Abstract
Introduction
Dialysis patients aged ≥70 years derive improved life expectancy through kidney transplantation compared with their waitlisted counterparts, but guidelines are not clear about how to identify appropriate transplantation candidates. We developed a clinical prediction score to identify elderly dialysis patients with expected 5-year survival appropriate for kidney transplantation (>5 years).
Methods
Incident dialysis patients in 2006–2009 aged ≥70 were identified from the United States Renal Data System database and divided into derivation and validation cohorts. Using the derivation cohort, candidate variables with a significant crude association with 5-year all-cause mortality were included in a multivariable logistic regression model to generate a scoring system. The scoring system was tested in the validation cohort and a cohort of elderly transplant recipients.
Results
Characteristics most predictive of 5-year mortality included age >80, body mass index <18, the presence of congestive heart failure, chronic obstructive pulmonary disease, immobility, and being institutionalized. Factors associated with increased 5-year survival were non-white race, a primary cause of end-stage renal disease other than diabetes, employment within 6 months of dialysis initiation, and dialysis start via arteriovenous fistula. Five-year mortality was 47% for the lowest risk score group (3.6% of the validation cohort) and >90% for the highest risk cohort (42% of the validation cohort).
Discussion
This clinical prediction score could be useful for physicians to identify potentially suitable candidates for kidney transplantation.
Keywords: elderly, kidney transplant referral, mortality, older adults, USRDS
Patients over the age of 70 years constitute a rapidly growing segment of the end-stage renal disease (ESRD) population; however, there is no consensus about which of these patients should be referred for kidney transplantation. Currently, guidelines do not include any age cutoff recommendations, and the practice of patient selection in the elderly population varies between centers.1 Similar to their younger counterparts, elderly patients who are transplanted have greater survival compared with those who remain on the waitlist, though the survival benefit is delayed compared with younger patients.2 Transplanted patients older than 70 do not achieve equal survival compared with those on the waitlist until 4 months after transplant and will not achieve equal survival time until 2 years from kidney transplantation.3 Older recipients tend to have an increased risk of adverse events, especially infection.4 Recipients’ baseline cardiovascular health also significantly impacts posttransplant survival.5 However, older patients exhibit considerable heterogeneity in comorbidities, functional status, and life expectancy. Thus, nephrologists and transplantation specialists are faced with the difficulty of selecting candidates who will benefit from transplantation from this growing population of older adults.
Prior work examining transplantation candidacy in the older adults has been limited. Grams et al.6 created a multivariable model to examine 3-year transplant survival in older adults. However, their model was based on older adults 65 years and older who were listed for transplantation, which was a preselected population.6 Also, their focus was on posttransplant outcomes, which is different from survival on dialysis. Recently, Dusseux et al.7 developed a clinical prediction score using baseline characteristics of incident dialysis patients 70 years or older to help nephrologists identify those elderly dialysis patients who should be referred for transplantation. Using the Renal Epidemiology and Information Network registry, Dusseux et al. examined 3-year mortality risk in the French population and created a score that identified roughly 20% of the French elderly population as having 3-year mortality of only 30% and thus suitable for transplant referral. However, their work cannot be applied to the US dialysis population for several reasons. First, the model does not include race and ethnicity because France does not collect these data. Second, the majority of France’s ESRD population uses peritoneal dialysis with different outcomes than the predominately hemodialysis population in the United States.8 Third, their score included variables such as congestive heart failure staging, behavioral diseases, and liver diseases that are not readily available in the US registry of dialysis patients. And lastly, they examined 3-year mortality, whereas the median wait time for a deceased donor kidney is 4.5 years in the United States.9 Hence, a scoring system needed to be created for the US population with a similar goal of assisting physicians in evaluating patient candidacy for kidney transplant referral.
The goal of this study was to develop a clinical prediction score that identifies incident elderly dialysis patients 70 years or older with a long-term prognosis appropriate for kidney transplant referral in the United States. Five-year survival was chosen because it approximates the median waitlist time for deceased donor kidneys in the United States.9
Methods
Population
We used data from the 2014 United States Renal Data System, which contains information about all patients with ESRD in the United States. Baseline patient information collected at the time of dialysis initiation was derived from the 2005 version of the Medical Evidence Form 2728.
