Abstract
Natural killer/T-cell (NK/T) lymphomas represent a group of rare tumors of NK and NK-T cells. The World Health Organization classifies NK-cell tumors into three types, extranodal NK/T-cell lymphomas (ENKL, nasal and non-nasal), NK-cell leukemias, and a blastic variant (CD4-positive, CD56-positive hematodermic neoplasms). We focus our review to the current concepts in biology and treatment of ENKL. Though considerable advances have been made in our understanding of NK-cell biology, malignant transformation including the role of Epstein–Barr virus, and prognosis, the rare nature of ENKL and its heterogeneity limit the ability to standardize therapy. Radiotherapy is fundamental to treatment of early-stage disease with a role for chemoradiotherapy among high-risk patients. The clinical course of advanced disease is highly aggressive with frequent chemotherapy resistance and a poor prognosis. Therapeutic approaches to advanced-stage or relapsed and refractory disease, including the appropriate sequence of chemotherapy, combined modality therapy, and stem cell transplantation is not well-established. International and multicenter clinical trials are needed for this rare and aggressive disease.
Keywords: Natural killer, T-cell, lymphoma, diagnosis, treatment
Introduction
Natural killer/T-cell (NK/T) lymphomas represent a group of rare tumors of NK and NK-T cells. NK and NK-T cells are critical components of the innate immune system responsible for anti-viral and anti-tumor immune responses as well as control of autoimmunity. NK cell function is uniquely independent of the T cell receptor (TCR) whereas NK-T cell activity is mediated through a restricted TCR repertoire. Surface markers of both cell types include CD16, CD56, and granzyme with variable expression of classical T cell markers (CD4 and CD8). The World Health Organization (WHO) classifies NK-cell tumors into three types, extranodal NK/T-cell lymphomas (ENKL, nasal and non-nasal), NK-cell leukemias, and a blastic variant (CD4-positive, CD56-positive hematodermic neoplasms) [1,2]. A potential fourth subtype has recently been reported with an immature NK-cell phenotype, known as precursor NK-cell neoplasm. For the purposes of this review, we will limit our discussion to the current concepts in biology and treatment of extranodal NK/T cell lymphoma. The complex nosology of ENKL includes prior description of this entity as lethal midline granuloma, polymorphic malignant reticulosis, or angiocentric immunoproliferative lesion.
The incidence of ENKL parallels the geographic distribution of Epstein–Barr virus (EBV) infection, which may be directly involved in lymphomagenesis. Histopathologic and molecular advances during the preceding three decades have resulted in improved diagnosis and treatment of NK/T-cell lymphomas. In most patients with advanced disease, the clinical course is highly aggressive with frequent chemotherapy resistance and a poor prognosis. Radiotherapy is fundamental to treatment of early-stage disease with a role for chemoradiotherapy among high-risk patients. Therapeutic approaches to advanced-stage or relapsed and refractory disease, including the appropriate sequence of chemotherapy, combined modality therapy, and stem cell transplantation are not well-established.
Pathobiology
Classic NK-cell tumor histology appears as a polymorphic neoplastic infiltrate with angioinvasion and cytoplasmic azurophilic granules. A diffuse lymphomatous infiltrate is commonly observed with areas of coagulative necrosis, apoptosis and background inflammatory infiltrate including plasma cells, small lymphocytes, histiocytes, and eosinophils. Tumor cells range in size with a mixture of small and large lymphoid cells with irregular nuclei, small nucleoli, and granular or vesicular chromatin. Immunohistochemical (IHC) stains demonstrate expression of T lineage antigens including CD2, CD7, and CD8, whereas NK lineage markers (such as CD56) are consistently expressed. For tumors suspected to be NK lineage it is important for diagnostic testing to be performed on fresh and not formalin-fixed tissue because of nonspecific staining following fixation (polyclonal CD3 is positive in both T and NK cells due to staining of both surface and cytoplasmic CD3 and CD3ε) [3,4]. Evidence of EBV involvement by in situ hybridization (ISH) staining for EBV-encoded small nuclear RNA-1 (EBER-1) within transformed cells is critical to diagnosis. Negative ISH for EBER-1 should prompt review of the primary pathology and consideration of an alternative diagnosis. Bone marrow involvement rarely demonstrates lymphoid aggregates, as observed in other lymphoma subtypes, by histology and IHC staining for CD56. However, it is important to evaluate the bone marrow for EBV by ISH for EBER-1. Bone marrow should be considered involved if explicit nuclear staining for EBER-1 is seen within a single non-B cell (EBER-1 positive B cells are observed in chronic EBV infection) [5].
The oncogenesis of ENKL is not well-understood. As other tumors in the nasal cavity and nasopharynx also have an EBV association, the increased incidence of EBV-positive staining within NK-cell lymphomas, particularly nasal-type, has been postulated to be a non-oncogenic marker of EBV within non-malignant cells of the nasal cavity and nasopharynx. However, comparative analysis of the EBV genome in normal cells from the nasal cavity and from ENKL argues for a direct role in pathogenesis. The EBV genome in normal cells from the nasopharynx demonstrates significant strain heterogeneity, while only a single common EBV strain is found among ENKL tumor cells [6]. In addition, the EBV strain within NK-cell tumors contains a unique, 30-base pair deletion in LMP1 (latent membrane protein 1) in 96–100% of ENKL cases compared to normal cells which were found to carry wildtype LMP1 [7,8]. Mutated LMP1 plays a critical role in cell transformation and immune system evasion because of EBV. Interestingly, cell culture studies have shown retained capacity of EBV to infect NK-cells despite lack of NK-cell surface expression of CD21, the receptor required for EBV adhesion and cell entry [9]. Further support of the role of EBV in the molecular pathogenesis of ENKL includes the observation that circulating EBV DNA titer directly correlates with disease activity and is prognostic with higher titers suggestive of extensive disease, unfavorable response to therapy, and poor survival [10].
Cytogenetic abnormalities are seen in up to 77% of NK-cell neoplasms, particularly in mature NK-cell tumors [11]. Karyotypes observed include pseudodiploidy (57%), hyperdiploidy (30%), and hypodiploidy (13%). Common cytogenetic abnormalities include loss of chromosome 6q, 11q, 13q, and 17p [11]. Deletion of chromosome 6q21–25 and i(6) (p10) are commonly observed by fluorescent in-situ hybridization (FISH) [11]. Molecular analysis of patient samples has also shown inactivation of multiple tumor suppressor genes including p16NK4A, p15NK4B, p14ARF, TP53, Rb, as well as mutations in FAS, B-catenin, and KIT [12]. On an epigenetic level, aberrant methylation of multiple tumor suppressor genes as well as other NK cell-associated genes has been observed. High levels of methylation have been identified in five genes including p15 (48%), RARbeta (56%), hMLH1 (61%), p16 (71%), and p73 (92%) [13]. Methylation patterns are not consistent at the primary tumor compared to metastatic sites, except for P73 which has similar expression patterns, and has recently been investigated as a marker of disease activity[13].
Clinical features
ENKL presents at a median age of 50 years with a male predominance and a geographic predilection with increased prevalence in Asia, Central and South America [14]. The International Peripheral T-cell Lymphoma Project reviewed 1314 cases and reported a four-fold higher relative frequency of ENKL among lymphomas in Asian countries compared to Western countries (22% vs. 5%) [15]. At diagnosis, 80% of cases involve the upper aerodigestive tract (UAT), inclusive anatomically of the nasal cavity, nasopharynx, oral cavity, oropharynx, and hypopharynx [16]. In the nasal type, 70% of patients present with localized disease resulting in symptoms at presentation including purulent rhinorrhea, epistaxis, and local swelling because of anatomical obstruction [16]. Extension into the hard palate leads to destruction with a characteristic mid-line perforation. Clinically, ENKL is the most common lymphoma subtype which presents within the nasal cavity, occurring along the midline at sites including the nasopharynx, paranasal sinus, tonsils, hypopharynx, larynx, and nasal cavity itself. However, in 10% ENKL may present in “extranasal” sites with a predilection for the gastrointestinal tract, skin, salivary glands, adrenals, spleen, and testis. Bone marrow and central nervous system involvement at presentation are rare, <3% and 7%, respectively [17]. The majority of patients with extranasal ENKL present with B symptoms, advanced stage, and evidence of hemophagocytosis with resultant cytopenias [15].
