Abstract
The incidence of acute kidney injury (AKI) among acutely ill patients is reportedly very high and has vexing consequences on patient outcomes and health care systems. The risks and impact of AKI differ between developed and developing countries. Among developing countries, AKI occurs in young individuals with no or limited comorbidities, and is usually due to environmental causes, including infectious diseases. Although several risk factors have been identified for AKI in different settings, there is limited information on how risk assessment can be used at population and patient levels to improve care in patients with AKI, particularly in developing countries where significant health disparities may exist. The Acute Disease Quality Initiative consensus conference work group addressed the issue of identifying risk factors for AKI and provided recommendations for developing individualized risk stratification strategies to improve care. We proposed a 5-dimension, evidence-based categorization of AKI risk that allows clinicians and investigators to study, define, and implement individualized risk assessment tools for the region or country where they practice. These dimensions include environmental, socioeconomic and cultural factors, processes of care, exposures, and the inherent risks of AKI. We provide examples of these risks and describe approaches for risk assessments in the developing world. We anticipate that these recommendations will be useful for health care providers to plan and execute interventions to limit the impact of AKI on society and each individual patient. Using a modified Delphi process, this group reached consensus regarding several aspects of AKI risk stratification.
Keywords: acute kidney injury, acute renal failure, developed countries, developing countries, risk assessment, outcomes
Acute kidney injury (AKI) is a common complication of acute illnesses in developed and developing countries.1 The impact of AKI on patient outcomes and the cost of health care are significant. AKI effects in the developing world are even more appreciable.2, 3 Identifying patients at risk of developing AKI allows health care providers to implement preventive interventions to avoid AKI, mitigate the effects of the injury, and limit consequences of acute illness, including volume overload, electrolyte and acid-base imbalances, de novo chronic kidney disease (CKD) development or its progression, or the need for long-term renal replacement therapy (RRT). These measures seek to alleviate the impact of AKI on all-cause mortality and health care costs.4 The relationship between AKI and CKD is another issue to be considered. On one hand, the risk of AKI is higher among those with baseline CKD; therefore, close monitoring of CKD patients is crucial in AKI prevention. On the other hand, providing close monitoring and appropriate care to patients with acute kidney disease (AKD) after an AKI episode could result in less incidence of progressive CKD, with a significant impact on overall AKI outcomes.5 There are major differences among the causes, incidence, and follow-up care of AKI between developed and developing countries. The frequency of AKI in developing countries is not well understood due to under-reporting and resource constraints that limit the identification of high-risk patients with AKI. It is also difficult in developing countries to escalate to higher levels of care for severely ill patients.6 It is estimated that 85% of AKI cases occur in developing countries, which causes tremendous impact on their public health and economy.7 In these areas, the estimated incidence of AKI differs from developed countries, and there are major differences in the age range of patients, risk factors, and the causes of this devastating and fatal syndrome.3 In contrast to developed countries where older patients with multiple comorbidities develop AKI that is frequently related to the multiorgan failure, AKI in developing countries may occur in younger and healthier individuals, primarily due to a single cause, including bacterial, viral, and parasitic infectious diseases.8 Pinpointing the major risk factors and causes of AKI in each region is necessary to provide optimized care for the adult and pediatric acutely ill patients. Therefore, the approach to AKI risk stratification should be individualized to each region and country based on multiple dimensions that affect the overall incidence and outcomes of AKI.
To achieve this goal, the steering committee of the 18th Acute Dialysis Quality Initiative (ADQI) conference dedicated a work group with the task of identifying elements that might affect the risk of AKI based on the availability of resources. Using a modified Delphi process, this group reached consensus regarding strategies to assess AKI risk in each region of the globe. The group addressed the following 4 questions that served as the basis for accompanying consensus statements:
-
1.
What are the recognized risk factors and exposures associated with AKI development in different regions of the globe?
-
2.
What are the differences between risk factors for community-acquired AKI (CAKI) versus hospital-acquired AKI (HAKI)?
-
3.
Can we identify populations and patients at high risk for AKI?
-
4.
How can high-risk patients be monitored to prevent AKI development or progression?
Methods
This consensus meeting followed the established ADQI process, as previously described.9 The broad objective of ADQI is to provide expert-based statements and interpretation of current knowledge for use by clinicians according to professional judgment and to identify clinical research priorities to address these gaps. The 18th ADQI Consensus Conference Chairs convened a diverse panel that represented relevant disciplines (i.e., adult and pediatric nephrology, critical care, and renal pathology) from several continents (e.g., Africa, Asia, North America, Latin America, and Europe) around the theme of “Management of Acute Kidney Injury in the Developing World” for a 2-1/2 day consensus conference in Hyderabad, India on September 27 to 30, 2016.
The preconference activities involved a search of the literature for evidence on the epidemiology, risk factor assessment, and management of AKI in developing countries and their differences with developed countries. Our work group was also tasked to summarize the scope, implementation, and evaluative strategies for AKI risk stratification based on the location, resource availability, and a critical evaluation of the relevant literature. A series of phone conferences and emails that involved work group members before the meeting identified the current state of knowledge to enable the formulation of main questions for which discussion and consensus would be developed. A formal systematic review was not conducted. During the conference, the work group developed consensus positions, and plenary sessions that involved all ADQI contributors were used to present, debate, and refine these positions. After the meeting, this summary report was generated, revised, and approved by all participants of the ADQI.
Supplementary Table S1 provides the definitions for “risk factor,” “exposure,” “community- and hospital-acquired AKI,” “developing country,” “Human Development Index,” and “prevention.”
Q1: What Are the Recognized Risk Factors and Exposures Associated With AKI Development in Different Regions of the Globe?
Consensus Statements
-
1.
