Abstract
IMPORTANCE
Novel approaches to perioperative surgical care focus on optimizing nutrition, mobility, and pain management to minimize adverse events after surgical procedures.
OBJECTIVE
To evaluate the outcomes of an enhanced recovery after surgery (ERAS) program among 2 target populations: patients undergoing elective colorectal resection and patients undergoing emergency hip fracture repair.
DESIGN, SETTING, AND PARTICIPANTS
A pre-post difference-in-differences study before and after ERAS implementation in the target populations compared with contemporaneous surgical comparator groups (patients undergoing elective gastrointestinal surgery and emergency orthopedic surgery). Implementation began in February and March 2014 and concluded by the end of 2014 at 20 medical centers within the Kaiser Permanente Northern California integrated health care delivery system.
EXPOSURES
A multifaceted ERAS program designed with a particular focus on perioperative pain management, mobility, nutrition, and patient engagement.
MAIN OUTCOMES AND MEASURES
The primary outcome was hospital length of stay. Secondary outcomes included hospital mortality, home discharge, 30-day readmission rates, and complication rates.
RESULTS
The study included a total of 3768 patients undergoing elective colorectal resection (mean [SD] age, 62.7 [14.1] years; 1812 [48.1%] male) and 5002 patients undergoing emergency hip fracture repair (mean [SD] age, 79.5 [11.8] years; 1586 [31.7%] male). Comparator surgical patients included 5556 patients undergoing elective gastrointestinal surgery and 1523 patients undergoing emergency orthopedic surgery. Most process metrics had significantly greater changes in the ERAS target populations after implementation compared with comparator surgical populations, including those for ambulation, nutrition, and opioid use. Hospital length of stay and postoperative complication rates were also significantly lower among ERAS target populations after implementation. The rate ratios for postoperative complications were 0.68 (95% CI, 0.46–0.99; P = .04) for patients undergoing colorectal resection and 0.67 (95% CI, 0.45–0.99, P = .05) for patients with hip fracture. Among patients undergoing colorectal resection, ERAS implementation was associated with decreased rates of hospital mortality (0.17; 95% CI, 0.03–0.86; P = .03), whereas among patients with hip fracture, implementation was associated with increased rates of home discharge (1.24; 95% CI, 1.06–1.44; P = .007).
CONCLUSIONS AND RELEVANCE
Multicenter implementation of an ERAS program among patients undergoing elective colorectal resection and patients undergoing emergency hip fracture repair successfully altered processes of care and was associated with significant absolute and relative decreases in hospital length of stay and postoperative complication rates. Rapid, large-scale implementation of a multidisciplinary ERAS program is feasible and effective in improving surgical outcomes.
To Err Is Human, the landmark Institute of Medicine report1 published in 1999, detailed the substantial toll that medical errors exact on patients, practitioners, and the health care system and elevated patient safety to a national priority. Ensuring patient safety has been particularly relevant in US surgical care, where more than 50 million in-patient procedures are performed annually2 and where perioperative complications are common and costly.3–11
To reduce complications and improve outcomes after surgery, bundled surgical care approaches have been proposed that aim to reduce the stress of surgery and maximize the potential for recovery.12–17 Enhanced recovery after surgery (ERAS) programs focus on several core elements, including optimizing pain control, nutrition and fluid management, and mobility, among others.13,17 Although prior studies14,18 have reported improved processes and outcomes after ERAS implementation, many have been limited by modest sample sizes and relatively few implementation target populations or sites.11–15,18–21
In this study, we assessed the concurrent implementation of an ERAS program among patients undergoing elective colorectal resection and patients undergoing emergency hip fracture surgery within the Kaiser Permanente Northern California (KPNC) integrated health care delivery system. We sought to evaluate how care and patient outcomes changed as a result of program implementation by comparing process and outcome measures in the ERAS target populations with those in similar surgical populations that received usual care before and after implementation.
Methods
This study was approved by the KPNC Institutional Review Board with a waiver of informed consent.
The KPNC ERAS Program
Program Design
In 2014, KPNC implemented an ERAS program across 20 hospitals in Northern California that targeted 2 surgical populations: patients undergoing elective colorectal resection and patients undergoing emergency hip fracture repair. Program design and implementation were led by a regional team composed of multidisciplinary subject matter experts, including clinicians, performance improvement staff, and patient engagement teams. The KPNC ERAS program aimed to standardize surgical care (Table 3) with a specific focus on 4 core perioperative elements: (1) pain management, (2) mobility, (3) nutrition, and (4) patient engagement. Pain management focused on opioid-sparing interventions through the use of multimodal analgesia, which included preoperative and postoperative intravenous acetaminophen, nonsteroidal anti-inflammatory medication, and perioperative intravenous lidocaine (colorectal) or peripheral nerve blocks (hip fracture). For mobility, ambulatory patients were encouraged to begin ambulation within 12 hours of surgery completion and maintain a daily goal of walking at least 21 ft in the first 3 postsurgical days. Nutrition was enhanced by reducing prolonged perioperative surgical fasting through the use of a preoperative high-carbohydrate beverage within 2 to 4 hours and/or solids within 8 to 12 hours before surgery. Postoperative nutrition was provided within 12 hours after surgery. To improve patient engagement in care, an infographic-based calendar was distributed to patients detailing expected care processes starting from the night before surgery through hospital discharge; an informational video series was also designed to improve patient education.