The diagnostic evaluation is similar to other lymphomas; however given the unique anatomic sites of involvement, dedicated imaging studies of the nasal cavity, hard palate, and anterior fossa are required. Panendoscopy including the oropharynx and stomach should be considered in all cases given the propensity for UAT involvement. The utility of fluorine-18 fluorodeoxyglucose positron emission tomography (PET) has demonstrated high sensitivity, greater than 95%, for extracutaneous disease, with the exception of cutaneous or bone marrow involvement [18,19]. Given this sensitivity, PET imaging may replace the role of panendoscopy. An MRI is also recommended as it provides a clearer distinction between soft tissue and bone involvement compared to PET-CT, and is useful in radiotherapy planning. Independent assessment of marrow involvement and a thorough physical exam remain necessary for adequate staging.
Prognostic factors
Accurate assessment of patient prognosis is important for identification of patients at low risk for recurrence with early-stage disease and selection of patients at high risk to receive high-dose, intensive therapies. The 5-year overall survival (OS) in early-stage disease is over two-fold superior to advanced-stage at diagnosis, 54% versus 20% [20]. Even within early-stage disease, patients with stage I disease have a long-term OS double that of stage II disease [21]. However, as ENKL often presents with extranodal disease, standard lymphoma staging, such as Ann Arbor stage, is limited in its applicability. Several earlier series suggest that anatomical location is prognostic including comparison of ENKL within the UAT versus non-UAT [22]. The UAT tumors have a higher rate of complete remission (CR) with initial therapy (60% versus 32%), and a 5-year OS approximately two-fold greater compared to non-UAT tumors (41–54% versus 20–22%) [20,23].
Multiple efforts have attempted to select new prognostic markers. In multivariate analyses across studies, independent predictors of OS include age over 60, lactate dehydrogenase (LDH)above the upper limit of normal, elevated C-reactive protein (CRP), eastern cooperative oncology group (ECOG) performance status greater than 2, presence of B symptoms, or histological evidence of high Ki-67 staining, hemophagocytosis, and local invasion (such as bone or skin invasion; Table I) [22,25,26]. Local tumor invasiveness increases the relative risk of progression and death by 7.3- and 2.8-fold, respectively [27].
Table I.
Prognostic factors in extranodal NK/T-cell lymphoma.
| Characteristic | % CR | Survival
|
|
|---|---|---|---|
| Median survival months | % 5y OS | ||
| Age* | |||
| ≥65 years | 50–61 | NR | 36–39 |
| <65 years | 44–64 | NR | 33–73 |
| Ann Arbor stage III/IV* | |||
| I | 50–86 | NR | 42–78 |
| II | 46–86 | NR | 19–48 |
| III/IV | 23–42 | NR | 20 |
| B symptoms* | |||
| Present | 38–63 | NR | 19–33 |
| Absent | 60–75 | NR | 41–49 |
| International Prognostic Index | |||
| Low (0–1) | 58–92 | >10y | 57.4 (20y) |
| Low intermediate (2) | 12 | 27.6 (20y) | |
| Nasal only | 50–91 | 82 (8y) | |
| High intermediate (3) | 12 | 27.6 (20y) | |
| Nasal only | 14–92 | 90 (8y) | |
| High (>3) | 4–6 | 27.6 (20y) | |
| Nasal only | 5–90 | 84 (8y) | |
| NK Lymphoma Prognostic Index† | |||
| Low (0) | NR | >10y | 80.9 |
| Low intermediate (1) | NR | 30 | 64.2 |
| Intermediate high (2) | NR | 9 | 34.4 |
| High (3–4) | NR | 4 | 6.6 |
| EBV viral load at diagnosis | |||
| <6.1 × 6107 copies/mL | NR | >54 | 80.9 |
| ≤6.1 × 6107 copies/mL | NR | 2.1 | 64.2 |
NR, not reported; y, year; CR, complete remission; OS, overall survival.
Prognostic significance supported by multiple series including in multivariate analysis.
Risk factors compose the NK cell lymphoma prognostic index proposed by Lee, et al, include Ann Arbor stage, LDH, B symptoms, regional lymphadenopathy [24].
There is ongoing debate regarding the applicability of the International Prognostic Index (IPI), a standard prognostic index in diffuse large B-cell lymphoma, in ENKL. Comparison of IPI between studies is limited by small sample size and heterogeneity of therapy. Series which support IPI as prognostic are weighted toward early-stage disease, and report a 20-year OS of 57% among patients with IPI ≤1 compared to 27.6% in patients with an IPI of 2 or more [28,29]. Because of the inability of the standard IPI to distinguish between low- and intermediate-risk patients a new prognostic model (NK/T cell PI) has been proposed [24,30,31]. This model was based on a retrospective review of 262 cases with low- and high-risk disease by IPI, and included four variables (presence of B symptoms, Ann Arbor stage III or IV, elevated LDH >normal, and presence of regional lymphadenopathy (N1 through N3, not M1)). Four risk groups were identified. The 5-year OS for patients with low-risk (0 factors) and low-intermediate risk (1 factor) were 80.9% and 64.2%, respectively. Patients with intermediate-high-risk (2 factors) and high-risk (3 or 4 factors) had a 5-year OS of 34.4% and 6.6%, respectively. Disappointingly, both the NK/T-cell PI and IPI were only prognostic for nasal type, with neither index predictive among patients with extranasal disease in the International Peripheral T-cell Lymphoma Project [15].
The inadequacies of clinical and laboratory-based prognostic models have led to the exploration of biological-based factors. Given prior implication of EBV in ENKL oncogenesis, Au et al. [10] evaluated whether EBV activity correlated with disease course and outcomes. Plasma EBV DNA levels by quantitative PCR correlated with stage of disease and response to therapy. In multivariate analysis, EBV DNA levels >6.1 ×107copies/mL were significantly associated with inferior disease-free survival (DFS). EBV DNA titer may also be evaluated within whole blood, where it has similarly been shown to correlate with stage at diagnosis, prognosis, and response to therapy [10,32]. However, whole blood EBV PCR may lead to possible inaccuracy due to detection of EBV-infected memory B cells. The effect of EBV status on prognosis may be modified by coexistent genetic mutations. Assessment of EBV by RNA ISH for EBERs and concurrent cytogenetic abnormalities has identified co-expression of EBER with a p53 mutation as highly predictive of treatment failure [33].
Expression of an emerging prognostic marker, CD94, a subtype of NK-cell antigen receptor correlates with increased NK-cell maturation as well as a better prognosis. In one series, eight of ten CD94-positive patients were alive beyond 1 year, compared to two of nine CD94-negative patients (60 versus 10-month median survival)[34]. S-phase kinase-associated protein 2 (Skp2, a subunit of the ubiquitin protein ligase complex) is found at increased levels in the majority of NK-cell lymphomas. Patients with Skp2 expression and loss of p27 have worse OS [35]. Other emerging prognostic markers include absence of granzyme B inhibitor PI9, expression of cutaneous lymphocyte antigen, missense mutations of TP53, and high serum levels of nm23-H1 protein [9]. At present, clinical and laboratory assessments remain the standards for risk assessment in NK lymphomas. EBV disease activity is a promising additional source of prognostic information. Newer molecular markers require additional study but offer hope for improved risk stratification and guiding treatment selection in the future.
