Abstract
Caffeine is the world’s most popular psychoactive drug and is also an active adulterant found in many drugs of abuse, including seized cocaine samples. Despite several studies which examine the effects of caffeine or cocaine administered as single agents, little data are available for these agents when given in combination. The purpose of the present study was to determine if combined intake of both psychostimulants can lead to maladaptive changes in striatal function. Mice were injected with a binge regimen (intermittent treatment for 13 days) of caffeine (3 × 5 mg/kg), cocaine (3 × 10 mg/kg), or combined administration. We found that chronic caffeine potentiated locomotion induced by cocaine and that both caffeine-treated groups showed sensitization. Striatal tissue was obtained 24 h and 7 days after last injection (withdrawal) for immunohistochemistry and mRNA expression. Our results show that combined intake of both psychostimulants can increase GFAP immunoreactivity in the striatum at both times post treatment. Gene expression analysis, targeted at dopamine, adenosine, and glutamate receptor subunit genes, revealed significant transcript down-regulation in the dorsal striatum of AMPA, NMDA, D1 and D2 receptor subunit mRNA expression in the group that received combined treatment, but not after individual administration. At withdrawal, we found increased D1 receptor mRNA expression along with increased A1, AMPA, NMDA, and metabotropic subunit expression. A2A mRNA showed decreased expression after both times in all experimental groups. Our study provides evidence that there are striatal alterations mediated by combined caffeine and cocaine administration, and highlights negative outcomes of chronic intake of both psychostimulants.
Keywords: Cocaine, Caffeine, Striatum, Locomotor sensitization, Dopamine, Astroglia
Introduction
The methylxanthine caffeine is the world’s most popular psychoactive drug. In recreational drug use settings, caffeine is found in a range of commercially available products such as energy drinks that may be consumed with other drugs (Reissig et al. 2009). The primary effect of caffeine is to relieve fatigue and enhance mental performance, but excessive ingestion might lead to intoxication and withdrawal symptoms if intake is discontinued (Dews et al. 2002). DSM-IV did not include caffeine dependence as a substance use disorder but caffeine withdrawal was included as a research diagnosis to encourage investigation (Hasin et al. 2013).
Apart from analyzing the potential clinical importance of caffeine by itself, another aspect that deserves consideration is its interactions with other addictive substances (Ferré 2013). It needs to be noted that forensic analyses of seized illicit drug samples have reported quantities of caffeine mixed with other stimulants, including cocaine and amphetamines (Cole et al. 2011). As an example relevant to the objectives of the present study, cocaine paste which is an intermediate product of the cocaine alkaloid extraction process from coca leaves has a high content of cocaine base mixed with other chemical substances and it is typically adulterated with caffeine (Lόpez-Hill et al. 2011).
Several preclinical and clinical studies have suggested that caffeine can augment the reinforcing (Schenk et al. 1994; Kuzmin et al. 1999), motor stimulant (Gasior et al. 2000), and subjective effects (Jones and Griffiths 2003, Liguori et al. 1997) of drugs of abuse, with potential implications for human drug users. It is also likely that concomitant consumption of caffeine with other stimulant drugs such as cocaine can profoundly alter the drug response and induce adverse interactive effects, both on the SNC and on the other organs. Our group has recently described that caffeine and cocaine administration can produce testicular toxicity and that caffeine can potentiate some of the detrimental changes induced by cocaine on testicular tissue (Gonzalez et al. 2015). Previous reports in animal models showed that high doses of caffeine are required to amplify the toxicity of d-amphetamine and cocaine as reported by Derlet et al. (1992), while lower doses of caffeine are sufficient to promote toxicity and lethality when combined with MDMA or MDA (McNamara et al. 2006; Vanattou-Saϊfoudine et al. 2012). Also, caffeine potentiated the toxic effect of methamphetamine in the striatum (Sinchai et al. 2011), a brain structure that is implicated in psychostimulant-induced neuroplasticity (Kalivas and O’Brien 2008). Furthermore, glial fibrillary acidic protein (GFAP), a cytoskeletal intermediate filament protein exclusively expressed in astrocytes (Bignami et al. 1972), has been previously demonstrated to be responsive to cocaine administration (Freeman et al. 2010; Blanco-Calvo et al. 2014). Little is known about glial responses evoked by chronic caffeine treatment.
The striatum receives synaptic inputs from many different sources. Glutamatergic afferents arrive from areas of the cortex and the thalamus, whereas the nigrostriatal pathway and intrinsic circuits provide the striatum with acetylcholine, GABA, dopamine (DA), nitric oxide, and adenosine (A) (Centonze et al. 2003, Ferré et al. 2010). The A1 receptor (A1R) and A2A receptor (A2AR) are primarily responsible for the central effects of adenosine (Dunwiddie and Masino 2001). A2AR are more concentrated in the striatum than anywhere else in the brain, while A1R are more widespread distributed (Fredholm et al. 2001; Rosin et al. 2003). The same receptors are also the main target of moderate, nontoxic doses of caffeine that acts as A1R and A2AR antagonist (Fredholm et al. 1999). In addition, caffeine is a well-known antagonist of inositol trisphosphate (IP3) receptors and an agonist of ryanodine receptors (RyRs) (McPherson et al. 1991). At the neuronal level, caffeine is capable of changing intracellular [Ca2+] levels through ryanodine intracellular stores (Garaschuk et al. 1997). On the other hand, cocaine blocks all three neuronal membrane monoamine transporters with approximately equal affinity. By blocking the dopamine transporter (DAT), cocaine allows released DA to persist in the extracellular space, extending DA receptor activation leading to behavioral activation (Volkow et al. 2012).
Despite several studies which examined caffeine or cocaine as single agents, little data are available for these agents given in combination. Complex interactions have now been clearly shown for A1R and dopamine receptor 1 (D1R) and A2AR and dopamine receptor 2 (D2R) in the striatum (Ferré et al. 2010). In fact, there are indications of the involvement of A2AR and dopamine receptors in the locomotor and sensitizing effects of cocaine (Filip et al. 2006). Moreover, a recent study suggested that caffeine might enhance DA signaling in the striatum by increasing D2/D3R levels or their affinity in the human brain (Volkow et al. 2015).
Several studies reported detrimental effects of caffeine when combined with other drugs of abuse (i.e., co-administration of caffeine with methamphetamine or MDMA). However, information is lacking on the impact of caffeine combined with cocaine on striatal function. Therefore, the purpose of the present study was to determine if combined intake of both psychostimulants leads to maladaptive changes and/or additive effects in the striatum. Particularly, neuroinflammation of the central nervous system (CNS) seems to participate in neuroplasticity changes and sensitizing effects of psychostimulants (Cadet and Bisagno 2014). Thus, in the present study treated mice were behaviorally tested for acute locomotor effects and sensitization after chronic intermittent treatment with caffeine, cocaine, or the combination of both psychostimulants. As mentioned before, information is scarce regarding potential neuroplastic changes involving striatal neuronal and glial cells produced by combined chronic treatment of caffeine and cocaine. Additional information is needed since striatal neuroadaptations could underlie some of the behavioral changes associated with chronic drug use (Cadet and Bisagno 2016) affecting cortico-striatal network physiology. Thus, we investigated the effect of psychostimulant treatments on reactive astrogliosis in the striatum at two different time points: (a) 24 h after the last binge injection and (b) 7 days after the last binge injection (withdrawal). Additionally, we measured mRNA expression of several dopaminergic, adenosinergic, and glutamatergic receptor subunits associated to psychostimulant-induced neuroplasticity on striatal tissue (Ferré et al. 2010).