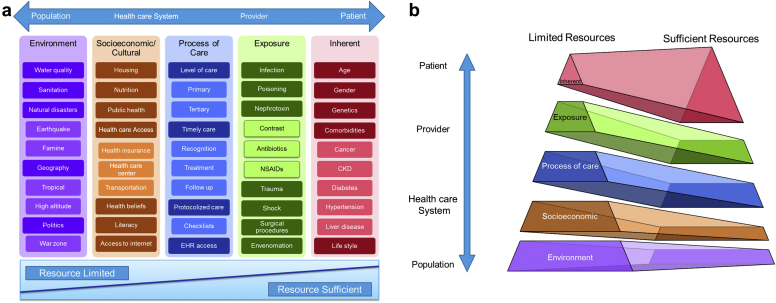
AKI risk is determined by multiple dimensions, and each dimension includes several factors. The dimensions are environmental, socioeconomic and/or cultural, the process of care, acute exposures, and inherent factors (Figure 1a). In resource-limited regions, environmental, socioeconomic and/or cultural, and the process of care risk dimensions play a more important role, both in adult and pediatric populations (Figure 1b).
-
2.
Each risk dimension needs to be evaluated at the population, health care system, provider, and patient levels (Table 1).10
-
3.
After the initial determination of the individual risk of AKI, the provider should periodically reassess the risk category of AKI, based on new exposures, throughout the medical visit. The frequency of these reevaluations depends on the resource availability and exposure intensity and rate.
Figure 1.
Risk dimensions and risk factors. (a) Provides a nonexhaustive list of risk factors within each risk dimension (from population to patient level) to highlight differences in their impact on the overall risk of acute kidney injury (AKI) risk, based on resource availabilities. Includes a nonexhaustive list of AKI risk factors, and additional factors may exist for each category. The factors listed may also span multiple dimensions but may be listed within only 1 risk dimension for simplicity. (b) Differences between resource-limited versus resource-sufficient regions. In resource-limited areas, the impact of environmental and socioeconomic and/or cultural risk dimensions on the overall risk of AKI is more than that in resource-sufficient areas. Mainly, in the absence of significant risk factors among exposure, process of care, socioeconomic dimensions, and environmental dimensions, inherent risk dimension gains more relevance in the development of appropriate AKI risk prediction. The bottom of the pyramid (environmental and socioeconomic and/or cultural risk dimensions) involves a larger cohort of individuals in each population. Although modifying these characteristics may require considerable effort, they would have a greater impact on the risk of AKI in the population. CKD, chronic kidney disease; EHR, electronic health record.
Table 1.
Interaction between the 5 risk dimensions and elements with impact on acute kidney injury outcomesa10
| Risk dimension | Population | Health care system | Provider | Patient |
|---|---|---|---|---|
| Inherent | Average age; societal norm of lifestyle | Comorbidity management policies | High-risk patient identification; awareness and desire to control comorbidities | Sex; personal comorbidities |
| Exposure | Poison or gun access; suicide incidence; tropical areas | Poison and gun control policies; antivenom availability | Adherence to care protocols and guidelines | Taking nephrotoxins (NSAIDs, etc.) |
| Process of care | Alternative medicine; transportation availability | Physician-to-patient ratio; policies to implement EHR | Trained providers; appropriate protocols; emphasis on informed decision | Trust in health care provider |
| Socioeconomic-cultural | Health beliefs, values, cultural practices; information access | Insurance coverage; disparity; quality standards | Heuristics; fear of malpractice litigation | Sedentary lifestyle due to personal or societal beliefs |
| Environmental | Campaign versus scientific driven legislations | Sanitation; clean drinking water | Emergency disaster preparedness | High-risk job (war-zone journalist, soldier, etc.) |
EHR, electronic health record; NSAID, nonsteroidal anti-inflammatory drug.
Includes a nonexhaustive list of acute kidney injury risk factors, and additional factors may exist for each category. The factors listed may also span multiple dimensions, but may be listed within only 1 risk dimension for simplicity.
AKI is a complex disorder associated with the interplay of patient-related factors within the environment where patients live. The U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion categorized the determinants of health in the following 5 groups: policymaking, social factors, health services, individual behavior, biology, and genetics.11 Each of these health determinants could be evaluated within 4 distinct levels of population, health care systems, providers, and patients (Table 1). The ADQI 18th annual work group determined 5 distinct dimensions of AKI risk factors inspired by the preceding categories. These dimensions are environmental, socioeconomic and cultural, processes of care, exposures, and the inherent risk elements (Figure 1a). Factors that can affect each of these dimensions could originate from the populations, health care systems, providers, or patients.10 In each dimension, several modifiable risk factors could be identified for the design and implementation of preventive interventions or further investigations. The nonmodifiable risk factors are also important in the assessment of AKI risk and escalate the level of care for higher risk patients, as needed.
The etiology and presentation of AKI in resource-limited regions differ from resource-sufficient areas. The impact of each dimension may vary based on resource availability. In previous epidemiological studies, AKI in developing countries with limited resources was usually community-acquired, affecting healthier and younger individuals, due to environmental and societal exposures. However, in resource-sufficient regions, AKI primarily occurred in older patients in the hospital setting and was associated with multiorgan failure in patients with a significant number of comorbidities.3, 12 Therefore, using a universal strategy to identify AKI in high-risk individual patients may not be appropriate at the global level. For example, the impact of diabetes as an AKI risk factor may vary from a quaternary health care institution in a developed country to a remote area in a developing country when experiencing a cholera pandemic. Also, the extent of risk modification impact differs based on the intervention in each region. Providing clean water prevents AKI in most individuals in a population with a diverse portfolio of health backgrounds, ages, and comorbidities, whereas tight glucose control in patients with diabetes can prevent AKI in a narrower group of individuals (Figure 1b).