Treatment
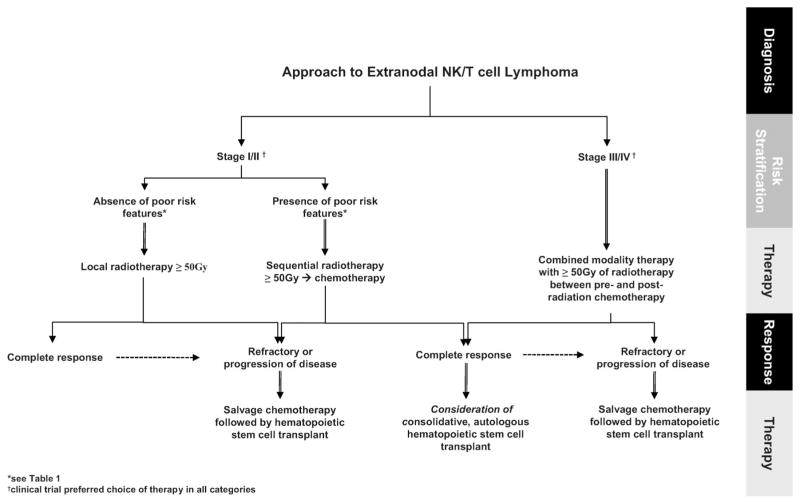
There remains a lack of consensus on treatment of ENKL and no therapy is considered standard. Most data are limited by small, non-randomized studies, inconsistent diagnostic criteria, and heterogeneous patient populations, with older series containing non-ENKL cases. Finally, as case series are reported from several countries, direct comparison of results is further limited by varying technology in tissue staining and processing, standards of medical care, and patient genetic makeup. Recognizing these limitations, for the purposes of this review, we have discussed treatment organized by stage and modality, and have suggested an algorithm for approach to management (Figure 1).
Figure 1.
Diagnostic and treatment approach to extranodal NK/T-cell lymphoma.
Early-stage
Experience with radiotherapy alone
Table II summarizes the experience with radiation therapy, chemotherapy, and combined modality therapy in the management of early-stage ENKL, both nasal and extranasal (non-nasal). There are no randomized trials comparing radiation therapy to chemotherapy. Prior to current diagnostic classification of NK-cell tumors, radiation was recognized as critical to inducing durable complete remissions in lethal midline granuloma and has remained the cornerstone of therapy for early-stage ENKL [42].
Table II.
Treatment and outcome for early-stage extranodal NK/T-cell lymphoma*.
| Author (year) | N (stage I/II) | Treatment regimen (n) | Outcomes
|
|
|---|---|---|---|---|
| % CR | % 5y OS | |||
| Aviles et al. (2000) [30] | 108 (108) | RT followed by CEOP-bleomycin | 92 | 86 (8y) |
| Kim et al. (2001) [36] | 143 (74) | RT (104) | 69 | 35 |
| CHOP or BACOP followed by RT (39) | 8 | 38 | ||
| Cheung et al. (2002) [21] | 79 (79) | Overall | 68 | 38 |
| RT (18) | NR | 29 | ||
| CMT(95% anthracycline-based regimen)(61) | NR | 40 | ||
| You et al. (2004) [26] | 42 (42) | Overall | 72 | 61 |
| RT (6) | NR | 83 | ||
| CHOP, CEOP, or m-BACOD(22) ± salvage RT (18) | NR | 29 | ||
| Li et al. (2004) [31] | 77 (56) | Overall | 63 | NR |
| RT (11) | 55 | 50 | ||
| CT(92% CHOP-based regimen)(18) | 50 | 15 | ||
| CMT(27) | 74 | 59 | ||
| Kim et al. (2005) [37] | 53 (29) | Overall | NR | 69 |
| RT (33) | 52 | 76 | ||
| CHOP, EPOCH, or COPBLAM-V + RT(22) | 38 | 59 | ||
| Li et al. (2006) [38] | 105 (105) | Overall stage I | 87 | 78 |
| Overall stage II | NR | 46 | ||
| RT (31) | 97 | 66 | ||
| RT followed by CT (34) | 71 | 77 | ||
| CT ± salvage RT (40) | 19 | 74 | ||
| Isobe et al. (2006) [39] | 35 (35) | Overall | 80 | 47 |
| RT (17) | NR | 44 | ||
| CMT(83% anthracycline-based regimen)(18) | NR | 52 | ||
| Huang et al. (2008) [25] | 82 (82) | Overall | 83 | 52 |
| RT (9) | 100 | 90 | ||
| CHOP(8) | 25 | NR | ||
| CMT (65), up-front RT (31), up-front CT (43) | NR | 48 | ||
| Kim et al. (2008) [22] | 280 (211) | Overall UAT | 60 | NR |
| Overall NUAT | 32 | NR | ||
| RT (17) or CT + RT (104) | NR | (90.3 month median) | ||
| CHOP, CEOP, or COPBLAM-V(144) | NR | (8.8 month median) | ||
| Li et al. (2008) [40] | 91 (71) | Overall, CMT (64), RT (13) or CT (14) monotherapy | 79 | 65 |
| Overall stage I | NR | 93 | ||
| Overall stage II | NR | 71 | ||
| Guo et al. (2008) [29] | 63 (57) | Overall | NR | 70 (2y) |
| CHOP (6) or CHOP + RT (53) | 49 | NR | ||
| RT +CHOP (4) | 100 | NR | ||
| Wang et al. (2008) [41] | 30 (28) | Overall (CHOP or CHOP + nitrosurea) | 33 | 69 (2y) |
| Overall P-gp positive | 20 | NR | ||
| Overall P-gp negative | 60 | NR | ||
Y, year; NR, not reported; CT, chemotherapy; RT, radiotherapy; CMT, combined modality therapy (chemoradiotherapy); CR, complete remission; DFS, disease-free survival; PFS, progression-free survival; OS, overall survival; UAT, upper aerodigestive tract; NUAT, non-upper aerodigestive tract; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CEOP, cyclophosphamide, epirubicin, vincristine, and prednisone; m-BACOD, mitoxantrone, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone; BACOP, bleomycin, doxorubicin, cyclophosphamide, vincristine, prednisolone; EPOCH, etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin; COPBLAM-V, cyclophosphamide, doxorubicin, vincristine, prednisolone, bleomycin, procarbazine; P-gp, p-glycoprotein.
Limited to series of at least 30 patients.
The largest series with radiation as a single modality treatment included 143 patients accrued over 20 years in which the majority had nasal-type, early-stage disease [36]. Of 143 patients, 104 received upfront radiation with a median dose of 50.4 Gy (range of 20–70 Gy). Sixty-nine percent of patients treated with radiotherapy alone achieved CR, while only 8% achieved a CR following chemotherapy administered prior to radiotherapy. Other smaller series have reported similar outcomes with CR rates between 52 and 100% [25,26,31,38–40]. Many of these support a potential OS benefit to radiation therapy alone compared to no treatment or chemotherapy alone with an absolute improvement in 5-year OS of up to 26% [25,26,31,37–40].
The benefit of radiation in early-stage disease is dose and field dependent. Influence of radiation dose on outcome has been examined with a significant benefit seen in patients receiving at least 54 Gy versus <54 Gy both in OS and DFS (5-year OS, 75.5% versus 46.1%, p = 0.019; 5-year DFS, 60.3% versus 33.4%, p = 0.004) [25]. This is largely due to superior locoregional control, 77% in patients receiving ≥50 Gy, compared to 33% in those treated with less than 50 Gy [39]. Systemic failure is seen in 25–30% of early-stage disease treated with radiotherapy, suggesting a role for chemotherapy added to radiation for control of clinically occult distant disease in high-risk, early-stage patients.