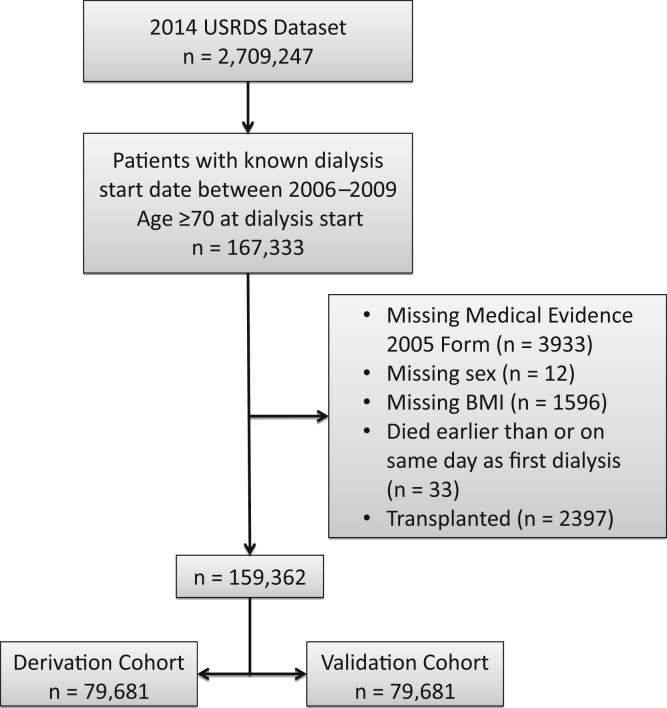
Our cohort included patients with ESRD in the United States Renal Data System database who initiated dialysis between 2006 and 2009 and were 70 years or older at the time of dialysis initiation. The year range was chosen to ensure that all patients included in the cohort had the same version of the Medical Evidence Form 2728 and a minimum of 5 years of follow-up information available in the database for 5-year mortality rate determination. Patients were excluded if they did not have a Medical Evidence Form 2728, died on the date of dialysis initiation, or if they were missing information on sex, body mass index (BMI), or date of first renal service (which was the basis of date of dialysis initiation). The rest of the patients included in the cohort did not have any additional missing data. All patients who received a kidney transplant between 2006 and 2014 but would have otherwise qualified for the cohort were excluded and placed into a separate transplant group. The main cohort was then divided using random number assignment into derivation and validation cohorts. Figure 1 shows the flowchart of the study population with inclusions and exclusions.
Figure 1.
Flowchart of the study population showing inclusion and exclusion criteria. BMI, body mass index; USRDS, United States Renal Data System.
Data
Baseline information obtained at dialysis initiation from the Medical Evidence Form 2728 included sex, race/ethnicity, BMI, primary cause of renal failure, employment status 6 months before dialysis initiation, care from a nephrologist before dialysis initiation, dialysis access/modality, and all listed comorbid conditions (Supplementary Appendix S1). Four age groups were created: 70–74, 75–79, 80–84, and 85 and above. Four BMI categories were created: less than 18, 18 to less than 25, 25 to less than 35, and 35 and above. Employment status before dialysis initiation was divided into 3 categories: retired (due to age/preference or disability), employed (full or part time), and other (unemployed, homemaker, medical leave of absence, student). Dialysis access and dialysis modality were combined to form one indicator variable: peritoneal dialysis, hemodialysis with fistula, hemodialysis with graft, and hemodialysis with a catheter.
Outcome
The outcome of interest is all-cause mortality occurring within 5 years of first dialysis service. Mortality and survival data are collected in the United States Renal Data System prospectively through either direct reporting or linkage with data from the Centers for Medicare and Medicaid Services, the National Death Index Files, and the Scientific Registry of Transplant Recipients.
Statistical Analysis
The derivation and validation cohorts were compared for their relevant baseline characteristics through chi-squared testing. The derivation cohort was used to develop the risk score. First, crude associations between baseline characteristics and 5-year mortality were assessed through single-variable logistic regression models for each baseline characteristic. Characteristics with a P-value less than 0.01 were then included in a multiple logistic regression model. Variables in the multivariable model with insignificant P-values (significance defined as P < 0.005) were further excluded. The model was then tested for multicollinearity through examination of the variance inflation factor of predictors within the multivariable model. Potential interactions between predictors were assessed through the addition of interaction terms and assessed by the P-value within the model. The model’s validity was checked through the Pearson chi-squared and Hosmer-Lemeshow goodness-of-fit tests, and through plots of residuals, standardized residuals, and leverage. Once the final multiple logistic regression model was defined, a scoring system was created using the beta coefficients. The smallest absolute value beta coefficient in the model was identified. Each predictor’s beta coefficient was then divided by this smallest beta coefficient’s absolute value and the result was rounded to the nearest integer.7, 10 Positive integer scores increase mortality risk status, whereas negative integer scores decrease mortality risk status. The scores of the derivation cohort were further divided into 5 groups representing 5 levels of mortality risk. The score divisions were arrived at based on percent mortality such that the 5-year mortality proportion was 50% in the lowest score group, 60% in the second lowest score group, 70% in the next score group, 80% in the second highest score group, and 90% in the highest score group.
The scoring system was then applied to the validation cohort. Mortality proportions were examined and compared in each of the 5 risk score strata to assess the score’s reproducibility to build a calibration curve. To assess the score’s discriminatory ability, the c-statistic was computed based on the predicted score and the actual mortality in the derivation and validation cohorts. Kaplan-Meier survival curves were constructed based on truncated 5-year survival data to assess how well the score separated the risk cohorts over time. A separate cohort of transplanted patients was also used to examine how well the risk score functioned in this group of patients. The distribution of scores was examined in this transplant population as well as 5-year mortality percentage by score category. The baseline characteristics of the transplanted cohort were further examined by the score group to determine which predictors were most influential in this cohort.
Of note, an alternative approach to deriving the prediction score using a Cox proportional hazards model was attempted treating time until death as the outcome. However, the proportional hazards assumption was violated across multiple predictors as well as in the multivariable model, so this approach was not adopted for the final score generation.
All statistical analyses were done using Stata MP 14 (StataCorp, College Station, TX).