Table 3.
Process and Outcome Metrics Before (Pre) and After (Post) Enhanced Recovery After Surgery Implementation for the Colorectal Resection and Hip Fracture Repair Cohorts
| Metric | No. of Patients | Patientsa | P Valueb | |
|---|---|---|---|---|
| Pre | Post | |||
| Colorectal Resection Cohort | ||||
| Mobility | ||||
| Early ambulation | 3687 | 415 (22.3) | 1032 (56.5) | <.001 |
| Sustained ambulation | 3685 | 1603 (86.2) | 1735 (95.0) | <.001 |
| Time to first ambulation, median (IQR), h | 3717 | 20.3 (13.5–26.1) | 10.3 (6.7–18.6) | <.001 |
| Nutrition | ||||
| No prolonged fast | 3767 | 69 (3.7) | 247 (13.2) | <.001 |
| Early nutrition | 3767 | 245 (13.0) | 736 (39.2) | <.001 |
| Time to first nutrition, median (IQR), h | 3680 | 27.0 (18.4–54.1) | 16.4 (7.0–23.2) | <.001 |
| Analgesia and sedation | ||||
| Any benzodiazepine use | 3767 | 267 (14.1) | 233 (12.4) | .12 |
| Morphine equivalents, median (IQR), mg | 3604 | 52.4 (23.0–102.0) | 30.6 (13.9–65.5) | <.001 |
| Mean acceptable pain scores, median (IQR), % | 3761 | 75.9 (60.0–88.7) | 73.8 (59.3–88.4) | .14 |
| Outcome metrics | ||||
| Hospital length of stay, median (IQR), d | 3768 | 5.1 (3.4–7.1) | 4.2 (3.1–6.2) | <.001 |
| Hospital mortality | 3768 | 16 (0.9) | 6 (0.3) | .03 |
| Discharge to home | 3768 | 1808 (95.7) | 1816 (96.7) | .10 |
| 30-d Readmission | 3768 | 348 (18.6) | 364 (19.4) | .50 |
| Postoperative complications | 2406 | 184 (18.1) | 204 (14.7) | .02 |
| Major complications | 2406 | 42 (4.1) | 39 (2.8) | .08 |
| Hip Fracture Repair Cohort | ||||
| Process metrics | ||||
| Mobility | ||||
| Early ambulation | 4619 | 66 (2.8) | 481 (21.2) | <.001 |
| Sustained ambulation | 4616 | 356 (15.2) | 719 (31.7) | <.001 |
| Time to first ambulation, median (IQR), h | 3189 | 25.3 (18.1–46.4) | 17.3 (11.6–25.5) | <.001 |
| Nutrition | ||||
| No prolonged fast | 5002 | 32 (1.3) | 122 (4.9) | <.001 |
| Early nutrition | 5002 | 1134 (45.6) | 1436 (57.1) | <.001 |
| Time to first nutrition, median (IQR), h | 4915 | 12.6 (6.5–17.5) | 10.3 (4.9–15.7) | <.001 |
| Analgesia and sedation | ||||
| Any benzodiazepine use | 5002 | 316 (12.7) | 251 (10.0) | .002 |
| Morphine equivalents, median (IQR), mg | 4887 | 38.9 (22.0–66.5) | 27.0 (13.5–48.3) | <.001 |
| Mean acceptable pain scores, median (IQR), % | 4988 | 68.2 (54.3–82.1) | 72.7 (57.1–85.7) | <.001 |
| Outcome metrics | ||||
| Hospital length of stay, median (IQR), d | 5002 | 3.6 (2.8–4.8) | 3.2 (2.6–4.7) | <.001 |
| Hospital mortality | 5002 | 35 (1.4) | 42 (1.7) | .45 |
| Discharge to home | 5002 | 541 (21.7) | 676 (26.9) | <.001 |
| 30-d Readmission | 5002 | 299 (12.2) | 342 (13.8) | .09 |
| Postoperative complications | 2287 | 240 (30.8) | 375 (24.9) | .003 |
| Major complications | 2287 | 25 (3.2) | 56 (3.7) | .53 |
Abbreviation: IQR, interquartile range.
Data are presented as number (percentage) of patients unless otherwise indicated. Not all data were available for all patients, so the total denominator differs based on the specific metric.
P values are based on χ2 or Wilcoxon rank sum tests.
