hourly average SBP for subjects who had neurologic deterioration or serious adverse events.
The graph presents maximum hourly SBP as blot dotted line, minimum SBP as black dotted line, and dose of nicardipine as green line. The solid black horizontal line represents the targeted SBP for the subject.
Subject 001-141 (ND at 12 hrs)
Subject 001-156 (ND at 15 mins)
Subject 001-173 (ND at 14.7 hrs)
Subject 001-178 (ND at 18.75 hrs)
Subject 001-183 (ND at 13.25 hrs)
Subject 002-177 (ND before infusion)
Subject 009-159 (ND At 15 mins)
ND, neurologic deterioration.
Footnotes
See also p. 731.
The authors have not disclosed any potential conflicts of interest.
References
- 1.Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation. 2008;118:176–187. doi: 10.1161/CIRCULATIONAHA.107.723874. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Suri MF, Nasar A, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38:2180–2184. doi: 10.1161/STROKEAHA.106.467506. [DOI] [PubMed] [Google Scholar]
- 3.Wallace JD, Levy LL. Blood pressure after stroke. JAMA. 1981;246:2177–2180. [PubMed] [Google Scholar]
- 4.Broderick JP, Brott TG, Tomsick T, et al. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72:195–199. doi: 10.3171/jns.1990.72.2.0195. [DOI] [PubMed] [Google Scholar]
- 5.Chen ST, Chen SD, Hsu CY, et al. Progression of hypertensive intracerebral hemorrhage. Neurology. 1989;39:1509–1514. doi: 10.1212/wnl.39.11.1509. [DOI] [PubMed] [Google Scholar]
- 6.Dandapani BK, Suzuki S, Kelley RE, et al. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke. 1995;26:21–24. doi: 10.1161/01.str.26.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Vemmos KN, Tsivgoulis G, Spengos K, et al. Association between 24-h blood pressure monitoring variables and brain oedema in patients with hyperacute stroke. J Hypertens. 2003;21:2167–2173. doi: 10.1097/00004872-200311000-00027. [see comment] [DOI] [PubMed] [Google Scholar]
- 8.Broderick JP, Diringer MN, Hill MD, et al. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38:1072–1075. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 9.Jauch EC, Lindsell CJ, Adeoye O, et al. Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke. 2006;37:2061–2065. doi: 10.1161/01.STR.0000229878.93759.a2. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi AI, Mohammad YM, Yahia AM, et al. A prospective multicenter study to evaluate the feasibility and safety of aggressive antihypertensive treatment in patients with acute intracerebral hemorrhage. J Intensive Care Med. 2005;20:34–42. doi: 10.1177/0885066604271619. [DOI] [PubMed] [Google Scholar]
- 12.Kim-Han JS, Kopp SJ, Dugan LL, et al. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke. 2006;37:2457–2462. doi: 10.1161/01.STR.0000240674.99945.4e. [DOI] [PubMed] [Google Scholar]
- 13.Powers WJ, Zazulia AR, Videen TO, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57:18–24. doi: 10.1212/wnl.57.1.18. [DOI] [PubMed] [Google Scholar]
- 14.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group Stroke. J Cerebral Circ. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 15.Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: Spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis. 2006;22:294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): Rationale and design. Neurocritical Care. 2007;6:56–66. doi: 10.1385/ncc:6:1:56. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi AI, Harris-Lane P, Kirmani JF, et al. Treatment of acute hypertension in patients with intracerebral hemorrhage using American Heart Association guidelines. Crit Care Med. 2006;34:1975–1980. doi: 10.1097/01.CCM.0000220763.85974.E8. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AI, Hutson AD, Harbaugh RE, et al. Methods and design considerations for randomized clinical trials evaluating surgical or endovascular treatments for cerebrovascular diseases. Neurosurgery. 2004;54:248–264. doi: 10.1227/01.neu.0000103446.26057.78. discussion 264–267. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi AI, Harris-Lane P, Kirmani JF, et al. Intra-arterial reteplase and intravenous ab-ciximab in patients with acute ischemic stroke: an open-label, dose-ranging, phase I study. Neurosurgery. 2006;59:789–796. doi: 10.1227/01.NEU.0000232862.06246.3D. discussion 796–797. [DOI] [PubMed] [Google Scholar]