Results
Baseline Characteristics
A total of 159,362 patients comprised the cohort, which was divided randomly into 79,681 patients for both the derivation and validation cohorts (Table 1). The 2 cohorts were well randomized except for a preponderance of peritoneal dialysis patients in the derivation cohort. The majority of the patients in this elderly dialysis population were non-Hispanic whites aged 70–79 with diabetes or hypertension as the primary cause of renal failure. The majority had BMI between 25 and 34, were retired, and were started on dialysis via a catheter. Their predominant comorbidities were cardiac or hypertension, and a large minority did not have a nephrologist care for them before dialysis initiation.
Table 1.
Baseline characteristics of the derivation and validation cohorts
| Variable | Derivation cohort (n = 79,681) | Validation cohort (n = 79,681) | P value |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 43,535 (54.64) | 43,037 (54.01) | 0.012 |
| Age groups, n (%) | |||
| 70–74 | 23,956 (30.06) | 24,274 (30.46) | 0.073 |
| 75–79 | 24,085 (30.23) | 23,759 (29.82) | |
| 80–84 | 19,487 (24.46) | 19,292 (24.21) | |
| ≥85 | 12,153 (15.25) | 12,356 (15.51) | |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 53,295 (66.89) | 53,038 (66.56) | 0.048 |
| Non-Hispanic black | 14,795 (18.57) | 15,190 (19.06) | |
| Hispanic | 8,076 (10.14) | 7,893 (9.91) | |
| Asian | 2938 (3.69) | 3012 (3.78) | |
| Other | 577 (0.72) | 548 (0.69) | |
| Primary disease, n (%) | |||
| Diabetes | 31,398 (39.40) | 31,368 (39.37) | 0.517 |
| Hypertension | 30,483 (38.26) | 30,488 (38.26) | |
| Glomerulonephritis | 3871 (4.86) | 4038 (5.07) | |
| Cystic | 713 (0.89) | 672 (0.84) | |
| Urologic | 1288 (1.62) | 1269 (1.59) | |
| Other cause | 8237 (10.34) | 8168 (10.25) | |
| Unknown cause | 3691 (4.63) | 3678 (4.62) | |
| BMI, n (%) | |||
| <18 | 2819 (3.54) | 2806 (3.52) | 0.936 |
| 18 to <25 | 30,867 (38.74) | 30,893 (38.77) | |
| 25 to <35 | 37,177 (46.66) | 37,093 (46.55) | |
| ≥35 | 8818 (11.07) | 8889 (11.16) | |
| Employment status before dialysis, n (%) | |||
| Retired | 67,705 (84.97) | 67,578 (84.81) | 0.409 |
| Employed | 2540 (3.19) | 2631 (3.30) | |
| Other (homemaker, unemployed) | 9436 (11.84) | 9472 (11.89) | |
| Nephrologist care before dialysis start, n (%) | |||
| Yes | 46,747 (58.67) | 46,755 (58.68) | 0.968 |
| No or unknown | 32,934 (41.33) | 32,926 (41.32) | |
| Access type/modality, n (%) | |||
| Arteriovenous fistula | 10,345 (12.98) | 10,170 (13.26) | <0.001 |
| Arteriovenous graft | 3041 (3.82) | 2996 (3.91) | |
| Catheter | 62,476 (78.41) | 62,592 (81.63) | |
| Peritoneal dialysis | 3819 (4.79) | 921 (1.20) | |
| Comorbidities, n (%) | |||
| Congestive heart failure | 34,161 (42.87) | 34,207 (42.93) | 0.816 |
| Atherosclerotic heart disease | 24,553 (30.81) | 24,649 (30.93) | 0.603 |
| Other cardiac disease | 18,693 (23.46) | 18,842 (23.65) | 0.379 |
| Cerebrovascular accident or transient ischemic attack | 9772 (12.26) | 9686 (12.16) | 0.511 |
| Peripheral vascular disease | 14,549 (18.26) | 14,478 (18.17) | 0.645 |
| Hypertension | 40,364 (50.66) | 40,119 (50.35) | 0.220 |
| Amputation | 1752 (2.20) | 1730 (2.17) | 0.706 |
| Diabetes, insulin | 23,626 (29.65) | 23,916 (30.01) | 0.112 |
| Diabetes, oral meds | 11,767 (14.77) | 11,628 (14.59) | 0.325 |
| Diabetes, no meds | 4510 (5.66) | 4527 (5.68) | 0.854 |
| Diabetic retinopathy | 4074 (5.11) | 4065 (5.10) | 0.918 |
| Chronic obstructive pulmonary disease | 10,287 (12.91) | 10,183 (12.78) | 0.436 |
| Tobacco | 2464 (3.09) | 2439 (3.06) | 0.717 |
| Cancer | 9132 (11.46) | 8941 (11.22) | 0.131 |
| Toxic nephropathy | 260 (0.33) | 287 (0.36) | 0.248 |
| Alcohol dependence | 440 (0.55) | 441 (0.55) | 0.973 |
| Drug dependence | 67 (0.08) | 70 (0.09) | 0.798 |
| Inability to ambulate | 7792 (9.78) | 8022 (10.07) | 0.054 |
| Inability to transfer | 4165 (5.23) | 4336 (5.44) | 0.057 |
| Needs assistance with daily activities | 13,192 (16.56) | 13,338 (16.74) | 0.326 |
| Institutionalized | 10,194 (12.79) | 10,328 (12.96) | 0.316 |
| Institutionalized, assisted living | 842 (1.06) | 852 (1.07) | 0.807 |
| Institutionalized, nursing home | 8963 (11.25) | 9049 (11.36) | 0.496 |
| Institutionalized, other | 439 (0.55) | 486 (0.61) | 0.121 |
| Nonrenal congenital abnormality | 72 (0.09) | 62 (0.08) | 0.387 |
| No comorbidities | 924 (1.16) | 960 (1.20) | 0.404 |
| Mortality, n (%) | |||
| Died within 5 yr of dialysis start | 64,167 (80.53) | 64,032 (80.36) | 0.394 |
Predictors of 5-Year Mortality
The 5-year mortality occurred in 80% of both the derivation and validation cohorts. The crude and multivariable logistic regression analyses resulted in 22 characteristics that were predictive of 5-year mortality, shown in Table 2. Crude associations for each of the candidate variables can be found in Supplementary Table S1.