Program Implementation
The ERAS program was implemented in a staggered, nonrandomized fashion across 20 medical centers throughout 2014 (eTable 1 and eTable 2 in the Supplement). Two pilot sites were chosen based on leadership interest, performance improvement expertise, and clinician engagement. Pilot site implementation was undertaken in February and March 2014, followed by secondary site implementation at an additional 6 centers between April and May 2014. In June 2014, a regional ERAS summit, including more than 400 regional and medical center staff members, detailed the full implementation elements and time line, the data analytic strategy and performance dashboards, and the patient education materials. All remaining medical centers implemented the ERAS program in both target populations by the end of 2014. To support implementation, new electronic tools were created, including (1) 13 electronic medical record order sets to facilitate standardized practice and (2) performance dashboards to facilitate low-latency case review at the patient, pathway, medical center, and regional levels.
Study Design
We used a pre-post difference-in-differences study design to compare changes in practices and outcomes between target and comparator groups after implementation of the ERAS program. Starting with the ERAS implementation month, standardized as time zero for each medical center and pathway, we collected practice and outcomes data from the preceding (pre) 12 months and subsequent (post) 12 months (eFigure in the Supplement).
ERAS Target and Comparator Groups
We identified ERAS target patients based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) hospital and diagnosis procedure codes (eTable 3 in the Supplement). We identified inpatient comparator groups undergoing similar surgical procedures within their first 3 days of hospital admission using ICD-9-CM procedure codes and Clinical Classification Software procedure level 2 categories (eTable 4 in the Supplement). For patients undergoing elective colorectal resection, comparator patients included those not admitted through the emergency department who also underwent gastrointestinal surgery. For patients undergoing emergency hip fracture repair, comparator patients included those admitted through the emergency department who underwent orthopedic surgery.
Hospitalization Data
We linked ERAS target and comparator patients with existing hospitalization data to include validated risk scores quantifying acute severity of illness at hospital admission (Laboratory and Acute Physiology Score, version 2) and comorbid disease burden (Comorbidity Point Score, version 2).22–24 We also identified patients admitted directly to the intensive care unit because patients requiring critical care have higher rates of adverse outcomes. We determined hospital death, length of stay, discharge disposition, and 30-day readmission based on previously described methods.22–26
Process of Care Metrics
We selected 3 process of care metrics within each ERAS perioperative care element, excluding patient engagement, in our analysis. For mobility, we evaluated (1) ambulation within 12 hours of surgery (early ambulation), (2) ambulation of 21 ft or more in the first 3 postoperative days (sustained ambulation), and (3) the elapsed time from surgery to first ambulation (time to ambulation). For nutrition, we evaluated (1) the provision of solids and/or liquids in the preoperative period (no prolonged fast), (2) the provision of postoperative oral nutrition within 12 hours of surgery (early nutrition), and (3) the elapsed hours from surgery to first nutrition (time to nutrition). For pain management and sedation, we evaluated (1) the use of any benzodiazepines in the first 3 postoperative days (benzodiazepine use), (2) the total intravenous morphine-equivalent dosage of opioid medication administered from hospitalization through the third postoperative day (opioid use), and (3) the proportion of time in which patients rated their current pain as acceptable (acceptable pain scores). We quantified these metrics based on medication administration records, preoperative checklists, and/or nursing shift assessments recorded in the electronic medical record.
Outcome Metrics
Our primary outcome metric was hospital length of stay. Our secondary outcomes included hospital mortality, discharge to home, and 30-day readmission. We evaluated surgical complication data, available for a subsample of patients (eTable 5 in the Supplement), based on data abstracted manually according to National Surgical Quality Improvement Program (NSQIP) guidelines and procedures.3 We quantified surgical complications based on NSQIP categories. The NSQIP data abstraction was regionalized in January 2014 along with a decision to standardize the NSQIP participation model to multispecialty or target the model for all medical centers, preferentially targeting patients undergoing colorectal and hip fracture surgery. In a post hoc analysis, we evaluated major complication rates, which included septicshock, myocardial infarction, stroke, cardiac arrest, progressive kidney injury or renal failure, respiratory failure requiring mechanical ventilation, and venous thromboembolic disease.
Statistical Analysis
Data are presented as mean (SD), median (interquartile range), and number (percentage). Unadjusted comparisons between groups are based on unpaired, 2-tailed t tests, Wilcoxon rank sum tests, or χ2 tests.
Process Measure Rate Ratios
To evaluate the comparative effect of implementation on process metrics after ERAS care vs usual care, we used generalized linear models to estimate the rate ratios for each metric, including a preperiod vs postperiod indicator flag, an ERAS vs comparator group indicator flag, and the interaction between the period and ERAS indicators. We reported rate ratios based on the interaction term indicating the postimplementation period among the ERAS-targeted group. For binary values, we used a Poisson distribution with a log-link function, whereas for continuous values, we used a gaussian distribution with a log-link function, both with robust SEs. We used the same models in an interrupted time-series approach to evaluate the monthly rates of process metric change between ERAS and comparator groups.
Outcome Measure Rate Ratios
To estimate rate ratios for the outcome metrics, we used generalized linear models while also including age, severity of illness at admission metrics (Laboratory and Acute Physiology Score, version 2, and Comorbidity Point Score, version 2), and direct admission to the intensive care unit as fixed effects and facility as a random effect. We considered 2-sided P ≤ .05 to be statistically significant. All analyses were performed in STATA/SE, version 14.1 (StataCorp).
