1. Introduction
The International Society for the Study of Vulvovaginal Disease (ISSVD) defines vulvodynia as chronic vulvar pain without visible dermatosis [44]. An estimated 10% to 15% of women will meet the diagnostic criteria at some time in their lives [31]. Two major vulvodynia categories have been defined by the ISSVD: “localized” and “generalized” subtypes. Displayed in Fig. 1A, localized provoked vulvodynia (LPV) presents with a characteristic pattern of mechanical allodynia localized to the vulvar vestibule, whereas generalized vulvodynia (GVD) shows a more diffuse pain pattern involving part or all of the pudendal nerve distribution and beyond. A number of researchers suspect that LPV and GVD may represent a continuum of the same condition rather than 2 distinct clinical entities. Clinically 2 subgroups of patients with LPV have been described: those with primary LPV, defined as dyspareunia from the first attempt of sexual intercourse; and those with secondary LPV, in which dyspareunia appears after a period of pain-free sexual intercourse.
Fig. 1.
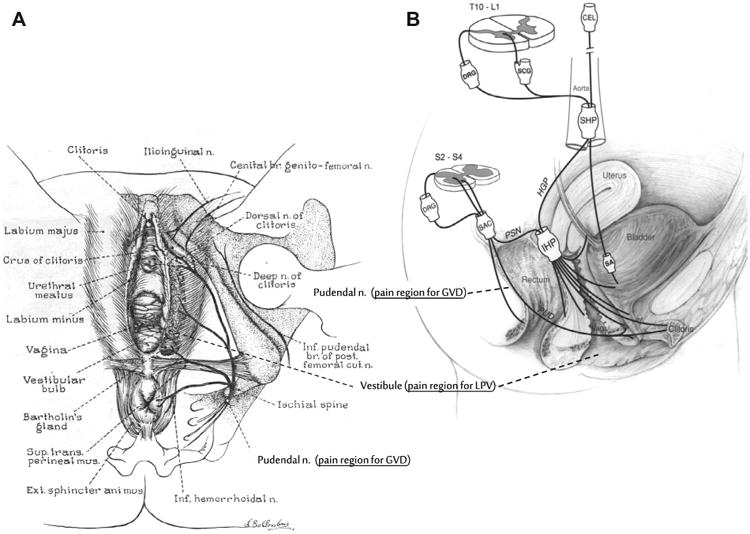
(A) Graphic illustration of female lower genital tract showing external vulva, vestibule, and lower vaginal tract. Associated innervation mediating sensory-motor activity of the pelvic region also represented. (B) Schematic drawing showing the innervation of the pelvic floor in females. Although this diagram attempts to show the innervation in humans, much of the anatomic information is derived from animal data. CEL, celiac plexus; DRG, dorsal root ganglion; HGP, hypogastric plexus; IHP, inferior hypogastric plexus; PSN, pelvic splanchnic nerve; PUD, pudendal nerve; SA, short adrenergic projections; SAC, sacral plexus; SCG, sympathetic chain ganglion; SHP, superior hypogastric plexus; Vag., vagina. (Source: Wesselmann U, Burnett AL, Heinberg LJ. The urogenital and rectal pain syndromes. PAIN® 1997;73:269-294 [62]. This figure has been reproduced with permission from the International Association for the Study of Pain (IASP). This figure may not be reproduced for any other purpose without permission.)
Vulvodynia often occurs in the context of other comorbid pain conditions [1,11,50], with irritable bowel syndrome and fibromyalgia being the most prevalent. These clinical observations and epidemiological studies highlight the heterogeneity of vulvar pain syndromes, suggesting different peripheral and central neurophysiological mechanisms relevant to the grouping with different comorbid pain conditions. As our understanding of vulvodynia evolves beyond the rudimentary classification of LPV, GVD, and primary and secondary types, nomenclature will be based on a clearer understanding of disease phenotype with improved diagnostic reliability and better-defined disease models.
This review will focus on progress in vulvodynia basic research. Because LPV is the more common subtype and has had a greater research emphasis, this review will reflect that focus. In addition to allodynia to light touch of the vulvar vestibule, a number of psychophysiologic events have been recognized to occur concurrently and to varying degrees in afflicted individuals, these include:
Pro-inflammatory cell migration to the vulvar vestibule [10,12,13,37].
