Abstract
Conventional CD4+ T cells are composed of naïve, pathogen-specific memory, and pathogen-independent memory-phenotype (MP) cells under steady state. Naïve and pathogen-specific memory cells play key roles in adaptive immunity, whereas the homeostatic mechanisms regulating the generation of MP cells and their biological functions are unclear. Here we show that MP cells are autonomously generated from peripheral naïve cells in the absence of infectious stimulation in a TCR- and CD28-dependent manner. We further demonstrate that MP cells contain a T-bethi subpopulation that is continuously generated by environmental IL-12 and rapidly produces IFN-γ in response to IL-12 in the absence of pathogen recognition. Importantly, these cells can provide nonspecific host resistance against Toxoplasma gondii infection while enhancing the adaptive CD4+ T cell responses. Together, these findings reveal that MP cells are continuously generated from naïve precursors and possess a previously undescribed innate immune function by which they produce an early, Th1-like protective response against pathogens.
Introduction
CD4+ T cells are essential for useful adaptive immune responses. Under steady state, conventional CD4+ T lymphocytes are comprised of naïve, pathogen-specific "authentic" memory, and pathogen-independent memory-phenotype (MP) cells (1). CD44lo CD62Lhi naïve and CD44hi CD62Llo pathogen-specific memory cells play critical roles in protection against primary and secondary infection, respectively (2). Naïve αβ CD4+ T cells, which are continuously generated in the thymus and are maintained by self antigens and γc cytokines in the periphery, react with cognate foreign antigens presented by MHC class II or CD1, robustly proliferate to become effector cells, and play a major role in host resistance to infection. After pathogen clearance, most effector cells die leaving a small residual population of antigen-specific memory cells that can more effectively respond to a subsequent infection. CD44hi CD62Llo cells are generally considered to represent foreign antigen-specific memory cells, but this concept has been called into question because some CD44hi CD62Llo cells rapidly divide in uninfected conditions whereas pathogen-specific memory cells only slowly proliferate (3), suggesting that "non-classical" memory cells exist in this population. This newly recognized, pathogen-independent set of memory cells has been called "MP" cells. Since MP cells can arise in germ-free (GF) and antigen-free (AF) mice (4, 5) as well as specific pathogen-free (SPF) animals, it is likely that MP cells develop in the absence of foreign antigen recognition and have distinct functions that are independent of overt antigenic stimulation. Indeed, there is a dearth of knowledge concerning the mechanisms by which MP cells are generated and maintained in steady state as well as their precise role in host defense.
MP CD4+ T cells are generated very efficiently from naïve cells under lymphopenic conditions such as those found in T cell-depleted, irradiated, and neonatal animals (6, 7). In these situations, MP cells arise as a subpopulation undergoing rapid homeostatic proliferation by a process that depends upon T cell receptor (TCR), costimulatory, and cytokine signaling (7–11). However, a recent report suggested that MP cell generation cannot be fully accounted for by lymphopenia-induced proliferation since the MP cells arising in adult mice have a large TCR repertoire while the MP cells generated by fast homeostatic proliferation in lymphopenic animals display limited TCR diversity (3). Therefore, it is possible that MP cells are a sum of those generated through both the lymphopenic neonatal and lymphoreplete adult periods.
The immunological function of MP CD4+ T cells primarily generated by rapid homeostatic proliferation is poorly understood. Although the classical view of the immune system divides effector responses into non-lymphoid innate and lymphoid adaptive (foreign antigen-specific) responses, there is increasing evidence that innate-like lymphocytes that are not triggered by cognate antigen recognition serve as an interface between these two types of responses. NK cells are an early-recognized member of this set of immune cells and more recently various other types of innate lymphoid cell (ILC) subpopulations have been described (12). In addition, it is also recognized that antigen-specific lymphocytes can mediate effector function based on cytokine stimulation in the absence of overt TCR engagement, a functionality that has been best examined for CD8+ T cells that adopt a memory-like state upon expansion (13, 14). However, little is known about whether CD4+ T lymphocytes, especially the MP cell subset, have this innate-like function, contributing in a foreign antigen-independent manner to host defense.
In the present study, we have analyzed the mechanisms by which MP CD4+ T cells are generated and maintained in the periphery of lymphoreplete adult as well as lymphopenic neonatal mice and asked whether these lymphocytes contain a subpopulation that has innate immunological function.
Results
MP cells are generated from naïve cells in the periphery
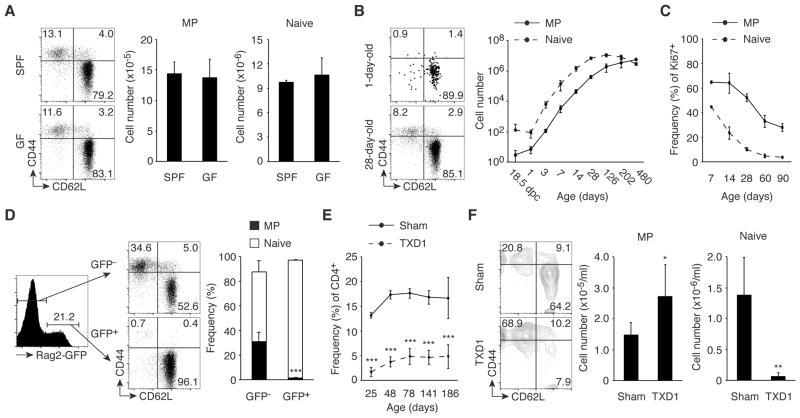
MP cells are qualitatively distinct from conventional antigen-specific memory cells (3). To see if the commensal microbiota is essential for MP cell generation, we first compared levels of CD44hi CD62Llo Foxp3− MP CD4+ T cells in SPF versus GF mice. Splenic MP cell numbers were indistinguishable in the two types of animals (Fig. 1A), suggesting that recognition of commensal antigens is dispensable for MP development. To study the kinetics of MP cell development, we quantitated MP and naïve CD4+ T cell numbers in SPF mice from 1 day prior to birth through 1 year of adult life. Both cell populations increased with age in the spleen (Fig. 1B) and lymph nodes (LNs; fig. S1A) at early times after birth, with MP cells continuing to grow in number after the levels of naïve cells reached a plateau. This difference was also reflected in the persistence of rapidly dividing Ki67+ MP cells after the naïve cell population had reached quiescence (Fig. 1C and fig. S1B).
Fig. 1. MP cells are generated from naïve cells in the periphery.
(A) SPF and GF mice have an equal number of MP cells. Splenic CD4+ T lymphocytes from 8-week-old wild-type mice housed in SPF or GF conditions were analyzed for expression of CD44 and CD62L. The representative dot plots indicate the frequency of MP and naïve cells among CD4+ T cells. The bar graphs indicate the number (mean ± SD) of MP and naïve CD4+ T cells from each group (n=3–4). Data are representative of 2 independent experiments. (B and C) Kinetics of splenic MP and naïve CD4+ T cell development and their proliferative capacity. At the indicated ages, (B) the absolute cell number (mean ± SD) of MP and naïve CD4+ T lymphocytes and (C) the frequency (mean ± SD) of Ki67+ cells among MP and naïve cells were monitored in cohort of wild-type mice (n=2–4 and 2–8 per time point in B and C, respectively). The representative dot plots show the frequency of MP and naïve cells among CD4+ T cells of 1-day-old and 28-day-old mice. Data shown are pooled from at least 2 independent experiments. (D) MP CD4+ T cells in Rag2-GFP reporter mice are GFP-. The histogram shows the GFP+ fraction among splenic CD4+ T cells in Rag2-GFP reporter mice, while the dot plots and the bar graph indicate the frequency of MP and naïve cells among GFP- and GFP+ CD4+ T cells (n=5). Data are representative of 2 independent experiments. (E and F) A normal-sized MP cell population is generated from peripheral CD4+ T cells in the absence of the thymus. (E) The graph shows the frequency (mean ± SD) of CD4+ T cells in the blood from sham-operated and TXD1 mice (n=3–17 per time point) over a 6-month period. (F) Numbers in the representative plots indicate the frequency of MP and naïve cells among peripheral blood CD4+ T lymphocytes on day 141. The bar graphs show the number (mean ± SD) of MP and naïve CD4+ T cells from each group (n=5–6). Data shown are pooled from at least 2 independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
Because conventional CD4+ T cells newly generated in the thymus are naïve, MP cells are likely to be generated from naïve cells in the periphery. On the other hand, it has been reported that MP cells can arise directly from the thymus (15). To examine where MP CD4+ T cells are generated, we used Rag2-GFP reporter mice, in which GFP marks recent thymic emigrants (RTEs) in the periphery (16, 17). When MP and naïve cell populations were examined in GFP+ and GFP− peripheral CD4+ T cells, all GFP+ cells were naïve while about 30% of GFP− cells had a MP (Fig. 1D), suggesting that rapidly proliferating MP cells derive from naïve RTEs. To further test if the continuous accumulation of peripheral MP cells requires the thymus, we performed thymectomy on 1 day following birth (TXD1) and followed the frequency of CD4+ T cells in the periphery for 6 months. In these TXD1 mice, all of the CD4+ T cells generated should theoretically derive from the peripheral CD4+ T cells pre-existing in 1-day-old neonates, which we found to consist of ~100 naïve CD4+ T lymphocytes with virtually no MP cells (Fig. 1B, day 1). In our analysis, we observed that with age CD4+ T cells from TXD1 mice reached a plateau of 5% among the total lymphocytes versus those from sham-operated mice, which increased to 15 – 20% (Fig. 1E). When the phenotype of these CD4+ T cells was determined at 141 days, TXD1 and sham-operated mice had an equivalent number of MP cells, while the TXD1 mice had almost no naïve cells (Fig. 1F). The above experiments argue that a substantial number of MP cells are generated from peripheral naïve cells in a thymus-independent fashion.
MP conversion of naïve cells occurs throughout life and requires TCR and CD28 signaling
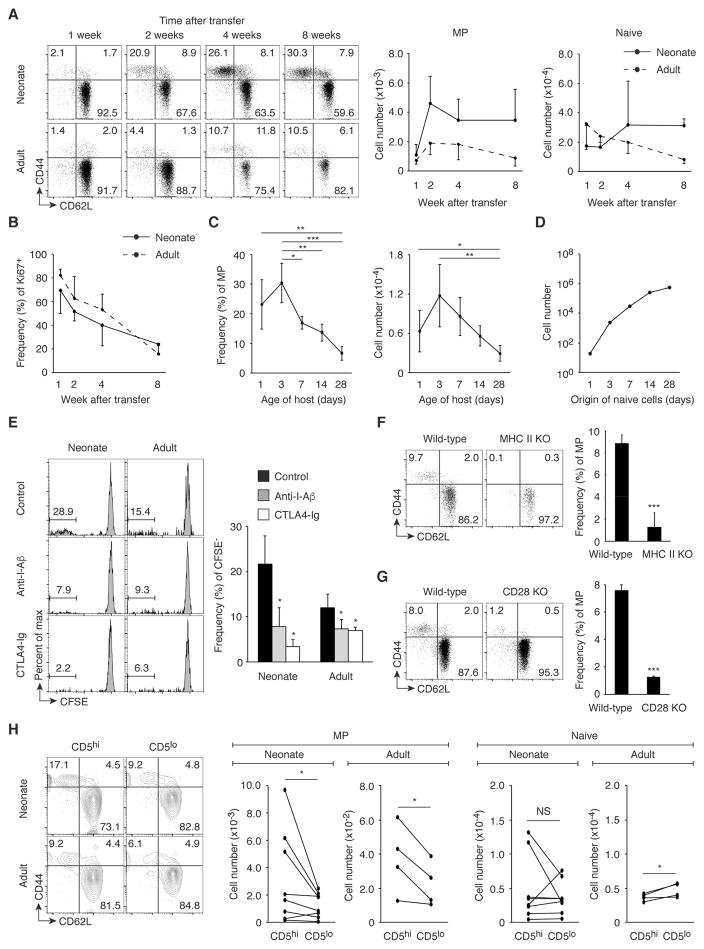
While the lymphopenic neonatal environment is important for MP cell generation in the periphery (7), our data indicated that MP cells continue to increase in number in the lymphoreplete adult environment as well (Fig. 1B). Indeed, it has been reported that lymphopenia-induced proliferation cannot fully explain the generation of MP cells seen in adult mice since adult MP cells have a large TCR diversity in contrast to MP cells generated by lymphopenia-induced proliferation that have a limited repertoire (3). These observations suggest that MP cells may be generated from peripheral naïve cells not only in lymphopenic but also in lymphoreplete conditions. To examine this issue, we transferred naïve CD4+ T cells from CD45.2 wild-type mice into CD45.1/2 neonatal and adult recipients and monitored the Foxp3− donor cells for up to 8 weeks after transfer. Importantly, MP cells were generated in the spleen (Fig. 2A) and, to a lesser extent, LNs (fig. S1C) of adult as well as neonatal mice. Most newly generated MP cells were Ki67+ and this fraction decreased with time after transfer (Fig. 2B and fig. S1D). These findings demonstrate that naïve cells convert to rapidly proliferating MP cells in adult lymphoreplete as well as neonatal lymphopenic environments.