Results
ERAS Cohorts
The study included a total of 3768 patients undergoing elective colorectal resection (mean [SD] age, 62.7 [14.1] years; 1812 [48.1%] male) and 5002 patients undergoing emergency hip fracture repair (mean [SD] age, 79.5 [11.8] years; 1586 [31.7%] male) (Table 2). Mean severity of illness and comorbid disease burden was higher among patients undergoing emergency hip fracture repair than among those undergoing elective colorectal resection.
Table 2.
Baseline Characteristics of Patients in the 1-Year Periods Before (Pre) and After (Post) the Enhanced Recovery After Surgery Implementation Across 20 Medical Centers in Kaiser Permanente Northern California
| Characteristic | Elective Colorectal Resection
|
Emergency Hip Fracture Repair
|
||||
|---|---|---|---|---|---|---|
| Pre (n = 1890) | Post (n = 1878) | P Valuea | Pre (n = 2488) | Post (n = 2514) | P Valuea | |
| Age, mean (SD), y | 63.2 (14.2) | 62.1 (14.0) | .02 | 79.3 (11.9) | 79.7 (11.7) | .23 |
|
| ||||||
| COPS2, mean (SD) | 27.5 (30.8) | 27.9 (30.1) | .64 | 43.9 (40.7) | 43.5 (40.0) | .72 |
|
| ||||||
| LAPS2, mean (SD) | 20.2 (13.0) | 19.7 (12.4) | .22 | 57.0 (29.5) | 58.0 (30.2) | .21 |
|
| ||||||
| Direct ICU admission, No. (%) | 5 (0.3) | 6 (0.3) | .76 | 16 (0.6) | 19 (0.8) | .63 |
|
| ||||||
| Elapsed time between admission and surgery, median (IQR), h | 2.2 (1.8–2.9) | 2.2 (1.7–2.9) | .63 | 15.9 (6.1–23.6) | 15.7 (6.3–23.0) | .52 |
|
| ||||||
| Duration of surgery, median (IQR), h | 2.6 (1.9–3.6) | 2.7 (2.0–3.9) | .01 | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | .53 |
Abbreviations: COPS2, Comorbidity Point Score, version 2; ICU, intensive care unit; IQR, interquartile range; LAPS2, Laboratory and Acute Physiology Score, version 2.
P values for pre-post comparisons are based on 2-tailed, unpaired t test; χ2 test; or Wilcoxon rank sum test.
ERAS Process and Outcome Metrics
Most process metrics among ERAS patients demonstrated significant changes between the preimplementation and post-implementation phases (Table 3). The rate of early ambulation increased from 22.3% to 56.5% (P < .001) among patients undergoing elective colorectal resection and from 2.8% to 21.2% (P < .001) among patients undergoing hip fracture repair. Similarly, the use of early nutrition increased from 13.0% to 39.2% in patients undergoing elective colorectal resection and from 45.6% to 57.1% in patients undergoing hip fracture repair (P < .001). The total dose of morphine equivalents also decreased significantly in both groups (52.4 vs 30.6 mg in the colorectal resection group and 38.9 vs 27.0 mg in the hip fracture group; P < .001). Hospital length of stay decreased significantly in both ERAS groups (5.1 to 4.2 days in the colorectal resection group and 3.6 to 3.2 days in the hip fracture group; P < .001), whereas postoperative complication rates decreased from 18.1% to 14.7% (P = .02) in patients undergoing elective colorectal resection and from 30.8% to 24.9% (P = .003) in patients undergoing hip fracture repair.