Chemotherapy alone
Few studies have included chemotherapy without radiation because of observations of early disease progression during chemotherapy treatment. A 10-fold worse OS has been reported for patients treated with chemotherapy alone compared to chemotherapy followed by radiation (median survival of 8.8 versus 90.3 months) [22]. Studies of chemotherapy regimens such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) without radiation are disappointing because of high rates of refractory or early relapsed disease with CR rates of less than 33%, and 2-year DFS and OS of 23 and 44%, respectively [43,44]. This has been the observation in patients treated with non-anthracycline containing regimens as well [45]. A mechanism postulated for the high failure rate and poor OS with chemotherapy alone is high P-glycoprotein (P-gp) expression in NK lymphoma cells compared to other lymphomas, which results in drug efflux and treatment resistance [46,47]. P-gp expression in ex-vivo and in-vitro studies is found on CD56+ human NK-cells, as well as in fresh tumor samples from patients with ENKL where expression P-gp/multidrug resistance (MDR) was detected at the initiation of therapy [46,47]. A recent series of 30 patients with early-stage ENKL receiving CHOP with or without a nitrosurea observed an overall CR of 33.3%. However, when stratified by P-gp expression, the CR rate was 60% among patients without expression of P-gp and 20% among patients with expression of P-gp [41]. Though several studies have confirmed a low response rate to chemotherapy associated with P-gp expression, a recent report describes expression of the short-length (rather than full-length) variant of P-gp in ENKL which does not confer capacity to efflux chemotherapies, including daunorubicin. The clinical impact of short-length variant P-gp in ENKL remains to be studied [41,48,49].
Combined modality therapy
The 5-year OS in early-stage disease with radiotherapy alone has ranged from 29 to >75% depending on the series, leading to continued debate regarding the benefit of adding chemotherapy to radiation (Table II). Combined modality therapy was anticipated to reduce distant failures and overall risk of relapse [50–52]. Promising results were reported in a series of 108 patients with early-stage ENKL receiving radiation followed by combination chemotherapy with cyclophosphamide, epirubicin, vincristine, and prednisone plus bleomycin. This regimen demonstrated significant efficacy with a 92% CR rate and 8-year OS of 86% [30]. Other series have conflicting results with significantly lower OS, 5-year OS of 28–49%, and a wide range of CR rates, 8–78% (Table II) [26,29,31,38,45]. A factor accounting for a portion of this variance is thought to be the sequence of combination therapy, with superior outcomes among studies with up-front radiation versus up-front chemotherapy. Collectively, these studies suggest that a subset of patients with high-risk features failed to achieve remission with up-front chemotherapy, translating to a lower OS as patients could not be salvaged with radiation or additional chemotherapy. This is supported by the report of Cheung et al. [21] in which only 21% of patients were successfully salvaged with radiation for relapsed disease during anthracycline-based chemotherapy.
Other smaller studies also support the importance of sequence in combined modality therapy. In a series of 63 patients with mostly early-stage disease receiving CHOP chemotherapy followed by 45 Gy of radiation, the CR rate was only 49.1%, compared to 100% in those who received radiation prior to CHOP [29]. In another report, only 6 of 17 patients completed radiation due to early progression during chemotherapy. In this study, patients who completed four cycles of CHOP and planned radiation had an OS rate of 100% compared to 27% among those who did not complete radiation [53]. The final results of JCOG0211, a phase I/II clinical trial in Japan, as recently reported, support the combined modality approach. Twenty-seven patients were enrolled and received 50 Gy of radiation during the first 6 weeks and reduced-dose DeVIC chemotherapy (carboplatin, etoposide, ifosfamide, and dexamethasone) [54,55]. The CR rate was 77% and overall response rate (ORR) 81%. Nine of 10 patients with recurrence following therapy failed at distant sites. In 2006, the International Lymphoma Project review of 136 cases, confirmed the benefit of radiation added to chemotherapy in early-stage disease with a statistically significant survival benefit [56]. Among 280 cases of nasal and extranasal ENKL with stage I disease, radiation-based, combination therapy improved median OS over fourfold (90.3 months versus 19.3 months, p = 0.045) and PFS almost eight-fold (66.0 versus 8.8 months) compared to chemotherapy alone [22].
Collectively, these studies demonstrate that if sequential, combined modality therapy is to be effective in early-stage disease, local control with radiation should precede systemic chemotherapy. Further, the addition of chemotherapy to radiation is likely only necessary in high-risk, early-stage disease. Prospective trials which stratify patients by low- and high-risk disease are required to determine the additional benefit of chemotherapy in low-risk disease.
Advanced-stage
There is a paucity of data to guide therapy in advanced-stage disease. In general, combined modality therapy is the most commonly employed approach for advanced-stage disease; however, encouraging results with regimens in early-stage disease have not translated to the advanced-stage setting. Further, due to the limited number and size of series which demonstrate a benefit with intensive therapy, it remains unclear whether the superior outcome is due, at least in part, to patient selection. Table III summarizes the experience with chemotherapy. In a small series of 24 patients treated with CHOP or bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone, and methotrexate (m-BA-COD) followed by radiation, 25% of patients with advanced-stage disease achieved a CR with OS of 2 months, compared to 75% with early-stage disease with OS of 12 months [57].
Table III.
Treatment and outcome for advanced-stage and relapsed or refractory extranodal NK/T-cell lymphoma.
| Author (year) | N (stage III/IV) | Treatment regimen (n) | Outcomes
|
|
|---|---|---|---|---|
| % CR | % 5y OS | |||
| Advanced-stage | ||||
| Kwong et al. (1997) [57] | 24 (4) | CT + RT, stage III/IV | 25 | (2-month median) |
| Yong et al. (2001) [43] | 37 (11) | Overall | NR | 43 (2y) |
| CHOP | 27 | NR | ||
| CHOP followed by CMT | 46 | NR | ||
| Kim et al. (2003) [44] | 59 (18) | CHOP or COPBLAM-V + salvage RT | 36 | 44 |
| Chim et al. (2004) [28] | 67 (11) | Overall | 64 | 37 (20y) |
| Up-front RT (7) | NR | 83 (20y) | ||
| Up-front anthracycline-based CT + RT(59) | NR | 32 (20y) | ||
| Li et al. (2004) [31] | 77 (21) | Overall | 43 | NR |
| RT (1) | 0 | 0 | ||
| CHOP-based regimen(10) | 60 | 30 | ||
| RT + CT (10) | 30 | 20 | ||
| Yong et al. (2006) [48] | 46 (19) | CHOP +RT | 65 | 65 |
| Lee et al. (2006) [45] | 26 (10) | IMEP (14) ± salvage RT (12), stage III/IV | 13 | 30 (3y) |
| Pagano et al. (2006) [58] | 26 (8) | CT (9), CT + RT (14), CT + surgery (2), surgery (1) | 23 | 18 |
| Aviles et al. (2007) [59] | 61 (61) | CMED +RT | 80 | 65 |
| Guo et al. (2008) [29] | 63 (6) | Overall stage I–IV | NR | 70 |
| Overall stage III/IV | NR | 50 | ||
| CHOP (6) or CHOP + RT (53) | 49 | NR | ||
| RT + CHOP (4) | 100 | NR | ||
| Li et al. (2008) [40] | 91 (20) | Overall stage I–IV | 79 | 65 |
| Overall stage III | NR | 36 | ||
| Overall stage IV | NR | 22 | ||
| RT (13) | 77 | NR | ||
| CT (14) | 30 | NR | ||
| CHOP or CHOP-bleomycin + RT (46) | 85 | NR | ||
| RT + CT (18) | 89 | NR | ||
| Relapsed or refractory disease | ||||
| Yong et al. (2003) [60] | 18 | L-asparaginase, vincristine, dexamethasone | 56 | 56 |
| Jaccard et al. (2008) [61] | 18 | L-asparaginase, methotrexate, and dexamethasone | 56 | 72 (8-month) |
| Yamaguchi et al. (2008) [62] | 6 | SMILE | 50 | NR |
Y, year; NR, not reported; CT, chemotherapy; RT, radiotherapy; CMT, combined modality therapy (chemoradiotherapy); CR, complete remission; OS, overall survival; SMILE, steroid dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CMED, cyclophosphamide, methotrexate, etoposide, and dexamethasone; COPBLAM-V, cyclophosphamide, doxorubicin, vincristine, prednisolone, bleomycin, procarbazine; IMEP, ifosfamide, methotrexate, etoposide, and prednisolone.