Fig. 2. MP cell conversion occurs in both neonates and adults dependently of TCR and CD28 signaling.
(A and B) Naïve CD4+ T lymphocytes give rise to MP cells in neonatal and adult mice. Sorted naïve CD4+ T cells from CD45.2 wild-type mice were transferred into 1-day-old neonatal or 8-week-old adult CD45.1/2 animals, and donor cells were analyzed in the spleen 1, 2, 4, and 8 weeks later. Representative dot plots show CD44 and CD62L expression by the donor cells and the graphs show (A) the number (mean ± SD) of MP and naïve donor cells and (B) the frequency (mean ± SD) of Ki67+ cells among MP donor cells (n=3–8). Data shown are pooled from 2 independent experiments. (C) The MP conversion rate correlates with age of host animals. Sorted naïve CD4+ T cells from CD45.2 mice were transferred into 1, 3, 7, 14, 28-day-old CD45.1/2 mice and the donor cells were analyzed for CD44 and CD62L expression 6 weeks later. The graphs show the frequency and the number (mean ± SD) of MP cells among the donor cells at each time point (n=3–7). Data shown are pooled from 3 independent experiments. (D) The MP conversion rate decreases, but the absolute number of generated MP cells increases with age. The number of MP cells that naïve CD4+ T cells from 1, 3, 7, 14, 28-day-old mice can generate 6 weeks later was calculated based on the absolute number of naïve CD4+ T lymphocytes obtained in Fig. 1B and the MP conversion rate obtained in Fig. 2C. (E) Fast proliferation of naïve CD4+ T lymphocytes requires TCR and CD28 signaling in neonates and adults. Sorted, CFSE-labeled naïve CD4+ T cells from CD45.2 wild-type mice were transferred into 1-day-old neonatal or 8-week-old adult CD45.1/2 recipients that were treated with anti-I-Aβ, CTLA4-Ig, or control IgG and were analyzed 3 weeks later. Representative histograms indicate CFSE dilution of the donor cells in the spleen, while the bar graph shows the frequency (mean ± SD) of CFSE− cells among donor cells from each group (n=2–3). (F) MP cell generation requires host MHC II expression. CD45.1 naïve CD4+ T cells were transferred into CD45.2 wild-type or MHC II KO mice and were analyzed 3 weeks later. Representative dot plots display CD44 and CD62L expression of donor cells, while the graph shows the frequency (mean ± SD) of MP population among donor cells (n=3). (G) MP cell generation depends upon CD28 signaling. The dot plots show expression of CD44 and CD62L by CD4+ T cells from wild-type and CD28 KO mice while the bar graph shows the frequency (mean ± SD) of MP cell population among CD4+ T cells (n=3). (H) CD5hi naive cells can generate more MP cells than CD5lo cells. Sorted CD5hi and CD5lo naïve CD4+ T cells from CD45.2 and CD45.1 mice, respectively, were transferred at a 1:1 ratio into 1-day- and 8-week-old CD45.1/2 recipients and were analyzed 3 weeks later. Representative plots indicate CD44 and CD62L expression by the donor cells in the spleen while the graphs show the number of MP and naïve cells generated from paired CD5hi and CD5lo naïve donor cells in an individual host mouse (n=4–8). Data are pooled from 2 independent experiments. * p<0.05, ** p<0.01, *** p<0.001, NS not significant.
To examine in greater depth the relationship between the MP conversion rate and host age, we transferred naïve CD4+ T cells into differently aged animals and examined the donor cell population 6 weeks later. As expected, MP conversion was observed even when naïve cells were transferred into 28-day-old lymphoreplete mice although the MP conversion rate decreased with the age of the recipients (Fig. 2C). To calculate how many MP cells can be generated from endogenous naïve cells 6 weeks later, we multiplied the total number of naive cells at different ages (obtained in Fig. 1B) by the MP conversion rate (obtained in Fig. 2C) at the same point. Naïve cells in 28-day-old animals generated many more MP cells than did those from neonates (Fig. 2D). Therefore, in terms of the absolute number, MP cells are continuously generated from naïve cells during both neonatal and adult life although the conversion rate decreases with age.
Under lymphopenic conditions, the fast proliferation that generates MP cells depends upon TCR and CD28 signals (7). To determine if this is also the case in lymphoreplete conditions, we labeled naïve CD4+ T cells with CFSE, transferred them into neonatal and adult recipients that received anti-I-Aβ monoclonal antibody (mAb), CTLA4-Ig, or control IgG, and analyzed the donor cells 3 weeks later. As expected, fast proliferating, CFSE− donor cells acquired a memory phenotype, whereas slowly proliferating, CFSE+ cells retained their naïve status (fig. S2). Blockade of I-Aβ or CD80/86 resulted in a significant reduction in the fraction of fast proliferating, CFSE− cells in both neonates and adults (Fig. 2E). To examine if MP conversion requires MHC II expression on host cells, naïve CD4+ T cells were transferred into MHC II knockout (KO) mice lacking I-Aα, I-Aβ, I-Eα, and I-Eβ (18). Three weeks later, few MP cells were detected in the MHC II KO recipients (Fig. 2F). To further determine if MP conversion depends upon CD28 expression, MP CD4+ T cells were compared in wild-type and CD28 KO mice. The frequency of MP cells was significantly lower in the KO animals (Fig. 2G). Thus, TCR and CD28 signaling appear to be essential for MP cell conversion in both lymphoreplete and lymphopenic conditions.
Recognition of self antigens plays a key role in T cell homeostasis (19). To seek the relationship between affinity of TCR to self antigens and MP conversion, we analyzed the influence of CD5 expression in naïve cells. This cell surface marker reflects the affinity of TCR for self antigens (20). Sorted CD5hi (upper 20%) and CD5lo (lower 20%) naïve CD4+ T cells from CD45.2 and CD45.1 mice, respectively, were mixed at a ratio of 1:1 and transferred into CD45.1/2 neonates and adults. When analyzed 3 weeks later, CD5hi naïve donor cells gave rise to more MP cells than did their CD5lo counterparts in both neonates and adults (Fig. 2H), suggesting that naïve cells with higher affinity to self antigens can generate more MP cells. One possible interpretation of this result is that CD5hi naïve cells react with foreign antigens to convert to MP cells given that affinity to self predicts foreign antigen reactivity (20). To examine the role of commensal antigens in MP generation, we transferred naïve CD4+ T cells into neonatal and adult mice treated with antibiotics using a regimen previously shown to dramatically deplete commensal bacteria (9). When analyzed 3 weeks following cell transfer, MP conversion was not significantly reduced in antibiotic-treated mice in donor as well as recipient cell populations (fig. S3, A and B). Therefore, these results indicate that there is a positive correlation between affinity of naïve cells to self antigens and MP productivity, and also suggest that commensal antigens are dispensable for this process.
The proliferation rate of MP cells correlates with TCR affinity to self antigens and is determined by CD28 signaling
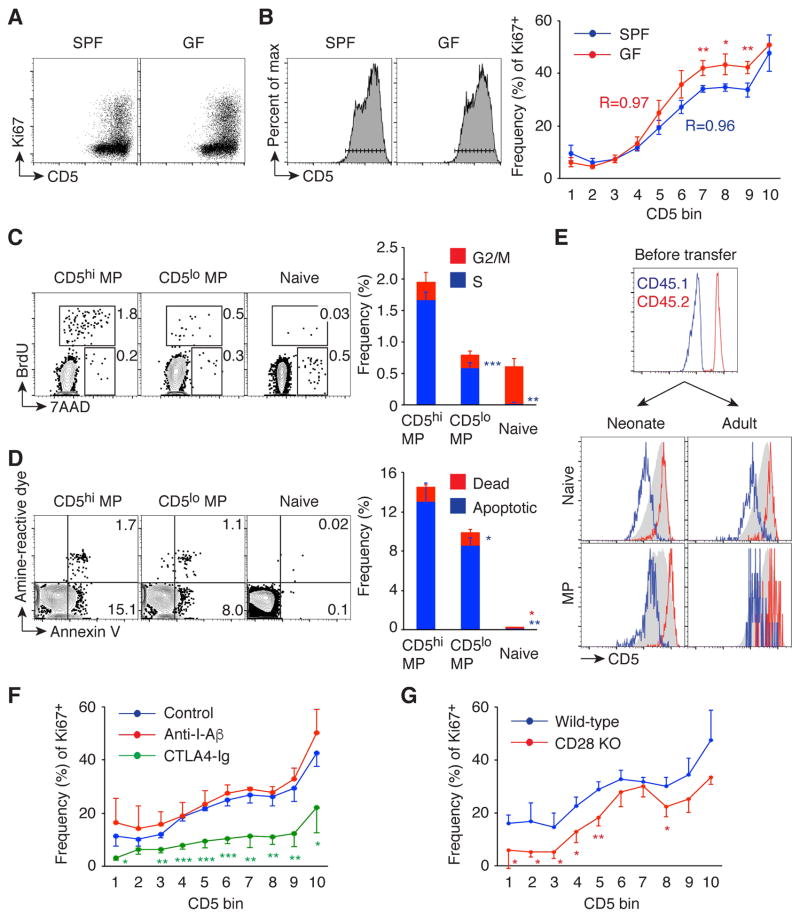
Since the affinity of TCR on naïve CD4+ T cells for available peptide-MHC (pMHC) ligands determined the MP conversion rate (Fig. 2H), we sought to examine the relationship between CD5 expression levels on MP cells and their proliferation rate. For this purpose, we measured Ki67 expression by CD5lo and CD5hi MP cells in SPF and GF mice (Fig. 3A). Interestingly, when CD5 expression was divided into 10 small bins and the frequency of Ki67+ cells in each bin was plotted, there was a clear positive correlation between CD5 and Ki67 expression (Fig. 3B). In addition, cell cycle analysis showed that CD5hi (top 20%) MP cells had a 2~3 fold greater fraction of cells in S phase as compared to their CD5lo (bottom 20%) counterparts (Fig. 3C), and Annexin V staining revealed that CD5hi cells had ~1.5 fold greater fraction of early apoptotic cells as compared to CD5lo cells (Fig. 3D), indicating that MP cells with higher CD5 expression are more rapidly proliferating and more rapidly dying than those with lower expression of this marker.
