Abstract
Introduction
Significant progress has been made in elucidating the physiological and pharmacological mechanisms of female sexual function through preclinical animal research. The continued development of animal models is vital for the understanding and treatment of the many diverse disorders that occur in women.
Aim
To provide an updated review of the experimental models evaluating female sexual function that may be useful for clinical translation.
Methods
Review of English written, peer-reviewed literature, primarily from 2000 to 2012, that described studies on female sexual behavior related to motivation, arousal, physiological monitoring of genital function and urogenital pain.
Main Outcomes Measures
Analysis of supporting evidence for the suitability of the animal model to provide measurable indices related to desire, arousal, reward, orgasm, and pelvic pain.
Results
The development of female animal models has provided important insights in the peripheral and central processes regulating sexual function. Behavioral models of sexual desire, motivation, and reward are well developed. Central arousal and orgasmic responses are less well understood, compared with the physiological changes associated with genital arousal. Models of nociception are useful for replicating symptoms and identifying the neurobiological pathways involved. While in some cases translation to women correlates with the findings in animals, the requirement of circulating hormones for sexual receptivity in rodents and the multifactorial nature of women’s sexual function requires better designed studies and careful analysis. The current models have studied sexual dysfunction or pelvic pain in isolation; combining these aspects would help to elucidate interactions of the pathophysiology of pain and sexual dysfunction.
Conclusions
Basic research in animals has been vital for understanding the anatomy, neurobiology, and physiological mechanisms underlying sexual function and urogenital pain. These models are important for understanding the etiology of female sexual function and for future development of pharmacological treatments for sexual dysfunctions with or without pain.
Keywords: Copulatory Behavior, Desire, Arousal, Orgasm, Neural Pathways, Pain
Introduction
Research and understanding of sexual function in women has expanded over the last few decades, with new models and criteria defining sexual disorders [1–6]. This increased knowledge of the human condition helps not only with diagnosis and treatment, but makes it possible to refine existing animal models and design more relevant techniques for preclinical research and etiology. Female sexual behavior comprises desire (interest to engage in sexual activity), arousal (physiological changes include an increased sensitivity of erogenous zones, vaginal lubrication, genital vasodilatation, and cognitive awareness), and orgasm (rhythmic muscle contractions and an increase in sympathetic activity). Relaxation and a feeling of well-being or reward usually follows orgasm [7]. The various stages of the sex cycle are modulated by neural pathways and circulating levels of hormones that might facilitate sexual desire, arousal, and orgasm, or might lead to the downregulation or inhibition of sexual behavior (see Figure 1). Female sexual dysfunction has been categorized into various types based on the phases of the sex cycle. In women, the most prevalent are hypoactive sexual desire disorder, hypoactive arousal disorder, anorgasmia, and pain with vaginal intercourse [8–11].
Figure 1.
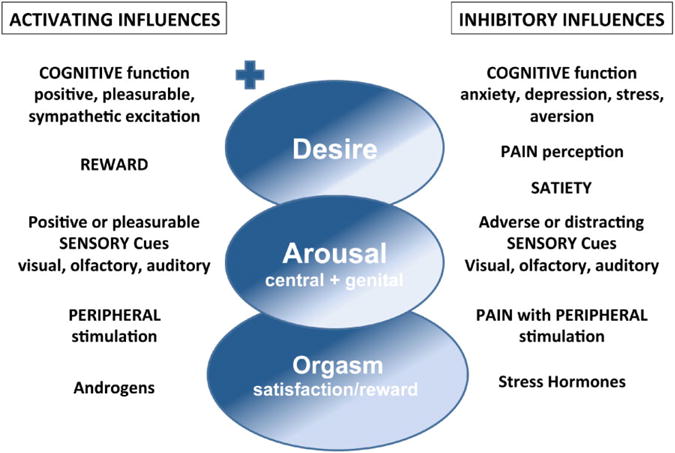
Female sexual behavior comprises desire, arousal, orgasm, and reward. Multiple factors activate and inhibit each stage of the sex cycle. Cognitive activation of hypothalamic and limbic brain regions can result in increased desire and arousal; while activation of brain inhibitory sites (cortex, hypothalamus, limbic system, and midbrain) either by stress, anxiety, or postorgasm (with satiety) can lead to inhibition or cessation of sexual behavior. Sensory cues, such as pleasing visual sexual images or desirable odors (pheromones), can enhance desire and arousal. In contrast, frightening visual or auditory stimuli, or loss of attention or focus can inhibit sexual behavior. Peripheral stimulation may lead to localized genital arousal alone or may produce central arousal and desire depending of the integrity and balance of excitatory (dopamine, oxytocin, noradrenaline, and melanocortin) and inhibitory (opioids, serotonin and endocannabinoids) neurotransmission from the peripheral nerves through the spinal cord to the brain. Androgens (testosterone and estradiol), present at normal levels, act both peripherally and centrally to enhance sexual function, while stress hormones (e.g., cortisol) inhibit sexual interest. Hormones such as oxytocin and progesterone are released into the circulation with orgasm. Pain arising from the genitals or reproductive organs can lead to sexual dysfunction. Peripheral pain activates inhibitory central spinal and brain pathways that may lead to aversion to sexual activity or a decrease in desire and arousal. As a result, an increased focus on activating the excitatory pathways of arousal and reward is required for sexual activity. + indicates activation or facilitation, - indicates inhibition or reduction.
Animal models used for studying sexual dysfunction in women must relate in a reliable and predictable way to the human condition. Past studies in rats, particularly, have provided considerable understanding of the pharmacology, neuroanatomy, and endocrine mechanisms of sexual function. Studies in other species have different advantages over rodents, for example, primates show more cognitive, complex behaviors without hormone priming, and may be more relevant to humans. Knockout mice allow for genetic traits to be studied and have helped document the importance of specific hormones (estrogen receptor alpha, oxytocin, GnRH, etc.) in specific brain regions that regulate female sexual behavior [12–16]. Other species, such as the Syrian hamster, have been valuable for understanding the role of hormones and brain pathways in social sexual behaviors [17–19]. Sexual arousal and hormonal regulation of sexual behavior have also been studied in birds [20] and monogamous prairie voles—these studies have been vital in elucidating the role of oxytocin and opioids in social bonding [21–23]. This review focuses on rodent animal models that correlate with clinical diagnoses and practices.
Behavioral Models of Sexual Desire and Motivation
There is no clear compartmental differentiation between desire, arousal, and reward in animal behavioral models, as these behaviors overlap and are dependent on each other. However, some aspects of female behavior, cues, and preferences can indicate changes in certain aspects of motivation (desire), arousal, and reward. These behaviors were categorized into consummatory (ability to engage in copulation) and appetitive or proceptive (motivation or desire for sexual behavior) types of behavior [24–26]. Researchers have utilized their understanding of these specific behaviors to design effective techniques that represent various aspects of female sexual behavior. Care should be taken when interpreting any animal’s behavior to consider whether the responses being measured are purely reflexive or motivationally driven, as well as the experimental conditions.
Copulatory Measures of Sexual Motivation or Desire
Female rats will display specific behaviors, such as ear wiggling, hopping, and darting, and will pace the number and frequency of approaches toward a male when they are sexually receptive [27–29]. This pacing of their preferred frequency of intromissions by the male has been useful to assess sexual motivation or sexual desire, and supports the importance of paced copulation in the female rat for reward. The proceptive behaviors can be recorded in multicompartmental pacing chambers, bilevel chambers, mazes, or choice boxes, and have been important in identifying the brain regions (medial preoptic area (MPOA), ventromedial hypothalamus (VMN), limbic regions, etc.) and neurotransmitters (such as the release of dopamine in anticipation of the rewards of sexual contact) important for the display of female sexual behavior [1,26,30–38].
One recent study examined proceptive behavior (hops/darts) and pacing behavior to examine the normal distribution of behaviors in female rats to see if individual differences exist. If individuals displayed high or low sexual motivation/behavior those females may mimic hyper- or hypoactive sexual desire and could be preselected and studied [39]. Three subgroups of females were observed, those that showed avoidance, a larger group that displayed normal approaches, and a group that displayed a high level of approaches to the male; the behaviors within each individual appeared to be stable over time. Some differences were observed between the avoiders and approachers with apomorphine treatment, which the authors suggest may be related to a difference in brain dopamine systems. Confirmation of this hypothesis and further studies on females that are avoiders is required to see if this is a good model of diminished sexual desire.
The relevance of monitoring female rat behavior is evident in a number of preclinical studies that have also demonstrated efficacy in measures of women’s sexual function. Using the bilevel pacing chamber, investigators were able to show a dramatic increase in solicitations made by the female when treated with the melanocortin agonist, bremelanotide (PT-141) [40–42]. This drug also showed increased vaginal arousal in women viewing an erotic film and increased the ability of women with lifelong hypoactive sexual desire to initiate sex with their partners [43]. Acute or chronic administration of selective serotonin reuptake inhibitors (SSRIs, e.g., fluoxetine or paroxetine) to female rats results in a reduction of lordosis behavior and sexual incentive motivation; SSRIs also reduce sexual behavior in women [44,45].