Comparator Groups
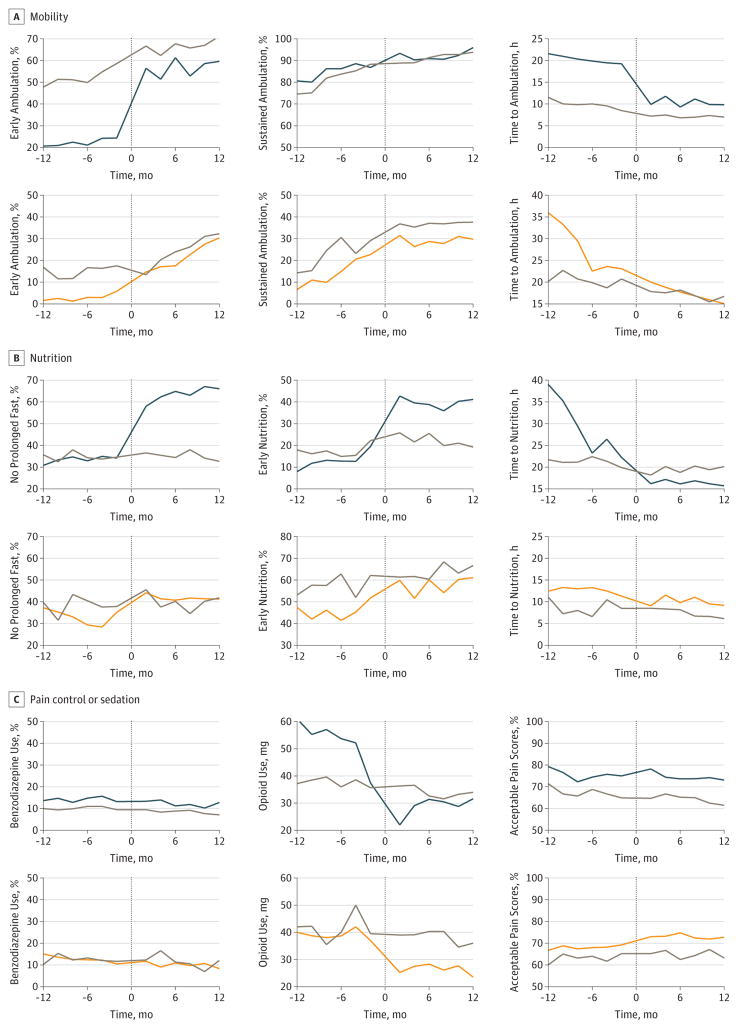
We identified 5556 patients for the colorectal resection comparator group and 1523 patients for the hip fracture repair comparator group (eTable 6 in the Supplement). Among the comparator group patients, many process metrics also changed significantly between the preimplementation and postimplementation phases, albeit more modestly (eTable 7 in the Supplement). The Figure shows the comparative attainment of process metrics between the ERAS target populations and their comparators and several patterns indicated by estimates from interrupted time-series analyses (eTable 8 in the Supplement), including similar changes over time (eg, benzodiazepine use in both groups), frameshifts after ERAS implementation that resulted in similar levels of attainment (eg, early ambulation in both groups), and frameshifts after implementation that resulted in differing levels of metric attainment (eg, early nutrition and no prolonged fasting among patients undergoing colorectal resection).
Figure. Bimonthly Process Metric Attainment Before and After Enhanced Recovery After Surgery (ERAS) Program Implementation.
Data are standardized to the month of implementation in each facility for mobility (A), nutrition (B), and pain control or sedation (C). Each point represents mean values during a 2-month period; data from the implementation month (month zero) are not included. Top rows represent ERAS colorectal resection (blue lines) and comparator (gray lines) groups; bottom rows represent ERAS hip fracture repair (orange lines) and comparator (gray lines) groups. The y-axes vary for each plot to improve clarity; percentage changes include a 50% interval, whereas changes in hours include a 25-hour interval.
Rate Ratios
We found favorable rate ratios in most process metrics among ERAS target populations compared with their respective comparator groups (eTable 9 in the Supplement). For example, ERAS implementation was associated with a significantly increased relative rate of early ambulation in patients undergoing colorectal resection (1.99; 95% CI, 1.80–2.21; P < .001) and hip fracture repair (4.44; 95% CI, 3.19–6.21; P < .001). Similarly, ERAS implementation was associated with a decreased relative rate of opioid use among patients undergoing colorectal resection (0.79; 95% CI, 0.71–0.89; P < .001) and patients undergoing hip fracture repair (0.73; 95% CI, 0.63–0.85; P < .001). There were no relative changes in the rate of benzodiazepine use in patients undergoing colorectal resection (1.11; 95% CI, 0.88–1.41; P = .35) or hip fracture repair (0.85; 95% CI, 0.62–1.17; P = .33).
In patients undergoing colorectal resection and hip fracture repair, ERAS implementation was associated with favorable reductions in hospital length of stay and postoperative complication rates (Table 4 and eTable 10 in the Supplement). Among patients undergoing colorectal resection, ERAS implementation was associated with decreased rates of hospital mortality (0.17; 95% CI, 0.03–0.86; P = .03) and major complications (0.28; 95% CI, 0.12–0.68; P = .005). Among patients undergoing hip fracture repair, implementation was associated with an increased rate of discharge to home (1.24; 95% CI, 1.06–1.44; P = .007). In neither patients undergoing colorectal resection (0.94; 95% CI, 0.74–1.20; P = .65) nor patients undergoing hip fracture repair (0.96; 95% CI, 0.74–1.16; P = .78) was ERAS implementation associated with relative differences in the rate of 30-day readmission.
Table 4.
Difference-in-Differences Risk Ratios for ERAS Patient Outcome Metrics After Program Implementation Among Colorectal Resection and Hip Fracture Repair Cohortsa
| Metric | Difference-in-Differences Estimates, Rate Ratio (95% CI) | |||
|---|---|---|---|---|
| Colorectal Resection vs Comparator | P Value | Hip Fracture vs Comparator | P Value | |
| Hospital length of stay, d | 0.92 (0.86–0.99) | .03 | 0.83 (0.77–0.91) | <.001 |
| Hospital mortality | 0.17 (0.03–0.86) | .03 | 1.12 (0.37–3.41) | .84 |
| Discharge to home | 1.01 (0.99–1.02) | .13 | 1.24 (1.06–1.44) | .007 |
| 30-d Readmission | 0.94 (0.74–1.20) | .65 | 0.96 (0.74–1.26) | .78 |
| Postoperative complications | 0.68 (0.46–0.99) | .04 | 0.67 (0.45–0.99) | .05 |
| Major complications | 0.28 (0.12–0.68) | .005 | 0.53 (0.15–1.84) | .32 |
Abbreviation: ERAS, Enhanced Recovery After Surgery.