Fig. 3. MP cells consist of rapidly proliferating CD5hi and relatively quiescent CD5lo cells, both of which require CD28 signaling for Ki67 expression.
(A and B) MP cells are composed of proliferating CD5hi and relatively quiescent CD5lo cells in both SPF and GF mice. (A) Representative dot plots of CD5 and Ki67 expression on splenic MP cells are shown. (B) Histograms of CD5 expression by MP cells used to define 10 bins. The graph depicts the frequency (mean ± SD) of Ki67+ fraction among MP cells of each CD5 bin (n=3–4). Data shown are representative of 2 independent experiments. (C and D) CD5hi MP cells die and proliferate more rapidly than CD5lo MP cells. (C) Splenic CD5hi and CD5lo MP and naive cells were analyzed for BrdU incorporation and 7AAD staining 1 hr after in vivo BrdU pulse of wild-type mice. The numbers in the dot plots show the frequency of BrdU+ (S phase) and BrdU− 7AAD+ (G2/M phase) cells among each CD4+ T cell subpopulation, while the bar graph indicates the frequency (mean ± SD) of cells in S and G2/M phase (n=3). (D) Splenic MP and naïve cells were stained with amine-reactive dye and Annexin V. Dot plots indicate the frequency of amine-reactive dye+ Annexin V+ (dead) and amine-reactive dye− Annexin V+ (early apoptotic) cells among each CD4+ T cell subpopulation, while the bar graph shows the frequency (mean ± SD) of dead and early apoptotic cells (n=3). (E) CD5 expression is maintained in MP conversion. CD5lo and CD5hi naïve CD4+ T cells from CD45.1 (blue line) and CD45.2 (red line) mice, respectively, were mixed at a 1:1 ratio and transferred into 1-day-old neonatal and 8-week-old adult CD45.1/2 mice, and 3 weeks later their CD5 expression was reanalyzed. Representative histograms show CD5 expression by naïve and MP donor cells and were overlaid with respective host populations shown in gray. Data shown are representative of 4–8 animals. (F) Short-term MP cell proliferation requires CD28 but not TCR signaling. Wild-type mice were injected ip with anti-I-Aβ, CTLA4-Ig, or control IgG on day 0 and 2, and splenic MP cells were analyzed on day 4. The graph indicates the frequency (mean ± SD) of Ki67+ cells among MP cells in each CD5 bin (n=3–4). Data shown are representative of 2 independent experiments. (G) CD28 is required for optimal Ki67 expression on MP cells. The graph shows the frequency (mean ± SD) of Ki67+ population among MP cells from wild-type and CD28 KO mice (n=3). * p<0.05, ** p<0.01, *** p<0.001.
CD5 expression is actively maintained by continuous ligation to self pMHC (21). Indeed, in vitro TCR stimulation of peripheral CD4+ T cells upregulates its expression levels (22). To examine if CD5 expression is altered when CD5hi / CD5lo MP cells are generated from naïve precursors, CD5lo and CD5hi naïve cells were sorted from CD45.1 and CD45.2 mice, respectively, mixed at a ratio of 1:1, and transferred into CD45.1/2 neonates and adults. The relative CD5 expression level was unchanged in both naïve and newly generated MP cells when examined 3 weeks later (Fig. 3E). To further determine whether quiescent CD5lo and rapidly dividing CD5hi MP cells maintain their CD5 level and proliferating status, sorted CD45.1 CD5lo and CD45.2 CD5hi MP cells were mixed at a 1:1 ratio and transferred into CD45.1/2 adult mice. When assayed 1 and 4 weeks later, CD45.2 donor cells maintained high CD5 expression level and their rapid proliferation status while CD45.1 cells exhibited low CD5 expression and remained quiescent (fig. S4). These findings suggest that CD5hi and CD5lo MP cells derive from CD5hi and CD5lo naïve precursors, respectively, in the long term, although it is still possible that CD5 expression is fluctuating upon pMHC recognition in the short term.
IL-7 signaling plays a role in MP cell division in the periphery (3). To look for additional signals that regulate the proliferation rate of MP cells as reflected by Ki67 expression, we injected normal mice with anti-I-Aβ mAb, CTLA4-Ig, or control IgG on day 0 and 2 and assayed Ki67+ MP cells on day 4. CTLA4-Ig but not anti-I-Aβ mAb treatment significantly decreased Ki67 expression in MP cells (Fig. 3F). Similarly, CD28 KO mice displayed a reduced frequency of Ki67+ MP cells compared to wild-type animals (Fig. 3G), indicating a critical role for CD28 signaling in determining optimal MP cell proliferation. To examine the role of TCR signaling in MP cell proliferation in more detail, we injected mice with cyclosporin A (CsA) which inhibits TCR signaling by binding to calcineurin (23) or with control vehicle every day and then analyzed the frequency of Ki67+ cells at day 4. There was only a minimal difference in Ki67+ frequency between the CsA-treated and control groups (fig. S5A). To examine whether Ki67 expression by MP cells depends on TCR signaling in the long term, sorted MP cells were transferred into wild-type or MHC II KO mice. When measured 2 weeks later, the frequency of Ki67+ cells among the donor cells was not significantly different between the two groups although there was a small difference in total donor-derived cell number (fig. S5B). Therefore, MP cell proliferation is less dependent on TCR signaling but highly dependent upon CD28 signaling, particularly over the short term. This contrasts with the role of MHC II in initiating the generation of MP cells from naïve precursors.
MP cells contain a T-bethi subpopulation that requires IL-12 for its expression
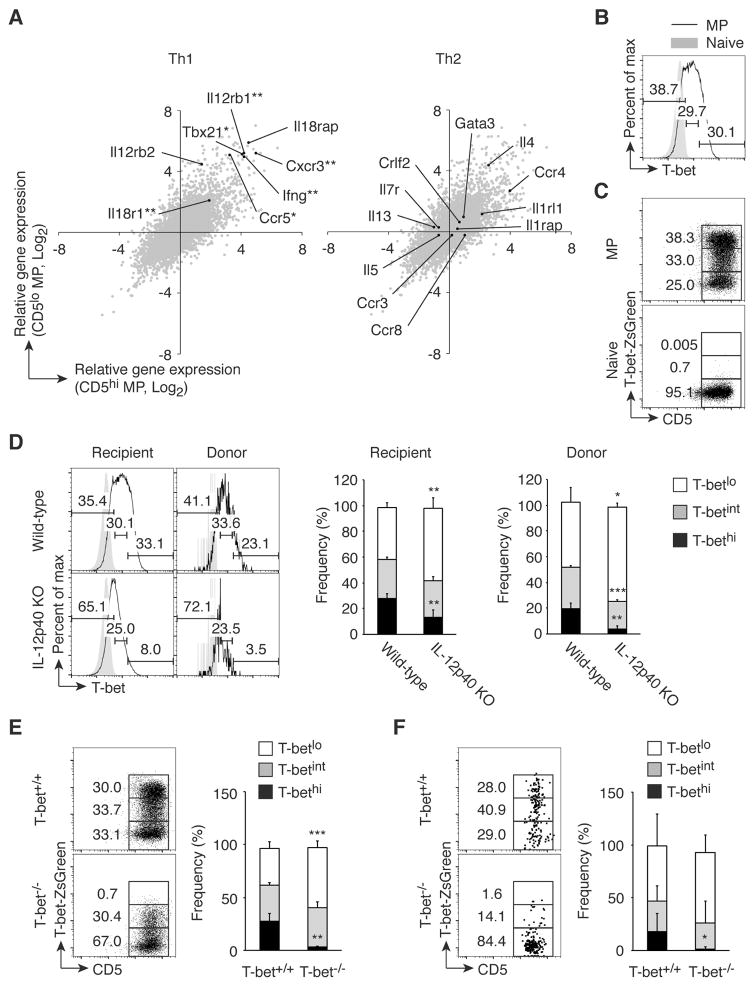
To gain insight into the function of MP cells, we compared gene expression profiles in unstimulated MP versus naïve cells by RNAseq. Interestingly, genes related to Th1 but not Th2 differentiation, such as Tbx21, Il12rb1, and Ifng, were significantly enriched in both CD5hi and CD5lo MP subpopulations compared to naïve cells (Fig. 4A). Consistent with these gene expression profiles, splenic MP but not naïve cells contained T-bethi and T-betint in addition to T-betlo subsets as detected by both intracellular staining and the use of T-bet-ZsGreen reporter mice (Fig. 4, B and C, respectively).
Fig. 4. MP cells contain T-bethi subpopulation, which is induced by IL-12.
(A) Genes associated with Th1 but not Th2 differentiation are highly expressed in MP cells. Gene expression by CD5hi and CD5lo MP and naïve CD4+ T lymphocytes was analyzed using RNAseq. Each gray dot shows the average of relative gene expression (log2) by CD5hi (x-axis) and CD5lo MP (y-axis) cells compared to naïve cell population, while black dots indicate genes associated with Th1- or Th2-type immune responses (n=3). (B and C) MP CD4+ T cells express T-bet. T-bet expression in MP and naïve cells was analyzed by (B) intracellular staining of wild-type CD4+ T lymphocytes and (C) detection of ZsGreen reporter expression in CD4+ T cells. Data shown are representative of 3 independent experiments. (D) Generation of T-bethi MP cells requires IL-12p40. FACS-sorted naïve CD4+ T cells from CD45.1 mice were transferred into CD45.2 wild-type or IL-12p40 KO animals and T-bet expression in MP cells from donor and recipient cells was analyzed 3 weeks later. Representative histograms of T-betlo, T-betint, and T-bethi subpopulations among MP cells from each group and bar graphs showing the corresponding frequency (mean ± SD) are depicted (n=3–5). Data shown are representative of 2 independent experiments. (E and F) Efficient transcription of T-bet requires T-bet expression. (E) ZsGreen expression in MP CD4+ T lymphocytes was analyzed in T-bet-ZsGreen reporter mice that were either T-bet+/+ or T-bet−/−. (F) Sorted naïve CD4+ T cells from CD45.2 T-bet+/+ or T-bet−/− T-bet reporter mice were transferred into CD45.1 wild-type mice and T-bet expression by the MP donor cells was analyzed 3 weeks later. The representative dot plots and the graph show the frequency (mean ± SD) of T-betlo, T-betint, and T-bethi cells among MP cells from each group (n=5). Data shown are representative of 2 independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
To investigate the mechanisms responsible for T-bethi cell generation, we examined the role of IL-12, which plays a critical role in Th1 immunity (24). Sorted naïve CD4+ T cells from CD45.1 mice were transferred into wild-type or IL-12p40 KO CD45.2 adult mice and T-bet expression by the MP cell populations from both donor and recipient cells examined 3 weeks later. The T-bethi cell population was significantly reduced in both the donor and recipient MP cells present in IL-12p40 KO versus wild-type mice (Fig. 4D), indicating that T-bethi cell generation is dependent upon IL-12. Also, T-bet expression is known to be augmented by a positive feedback loop (25), suggesting that efficient transcription of T-bet might require pre-existing T-bet protein. To test this possibility, T-bet+/+ and T-bet−/− mice carrying T-bet-ZsGreen reporter which reads out T-bet transcription were analyzed for T-bet-ZsGreen expression by MP cells. In the T-bet−/− mice, the T-bethi cell population (assessed by reporter expression) was specifically lost, whereas the T-betint cells remained intact (Fig. 4E). Furthermore, when sorted naïve CD4+ T cells from T-bet+/+ or T-bet−/− reporter mice were transferred into CD45.1 adult mice, T-bethi MP cells were detected 3 weeks later in the recipients of T-bet+/+ but not T-bet−/− donor cells (Fig. 4F). Taken together, these results imply that IL-12 plays a critical role in the generation of T-bethi MP cells from naïve cells by facilitating the positive feedback loop of T-bet expression.