Lack of access to clean drinking water and sanitation, inadequate control of infection-carrying vectors, living in an area with a geographical propensity for natural or man-made disasters (famine, flood, earthquakes), and a high incidence of tropical infections and/or venomous snake accidents are examples of environmental risk factors.13, 14, 15, 16 Governments with political will for improvement in public health can mitigate the development of AKI at the population level. Living in a war zone increases the risk of trauma and infectious diseases epidemics, and therefore, may increase the incidence of AKI.17
Within the socioeconomic and cultural dimension, risk factors, such as deficient housing, nutrition, and hygiene; lack of access to health services and health insurance; difficulty in transporting patients to a higher level health service; low health care budgets; insufficient health foundations, including scarce human resources; and inadequate physical infrastructure are important factors to consider.13, 14 The International Society for Peritoneal Dialysis used a questionnaire during the 2014 society meeting in Madrid, with the aim of understanding the barriers of increasing awareness regarding AKI in resource-limited regions.18 The respondents confirmed that limited resources were available for the diagnosis of AKI in rural health centers, where assessment is made primarily through clinical judgment. The management of dehydration, hypovolemia, and febrile infectious diseases was suboptimal, because approximately one-half of rural health centers had no access to i.v. fluids or antibiotics. Also, when appropriate treatment was available, patients were often required to pay for such services, rendering RRT out of reach for most of them. The tendency of communities to prefer to use alternative medicine and a mistrust of modern medicine could result in the late presentation of patients with acute illnesses to health care providers or might increase their exposure to toxins and infections.12 In West Africa, burial practices and cemetery management affected the spread of Ebola virus disease. This cultural practice is an example of how the cultural dimension can affect the overall risk of acute diseases and their complications (e.g., AKI) in the population.19 Literacy and general awareness, as well as access to electronic media, including the internet, can also affect the risk and acquisition of acute illnesses, including AKI. A higher level of awareness and literacy is associated with earlier and more impactful medical attention.13, 14, 20
The process of care has a bearing on the causation and outcomes of AKI. Delayed recognition and treatment of sepsis; the unavailability of diagnostics tools or a higher level of care; limited access to antivenom and antibiotics, including highly active antiretroviral therapy; and the inability to provide timely and monitored management of hyperkalemia, acidosis, and fluid overload with diuretics increase AKI incidence, likely escalate the requirements for dialysis treatment, and lead to higher mortality.13, 14 Tropical infections,16, 21 community-acquired pneumonia or meningitis, pregnancy-related complications (bleeding, eclampsia, septic abortion),22, 23, 24, 25, 26 dehydration due to inadequate access to fluids in frail older adults and young children, exposure to nephrotoxins (e.g., nonsteroidal anti-inflammatory drugs, calcineurin blockers, antiretroviral therapy, antibiotics, or contrast media),27, 28, 29 poisons (e.g., arsenic poisoning),30 drug interactions (e.g., calcium-channel blocker plus clarithromycin),31 animal venoms (e.g., snake venom),20 trauma-induced rhabdomyolysis,32 and shock states due to heart failure, hypovolemia, or sepsis1, 16, 33 are all factors that can result in AKI. AKI following polypharmacy and nephrotoxin exposures in developing countries may be more prevalent, particularly when oversights of pharmacies are not robust due to more limited resources.
The inherent factors associated with AKI include age1, 22 and sex22; genetic susceptibility34, 35; and other comorbidities,16, 22 such as diabetes mellitus, hypertension, cancer, liver cirrhosis, chronic obstructive pulmonary disease,36, 37 previous episodes of AKI (AKD),5 and CKD.36, 38, 39 In a large multicenter international cross-sectional study (Acute Kidney Injury–Epidemiologic Prospective Investigation study), AKI in patients in the intensive care unit was associated with hypertension, diabetes, cardiovascular causes of admission, neurosurgery, and severity of illness at the time of admission.1
The etiology of AKI in children in developing countries is heterogeneous, ranging from perinatal asphyxia, sepsis, and dehydration in neonates and infancy, to severe systemic infections, diarrheal dehydration, hemolytic uremic syndrome, and infection-related glomerulonephritis in older children. Episodes of AKI may follow infections due to vivax or falciparum malaria, leptospirosis, dengue, and chikungunya, in both adults and children.40
Following the initial medical visit and determination of AKI risk category, clinicians need to reevaluate the risk of AKI whenever new patients have new exposures (e.g., sepsis, nephrotoxins, surgery, and so on). The previously mentioned surveillance could be facilitated via bedside examination, electronic records, and mobile tools. The frequency of such reevaluations depends on the resource availability and the extent and severity of new exposures. Future investigations should focus on the impact, optimal frequency, and the degree of resource dependency of such surveillance studies.
A prospective observational study of critically ill patients that compared AKI profiles in developing versus developed countries demonstrated that sepsis was the most common cause of AKI in developed countries, whereas glomerular and interstitial diseases accounted for most of the AKI cases in developing countries.38 Comparative data on the impact of individual components on AKI risk from resource-limited and resource-sufficient countries are few.14
Collecting information at large multinational levels to evaluate the global burden of health seems a necessary step to have a more precise view on the impact of each AKI risk dimension and factor on patients outcomes.41
Research Recommendation
Because data are limited with regard to the impact of each dimension in different regions of the globe, we recommend studies record risk factors and their effects on overall AKI outcomes from each level of individual dimensions in each region.
Q2: What Are the Differences Between Risk Factors for Community-Acquired Versus Hospital-Acquired AKI?
Consensus Statements
-
1.
Risk dimensions and factors vary between CAKI and HAKI.
-
2.
We recommend that health care providers identify risk factors at the population, health care system, provider, and the individual patient levels for each type of AKI (community-acquired vs. hospital-acquired).