Another study used marmoset monkeys, in which mating occurs in stable male–female pairs, to examine sexual and social behavior with flibanserin (a 5-HT1A agonist and 5-HT2A antagonist), or 8-OH-DPAT (a 5-HT1A agonist) treatment. Many studies have previously shown that 5HT1A receptor activation inhibits lordosis behavior in female rodents [46,47]. The study performed in monkeys showed that flibanserintreated females attracted more male sexual interest, and triggered increased grooming between partners. In contrast, 8-OH-DPAT-treated females showed an increased rejection of the male’s sexual advances, a tendency for decreased male sexual interest, and increased aggression with their male partners. Flibanserin has been shown to increase solicitations and sexual receptivity in female rats [37,41,48]. Flibanserin improved sexual function in women with depression and hypoactive sexual desire disorder [49,50].
Motivation Utilizing Operant Response
In these studies, the rat is trained to complete a task, such as a lever press or nose poke, in order to receive a reward. The effort and attention made to obtain the reward is thought to provide a meaningful measure of the expression of desire or wanting. This paradigm has been used to train female rats to bar press for their preferred partner or subsequent to a mount or intromission.
A recent study conducted by Cummings and Becker was able to quantify female sexual motivation using the number of nose pokes in a two-chamber apparatus in which the female’s strength of motivation to access and mate with a male was measured [51]. These studies have the advantage that female pacing and solicitation measures (hop/darts and ear wiggles) and strength of receptivity (lordosis) can be made simultaneously. During this first study, the authors found that hormonally primed female rats were significantly more motivated to obtain access to a sexually active male, approach the male after a shorter duration, and spend more time in direct contact with the male rat, compared with when they were not hormonally primed (not sexually receptive). This technique provides a new method to examine female sexual behavior; hopefully, further studies will confirm the relevance of this model to female sexual motivation.
Motivation and Reward Using Preference Testing
Animal models of a choice paradigm can measure solicitations, conditioned locomotion in anticipation of sex, time spent near a sexual incentive, choices made between two or more incentives, and are all used to measure sexual desire. Increase or decrease in the strength of the behaviors are measured and are based on the assumption that females with more desire will display more robust behaviors than animals with less desire [2,37,38,52–54].
Sexual preference paradigms
Sexual preference paradigms utilize preferences that are learned experiences paired with sexual reward, such as pacing behavior in females, or stimuli associated with sexual experience. These preferences are typically displayed prior to sexual interaction, allowing the female to focus their effort toward the sexual incentives. Some of these paradigms require differing degrees of training and consequently have only been used in a few studies [55,56].
This technique has also been used to study the brain sites in rats that are activated with olfactory stimulation paired with female-paced copulatory behavior. When females are paired with almond-scented males in a paced testing paradigm, the females will subsequently select to solicit and receive ejaculations from an almond-scented male rather than an unscented male. This preference is not displayed when paired in a nonpaced behavioral paradigm [35,36]. The brain sites that were activated in rats were similar to those reported with arousal in women during brain imaging studies with visual sexual stimulation and orgasm [35,57–63]. The brain regions include cortex, striatum, nucleus accumbens, MPOA, ventral tegmental area, paraventricular nucleus, and medial amygdala; these brain regions are regulated by various neurotransmitters including serotonin, opioids, dopamine, norepinephrine, and oxytocin (see figure 7 in reference [37] for details).
Since sexual reward in female rats occurs when females are allowed to pace their copulations [27,64,65], these reward states can be measured using a conditioned place preference paradigm, where females show a strong distinct preference for an environment only if placed in the conditioned place preference box immediately after paced copulation [36,55,66]. Artificial vaginocervical stimulation (with glass rod or plunger at a force or frequency that mimics intromissions) provides a stimulus for reward and reproduction that mimics intromissions from the male [67,68]. For example, vaginocervical stimulation at 30-second intervals for 15 stimulations induced a reliable conditioned place preference in ovariectomized rats primed with estrogen and progesterone [67,69]. Other studies looked at clitoral stimulation alone—via a lubricated paintbrush or small cotton-tipped vibrator—and showed that conditioned place preference occurred if the stimulus frequency was 1 stimulation every 5 seconds for five trials [70,71]. Therefore, the pattern/frequency of the stimulus is important to produce changes in sexual motivation and conditioned place preference and does not occur if the stimulus is of a regular, nonpaced frequency. Both vaginocervical and clitoral stimuli induced robust conditioned place preference. However, clitoral-induced reward was also seen in ovariectomized females without hormone priming, indicating that clitoral induced sexual reward may be independent of the circulating levels of estrogen and progesterone. In female rodents, hormonal priming is required for lordosis, which allows clitoral stimuli from the male during mounting and intromission behavior. Systemic or central administration of naloxone can block pacing-related conditioned place preference [37,72,73]. Thus, activation of the opioid pathways is necessary for sexual reward.
Animal Models of Sexual Arousal
Central Arousal
Generalized arousal involves activation brain pathways (cortex, hypothalamus, and reticular formation) to increase heart rate, blood pressure, and blood flow, and provides the sensory alertness so that the motor system can respond through descending pathways from the brain to the spinal cord, peripheral nerves, and muscles. Thus, sexual arousal comprises some form of central arousal [74,75]. A few studies have examined the relationship between central arousal and sexual function [75–80]. Methamphetamine has been reported to increase sexual thoughts and behavior in women by increasing generalized arousal; when given to female rats, methamphetamine also increased the incidence of lordosis and increased sexual motivation [81].
Central and sexual arousal involves increased activity of particular brain pathways. Mapping the location of activated brain neurons using the immediate early gene, c-fos, has been reported after the female received various types of stimuli from the male, such as mounts only, intromissions or ejaculations [62,63,82–87]. These studies reported an increase in c-fos expression in various brain regions, including the MPOA, VMN, bed nucleus of the stria terminals, and medial amygdala, that are activated with female sexual behavior.
The cellular mechanisms regulating the activity of the MPOA, VMN, and brainstem reticular neurons, which are all known to be involved in female sexual behavior, have recently been examined using electrophysiology and patch clamp techniques [76,88–93]. These studies demonstrated, for example, that norepinephrine increases the excitability of VMN neurons; while histamine depolarizes VMN neurons via decrease in potassium current. Understanding the cellular mechanisms behind neuronal activation and deactivation may provide new insight to the excitatory and inhibitory processes of central arousal that may, one day, be used to understand new psychotherapies, such as mindfulness in the treatment of sexual dysfunction.
Genital Arousal
Autonomic (pelvic and hypogastric nerves) and somatic peripheral nerves (pudendal sensory nerve) mediate arousal and sensation of the genitals (see Figure 2) [2,94–97]. Genital arousal in females is associated with an increase in vaginal lubrication and blood flow to the vagina and clitoris, which facilitates clitoral erection and vaginal engorgement. Improved sexual function has been reported in women after sacral nerve stimulation (the nerve fibers of the pudendal and pelvic nerves are contained within S2-S4 in humans) [99,100]. Nerve stimulation studies in animal models may be useful in understanding the mechanisms of afferent nerve stimulation and their role in modulating sexual arousal and reducing pelvic pain. Animal models using rats and rabbits have been developed to mimic the physiological changes that occur with genital arousal [1,101–112]. Stimulation of the pelvic nerve increases vaginal and clitoral blood flow, vaginal length, clitoral intracavernosal pressure, and vaginal luminal pressure. Similar responses can be evoked by pudendal sensory nerve stimulation— the major sensory nerve innervating the clitoris and vagina [105]. Sensory input is then relayed through the dorsal horn and spinal interneurons to modify parasympathetic output, which leads to increased vaginal blood flow [94,95,105,113]. Stimulation of the MPOA also resulted in increased vaginal blood flow, indicating a possible pathway from brain arousal centers to induce genital arousal responses [104]. Interestingly, MPOA stimulation also evoked ejaculatory-like reflexes in males [114].
Figure 2.
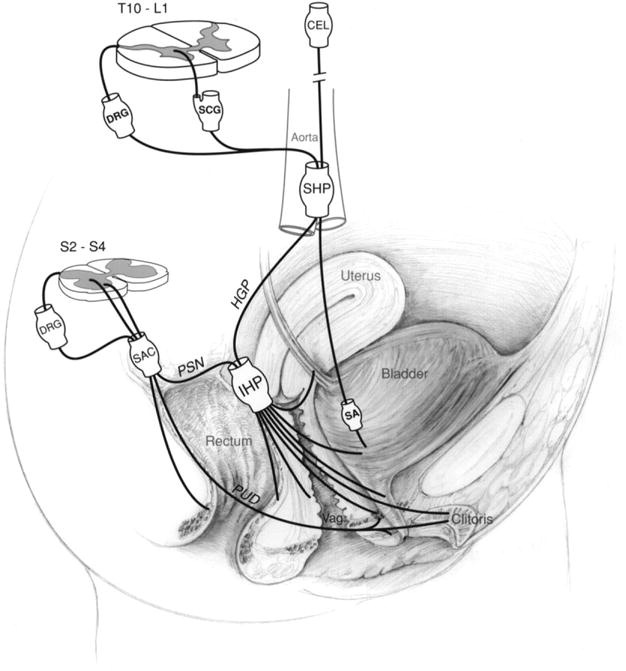
Schematic drawing showing the innervation of the urogenital and rectal area in females. Although this diagram attempts to show the innervation in humans, much of the anatomical information is derived from animal data (see text). CEL, celiac plexus; DRG, dorsal root ganglion; HGP, hypogastric nerve; IHP, inferior hypogastric plexus; PSN, pelvic splanchnic nerve; PUD, pudendal nerve; SA, short adrenergic projections; SAC, sacral plexus; SCG, sympathetic chain ganglion; SHP, superior hypogastric plexus; vag, vagina. This figure has been reproduced with permission of the International Association for the Study of PainR (IASP) from reference [98]. The figure may not be reproduced for any other purpose without permission.