Efforts to develop effective regimens for advanced-stage disease have focused on overcoming the MDR-mediated chemotherapy resistance of ENKL with the use of P-gp substrates. Improved outcomes have been achieved with high-intensity chemotherapy combined with radiation. In a study of 32 patients with stage IV, nasal ENKL treated with six cycles of cyclophosphamide, methotrexate, etoposide, and dexamethasone (CMED, with growth factor support) administered every 14 days with radiation (55 Gy to the nasal cavity and nasopharynx) following the third cycle, 21 achieved a CR with a 5-year OS of 65% [63]. Similar efficacy of CMED was reported in another study of 61 patients (97% with stage IV disease), with a CR rate of 80%. At a median follow-up of 46 months, the failure-free survival and OS were 81% and 65%, respectively [59].
Another novel approach is the use of L-asparaginase. L-asparaginase in-vitro inhibits tumor cell growth by amino acid deprivation and subsequent inhibition of both protein synthesis as well as DNA and RNA synthesis. In-vitro, NK-cell tumors appear highly sensitive to L-asparaginase, as NK cells express low levels of asparagine synthase. As a result of dependence on exogenous asparagine relative to other cells, NK-cell tumors are selectively impacted by this therapy, which has efficacy even in the setting of high-level MDR expression [64]. Efficacy of L-asparaginase was first suggested in 2001 and again in 2003 in two case reports of relapsed ENKL following autologous stem cell transplantation who obtained CRs following 6000 μ/m2/day of L-asparaginase [65,66]. Subsequently, in a series of 15 patients with relapsed or refractory ENKL treated with L-asparaginase monotherapy, seven achieved a CR, with an ORR of 86.7% [67]. Combination chemotherapy with L-asparaginase, vincristine, and dexamethasone, followed by radiotherapy has been evaluated in patients with refractory disease [60]. Ten of 18 patients (55.6%) achieved a CR, and 5 additional patients achieved a PR with a 5-year OS of 55.6%. This series has been updated with 46 evaluable patients, confirming the initial efficacy data with 51.5% of patients achieving a CR with 5-year OS of 64.5% after failing CHOP chemotherapy and radiation, to which only 28.3% achieved a CR [48]. In yet another series, 18 patients with relapsed or refractory disease received up to six cycles of L-asparaginase-based combination chemotherapy followed by consolidative stem cell transplant. The ORR was 94.4% with over half, 55.6%, obtaining a CR and 72.2% of patients alive at a median follow-up of 8 months [61].
These concepts have now been integrated into a novel regimen, steroids, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE), which was designed to incorporate L-asparaginase with chemotherapeutic agents known to be independent of MDR. Etoposide was incorporated for its activity in EBV-associated diseases including hemophagocytic syndrome and lymphoproliferative disorders. In a phase I study, six patients were enrolled with stage IV, relapsed, or refractory disease [62]. The first three patients experienced dose-limiting toxicity and one died of sepsis with grade 4 neutropenia, leading to a protocol modification and inclusion of granulocyte colony-stimulating factor (G-CSF). Following this modification, no significant infectious complications occurred. With six patients treated, the reported ORR following two cycles of SMILE was 67% with 50% achieving CR. Three patients received additional SMILE chemotherapy followed by autologous hematopoietic stem cell transplantation. A phase II study of SMILE with G-CSF began in 2007 and is currently ongoing in Asia. These results need to be confirmed in other populations and, if promising, need evaluation in an upfront setting as well.
Hematopoietic cell transplantation
Autologous and allogeneic hematopoietic cell transplantation (HCT) have been evaluated for consolidation, as well as for relapsed or refractory disease. For patients who achieve a CR, autologous HCT may prolong DFS as a consolidative approach, however, only limited studies have directly addressed superiority over conventional therapy. In a case series of 18 consecutive patients with primarily early-stage disease, seven remained in CR following consolidative autologous HCT with follow-up over 5 years (Table IV). Patients in first CR with early-stage nasal disease receiving autologous HCT were compared to historical controls with similar stage and disease status. A non-statistically significant trend toward improved survival was observed for patients with early-stage disease in first CR undergoing consolidative, autologous transplantation [69]. This has now been confirmed in two retrospective reviews including 38 patients, of whom 25 were in CR at time of consolidative HCT [71,72]. The 4-year OS for 22 patients in first CR at time of HCT was 68%, demonstrating a significant benefit compared to 188 historical controls with 4-year OS of 21% [72]. In the second series, nine patients transplanted in first CR were compared to 246 historical controls and demonstrated a statistically significant benefit from autologous HCT [71].
Table IV.
Treatment and outcome for extranodal NK/T-cell lymphoma with hematopoietic cell transplantation.
| Author (year) | N | Transplant regimen (n) | Patient characteristics at transplant (n) | Outcomes
|
|
|---|---|---|---|---|---|
| % CR | % OS (y) | ||||
| Takenaka et al. (2001) [68] | 3 | Auto (2), Allo (1) | SD or PD (3) | 67 | 67 (5y) |
| Au et al. (2003) [69] | 18 | Auto (18) | CR1 (7), CR2 (5) | 58 | NR |
| Murashige (2005) [70] | 28 | Allo (28) | 22 extranodal, CR (8), SD or PD (20) | NR | 40 (2y) |
| Kim et al. (2006) [71] | 16 | Auto (16) | CR1 or CR2 (9), SD or PD (7) | 75 | 71 (2y) |
| Suzuki et al. (2006) [72] | 40 | Auto (25), Allo (15) | 22 extranodal, SD or PD (18) | NR | 39 (4y) |
| CR (22) | NR | 68 (4y) | |||
Y, year; Auto, autologous; Allo, allogeneic; CR, complete remission; SD, stable disease; PD, progression of disease; OS, overall survival; NR, not reported.
Because of inadequate number of cases reporting outcomes following HCT as salvage therapy, the ability to define its role as therapy for relapsed or refractory disease remains limited. In a series of seven patients with relapsed or refractory disease undergoing autologous HCT, four achieved a CR and median OS was 9 months [71]. The efficacy of this high-dose myeloablative approach either with autologous or allogeneic HCT is limited by a 15% treatment-related mortality and, with 4-year follow-up, OS of only 24% (similar to 21% OS of 188 historical controls) [72]. Since 1996, a total of 43 cases have been reported with salvage HCT in patients with relapsed ENKL, 17 (39.5%) of whom achieved CRs following allogeneic HCT [70,73–77]. In 2000, a successful double-autologous HCT for a patient with advanced-stage, refractory ENKL was reported [78]. Following a pre-transplant conditioning regimen of high-dose ranimustine, carboplatin, etoposide, and cyclophosphamide followed by high-dose ifosfamide, etoposide, and carboplatin, the patient achieved a CR and remained free of disease after 36 months of follow-up. Despite limited sample size, comparison to historical controls, and potential bias due to patient selection, these studies argue for consideration of autologous HCT for patients in first or second CR, and acknowledging less evidence, for consideration of allogeneic HCT for patients with relapsed or refractory disease. If possible, HCT should be considered in the context of a clinical trial.
Novel approaches under consideration
Uniform expression of surface CD56, neural cell adhesion molecule (NCAM), by NK-cell tumors provides a potential targeted approach. IMGN901 is an immunoconjugate of a cytotoxic derivative of DM1 (N-deacetyl-N-3-mercapto-1-oxopropyl-maytansine, which inhibits tubule polymerization leading to cell death) conjugated to the antibody, huN901, that binds with high affinity to CD56 [79]. IMGN901 must be internalized after binding to CD56 in order to release DM1. Co-culture of IMGN901 with an NK-cell line results in cell growth inhibition and direct cytotoxicity. A limitation is the lack of specificity to malignant NK-cells as expression of CD56 is seen on benign NK-cells, a subset of T-cells, and within the brain, cerebellum, and at neuromuscular junctions. IMGN901 is currently in human phase I/II clinical trials for ENKL [79].