Materials and Methods
Animals
Male C57BL/6 mice (20–25 g) from the School of Exact and Natural Sciences of the University of Buenos Aires were housed in a light- and temperature-controlled room. Mice had free access to food and water. Principles of animal care were followed in accordance with “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council, 2003), and approved by Universidad de Buenos Aires authorities (Protocol Number: A5801-01) using OLAW and ARENA directives (NIH, Bethesda, USA).
Pharmacological and Physiological Procedures
Cocaine hydrochloride and caffeine (Sigma-Aldrich, St Louis, MO) were administered in a binge-like regimen of three i.p. injections per day, 1 h apart. Animals were assigned to four different groups: Coc (Cocaine 3 × 10 mg/kg), Caf (Caffeine 3 × 5 mg/kg), Caf-Coc (3× Cocaine + Caffeine combined solution: Coc 10 mg/ kg +Caf 5 mg/kg, both dissolved in the same sterile saline solution and co-administered in a single injection), or Veh (3× sterile saline), see Fig. 1.
Fig. 1.
Schematic representation of the experimental protocol used in this study. Male C57BL/6 mice were subjected to an intermittent chronic treatment (one day on/one day off for 13 days) of caffeine 5 mg/Kg (Caf), cocaine 10 mg/kg (Coc), and their combination (5 mg/kg Caf + 10 mg/kg Coc) in a binge protocol: 3 i.p. injections per day, 1 h apart. Locomotor activity was evaluated at Day 1 and Day 13 in an open field with 30 min of habituation before the first injection. Tissue samples were taken 1 day and 7 days after the last binge injection (Days 14 and 21, respectively)
The induction of locomotor sensitization depends on the temporal pattern of drug exposure. Repeated intermittent treatments with moderate doses of drugs are more effective for inducing locomotor sensitization than continuous exposure to high or escalating drug doses (Robinson and Berridge 1993; Vanderschuren and Kalivas 2000). Accordingly, we selected an intermittent protocol of psychostimulant administration, i.e., mice received binge injections 1 day on/1 day off during 13 days for a total of 7 binges, see Fig. 1. Striatal tissue for gene and protein expression was obtained 24 h after the last injection (Experiment #1), to investigate the effect of chronic treatment using both psychostimulants. It should be noted that striatal tissue obtained 24 h after the last binge injection likely reflects the consequences of chronic administration of both caffeine and cocaine. Since the mice were subjected to an intermittent chronic treatment (one day on/1 day off for 13 days), it is unlikely that the mice would perceive this time as “withdrawal” (since mice were already used to intermittent injections).
For Experiment #2, we also used the same protocol as Experiment #1, but in this case mice were sacrificed 7 days after last binge injection. At that time, striatal tissue was also collected to investigate the effect of withdrawal from chronic treatment with both psychostimulants.
Behavioral Studies
Locomotor Activity
Mice were housed in plastic cages for at least 1 week prior to the initiation of locomotor experiments. Mice were moved to an experimental room 12 h before drug treatment. Food and water were provided ad libitum in a temperature-con-trolled room, with a 14/10-h light/dark cycle. Locomotor activity (total distance in cm) was recorded using a CCD camera (Sony, U.S.A.) in custom-designed open-field boxes located in a sound-attenuated room. For acquisition and analysis, we used Ethovision XT 7.0 software (Noldus, The Netherlands). Each box consisted of an open-field compartment (19 × 40 × 40 cm). On injection Days 1 and 13, animals were placed in open-field boxes for 30 min (recorded as baseline), and later injected with drugs or saline. Total distance traveled (in cm) was quantified in 5-min bins for a total of 180 min after the first injection (i.e., 60 min were recorded after each of the three drug injections). Behavioral recordings were made simultaneously in four open-field arenas using the Ethovision XT multiple arena feature from 9 AM to 4 PM of the light period of the light/dark cycle, similar to a previously published study (Bisagno et al. 2010). Injection time and arenas (right and left) were counterbalanced across experimental groups.
Immunohistochemistry for GFAP
Animals were deeply anesthetized 24 h or 7 days after treatments with ketamine–xylazine (0.5 ml/kg i.p.; 1.4 ml/ kg i.p., respectively) and then transcardially perfused with 30 ml of phosphate buffer saline (PBS) 10 mM (pH 7.4) followed by 80 ml of ice-cold paraformaldehyde 4 % (Sigma, USA) diluted in 0.1 M (pH 7.4) PBS. Brains were dissected and placed in the same fixative solution for 16 h at 4 °C, and put in PBS/sucrose 30 % for cryoprotection until coronal sections were cut throughout the striatum.
Immunostaining for GFAP was performed on 25-µm free-floating coronal serial slices from striatal sections. During all staining procedures 0.1 M PBS containing 0.3 % Triton X-100 (PBS-T) was used for diluting all immunoreagents and for washing after incubating with antibodies. Sections were incubated in 2 % H2O2 followed by 2 % normal goat serum (NGS) in PBS-T and exposed to a rabbit anti-GFAP antibody (1:500, Sigma, USA) overnight at 4° C. After washing, sections were incubated for 2 h in biotinylated goat antirabbit IgG antisem (1:250, Sigma, USA) followed by the avidin–biotin peroxidase complex (1:125, Vectastain, ELITE ABC kit, Vector Laboratories). Chromogenic reactions were induced with 0.5 mg/ml DAB and 0.015 % H2O2. Sections were then rinsed, mounted on gelatin-coated slides, air-dried, dehydrated, cleared, and cover slipped.
GFAP-immunoreactive area was quantified as follows: light microscopic examination of microtome sections revealed GFAP-positive cell staining throughout the striatum, with scattered distribution. Data were collected with the help of mapping software (Mercator Pro; Explora Nova, France), coupled to a Nikon microscope. This software has an embedded navigation system that allowed us to draw maps of the entire dorsal striatum (left and right hemispheres) at low magnification (×4), identify a GFAP-stained cell, and then use a higher magnification (×60) to confirm GFAP-positive astrocytes and mark them. At the end of each map quantification, Mercator software displays an excel file with the # of GFAP-positive astrocytes. Cells were quantified on 3 different striatal sections, bilaterally. Data were averaged in order to have a unique estimative count per animal.
Real-Time PCR
Striatal tissue was extracted 24 h or 7 days after treatments: mouse brains were rapidly removed, and striatal tissues were dissected, placed on dry ice, and then stored at −70 °C in RNAlater (Qiagen) until further assays. Total RNA was isolated using TRIZOL reagent (Invitrogen) according to the manufacturer’s protocol. Five hundred nanograms of RNA were treated with DNAseI (Invitrogen) and reverse-transcribed in a 20 µL reaction using M-MLV reverse transcriptase (Promega) and random hexamers (Biodynamics). For quantitative real-time PCR, primer sets were designed for the specific amplification of murine Drd1a, Drd2, Adora1, Adora2a, Gria1, Gria2, Grin1, Grin2a, Grin2b, Grtn1, Grm5, and Gapdh as a housekeeping control gene (sequences listed in Table 1). Each sample was assayed in duplicate using 4 pmol of each primer, 1X SYBR Green Master Mix (Applied Biosystems), and 2–20 ng of cDNA in a total volume of 13 µL. Amplification was carried out in an ABI PRISM 7500 Sequence Detection System (Applied Biosystems).
Table 1.