Evidence that clitoral erection is mediated by similar mechanisms as penile erection has been provided. The neuroanatomical pathways of the dorsal clitoral nerve are similar to the dorsal nerve of the penis, which forms part of the sensory branch of the pudendal nerve to regulate clitoral sensations and engorgement. In addition, evidence for endothelial and neuronal nitric oxide pathways have been documented [105,113,115–124]. However, in clinical studies, sildenafil did not improve sexual function or significantly increase clitoral engorgement in women with female sexual arousal disorder [125,126]. Phosphodiesterase type 5 inhibitors may be beneficial in women with sexual problems related to antidepressant therapy, but appear to be less effective or ineffective in women with neurologically induced sexual dysfunction or sexual desire disorders [127].
In vitro smooth muscle preparations that examine contractile and relaxant effects of neurotransmitters or electrical field stimulation on vaginal or clitoral tissue have the potential of furthering our understanding of the peripheral mechanisms regulating sexual arousal responses and strength of muscle contractions that occur with orgasm [120,128–130]. Fewer studies have looked at cutaneous receptors and epithelial tissue [131]. Since genital stimulation and stimulation of erogenous skin can increase sexual arousal, this may be an opportunity for future research models.
Understanding the peripheral and spinal mechanisms mediating genital sensations and arousal is not only important for helping understand decreased genital arousal disorders, but is essential for development of treatments to aid women with persistent genital arousal that can cause severe distress in women [3,5].
Animal Models of Orgasm
Physiological responses that occur with orgasm in women include contractions of the pelvic floor, anal sphincter, and vagina, increases in respiration, blood pressure, and release of oxytocin [132–135]. The only animal model developed to mimic the physiological responses seen during orgasm is the urethrogenital reflex (UGR) [136,137]. Activation of the UGR, by brief distention of urethra or stimulation of pudendal nerve afferents in female rats, produces rhythmic contractions of the pudendal motor nerve, which regulates the pelvic floor and sphincter muscles, vagina, anal sphincter, and uterus. The sensory threshold required to evoke the UGR is reduced with infusion of intraurethral serotonin [137]. This model has been used to map the spinal and brain neurons and pathways activated with the UGR. The spinal pathways that innervate the genitalia and are activated with the UGR comprise a network that includes afferent inputs to the lumbar sacral dorsal horn, the dorsal gray commissure, and medial and lateral gray, specifically in regions that overlap with the sympathetic and parasympathetic preganglionic neurons of the hypogastric and pelvic nerves. In the brain, the nucleus paragigantocellularis, paraventricular nucleus of the hypothalamus, VMN, periaqueductal gray, MPOA, medial amygdala, bed nucleus of the stria terminalis, and cortex all have been shown to contain neurons whose activity is modulated by genital sensory stimulation [1,62,82]. These sites overlap with regions seen during functional MRI and PET scan mapping of the brain during orgasm in humans [57,59,138–140].
Models of Pain Associated with Potential Sexual Dysfunction
Genital pain can lead to sexual dysfunction by reducing desire, decreasing arousal, and increasing sexual inhibition. While pain with vaginal intercourse can arise from vaginal dryness, in which applied lubrications and estrogen cream can be helpful, the fear of pain associated with intercourse can lead to decreased sexual desire and arousal. Pain originating from the female reproductive organs is a major complaint during the fertile years [98,141]. Primary or secondary dysmenorrhea, pelvic inflammatory disease, or chronic pelvic pain, are just a few examples, whose causes are still largely unknown. In postmenopausal women vaginal dryness, vaginal and vulvar irritation and pain are frequent complaints related to estrogen deficiency. In addition, hyperactive genital arousal or persistent or spontaneous orgasms can be painful, frustrating, and debilitating. The development of animal models to address many of these painful disorders is lacking. Several animal models of pain have been developed that employ nociceptive distension, inflammation, or endometriosis, and are reviewed below. These models are useful for replicating the symptoms and dissecting the neuroanatomical and pharmacological pathways involved in pain. However, animal models of pain may have limited value for studying treatment responses for translational purposes due to species differences in receptor systems and immunological reactions to pain, as well as the unknown cognitive experience of pain perception. One exception to this may be the spontaneous pain model development by Giamberardino and coworkers described below [142,143].
Mechanical Models of Pain
Vaginal
Vaginal hypersensitivity can be measured by distension of the vagina in female rats [144]. Under anesthesia, a lubricated balloon is inserted into the vagina, avoiding the cervix, and hyperalgesia develops over time. Subsequently, escape responses are measured at different distension volumes [144,145]. Berkley and coworkers have combined this distension paradigm in rats with ovariectomy, to propose a model for dyspareunia associated with ovarian function loss. This hypersensitivity was reversed by estrogen replacement.
Uterine
Uterine distension has been used to evoke uterine pain in rats [144]. In a similar fashion to the vaginal hypersensitivity technique, animals can be tested for the probability to produce an escape response to a noxious tail pinch with distension of one uterine horn.
Distension of the uterine cervix has also been applied in anesthetized rats, to mimic acute pain that women experience during labor. The perceived visceral pain is monitored via electromyographic (EMG) response in the rectus abdominis muscle, mean blood pressure, and heart rate changes in response to uterine cervical distension. Morphine and peripherally restricted kappa opioid receptor agonists attenuate these responses; but the presence of estrogen renders the morphine treatment ineffective [146,147]. The EMG activity induced by uterine cervix distension can be blocked by COX inhibitors (SC58238 and indomethacin), but the cardiovascular responses remain [148].
An ovarian ligament nociceptive technique was proposed, by the authors, to provide a humane mechanism to study the effectiveness of analgesics for acute ovarian pain [149]. Under anesthesia, the right ovary is accessed via laparoscopy, and a suture is placed around the ovarian ligament and exteriorized through the abdominal wall for stimulation. The noxious stimulus consists of pulling the ovary and ovarian ligament with a force transducer. The response to noxious stimulation is determined using the anesthetic minimum alveolar concentration requirement (MAC) for sevoflurane (minimum concentration required to suppress the dog’s purposeful movement after 1 minute of ovarian stimulation).
It is important to note that in the mechanical distension models, not all the manipulated animals show hypersensitive responses, and the amount of mechanical force necessary to evoke the nociception is probably beyond the range of any natural event. Thus, it is unclear how useful these models are in relation to the clinical situation [144,150]. However, they have the advantage of establishing a precise relationship between the noxious stimulus and the evoked responses, and have shown reversal with commonly used analgesics. The inflammatory and endometrial methods (see below) appear to mimic the clinical situations more closely, and are thus suitable to investigate and interpret the pain phenomena observed in clinical pain syndromes in patients.
Inflammatory Techniques
Vulvodynia
Repeated exposure of the vulva to Candida albicans in mice produces long-lasting (>3 weeks after resolution of infection/inflammation) localized mechanical allodynia and hyperinnervation of peptidergic nociceptor and sympathetic fibers [151,152]. Around 40% of the infected animals (Candida + fluconazole) were allodynic vs. 5.5% of the fluconazole controls (saline + fluconazole). The increased nerve innervation of the vagina was measured by comparing protein gene product 9.5, calcitonin gene-related peptide, and vesicular monoamine transporter 2 immunoreactivity in infected and noninfected mice. Long-lasting behavioral allodynia in a subset of mice was also observed after a single, extended Candida infection, as well as after repeated vulvar inflammation induced with zymosan, a mixture of fungal antigens. This model resembles provoked vestibulodynia in women, the most common form of vulvodynia, an idiopathic pain disorder associated with a history of recurrent candidiasis, which is characterized by vulvar allodynia and hyperinnervation [152,153].
Pelvic Visceral/Muscle Pain
Uterine inflammation
Uterine inflammation in female rodents can be initiated by injection of mustard oil into one uterine horn. This induces behaviors that mimic pelvic pain and referred muscle hyperalgesia in women with inflammatory conditions of their reproductive area. After 2–4 days, the majority of rats show spontaneous pain behavior (major episodes of movements/postures indicative of pelvic pain) and referred hyperalgesia (measured by vocalization) in the ipsilateral flank muscles, in response to stimulation [154,155]. The areas of referred muscle hyperalgesia are also the site of neurogenic plasma extravasation in the skin, which is the first experimental evidence of trophic changes in sites of referred pain from viscera, a well-known phenomenon in the clinical setting [156].
Ovariectomy
Ovariectomy has been used in mice and rats to produce a condition of visceral pain/hypersensitivity of the pelvic area, which has been proposed as a model of a hormonally dependent hyperalgesia resembling functional pain in women [157–160]. Ovariectomy also increases depression in rodents [158–160]. Ovariectomized mice and rats present a hyperalgesic state (a robust mechanical and thermal hyperalgesia in the abdominal and pelvic regions) of slow onset (4 weeks) and long duration, as well as visceral hypersensitivity. Hormone replacement with 17beta-estradiol prevents and reverses the development of hyperalgesia but does not stop the involution of the internal reproductive organs.
Experiments in mice have shown that spinal ERK 1/2 is involved in the estrogen-dependent chronic visceral hyperalgesia [161]. Ovariectomized mice show a significant increase in the activation of ERK 1/2 (the extracellular signal-regulated kinases 1 and 2, members of the MAPK [mitogen-activated protein kinases] family) in the lumbosacral spinal cord which followed the time course of the hyperalgesia. Estrogen replacement reversed both the development of the hyperalgesia and the enhanced activation of ERK 1/2, while intrathecal injections of the ERK 1/2 inhibitor, U0126, significantly attenuated the abdominal hyperalgesia (up to 24 hours after the injection) and reversed the enhanced expression of ERK 1/2 [161].