A difference-in-differences risk ratio of 0.67 (postoperative complications; hip fracture) can be interpreted as a 33% lower rate of postoperative complications among patients undergoing hip fracture repair after ERAS program implementation compared with before ERAS implementation relative the orthopedics comparator cohort during the same intervals.
Discussion
System-level implementation of an ERAS program resulted in significant practice changes across a heterogeneous set of patients, clinicians, and hospitals. Compared with other surgical populations treated at the same hospitals over identical periods, ERAS-driven practice changes were associated with significantly decreased hospital length of stay and rates of postoperative complications. In patients undergoing elective colorectal resection, program implementation was associated with decreased surgical mortality and major complications. In patients undergoing emergency hip fracture repair, implementation was associated with improved rates of discharge to home.
Most prior studies12,14–18,27 that evaluated ERAS implementation have included patients undergoing colorectal surgery. A Cochrane systematic review12 of ERAS in 2011 identified 4 randomized clinical trials including a total of 237 patients who met the review criteria. Similar to our results, length of stay and overall complication rates decreased with ERAS implementation without significant differences in readmission rates. A more recent meta-analysis by Greco et al14 identified 16 trials that enrolled a total of 2376 patients and reported a significant reduction in length of stay and nonsurgical complication rates without an accompanying difference in readmission rates. Although these prior studies12,14–18,27 are broadly consistent with our findings, we also identified a significant reduction in hospital mortality after ERAS implementation in our population undergoing elective colorectal resection.
Considerably fewer studies have robustly evaluated ERAS implementation outside colorectal surgery. A meta-analysis28 of ERAS programs in several surgical domains that included 38 trials and 5099 patients found significant reductions in length of stay and 30–day complication rates, without significant differences in mortality or readmission. Of these trials, only 3 included patients undergoing lower extremity joint replacement. Macfie et al29 evaluated 232 patients with femoral neck fractures and found that an ERAS program reduced postoperative complications without significant differences in mortality or length of stay. With a pre-post study design, Pedersen et al30 reported significant reductions in inpatient complication rates with a significant reduction in hospital mortality among community-dwelling patients. We identified a similar reduction in complication rates along with a modest reduction in length of stay.
Strengths and Limitations
This study has several strengths compared with prior studies. First, we evaluated 2 heterogeneous surgical populations to assess for similar and differential effects of implementation. Patients undergoing colorectal resection were younger and healthier than patients undergoing hip fracture repair; they also underwent elective procedures. Before implementation, their care processes differed as well. For example, the rates of early ambulation were much higher among the patients undergoing colorectal resection, whereas the rates of postoperative nutrition were much higher among patients undergoing hip fracture repair. Despite these substantial differences in baseline patient and care characteristics, the effect of program implementation on complications was a similar one-third relative decrease in complication rates; length of stay also decreased in both groups. Most prior studies12–15,18–21 have been limited to more homogeneous target populations. This study demonstrates the effectiveness of a systems-level approach to ERAS program implementation even across widely divergent target populations.
Second, we included contemporaneous comparator patients to isolate the effect of program implementation rather than effects that arise from secular change in surgical practice. Despite the fact that comparator patients were treated by the same surgical teams and experienced some modest changes in process metrics, their outcomes were largely unchanged before and after implementation, strengthening the assertion that the changes in outcomes in the target populations resulted from the implementation of the ERAS program itself. Most prior multicenter studies14,18 have not used contemporaneous comparator groups, limiting their study conclusions because of inherent limitations of standard pre-post study designs.
Third, prior studies12,20 have had limited ability to evaluate program implementation at scale in real-world settings. During a 2-year period, we were able to evaluate care patterns in more than 15 000 surgical patients. Program implementation affected the practice of thousands of clinicians and occurred in a diverse set of hospitals that differ significantly in size, patient case mix, teaching status, geographic location, and on-site specialty service availability, strengthening the generalizability of our findings. Our study was aided by evaluating electronic medical record–based metrics that were already used by clinicians, allowing us to precisely quantify a large panel of performance measures populated during routine care delivery. Thus, we were able to demonstrate the feasibility of large-scale ERAS program implementation during a relatively short interval.
The study also has important limitations. First, the evaluation was not a randomized clinical trial, and the program rollout was not randomized by site. Thus, the results may be affected by residual confounding and baseline differences in intervention and usual care patients. Our comparator groups also underwent different surgical procedures than the target populations, limiting our ability to directly compare 2 identical groups. Second, we did not have standardized and validated complications data on all patients; there was also an imbalance in the acquisition of these data over time and between the target and comparator groups. Because of the size and speed of the implementation effort, program resources were not available to perform data abstraction for every surgical patient. Third, as a highly integrated health care delivery system, the KPNC may differ from other health care systems in the United States. Thus, implementation and results may vary depending on the particular model of surgical practice and performance improvement method. Fourth, our study evaluated short-term outcomes; future studies are necessary to evaluate the effect of enhanced recovery on longer-term outcomes, especially functional and cognitive ability after surgery.