Local production and release of pro-inflammatory, pain-inducing substances [9,20].
Regional lowering thresholds to varied stimuli [7,48,53,66].
Manifestation of somatization, depression, anxiety, and hypervigilance [41,47,54].
Superimposed pelvic floor muscle hypercontractility resulting in introital narrowing and muscle pain [23,24,29,30].
2. Genetics of vulvodynia
Several studies suggest a genetic predisposition to the development of LPV, potentially based on 3 mechanisms:
an influence on the risks of recurrent vulvovaginal candidiasis (RVVC),
an altered inflammatory response,
an increased sensitivity to pain.
Vulvovaginal infections are frequently identified as the inciting event for the development of LPV. It has been postulated that an inability to clear these infections and the subsequent inflammation produced may lead to the development of this condition. Both mannose-binding lectin (MBL) and NALP3 inflammosome are associated with defense against Candida species, and the genes that code for each are polymorphic. Women with RVVC have a higher MBL variant allele frequency than do controls [2]. Possession of homozygous variant alleles for NALP3 is more common in women with LPV associated with RVVC than in women without LPV or in women with LPV who have no history of RVVCs [38]. Genetic polymorphisms have been linked to alterations in the function of interleukin (IL)-1β and IL-1 receptor antagonist, and their variant alleles, which have been associated with an increased and prolonged inflammatory response, are found more commonly in women with LPV [26]. Melanocortin-1 receptor (MC1R), when bound to melanocyte-stimulating hormone, has anti-inflammatory effects, and the presence of loss-of-function mutations of this gene is seen more commonly in women with LPV. The combination of a loss-of-function mutation in the MC1R gene with the variant allele of the IL-1β receptor antagonist gene leads to additive risks [18]. Polymorphisms of guanosine triphosphate cyclohydrolase (GCH1) have been associated with overall increased pain sensitivity and a susceptibility to several long-standing pain conditions; however, no increase in this polymorphism has been noted in women with LPV [35].
Although not yet found in vulvodynia research, genetic research of other conditions is rapidly moving from analysis of single “candidate” gene research to genome-wide association studies. This has been motivated by several observations, including the following:
“Candidate” gene allele frequency commonly is low.
The inverse relationship between allele frequency and effect size is a biologic reality.
The chance of finding the candidate gene for vulvodynia is extremely remote.
3. Neuroinflammatory hypothesis
Hypothetically, regional changes in the biochemical milieu via changes in cytokine, neurokine, chemokine, or prostanoid signaling could alter ion channel activity of the peripheral pre- terminal axon. This could lead to a lowering of mechanical, thermal, or chemical threshold in the primary nociceptors. Vulvodynia-affected mucosa demonstrates various degrees of alterations in neurokines, cytokines, and neural responsiveness. As a result, vulvodynia has been proposed by some to have a neuro-inflammatory pathogenesis, similar to other chronic pain conditions [45]. Studying vulvodynia histology, several groups report mast cell-predominant inflammation [10,13,37]; another group reports inflammation without mast cell predominance [12]; and others report a total absence of inflammatory cell infiltration [33,39]. Assays for pro-inflammatory cytokines/neurokines report similar inconsistent results, with some studies reporting an increase in vulvovaginal proinflammatory cytokines [20], increased vulvovaginal neurokine CGRP [9], and increased systemic IL1-RA to post-heat shock protein provocation [25].
How and why inflammatory pain localizes to the vulvar vestibule in LPV remains a potentially fruitful area of future research. Reports using either Doppler flow or a novel visualization system have found the cardinal inflammatory sign of “rubor” or erythema to be greater in the vestibule (introitus) compared to the external vulva in all women and greatest in the introitus of vulvodynia-affected women compared to pain-free controls [6,15]. Selective sampling of fibroblasts from painful and adjacent nonpainful sites demonstrates enhanced pro-inflammatory cytokine production after yeast extract stimulation [21]. Other studies using immunohistochemistry (IHC)-based assays find no difference in proinflammatory cytokines from painful and nonpainful tissue sites [5,14]. As a result of the divergent findings described above, a neuroinflammatory pathogenesis has yet to be universally accepted. Improved consensus may result from future developments in several areas:
Greater reliance on prospective “natural history” vulvodynia studies.