Endometriosis
Endometriosis can result in pain, related to secondary dysmenorrhea or a more generalized chronic pelvic pain syndrome; in addition, vaginal hyperalgesia can occur that results in sexual dysfunction [162]. In women, the intensity of painful symptoms is not related to the size or location of the lesions [163]. A number of nonhuman and rodent models of endometriosis have been developed to study subfertility; more recently, these techniques are being used to study pain [164]. For example, endometriosis can be induced in female rats by grafting pieces of autologous endometrium (from one uterine horn) in different locations of the abdominal cavity: on alternate cascade mesenteric arteries that supply the caudal small intestine, at the level of the ovary and inner surface of abdominal muscles. Two to three weeks later, fluid-filled cysts develop at the implantation sites. Estradiol is required for cyst maintenance, and the severity of vaginal hyperalgesia varies with the estrus cycle. Interestingly, the cysts develop their own sensory and sympathetic nerve supply, which may be useful for future vascular studies. Evidence has been provided for a role of local cannabinoids (CB1) in the expression of the hyperalgesia associated with the endometrial cysts [163]. Mouse studies of this endometrial model have been developed [165] allowing future studies to utilize the availability of the many transgenic mouse strains. When combined with an artificial ureteral calculosis, endometriosis in rats produces a notable enhancement of the poststone spontaneous pain behavior (increase in both “ureteral” and “uterine” typical behavior, monitored over several days at videotape recordings, and increase in pelvic muscle hyperalgesia) [142]. This combination model closely resembles the clinical condition of “viscero-visceral hyperalgesia” (i.e., increased spontaneous pelvic and urinary pain, as well as in referred pelvic muscle hyperalgesia) extensively documented in women with endometriosis plus ureteral calculosis, and therefore is particularly suitable for studies understanding the pathophysiological mechanisms of this condition in women [143,166].
Summary
Many in vivo animal models of female sexual behavior (desire, arousal, motivation, and orgasm) and pelvic pain have been developed, some employing simple paradigms, such as monitoring proceptive behavior, or pelvic organ distension, others involving more complex learning paradigms or surgical interventions. Each model or technique has its advantages and limitations. Nevertheless, valuable information on the mechanisms, development, and occurrence of sexual function and dysfunction with or without pain have been gained that can be translated to women. New research understanding genital sensory inputs and their regulation in females should help aid our understanding and development of treatments for female sexual dysfunction that are not primarily based on the research conducted in males. More information on the etiology of women’s sexual dysfunction disorders would aid development and application of the preclinical models.
Future Directions
Studies on gonadal steroid hormones have been conducted, but there remains a lack of studies in females examining other hormones, such as thyroid and stress hormones. Animal models for pathological and disease states are lacking, such as cancer, stroke, cardiovascular function, diabetes, and aging. The pelvic pain models should also be used to test the impact of pain on sexual dysfunction; effective pharmacologic treatments (classic and newer) for the pain should also be tested with respect to their effectiveness on the sexual dysfunction parameters. The study of comorbidities (e.g., bladder inflammation, fibromyalgia, and myofascial pain syndromes) related to pelvic pain that may lead to sexual dysfunction in females has yet to be fully explored. These types of techniques may mimic the clinical condition of extensive cooccurrence of several pain conditions in the urogenital area observed in patients [166–169].
Acknowledgments
Ursula Wesselmann’s work has been supported by NIH grant HD39699 (NICHD) and the Office of Research on Women’s Health (ORWH).
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Giraldi A, Marson L, Nappi R, Pfaus J, Traish AM, Vardi Y, Goldstein I. Physiology of female sexual function: Animal models. J Sex Med. 2004;1:237–53. doi: 10.1111/j.1743-6109.04037.x. Epub 2006/01/21. eng. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano F, Pfaus J, Srilatha B, Hedlund P, Hisasue S, Marson L, Wallen K. Experimental models for the study of female and male sexual function. J Sex Med. 2010;7:2970–95. doi: 10.1111/j.1743-6109.2010.01960.x. Epub 2010/11/06.eng. [DOI] [PubMed] [Google Scholar]
- 3.Basson R, Leiblum S, Brotto L, Derogatis L, Fourcroy J, Fugl-Meyer K, Graziottin A, Heiman JR, Laan E, Meston C, Schover L, van Lankveld J, Shultz WW. Revised definitions of women’s sexual dysfunction. J Sex Med. 2004;1:40–8. doi: 10.1111/j.1743-6109.2004.10107.x. Epub 2006/01/21. eng. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann GA, Rosen R, Pinn VW, Utian WH, Ayers C, Basson R, Binik YM, Brown C, Foster DC, Gibbons JM, Jr, Goldstein I, Grazziottin A, Haefner HK, Harlow BL, Spaot SR, Leiblum SR, Masheb RM, Reed BD, Sobel JD, Veasly C, Wesselmann U, Witkin SS. Vulvodynia: A state-of-the-art consensus on definitions, diagnosis and management. J Reprod Med. 2006;51:447–56. [PubMed] [Google Scholar]
- 5.Basson R, Althof S, Davis S, Fugl-Meyer K, Goldstein I, Leiblum S, Meston C, Rosen R, Wagner G. Summary of the recommendations on sexual dysfunctions in women. J Sex Med. 2004;1:24–34. doi: 10.1111/j.1743-6109.2004.10105.x. [DOI] [PubMed] [Google Scholar]
- 6.Kingsberg S, Althof SE. Evaluation and treatment of female sexual disorders. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(suppl 1):S33–43. doi: 10.1007/s00192-009-0833-x. [DOI] [PubMed] [Google Scholar]
- 7.Masters WH, Johnson VE. In human sexual response. Boston: Little, Brown & Co.; 1966. [Google Scholar]
- 8.Tan O, Bradshaw K, Carr BR. Management of vulvovaginal atrophy-related sexual dysfunction in postmenopausal women: An up-to-date review. Menopause. 2012;19:109–17. doi: 10.1097/gme.0b013e31821f92df. Epub 2011/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 9.Giraldi A, Rellini AH, Pfaus J, Laan E. Female sexual arousal disorders. J Sex Med. 2013;10:58–73. doi: 10.1111/j.1743-6109.2012.02820.x. Epub 2012/09/15. Eng. [DOI] [PubMed] [Google Scholar]
- 10.Laan E, Rellini AH, Barnes T. Standard operating procedures for female orgasmic disorder: Consensus of the international society for sexual medicine. J Sex Med. 2013;10:74–82. doi: 10.1111/j.1743-6109.2012.02880.x. Epub 2012/09/14. Eng. [DOI] [PubMed] [Google Scholar]
- 11.Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders. J Sex Med. 2010;7(1 Pt 2):586–614. doi: 10.1111/j.1743-6109.2009.01630.x. Epub 2010/01/23. eng. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. Epub 2012/02/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm Behav. 2012;61:266–76. doi: 10.1016/j.yhbeh.2011.11.001. Epub 2011/11/24. eng. [DOI] [PubMed] [Google Scholar]
- 14.Wen S, Gotze IN, Mai O, Schauer C, Leinders-Zufall T, Boehm U. Genetic identification of GnRH receptor neurons: A new model for studying neural circuits underlying reproductive physiology in the mouse brain. Endocrinology. 2011;152:1515–26. doi: 10.1210/en.2010-1208. Epub 2011/02/10. eng. [DOI] [PubMed] [Google Scholar]
- 15.Spiteri T, Ogawa S, Musatov S, Pfaff DW, Agmo A. The role of the estrogen receptor alpha in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav Brain Res. 2012;230:11–20. doi: 10.1016/j.bbr.2012.01.048. Epub 2012/02/11. eng. [DOI] [PubMed] [Google Scholar]
- 16.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A. Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology. 2010;91:142–54. doi: 10.1159/000255766. Epub 2009/11/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–9. doi: 10.1111/j.1601-183X.2009.00491.x. Epub 2009/07/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–5. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- 19.Martinez LA, Albers HE, Petrulis A. Blocking oxytocin receptors inhibits vaginal marking to male odors in female Syrian hamsters. Physiol Behav. 2010;101:685–92. doi: 10.1016/j.physbeh.2010.08.007. Epub 2010/08/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–46. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–7. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- 22.Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–8. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 24.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–38. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 25.Beach FA. Cerebral and hormonal control of reflexive mechanisms involved in copulatory behavior. Physiol Rev. 1967;47:289–316. doi: 10.1152/physrev.1967.47.2.289. [DOI] [PubMed] [Google Scholar]