Primer sequences
| Gene | Ac. Number | Primer forward | Primer reverse |
|---|---|---|---|
| Drd1a | NM_010076 | TTCTTCCTGGTATGGCTTGG | GCTTAGCCCTCACGTTCTTG |
| Drd2 | NM_010077 | TATGCCCTGGGTCGTCTATC | AGGACAGGACCCAGACAATG |
| Adora1 | NM_001039510 | TAGACAGTTCAGGTGGCCAG | AGTACATTTCCGGGCACAGA |
| Adora2a | NM_009630 | CGCAGAGTTCCATCTTCAGC | ACGTCCTCAAACAGACAGGT |
| Gria1 | NM_001113325 | CTGTGAATCAGAACGCCTCA | TCACTTGTCCTCCACTGCTG |
| Gria2 | NM_001083806 | ATTTCGGGTAGGGATGGTTC | GTTGGGAAGCTTGGTGTGAT |
| Grm1 | NM_016976 | CGAGAAGAGCTTTGATCGGC | TGTCTGCCCATCCATCACTT |
| Grm5 | NM_001081414 | CAGCCTAGTCAACCTGTGGA | TCTCTGTGCTCTTGGGGAAG |
| Grin1 | NM_008169 | ACTCCCAACGACCACTTCAC | GTAGACGCGCATCATCTCAA |
| Grin2a | NM_008170 | TTGTCTCTGCCATTGCTGTC | CAAAGAAGGCCCACACTGAT |
| Grin2b | NM_008171 | CCACACCCTGAGATTCCCTT | TCTTCAGCTCGTCGACTCTC |
| Gapdh | NM_008084 | AGTGCCAGCCTCGTCCCGTAG | GTGCCGTTGAATTTGCCGTGAGTG |
Statistical Analysis
InfoStat 2010 software (www.infostat.com.ar) was used for statistical analysis. Statistics were performed using oneway ANOVA followed by Fisher’s LSD post hoc tests. Data were transformed when required using logarithmic transformations in order to comply with ANOVA assumptions. For the analysis of locomotor sensitization, two-way repeated measures ANOVA followed by Fisher’s LSD was performed. Data are expressed as the mean ± SEM. Differences were considered significant if p < 0.05.
Results
Acute and Chronic Effects of Caffeine, Cocaine, and Their Combination on Locomotor Activity and Sensitization
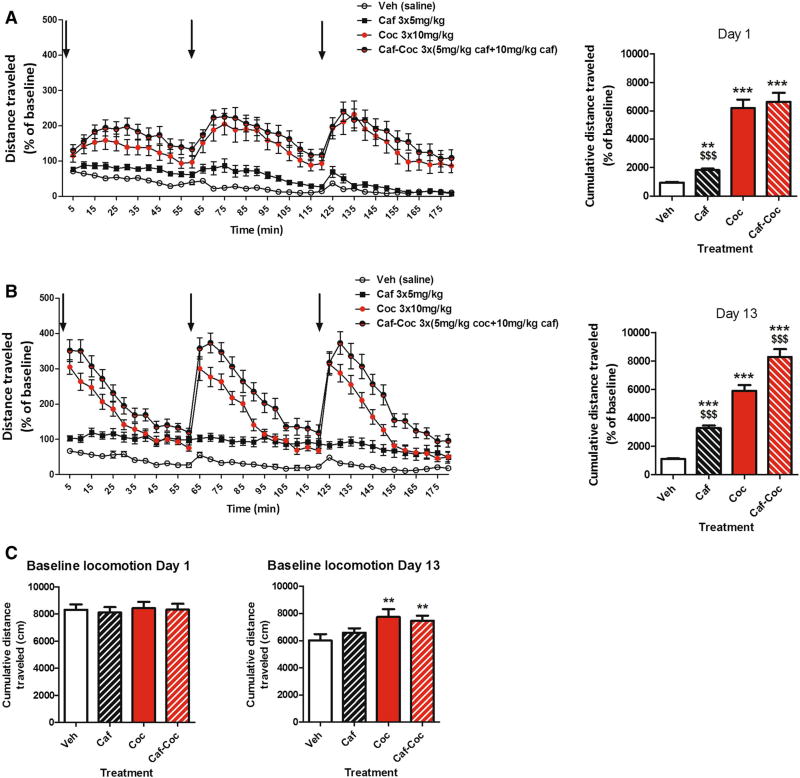
Locomotor activity was measured after acute (Day 1) and chronic (Day 13) administration of caffeine, cocaine, and the combination of both psychostimulants (administered in binge-like form, i.e., three injections, see protocol on Fig. 1). On experimental Days 1 and 13, locomotor activity was measured in all treated groups for 30 min before the first injection (baseline locomotion), and 60 min after each one of the three injections (for a total of 180 min), see Fig. 2.
Fig. 2.
Effect of chronic treatment with caffeine, cocaine, and their combination on locomotor activity. a. Acute effect. Left panel locomotor activity induced by the 3 injections of caffeine (Caf), cocaine (Coc), and their combination (Caf–Coc) during binge treatment on Day 1. Right panel cumulative distance traveled (expressed as % baseline) during binge treatment on Day 1 (180 min). b. Chronic effect. Left panel locomotor activity induced by the 3 injections of Caf, Coc, and their combination (Caf–Coc) during binge treatment on Day 13. Right panel cumulative distance traveled (expressed as % baseline) on Day 13 (180 min). c. Basal locomotor activity. Distance traveled during 30-min habituation to the locomotor arena prior to binge treatment on Day 1 and Day 13. Data are expressed as mean ± SEM (n = 14–20). The arrows indicate the time of injection. The values indicate mean ± SEM (N = 14–20). One-way ANOVA–Fisher’s LSD: *p < 0.05, **p < 0.01, ***p < 0.001 different from Veh; $p < 0.05, $$p < 0.01, $$$p< 0.001 different from Coc
Acute effects
As shown in Fig. 2a, caffeine, cocaine, and their combination induced increases in total locomotion after acute administration compared to Veh (Fig. 2a left). Figure 2a right shows accumulated distance traveled throughout Day 1 binge (for a total time of 180 min after the first injection). ANOVA–Fisher’s LSD showed significant differences among treatments (F(3,59) = 136.20, p < 0.0001), where all groups injected with psychostimulants showed increased total locomotion compared to Veh values (p < 0.01). Also, the Coc group showed higher locomotion than the Caf group (p < 0.001), and was not different from the Caf–Coc group (Fig. 2a right).
Chronic effects
As shown in Fig. 2b, after chronic psychostimulant administration, caffeine, cocaine, and their combination induced increases in total locomotion after acute administration compared to Veh (Fig. 2b left). Figure 2B right shows accumulated distance traveled throughout Day 13 binge. ANOVA–Fisher’s LSD also showed significant differences across treatments (F(3,64) = 205.84, p < 0.0001), where all groups injected with psychostimulants showed increased total locomotion compared to Veh values (p < 0.001). Also, the Coc group showed higher locomotion than the Caf group (p < 0.001), and interestingly the group that received the combination of Caf–Coc showed significantly higher values compared to the Coc group (p < 0.05).
We also quantified and analyzed baseline levels of locomotor activity on Days 1 and 13 (measured 30 min before the first injection, Fig. 2c). As expected, baseline locomotion on Day 1 showed no significant differences across treatments. However, at Day 13 ANOVA–Fisher’s LSD showed significant differences across treatments (F(3,60) = 3.95, p < 0.05). Coc and Caf–Coc groups showed higher values of basal locomotion compared to Veh (p < 0.05). Repetitive treatment was administered in the animal’s cages during the protocol with the exception of Days 1 and 13 when injections were done in the open- field cages in order to record behavior. Significant differences on Day 13 observed at baseline might be related to the fact that vehicle-treated animals showed lower baseline values on Day 13 (compared to the first exposure to open- field cages on Day 1). Open-field arenas and procedures on Day 13 might have not been seen as “novelty” for vehicle-treated animals and thus less locomotion was observed in that group.