Table 2.
Scoring model generated from the derivation cohort
| Variables | Beta coefficient | Pointsa |
|---|---|---|
| Male | ||
| Baseline = female | 0.14 | +2 |
| Age group | ||
| 70–74 | Baseline | |
| 75–79 | 0.27 | +3 |
| 80–84 | 0.66 | +8 |
| ≥85 | 1.18 | +14 |
| Race/ethnicity | ||
| Non-Hispanic white | Baseline | |
| Non-Hispanic black | −0.52 | −6 |
| Hispanic | −0.57 | −7 |
| Asian | −0.73 | −9 |
| Primary disease | ||
| Diabetes | Baseline | |
| Hypertension | −0.37 | −4 |
| Glomerulonephritis | −0.40 | −5 |
| Cystic | −0.66 | −8 |
| Urologic | −0.55 | −7 |
| Other cause | −0.19 | −2 |
| Unknown | −0.20 | −2 |
| BMI | ||
| 25, <35 | Baseline | |
| <18 | 0.71 | +8 |
| ≥18, <25 | 0.29 | +3 |
| ≥35 | Nonsignificantb | 0 |
| Previous employment | ||
| Retired | Baseline | |
| Employed | −0.30 | −4 |
| Previous renal care | ||
| Nephrologist care | Baseline | |
| No nephrologist care | 0.10 | +1 |
| Access type/modality | ||
| Catheter | Baseline | |
| Arteriovenous fistula | −0.50 | −6 |
| Arteriovenous graft | −0.27 | −3 |
| Peritoneal dialysis | Nonsignificantb | 0 |
| Comorbidities | ||
| Congestive heart failure | 0.43 | +5 |
| Atherosclerotic heart disease | 0.08c | +1 |
| Other cardiac | 0.20 | +2 |
| Cerebrovascular accident or temporary vascular accident | 0.20 | +2 |
| Peripheral vascular disease | 0.15 | +2 |
| Hypertension | −0.28 | −3 |
| Amputation | 0.24 | +3 |
| Insulin-dependent diabetes mellitus | 0.16 | +2 |
| Chronic obstructive pulmonary disease | 0.45 | +5 |
| Tobacco | 0.25 | +3 |
| Cancer | 0.29 | +3 |
| Immobility | 0.36 | +4 |
| Needs assistance w activities of daily living | 0.23 | +3 |
| Institutionalized | 0.48 | +6 |
BMI, body mass index.
Point values greater than zero confer increased the risk of 5-yr mortality. Point values less than zero confer the decreased risk of 5-yr mortality.
Nonsignificant categories mean that the beta coefficients in the model were nonsignificant, and hence these categories are considered the same as the baseline category.
Smallest beta coefficient used to derive the scores.
Table 2 shows the scoring system that was created based on multivariable logistic regression modeling using significant predictors. Positive points indicate a higher risk of mortality, whereas negative points indicate a lower risk of mortality. The predictor with the strongest association with increased mortality was age 85 or above, which resulted in +14 points. Other factors strongly associated with increased mortality included chronologic age 80 to 84, BMI less than 18, and being institutionalized. The factors most associated with reduced mortality were any race other than white, a primary cause of ESRD other than diabetes, being employed within 6 months of dialysis initiation, and having an arteriovenous fistula at dialysis initiation. Comorbid hypertension (other than as the primary cause of ESRD) was also found to be associated with increased 5-year survival.