- 26.Pfaus JG. Neurobiology of sexual behavior. Curr Opin Neurobiol. 1999;9:751–8. doi: 10.1016/s0959-4388(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 27.Erskine MS. Solicitation behavior in the estrous female rat: A review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 28.McClintock MK, Adler NT. The role of the female during copulation in wild and domestic Norway rats (Rattus noregicus) Behaviour. 1977;18:379–86. [Google Scholar]
- 29.McClintock MK, Anisko JJ. Group mating among Norway rats: I. Sex differences in the pattern and neuroendocrine consequences of copulation. Anim Behav. 1982;30:398–409. [Google Scholar]
- 30.Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. Epub 2003/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 31.Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors I. Behavioral effects of glutamate and its selective receptor agonists AMPA, NMDA and kainate. Pharmacol Biochem Behav. 2006;83:322–32. doi: 10.1016/j.pbb.2006.02.016. Epub 2006/03/25. eng. [DOI] [PubMed] [Google Scholar]
- 32.Baskerville TA, Douglas AJ. Interactions between dopamine and oxytocin in the control of sexual behaviour. Prog Brain Res. 2008;170:277–90. doi: 10.1016/S0079-6123(08)00423-8. [DOI] [PubMed] [Google Scholar]
- 33.Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behavior. In: Knobil E, Neill J, editors. The physiology of reproduction. Vol. 1994. New York: Raven Press, Ltd.; pp. 107–220. [Google Scholar]
- 34.Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73:179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Coria-Avila GA, Pfaus JG. Neuronal activation by stimuli that predict sexual reward in female rats. Neuroscience. 2007;148:623–32. doi: 10.1016/j.neuroscience.2007.05.052. Epub 2007/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 36.Coria-Avila GA, Ouimet AJ, Pacheco P, Manzo J, Pfaus JG. Olfactory conditioned partner preference in the female rat. Behav Neurosci. 2005;119:716–25. doi: 10.1037/0735-7044.119.3.716. Epub 2005/07/07. eng. [DOI] [PubMed] [Google Scholar]
- 37.Pfaus JG, Kippin TE, Coria-Avila GA, Gelez H, Afonso VM, Ismail N, Parada M. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch Sex Behav. 2012;41:31–62. doi: 10.1007/s10508-012-9935-5. Epub 2012/03/10. eng. [DOI] [PubMed] [Google Scholar]
- 38.Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6:1506–33. doi: 10.1111/j.1743-6109.2009.01309.x. Epub 2009/05/21. eng. [DOI] [PubMed] [Google Scholar]
- 39.Snoeren EM, Chan JS, de Jong TR, Waldinger MD, Olivier B, Oosting RS. A new female rat animal model for hypoactive sexual desire disorder; behavioral and pharmacological evidence. J Sex Med. 2011;8:44–56. doi: 10.1111/j.1743-6109.2010.01998.x. Epub 2010/09/03. eng. [DOI] [PubMed] [Google Scholar]
- 40.Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc Natl Acad Sci U S A. 2004;101:10201–4. doi: 10.1073/pnas.0400491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaus J, Giuliano F, Gelez H. Bremelanotide: An overview of preclinical CNS effects on female sexual function. J Sex Med. 2007;4(suppl 4):269–79. doi: 10.1111/j.1743-6109.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 42.Rossler AS, Pfaus JG, Kia HK, Bernabe J, Alexandre L, Giuliano F. The melanocortin agonist, melanotan II, enhances proceptive sexual behaviors in the female rat. Pharmacol Biochem Behav. 2006;85:514–21. doi: 10.1016/j.pbb.2006.09.023. Epub 2006/11/23. eng. [DOI] [PubMed] [Google Scholar]
- 43.Diamond LE, Earle DC, Heiman JR, Rosen RC, Perelman MA, Harning R. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med. 2006;3:628–38. doi: 10.1111/j.1743-6109.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaspersen H, Agmo A. Paroxetine-induced reduction of sexual incentive motivation in female rats is not modified by 5-HT1B or 5-HT2C antagonists. Psychopharmacology (Berl) 2012;220:269–80. doi: 10.1007/s00213-011-2475-1. Epub 2011/09/13. eng. [DOI] [PubMed] [Google Scholar]
- 45.Miryala CS, Hiegel C, Uphouse L. Sprague-Dawley and Fischer female rats differ in acute effects of fluoxetine on sexual behavior. J Sex Med. 2013;10:350–61. doi: 10.1111/j.1743-6109.2012.02981.x. Epub 2012/11/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guptarak J, Sarkar J, Hiegel C, Uphouse L. Role of 5-HT(1A) receptors in fluoxetine-induced lordosis inhibition. Horm Behav. 2010;58:290–6. doi: 10.1016/j.yhbeh.2010.03.003. Epub 2010/03/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinclair-Worley L, Uphouse L. Effect of estrogen on the lordosis-inhibiting action of ketanserin and SB 206553. Behav Brain Res. 2004;152:129–35. doi: 10.1016/j.bbr.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 48.Gelez H, Poirier S, Facchinetti P, Allers KA, Wayman C, Alexandre L, Guiliano F. Neuroanatomical evidence for a role of central melanocortin-4 receptors and oxytocin in the efferent control of the rodent clitoris and vagina. J Sex Med. 2010;40:310–24. doi: 10.1111/j.1743-6109.2010.01760.x. [DOI] [PubMed] [Google Scholar]
- 49.Stahl SM, Sommer B, Allers KA. Multifunctional pharmacology of flibanserin: Possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011;8:15–27. doi: 10.1111/j.1743-6109.2010.02032.x. Epub 2010/09/16. eng. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy S. Flibanserin: Initial evidence of efficacy on sexual dysfunction, in patients with major depressive disorder. J Sex Med. 2010;7:3449–59. doi: 10.1111/j.1743-6109.2010.01938.x. Epub 2010/07/22. eng. [DOI] [PubMed] [Google Scholar]
- 51.Cummings JA, Becker JB. Quantitative assessment of female sexual motivation in the rat: Hormonal control of motivation. J Neurosci Methods. 2012;204:227–33. doi: 10.1016/j.jneumeth.2011.11.017. Epub 2011/11/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham MD, Pfaus JG. Differential regulation of female sexual behaviour by dopamine agonists in the medial preoptic area. Pharmacol Biochem Behav. 2010;97:284–92. doi: 10.1016/j.pbb.2010.08.012. Epub 2010/09/03. eng. [DOI] [PubMed] [Google Scholar]
- 53.Graham MD, Pfaus JG. Differential effects of dopamine antagonists infused to the medial preoptic area on the sexual behavior of female rats primed with estrogen and progesterone. Pharmacol Biochem Behav. 2012;102:532–9. doi: 10.1016/j.pbb.2012.06.020. Epub 2012/07/04. eng. [DOI] [PubMed] [Google Scholar]
- 54.Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: A review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- 55.Pfaus JG, Kippin TE, Coria-Avila G. What can animal models tell us about human sexual response? Annu Rev Sex Res. 2003;14:1–63. Epub 2004/08/04. eng. [PubMed] [Google Scholar]
- 56.Gilman DP, Hitt JC. Effects of gonadal hormones on pacing of sexual contacts by female rats. Behav Biol. 1978;24:77–87. doi: 10.1016/s0091-6773(78)92926-7. [DOI] [PubMed] [Google Scholar]
- 57.Georgiadis JR, Kortekaas R, Kuipers R, Nieuwenburg A, Pruim J, Reinders AA, et al. Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur J Neurosci. 2006;24:3305–16. doi: 10.1111/j.1460-9568.2006.05206.x. Epub 2006/12/13. eng. [DOI] [PubMed] [Google Scholar]
- 58.Georgiadis JR, Reinders AA, Paans AM, Renken R, Kortekaas R. Men versus women on sexual brain function: Prominent differences during tactile genital stimulation, but not during orgasm. Hum Brain Mapp. 2009;30:3089–101. doi: 10.1002/hbm.20733. Epub 2009/02/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komisaruk BR, Whipple B. Functional MRI of the brain during orgasm in women. Annu Rev Sex Res. 2005;16:62–86. Epub 2006/08/18. eng. [PubMed] [Google Scholar]
- 60.Borg C, de Jong PJ, Georgiadis JR. Subcortical BOLD responses during visual sexual stimulation vary as a function of implicit porn associations in women. Soc Cogn Affect Neurosci. 2012 Oct 29; doi: 10.1093/scan/nss117. Epub 2012/10/12 Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Georgiadis JR, Kringelbach ML. The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Prog Neurobiol. 2012;98:49–81. doi: 10.1016/j.pneurobio.2012.05.004. Epub 2012/05/23. eng. [DOI] [PubMed] [Google Scholar]
- 62.Pfaus JG, Manitt C, Coopersmith CB. Effects of pelvic, pudendal, or hypogastric nerve cuts on Fos induction in the rat brain following vaginocervical stimulation. Physiol Behav. 2006;89:627–36. doi: 10.1016/j.physbeh.2006.07.022. Epub 2006/09/09. eng. [DOI] [PubMed] [Google Scholar]