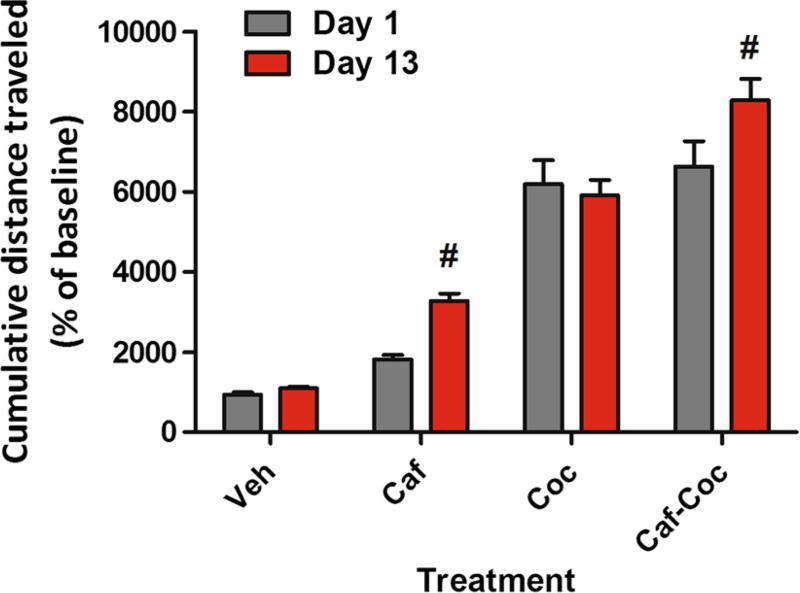
Sensitization
Locomotor sensitization to psychostimulants was defined as a greater total distance traveled after drug injections on the last administration (Day 13) compared to the first drug exposure (Day 1). Repeated measures two-way ANOVA followed by Fisher’s LSD showed significant treatment (F(3,60) = 71.77, p < 0.0001), and day (F(1,60) = 8.07, p < 0.01) effects, with no significant interaction effects (F(2,60) = 2.05, p > 0.05). As shown in Fig. 3, both the Caf-and Caf–Coc-treated groups showed increased locomotor activity on Day 13 compared to the values observed after the injections on Day 1, suggesting locomotor sensitization.
Fig. 3.
Effect of chronic treatment with caffeine, cocaine, and their combination on locomotor sensitization. Cumulative distance traveled (expressed as % baseline) on Day 1 and Day 13. The values indicate mean ± SEM (N = 14–20). Two-way repeated measures ANOVA-Fisher’s LSD: #p < 0.05 different from Day 1 of the respective treatment
It should to be noted that our treatment involved intermittent administration of low doses of caffeine in a binge form that was able to show locomotor sensitization. However, no incidence of seizures or death was observed in our study in any experimental group, unlike previous studies involving higher doses of caffeine (100 mg/kg) (Derlet et al. 1992).
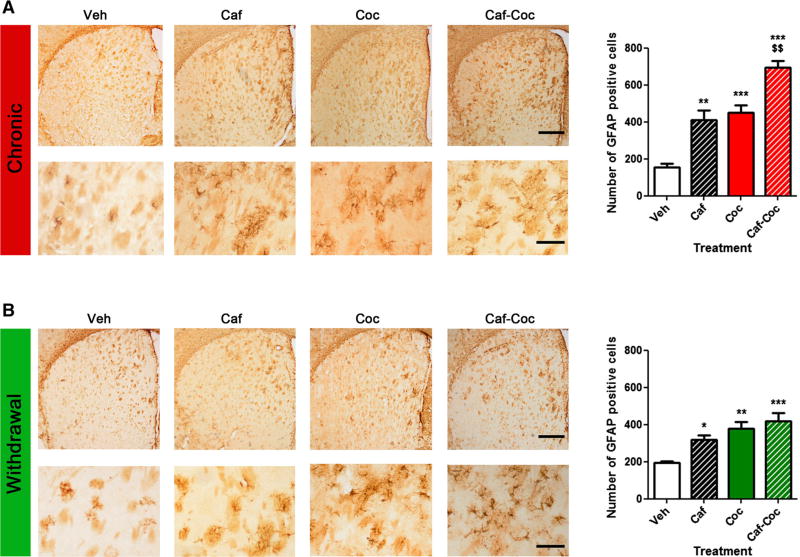
Chronic effects of caffeine and cocaine on GFAP immunoreactivity in the striatum
Astrocytes have the ability to change their molecular expression and morphology in response to different CNS insults (Sofroniew 2009). These changes on astrocytes are called reactive astrogliosis and are a hallmark of all CNS pathologies (Sofroniew 2009). Immuno-detection of GFAP, a standard marker for astrogliosis, was analyzed following chronic administration with both psychostimulants. GFAP levels have been previously demonstrated to respond to cocaine exposure (Freeman et al. 2010). However, no reports are available showing cocaine treatment increasing reactive astrogliosis in the dorsal striatum. Moreover, other conditions where other psychostimulants were co-administered with caffeine reported that caffeine can potentiate astrogliosis in different SNC areas (Khairnar et al. 2010; Frau et al. 2015). We have found increased GFAP-positive astrocytes in the dorsal striatum after chronic psychostimulant treatment and after 7 days of withdrawal. Figure 4a shows the effect of chronic treatment with both psychostimulants and their combination. ANOVA–Fisher’s LSD showed significant differences across treatments (F(3,15) = 32.81, p < 0.0001), all psychostimulant-treated groups showed significant increase in GFAP expression (p < 0.05), with the group that received the combination of caffeine and cocaine showing the highest GFAP-positive astrocyte values (p < 0.001). We also measured GFAP expression after withdrawal (Fig. 4b). ANOVA-I isher’s LSD showed significant differences across treatments (F(3, 15) = 9.69, p < 0.01); at this time, all psy- chostimulant-treated groups also showed increased GFAP immunoreactivity, (p < 0.05), but following drug washout possible additive effects of combined treatment with both psychostimulants were no longer statistically different.
Fig. 4.
Effect of caffeine, cocaine, and their combination on striatal GFAP immunoreactivity after chronic treatment or withdrawal. The values (number of GFAP-positive cells) indicate mean ± SEM (N= 4) from striatal tissue extracted after chronic treatment a or after a 7-day withdrawal period b. One-way ANOVA–Fisher’s LSD: *p < 0.05, **p < 0.01, ***p < 0.001 different from Veh; $$p < 0.01 different from Coc. Representative images of striatal sections from animals treated with either Veh, Caf, Coc, or Caf + Coc; scale bar 500 µm, and their respective higher magnification photomicrographs, scale bar 150 µm
It should to be noted that the pattern of GFAP-positive astroglia found in this study was different than the massive GFAP reaction that is usually seen after toxic doses of methamphetamine (Raineri et al. 2012, 2015). This difference in the astrogliosis produced by toxic methamphetamine and cocaine might be related to the different doses assayed in both studies (methamphetamine toxic regime involves repetitive high-dose administration) or to the toxic profile that cocaine or methamphetamine can display. GFAP-pos-itive cells using our protocol of intermittent caffeine and cocaine administration followed a scattered distribution through the striatal tissue, with more activation found in dorsal/medial striatal areas, adjacent to ventricles.