Scoring System Performance
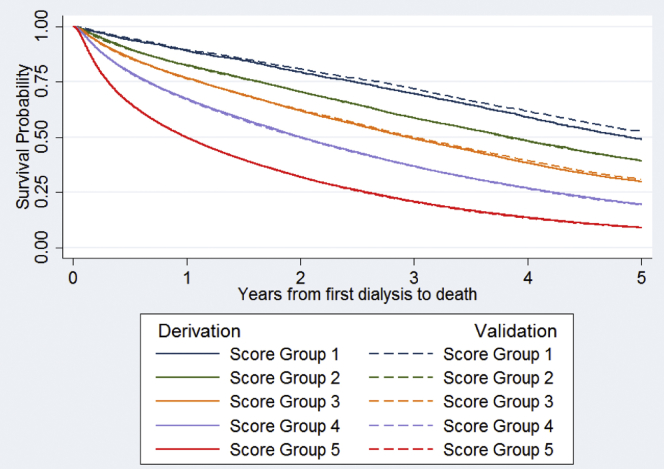
Table 3 shows the number of patients and the proportion of 5-year mortality outcomes in each score group in both the derivation and validation cohorts. The range of scores in the derivation cohort was from −25 to +50 with a median of 7 and an SD of 9.86. The range of scores in the validation cohort was from −27 to +51 with a median of 7 and an SD of 9.93. In the derivation cohort, score group 1 (score ≤ 9) comprised 3.5% of the cohort and had 51% 5-year mortality. In the validation cohort, score group 1 comprised 3.6% of the cohort and had 47% 5-year mortality. Score group 5 (score ≥ 10) comprised the largest proportion of both cohorts (42%) and experienced the highest 5-year mortality (more than 90%). The 2 cohorts had similar numbers of patients in each score group as well as similar mortality proportions, indicating good calibration and predictive function of the scoring system. The c-statistic for the derivation cohort was 0.71 (95% confidence interval 0.70–0.71), and the c-statistic for the validation cohort was 0.71 (0.71, 0.72), indicating a satisfactory discriminatory function. The score’s ability to distinguish between 5-year mortality risk groups can also be seen in the Kaplan-Meier plot of survival over the 5-year period by the cohort and score group (Figure 2). The derivation and validation cohorts had similar survival curves by the score group.
Table 3.
Five-year mortality rates by patients’ risk scores in the derivation and validation cohorts
| Score group | Actual score | Derivation cohort |
Validation cohort |
||
|---|---|---|---|---|---|
| Number at risk, n (%) | Number of deaths, n (%) | Number at risk, n (%) | Number of deaths, n (%) | ||
| 1 | ≤ −9 | 2756 (3.46) | 1342 (50.94) | 2856 (3.58) | 1345 (47.09) |
| 2 | −8 to −4 | 6932 (8.70) | 4222 (60.79) | 6965 (8.74) | 4222 (60.62) |
| 3 | −3 to +1 | 12,034 (15.10) | 8328 (70.17) | 12,045 (15.12) | 8328 (69.14) |
| 4 | +2 to +9 | 24,691 (30.99) | 19,757 (80.42) | 24,483 (30.73) | 19,757 (80.70) |
| 5 | ≥10 | 33,268 (41.75) | 30,380 (90.93) | 33,332 (41.83) | 30,380 (91.14) |
| All | 79,681 | 64,032 (80.53) | 79,681 | 64,032 (80.36) | |
| c-statistic (95% confidence interval) | 0.71 (0.70, 0.71) | 0.71 (0.71, 0.72) | |||
Figure 2.
Kaplan-Meier plot of survival probabilities in the derivation and validation cohorts by the score group.
Scoring System in the Kidney Transplant Population
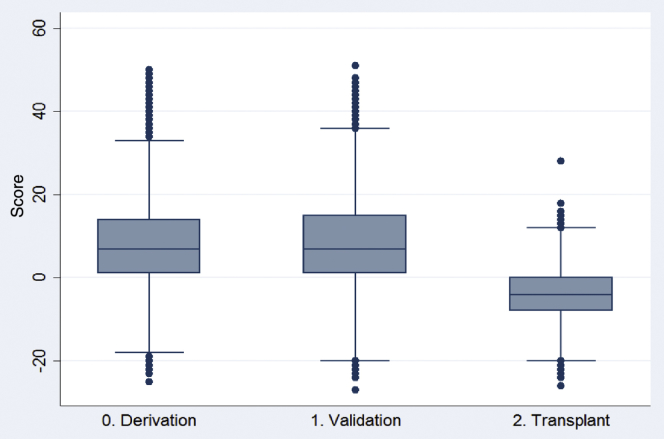
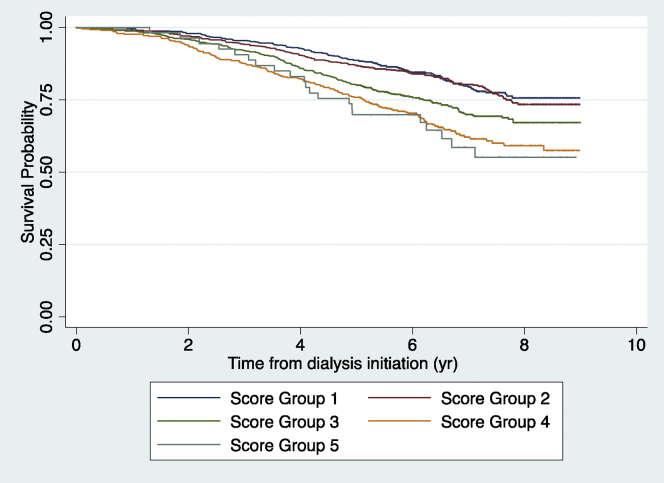
A total of 2397 patients 70 years or older between 2006 and 2009 received a kidney transplant before 2014, the majority of them before 5 years of dialysis. Of those, 1624 (67.75%) received deceased donor kidney transplants and 603 of them died within 5 years of beginning renal replacement therapy (be that dialysis or transplantation). When the scoring system was applied to this cohort of kidney transplant recipients who would otherwise have qualified for the derivation or validation cohort, the resulting score distributions were as can be found in Table 4. The range in scores for this cohort was −26 to +28 with a median of −4 and an SD of 6.42. More than 50% of these patients were in the lowest 2 score groups (all negative scores) and only 2% were in the highest score group. The c-statistic for the prediction of 5-year mortality in this kidney transplant cohort was 0.60 (95% confidence interval 0.57–0.63). Figure 3 demonstrates the difference in the distribution of scores between the transplant cohort and the other 2 cohorts. Whereas the distributions of the derivation and validation cohorts basically overlap, the distribution of the transplant cohort is shifted to the left. The transplanted group generally had fewer comorbidities, were younger, and had less diabetes than the other 2 cohorts (Supplementary Table S2). They also had much larger proportions receiving nephrologist care before dialysis initiation or with hypertension. Figure 4 shows the Kaplan Meier plot of survival in the transplanted cohort by the score group, which showed a significant survival difference between score groups by the log rank test (P < 0.001).