-
3.
After identification of modifiable risk factors in each setting, we recommend the implementation of primary and secondary preventive strategies, based on each context, should be considered.42 Continuous reassessment of patients after each risk modification or any new exposure should be regarded as care continuum to improve patient outcome and safety.
Supplementary Table S1 provides definitions for CAKI and HAKI. In the developing world, the clinical outcomes of CAKI are different from HAKI.43 The marked social, political, and economic heterogeneity, frequent illness pattern changes, and disparities in access to products and services found in the developing world profoundly affect the relative burden of the 5 dimensions of AKI risk factors in the community and hospital settings. Even in a single country, large, wealthy urban locations may have extremely well-developed areas, whereas isolated smaller urban and rural zones could have poor services and limited infrastructure. Furthermore, in each city, the medical care provided by the university, public, and private hospitals alter from high-tech, tertiary-level care to extremely inadequate or even a nonexistent health system foundation.13, 14 These disparities among different locations in each country reflect the challenges of identifying the AKI risk profiles of each patient or population. Using well-developed measures of disparity to evaluate its impact on AKI risk should be assessed in future investigations (e.g., the Gini coefficient commonly used measure of inequality to detect the income distribution of residents in each country).44
Community-Acquired AKI
In areas with poor infrastructure, most of the patients present to the health care system late and often in advanced disease stages, which in turn imposes a significant additional burden of CAKI in deficient economies.
Environmental exposures (e.g., infection) play the most important role in CAKI among developing communities with poor infection control infrastructures. At the community level, the presence of chronic anemia, undernutrition, and parasitic diseases impose additional, potentially modifiable inherent risk factors in this population. In a large-scale epidemiology study in east India, authors reported that the impact of risk factors on the individual patient risk of CAKI changed course during the last quarter century. They found, although CAKI due to pregnancy, surgery, and diarrhea decreased significantly, the role of malaria, sepsis, nephrotoxic drugs, and liver disease in AKI predisposition increased.45 Because basic primary health infrastructure may be lacking in rural areas, an underdeveloped and underfunded public health foundation in these regions increases the chance of CAKI.13, 14, 25, 46
Hospital-Acquired AKI
The characteristics of patients with AKI in tertiary and quaternary care hospitals in developing and developed countries are most likely similar. The patients are typically older, affected by multiorgan failure, have preexisting chronic comorbidities, have sepsis, and/or use nephrotoxic drugs, which can be the cause of AKI.47
AKI risk factors among each population are different for CAKI as in HAKI. The environment and socioeconomic and/or cultural factors play a more limited role in HAKI. In the hospital setting, the process of care gains more significance. Inadequate identification of risk factors, delayed or missing AKI diagnosis, late or noninstallation of preventive measures or early therapy, lack of protocolized care, limited access to nephrology care, and poor awareness of the impact of AKI on morbidity and mortality have all been previously documented in developed and developing countries.48, 49, 50
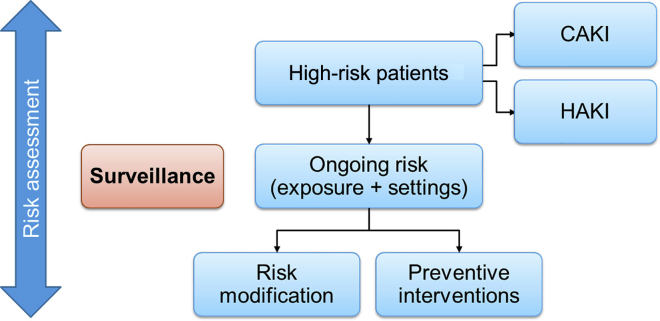
Exposures that increase the risk of HAKI are often completely or partially modifiable, and include sepsis, intravascular volume depletion, hypotension and/or shock, anemia, hypoxia, use of nephrotoxic drugs (antibiotics, iodinated contrast agents, nonsteroidal anti-inflammatory drugs, anticancer drugs, antiretroviral drugs, and calcineurin blockers), cardiac surgeries, and other major noncardiac surgeries.14 Inherent, nonmodifiable risk factors, such as the presence of previous comorbidities (CKD, diabetes, cancer, chronic heart disease, chronic lung disease, and chronic gastrointestinal disease, among others), genetic susceptibilities, sex, and age have been consistently demonstrated as relevant in this setting.14 Surveillance studies following each risk modification or new risk exposures, to re-strategize preventive measures, are necessary steps in improving patient outcomes (Figure 2).
Figure 2.
The process of ongoing risk assessment and surveillance. Among high-risk patients within the community and hospital settings, a subgroup of patients proceeds to develop acute kidney injury (AKI). In this group, in addition to risk modification and prevention, providing management measures like renal replacement therapies is essential. Risk assessment and modification can decrease the incidence of AKI in the community and hospital. Following the initial risk evaluation, the risk of AKI for each population or patient needs to be reassessed after any new exposure, risk modification, or preventive measure implementation. Community-based risk surveillance includes monitoring the occurrence of infection or drug use pandemics, nutrition status among children, and women of childbearing age, and so on. At the hospital setting, surveillance could include monitoring the incidence of antibiotic-resistant infections, inappropriate antibiotic use, compliance with sepsis management protocols, and so on. CAKI, community-acquired acute kidney injury; HAKI, hospital-acquired acute kidney injury.
Research Recommendations
-
•
The impact of AKI risk identification and modification to prevent acute kidney disease, kidney function nonrecovery, development or progress of CKD, quality of life, and mortality needs to be studied in both community and hospital settings.
-
•
In addition to the risk assessment tools for HAKI, investigators need to drive and validate risk assessment scores for CAKI.
Q3: Can We Identify Populations and Patients Who Have a High Risk for AKI?
Consensus Statement
-
1.