MP cells rapidly produce IFN-γ in response to Toxoplasma infection in the absence of cognate pathogen recognition
The above results (Fig. 4) raised the possibility that T-bet+ MP cells might play a role in Th1-type immunity. The intracellular protozoan parasite, Toxoplasma gondii, is well known to trigger Th1 responses in peritoneal cavity (PC) and spleen (26, 27). We used this model to ask whether MP cells present in the same tissue sites might provide protection against pathogen challenge. In initial experiments, we detected substantial numbers of T-bet+ MP cells in the PC of naïve mice and these cells in common with the T-bet+ MP cells present in spleen required both IL-12 and an initial low level of T-bet induction for their generation (fig. S6, A and B).
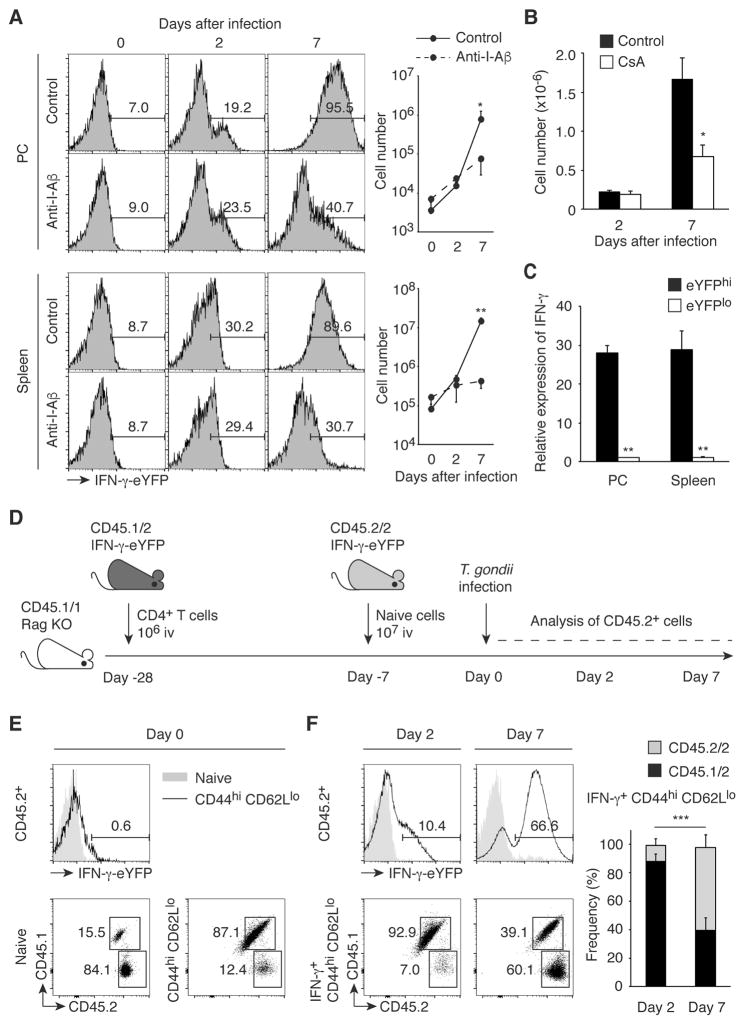
We then infected IFN-γ-eYFP reporter mice (28) with tissue cysts of T. gondii and monitored the kinetics of IFN-γ production by CD44hi CD62Llo CD25lo CD4+ cells that hypothetically could include antigen-specific effector CD4+ T cells as well as pathogen-independent MP. To exclude the possible contribution by NK and NKT cells, we gated on the NK1.1− CD3hi cell population. Interestingly, we observed IFN-γ production by MP cells as early as 2 days after T. gondii infection. Pretreatment of the animals with anti-I-Aβ failed to block this response, suggesting that it occurred independently of conventional MHC II-TCR interaction (Fig. 5A). As an internal positive control, we confirmed that this anti-I-Aβ mAb significantly inhibited IFN-γ production by CD44hi CD62Llo cells at 7 days when the adaptive antigen-specific CD4+ T cell response is fully developed in the same animals. To further test whether IFN-γ production by MP cells at day 2 requires TCR signaling, we treated IFN-γ-eYFP reporter mice with CsA and then infected them with T. gondii. As expected, CsA treatment substantially inhibited the development of IFN-γ+ effector cells at day 7 while failing to reduce the number of IFN-γ+ MP cells present at day 2 (Fig. 5B). To confirm that eYFP faithfully reports IFN-γ expression in IFN-γ-eYFP reporter mice, we sorted on the eYFPhi (upper 20%) and eYFPlo (lower 20%) populations present at day 2 post infection and measured their IFN-γ mRNA levels by qPCR. As shown in Fig. 5C, IFN-γ mRNA expression was far greater in the eYFPhi than in the eYFPlo cells. Taken together, these experiments reveal that during early T. gondii infection MP cells produce IFN-γ without a requirement for explicit TCR stimulation.
Fig. 5. MP cells, but not pathogen-elicited effector CD4+ T lymphocytes, rapidly produce IFN-γ during T. gondii infection independently of pathogen antigens.
(A to C) MP cells rapidly produce IFN-γ during T. gondii infection in the absence of pathogen recognition. (A) IFN-γ-eYFP reporter mice treated with anti-I-Aβ mAb or control IgG were infected with T. gondii and CD44hi CD62Llo CD25lo CD4+ T cells from PC and spleen were analyzed at the indicated days. The numbers in the representative histograms indicate the frequency of IFN-γ-eYFP+ cells among each group. The graphs show the number (mean ± SD) of IFN-γ-YFP+ cells from each group (n=2–3). Data shown are representative of 2 independent experiments. (B) T. gondii-infected IFN-γ-eYFP reporter mice were either treated with or without CsA and splenic CD44hi CD62Llo CD25lo CD4+ T cells were analyzed for IFN-γ-eYFP expression on day 2 and 7. The graph shows the number (mean ± SD) of IFN-γ+ cells (n=2–3). (C) IFN-γ mRNA expression in sorted eYFPhi and eYFPlo MP cells obtained from PC and spleen was measured by qPCR. The graph shows its expression (mean ± SD) relative to β-actin (n=3). (D to F) Existing MP cells are the major source of IFN-γ on day 2 after T. gondii infection. (D) Experimental design. CD45.1/1 Rag KO mice, which received first 1x106 total CD4+ T cells from CD45.1/2 IFN-γ-eYFP reporter mice (Day -28) and 3 weeks later 107 naïve CD4+ T cells from CD45.2/2 IFN-γ-eYFP reporter mice (Day -7), were infected with T. gondii (Day 0), and CD45.2+ donor cells including both CD45.1/2 and CD45.2/2 populations were analyzed on day 0, 2, and 7. (E) The histogram shows IFN-γ-eYFP expression by naïve and MP CD45.2+ CD4+ T cells, while the numbers in the representative dot plots show the frequency of CD45.1/2 and CD45.2/2 cells among naïve and MP CD45.2+ CD4+ T lymphocytes on day 0 (n=6). (F) IFN-γ-eYFP expression by naïve and MP CD45.2+ CD4+ T cells is shown in the histograms, while the dot plots show the frequency of CD45.1/2 and CD45.2/2 donor cell populations among IFN-γ+ CD44hi CD62Llo CD25lo CD45.2+ CD4+ T cells in the spleen on day 2 and 7. The graph shows the frequency (mean ± SD) of CD45.1/2 and CD45.2/2 donor cells among each group (n=4). Data shown are pooled from 4 independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
To further examine whether the IFN-γ+ MP cells detected at day 2 post infection are distinct from pathogen-elicited effector cells, we asked whether they derive from a naïve T cell or pre-existing MP cell population. To do so, we performed an experiment in which CD45.1/1 Rag KO mice were sequentially transferred with CD45.1/2 total CD4+ T cells (at day -28) and purified CD45.2/2 naïve cells (at day -7) from IFN-γ-eYFP reporter mice (Fig. 5D). When analyzed at day 0, the MP and naïve populations were dominated by CD45.1/2 and CD45.2/2 populations, respectively (Fig. 5E), presumably because the first donor cells occupying the MP cell niche via robust lymphopenia-induced proliferation inhibit MP conversion of the second donor cohort (29). These mice were then infected with T. gondii and the ratio of CD45.1/2 and CD45.2/2 populations among IFN-γ+ CD44hi CD62Llo cells examined at day 2 and 7 after infection. We found that most of the IFN-γ+ population present at day 2 was CD45.1/2, while at day 7 the CD45.2/2 cells predominated instead (Fig. 5F). These findings argue that the IFN-γ+ cells detected at day 2 post T. gondii infection derive from pre-existing MP cells while those present at day 7 are pathogen-induced effector cells that have differentiated from naïve T cells.
Innate-like lymphocytes are able to promptly deliver effector functions upon activation by receptor ligand or cytokine signaling (12). To examine the relative contribution of NK, NKT, γδT, and MP CD4+ and CD8+ T cells to early IFN-γ production in T. gondii infection, the fraction and the number of IFN-γ+ cells in each cell subset was determined on day 0 and 2 post infection. IFN-γ+ MP CD4+ T cells constituted the first and second largest populations of responding cells in spleen and PC, respectively (fig. S7). Thus, MP CD4+ T cells in collaboration with other innate-like lymphocytes contribute to early IFN-γ production during Toxoplasma infection.
IL-12 drives IFN-γ production by T-bethi MP cells
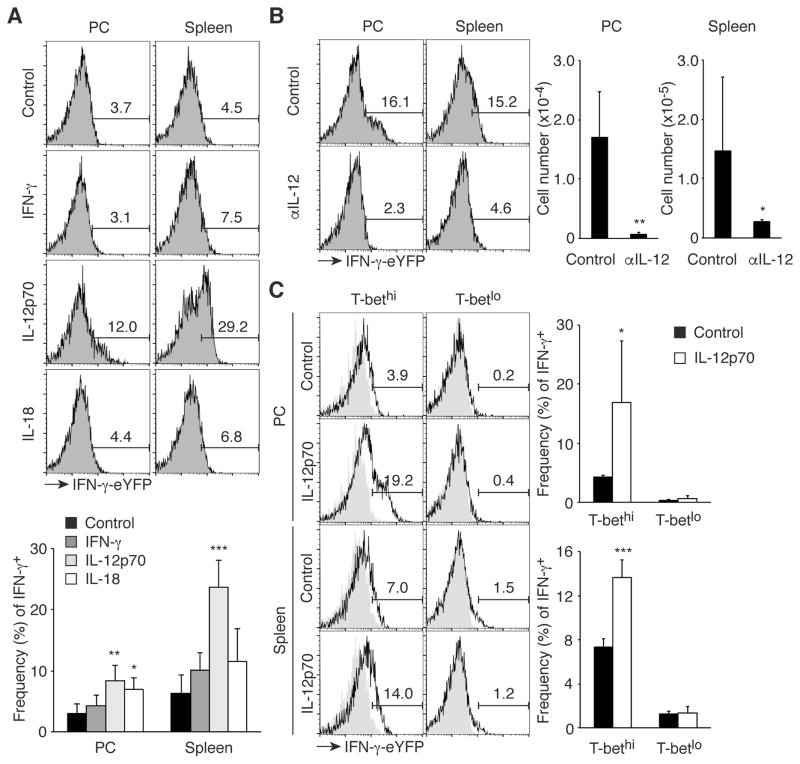
Some memory T cells produce effector cytokines in a cytokine-driven manner (30). To determine the mechanism underlying IFN-γ production by MP cells, we administered cytokines promoting Th1 differentiation, including IFN-γ, IL-12p70, and IL-18 to IFN-γ-eYFP reporter mice on two consecutive days and measured IFN-γ expression by peritoneal and splenic MP cells 24 hours after the second injection. Interestingly, IL-12p70 induced IFN-γ expression (Fig. 6A), consistent with the finding that MP cells expressed IL-12Rβ1/2 mRNA (Fig. 4A). To determine if IL-12 is essential for IFN-γ production by MP cells at day 2 of T. gondii infection, anti-IL-12 mAb or control IgG was administered to IFN-γ-eYFP reporter mice. Blockade of IL-12 almost completely abolished IFN-γ production by MP cells (Fig. 6B). These data indicate that IL-12 induces IFN-γ expression by the MP cells present at day 2 post Toxoplasma infection.