Research into “nonclassical” pathways to neuro-inflammation.
Greater stringency in research methods.
4. Hyperinnervation in vulvodynia
A number of studies report an increase in nerve fiber density in LPV corresponding to regional heightened mechanical allodynia and hyperalgesia. Westrom et al. reported that stromal nerve fiber density per square unit was significantly correlated with the level of inflammation but not significantly correlated with the presence or absence of pain [64]. Bohm-Starke et al. used a semi-quantitative measure and reported a significantly higher number of PGP9.5(+) intraepithelial nerve fibers in LPV patients than in asymptomatic controls [8]. Tympanidis et al. reported a significant increase in density of PGP 9.5 immunoreactive fibers in patients [59]. The density difference was seen at the dermal epidermal border. In a subsequent article, Tympanidis et al. reported a quantitatively increased density of vanilloid receptors, VR-1 but scant evidence of SNS1 / PN3 immunoreactivity in LPV [58]. Similar to studies of neuro-inflammation pathogenesis, the role of neural proliferation in the pathogenesis of vulvodynia are clouded by several issues that will need to be clarified in future research:
C-fiber proliferation is not unique to LPV but is also found with the “itch” of atopic dermatitis. A neurophysiologic difference in the neural proliferation that produces pain versus itch remains undefined.
Neural proliferation is reversible in atopic dermatitis, but in vulvodynia this is unstudied.
Immunohistochemical (IHC) techniques for neural quantification need to be standardized, including the development of 3-dimensional methods for peripheral nerve quantification, as well as refined quantitative counting methods.
5. Psychophysical studies
The results of several psychophysical studies suggest that LPV is associated with sensory abnormalities, and evidence of both central and peripheral sensitization exist. LPV patients have been found to have both lower sensory thresholds and lower pain thresholds than asymptomatic women and have increased pain intensity at suprathreshold levels [7,48,53]. Somatosensory dysfunction is higher in the vestibule of women with LPV than in controls, and this is most pronounced in the posterior vestibule [7,66]. In asymptomatic women, the tactile threshold in the vestibule and at extragenital sites is similar, but in women with LPV the vestibule is significantly more sensitive [48]. Although studies have demonstrated alterations in sensitivity at the vestibule, there also is evidence of more generalized alterations in sensory processing. Women with LPV have increased pain perception with tender point examination, lower pain thresholds, and augmented pain perception at nongenital sites throughout the body [28,32,47]. These women have also demonstrated increased pain sensitivity, punctate hyperalgesia, and dynamic allodynia compared with pain-free women after the administration of intradermal capsaicin at nongenital sites [19]. A novel method of digital vibratory amplitude discrimination after a vibratory conditioning stimulus found that vulvodynia of longer duration reduced adaptation to vibratory conditioning stimulus found in controls and shorter-duration vulvodynia case patients [65]. These findings at sites distant from symptom location suggest some contribution of central sensitization, and might provide a neurophysiological explanation for the clinical observation that patients with vulvodynia often present with additional pain comorbidities. Diffuse noxious inhibitory control (DNIC) function refers to the phenomenon of reduced pain perception at a given location in the presence of different painful stimulus at a different location. A reduction in DNIC function has been noted in several pain syndromes; however, studies have shown no difference in the DNIC response between women with LPV and asymptomatic controls [55]. Few studies have explored the correlations of psychophysical parameters and psychosocial as well as sexual function in vulvodynia. In women with LPV, decreased sexual function and sexual self-efficacy were associated with higher vulvar pressure-pain ratings [55].
Improvement in psychophysical methods for vulvodynia may include the following (adapted from Price) [46]:
Psychophysical methods with ratio scale properties.
Generalizable techniques for experimental and clinical settings.
Methods with good reliablity and sensitivity to change in pain intensity.
Methods that are simple to use.
Techniques that separately assess pain dimensions of intensity and affective qualities (how the pain makes one feel).