In addition, since we found increased GFAP expression after chronic treatment and withdrawal, we also evaluated the pro-apoptotic marker Bax that was previously shown to be increased in toxic psychostimulant protocols (Jayanthi et al. 2001; Raineri et al. 2012). We did not find a significant difference in Bax expression across treatments after chronic treatment or after withdrawal (data not shown).
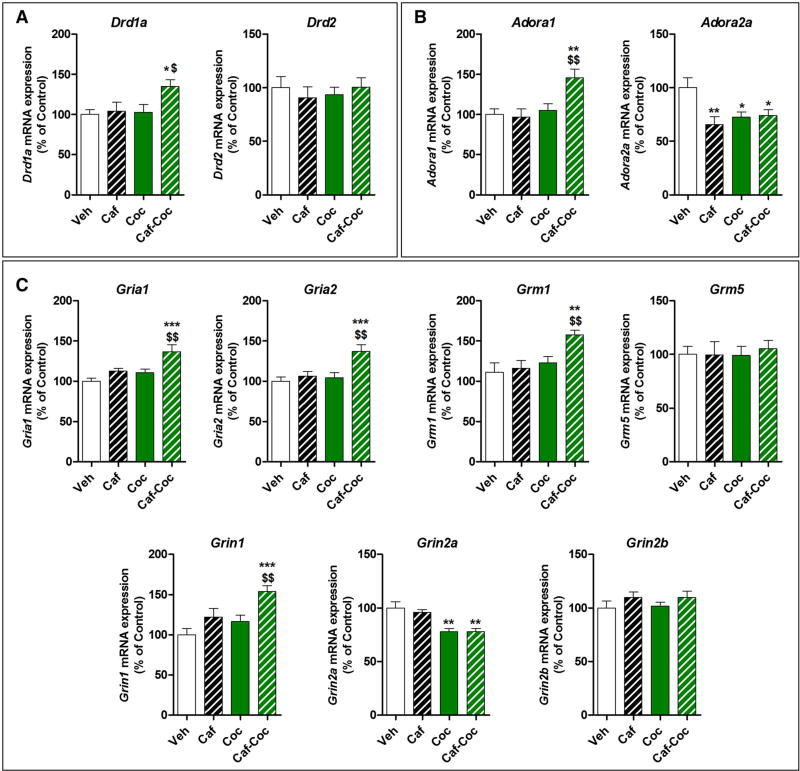
Effects of Chronic Caffeine and Cocaine Treatments on Striatal Gene Expression: Dopamine, Adenosine, and Glutamate Receptor Subunit mRNA Expression
Considering the central role of striatal circuitry in behaviors related to reward processing, motivation, emotion, and motor activity (Volkow et al. 2012), we characterized relevant gene expression in the dorsal striatum. We were interested in investigating whether dopamine, adenosine, and glutamate receptor expression would be altered after chronic psychostimulant treatments. Thus, we analyzed the effect of chronic combined treatment with caffeine and cocaine on mRNA expression of i) dopamine receptor subunits D1 (Drd1a) and D2 (Drd2), ii) adenosine receptor subunits A1 (Adora1) and A2AR (Adora2a), and iii) glutamate receptor subunits AMPA-type (Gria1 and Gria2), NMDA-type (Grin1, Grin2a, and Grin2b), and metabotropic group I (Grm1 and Grm5) in the striatum.
Chronic treatment
We found that mice receiving the combined Caf – Coc treatment showed specific changes in DA and glutamate receptor subunit expression that were not seen in the Veh, Caf, or Coc groups (Fig. 5). Specifically, ANOVA – Fish- er’s LSD showed significant differences across treatments for Drd1a (F(3,19) = 4.19, p < 0.05), Drd2 (F(3,19) = 8.03, p < 0.01), AMPA-type Gria1 (F(3,19) = 8.02, p < 0.01), and NMDA-type Grin2b (F(3,19) = 7.83, p < 0.01). We found reduced expression of these markers in the Caf – Coc group compared to Veh values (p < 0.05). Interestingly, all the psychostimulant-treated mice showed reduced expression of Adora2a after chronic treatment (F(3,19) = 11.74, p < 0.0001). Additionally, we detected a specific effect of chronic caffeine on Grin2a expression (F(3,19) = 7.23, p < 0.01). We found no significant changes in metabotropic group I glutamate receptors after any treatment.
Fig. 5.
Effect of chronic treatment with caffeine, cocaine, and their combination on striatal dopamine, adenosine, and glutamate receptor subunit mRNA expression. a Dopamine receptor subunits Drd1a (D1R) and Drd2 (D2r). b Adenosine receptor subunits Adoral (A1R) and Adora2a (A2aR). c Glutamate receptors subunits AMPA-type Grial (GluA1) and Gria2 (GluA2), NMDA-type Grin1 (GluN1), Grin2a (GluN2B), and Grin2b (GluN2B), and metabotropic group I Grm1 (mGluR1) and Grm5 (mGluR5). The values indicate mean ± SEM (N = 5). One-way ANOVA–Fisher’s LSD: *p < 0.05, **p < 0.01, ***p < 0.001 different from Veh; $p < 0.05, $$p < 0.01, $$$p < 0.001 different from Coc
Withdrawal
Following a withdrawal period of 7 days, several changes in gene expression in the striatum were observed. Mice treated with the combination of caffeine and cocaine manifested specific changes in DA and glutamate receptor subunit expression that were not seen in the Veh, Caf, or Coc groups (Fig. 6), but with a different profile of gene expression changes in comparison to the one obtained 24 h after the last injections. Specifically, ANOVA–Fisher’s LSD showed significant differences across treatments for Drd1a (F(3,21) = 3.28,p < 0.05), Adora1 (F(3,21) = 6.67,p < 0.01), AMPA-type Gria1 and Gria2 (F(3,21) = 8.02 and 7.15, respectively, p < 0.01), metabotropic Grm1 (F(3,21) = 4.8, p < 0.05), and NMDA-type Grin1 (F(3,21) = 6.72,P < 0.01), where we found increased expression of these markers in the Caf–Coc group compared to Veh values (P < 0.05). Interestingly, psychostimulant-treated mice showed reduced expression of Adora2a (F(3,21) = 4.56,P < 0.05), in a similar manner to the effects found 24 h after chronic treatment. Additionally, we observed an effect of chronic cocaine withdrawal, alone or when combined with caffeine, since both groups that received cocaine showed decreased Grin2a expression (F(3,21) = 8.64, P < 0.001).
Fig. 6.
Effect of 7-day withdrawal from chronic treatment with caffeine, cocaine, and their combination on striatal dopamine, adenosine, and glutamate receptor subunit mRNA expression. a Dopamine receptor subunits Drd1a (D1R) and Drd2 (D2r). b Adenosine receptor subunits Adoral (A1R) and Adora2a (A2aR). c Glutamate receptor subunits AMPA-type Gria1 (GluA1) and Gria2 (GluA2), NMDA-type Grin1 (GluN1), Grin2a (GluN2B), and Grin2b (GluN2B), and metabotropic group I Grm1 (mGluR1) and Grm5 (mGluR5). The values indicate mean ± SEM (N =6). One-way ANOVA–Fisher’s LSD: *p < 0.05, **p < 0.01, ***p < 0.001 different from Veh; $p < 0.05, $$p < 0.01, $$$p < 0.001 different from Coc
Discussion
The results from this study provide evidence that there are significant striatal alterations mediated by the combined caffeine and cocaine administration and highlight the potential negative outcome of chronic intake of both psychostimulants. Combined treatment was associated with increased reactive astrogliosis in striatal tissue and altered gene expression involved in dopaminergic, adenosinergic, and glutamatergic transmission. We also show that some of these detrimental effects persist into withdrawal.