Conclusions
Implementation of the ERAS program successfully altered the process-of-care metrics for patients undergoing emergency hip fracture repair and patients undergoing elective colorectal resection across 20 hospitals. In this study of more than 15 000 surgical patients, program implementation was associated with significant absolute and relative improvements in hospital length of stay and surgical complication rates.
Supplementary Material
Table 1.
Care Processes Included in the Kaiser Permanente Northern California Enhanced Recovery After Surgery Program
| Care Process | Description |
|---|---|
| Preoperative | |
| Patient education | Verbal counseling and written brochures provided preoperatively |
| No prolonged fasting | Clear liquids allowed up to 2 h and solids up to 8 h before surgery |
| Carbohydrate loading | Carbohydrate-rich drink or apple juice 2 to 4 h preoperatively |
| Decreased sedative medications | Recommended premedication limited to 2 mg of midazolam |
| Regional anesthesia | Peripheral nerve blocks in emergency department (hip fracture) |
| Intraoperative | |
| Antimicrobial prophylaxis | Chlorhexidine skin preparation and antibiotics within 1 h before incision |
| Postoperative nausea and vomiting prophylaxis | Multimodal prophylaxis based on risk |
| Multimodal analgesia | Gabapentin, acetaminophen, intravenous lidocaine infusion, nerve blocks, or thoracic epidural |
| Standard anesthetic protocol | Hip fracture: neuraxial anesthesia preferred Colorectal: thoracic epidural, nerve block, or intravenous lidocaine infusion |
| Minimally invasive surgery | Laparoscopic approach preferred (colorectal) |
| Avoidance of drains and tubes | Routine nasogastric tubes and drains discouraged |
| Perioperative fluid management | Restrictive or goal-directed fluid replacement |
| Prevention of hypothermia | Active warming devices |
| Postoperative | |
| Multimodal analgesia | Scheduled acetaminophen and ketorolac, intravenous lidocaine infusion (colorectal), or regional anesthesia |
| Early oral nutrition | Full liquid or regular diet within 12 h after surgery |
| Early and sustained ambulation | Ambulation accomplished within 12 h after surgery and twice daily thereafter |
| Early urinary catheter removal | Removal of urinary catheter within 24 h after surgery |
| Deep vein thrombosis prevention | Sequential compression devices unless ambulating |
| Restoration of gut function | Chewing gum (colorectal) |
Key Points.
Question
What is the influence of implementation of an enhanced recovery after surgery program on outcomes among patients undergoing elective colorectal resection and emergency hip fracture repair?
Findings
In this pre-post difference-in-differences study of 15 849 surgical patients at 20 medical centers in Northern California, implementation of a multifaceted enhanced recovery program was associated with a one-third reduction in postoperative complication rates in the target population relative to comparator surgical populations. The program was also associated with decreased hospital mortality among patients undergoing colorectal resection and increased rates of discharge to home among patients undergoing hip fracture repair.
Meaning
Large-scale implementation of an enhanced recovery after surgery program significantly improved many processes and outcomes of surgical care in 2 distinct populations.
Acknowledgments
Funding/Support: This study was funded by the Gordon and Betty Moore Foundation, The Permanente Medical Group, and Kaiser Foundation Health Plan. Dr Liu was supported by grant K23GM112018 from the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the Enhanced Recovery After Surgery executive sponsors, steering committee, regional mentors, local champion committees, KP HealthConnect, and regional health education teams for their dedication to improving surgical care. We also thank the thousands of clinicians and staff members who contributed to make this work a reality.
Author Contributions: Dr Liu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Liu, Rosas, Hwang, Cain, Foss-Durant, Clopp, Huang, Parodi.
Acquisition, analysis, or interpretation of data: Liu, Cain, Huang, Lee, Mustille, Kipnis, Parodi.
Drafting of the manuscript: Liu, Clopp, Lee, Parodi.
Critical revision of the manuscript for important intellectual content: Liu, Rosas, Hwang, Cain, Foss-Durant, Huang, Lee, Mustille, Kipnis, Parodi.
Statistical analysis: Liu, Mustille, Kipnis.
Obtained funding: Liu, Foss-Durant, Parodi.
Administrative, technical, or material support: Liu, Rosas, Cain, Clopp, Huang, Lee, Mustille, Parodi.