Fig. 6. T-bethi MP cells produce IFN-γ in response to IL-12.
(A) IL-12p70 induces IFN-γ production by MP cells. IFN-γ-eYFP reporter mice were treated i.p. with IFN-γ, IL-12p70, or IL-18 on day 0 and 1 and on day 2 MP cells were analyzed for IFN-γ expression in PC and spleen. The representative histograms indicate the frequency of IFN-γ+ cells among MP cells and the bar graph shows the frequency (mean ± SD) of IFN-γ+ cells (n=4). Data shown are pooled from 2 independent experiments. (B) Neutralization of IL-12 abolishes IFN-γ production by MP cells during T. gondii infection. IFN-γ-eYFP reporter mice infected with T. gondii were treated with blocking anti-IL-12 mAb or control IgG and MP cells in PC and spleen were analyzed for IFN-γ expression on day 2. The numbers in the histograms show the frequency of IFN-γ+ cells among MP cells and the graphs indicate the number (mean ± SD) of IFN-γ+ MP cells from each group (n=3–6). Data shown are pooled from 2 independent experiments. (C) T-bethi but not T-betlo MP cells produce IFN-γ in response to IL-12p70. IFN-γ-eYFP T-bet-AmCyan double reporter mice were treated with IL-12p70 as described in (A), and MP cells from PC and spleen were analyzed for IFN-γ-eYFP and T-bet-AmCyan expression. The representative histograms indicate the frequency of IFN-γ+ cells among T-bethi and T-betlo MP cells and the bar graphs show the frequency (mean ± SD) of IFN-γ+ cells among each MP subset (n=3–5). Data shown are pooled from 2 independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
As shown in Fig. 4, B and C, MP cells contained T-bethi cells. To determine whether this population produces IFN-γ in response to IL-12, we injected uninfected T-bet-AmCyan IFN-γ-eYFP double reporter mice with IL-12p70 or control PBS at day 0 and 1 and analyzed IFN-γ production at day 2. As expected, T-bethi but not T-betlo MP cells were triggered to produce IFN-γ (Fig. 6C). This finding suggests that in T. gondii infection the T-bethi subpopulation of MP cells is likely to be the main contributor of IL-12-dependent IFN-γ production.
MP cells provide nonspecific, IL-12-dependent resistance against infection and promote the development of adaptive Th1 immunity
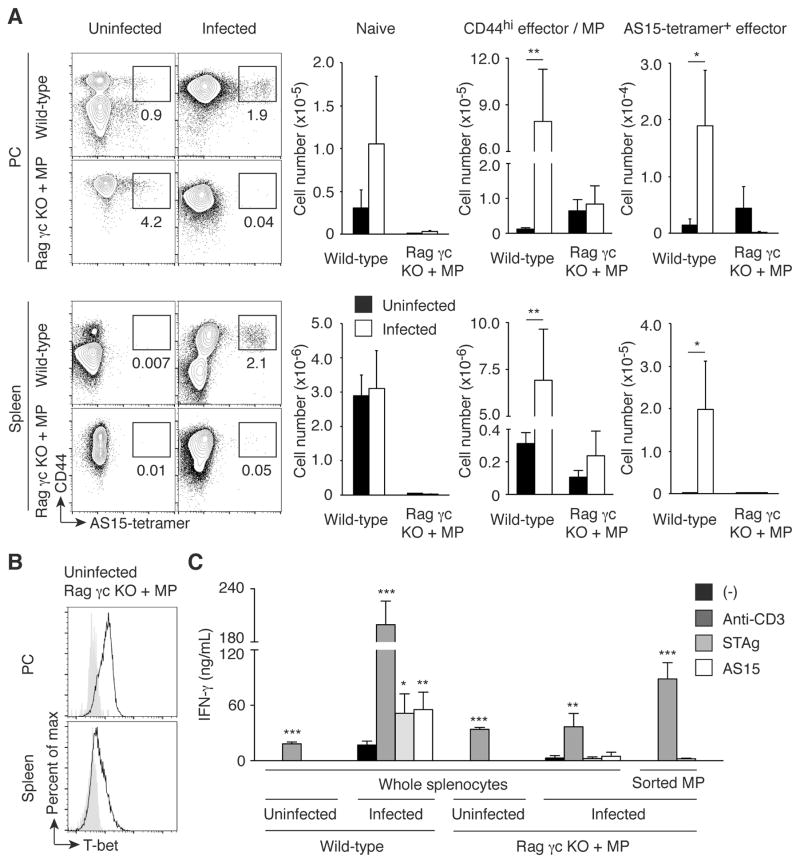
The detection of early IFN-γ production by MP cells in T. gondii-infected animals raised the question of whether this pathogen-unspecific, IL-12-driven cytokine response contributes significantly to host defense against this parasite. As an approach to address this question, we decided to generate animals that possess an MP cell population but lack the other cells required for conventional adaptive and innate lymphocyte-mediated immunity. For this purpose, we transferred CD4+ T cells into Rag2 common γ chain double KO (Rag γc KO) mice, which are deficient in T/B/NK cells and thus can promote MP cell generation via lymphopenia-induced proliferation. We then waited several weeks so that the transferred CD4+ donor cells could acquire a CD44hi phenotype. When assayed 4 weeks after transfer, a considerable number of T-bet+ CD44hi CD4+ T cells were generated from the donor T lymphocytes while almost no naïve cells were detectable in PC and spleen (Fig. 7, A and B, Uninfected Rag γc KO + MP). Theoretically, this CD44hi cell population should contain T cells that recognize both T. gondii-specific and unrelated antigens. To check the relative contribution of these two subpopulations, Rag γc KO mice that had received CD4+ T cells 4 weeks previously were challenged with T. gondii cysts. Seven days after infection, the numbers of total CD44hi CD4+ T cells as well as the tetramer+ subpopulation specific for the major T. gondii epitope AS15 were not significantly increased in the infected CD4+-reconstituted Rag γc KO mice compared to uninfected animals (Fig. 7A). Furthermore, in vitro stimulation of total splenocytes as well as sorted CD44hi CD62Llo cells from infected, CD4+-reconstituted Rag γc KO mice with a soluble antigen extract of Toxoplasma tachyzoites (STAg) or with AS15 peptide failed to induce high levels of IFN-γ (Fig. 7C). These results suggest that the CD44hi MP cells established in Rag γc KO mice using this protocol do not contain a substantial number of T lymphocytes specific for major Toxoplasma antigens, presumably because only a relatively small number of donor cells robustly proliferate in empty host animals thereby giving rise to MP cells with a limited TCR repertoire diversity (3).
Fig. 7. MP cells established in Rag γc KO mice are Toxoplasma antigen-unspecific T-bet+ population.
(A) MP cells established in Rag γc KO mice do not contain a major Toxoplasma antigen AS15-specific population. Wild-type and Rag γc KO mice that had received CD4+ T cells 4 weeks earlier were infected with T. gondii or left uninfected, and 7 days later CD4+ T lymphocytes from PC and spleen of these animals were analyzed for CD44 expression and AS15-tetramer binding. The representative plots depict CD44 expression and AS15-tetramer binding for each group, while the bar graphs show the number (mean ± SD) of naïve, CD44hi, and CD44hi AS15-tetramer+ cells (n=5). Data shown are representative of 2 independent experiments. (B) MP cells generated in Rag γc KO mice express T-bet. The histograms show T-bet expression by MP cells generated in Rag γc KO mice as shown in (A). Filled histograms show negative control staining. Representative data from 3 animals analyzed. (C) MP cells established in Rag γc KO mice do not produce IFN-γ in response to Toxoplasma antigen stimulation. Wild-type and Rag γc KO mice reconstituted with MP cells as shown in (A) were infected with T. gondii, and on day 7 post infection whole splenocytes as well as sorted CD44hi CD62Llo CD4+ T cells from the indicated groups were stimulated with medium, anti-CD3 mAb, STAg, or AS15 peptide in vitro. The graph indicates the concentration (mean ± SD) of IFN-γ in the culture supernatant from each group (n=5). Data shown are representative of 2 independent experiments performed. * p<0.05, ** p<0.01, *** p<0.001.
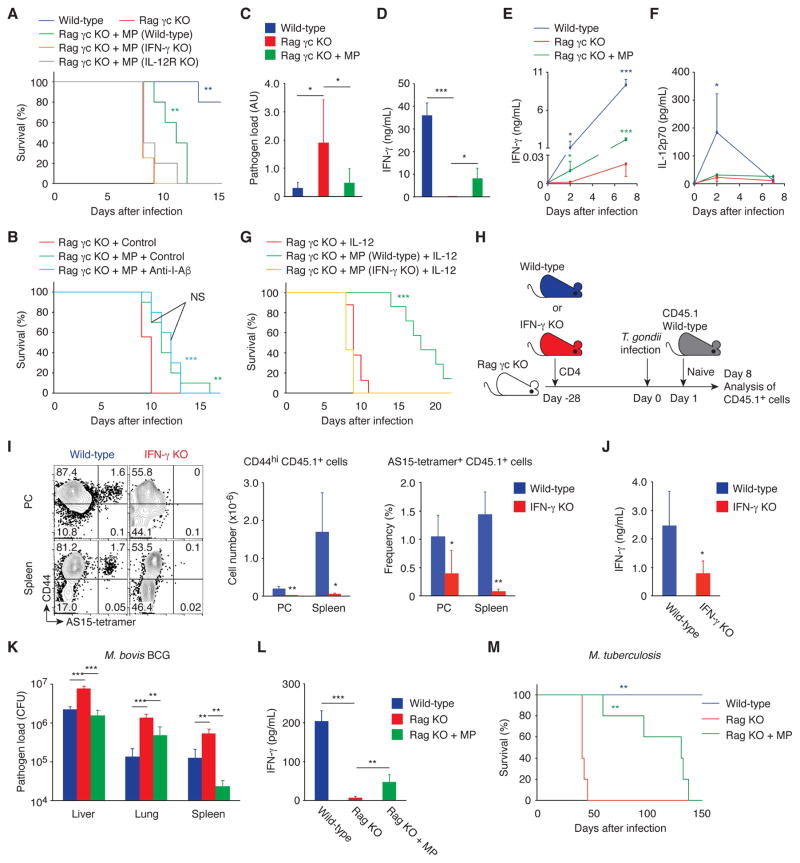
We then examined whether these MP cells provide protection against Toxoplasma infection. Wild-type, Rag γc KO, and Rag γc KO mice that had been given CD4+ T cells 4 weeks before were infected with T. gondii and checked for survival. As shown in Fig. 8A, the presence of MP cells in Rag γc KO recipients was associated with prolonged survival of the infected animals. To examine whether pathogen antigen recognition is required for this protective effect of MP cells, MP-containing Rag γc KO mice were treated with anti-I-Aβ mAb or control IgG and then challenged with T. gondii. MP cells prolonged survival regardless of anti-I-Aβ treatment (Fig. 8B), suggesting that MP cells can confer host resistance independent of TCR stimulation by the pathogen. To further examine whether or not cognate foreign antigen recognition contributes to this protection, Rag γc KO mice received CD4+ T cells from OT-II Rag1 KO animals. Four weeks after injection, some of the donor naïve OT-II cells had converted to MP cells and acquired T-bet expression in PC and spleen (fig. S8, A and B). When these animals were challenged with T. gondii and checked for survival, the OT-II MP cells were found to provide significant protection (fig. S8C), confirming that cognate antigen recognition is not essential for the MP cell-dependent prolongation of survival. Taken together, these data indicate that a significant proportion of MP cells support early innate resistance toward Toxoplasma infection independently of specific pathogen antigen recognition.