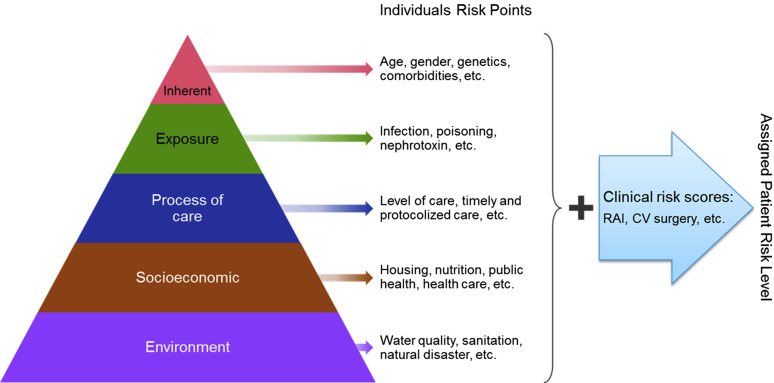
We recommend assessing the AKI risk profiles to be determined based on all of the 5 dimensions of AKI risk: environment, socioeconomic and/or cultural, the process of care, acute exposures, and inherent factors (Figure 3 and Table 1). The risk calculation needs to determine categories at the population, health care system, provider, and patient levels.
Figure 3.
Suggested checklist for patient risk–level determination based on the 5 risk dimensions and available literature risk stratification scores. Includes a nonexhaustive list of acute kidney injury risk factors, and additional factors may exist for each category. The factors listed may also span multiple dimensions, but may be listed within only 1 risk dimension for simplicity. CV cardiovascular surgery; RAI, renal angina index.
Risk assessment tools have been used to assist in determining patients at risk of AKI in some specific clinical settings. Most studies have identified risk in critically ill patients—those undergoing general surgical procedures or patients who receive iodinated contrast.51, 52, 53, 54 The factors included in most of the AKI risk scores are related to patient-inherent characteristics and comorbidities (e.g., age, diabetes, heart failure, hypertension, and the presence of CKD). Occasionally, some physiological factors, such as hypovolemia, oliguria, and hypoxia, have also been included in AKI risk stratification scores. Only a few risk assessment scores have incorporated the process of care factors, such as the use of diuretics (loop diuretics, thiazides, and potassium-sparing diuretics), angiotensinogen-converting enzyme inhibitor and/or angiotensin receptor blockers, or β-blockers.52, 55 Recent studies have suggested that estimation of the renal angina index, a composite measure of risk strata and signs of kidney injury in a critically ill pediatric population, may predict the occurrence of AKI.56 The performance of these risk stratification scores in the developing countries needs to be validated.
Although hospitalized and acutely ill patients are certainly those who would benefit most from risk determination, the concept of AKI risk needs to be further extended to other settings and populations. There are several potential advantages of an AKI risk assessment tool. Although evidence regarding the use of risk assessment tools for HAKI is of average quality, we believe that an accurate AKI risk assessment might alter clinical management, allow more informed decision-making, and might lead to earlier involvement of a specialist or referral to a higher level of care.
The assessment of risk factors and application of risk scores in clinical preventive strategies should be equally emphasized and recommended in patients within the primary care setting. All individuals and health care providers should be aware of the consequences of AKI and recognize the risk of AKI in primary and outpatient care settings, as well as urban and rural areas.
AKI risk profiling involves an integrated assessment of population, health care system, provider, and patient characteristics. Because of the complexity of the dimensions and risk factors involved in AKI development, a broad assessment is fundamental to build a risk profile in each region of the world. The assessment of environmental, socioeconomic and/or cultural, and the process of care dimensions are of particular importance in primary and outpatient clinics, especially in resource-limited regions, where these dimensions largely contribute to the overall AKI risk. In the context of resource-limited areas where AKI occurs in younger and generally healthier individuals and is caused by infections, volume depletion associated with severe diarrhea, pregnancy-related events, or animal envenomation,14 limited access to clean water and basic health care, lack of awareness about the impact of AKI on clinical outcomes, and scarcity of supervision for nephrotoxin and polypharmacy exposure constitute important risk factors and therefore need to be included in the risk profile of the population. At the patient level, the risk associated with environmental, socioeconomic and/or cultural, and the process of care dimensions, constitute potentially modifiable factors. Therefore, these could be the key targets in risk assessment tools for AKI prevention.
Although we currently have no evidence of the economic benefit of assessing the risk of AKI, the application of risk scores is not costly. Identification of patients at risk may result in the earlier initiation of preventive therapies, avoidance of harmful drugs, and early interventions, which may potentially change the course of patients with AKI. The potential harm of falsely classifying a patient as high risk for AKI cannot be ignored. Overdiagnosis of AKI could result in unnecessary interventions that could be associated with higher cost and worse patient-related outcomes.11 Costly, intensive and frequent monitoring, unnecessary investigations, and complex treatments can negatively affect patients and add to the cost of care related to population, health care systems, providers, and patients (Table 1).
Research Recommendations
-
•
We recommend that future research should address the development of a risk score for AKI prediction and implementation of appropriate preventive strategies suitable for each region.
-
•
Subsequent studies should provide an evaluation of the usefulness of risk assessment−driven preventive measures on patient-centered outcomes, such as progression to more severe stages, renal recovery, functional status, the length of hospital stay, and mortality.
Q4: How Can High-Risk Patients Be Monitored to Prevent AKI Development or Progression?
Consensus Statement
-
1.Prevention of AKI should be provided at the population, health care system, provider, and patient levels. Within each level, we recommend that efforts be made to recognize and monitor risk factors within the each of the 5 dimensions for each region. In the resource- limited areas, general practitioners, and allied health staff or public health operatives should be trained to assess AKI and provide preventive measures.