Table 4.
Score distributions and mortality in the cohort of patients who received a kidney transplant
| Score group | Actual score | Transplanted cohort |
|
|---|---|---|---|
| Number at risk, n (%) | Number of deaths, n (%) | ||
| 1 | ≤ −9 | 571 (23.82) | 109 (19.09) |
| 2 | −8 to −4 | 676 (28.20) | 138 (20.41) |
| 3 | −3 to +1 | 712 (29.70) | 198 (27.81) |
| 4 | +2 to +9 | 385 (16.06) | 137 (35.58) |
| 5 | ≥ 10 | 53 (2.21) | 21 (39.62) |
| All | 2397 | 603 (25.16) | |
| c-statistic (95% confidence interval) | 0.60 (0.57, 0.63) | ||
Figure 3.
Box-and-whisker plot of score distributions for each cohort.
Figure 4.
Kaplan-Meier plot of survival probabilities of the transplanted cohort from the date of dialysis initiation.
Discussion
We developed a scoring system to assess 5-year mortality risk in US incident dialysis patients 70 years or older to determine clinical appropriateness for transplant referral. The 5-year mortality event rate in the population was 80%, which is much higher than that of the general dialysis population in the United States that has a 5-year mortality event rate of approximately 60%.11 However, the older ESRD population demonstrated significant variability in mortality—40% of this population had a 90% 5-year mortality rate, whereas approximately 3% had a 50% 5-year mortality rate.
Our goal was to identify the group of older patients who would derive the greatest benefit from transplantation. A score of −4 or less could be used to identify patients for transplantation referral. This cutoff identifies the top 12% (those in score groups 1 and 2) of older dialysis patients. The first group includes 3.5% of this population with a 50% 5-year mortality risk and a second group that contains nearly 9% of this population with approximately 60% 5-year mortality risk. These mortality risks are comparable to the 5-year mortality risk of the general ESRD population that is approximately 60% for hemodialysis patients.11 Because this score is a screening tool for referral to transplantation evaluation, we should aim to be more inclusive rather than more exclusive. For those falling outside of the lowest score groups, individual considerations may still take precedence, especially if most of the score derives from chronologic age alone rather than other comorbidities. Apart from its use in transplant referral, calculating this prediction score for each individual allows the health professional to have a starting place for comparison and a framework with which to approach patients and initiate patient-care discussions.
We found that the characteristics with the strongest association with 5-year mortality were chronologic age greater than 80, BMI less than 18, the presence of congestive heart failure, chronic obstructive pulmonary disease, immobility, or being institutionalized. Increasing age, heart failure and chronic obstructive pulmonary disease have also been correlated with increased first year mortality in incident dialysis patients in other countries.12, 13 Immobility or institutionalized status has been found to be associated with mortality in the ESRD or elderly population, and dialysis initiation portends worsening functional status in institutionalized dialysis patients.7, 14 Low BMI has been found to be correlated with increased mortality risk in the general white population in the United States and Switzerland.15, 16 It is hypothesized that low BMI in the general population is an indicator of comorbid conditions like cancer or respiratory disease, though the Swiss study showed that mortality in the low BMI group is largely due to external causes rather than comorbid conditions.16 BMI less than 18 has also been found in retrospective studies of US and European dialysis patients to be highly correlated with increased mortality.17 In our study population, BMI less than 25, which is typically considered the cutoff for normal, was also found to be associated with increased 5-year mortality, though the strength of the association was weaker than that for the BMI less than 18. These findings are suggestive of the fact that “normal” or “healthy” BMI of the dialysis population may be higher than that of the general population. Dialysis patients are known to experience higher rates of protein-calorie malnutrition and generally have lower BMI than their age- and sex-matched controls in the general population.18 Older adults may be particularly vulnerable to malnutrition and thus exhibit a more pronounced mortality difference at what are considered low and normal BMI in the general population. Furthermore, those with BMI higher than 35 in our study did not exhibit a significantly different mortality risk from those with BMI in the 25–35 range. These findings are consistent with the “obesity paradox” observed among older patients in the general population and also corroborated in the dialysis population.17, 19 In fact, studies have shown that patients who are obese before transplant actually do better after transplant than those with low BMI, though they do have higher wound complications.20
The strongest associations to 5-year survival were any race other than white, a primary cause of ESRD other than diabetes, being employed within 6 months of dialysis initiation, and having an arteriovenous fistula at the time of dialysis start. Hypertension was also predictive of 5-year survival, which is consistent with other publications’ finding that hypotension, particularly during dialysis, is correlated with worse outcomes in the ESRD population.21 A Canadian study also showed improved 1-year mortality in patients with hypertension.13 Furthermore, hypertension was present in a much higher proportion of the transplanted cohort, also suggesting that hypertension is associated with better prognosis. These findings of hypertension in the dialysis population actually portending better outcomes will need further delineation to distinguish the possible mechanism of protection. Patients may be better able to tolerate dialysis treatments because they have higher blood pressures. Patients with hypertension may also be on blood pressure medications that may protect them against cardiovascular comorbidities. We also found that older racial and ethnic minority patients with ESRD all have higher 5-year survival than white patients. Our work is consistent with prior research on older adults that shows that compared with whites, racial and ethnic minorities have greater survival on dialysis and improved post-transplant survival.22, 23, 24 However, despite improved survival of older Hispanics, blacks, and Asians compared with their white counterparts, whites are still over-represented in the transplanted cohort.