Fig. 8. MP cells can mediate resistance in infectious models that induce Th1-type immunity.
(A to G) MP cells ameliorate toxoplasmosis via the IL-12-IFN-γ axis in the absence of pathogen antigen recognition. (A) Survival of T. gondii-infected wild-type, Rag γc KO, and Rag γc KO animals that had received CD4+ T cells from wild-type, IFN-γ KO, or IL-12Rβ2 KO mice 4 weeks earlier was monitored daily (n=4–5). (B) T. gondii-infected Rag γc KO and Rag γc KO mice with MP cells were treated with anti-I-Aβ mAb or control IgG and survival was assessed (n=9–10). (C) The relative pathogen load (mean ± SD) and (D) concentration of IFN-γ in PC of T. gondii-infected wild-type, Rag γc KO, and Rag γc KO animals that received CD4+ T cells 4 weeks earlier (n=3–10). (E and F) Serum IFN-γ and IL-12p70 concentration (mean ± SD) at the indicated days after infection (n=3–5). (G) T. gondii-infected Rag γc KO mice with or without MP cells from wild-type or IFN-γ KO mice were treated with IL-12 and survival was assessed (n=7–8). Data shown are (A, D to F) representative of 2, (B, C) pooled from 2, and (G) pooled from 3 independent experiments performed. (H to J) IFN-γ produced by MP cells augments antigen-specific effector CD4+ T cell responses. (H) Experimental design. Rag γc KO mice that had received CD4+ T cells from wild-type or IFN-γ KO mice at day -28 were infected with T. gondii at day 0 and transferred with sorted naïve CD4+ T cells from CD45.1 mice at day 1. (I) The representative dot plots show CD44 expression versus AS15-tetramer staining by CD45.1 donor cells from each group at day 8, while the bar graphs show the number (mean ± SD) of CD44hi CD45.1 donor cells (left) and the frequency (mean ± SD) of CD44hi AS15-tetramer+ cell population among CD45.1 donor cells (right, n=5). (J) CD44hi CD62Llo CD45.1 donor cell population sorted from splenocytes of each group was stimulated with STAg. The bar graph shows the concentration (mean ± SD) of IFN-γ in the culture supernatant from each group (n=4–5). (K and L) MP cells produce IFN-γ and control bacterial growth in M. bovis BCG infection. Wild-type, Rag KO, and Rag KO mice that had received CD4+ T cells 4 weeks earlier were infected with M. bovis BCG and (K) bacterial burden (CFU, mean ± SD) in the indicated organs and (L) concentration (mean ± SD) of IFN-γ in the spleen were analyzed 17 days after infection (n=4–5). (M) MP cells are protective in M. tuberculosis infection. Wild-type, Rag KO, and Rag KO mice that had received CD4+ T cells were infected with M. tuberculosis and survival was assessed (n=5). * p<0.05, ** p<0.01, *** p<0.001, NS not significant.
Our prior results revealed that MP cells express IFN-γ in response to IL-12p70 (Fig. 6). In fact, MP cells established in Rag γc KO mice produced IFN-γ in PC and serum at day 2 and 7 post infection, which was paralleled by a significant reduction in T. gondii pathogen load in the PC (Fig. 8, C to E). To assess the roles of IFN-γ and IL-12 in survival prolongation, we established MP cells using donor CD4+ T cells from wild-type, IFN-γ KO, or IL-12Rβ2 KO mice in Rag γc KO recipients using the above protocol. When these animals were infected with T. gondii, the MP cells from wild-type but not IFN-γ KO or IL-12Rβ2 KO animals were found to generate protection (Fig. 8A), indicating that MP cells can contribute to early resistance to T. gondii infection via the IL-12-IFN-γ axis. Furthermore, when serum IL-12p70 concentrations were measured, Rag γc KO mice were found to have lower IL-12p70 levels compared to wild-type animals (Fig. 8F). This finding prompted us to test whether exogenous IL-12p70 boosts MP activity. Indeed, wild-type MP-reconstituted Rag γc KO mice showed a dramatic increase in survival relative to unreconstituted or IFN-γ KO MP-reconstituted animals as a consequence of exogenous IL-12p70 administration (Fig. 8G). Thus, IL-12p70 is an important cytokine in the induction of IFN-γ-mediated MP cell function during infection.
To examine the possibility that this apparently pathogen-nonspecific, IL-12-driven early IFN-γ production might contribute to the acquisition of Toxoplasma-specific CD4+ T cell effector function, CD45.2 Rag γc KO mice that had received CD45.2 CD4+ T cells from wild-type or IFN-γ KO mice at day -28 were infected with T. gondii at day 0 and then were transferred 1 day later with naïve CD45.1 CD4+ T cells (Fig. 8H). On day 8 post infection, the number of newly generated CD44hi effector cells among the CD45.1 donor-derived cells was dramatically lower in the IFN-γ KO MP-reconstituted mice than in the wild-type MP-reconstituted animals as was the frequency of AS15-specific donor cells (Fig. 8I). Moreover, IFN-γ production in response to in vitro stimulation with STAg was significantly reduced in CD44hi CD62Llo CD45.1+ donor cells in mice reconstituted with IFN-γ KO MP cells versus those in mice reconstituted with wild-type MP cells (Fig. 8J). These results imply that early IFN-γ production by MP cells plays a critical role in augmenting Th1-type responses of antigen-specific CD4+ T cells later in the infection.
Finally, to test if MP cells are also functional in other infectious settings, wild-type and Rag KO mice that had been reconstituted with MP cells by transfer of CD4+ T cells 4 weeks in advance were infected with Mycobacterium bovis BCG, an avirulent bacterium which in common with T. gondii induces Th1-type immune responses. When assayed on day 17 post infection, bacterial burdens in liver, lung, and spleen were decreased while splenic IFN-γ concentrations were increased in Rag KO mice reconstituted with MP cells (Fig. 8, K and L), suggesting increased bacterial control by the reconstituted MP cells. To determine whether the anti-bacterial response can protect against lethal mycobacterial infection, MP-reconstituted Rag KO mice were challenged by aerosol exposure to virulent M. tuberculosis. As shown in Fig. 8M, these animals survived significantly longer than unreconstituted Rag KO mice. Therefore, MP cells may be functional in multiple infectious settings.
Discussion
In the present study, we addressed the mechanisms underlying MP CD4+ T cell homeostasis and their role in host resistance to infection. We found that MP cells are spontaneously generated from naïve cells in lymphoreplete adults in addition to lymphopenic neonatal animals in a TCR- and CD28-dependent manner. Once MP cells are generated, they become less dependent on TCR signaling for their maintenance while retaining a requirement for CD28 signaling. Furthermore, a subpopulation of autonomously generated MP cells express T-bet, which is induced by environmental IL-12 under steady state conditions. This T-bethi subpopulation rapidly produces IFN-γ in response to IL-12 in a pathogen antigen-independent fashion to enhance antigen-specific effector CD4+ T cell responses involved in host resistance to infection. Together, these observations reveal a previously unknown innate immune function of MP CD4+ T cells in providing early, foreign antigen-independent protection against invading pathogens. They add susbstantially to the emerging story of how the immune system has evolved a layer of host defense that is intermediate between the well-recognized myeloid cell innate activities and the antigen-driven actions of conventional T and B cells.
Previous studies have shown that innate-like lymphocytes such as NK, NKT, γδT cells, and various types of ILCs generate effector functions in response to inflammatory cytokines in the absence of foreign antigen recognition (12, 31–34). In addition, antigen-specific memory CD8+ T cells have been shown to have similar innate activity (35), and more recently conventional CD8+ T lymphocytes have been found to contain a subpopulation with a MP that is induced and maintained by various types of cytokines (36–39). Both CD8+ T cell subsets produce IFN-γ in response to IL-12 and IL-18. Here we provide evidence that CD4+ T lymphocytes have a similar functional subset, which contributes to host defense against Th1-inducing pathogens. Notably, in the case of Toxoplasma infection, MP CD4+ T lymphocytes together with NK and NKT cells (but not γδT or MP CD8+ T cells) are the main contributors to early IFN-γ production. In contrast, in the case of Listeria monocytogenes infection, MP CD8+ T cells appear to exert innate immune effector function, suggesting a division of labor among these innate-like cells in different infectious settings.
It is well known that differentiated CD4+ T cells produce effector cytokines in response to STAT activators and NF-κB-activating IL-1 family cytokines in vitro. Th1 cells produce IFN-γ in response to IL-12 (STAT4 activator) and IL-18, Th2 cells produce IL-13 in response to TSLP (STAT5 activator) and IL-33, and Th17 cells produce IL-17 in response to IL-23 (STAT3 activator) and IL-1 (40–42). Consistent with these in vitro findings, a recent study reported that in the absence of antigenic stimulation, Th2 memory cells produce IL-13 following challenge with STAT5 activator and IL-33 in vivo (30). Similarly, we found that MP CD4+ T cells produce IFN-γ in response to IL-12 in an antigen-nonspecific manner. Together, these findings suggest the existence in vivo of a cytokine-driven effector cytokine circuit in CD4+ T cells.
Importantly, the IL-12-driven IFN-γ production by MP cells plays a critical role in host defense, which is likely to be accomplished in two different ways; first, IFN-γ directly works on peritoneal macrophages to eliminate intracellular parasites (43); and second, IFN-γ enhances antigen-specific Th1 responses by inducing IL-12 production by dendritic cells (DC) and by synergizing with TCR signaling to increase T-bet expression by T cells (44, 45). These functions of MP cells appear to overlap with those of NK cells to some extent (46). What then is the functional significance of MP CD4+ T cells compared to NK cells in T. gondii infection? NK cells are known to strongly produce IFN-γ and rapidly die after infection (47, 48). By contrast, MP cells appear to produce lower levels of IFN-γ (Fig. 5A) and do so for a longer period under inflammatory conditions (Fig. 8G). Therefore, MP cells may contribute to innate protection particularly after NK cells have been deleted following acute infection. To test this hypothesis, we plan to analyze the frequencies of IFN-γ+ MP and NK cells over a longer time interval post infection.
In our experiments, the T-bethi subpopulation of MP CD4+ T cells was the major source of innate IFN-γ and its production was shown to be IL-12p40-dependent. The latter is likely to reflect a requirement for IL-12p70 rather than IL-23 since MP cells do not express IL-23R mRNA (RNAseq data). One potential source of IL-12p70 in steady state might be peripheral DC since DC-T interaction through CD40/CD40L induces IL-12p70 production by DC (49) and since MP cells have high levels of CD40L mRNA (RNAseq data). Commensal and other foreign antigens are unlikely to be the major stimuli for IL-12p70 expression under steady state since T-bet+ MP cells are equally present in SPF, GF, and AF mice (4). Furthermore, in addition to IL-12p70, efficient transcription of T-bet requires intrinsic T-bet expression. Since naïve cells are T-bet−, it is possible that the initial T-bet induction, which depends upon TCR and CD28 signaling, is essential for these cells to be further polarized into T-bethi cells by a positive feedback loop in the presence of IL-12p70 in steady state conditions as well. Such a mechanism is known to operate in conventional effector T cell responses (25, 45, 50).