Other novel therapies have focused on epigenetic modulation of gene expression within the tumor. Preclinical studies of ENKL tumors have observed high levels of aberrant methylation of promoter CpG regions leading to tumor suppressor gene inactivation [80,81]. In 33 patient samples, p73 was methylated in 94% of cases. Treatment of an in-vitro ENKL cell line with 5-azacytidine, a hypomethylating agent, resulted in demethylation and reinduction of p73 gene expression. Combination therapy trials with hypomethylating agents and histone deacetylase inhibitors which are currently underway in T cell lymphomas are awaited.
Another promising strategy is targeting of EBV, given its role in oncogenesis, in ENKL [82]. An early phase trial of adoptive transfer of EBV-specific cytotoxic T lymphocytes in seven patients has recently been reported [83]. Three patients with nasal ENKL received either transferred autologous EBV-specific, cytotoxic T lymphocytes (CTL) or allogeneic EBV-specific CTLs from an HLA-matched, sibling. One patient achieved a CR, one maintained stable disease, and one died of disease progression after 15 months. Peripheral blood samples assayed during and following CTL infusions confirmed increased EBV-specific lymphocyte populations. A second adoptive transfer strategy used gene transfer to enhance the expression and immunogenicity of LMP2, an EBV-specific peptide, on the surface of antigen presenting cells (APC) [84]. Autologous CTLs were infused into four patients with nasal ENKL as active therapy in one patient and maintenance in three patients following stimulation and expansion in culture with the modified APCs. As active therapy, CTL infusion resulted in a five-fold expansion of LMP2-specific lymphocytes for greater than 3 months and a corresponding fall in the EBV viral load occurred. After 9 months the patient relapsed and subsequently died at 18 months. All three patients who received maintenance autologous lymphocyte infusions remained in remission for 2–6 months, and post-infusion peripheral blood studies confirmed a five-fold increase in LMP2-specific CTLs.
Conclusion
Though considerable advances have been made in our understanding of NK-cell biology, malignant transformation including the role of EBV, and prognosis, the rare nature of extranodal NK/T-cell lymphoma and its heterogeneity limit the ability to standardize therapy. Without randomized, controlled trials, treatment recommendations are based on review of case series, with their inherent limitations. When possible, every effort should be made to enroll patients in clinical trials and national registries. International, multicenter clinical trials for patients with extranodal NK/T-cell lymphoma are desperately needed to improve outcomes for this rare and aggressive entity.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Liang X, Graham DK. Natural killer cell neoplasms. Cancer. 2008;112:1425–1436. doi: 10.1002/cncr.23316. [DOI] [PubMed] [Google Scholar]
- 2.Chan JKC, Quintanilla-Martinez L, Ferry JA, et al. Extranodal N K/T-cell lymphoma, nasalt type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon (France): IARC Press; 2008. pp. 285–288. [Google Scholar]
- 3.Ferry JA. Extranodal lymphoma. Arch Pathol Lab Med. 2008;132:565–578. doi: 10.5858/2008-132-565-EL. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz EJ, Molina-Kirsch H, Zhao S, et al. Immunohistochemical characterization of nasal-type extranodal NK/T-cell lymphoma using a tissue microarray: an analysis of 84 cases. Am J Clin Pathol. 2008;130:343–351. doi: 10.1309/V561QTM6854W4WAV. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Suh C, Huh J, et al. Effect of positive bone marrow EBV in situ hybridization in staging and survival of localized extranodal natural killer/T-cell lymphoma, nasal-type. Clin Cancer Res. 2007;13:3250–3254. doi: 10.1158/1078-0432.CCR-06-2373. [DOI] [PubMed] [Google Scholar]
- 6.Chiang AK, Wong KY, Liang AC, et al. Comparative analysis of Epstein–Barr virus gene polymorphisms in nasal T/NK-cell lymphomas and normal nasal tissues: implications on virus strain selection in malignancy. Int J Cancer. 1999;80:356–364. doi: 10.1002/(sici)1097-0215(19990129)80:3<356::aid-ijc4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Nagamine M, Takahara M, Kishibe K, et al. Sequence variations of Epstein-Barr virus LMP1 gene in nasal NK/T-cell lymphoma. Virus Genes. 2007;34:47–54. doi: 10.1007/s11262-006-0008-5. [DOI] [PubMed] [Google Scholar]
- 8.Suzumiya J, Ohshima K, Takeshita M, et al. Nasal lymphomas in Japan: a high prevalence of Epstein–Barr virus type A and deletion within the latent membrane protein gene. Leuk Lymphoma. 1999;35:567–578. doi: 10.1080/10428199909169621. [DOI] [PubMed] [Google Scholar]
- 9.Isobe Y, Sugimoto K, Yang L, et al. Epstein–Barr virus infection of human natural killer cell lines and peripheral blood natural killer cells. Cancer Res. 2004;64:2167–2174. doi: 10.1158/0008-5472.can-03-1562. [DOI] [PubMed] [Google Scholar]
- 10.Au WY, Pang A, Choy C, et al. Quantification of circulating Epstein–Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. 2004;104:243–249. doi: 10.1182/blood-2003-12-4197. [DOI] [PubMed] [Google Scholar]
- 11.Wong KF, Zhang YM, Chan JK. Cytogenetic abnormalities in natural killer cell lymphoma/leukaemia – is there a consistent pattern? Leuk Lymphoma. 1999;34:241–250. doi: 10.3109/10428199909050949. [DOI] [PubMed] [Google Scholar]
- 12.Sakajiri S, Kawamata N, Egashira M, et al. Molecular analysis of tumor suppressor genes, Rb, p53, p16INK4A, p15INK4B and p14ARF in natural killer cell neoplasms. Jpn J Cancer Res. 2001;92:1048–1056. doi: 10.1111/j.1349-7006.2001.tb01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siu LL, Chan JK, Wong KF, et al. Aberrant promoter CpG methylation as a molecular marker for disease monitoring in natural killer cell lymphomas. Br J Haematol. 2003;122:70–77. doi: 10.1046/j.1365-2141.2003.04396.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki R, Nakamura S, Suzumiya J, et al. Blastic natural killer cell lymphoma/leukemia (CD56-positive blastic tumor): prognostication and categorization according to anatomic sites of involvement. Cancer. 2005;104:1022–1031. doi: 10.1002/cncr.21268. [DOI] [PubMed] [Google Scholar]
- 15.Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal NK/T-cell lymphoma: a study of 136 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 16.Oshimi K, Kawa K, Nakamura S, et al. NK-cell neoplasms in Japan. Hematology. 2005;10:237–245. doi: 10.1080/10245330400026162. [DOI] [PubMed] [Google Scholar]
- 17.Oshimi K. Progress in understanding and managing natural killer-cell malignancies. Br J Haematol. 2007;139:532–544. doi: 10.1111/j.1365-2141.2007.06835.x. [DOI] [PubMed] [Google Scholar]
- 18.Kako S, Izutsu K, Ota Y, et al. FDG-PET in T-cell and NK-cell neoplasms. Ann Oncol. 2007;18:1685–1690. doi: 10.1093/annonc/mdm265. [DOI] [PubMed] [Google Scholar]