- 63.Shelley DN, Meisel RL. The effects of mating stimulation on c-Fos immunoreactivity in the female hamster medial amygdala are region and context dependent. Horm Behav. 2005;47:212–22. doi: 10.1016/j.yhbeh.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 64.Paredes RG, Vazquez B. What do female rats like about sex? Paced mating Behav Brain Res. 1999;105:117–27. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 65.Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci. 1997;111:123–8. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- 66.Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43:503–7. doi: 10.1016/s0018-506x(03)00031-x. Epub 2003/06/06. eng. [DOI] [PubMed] [Google Scholar]
- 67.Meerts SH, Clark AS. Artificial vaginocervical stimulation induces a conditioned place preference in female rats. Horm Behav. 2009;55:128–32. doi: 10.1016/j.yhbeh.2008.09.003. Epub 2008/10/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lehmann ML, Erskine MS. Induction of pseudopregnancy using artificial VCS: Importance of lordosis intensity and prestimulus estrous cycle length. Horm Behav. 2004;45:75–83. doi: 10.1016/j.yhbeh.2003.09.011. Epub 2004/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 69.Meerts SH, Clark AS. Conditioned place preference for mating is preserved in rats with pelvic nerve transection. Behav Neurosci. 2009;123:539–46. doi: 10.1037/a0015267. Epub 2009/06/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cibrian-Llanderal T, Tecamachaltzi-Silvaran M, Triana-Del Rio R, Pfaus JG, Manzo J, Coria-Avila GA. Clitoral stimulation modulates appetitive sexual behavior and facilitates reproduction in rats. Physiol Behav. 2010;100:148–53. doi: 10.1016/j.physbeh.2010.02.015. Epub 2010/03/02. eng. [DOI] [PubMed] [Google Scholar]
- 71.Parada M, Chamas L, Censi S, Coria-Avila G, Pfaus JG. Clitoral stimulation induces conditioned place preference and Fos activation in the rat. Horm Behav. 2010;57:112–8. doi: 10.1016/j.yhbeh.2009.05.008. Epub 2009/06/13. eng. [DOI] [PubMed] [Google Scholar]
- 72.Coria-Avila GA, Solomon CE, Vargas EB, Lemme I, Ryan R, Menard S, Gavrila AM, Pfaus JG. Neurochemical basis of conditioned partner preference in the female rat: I. Disruption by naloxone. Behav Neurosci. 2008;122:385–95. doi: 10.1037/0735-7044.122.2.385. Epub 2008/04/16. eng. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Horsman SP, Agmo A, Paredes RG. Infusions of naloxone into the medial preoptic area, ventromedial nucleus of the hypothalamus, and amygdala block conditioned place preference induced by paced mating behavior. Horm Behav. 2008;54:709–16. doi: 10.1016/j.yhbeh.2008.07.011. Epub 2008/08/30. eng. [DOI] [PubMed] [Google Scholar]
- 74.Agmo A. On the intricate relationship between sexual motivation and arousal. Horm Behav. 2011;59:681–8. doi: 10.1016/j.yhbeh.2010.08.013. Epub 2010/09/08. eng. [DOI] [PubMed] [Google Scholar]
- 75.Weil ZM, Zhang Q, Hornung A, Blizard D, Pfaff DW. Impact of generalized brain arousal on sexual behavior. Proc Natl Acad Sci U S A. 2010;107:2265–70. doi: 10.1073/pnas.0914014107. Epub 2010/01/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pfaff DW, Martin EM, Faber D. Origins of arousal: Roles for medullary reticular neurons. Trends Neurosci. 2012;35:468–76. doi: 10.1016/j.tins.2012.04.008. Epub 2012/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 77.Devidze N, Lee AW, Zhou J, Pfaff DW. CNS arousal mechanisms bearing on sex and other biologically regulated behaviors. Physiol Behav. 2006;88:283–93. doi: 10.1016/j.physbeh.2006.05.030. Epub 2006/06/14. eng. [DOI] [PubMed] [Google Scholar]
- 78.Guarraci FA, Clark AS. Amphetamine modulation of paced mating behavior. Pharmacol Biochem Behav. 2003;76:505–15. doi: 10.1016/j.pbb.2003.09.003. Epub 2003/12/04. eng. [DOI] [PubMed] [Google Scholar]
- 79.Afonso VM, Mueller D, Stewart J, Pfaus JG. Amphetamine pretreatment facilitates appetitive sexual behaviors in the female rat. Psychopharmacology (Berl) 2009;205:35–43. doi: 10.1007/s00213-009-1511-x. Epub 2009/03/14. eng. [DOI] [PubMed] [Google Scholar]
- 80.Pfaus JG, Wilkins MF, Dipietro N, Benibgui M, Toledano R, Rowe A, Couch MC. Inhibitory and disinhibitory effects of psychomotor stimulants and depressants on the sexual behavior of male and female rats. Horm Behav. 2010;58:163–76. doi: 10.1016/j.yhbeh.2009.10.004. Epub 2009/10/20. eng. [DOI] [PubMed] [Google Scholar]
- 81.Holder MK, Mong JA. Methamphetamine enhances paced mating behaviors and neuroplasticity in the medial amygdala of female rats. Horm Behav. 2010;58:519–25. doi: 10.1016/j.yhbeh.2010.04.006. Epub 2010/04/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pfaus JG, Marcangione C, Smith WJ, Manitt C, Abillamaa H. Differential induction of Fos in the female rat brain following different amounts of vaginocervical stimulation: Modulation by steroid hormones. Brain Res. 1996;741:314–30. doi: 10.1016/s0006-8993(96)00985-7. [DOI] [PubMed] [Google Scholar]
- 83.Normandin JJ, Murphy AZ. Nucleus paragigantocellularis afferents in male and female rats: Organization, gonadal steroid receptor expression, and activation during sexual behavior. J Comp Neurol. 2008;508:771–94. doi: 10.1002/cne.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang JJ, Oberlander JG, Erskine MS. Expression of FOS, EGR-1, and ARC in the amygdala and hippocampus of female rats during formation of the intromission mnemonic of pseudopregnancy. Dev Neurobiol. 2007;67:895–908. doi: 10.1002/dneu.20376. [DOI] [PubMed] [Google Scholar]
- 85.Michael RP, Clancy AN, Zumpe D. Mating activates estrogen receptor-containing neurons in the female monkey brain. Physiol Behav. 2005;85:404–13. doi: 10.1016/j.physbeh.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 86.Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- 87.Pfaus JG, Kleopoulos SP, Mobbs CV, Gibbs RB, Pfaff DW. Sexual stimulation activates c-fos within estrogen-concentrating regions of the female rat forebrain. Brain Res. 1993;624:253–67. doi: 10.1016/0006-8993(93)90085-2. [DOI] [PubMed] [Google Scholar]
- 88.Pitchers KK, Schmid S, Di Sebastiano AR, Wang X, Laviolette SR, Lehman MN, et al. Natural reward experience alters AMPA and NMDA receptor distribution and function in the nucleus accumbens. PLoS ONE. 2012;7:e34700. doi: 10.1371/journal.pone.0034700. Epub 2012/04/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin EM, Devidze N, Shelley DN, Westberg L, Fontaine C, Pfaff DW. Molecular and neuroanatomical characterization of single neurons in the mouse medullary gigantocellular reticular nucleus. J Comp Neurol. 2011;519:2574–93. doi: 10.1002/cne.22639. Epub 2011/04/02. eng. [DOI] [PubMed] [Google Scholar]
- 90.Dupre C, Lovett-Barron M, Pfaff DW, Kow LM. Histaminergic responses by hypothalamic neurons that regulate lordosis and their modulation by estradiol. Proc Natl Acad Sci U S A. 2010;107:12311–6. doi: 10.1073/pnas.1006049107. Epub 2010/06/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Devidze N, Zhang Q, Zhou J, Lee AW, Pataky S, Kow LM, et al. Presynaptic actions of opioid receptor agonists in ventromedial hypothalamic neurons in estrogen- and oil-treated female mice. Neuroscience. 2008;152:942–9. doi: 10.1016/j.neuroscience.2008.01.033. Epub 2008/03/18. eng. [DOI] [PubMed] [Google Scholar]
- 92.Lee AW, Kyrozis A, Chevaleyre V, Kow LM, Devidze N, Zhang Q, Etgen AM, Pfaff DW. Estradiol modulation of phenylephrine-induced excitatory responses in ventromedial hypothalamic neurons of female rats. Proc Natl Acad Sci U S A. 2008;105:7333–8. doi: 10.1073/pnas.0802760105. Epub 2008/05/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou J, Lee AW, Devidze N, Zhang Q, Kow LM, Pfaff DW. Histamine-induced excitatory responses in mouse ventromedial hypothalamic neurons: Ionic mechanisms and estrogenic regulation. J Neurophysiol. 2007;98:3143–52. doi: 10.1152/jn.00337.2007. Epub 2007/10/19. eng. [DOI] [PubMed] [Google Scholar]
- 94.Wiedey J, Alexander MS, Marson L. Spinal neurons activated in response to pudendal or pelvic nerve stimulation in female rats. Brain Res. 2008;4:106–14. doi: 10.1016/j.brainres.2007.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: A dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R419–28. doi: 10.1152/ajpregu.00864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marson L, Foley KA. Identification of neural pathways involved in genital reflexes in the female: A combined anterograde and retrograde tracing study. Neuroscience. 2004;127:723–36. doi: 10.1016/j.neuroscience.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 97.Marson L, Cai R, Makhanova N. Identification of spinal neurons involved in the urethrogenital reflex in the female rat. J Comp Neurol. 2003;462:355–70. doi: 10.1002/cne.10732. [DOI] [PubMed] [Google Scholar]