-
•At the population level, raising awareness regarding AKI and its risk factors, preventive measures, raising the level of education and literacy, and correction of potentially harmful beliefs could affect the overall incidence of AKI and its effects on patients, health systems, and the economy.
-
•At the health care system level, enactment of appropriate policies and laws for risk control, investing in public health, and health care, improvement in health care access (including RRTs) are steps to affect AKI and its effects on patients, health systems, and the economy.
-
•At the provider level, training of primary care providers and ancillary personnel, plus supplying tools to record and convey information about high-risk individuals is recommended.
- •
-
•
To deliver AKI primary prevention, health care providers, together with public health policymakers and politicians, need to collaborate to devise and implement plans to affect risk factors in all 5 risk dimensions at the population, health care system, providers, and patient levels. The first step is to raise awareness regarding AKI, and its risk factors and consequences among each population and all health care providers.2, 13, 14
At the population level, interventions like mosquito control can prevent AKI and death from malaria due to Plasmodium falciparum. Epidemiological studies have shown that the incidence of malaria dropped by 18% around the world from 2000 to 2015.57 Malaria-related mortality in Africa decreased from 764,000 to 395,000 during this period. There have been concerted efforts to provide insecticide-treated mosquito nets (ITN) to countries in sub-Saharan Africa (2% in 2000 and 55% in 2015), where most deaths were reported. Seventy percent of the decreased malaria-related deaths in sub-Saharan Africa was due to simple interventions like ITNs and indoor residual spraying rather than artemisinin-based combination therapy. In this case, funding came from the Global Fund and domestic government expenditures.58 The cost was approximately 2.5 billion United States Dollars (USD) in 2014 ($1.9 billion USD from international donors and $550,000 USD from local governments).57 Despite the initial cost, controlling malaria was proven to be associated with faster economic growth.59 Approximately 1% of malaria infection episodes lead to AKI,40 which contributes to the fatality due to malaria because dialysis support is limited.7, 60 It is logical to note that increasing malaria control may result in a significant reduction in AKI incidence in the whole population.
An example of a successful government (health care system) intervention was the imposition of a “sugar tax.” A tax on sugar-sweetened beverages was imposed in Mexico in 2014 in an attempt to prevent obesity and type 2 diabetes mellitus.61 There was a 7.3% decline in per capita sales of sugar-sweetened beverages from 2014 to 2015 compared with from 2007 to 2013. The long-term effects of such reduction in sugar use would most likely affect the risk of obesity, diabetes, CKD, and therefore, AKI. The provision of clean drinking water to prevent diarrheal disease in developing countries and to provide oral rehydration solutions could significantly affect AKI incidence.62 Providing appropriate access to health care and education, improving housing, transportation, and mitigating racial and economic disparity, as well as unemployment could affect the risk of AKI.10 Supporting public health facilities to improve prenatal care, vaccination, prevention of anemia, and malnutrition are essential steps in AKI prevention at the health care system level. Obviously, primary prevention is not complete without limiting exposures like infection, traffic accidents, snake bites, or alternative nephrotoxic medicines.7
Providers can prevent AKI development and progression with proper risk assessment, timely management, and ongoing monitoring. In the hospital setting, the known methods of AKI primary prevention include fluid resuscitation with i.v. balanced crystalloids, prevention of hypotension with inotropes after volume repletion, adjustment of medication doses for kidney function, and limiting exposures to nephrotoxic drugs. In the community setting, rapid and adequate rehydration, early antibiotic therapy for febrile infectious diseases, and early antivenom administration after snakebites may have a profound effect on the incidence of AKI.
For each individual patient, the inherent risk factors should be taken into account for assessing the risk of AKI in individual patients—age, sex, diabetes, CKD, and substance abuse.63 Educating each high-risk member of the community to improve lifestyle or control modifiable comorbidities (e.g., hypertension, diabetes) could be considered as preventive measures.
Research Recommendations
-
•
We recommend the evaluation of preventive measures effects on high-risk AKI individuals as determined by regionally specific AKI risk scores based on the 5 dimensions.
-
•
We recommend that health care providers in each region evaluate the impact of population, health care system, provider, and patient-level risk modification on patient outcomes.
-
•
We recommend that the role of mobile health technology for surveillance and tracking of high-risk individuals be evaluated in resource-limited areas.
Conclusion
The identification of risk factors that predispose to AKI is a crucial aspect of care. Application of a 5 dimension risk approach is advisable for adequate flow and sequence of actions. Risk factors in the environment and socioeconomic and/or cultural dimensions must be correctly and precisely identified. Knowledge of modifiable exposures, especially in populations and patients with high-risk profiles, is crucial for prevention, early diagnosis, and/or attenuation of AKI. Political efforts for the modification of risk factors within each dimension in conjunction with increasing awareness among health care providers is likely to significantly affect patient outcomes.64, 65, 66
Disclosure
All the authors declared no competing interests.
Author Contributions
KK, EM, EB, DK, LH, RM, and RC all participated in the consensus-building process and drafting of this article. RM, RC, and AB provided a critical review of this article.
Acknowledgment
Supported through the UAB-UCSD O’Brien Center NIH-NIDDK Grant DK079337.
Footnotes
Table S1. Definitions of terms used in the article.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Definitions of terms used in the article.
References
- 1.Hoste E.J., Bagshaw S., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 2.Lewington A.J.P., Cerda J., Mehta R.L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lameire N.H., Bagga A., Cruz D. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 4.Venkataraman R., Kellum J.A. Prevention of acute renal failure. Chest. 2007;131:300–308. doi: 10.1378/chest.06-1246. [DOI] [PubMed] [Google Scholar]
- 5.ADQI-XVII: Recovery from AKI. Available at: http://www.adqi.org. 2015.