- 19.Khong PL, Pang CB, Liang R, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87:613–621. doi: 10.1007/s00277-008-0494-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Park YH, Kim WS, et al. Extranodal nasal type NK/T-cell lymphoma: elucidating clinical prognostic factors for risk-based stratification of therapy. Eur J Cancer. 2005;41:1402–1408. doi: 10.1016/j.ejca.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Cheung MM, Chan JK, Lau WH, et al. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys. 2002;54:182–190. doi: 10.1016/s0360-3016(02)02916-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim TM, Lee SY, Jeon YK, et al. Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol. 2008;19:1477–1484. doi: 10.1093/annonc/mdn147. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Kim WS, Park YH, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226–1230. doi: 10.1038/sj.bjc.6602502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612–618. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 25.Huang M-J, Jiang Y, Liu W-P, et al. Early or up-front radiotherapy Improved survival of localized extranodal NK/T-Cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70:166–174. doi: 10.1016/j.ijrobp.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 26.You JY, Chi KH, Yang MH, et al. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: a single institute survey in Taiwan. Ann Oncol. 2004;15:618–625. doi: 10.1093/annonc/mdh143. [DOI] [PubMed] [Google Scholar]
- 27.Kim TM, Park YH, Lee SY, et al. Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage I(E)/II(E) extranodal NK/T-cell lymphoma, nasal type. Blood. 2005;106:3785–3790. doi: 10.1182/blood-2005-05-2056. [DOI] [PubMed] [Google Scholar]
- 28.Chim CS, Ma SY, Au WY, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004;103:216–221. doi: 10.1182/blood-2003-05-1401. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, Lu JJ, Ma X, et al. Combined chemoradiation for the management of nasal natural killer (NK)/T-cell lymphoma: elucidating the significance of systemic chemotherapy. Oral Oncol. 2008;44:23–30. doi: 10.1016/j.oraloncology.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Aviles A, Diaz NR, Neri N, et al. Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol. 2000;22:215–220. doi: 10.1046/j.1365-2257.2000.00307.x. [DOI] [PubMed] [Google Scholar]
- 31.Li CC, Tien HF, Tang JL, et al. Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer. 2004;100:366–375. doi: 10.1002/cncr.11908. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Kim KH, Chang MH, et al. Whole blood Epstein–Barr virus DNA load as a diagnostic and prognostic surrogate: extranodal natural killer/T-cell lymphoma. Leuk Lymphoma. 2009;50:757–763. doi: 10.1080/10428190902803669. [DOI] [PubMed] [Google Scholar]
- 33.Jung CK, Lee KY, Kim Y, et al. Epstein–Barr virus infection, drug resistance and prognosis in Korean T- and NK-cell lymphomas. Pathol Int. 2001;51:355–363. doi: 10.1046/j.1440-1827.2001.01214.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin CW, Chen YH, Chuang YC, et al. CD94 transcripts imply a better prognosis in nasal-type extranodal NK/T-cell lymphoma. Blood. 2003;102:2623–2631. doi: 10.1182/blood-2003-01-0295. [DOI] [PubMed] [Google Scholar]
- 35.Xiang-Lan M, Zu-Lan S, Dan H, et al. Skp2/p27 expression profile is correlated with Epstein–Barr virus status in extranodal nasal-type natural killer cell lymphoma. Transl Res. 2008;151:303–308. doi: 10.1016/j.trsl.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Kim GE, Lee SW, Chang SK, et al. Combined chemotherapy and radiation versus radiation alone in the management of localized angiocentric lymphoma of the head and neck. Radiother Oncol. 2001;61:261–269. doi: 10.1016/s0167-8140(01)00428-5. [DOI] [PubMed] [Google Scholar]
- 37.Kim K, Chie EK, Kim CW, et al. Treatment outcome of angiocentric T-cell and NK/T-cell lymphoma, nasal type: radiotherapy versus chemoradiotherapy. Jpn J Clin Oncol. 2005;35:1–5. doi: 10.1093/jjco/hyi006. [DOI] [PubMed] [Google Scholar]
- 38.Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–189. doi: 10.1200/JCO.2005.03.2573. [DOI] [PubMed] [Google Scholar]
- 39.Isobe K, Uno T, Tamaru J, et al. Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer. 2006;106:609–615. doi: 10.1002/cncr.21656. [DOI] [PubMed] [Google Scholar]
- 40.Li YX, Fang H, Liu QF, et al. Clinical features and treatment outcome of nasal-type NK/T cell lymphoma of Waldeyer’s ring. Blood. 2008;112:3057–3064. doi: 10.1182/blood-2008-05-160176. [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Li XQ, Ma X, et al. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol. 2008;83:795–799. doi: 10.1002/ajh.21256. [DOI] [PubMed] [Google Scholar]
- 42.Sakata K, Hareyama M, Ohuchi A, et al. Treatment of lethal midline granuloma type nasal T-cell lymphoma. Acta Oncol. 1997;36:307–311. doi: 10.3109/02841869709001268. [DOI] [PubMed] [Google Scholar]
- 43.Yong W, Zheng W, Zhang Y. Clinical characteristics and treatment of midline nasal and nasal type NK/T cell lymphoma. Zhonghua Yi Xue Za Zhi. 2001;81:773–775. [PubMed] [Google Scholar]
- 44.Kim BS, Kim TY, Kim CW, et al. Therapeutic outcome of extranodal NK/T-cell lymphoma initially treated with chemotherapy – result of chemotherapy in NK/T-cell lymphoma. Acta Oncol. 2003;42:779–783. doi: 10.1080/02841860310010682. [DOI] [PubMed] [Google Scholar]
- 45.Lee KW, Yun T, Kim DW, et al. First-line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/− radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leuk Lymphoma. 2006;47:1274–1282. doi: 10.1080/10428190600562823. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes [see comments] Blood. 1992;80:2735–2739. [PubMed] [Google Scholar]
- 47.Yamaguchi M, Kita Kenkichi, Miwa Hiroshi, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76:2351–2356. doi: 10.1002/1097-0142(19951201)76:11<2351::aid-cncr2820761125>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Yong W, Zheng W, Zhu J, et al. Midline NK/T-cell lymphoma nasal-type: treatment outcome, the effect of L-asparaginase based regimen, and prognostic factors. Hematol Oncol. 2006;24:28–32. doi: 10.1002/hon.765. [DOI] [PubMed] [Google Scholar]
- 49.Kim GE, Yang WI, Lee SW, et al. Lack of correlation between P-glycoprotein and chemotherapy resistance in nasal NK/T-cell lymphomas. Leuk Lymphoma. 2004;45:1857–1864. doi: 10.1080/10428190410001693524. [DOI] [PubMed] [Google Scholar]
- 50.Liang R, Todd D, Chan TK, et al. Treatment outcome and prognostic factors for primary nasal lymphoma. J Clin Oncol. 1995;13:666–670. doi: 10.1200/JCO.1995.13.3.666. [DOI] [PubMed] [Google Scholar]
- 51.Li YX, Coucke PA, Li JY, et al. Primary non-Hodgkin’s lymphoma of the nasal cavity: prognostic significance of paranasal extension and the role of radiotherapy and chemotherapy. Cancer. 1998;83:449–456. doi: 10.1002/(sici)1097-0142(19980801)83:3<449::aid-cncr13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 52.Kim GE, Cho JH, Yang WI, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol. 2000;18:54–63. doi: 10.1200/JCO.2000.18.1.54. [DOI] [PubMed] [Google Scholar]