MP cells are generated by proliferation of naïve precursors. Fast T cell proliferation had been thought to occur only in lymphopenic settings (19). The availability of “niche space” is an important factor regulating such fast proliferation and is hypothesized to be determined by the relative availability to individual T cells of self/foreign antigens, costimulatory molecules, and γc cytokines. By contrast, the present study demonstrates that rapid proliferation of naïve cells can also occur in a lymphoreplete environment, although at a lower rate. This suggests that MP conversion can be induced even when the niche space is small. Importantly, the generation of MP cells also requires TCR and CD28 signaling in lymphoreplete conditions, indicating that this process is dependent upon antigen recognition. How then do naïve cells find their cognate weak antigens in a crowded environment? We have previously shown that the repertoire complexity of pre-existing MP cells determines the MP conversion rate of naïve cells, suggesting that naïve cells can sense a “hole” in the MP repertoire and proliferate to become MP cells that fill this hole (29). Since MP cells are rapidly or slowly dying, it is conceivable that this rapid expansion is induced to occupy the niche space of MP cells in a lymphosufficient environment as well, depending upon the complexity of each individual’s MP cell repertoire.
Whether self or foreign antigens drive MP conversion is an important question that is still unanswered. Since MP cells are present not only in SPF and GF but also in AF mice (4), it is highly likely that the MP conversion is mainly driven by self antigens in normal settings. However, previous papers have shown that commensal antigens provide a major stimulus for the induction of lymphopenia-induced rapid proliferation in T cell-deficient as well as sublethally irradiated mice (9, 51). The latter findings suggest the existence of a specific MP subpopulation that is induced by commensal antigen recognition. This hypothesis is worthy of consideration since the comparable numbers of MP cells in SPF and GF mice do not rule out the possible contribution of commensal antigens to MP cell production in SPF mice. Thus, the repertoire of MP cells in GF mice may be sparse, which would allow additional expansion of a smaller initial set of CD4+ T cells resulting in the total number equal to that seen in the SPF mice. Deep sequencing of TCR expressed by MP cells could be employed to determine the exact ratio of MP cells induced by self antigens versus those generated by commensal and food antigens.
Here we have shown that in contrast to the clear requirement of self / foreign antigen recognition in MP cell development from naïve precursors, proliferation of MP cells for their maintenance is less dependent upon TCR signaling once they have been generated. This is similar to the process of antigen-specific memory CD4+ T cell formation, where MHC II-TCR interaction is dispensable for memory cell survival while it is essential for effector generation (52). Such alteration in TCR dependency has been proposed to promote the survival of memory cells since repetitive TCR stimulation drives activation-induced cell death (AICD) (53). Based on this hypothesis, it is likely that MP cells are also desensitized to TCR signals to avoid AICD thus preserving the preformed MP population while self and environmental antigens continue to drive MP development throughout life. Instead of TCR signaling, we found that CD28 and IL-7 but not IL-15 or IL-2 (3) drive proliferation of preformed MP cells. To what extent desensitization of TCR signals provided by self and possibly environmental antigens and responsiveness to CD80/86 and IL-7 differentially contribute to pre-existing MP cell maintenance needs further investigation.
Given that self antigen recognition is used to select mature naïve cells in the thymus, is the destiny of naïve cells to eventually convert to MP cells predetermined at the positive selection stage? This would appear to be the case since we found that CD5hi naïve cells with high affinity to self antigens convert to MP cells at a higher frequency than their CD5lo counterparts with low affinity. Even if recognition of environmental antigens in the periphery generates a subpopulation among MP cells, this notion would still be valid since the affinity of TCR to self is positively correlated with that to foreign antigens (20). Nevertheless, because CD5hi cells were only slightly better than CD5lo cells in generating MP cells, it is likely that there are additional factors that govern MP conversion and in particular, prevent the overproduction of the CD5hi MP subpopulation. In this regard, we observed that CD5hi MP cells die in greater frequency than their CD5lo counterparts under steady state conditions. Moreover, MP cells are considered to be generated via interaction with peripheral antigen-presenting cells (APCs) and during steady state, individual APCs are proposed to express a random set of self antigens in the periphery (54). If so, the probability that individual naïve cells find their cognate antigens might be governed by stochastic mechanisms as well, which may prevent excess CD5hi MP cell generation. Why then does the overproduction of CD5hi MP cells need to be avoided? Since CD5hi MP cells are thought to have high affinity for foreign antigens (20) and thus may decrease in number after resolution of severe infection, these pathways might be helpful in ensuring the production of the minimal number of T-bethi MP cells through the simultaneous generation of CD5lo cells.
Based on the findings reported here, we propose that T-bethi MP CD4+ T cells are a continuously generated population of innate effector cells that provide rapid and broad protection and also enhance antigen-specific Th1 immunity to infection with pathogens. However, several further issues need to be addressed. First, it will be important to determine if there are specific markers or transcription factors that uniquely define MP CD4+ T cells. Identification of these molecules would not only help better characterize this population but such knowledge would also enable the development of gene manipulated animals and/or depleting antibodies to directly assess their immunological function. A second issue concerns whether or not MP cells always function in a pathogen-independent manner. In T. gondii infection, MP cells produce IFN-γ in the absence of pathogen recognition, but their broad repertoire (3) suggests that in other infectious settings, MP cells may also exert effector function in an antigen-driven manner. A third concern is whether all MP cells are generated in the periphery. In the case of MP CD8+ T cells, a minor subpopulation is known to be generated in the thymus (55), and the data presented here do not rule out this possibility for MP CD4+ T cells. Furthermore, it is possible that thymic T cell generation enhances the total complexity of the MP cell repertoire as well as the diversity of their tissue distribution throughout the body. A final question concerns the existence of a homologous MP CD4+ population in humans. If such a functional subset is present, it could provide a target for boosting or blocking natural immunity in various clinical situations.
Materials and Methods
Study design
The aim of this study was to determine the mechanisms of generation and maintenance of MP CD4+ T lymphocytes and to elucidate the role of these cells in infection with pathogens. For this purpose, in vivo and in vitro experiments using mice were performed as written in this Materials and Methods section. The animal experiments were not randomized. The investigators were not blinded to the allocation during experiments and analyses. Experimental replication is indicated in the figure legends.
Mice
C57BL/6 CD45.2 wild-type mice were purchased from Taconic Farms. CD45.1 and CD45.1/2 wild-type, CD45.1 and CD45.2 Rag1 KO, OT-II Rag1 KO, Rag2 γc KO, T-bet+/+ and T-bet−/− T-bet-ZsGreen reporter, IL-12p40 KO, and IFN-γ KO mice were obtained from the National Institute of Allergy and Infectious Diseases (NIAID) contract facility at Taconic Farms. IFN-γ-eYFP reporter, IL-12Rβ2 KO, CD28 KO, and MHC II KO mice were purchased from the Jackson Laboratory. C57BL/6 Rag2-GFP reporter mice were kindly provided by Dr. R J Hodes (National Institute on Aging, National Institutes of Health (NIH)). CD45.1/2 IFN-γ-eYFP reporter mice were obtained by crossing IFN-γ-eYFP reporter with CD45.1 mice. T-bet / IFN-γ double reporter mice were obtained by crossing T-bet-AmCyan (56) with IFN-γ-eYFP reporter mice. Fetal and neonatal mice of defined age were obtained through examination for vaginal plugs (0.5 dpc). In experiments analyzing KO animals, wild-type mice with a matching genetic background as KO mice were used as controls and maintained in adjacent caging in a room with standardized commensal flora. All mice were maintained in NIAID animal facilities. GF mice were obtained and maintained at the NIAID GF facility. The care and handling of the animals used in our studies was in accordance with the animal study protocols approved by the NIAID Animal Care and Use Committee except in surgery of neonatal thymectomy, where the procedure was performed following the protocols approved by the Institutional Committee for the Use and Care of Laboratory Animals of Tohoku University.
Pathogens and murine infection
Type II avirulent strain ME49 cysts of T. gondii were obtained from the brains of chronically infected C57BL/6 wild-type mice. Cyst preparations were treated with pepsin to eliminate host cell contamination. For infection, mice were inoculated by the intraperitoneal route (ip) with ~15 cysts. Avirulent M. bovis BCG (Pasteur strain) and virulent M. tuberculosis H37Rv were expanded to log phase on Middlebrook 7H9 liquid medium supplemented with albumin-dextrose-catalase (Difco), washed, aliquoted in PBS, and stored at −80°C before use. Bacterial titers were quantified by plating out bacteria on 7H11 agar supplemented with oleic acid-albumin-dextrose-catalase (Difco). For M. bovis BCG infection, mice were injected intravenously (iv) with 106 CFU per animal. For M. tuberculosis infection, animals were exposed by aerosol for 15 min to approximately ~100 CFU per mouse.
Neonatal thymectomy
Neonatal thymectomy was performed on day 1 neonates. Mice were anesthesized by keeping them on crushed ice for 2–3 min and the thymus removed under a microscope with a cottonhead toothpick. Sham operated mice were incised at the sternum without removal of the thymus. After suture of the wounds, the animals were returned to their mothers.
Antibiotic treatment
Mice were treated with ampicillin (1 g/L), neomycin (1 g/L), vancomycin (0.5 g/L), and metronidazole (1 g/L) (Sigma-Aldrich) in drinking water for 3 weeks (9). For generation of antibiotic-treated neonatal mice, female and male mice that had been separately given the above antibiotics for 1 week were cohoused for breeding while being treated with antibiotics. The antibiotic treatment was continued after birth.
In vivo mAb, cytokine, and chemical treatment
To block TCR and CD28 signaling in transfer experiments, anti-I-Aβ mAb (Y3P; 300 μg/20g body weight; BioXCell), CTLA4-Ig (300 μg/20g body weight; BioLegend), or control mouse IgG was administered ip to neonatal and adult mice every 3 days, or in some experiments, every other day. To block TCR signaling, CsA (20 mg/kg body weight; Cayman Chemical) dissolved in corn oil (Sigma-Aldrich), or corn oil alone, was administered ip to mice every day. For cytokine injection, 1 μg of IFN-γ, IL-12p70, or IL-18 (R&D Systems) dissolved in PBS, or control PBS alone, was injected ip every day. To block IL-12, 1 mg of anti-IL-12 mAb (C17.8; BioXCell) or control IgG was administered once on the day of infection.
Adoptive transfer
Naïve CD4+ T cells were purified from pooled splenocytes and LN cells of CD45.2 wild-type mice by sorting for CD4+ CD25− CD44lo CD62Lhi cells using FACSAria II (BD Biosciences) as shown in fig. S9A. Purity was >99%. In some experiments, total CD4+ and naïve CD4+ T cells were obtained from splenocytes and LN cells by use of CD4+ and Naïve CD4+ T Cell Isolation Kits (both from Miltenyi Biotec). Purity was >95%. For CFSE analysis, the sorted cells were labeled with 1 μM of CFSE (eBiosciences). Depending on the experiment, 106 ~ 107 donor cells were injected into CD45.1/2 neonates or adults, by ip or iv injection, respectively.