This study has several strengths. It is based on a large cohort of patients that includes the entire US dialysis population with a very small percentage excluded. The characteristics examined are derived from information readily available to the nephrologist on a patient’s initiation at their dialysis unit, making the score applicable to patients who initiate dialysis and easy to use by nephrologists. The score developed captures not only baseline demographic and medical information, but also some information about the patient’s functional status (mobility, assistance with activities of daily living), which are increasingly recognized as important predictors of outcomes in the elderly population.25, 26
Furthermore, although this score is more useful for potential deceased donor kidney transplant candidates, it can be applied to older patients with potential living donors as well as to help prognosticate posttransplant survival. When applied to the transplanted cohort, our score had a c-statistic of 0.60 for the prediction of posttransplant mortality, which is unsurprising because mortality risk after receipt of a kidney transplant includes different predictors than the current model. This c-statistic is also similar to the performance of the Estimated Post Transplant Survival score currently used to risk stratify potential kidney recipients, which had a c-statistic of 0.67 to 0.69 in an external validation study.27 Furthermore, because the transplantation surgery itself is not without risk, the scoring system may help identify patients who may not be able to tolerate the surgery. For example, those patients in the highest risk score group may need further scrutiny to determine their viability for surgical intervention regardless of the donor type. The use of this score may also be adjusted in those potential recipients with living donors such that perhaps an even more inclusive cutoff may be used to identify potential recipients to maximize the benefit of transplantation in relation to the risk of surgery for both recipients and donors.
One potential critique of this study is the exclusion of the kidney transplant recipients from the derivation and validation cohorts because their information could have contributed to a better, more discriminatory scoring system. However, transplant recipients needed to be excluded because we used logistic regression modeling for score development. Although a survival analysis approach like the Cox proportional hazards model would have allowed their inclusion through censoring of transplant patients at the time of transplantation, the data violated the basic proportional hazards assumption. In addition, the majority of patients who received transplants were in the lowest 2 score categories. Furthermore, given the small number of patients in the transplanted cohort (n = 2297) compared with the number of patients in the derivation and validation cohorts (n = 159,362), we are confident that the transplant cohort would have had a very small effect on the ultimate score model.
This study is limited in that the data used are derived from the Medical Evidence Form 2728, which is typically completed by the physician, nurse, or social worker at the patient’s dialysis unit. The comorbidities examined are reported in the form of check boxes, which may be completed with varying degrees of accuracy.28 Also, because of the check-box way that comorbidities are listed on the Medical Evidence Form, the severity of certain important comorbidities could not be assessed. For example, the degree of congestive heart failure is not noted, nor the type or stage of cancer, both of which may preclude transplantation immediately without consideration of any other characteristic. This form also does not include potentially relevant information such as behavioral disorders or liver disease, which may impact survival.7 The lack of granularity in this dataset precludes a very robust discriminatory ability in the scoring system. Furthermore, this scoring system does not include discriminators such as gait speed, frailty, cognitive impairment, or social support that can be obtained from a comprehensive geriatric assessment and that have been found to correlate with mortality.25, 29, 30
In conclusion, we developed a predictive risk score to evaluate elderly incident dialysis patients and determine their suitability for referral for kidney transplantation. Given the heterogeneity of this patient group, the score can help broaden referral decisions and provide a clinical rationale for nonreferral. The score is not meant to be all encompassing, but aims to provide guidance for referring physicians and transplant professionals when making important clinical decisions in a heterogeneous population. Further work evaluating the performance of this score in a prospective manner still needs to be done.
Disclosure
All the authors declared no competing interests. MRS is supported by a career development award from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK K23 DK103111). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Acknowledgment
An earlier version of this work was presented as an oral abstract at the American Transplant Congress in Boston, MA, in June 2016.