- 20.Cuzick J, Howell A, Forbes J. Early stopping of clinical trials. Breast Cancer Res. 2005;7:181–183. doi: 10.1186/bcr1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelmsen L. Role of the Data and Safety Monitoring Committee (DSMC) Stat Med. 2002;21:2823–2829. doi: 10.1002/sim.1286. [DOI] [PubMed] [Google Scholar]
- 22.Asplund K. The role of the data safety and monitoring committee in stroke trials. Eur Neurol. 2003;49:115–119. doi: 10.1159/000068511. [DOI] [PubMed] [Google Scholar]
- 23.Sydes MR, Spiegelhalter DJ, Altman DG, et al. Systematic qualitative review of the literature on data monitoring committees for randomized controlled trials. Clin Trials. 2004;1:60–79. doi: 10.1191/1740774504cn004rr. [DOI] [PubMed] [Google Scholar]
- 24.Broderick J, Brott T, Tomsick T, et al. Management of intracerebral hemorrhage in a large metropolitan population. Neurosurgery. 1994;34:882–887. doi: 10.1227/00006123-199405000-00015. discussion 887. [DOI] [PubMed] [Google Scholar]
- 25.Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Safdar K, Weil J, et al. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26:1764–1767. doi: 10.1161/01.str.26.10.1764. [DOI] [PubMed] [Google Scholar]
- 27.Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Valiente RA, de Miranda-Alves MA, Silva GS, et al. Clinical features associated with early hospital arrival after acute intracerebral hemorrhage: Challenges for new trials. Cere-brovasc Dis. 2008;26:404–408. doi: 10.1159/000151681. [DOI] [PubMed] [Google Scholar]
- 29.Wallin JD, Fletcher E, Ram CV, et al. Intravenous nicardipine for the treatment of severe hypertension. A double-blind, placebo-controlled multicenter trial. Arch Intern Med. 1989;149:2662–2669. [PubMed] [Google Scholar]
- 30.Halpern NA, Goldberg M, Neely C, et al. Post-operative hypertension: A multicenter, prospective, randomized comparison between intravenous nicardipine and sodium nitro-prusside. Crit Care Med. 1992;20:1637–1643. [PubMed] [Google Scholar]
- 31.Efficacy and safety of intravenous nicardipine in the control of postoperative hypertension. IV Nicardipine Study Group. Chest. 1991;99:393–398. doi: 10.1378/chest.99.2.393. [DOI] [PubMed] [Google Scholar]
- 32.Flamm ES, Adams HP, Jr, Beck DW, et al. Dose-escalation study of intravenous nicardipine in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1988;68:393–400. doi: 10.3171/jns.1988.68.3.0393. [DOI] [PubMed] [Google Scholar]
- 33.Haley EC, Jr, Kassell NF, Torner JC, et al. A randomized trial of two doses of nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1994;80:788–796. doi: 10.3171/jns.1994.80.5.0788. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama T, Yokoyama T, Matsukawa T, et al. Continuous nicardipine infusion to control blood pressure after evacuation of acute cerebral hemorrhage. Can J Anaesthesia. 2000;47:1196–1201. doi: 10.1007/BF03019868. [DOI] [PubMed] [Google Scholar]
- 35.Anderson CS, Huang Y, Wang G, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): A pilot randomised trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 36.Sorimachi T, Fujii Y, Morita K, et al. Rapid administration of antifibrinolytics and strict blood pressure control for intracerebral hemorrhage. Neurosurgery. 2005;57:837–844. doi: 10.1227/01.neu.0000180815.38967.57. [DOI] [PubMed] [Google Scholar]
- 37.Ohwaki K, Yano E, Nagashima H, et al. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004;35:1364–1367. doi: 10.1161/01.STR.0000128795.38283.4b. [DOI] [PubMed] [Google Scholar]
- 38.Broderick JP, Adams HP, Jr, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi AI, Wilson DA, Hanley DF, et al. Pharmacologic reduction of mean arterial pressure does not adversely affect regional cerebral blood flow and intracranial pressure in experimental intracerebral hemorrhage. Crit Care Med. 1999;27:965–971. doi: 10.1097/00003246-199905000-00036. [DOI] [PubMed] [Google Scholar]
- 40.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 41.Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24:719–725. doi: 10.1016/j.cct.2003.08.012. [DOI] [PubMed] [Google Scholar]