Disturbances of the dorsolateral striatum glutamatergic system are well documented to influence stimulus response, habit learning, and compulsive behaviors (Vollstädt-Klein et al. 2010). Astrocytes can regulate synaptic transmission between neurons by modifying the concentration of extracellular potassium, controlling local blood flow, by releasing and/or taking up neurotransmitters or neuromodulators, by delivering nutrients to neurons, and by altering the geometry and volume of the brain extracellular space (Araque et al. 2014). Therefore, astrocytes are major contributors to synaptic plasticity induced by drugs of abuse (Cadet and Bisagno 2014), and can react to various neurodegenerative insults rapidly, leading to vigorous astrogliosis (Eng et al. 2000). Also, it has been shown that glutamate receptor activation can increase the expression of GFAP (Bowers and Kalivas 2003). It has been suggested that reactive astrogliosis might result following altered neuronal activity, particularly synaptic activity (Maragakis and Rothstein 2006). In our study, all psychostimulant-treated groups showed increased astrogliosis after chronic treatment. Even if many studies are not available reporting cocaine effects on astrocyte reactivity, GFAP has been previously demonstrated to be responsive to cocaine administration (Freeman et al. 2010; Blanco-Calvo et al. 2014). In addition, it was previously shown that caffeine enhanced astroglia and microglia reactivity induced by MDMA in the mouse striatum (Khairnar et al. 2010) and that caffeine can potentiate MDMA-induced neurotoxicity (Frau et al. 2015). Moreover, caffeine potentiated the neurotoxic effects of methamphetamine (Sinchai et al. 2011). Caffeine detrimental effects when given in combination with other psychostimulants might not be exclusive on CNS physiology. Recently, our group (using the same experimental protocol and drug doses) has shown that caffeine can potentiate cocaine toxicity in different cellular components of the mouse testicular tissue (Gonzalez et al. 2015) and that combined administration of both psychostimulants also led to increased lipid peroxidation and altered components of a local dopaminergic system in the testis.
Central effects elicited by caffeine at pharmacologically relevant doses are believed to be principally mediated by an interaction with A1 or A2 subtypes of adenosine receptors since the putative brain concentration is attained with moderate doses (doses not high enough to elicit blockade of adenosine cAMP-phosphodiesterase activity (Fredholm 1995). Adenosine, acting on type-2 receptors, is an effective endogenous anti-inflammatory agent that can modulate inflammation both in the periphery and the brain (Rosi et al. 2003). It is possible that continuous blockade of A2 receptors by chronic caffeine treatment may have disrupted inflammatory responses involving astrocyte reactivity in striatal tissue. In addition, A2AR was found in astrocytes, which co-localized with different glutamate transporters, suggesting the potential role of astrocytic A2AR in the control of pathophysiological processes known to be influenced by glial activity (Matos et al. 2012). It should be noted that chronic cocaine was found to increase reactive oxygen species production in the striatum (Dietrich et al. 2005). We cannot rule out that the cocaine doses used in the present study may have increased reactive oxygen species to some extent, or that when combined with caffeine some oxidative stress might have contributed to astrocyte activation in the striatum.
Repeated exposure to drugs of abuse results in a progressive and long-lasting enhancement of the locomotor response, a phenomenon termed psychomotor (or locomotor) sensitization (Robinson and Berridge 1993). In rodents, sensitization has been shown to correlate with enhanced predisposition to self-administer psychostimulants (Schenk and Partridge 1997). Of relevance to this study, cocaine intake was found to be increased by caffeine administration, and the combination of both drugs showed acutely increased c-Fos expression in the basal ganglia (Kuzmin et al. 2000b). Our study shows that chronic intermittent treatment with caffeine is able to induce locomotor sensitization, in agreement with previous reports showing that caffeine itself may induce sensitization and cross-sensitization with other psychostimulants (Cauli et al. 2003, Hsu et al. 2009). We also showed that chronic treatment with caffeine and cocaine induced locomotor sensitization, while treatment with cocaine alone did not show sensitization. The induction of locomotor sensitization depends on several factors such as the temporal pattern of drug exposure and dose regimen (discussed in Valjent et al. 2010). In addition, the cocaine dose used in this study was previously used to show that Disulfiram was able to facilitate cocaine sensitization, while no sensitization was observed for some variables (distance traveled) after a withdrawal period from chronic 10 mg/kg cocaine (Haile et al. 2003). Moreover, Eisener-Dorman et al. (2011) used a 20 mg/kg cocaine dose in order to investigate cocaine sensitization in several mouse strains suggesting that cocaine doses lower than 20 mg/kg might not be sufficient to induce locomotor sensitization. Thus, in our study, we intentionally decided to use a low dose of cocaine (10 mg/ kg), a dose that would have low probability of inducing locomotor sensitization on its own, but that would allow us to search for possible additive effects while combined with caffeine.
Our results are in agreement with a recent study by Prieto et al. (2015) that demonstrated that caffeine can enhance and accelerate the expression of sensitization induced by coca paste in rats. It was previously suggested that caffeine and cocaine stimulate locomotion through different mechanisms (Kuzmin et al. 2000a). Caffeine-stimulated motor responses appear to be mediated by A2AR blockade and to involve dopamine-dependent as well as dopamine independent mechanisms (El Yacoubi et al. 2000). Caffeine could increase motor activity by blocking A2AR and reduce tonic activation of the cAMP/PKA pathway in striatopallidal neurons. This effect would result in reduced phosphorylation of downstream target proteins, thereby affecting the activity of striatopallidal neurons and ultimately enhancing locomotion (Fisone et al. 2007), possibly involving the D2R. Additionally, it has been shown that caffeine alters D2 expression in neurons (Stonehouse et al. 2003). Cocaine effects are linked to both D2 (Ujike et al. 1990; Collins and France 2015) and D1 receptor activation (Wang et al. 2014). Of related interest to this discussion is the fact that a critical aspect of the mechanisms underlying caffeine’s psychostimulant effects is the release of the pre- and postsynaptic brakes that adenosine imposes on striatal dopaminergic neurotransmission (Ferré et al. 2010). In addition, caffeine appears to prime reward-relevant dopaminergic circuits implicated in cocaine misuse, and has been found to reinstate extinguished cocaine-taking behavior in rats (Worley et al. 1994).
An interesting feature of the molecular markers studied in the striatum was that the combined treatment showed a biphasic, time-dependent effect on dopaminergic and glutamatergic markers, with decreased gene expression after chronic treatment and increased expression at withdrawal, suggesting distinct and compensatory changes in chronic administration and following withdrawal. Combined treatment with both psychostimulants showed reduced AMPA and NMDA glutamate receptor subunit expression, along with reduced D1 and D2 mRNA expression. On the contrary, at withdrawal, we found increased D1 mRNA expression along with increased A1, AMPA, NMDA, and metabotropic subunit expression. The exception to this trend was Adora2a mRNA that showed decreased expression after both chronic treatment and withdrawal in all experimental groups. Similarly to the observations in this study, down-regulation of synaptic plasticity-related genes (e.g., Gria1, Drd2, and other molecules that regulate glutamate receptor function) has been reported in the dorsal striatum of relapse-vulnerable rats following chronic cocaine self-administration (Brown et al. 2011). While the precise nature of the changes that occur at the protein level remains to be fully elucidated, our data imply the presence of altered transcriptional control that may contribute to abnormal synaptic AMPA/NMDA subunit composition following chronic treatment and withdrawal. Interestingly, concomitant administration of cocaine and A2AR antagonists reduced glutamatergic synaptic transmission in striatal spiny neurons, while these drugs failed to produce the effect when given in isolation (Tozzi et al. 2012). The contrasting effects on gene expression that we found in this study could reflect changes in glutamate signaling in cor- tico-striatal synapses. Of interest to this discussion, both D1 and D2, the most abundant subtypes of DA receptors in vivo, are critically involved in drug addiction-related neuroplastic events in the striatum (Kalivas and O’Brien 2008). These results only indicate alterations at the transcriptional level and should then be interpreted with caution since protein levels may not follow the same trend.