Footnotes
Appendix S1. ESRD medical evidence report Medicare entitlement and/or patient registration.
Table S1. Crude associations between candidate predictors and 5-year mortality.
Table S2. Baseline characteristics of the transplanted cohort by the score group.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
ESRD medical evidence report Medicare entitlement and/or patient registration.
Crude associations between candidate predictors and 5-year mortality.
Baseline characteristics of the transplanted cohort by the score group.
References
- 1.Abramowicz D., Cochat P., Claas F.H.J. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant. 2015;30:1790–1797. doi: 10.1093/ndt/gfu216. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.Rao P.S., Merion R.M., Ashby V.B. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche H., Ojo A., Hanson J. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69:885–889. doi: 10.1097/00007890-200003150-00037. [DOI] [PubMed] [Google Scholar]
- 5.Gill J.S., Schaeffner E., Chadban S. Quantification of the early risk of death in elderly kidney transplant recipients. Am J Transplant. 2013;13:427–432. doi: 10.1111/j.1600-6143.2012.04323.x. [DOI] [PubMed] [Google Scholar]
- 6.Grams M.E., Kucirka L.M., Hanrahan C.F. Candidacy for kidney transplantation of older adults. J Am Geriatr Soc. 2012;60:1–7. doi: 10.1111/j.1532-5415.2011.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusseux E., Albano L., Fafin C. A simple clinical tool to inform the decision-making process to refer elderly incident dialysis patients for kidney transplant evaluation. Kidney Int. 2015;88:121–129. doi: 10.1038/ki.2015.25. [DOI] [PubMed] [Google Scholar]
- 8.Goodkin D.A., Bragg-Gresham J.L., Koenig K.G. Association of Comorbid Conditions and Mortality in Hemodialysis Patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 9.Matas A.J., Smith J.M., Skeans M.A. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;15(suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 10.Pavlou M., Ambler G., Seaman S.R. How to develop a more accurate risk prediction model when there are few events. BMJ. 2015;351:h3868. doi: 10.1136/bmj.h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 12.Chua H.R., Lau T., Luo N. Predicting first-year mortality in incident dialysis patients with end-stage renal disease—The UREA5 Study. Blood Purif. 2014;37:85–92. doi: 10.1159/000357640. [DOI] [PubMed] [Google Scholar]
- 13.Quinn R.R., Laupacis A., Hux J.E. Predicting the risk of 1-year mortality in incident dialysis patients: accounting for case-mix severity in studies using administrative data. Med Care. 2011;49:257–266. doi: 10.1097/MLR.0b013e318202aa0b. [DOI] [PubMed] [Google Scholar]
- 14.Kurella Tamura M., Covinsky K.E. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrington de Gonzalez A., Hartge P., Cerhan J.R. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roh L., Braun J., Chiolero A. Mortality risk associated with underweight: a census-linked cohort of 31,578 individuals with up to 32 years of follow-up. BMC Public Health. 2014;14:1–9. doi: 10.1186/1471-2458-14-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leavey S.F., McCullough K., Hecking E. Body mass index and mortality in 'healthier' as compared with 'sicker' haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16:2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 18.Kopple J.D., Zhu X., Lew N.L., Lowrie E.G. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 19.Oreopoulos A., Kalantar-Zadeh K., Sharma A.M., Fonarow G.C. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009;25:643–659. doi: 10.1016/j.cger.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Chung H., Lam V.W.T., Yuen L.P.K. Renal transplantation: better fat than thin. J Surg R. 2015;194:644–652. doi: 10.1016/j.jss.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Shoji T., Tsubakihara Y., Fujii M., Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 22.Ilori T.O., Adedinsewo D.A., Odewole O. Racial and ethnic disparities in graft and recipient survival in elderly kidney transplant recipients. J Am Geriatr Soc. 2015;63:2485–2493. doi: 10.1111/jgs.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucirka L.M., Grams M.E., Lessler J. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306:620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee C.M., Lertdumrongluk P., Streja E. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39:183–194. doi: 10.1159/000358497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallenberg M.H., Kleinveld H.A., Dekker F.W. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD—a systematic review. Clin J Am Soc Nephrol. 2016;11:1624–1639. doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thamer M., Kaufman J.S., Zhang Y. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis. 2015;66:1024–1032. doi: 10.1053/j.ajkd.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton P.A., McDonald S.P., Snyder J.J. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am J Transplant. 2014;14:1922–1926. doi: 10.1111/ajt.12761. [DOI] [PubMed] [Google Scholar]
- 28.Longenecker J.C., Coresh J., Klag M.J. Validation of comorbid conditions on the end-stage renal disease medical evidence report. The CHOICE Study. J Am Soc Nephrol. 2000;11:520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 29.Kutner N.G., Zhang R., Huang Y., Painter P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US Renal Data System Special Study. Am J Kidney Dis. 2015;66:297–304. doi: 10.1053/j.ajkd.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIsaac D.I., Bryson G.L., van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg. 2016;151:538–545. doi: 10.1001/jamasurg.2015.5085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ESRD medical evidence report Medicare entitlement and/or patient registration.
Crude associations between candidate predictors and 5-year mortality.
Baseline characteristics of the transplanted cohort by the score group.