- 6.Adhikari N.K.J., Rubenfeld G.D. Worldwide demand for critical care. Curr Opin Crit Care. 2011;17:620–625. doi: 10.1097/MCC.0b013e32834cd39c. [DOI] [PubMed] [Google Scholar]
- 7.Ponce D., Balbi A. Acute kidney injury: risk factors and management challenges in developing countries. Int J Nephrol Renovasc Dis. 2016;9:193–200. doi: 10.2147/IJNRD.S104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerda J., Bagga A., Kher V. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–153. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 9.Kellum J.A., Bellomo R., Ronco C. Acute Dialysis Quality Initiative (ADQI): methodology. Int J Artif Organs. 2008;31:90–93. doi: 10.1177/039139880803100202. [DOI] [PubMed] [Google Scholar]
- 10.Elmore J.G. Solving the problem of overdiagnosis. N Engl J Med. 2016;375:1483–1486. doi: 10.1056/NEJMe1608683. [DOI] [PubMed] [Google Scholar]
- 11.Office of Disease Prevention and Health Promotion. Healthypeople.gov: Determinants of Health. Available at: https://www.healthypeople.gov/2020/about/foundation-health-measures/Determinants-of-Health. Accessed October 17, 2016.
- 12.Mathew A.J., George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31:451–456. doi: 10.4103/0256-4947.84620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta R.L., Cerdá J., Burdmann E.A. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 14.Mehta R.L., Burdmann E.A., Cerdá J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L., Fu P., Wang L. The clinical features and outcome of crush patients with acute kidney injury after the Wenchuan earthquake: differences between elderly and younger adults. Injury. 2012;43:1470–1475. doi: 10.1016/j.injury.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Hu Z., Zeng X., Fu P. Predictive factors for acute renal failure in crush injuries in the Sichuan earthquake. Injury. 2012;43:613–618. doi: 10.1016/j.injury.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Chen X., Zhong H., Fu P. Infections in crush syndrome: a retrospective observational study after the Wenchuan earthquake. Emerg Med J. 2011;28:14–17. doi: 10.1136/emj.2009.077859. [DOI] [PubMed] [Google Scholar]
- 18.Lunyera J., Kilonzo K., Lewington A. Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis. 2016;67:834–840. doi: 10.1053/j.ajkd.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen C.F., Kidd S., Sillah A.R. Improving burial practices and cemetery management during an Ebola virus disease epidemic - Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:20–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Harshavardhan L., Lokesh A., Tejeshwari H. A study on the acute kidney injury in snake bite victims in a tertiary care centre. J Clin Diagn Res. 2013;7:853–856. doi: 10.7860/JCDR/2013/5495.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daher E.F., Junior Silva G.B., Vieira A.P. Acute kidney injury in a tropical country: a cohort study of 253 patients in an infectious diseases intensive care unit. Rev Soc Bras Med Trop. 2014;47:86–89. doi: 10.1590/0037-8682-0223-2013. [DOI] [PubMed] [Google Scholar]
- 22.Shacham Y., Gal-Oz A., Leshem-Rubinow E. Association of admission hemoglobin levels and acute kidney injury among myocardial infarction patients treated with primary percutaneous intervention. Can J Cardiol. 2015;31:50–55. doi: 10.1016/j.cjca.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Wonnacott A., Meran S., Amphlett B. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol. 2014;9:1007–1014. doi: 10.2215/CJN.07920713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombardi R., Yu L., Younes-Ibrahim M. Epidemiology of acute kidney injury in Latin America. Semin Nephrol. 2008;28:320–329. doi: 10.1016/j.semnephrol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Jha V., Parameswaran S. Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol. 2013;9:278–290. doi: 10.1038/nrneph.2013.36. [DOI] [PubMed] [Google Scholar]
- 26.Paula Santos U., Zanetta D.M., Terra-Filho M. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87:792–799. doi: 10.1038/ki.2014.306. [DOI] [PubMed] [Google Scholar]
- 27.Goldfarb S., McCullough P.A., McDermott J. Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clinic Proc. 2009;84:170–179. doi: 10.4065/84.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goussot S., Mousson C., Guenancia C. N-terminal fragment of pro B-type natriuretic peptide as a marker of contrast-induced nephropathy after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 2015;116:865–871. doi: 10.1016/j.amjcard.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Inohara T., Kohsaka S., Abe T. Development and validation of a pre-percutaneous coronary intervention risk model of contrast-induced acute kidney injury with an integer scoring system. Am J Cardiol. 2015;115:1636–1642. doi: 10.1016/j.amjcard.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Lee J.Y., Eom M., Yang J.W. Acute kidney injury by arsine poisoning: the ultrastructural pathology of the kidney. Renal Fail. 2013;35:299–301. doi: 10.3109/0886022X.2012.745117. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi S., Fleet J.L., Bailey D.G. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA. 2013;310:2544–2553. doi: 10.1001/jama.2013.282426. [DOI] [PubMed] [Google Scholar]