- 98.Wesselmann U, Burnett AL, Heinberg LJ. The urogenital and rectal pain syndromes. Pain. 1997;73:269–94. doi: 10.1016/S0304-3959(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 99.Fariello JY, Whitmore K. Sacral neuromodulation stimulation for IC/PBS, chronic pelvic pain, and sexual dysfunction. Int Urogynecol J. 2010;21:1553–8. doi: 10.1007/s00192-010-1281-3. Epub 2010/10/26. eng. [DOI] [PubMed] [Google Scholar]
- 100.Gill BC, Swartz MA, Firoozi F, Rackley RR, Moore CK, Goldman HB, et al. Improved sexual and urinary function in women with sacral nerve stimulation. Neuromodulation. 2011;14:436–43. doi: 10.1111/j.1525-1403.2011.00380.x. discussion 43. Epub 2011/08/23. eng. [DOI] [PubMed] [Google Scholar]
- 101.Waxman SE, Pukall CF. Laser Doppler imaging of genital blood flow: A direct measure of female sexual arousal. J Sex Med. 2009;6:2278–85. doi: 10.1111/j.1743-6109.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 102.Traish AM, Kim SW, Stankovic M, Goldstein I, Kim NN. Testosterone increases blood flow and expression of androgen and estrogen receptors in the rat vagina. J Sex Med. 2007;4:609–19. doi: 10.1111/j.1743-6109.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 103.Munarriz R, Kim SW, Kim NN, Traish A, Goldstein I. A review of the physiology and pharmacology of peripheral (vaginal and clitoral) female genital arousal in the animal model. J Urol. 2003;170(2 Pt 2):S40–4. doi: 10.1097/01.ju.0000075352.03144.15. discussion S4–5. Epub 2003/07/11. eng. [DOI] [PubMed] [Google Scholar]
- 104.Giuliano F, Allard J, Compagnie S, Alexandre L, Droupy S, Bernabe J. Vaginal physiological changes in a model of sexual arousal in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R140–9. doi: 10.1152/ajpregu.2001.281.1.R140. Epub 2001/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 105.Cai RS, Alexander MS, Marson L. Activation of somatosensory afferents elicit changes in vaginal blood flow and the urethrogenital reflex via autonomic efferents. J Urol. 2008;180:1167–72. doi: 10.1016/j.juro.2008.04.139. [DOI] [PubMed] [Google Scholar]
- 106.Min K, Munarriz R, Kim NN, Choi S, O’Connell L, Goldstein I, Traish AM. Effects of ovariectomy and estrogen replacement on basal and pelvic nerve stimulated vaginal lubrication in an animal model. J Sex Marital Ther. 2003;29(suppl 1):77–84. doi: 10.1080/713847131. [DOI] [PubMed] [Google Scholar]
- 107.Vachon P, Simmerman N, Zahran AR, Carrier S. Increases in clitoral and vaginal blood flow following clitoral and pelvic plexus nerve stimulations in the female rat. Int J Impot Res. 2000;12:53–7. doi: 10.1038/sj.ijir.3900480. [DOI] [PubMed] [Google Scholar]
- 108.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–31. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 109.Min K, Munarriz R, Kim NN, Goldstein I, Traish A. Effects of ovariectomy and estrogen and androgen treatment on sildenafil-mediated changes in female genital blood flow and vaginal lubrication in the animal model. Am J Obstet Gynecol. 2002;187:1370–6. doi: 10.1067/mob.2002.126641. Epub 2002/11/20. eng. [DOI] [PubMed] [Google Scholar]
- 110.Traish AM, Kim NN, Munarriz R, Moreland R, Goldstein I. Biochemical and physiological mechanisms of female genital sexual arousal. Arch Sex Behav. 2002;31:393–400. doi: 10.1023/a:1019831906508. Epub 2002/09/20. eng. [DOI] [PubMed] [Google Scholar]
- 111.Kim NN, Min K, Huang YH, Goldstein I, Traish AM. Biochemical and functional characterization of alpha-adrenergic receptors in the rabbit vagina. Life Sci. 2002;71:2909–20. doi: 10.1016/s0024-3205(02)02162-8. Epub 2002/10/16. eng. [DOI] [PubMed] [Google Scholar]
- 112.Min K, Munarriz R, Berman J, Kim NN, Goldstein I, Traish AM, Stankovic MR. Hemodynamic evaluation of the female sexual arousal response in an animal model. J Sex Marital Ther. 2001;27:557–65. doi: 10.1080/713846801. Epub 2001/09/14. eng. [DOI] [PubMed] [Google Scholar]
- 113.Tovar A, Lara-Garcia M, Cruz Y, Pacheco P. Dorsal root activity evoked by stimulation of vagina-cervix-uterus junction in the rat. Brain Res. 2013;1496:49–54. doi: 10.1016/j.brainres.2012.12.023. Epub 2012/12/29. Eng. [DOI] [PubMed] [Google Scholar]
- 114.Marson L, McKenna KE. Stimulation of the hypothalamus initiates the urethrogenital reflex in male rats. Brain Res. 1994;638:103–8. doi: 10.1016/0006-8993(94)90638-6. Epub 1994/02/28. eng. [DOI] [PubMed] [Google Scholar]
- 115.Musicki B, Liu T, Strong TD, Lagoda GA, Bivalacqua TJ, Burnett AL. Post-translational regulation of endothelial nitric oxide synthase (eNOS) by estrogens in the rat vagina. J Sex Med. 2010;7:1768–77. doi: 10.1111/j.1743-6109.2010.01750.x. Epub 2010/03/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Musicki B, Liu T, Lagoda GA, Bivalacqua TJ, Strong TD, Burnett AL. Endothelial nitric oxide synthase regulation in female genital tract structures. J Sex Med. 2009;6(suppl 3):247–53. doi: 10.1111/j.1743-6109.2008.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martin-Alguacil N, Pfaff DW, Shelley DN, Schober JM. Clitoral sexual arousal: An immunocytochemical and innervation study of the clitoris. BJU Int. 2008;101:1407–13. doi: 10.1111/j.1464-410X.2008.07625.x. Epub 2008/05/06. eng. [DOI] [PubMed] [Google Scholar]
- 118.Gonzalez-Flores O, Beyer C, Lima-Hernandez FJ, Gomora-Arrati P, Gomez-Camarillo MA, Hoffman K, et al. Facilitation of estrous behavior by vaginal cervical stimulation in female rats involves alpha1-adrenergic receptor activation of the nitric oxide pathway. Behav Brain Res. 2007;176:237–43. doi: 10.1016/j.bbr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Werkstrom V, Svensson A, Andersson KE, Hedlund P. Phosphodiesterase 5 in the female pig and human urethra: Morphological and functional aspects. BJU Int. 2006;98:414–23. doi: 10.1111/j.1464-410X.2006.06217.x. Epub 2006/04/22. eng. [DOI] [PubMed] [Google Scholar]
- 120.Uckert S, Ehlers V, Nuser V, Oelke M, Kauffels W, Scheller F, Jonas U. In vitro functional responses of isolated human vaginal tissue to selective phosphodiesterase inhibitors. World J Urol. 2005;23:398–404. doi: 10.1007/s00345-005-0014-6. [DOI] [PubMed] [Google Scholar]
- 121.Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. An in vivo rat model to investigate female vaginal arousal response. J Urol. 2004;171:1357–61. doi: 10.1097/01.ju.0000109868.19569.d7. [DOI] [PubMed] [Google Scholar]
- 122.Tajkarimi K, Burnett AL. The role of genital nerve afferents in the physiology of the sexual response and pelvic floor function. J Sex Med. 2011;8:1299–312. doi: 10.1111/j.1743-6109.2011.02211.x. Epub 2011/02/18. eng. [DOI] [PubMed] [Google Scholar]
- 123.Yuan SY, Gibbins IL, Zagorodnyuk VP, Morris JL. Sacro-lumbar intersegmental spinal reflex in autonomic pathways mediating female sexual function. J Sex Med. 2011;8:1931–42. doi: 10.1111/j.1743-6109.2010.02160.x. Epub 2011/01/08. eng. [DOI] [PubMed] [Google Scholar]
- 124.Martin-Alguacil N, Schober JM, Sengelaub DR, Pfaff DW, Shelley DN. Clitoral sexual arousal: Neuronal tracing study from the clitoris through the spinal tracts. J Urol. 2008;180:1241–8. doi: 10.1016/j.juro.2008.06.009. Epub 2008/08/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leddy LS, Yang CC, Stuckey BG, Sudworth M, Haughie S, Sultana S, Maravilla KR. Influence of sildenafil on genital engorgement in women with female sexual arousal disorder. J Sex Med. 2012;9:2693–7. doi: 10.1111/j.1743-6109.2012.02796.x. Epub 2012/05/25. eng. [DOI] [PubMed] [Google Scholar]
- 126.Alexander MS, Rosen RC, Steinberg S, Symonds T, Haughie S, Hultling C. Sildenafil in women with sexual arousal disorder following spinal cord injury. Spinal Cord. 2011;49:273–9. doi: 10.1038/sc.2010.107. Epub 2010/08/25. eng. [DOI] [PubMed] [Google Scholar]
- 127.Chivers ML, Seto MC, Lalumiere ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: A meta-analysis. Arch Sex Behav. 2010;39:5–56. doi: 10.1007/s10508-009-9556-9. Epub 2010/01/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Giraldi A, Alm P, Werkstrom V, Myllymaki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002;14:271–82. doi: 10.1038/sj.ijir.3900886. [DOI] [PubMed] [Google Scholar]
- 129.Rahardjo HE, Brauer A, Magert HJ, Meyer M, Kauffels W, Taher A, Rahardjo D, Jonas U, Kuczyk MA, Uckert S. Endogenous vasoactive peptides and the human vagina—A molecular biology and functional study. J Sex Med. 2011;8:35–43. doi: 10.1111/j.1743-6109.2010.01923.x. Epub 2010/06/30. eng. [DOI] [PubMed] [Google Scholar]
- 130.Pessina MA, Hoyt RF, Jr, Goldstein I, Traish AM. Differential effects of estradiol, progesterone, and testosterone on vaginal structural integrity. Endocrinology. 2006;147:61–9. doi: 10.1210/en.2005-0870. [DOI] [PubMed] [Google Scholar]