- 53.Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001;12:349–352. doi: 10.1023/a:1011144911781. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi M, Oguchi M, Tobinai K, et al. Phase I/II study of concurrent chemoradiotherapy for newly-diagnosed, localized nasal NK/T-cell lymphoma: results of a phase I portion of JCOG0211-DI. Blood (Suppl) 2005;106:2685. (abstract) [Google Scholar]
- 55.Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal NK/T-cell lymphoma: final results of JCOG0211. J Clin Oncol (Meeting Abstracts) 2009;27:8549. doi: 10.1200/JCO.2009.23.8295. (abstract) [DOI] [PubMed] [Google Scholar]
- 56.Au W, Intragumtornchai T, Nakamura S, et al. Clinical and pathological differences between nasal and nasal-type NK/T cell lymphomas: a summary of 136 cases from the International T Cell Lymphoma (ITCL) project. Blood (Suppl) 2006;108:292. (abstract) [Google Scholar]
- 57.Kwong YL, Chan AC, Liang R, et al. CD56+ NK lymphomas: clinicopathological features and prognosis. Br J Haematol. 1997;97:821–829. doi: 10.1046/j.1365-2141.1997.1462962.x. [DOI] [PubMed] [Google Scholar]
- 58.Pagano L, Gallamini A, Trape G, et al. NK/T-cell lymphomas ‘nasal type’: an Italian multicentric retrospective survey. Ann Oncol. 2006;17:794–800. doi: 10.1093/annonc/mdl015. [DOI] [PubMed] [Google Scholar]
- 59.Aviles A, Cleto S, Castaneda C, et al. CMED in the treatment of nasal natural killer cell lymphoma with distant metastases. Hematology. 2007;12:241–244. doi: 10.1080/10245330701214327. [DOI] [PubMed] [Google Scholar]
- 60.Yong W, Zheng W, Zhang Y, et al. L-asparaginase-based regimen in the treatment of refractory midline nasal/nasal-type T/NK-cell lymphoma. Int J Hematol. 2003;78:163–167. doi: 10.1007/BF02983387. [DOI] [PubMed] [Google Scholar]
- 61.Jaccard A, Gachard N, Coppo P, et al. A prospective phase II trial of an L-asparaginase containing regimen in patients with refractory or relapsing extra nodal NK/T-cell lymphoma. Blood (Suppl) 2008;112:579. (abstract) [Google Scholar]
- 62.Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. 2008;99:1016–1020. doi: 10.1111/j.1349-7006.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aviles A, Neri N, Fernandez R, et al. Nasal NK/T-cell lymphoma with disseminated disease treated with aggressive combined therapy. Med Oncol. 2003;20:13–17. doi: 10.1385/MO:20:1:13. [DOI] [PubMed] [Google Scholar]
- 64.Ando M, Sugimoto K, Kitoh T, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005;130:860–868. doi: 10.1111/j.1365-2141.2005.05694.x. [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto Y, Nomura K, Kanda-Akano Y, et al. Successful treatment with Erwinia L-asparaginase for recurrent natural killer/T cell lymphoma. Leuk Lymphoma. 2003;44:879–882. doi: 10.1080/1042819031000067873. [DOI] [PubMed] [Google Scholar]
- 66.Nagafuji K, Fujisaki T, Arima F, et al. L-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol. 2001;74:447–450. doi: 10.1007/BF02982090. [DOI] [PubMed] [Google Scholar]
- 67.Jaccard A, Petit B, Girault S, et al. L-Asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol. 2009;20:110–116. doi: 10.1093/annonc/mdn542. [DOI] [PubMed] [Google Scholar]
- 68.Takenaka K, Shinagawa K, Maeda Y, et al. High-dose chemotherapy with hematopoietic stem cell transplantation is effective for nasal and nasal-type CD56+ natural killer cell lymphomas. Leuk Lymphoma. 2001;42:1297–1303. doi: 10.1080/10428190127500. [DOI] [PubMed] [Google Scholar]
- 69.Au WY, Lie AK, Liang R, et al. Autologous stem cell transplantation for nasal NK/T-cell lymphoma: a progress report on its value. Ann Oncol. 2003;14:1673–1676. doi: 10.1093/annonc/mdg458. [DOI] [PubMed] [Google Scholar]
- 70.Murashige N, Kami M, Kishi Y, et al. Allogeneic haematopoietic stem cell transplantation as a promising treatment for natural killer-cell neoplasms. Br J Haematol. 2005;130:561–567. doi: 10.1111/j.1365-2141.2005.05651.x. [DOI] [PubMed] [Google Scholar]
- 71.Kim HJ, Bang SM, Lee J, et al. High-dose chemotherapy with autologous stem cell transplantation in extranodal NK/T-cell lymphoma: a retrospective comparison with non-transplantation cases. Bone Marrow Transplant. 2006;37:819–824. doi: 10.1038/sj.bmt.1705349. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki R, Suzumiya J, Nakamura S, et al. Hematopoietic stem cell transplantation for natural killer-cell lineage neoplasms. Bone Marrow Transplant. 2006;37:425–431. doi: 10.1038/sj.bmt.1705244. [DOI] [PubMed] [Google Scholar]
- 73.Okamura T, Hatsukawa Y, Arai H, et al. Blood stem-cell transplantation for chronic active Epstein–Barr virus with lymphoproliferation. Lancet. 2000;356:223–224. doi: 10.1016/S0140-6736(00)02488-0. [DOI] [PubMed] [Google Scholar]
- 74.Teshima T, Miyaji R, Fukuda M, et al. Bone-marrow transplantation for Epstein–Barr-virus-associated natural killer cell-large granular lymphocyte leukaemia. Lancet. 1996;347:1124. doi: 10.1016/s0140-6736(96)90325-6. [DOI] [PubMed] [Google Scholar]
- 75.Kawa K, Okamura T, Yasui M, et al. Allogeneic hematopoietic stem cell transplantation for Epstein–Barr virus-associated T/NK-cell lymphoproliferative disease. Crit Rev Oncol Hematol. 2002;44:251–257. doi: 10.1016/s1040-8428(02)00116-6. [DOI] [PubMed] [Google Scholar]
- 76.Okamura T, Kishimoto T, Inoue M, et al. Unrelated bone marrow transplantation for Epstein–Barr virus-associated T/NK-cell lymphoproliferative disease. Bone Marrow Transplant. 2003;31:105–111. doi: 10.1038/sj.bmt.1703796. [DOI] [PubMed] [Google Scholar]
- 77.Sato E, Ohga S, Kuroda H, et al. Allogeneic hematopoietic stem cell transplantation for Epstein–Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol. 2008;83:721–727. doi: 10.1002/ajh.21247. [DOI] [PubMed] [Google Scholar]
- 78.Sasaki M, Matsue K, Takeuchi M, et al. Successful treatment of disseminated nasal NK/T-cell lymphoma using double autologous peripheral blood stem cell transplantation. Int J Hematol. 2000;71:75–78. [PubMed] [Google Scholar]
- 79.Ishitsuka K, Jimi S, Goldmacher VS, et al. Targeting CD56 by the maytansinoid immunoconjugate IMGN901 (huN901-DM1): a potential therapeutic modality implication against natural killer/T cell malignancy. Br J Haematol. 2008;141:129–131. doi: 10.1111/j.1365-2141.2008.07000.x. [DOI] [PubMed] [Google Scholar]
- 80.Fu L, Gao Z, Zhang X, et al. Frequent concomitant epigenetic silencing of the stress-responsive tumor suppressor gene CADM1, and its interacting partner DAL-1 in nasal NK/T-cell lymphoma. Int J Cancer. 2009;124:1572–1578. doi: 10.1002/ijc.24123. [DOI] [PubMed] [Google Scholar]
- 81.Siu LL, Chan JK, Wong KF, et al. Specific patterns of gene methylation in natural killer cell lymphomas: p73 is consistently involved. Am J Pathol. 2002;160:59–66. doi: 10.1016/s0002-9440(10)64349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merlo A, Turrini R, Dolcetti R, et al. Adoptive cell therapy against EBV-related malignancies: a survey of clinical results. Expert Opin Biol Ther. 2008;8:1265–1294. doi: 10.1517/14712598.8.9.1265. [DOI] [PubMed] [Google Scholar]
- 83.Cho HI, Hong YS, Lee MA, et al. Adoptive transfer of Epstein–Barr virus-specific cytotoxic T-lymphocytes for the treatment of angiocentric lymphomas. Int J Hematol. 2006;83:66–73. doi: 10.1532/IJH97.A30505. [DOI] [PubMed] [Google Scholar]
- 84.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]