- 32.Stewart I.J., Faulk T.I., Sosnov J.A. Rhabdomyolysis among critically ill combat casualties: associations with acute kidney injury and mortality. J Trauma Acute Care Surg. 2016;80:492–498. doi: 10.1097/TA.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 33.Vincent J.-L., Marshall J.C., Ñamendys-Silva S.A. Assessment of the worldwide burden of critical illness: the Intensive Care Over Nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 34.Stafford-Smith M., Li Y.J., Mathew J.P. Genome-wide association study of acute kidney injury after coronary bypass graft surgery identifies susceptibility loci. Kidney Int. 2015;88:823–832. doi: 10.1038/ki.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varrier M., Ostermann M. Novel risk factors for acute kidney injury. Curr Opin Nephrol Hyperten. 2014;23:560–569. doi: 10.1097/MNH.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 36.James M.T., Grams M.E., Woodward M. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66:602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darmon M., Vincent F., Canet E. Acute kidney injury in critically ill patients with haematological malignancies: results of a multicentre cohort study from the Groupe de Recherche en Reanimation Respiratoire en Onco-Hematologie. Nephrol Dial Transplant. 2015;30:2006–2013. doi: 10.1093/ndt/gfv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouchard J., Acharya A., Cerda J. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324–1331. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grams M.E., Astor B.C., Bash L.D. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerda J., Lameire N., Eggers P. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881–886. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 41.Christopher M., Alan L. Vol. 1. Harvard SchooL of Public Health on behalf of the World Health Organization and the World Bank; Boston: 1997. (The Global Burden of Disease). [Google Scholar]
- 42.Miquel P. 6th ed. Oxford University Press; Oxford, UK: 2008. A Dictionary of Epidemiology. [Google Scholar]
- 43.Daher E.F., Silva Junior G.B., Santos S.Q. Differences in community, hospital and intensive care unit-acquired acute kidney injury: observational study in a nephrology service of a developing country. Clin Nephrol. 2012;78:449–455. doi: 10.5414/CN107167. [DOI] [PubMed] [Google Scholar]
- 44.Darkwah K.A., Nortey E.N.N., Lotsi A. Estimation of the Gini coefficient for the lognormal distribution of income using the Lorenz curve. SpringerPlus. 2016;5:1196. doi: 10.1186/s40064-016-2868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prakash J., Singh T.B., Ghosh B. Changing epidemiology of community-acquired acute kidney injury in developing countries: analysis of 2405 cases in 26 years from eastern India. Clin Kidney J. 2013;6:150–155. doi: 10.1093/ckj/sfs178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Xing G, Wang L, et al. Acute kidney injury in China: a cross-sectional survey. Lancet. 386:1465–1471. [DOI] [PubMed]
- 47.Santos W.J., Zanetta D.M., Pires A.C. Patients with ischaemic, mixed and nephrotoxic acute tubular necrosis in the intensive care unit–a homogeneous population? Crit Care. 2006;10:R68. doi: 10.1186/cc4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aitken E., Carruthers C., Gall L. Acute kidney injury: outcomes and quality of care. QJM. 2013;106:323–332. doi: 10.1093/qjmed/hcs237. [DOI] [PubMed] [Google Scholar]
- 49.Finlay S., Bray B., Lewington A.J. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med (Lond) 2013;13:233–238. doi: 10.7861/clinmedicine.13-3-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponce D., Zorzenon CdPF, Santos NYd Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol Dialysis Transplant. 2011;26:3202–3206. doi: 10.1093/ndt/gfr359. [DOI] [PubMed] [Google Scholar]
- 51.Thakar C.V., Arrigain S., Worley S. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 52.Kashani K., Steuernagle Iv J.H., Akhoundi A. Vascular surgery kidney injury predictive score (vKIPS): a historical cohort study. J Cardiothor Vasc Anesthesia. 2015;29:1588–1595. doi: 10.1053/j.jvca.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Kheterpal S., Tremper K.K., Heung M. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110:505–515. doi: 10.1097/ALN.0b013e3181979440. [DOI] [PubMed] [Google Scholar]
- 54.Mehran R., Aymong E.D., Nikolsky E. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 55.Palevsky P.M., Zhang J.H., Seliger S.L. Incidence, severity, and outcomes of AKI associated with dual renin-angiotensin system blockade. Clin J Am Soc Nephrol. 2016;11:1944–1953. doi: 10.2215/CJN.03470316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu R.K., Zappitelli M., Brunner L. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85:659–667. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. World Malaria Report 2015. Available at: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en. Accessed October 17, 2016.
- 58.Korenromp E.L., Hosseini M., Newman R.D. Progress towards malaria control targets in relation to national malaria programme funding. Malar J. 2013;12:18. doi: 10.1186/1475-2875-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breman J., Egan A., Keusch G. The intolerable burden of malaria: a new look at the numbers. Am J Trop Med Hyg. 2001;64(1-2 Suppl):iv–vii. doi: 10.4269/ajtmh.2001.64.iv. [DOI] [PubMed] [Google Scholar]
- 60.Prasad R., Mishra O.P. Acute kidney injury in children with Plasmodium falciparum malaria: determinants for mortality. Perit Dial Int. 2016;36:213–217. doi: 10.3747/pdi.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colchero M.A., Guerrero-Lopez C.M., Molina M. Beverages sales in Mexico before and after implementation of a sugar sweetened beverage tax. PLoS One. 2016;11:e0163463. doi: 10.1371/journal.pone.0163463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf J., Pruss-Ustun A., Cumming O. Assessing the impact of drinking water and sanitation on diarrhoeal disease in low- and middle-income settings: systematic review and meta-regression. Trop Med Int Health. 2014;19:928–942. doi: 10.1111/tmi.12331. [DOI] [PubMed] [Google Scholar]
- 63.Kellum J.A., Lameire N., Group KAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho A., Lee J.E., Yoon J.Y. Effect of an electronic alert on risk of contrast-induced acute kidney injury in hospitalized patients undergoing computed tomography. Am J Kidney Dis. 2012;60:74–81. doi: 10.1053/j.ajkd.2012.02.331. [DOI] [PubMed] [Google Scholar]
- 65.Brady P., Gorham J., Kosti A. “SHOUT” to improve the quality of care delivered to patients with acute kidney injury at Great Western Hospital. BMJ Qual Improv Rep. 2015;4 doi: 10.1136/bmjquality.u207938.w3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldstein S.L., Mottes T., Simpson K. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90:212–221. doi: 10.1016/j.kint.2016.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definitions of terms used in the article.