- 131.Kim NN, Stankovic M, Armagan A, Cushman TT, Goldstein I, Traish AM. Effects of tamoxifen on vaginal blood flow and epithelial morphology in the rat. BMC Womens Health. 2006;6:14. doi: 10.1186/1472-6874-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Exton MS, Bindert A, Kruger T, Scheller F, Hartmann U, Schedlowski M. Cardiovascular and endocrine alterations after masturbation-induced orgasm in women. Psychosom Med. 1999;61:280–9. doi: 10.1097/00006842-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 133.Meston CM, Gorzalka BB. Differential effects of sympathetic activation on sexual arousal in sexually dysfunctional and functional women. J Abnorm Psychol. 1996;105:582–91. doi: 10.1037//0021-843x.105.4.582. [DOI] [PubMed] [Google Scholar]
- 134.Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- 135.Bohlen JG, Held JP, Sanderson MO, Ahlgren A. The female orgasm: Pelvic contractions. Arch Sex Behav. 1982;11:367–86. doi: 10.1007/BF01541570. [DOI] [PubMed] [Google Scholar]
- 136.McKenna KE, Chung SK, McVary KT. A model for the study of sexual function in anesthetized male and female rats. Am J Physiol. 1991;261(5 Pt 2):R1276–85. doi: 10.1152/ajpregu.1991.261.5.R1276. [DOI] [PubMed] [Google Scholar]
- 137.McKenna KE, Knight KC, Mayers R. Modulation by peripheral serotonin of the threshold for sexual reflexes in female rats. Pharmacol Biochem Behav. 1991;40:151–6. doi: 10.1016/0091-3057(91)90336-z. Epub 1991/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 138.Whipple B, Komisaruk BR. Brain (PET) responses to vaginal-cervical self-stimulation in women with complete spinal cord injury: Preliminary findings. J Sex Marital Ther. 2002;28:79–86. doi: 10.1080/009262302317251043. [DOI] [PubMed] [Google Scholar]
- 139.Janszky J, Szucs A, Halasz P, Borbely C, Hollo A, Barsi P, Mirnics Z. Orgasmic aura originates from the right hemisphere. Neurology. 2002;58:302–4. doi: 10.1212/wnl.58.2.302. [DOI] [PubMed] [Google Scholar]
- 140.Exton MS, Kruger TH, Koch M, Paulson E, Knapp W, Hartmann U, Schedlowski M. Coitus-induced orgasm stimulates prolactin secretion in healthy subjects. Psychoneuroendocrinology. 2001;26:287–94. doi: 10.1016/s0306-4530(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 141.Wesselmann U, Czakanski PP. Pelvic pain: A chronic visceral pain syndrome. Curr Pain Headache Rep. 2001;5:13–9. doi: 10.1007/s11916-001-0005-2. [DOI] [PubMed] [Google Scholar]
- 142.Giamberardino MA, Berkley KJ, Affaitati G, Lerza R, Centurione L, Lapenna D, Vecchiet L. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95:247–57. doi: 10.1016/S0304-3959(01)00405-5. Epub 2002/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 143.Giamberardino MA, De Laurentis S, Affaitati G, Lerza R, Lapenna D, Vecchiet L. Modulation of pain and hyperalgesia from the urinary tract by algogenic conditions of the reproductive organs in women. Neurosci Lett. 2001;304:61–4. doi: 10.1016/s0304-3940(01)01753-0. Epub 2001/05/04. eng. [DOI] [PubMed] [Google Scholar]
- 144.Berkley KJ, Wood E, Scofield SL, Little M. Behavioral responses to uterine or vaginal distension in the rat. Pain. 1995;61:121–31. doi: 10.1016/0304-3959(94)00150-D. [DOI] [PubMed] [Google Scholar]
- 145.Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–97. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 146.Sandner-Kiesling A, Pan HL, Chen SR, James RL, DeHaven-Hudkins DL, Dewan DM, et al. Effect of kappa opioid agonists on visceral nociception induced by uterine cervical distension in rats. Pain. 2002;96:13–22. doi: 10.1016/s0304-3959(01)00398-0. Epub 2002/04/05. eng. [DOI] [PubMed] [Google Scholar]
- 147.Shin SW, Sandner-Kiesling A, Eisenach JC. Systemic, but not intrathecal ketorolac is antinociceptive to uterine cervical distension in rats. Pain. 2003;105:109–14. doi: 10.1016/s0304-3959(03)00172-6. Epub 2003/09/23. eng. [DOI] [PubMed] [Google Scholar]
- 148.Du D, Eisenach JC, Ririe DG, Tong C. The antinociceptive effects of spinal cyclooxygenase inhibitors on uterine cervical distension. Brain Res. 2004;1024:130–6. doi: 10.1016/j.brainres.2004.07.056. Epub 2004/09/29. eng. [DOI] [PubMed] [Google Scholar]
- 149.Boscan P, Monnet E, Mama K, Twedt DC, Congdon J, Eickhoff JC, Steffey EP. A dog model to study ovary, ovarian ligament and visceral pain. Vet Anaesth Analg. 2011;38:260–6. doi: 10.1111/j.1467-2995.2011.00611.x. Epub 2011/04/16. eng. [DOI] [PubMed] [Google Scholar]
- 150.Taneja A, Di Iorio VL, Danhof M, Della Pasqua O. Translation of drug effects from experimental models of neuropathic pain and analgesia to humans. Drug Discov Today. 2012;17:837–49. doi: 10.1016/j.drudis.2012.02.010. Epub 2012/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 151.Farmer MA, Taylor A, MacIntyre LC, Binik YM, Bennett GJ, Ribiero-da-Silva A, et al. Of mice and women: A mouse model of vestibulodynia following recurrent vulvovaginal candidiasis. Glasgow, Scotland: International Association for the Study of Pain(IASP) meeting; 2008. [Google Scholar]
- 152.Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, Crissman HP, Bennett GJ, Ribeiro-da-Silva A, Binik YM, Mogil JS. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Transl Med. 2011;3:101ra91. doi: 10.1126/scitranslmed.3002613. Epub 2011/09/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: A historical perspective. J Reprod Med. 2004;49:772–7. Epub 2004/12/01. eng. [PubMed] [Google Scholar]
- 154.Wesselmann U, Czakanski PP, Affaitati G, Giamberardino MA. Uterine inflammation as a noxious visceral stimulus: Behavioral characterization in the rat. Neurosci Lett. 1998;246:73–6. doi: 10.1016/s0304-3940(98)00234-1. Epub 1998/06/17. eng. [DOI] [PubMed] [Google Scholar]
- 155.Giamberardino MA, Berkley KJ, Iezzi S, de Bigontina P, Vecchiet L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain. 1997;71:187–97. doi: 10.1016/s0304-3959(97)03362-9. Epub 1997/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 156.Wesselmann U, Lai J. Mechanisms of referred visceral pain: Uterine inflammation in the adult virgin rat results in neurogenic plasma extravasation in the skin. Pain. 1997;73:309–17. doi: 10.1016/S0304-3959(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 157.Sanoja R, Cervero F. Estrogen-dependent changes in visceral afferent sensitivity. Auton Neurosci. 2010;153:84–9. doi: 10.1016/j.autneu.2009.07.001. Epub 2009/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 158.Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–16. doi: 10.1016/j.psyneuen.2009.01.004. Epub 2009/02/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–26. doi: 10.1016/s0018-506x(03)00159-4. Epub 2003/11/14. eng. [DOI] [PubMed] [Google Scholar]
- 160.Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: Prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology (Berl) 2005;183:300–7. doi: 10.1007/s00213-005-0179-0. Epub 2005/10/18. eng. [DOI] [PubMed] [Google Scholar]
- 161.Klinger MB, Sacks S, Cervero F. A role for extracellular signal-regulated kinases 1 and 2 in the maintenance of persistent mechanical hyperalgesia in ovariectomized mice. Neuroscience. 2011;172:483–93. doi: 10.1016/j.neuroscience.2010.10.043. Epub 2010/10/26. eng. [DOI] [PubMed] [Google Scholar]
- 162.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–9. doi: 10.1016/j.fertnstert.2012.06.029. Epub 2012/07/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dmitrieva N, Nagabukuro H, Resuehr D, Zhang G, McAllister SL, McGinty KA, Mackie K, Berkley KJ. Endocannabinoid involvement in endometriosis. Pain. 2010;151:703–10. doi: 10.1016/j.pain.2010.08.037. Epub 2010/09/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grummer R. Animal models in endometriosis research. Hum Reprod Update. 2006;12:641–9. doi: 10.1093/humupd/dml026. Epub 2006/06/16. eng. [DOI] [PubMed] [Google Scholar]
- 165.Pelch KE, Sharpe-Timms KL, Nagel SC. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J Vis Exp. 2012;(59):e3396. doi: 10.3791/3396. Epub 2012/01/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Giamberardino MA, Costantini R, Affaitati G, Fabrizio A, Lapenna D, Tafuri E, Mezzetti A. Viscero-visceral hyperalgesia: Characterization in different clinical models. Pain. 2010;151:307–22. doi: 10.1016/j.pain.2010.06.023. Epub 2010/07/20. eng. [DOI] [PubMed] [Google Scholar]
- 167.Giamberardino MA, Affaitati G, Fabrizio A, Costantini R. Myofascial pain syndromes and their evaluation. Best practice & research. Clin Rheumatol. 2011;25:185–98. doi: 10.1016/j.berh.2011.01.002. Epub 2011/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 168.Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123–31. doi: 10.1016/j.juro.2009.07.036. Epub 2009/09/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith KA, Pearson CB, Hachey AM, Xia DL, Wachtman LM. Alternative activation of macrophages in rhesus macaques (Macaca mulatta) with endometriosis. Comp Med. 2012;62:303–10. Epub 2012/10/10.eng. [PMC free article] [PubMed] [Google Scholar]