There is evidence that patients who are addicted to other drugs use more caffeine than general psychiatric patients (Hays et al. 1998). Our study shows that chronic treatment with caffeine can have deleterious effects on striatal function, and that caffeine intake should be monitored and taken into account in clinical settings aimed to treat cocaine-dependent patients. Caffeine is not only the most consumed psychoactive drug in the world but also a common additive of cocaine (Cole et al. 2011). Also, it was previously reported that cocaine and caffeine are the main components of cocaine paste base and responsible for the CP-induced stimulant action (Lόpez-Hill et al. 2011). Cocaine-dependent subjects commonly abuse multiple substances and many of them drink coffee before and after cocaine use. The high frequency of simultaneous exposure to both the drugs may influence the progression to cocaine addiction and the consequences of cocaine exposure. Also, caffeine and cocaine combined exposure may be behind the profound neuropathological alterations found in the brains of cocaine addicts (Cadet et al. 2014). Even after long periods of abstinence, addicts remain vulnerable to drug craving and/or relapses that can be triggered by stimuli previously associated with drugs (Volkow et al. 2012). These features of addiction suggest that drugs might cause a form of persistent neuroplasticity that is acutely responsive to environmental stimuli, with consequent compulsive drug-seeking and drug-taking behaviors. Our report reveals some of the effects that might influence behavioral responses to cocaine, but also highlights interactive effects of the combination of both drugs on neuroplastic changes in the striatum. These effects on striatal gene expression on withdrawal were different compared to the ones obtained 24 h after the last injection suggesting that some of the effects on dopaminergic and glutamatergic synapses in the striatum may actually persist after a washout period.
In conclusion, our study provides novel evidence of detrimental mechanisms activated by combined exposure to caffeine and cocaine. Understanding the underlying mechanisms will guide appropriate intervention strategies for the management of severe reactions and drug-related adverse effects resulting from concomitant caffeine consumption.
Acknowledgments
Dr. Bisagno has been authorized to study drug abuse substances in animal models by A.N.M.A.T. (National Board of Medicine Food and Medical Technology, Ministerio de Salud, Argentina). This work was supported by FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. T 2012-0924 Argentina (to Dr. Bisagno) and FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT- 2012-1769, Argentina, and UBACYT 2014-2017 #201201301013 05BA (to Dr. Urbano). In addition, this work was supported by core facilities of the Center for Translational Neuroscience, UAMS, USA, supported by NIH award P20 GM110702.
References
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Raineri M, Peskin V, Wikinski SI, Uchitel OD, Llinás RR, Urbano FJ. Effects of T-type calcium channel blockers on cocaine-induced hyperlocomotion and thalamocortical GABAergic abnormalities in mice. Psychopharmacology. 2010;212:205–214. doi: 10.1007/s00213-010-1947-z. [DOI] [PubMed] [Google Scholar]
- Blanco-Calvo E, Rivera P, Arrabal S, Vargas A, Pavón FJ, Serrano A, Castilla-Ortega E, Galeano P, Rubio L, Suárez J, Rodriguez de Fonseca F. Pharmacological blockade of either cannabinoid CB1 or CB2 receptors prevents both cocaine-induced conditioned locomotion and cocaine-induced reduction of cell proliferation in the hippocampus of adult male rat. Front Integr Neurosci. 2014;8(7):106. doi: 10.3389/fnint.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Kalivas PW. Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neurosci. 2003;17:1273–1278. doi: 10.1046/j.1460-9568.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- Brown AL, Flynn JR, Smith DW, Dayas CV. Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int J Neuropsy- chopharmacol. 2011;14:1099–1110. doi: 10.1017/S1461145710001367. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V. Glial-neuronal ensembles: partners in drug addiction-associated synaptic plasticity. Front Pharmacol. 2014;2(5):204. doi: 10.3389/fphar.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016 doi: 10.3389/fpsyt.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127:91–107. doi: 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli O, Pinna A, Valentini V, Morelli M. Subchronic caffeine exposure induces sensitization to caffeine and cross-sensitization to amphetamine ipsilateral turning behavior independent from dopamine release. Neuropsychopharmacology. 2003;28:1752–1759. doi: 10.1038/sj.npp.1300240. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Beilis M. Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal. 2011;3:89–96. doi: 10.1002/dta.220. [DOI] [PubMed] [Google Scholar]
- Collins GT, France CP. Determinants of conditioned reinforcing effectiveness: dopamine D2-like receptor agonist-stimulated responding for cocaine-associated stimuli. Eur J Pharmacol. 2015;769:242–249. doi: 10.1016/j.ejphar.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derlet RW, Tseng JC, Albertson TE. Potentiation of cocaine and d-amphetamine toxicity with caffeine. Am J Emerg Med. 1992;10:211–216. doi: 10.1016/0735-6757(92)90211-F. [DOI] [PubMed] [Google Scholar]
- Dews PB, O’Brien CP, Bergman J. Caffeine: behavioral effects of withdrawal and related issues. Food ChemToxicol. 2002;40:1257–1261. doi: 10.1016/s0278-6915(02)00095-9. [DOI] [PubMed] [Google Scholar]
- Dietrich JB, Mangeol A, Revel MO, Burgun C, Aunis D, Zwiller J. Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology. 2005;48:965–974. doi: 10.1016/j.neuropharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Eisener-Dorman AF, Grabowski-Boase L, Tarantino LM. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behav Brain Funct. 2011;1(7):29. doi: 10.1186/1744-9081-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghimikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Ferré S. Caffeine and substance use disorders. J Caffeine Res. 2013;3:57–58. doi: 10.1089/jcr.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, Canela El, Franco R, Goldberg SR. Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol. 2010;160:443–153. doi: 10.1111/j.1476-5381.2010.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegaliński E, Muller CE, Agnati L, Franco R, Roberts DC, Fuxe K. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 2006;1077:67–80. doi: 10.1016/j.brainres.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Fisone G, Håkansson K, Borgkvist A, Santini E. Signaling in the basal ganglia: postsynaptic and presynaptic mechanisms. Physiol Behav. 2007;92:8–14. doi: 10.1016/j.physbeh.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Frau L, Costa G, Porceddu PF, Khairnar A, Castelli MP, Ennas MG, Madeddu C, Wardas J, Morelli M. Influence of caffeine on 3,4-methylenedioxymethamphetamine-induced dopaminergic neuron degeneration and neuroinflammation is age-dependent. J Neurochem. 2015 doi: 10.1111/jnc.13377. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Lull ME, Patel KM, Brucklacher RM, Morgan D, Roberts DC, Vrana KE. Gene expression changes in the medial prefrontal cortex and nucleus accumbens following abstinence from cocaine self-administration. BMC Neurosci. 2010;11:29. doi: 10.1186/1471-2202-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol (Lond) 1997;502(Pt 1):13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Peters J, Goldberg SR. Changes in the ambulatory activity and discriminative stimulus effects of psychostimulant drugs in rats chronically exposed to caffeine: effect of caffeine dose. J Pharmacol Exp Ther. 2000;295:1101–1111. [PubMed] [Google Scholar]