Flow cytometric analysis
Single cell suspensions were prepared from spleens and peripheral LNs. Peritoneal cells were obtained by washing the peritoneal cavity with 10 ml of PBS. In some experiments, splenic and LN cells were further enriched for CD4+ T cells using CD4+ or Pan T Cell Isolation Kits. Cells were then incubated with CD16/32 mAb (2.4G2; Harlan Bioproducts) and stained with mAbs against cell surface markers for 20 min on ice. mAbs employed (all purchased from BioLegend) were directed against CD3 (17A2), CD4 (RM4-5), CD5 (53-7.3), CD8 (53-6.7), CD11b (M1/70), CD11c (N418), CD25 (PC61), CD44 (IM7), CD45.1 (A20), CD45R/B220 (RA3-6B2), CD45.2 (104), CD62L (MEL-14), NK1.1 (PK136), and TCRβ (H57-597). Tetramer staining employed CD1d (PBS-57) and I-Aβ-AS15 tetramers, obtained from NIH Tetramer Core Facility (Emory University). Apoptotic cells were stained using an Annexin V Apoptosis Detection Kit (eBioscience). To detect intracellular antigens, cells were fixed and permeabilized using Foxp3 / Transcription Factor Staining Buffer Set for 30 min on ice after surface staining and then stained with anti-Foxp3 (FJK-16s) and anti-Ki67 (SolA15) (both eBioscience) mAbs for 20 min on ice. For T-bet detection, fixed cells were stained with anti-T-bet (O4-46; BD Biosciences) mAb for 2 hr at room temperature. Flow cytometry was performed using a LSR II or Fortessa (BD Biosciences) and the data analyzed with FlowJo software (TreeStar). The representative gating strategies in transfer and IFN-γ-eYFP detection experiments are shown in fig. S9, B and C, respectively.
BrdU incorporation analysis
For BrdU incorporation analysis, 1 mg of BrdU was ip injected to each animal. 1 hr later, cells were stained with anti-BrdU and other mAbs and 7AAD using BrdU Flow Kit (BD Biosciences).
RNAseq analysis
Total RNA was prepared from sorted naïve and CD5hi/CD5lo MP cells using a RNeasy Mini Kit (Qiagen) and 300 ng to 3 μg of total RNA subsequently used to prepare RNAseq libraries by means of TruSeq Stranded mRNA Library Prep Kit (Illumina). The libraries were sequenced utilizing a NextSeq500 v2 (Illumina).
Quantitative real-time PCR
For detection of IFN-γ mRNA, total RNA was extracted from sorted cells using a RNeasy Mini Kit (Qiagen) and reverse transcribed with SuperScript III Reverse Transcriptase (Thermofischer Scientific). For detection of T. gondii, total DNA was isolated from peritoneal cells using a DNeasy Blood & Tissue Kit (Qiagen) (57). Real-time PCR was performed utilizing TaqMan Gene Expression Assays (ThermoFisher Scientific) or FastStart Universal SYBR Green Master (ROX) (Roche). Quantitative PCR analysis was carried out using a QuantStudio 7 Flex Real-Time PCR System (ThermoFisher Scientific). Relative gene expression was calculated by the ΔCt method and normalized to the amount of β-actin (IFN-γ detection) or GAPDH (T. gondii detection). The following primer sets were used: T. gondii, 5’-AATATTGGAAGCCAGTGCAGG-3’ and 5’-CAATCTTTCACTCTCTCTCAA-3’; GAPDH, 5’-CCAGGTTGTCTCCTGCGACTT-3’ and 5’-CCTGTTGCTGTAGCCGTATTCA-3’. TaqMan probes (from ThermoFisher Scientific) were used for measurement of IFN-γ (Mm01168134_m1) and β-actin (Mm00607939_s1) expression.
Cytokine measurement
Serum samples were collected at the indicated days after T. gondii infection. For cytokine detection in PC and spleen, peritoneal fluid was obtained by lavage with 3 ml PBS while spleens were dissociated with 5 ml PBS. Cells were then centrifuged and supernatants stored before use. Levels of IFN-γ and IL-12p70 were then measured using Mouse Quantikine ELISA Kits for IFN-γ and IL-12p70 (R&D Systems).
In vitro cell culture
Total splenocytes (3x106 cells/ml) or FACS-sorted CD44hi CD62Llo CD4+ T cells (1.0x106 and 1.5x105 cells/ml in Fig. 7C and Fig. 8J, respectively) were stimulated with anti-CD3 mAb (2C11; 0.3 μg/mL), STAg (5 μg/ml), or AS15 peptide (5 μg/ml) in the presence of irradiated splenocytes (1x107 cells/ml) in RPMI complete media in 96 well plates for 3 days. Cells were then centrifuged and IFN-γ levels in culture supernatants measured by ELISA.
Statistical analysis
In survival experiments, a Log-rank test was employed to establish statistical significance. In the RNAseq analysis, raw and adjusted p values were calculated using one-way ANOVA and the false discovery rate method (Benjamini and Hochberg procedure), respectively. In all other instances, a Student’s t test was applied. p values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Y. Belkaid (NIAID, NIH) for GF mice, K. S. Hathcock (National Cancer Institute, NIH) and R. J. Hodes (National Institute on Aging, NIH) for Rag2-GFP mice, N. Ishii (Tohoku University, Japan) for research support, T. G. Myers (NIAID, NIH) for RNAseq, E. M. Shevach (NIAID, NIH) for thoughtful discussions, T. Takahashi (Central Institute for Experimental Animals, Japan) for experiments using neonatal animals, NIH Tetramer Core Facility for tetramers, D. L. Costa and S. Sakai for M. bovis BCG, J. Hu-Li, X. Chen, S. Hieny, S. D. Oland, and L. Mittereder for technical assistance, and K. L. Holmes, C. C. Henry, and K. Weng for cell sorting. We deeply regret the loss of W. E. Paul who passed away on September 18, 2015.
Funding: This work was supported by the Intramural Research Program of the NIAID, NIH. T.K. was supported by the grant from Japan Society for the Promotion of Science.
Footnotes
Author contributions: T.K., R.N.G., and W.E.P. designed the research, T.K. and S.K. performed experiments, T.K. analyzed the data and carried out statistical analyses, D.J., Y.H., P.H.L., H.Y., and J.Z. assisted some experiments, T.K., D.J., H.Y., A.S., and R.N.G. wrote the manuscript.
Competing interests: The authors declare no competing financial interests.
Publisher's Disclaimer: This manuscript has been accepted for publication in Science Immunology. This version has not undergone final editing. Please refer to the complete version of record at http://immunology.sciencemag.org. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 3.Younes SA, Punkosdy G, Caucheteux S, Chen T, Grossman Z, Paul WE. Memory phenotype CD4 T cells undergoing rapid, nonburst-like, cytokine-driven proliferation can be distinguished from antigen-experienced memory cells. PLoS Biol. 2011;9:e1001171. doi: 10.1371/journal.pbio.1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858–863. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 5.Dobber R, Hertogh-Huijbregts A, Rozing J, Bottomly K, Nagelkerken L. The involvement of the intestinal microflora in the expansion of CD4+ T cells with a naive phenotype in the periphery. Dev Immunol. 1992;2:141–150. doi: 10.1155/1992/57057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 7.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 8.Martin B, Bourgeois C, Dautigny N, Lucas B. On the role of MHC class II molecules in the survival and lymphopenia-induced proliferation of peripheral CD4+ T cells. Proc Natl Acad Sci U S A. 2003;100:6021–6026. doi: 10.1073/pnas.1037754100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabe T, Sun SL, Fujita T, Yamaki S, Asao A, Takahashi T, So T, Ishii N. Homeostatic proliferation of naive CD4+ T cells in mesenteric lymph nodes generates gut-tropic Th17 cells. J Immunol. 2013;190:5788–5798. doi: 10.4049/jimmunol.1203111. [DOI] [PubMed] [Google Scholar]
- 10.Zhang N, Bevan MJ. TGF-beta signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol. 2012;13:667–673. doi: 10.1038/ni.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med. 2007;204:1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 13.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, August A. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol. 2008;180:6544–6552. doi: 10.4049/jimmunol.180.10.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu W, Misulovin Z, Suh H, Hardy RR, Jankovic M, Yannoutsos N, Nussenzweig MC. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5' of RAG2. Science. 1999;285:1080–1084. doi: 10.1126/science.285.5430.1080. [DOI] [PubMed] [Google Scholar]
- 17.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 18.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandl JN, Liou R, Klauschen F, Vrisekoop N, Monteiro JP, Yates AJ, Huang AY, Germain RN. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naive CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 2012;109:18036–18041. doi: 10.1073/pnas.1211717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med. 2001;194:1253–1261. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urdahl KB, Pardoll DM, Jenkins MK. Cyclosporin A inhibits positive selection and delays negative selection in alpha beta TCR transgenic mice. J Immunol. 1994;152:2853–2859. [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 25.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 26.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 27.Kugler DG, Mittelstadt PR, Ashwell JD, Sher A, Jankovic D. CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection. J Exp Med. 2013;210:1919–1927. doi: 10.1084/jem.20122300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci U S A. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Jr, Paul WE. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16:1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aste-Amezaga M, D'Andrea A, Kubin M, Trinchieri G. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell Immunol. 1994;156:480–492. doi: 10.1006/cimm.1994.1192. [DOI] [PubMed] [Google Scholar]
- 33.Skeen MJ, Ziegler HK. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 34.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 35.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White JT, Cross EW, Burchill MA, Danhorn T, McCarter MD, Rosen HR, O'Connor B, Kedl RM. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun. 2016;7:11291. doi: 10.1038/ncomms11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinet V, Tonon S, Torres D, Azouz A, Nguyen M, Kohler A, Flamand V, Mao CA, Klein WH, Leo O, Goriely S. Type I interferons regulate eomesodermin expression and the development of unconventional memory CD8(+) T cells. Nat Commun. 2015;6:7089. doi: 10.1038/ncomms8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurzweil V, LaRoche A, Oliver PM. Increased peripheral IL-4 leads to an expanded virtual memory CD8+ population. J Immunol. 2014;192:5643–5651. doi: 10.4049/jimmunol.1301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 41.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Wilson D, Matthews S, Yap GS. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect Immun. 2007;75:4799–4803. doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 45.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 47.Min-Oo G, Bezman NA, Madera S, Sun JC, Lanier LL. Proapoptotic Bim regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. J Exp Med. 2014;211:1289–1296. doi: 10.1084/jem.20132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivanova DL, Fatima R, Gigley JP. Comparative Analysis of Conventional Natural Killer Cell Responses to Acute Infection with Toxoplasma gondii Strains of Different Virulence. Front Immunol. 2016;7:347. doi: 10.3389/fimmu.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G, Feigenbaum L, Zhao K, Paul WE. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37:660–673. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, Dummer W, Shen H, Cebra JJ, Surh CD. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 52.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Brunner T, Carter L, Dutton RW, Rogers P, Bradley L, Sato T, Reed JC, Green D, Swain SL. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul WE, Milner JD, Grossman Z. Pathogen-sensing, regulatory T cells, and responsiveness-tuning collectively regulate foreign- and self-antigen mediated T-cell responses. Cold Spring Harb Symp Quant Biol. 2013;78:265–276. doi: 10.1101/sqb.2013.78.020198. [DOI] [PubMed] [Google Scholar]
- 55.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu F, Sharma S, Edwards J, Feigenbaum L, Zhu J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol. 2015;16:197–206. doi: 10.1038/ni.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahumatullah A, Khoo BY, Noordin R. Triplex PCR using new primers for the detection of Toxoplasma gondii. Exp Parasitol. 2012;131:231–238. doi: 10.1016/j.exppara.2012.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.