Supervision: Rosas, Cain, Foss-Durant, Huang, Parodi.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
References
- 1.Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 2.Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. Centers for Disease Control and Prevention; [Accessed October 5, 2016]. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. [Google Scholar]
- 3.Maggard-Gibbons M. The use of report cards and outcome measurements to improve the safety of surgical care: the American College of Surgeons National Surgical Quality Improvement Program. BMJ Qual Saf. 2014;23(7):589–599. doi: 10.1136/bmjqs-2013-002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vonlanthen R, Slankamenac K, Breitenstein S, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg. 2011;254(6):907–913. doi: 10.1097/SLA.0b013e31821d4a43. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Gust C, Dimick JB, Birkmeyer NJ, Skinner JS. Hospital quality and the cost of inpatient surgery in the United States. Ann Surg. 2012;255(1):1–5. doi: 10.1097/SLA.0b013e3182402c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA., Jr Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199(4):531–537. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 7.Healy MA, Mullard AJ, Campbell DA, Jr, Dimick JB. Hospital and payer costs associated with surgical complications. JAMA Surg. 2016;151(9):823–830. doi: 10.1001/jamasurg.2016.0773. [DOI] [PubMed] [Google Scholar]
- 8.Khuri SF, Henderson WG, Daley J, et al. Principal Investigators of the Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329–336. doi: 10.1097/SLA.0b013e3181823485. [DOI] [PubMed] [Google Scholar]
- 9.Eappen S, Lane BH, Rosenberg B, et al. Relationship between occurrence of surgical complications and hospital finances. JAMA. 2013;309(15):1599–1606. doi: 10.1001/jama.2013.2773. [DOI] [PubMed] [Google Scholar]
- 10.Silber JH, Rosenbaum PR, Trudeau ME, et al. Changes in prognosis after the first postoperative complication. Med Care. 2005;43(2):122–131. doi: 10.1097/00005650-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Healey MA, Shackford SR, Osler TM, Rogers FB, Burns E. Complications in surgical patients. Arch Surg. 2002;137(5):611–617. doi: 10.1001/archsurg.137.5.611. [DOI] [PubMed] [Google Scholar]
- 12.Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;(2):CD007635. doi: 10.1002/14651858.CD007635.pub2. [DOI] [PubMed] [Google Scholar]
- 13.McLeod RS, Aarts MA, Chung F, et al. Development of an enhanced recovery after surgery guideline and implementation strategy based on the knowledge-to-action cycle. Ann Surg. 2015;262(6):1016–1025. doi: 10.1097/SLA.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 14.Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(6):1531–1541. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 15.Lassen K, Soop M, Nygren J, et al. Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 16.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Kehlet H. Fast-track colorectal surgery. Lancet. 2008;371(9615):791–793. doi: 10.1016/S0140-6736(08)60357-8. [DOI] [PubMed] [Google Scholar]
- 18.Wind J, Polle SW, Fung Kon Jin PH, et al. Laparoscopy and/or Fast Track Multimodal Management Versus Standard Care (LAFA) Study Group; Enhanced Recovery after Surgery (ERAS) Group. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93(7):800–809. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 19.Mortensen K, Nilsson M, Slim K, et al. Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101(10):1209–1229. doi: 10.1002/bjs.9582. [DOI] [PubMed] [Google Scholar]
- 20.Bagnall NM, Malietzis G, Kennedy RH, Athanasiou T, Faiz O, Darzi A. A systematic review of enhanced recovery care after colorectal surgery in elderly patients. Colorectal Dis. 2014;16(12):947–956. doi: 10.1111/codi.12718. [DOI] [PubMed] [Google Scholar]
- 21.Rawlinson A, Kang P, Evans J, Khanna A. A systematic review of enhanced recovery protocols in colorectal surgery. Ann R Coll Surg Engl. 2011;93(8):583–588. doi: 10.1308/147870811X605219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395. doi: 10.1002/jhm.1929. [DOI] [PubMed] [Google Scholar]
- 23.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 24.Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 25.Escobar GJ, Fireman BH, Palen TE, et al. Risk adjusting community-acquired pneumonia hospital outcomes using automated databases. Am J Manag Care. 2008;14(3):158–166. [PubMed] [Google Scholar]
- 26.Liu V, Turk BJ, Ragins AI, Kipnis P, Escobar GJ. An electronic Simplified Acute Physiology Score–based risk adjustment score for critical illness in an integrated healthcare system. Crit Care Med. 2013;41(1):41–48. doi: 10.1097/CCM.0b013e318267636e. [DOI] [PubMed] [Google Scholar]
- 27.Spanjersberg WR, van Sambeeck JD, Bremers A, Rosman C, van Laarhoven CJ. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc. 2015;29(12):3443–3453. doi: 10.1007/s00464-015-4148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101(3):172–188. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 29.Macfie D, Zadeh RA, Andrews M, Crowson J, Macfie J. Perioperative multimodal optimisation in patients undergoing surgery for fractured neck of femur. Surgeon. 2012;10(2):90–94. doi: 10.1016/j.surge.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen SJ, Borgbjerg FM, Schousboe B, et al. Hip Fracture Group of Bispebjerg Hospital. A comprehensive hip fracture program reduces complication rates and mortality. J Am Geriatr Soc. 2008;56(10):1831–1838. doi: 10.1111/j.1532-5415.2008.01945.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.