- Gonzalez CR, Gonzalez B, Matzkin ME, Muñiz JA, Cadet JL, Garcia-Rill E, Urbano FJ, Vitullo AD, Bisagno V. Psychostim- ulant-induced testicular toxicity in mice: evidence of cocaine and caffeine effects on the local dopaminergic system. PLoS One. 2015;10(11):e0142713. doi: 10.1371/journal.pone.0142713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA. Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biol Psychiatry. 2003;54:915–921. doi: 10.1016/s0006-3223(03)00241-5. [DOI] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays LR, Farabee D, Miller W. Caffeine and nicotine use in an addicted population. J Addict Dis. 1998;17:47–54. doi: 10.1300/J069v17n01_05. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Chen CY, Wang CS, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75- DARPP-32 in mice. Psychopharmacology (Berl) 2009;204:313–325. doi: 10.1007/s00213-009-1461-3. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- Jones HE, Griffiths RR. Oral caffeine maintenance potentiates the reinforcing and stimulant subjective effects of intravenous nicotine in cigarette smokers. Psychopharmacology. 2003;165:280–290. doi: 10.1007/s00213-002-1262-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33(1):166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Khairnar A, PlumitalloA Frau L, Schintu N, Morelli M. Caffeine enhances astroglia and microglia reactivity induced by 3,4-methylenedioxymethamphetamine (‘ecstasy’) in mouse brain. Neurotox Res. 2010;17:435–439. doi: 10.1007/s12640-009-9125-y. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Zvartau EE, Fredholm BB. Caffeine, acting on adenosine A(1) receptors, prevents the extinction of cocaine-seeking behavior in mice. J PharmacolExpTher. 1999;290:535–542. [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Fredholm BB, Ögren SO. Genetic evidence that cocaine and caffeine stimulate locomotion in mice via different mechanisms. Life sciences. 2000a;66(8):PL113–PL118. doi: 10.1016/s0024-3205(99)00647-5. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Semenova S, Fredholm BB. Differences in the effect of chronic and acute caffeine on selfadministration of cocaine in mice. Eur J Neurosci. 2000b;12:3026–3032. doi: 10.1046/j.1460-9568.2000.00179.x. [DOI] [PubMed] [Google Scholar]
- Liguori A, Hughes JR, Goldberg K, Callas P. Subjective effects of oral caffeine in formerly cocaine-dependent humans. Drug Alcohol Depend. 1997;49:17–24. doi: 10.1016/s0376-8716(97)00133-6. [DOI] [PubMed] [Google Scholar]
- López-Hill X, Prieto JP, Meikle MN, Urbanavicius J, Abin-Carriquiry JA, Prunell G, Umpiérrez E, Scorza MC. Coca-paste seized samples characterization: chemical analysis, stimulating effect in rats and relevance of caffeine as a major adulterant. Behav Brain Res. 2011;221:134–141. doi: 10.1016/j.bbr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat ClinPractNeurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Santos-Rodrigues AD, Schwarzschild MA, Chen JF, Cunha RA, Agostinho P. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia. 2012;60:702–716. doi: 10.1002/glia.22290. [DOI] [PubMed] [Google Scholar]
- McNamara R, Kerans A, O’Neill B, Harkin A. Caffeine promotes hyperthermia and serotonergic loss following coadministration of the substituted amphetamines, MDMA (“Ecstasy”) and MDA (“Love”) Neuropharmacology. 2006;50:69–80. doi: 10.1016/j.neuropharm.2005.08.006. [DOI] [PubMed] [Google Scholar]
- McPherson PS, Kim YK, Valdivia H, Knudson CM, Takekura H, Franzini-Armstrong C, Coronado R, Campbell KP. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991;7:17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Prieto JP, Galvalisi M, López-Hill X, Meikle MN, Abin-Carriquiry JA, Scorza C. Caffeine enhances and accelerates the expression of sensitization induced by coca paste indicating its relevance as a main adulterant. Am J Addict. 2015;24:475–481. doi: 10.1111/ajad.12245. [DOI] [PubMed] [Google Scholar]
- Raineri M, Gonzalez B, Goitia B, Garcia-Rill E, Krasnova IN, Cadet JL, Urbano FJ, Bisagno V. Modafinil abrogates metham- phetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS One. 2012;7:e46599. doi: 10.1371/journal.pone.0046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks–a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rosi S, McGann K, Hauss-Wegrzyniak B, Wenk GL. The influence of brain inflammation upon neuronal adenosine A2B receptors. J Neurochem. 2003;86:220–227. doi: 10.1046/j.1471-4159.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61(11 Suppl 6):S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav. 1997;57:543–550. doi: 10.1016/s0091-3057(96)00447-9. [DOI] [PubMed] [Google Scholar]
- Schenk S, Valadez A, Horger BA, Snow S, Wellman PJ. Interactions between caffeine and cocaine in tests of selfadministration. Behav Pharmacol. 1994;5:153–158. doi: 10.1097/00008877-199404000-00006. [DOI] [PubMed] [Google Scholar]
- Sinchai T, Plasen S, Sanvarinda Y, Jaisin Y, Govitrapong P, Morales NP, Ratanachamnong P, Plasen D. Caffeine potentiates methamphetamine-induced toxicity both in vitro and in vivo. Neurosci Lett. 2011;502:65–69. doi: 10.1016/j.neulet.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonehouse AH, Adachi M, Walcott EC, Jones FS. Caffeine regulates neuronal expression of the dopamine 2 receptor gene. Mol Pharmacol. 2003;64:1463–1473. doi: 10.1124/mol.64.6.1463. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de lure A, Marsili V, Romano R, Tantucci M, Di Filippo M, Costa C, Napolitano F, Mercuri NB, Borsini F, Giampà C, Fusco FR, Picconi B, Usiello A, Calabresi P. A2A adenosine receptor antagonism enhances synaptic and motor effects of cocaine via CB1 cannabinoid receptor activation. PLoS One. 2012;7(6):e38312. doi: 10.1371/journal.pone.0038312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Akiyama K, Otsuki S. D-2 but not D-1 dopamine agonists produce augmented behavioral response in rats after subchronic treatment with methamphetamine or cocaine. Psychopharmacology. 1990;102:459–464. doi: 10.1007/BF02247125. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanattou-Saïfoudine N, McNamara R, Harkin A. Caffeine provokes adverse interactions with 3,4-methylenedioxymetham- phetamine (MDMA, ‘ecstasy’) and related psychostimulants: mechanisms and mediators. Br J Pharmacol. 2012;167:946–959. doi: 10.1111/j.1476-5381.2012.02065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev PharmacolToxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Logan J, Alexoff D, Fowler JS, Thanos PK, Wong C, Casado V, Ferre S, Tomasi D. Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl Psychiatry v. 2015;5:e549. doi: 10.1038/tp.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wang N, Su P, Zhang Y, Lu J, Xing B, Kang K, Li W, Wang Y. Protein kinase Dl-dependent phosphorylation of dopamine D1 receptor regulates cocaine-induced behavioral responses. Neuropsychopharmacology. 2014;39:1290–1301. doi: 10.1038/npp.2013.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48:217–221. doi: 10.1016/0091-3057(94)90519-3. [DOI] [PubMed] [Google Scholar]