6. Muscle dysfunction
It has long been recognized that LVD is often associated with some degree of pelvic floor muscle dysfunction. Women with LPV have been shown to have lower pelvic muscle pressure pain thresholds and tolerance when compared to asymptomatic controls [66]. Using electromyography (EMG) recordings, several studies have demonstrated increased resting muscle tone, impaired voluntary relaxation, decreased voluntary muscle contractile ability, and reduced pelvic floor muscle flexibility in women with LPV [23,24,29,30]. Greater urethral pressure variability than seen in women without LPV has also been noted, and it is hypothesized that this is the result of variations in urethral muscle tone [22]. This pelvic floor muscle dysfunction may be the result of a protective reflex to avoid penetration that the woman finds painful or that she fears. Whether this dysfunction is a cause or a result of the pain experienced with LPV is unclear, but it is likely maintained by ongoing mechanical allodynia.
Points and directions in future research of motor dysfunction in vulvodynia:
Is motor hypertonus a separable condition or intrinsic to vulvodynia?
Results are highly operator dependent, and evaluation by same examiner may result in better reliability.
Assessment of pressure point tenderness of pelvic floor muscles should be limited to measure once per visit for better reliability, particularly in women with significant vaginismus [57].
Nonpenetrative methods of muscle assessment, such as transperineal ultrasound, may improve upon methodological limitations of intravaginal digital examination [43].
7. Animal models
Compared to other chronic pain conditions, very few animal models have been developed to replicate relevant clinical pain conditions from the urogenital tract [27,40]. Translation of findings in these models to sexual pain in women might be more difficult than in other pain models because of the requirement of circulating hormones for sexual receptivity in rodents and the multifactorial nature of women's sexual function. The innervation of the urogenital floor is served by both components of the autonomic nervous system, the sympathetic and parasympathetic divisions, as well as the somatic nervous systems [62] (Fig. 1B). Studies in rats have demonstrated that the extent of the vaginal innervation varies as a function of the gonadal hormonal status [56]. This plasticity of the innervation is not restricted to the vagina, but it has been reported in other parts of the female reproductive tract as well [36]. Mimicking repeated vaginal candidiasis that some women report experiencing provoked vestibulodynia, a rodent model of long-lasting mechanical allodynia after repeated exposure of the vulva to Candida albicans, has been developed in mice. Allodynia persists for at least 3 weeks after resolution of infection in a subset of female mice in this model and produces hyperinnervation [17]. Berkley et al. have developed a model of vaginal nociceptive distension in rats, and have explored this model to study vaginal hypersensitivity both after ovariectomy, as a model for dyspareunia in women after loss of ovarian function, and in the context of a rodent model of endometriosis, as a model for secondary dysmenorrhea and chronic pelvic pain reported by women diagnosed with endometriosis [4]. Vaginal hypersensitivity after ovariectomy is reversed by estrogen replacement in this model and histologically characterized by increased sympathetic innervation [56]. The endometriosis/vaginal hyperalgesia model has been explored to assess the pharmacological interactions of the endocannabinoid system in endometriosis-associated pain [27]. Using a rodent model of uterine inflammation [63], vaginal nociceptive pathways have been studied in rats that had recovered from previous uterine inflammation [61]. Vaginal inflammation in rodents that had recovered from uterine inflammation resulted in a significant increase in FOS-ir neurons in spinal cord segments T10-L5 (outside of the vaginal innervation area), suggesting that previous uterine inflammation modifies central nervous system processing from the vagina. These studies are relevant to the clinical observation that women who have recovered from pelvic inflammatory disease (PID) often develop chronic pelvic pain and dyspareunia after they have initially recovered from PID, sometimes triggered by a seemingly trivial vaginal infection.
Challenges in developing a clinically relevant animal model of vulvodynia include the following:
Clinically relevant stimuli need to be identified in an animal model that reflect the pain and sexual dysfunction that women with vulvodynia experience.
The heterogenous pathology and multifactorial presentation of vulvodynia in women, which make it difficult to develop clinically relevant animal models for vulvodynia to study the pathophysiological mechanism and to identify treatment targets.
An endogenous animal model of vulvodynia needs to be developed.
Animal models provide a tool to investigate the contribution of background genetics in a controlled environment to the functional and molecular mechanisms of the disease. What laboratory animal/strain will represent the vulvodynia reality?
The significant neuronal plasticity of the reproductive tract as a function of the hormonal milieu and differences in the gonadal profiles of rodents and humans make it difficult to translate findings in these animal models to women with vulvodynia and to develop clinically relevant targets for treatment.
8. Neuroimaging
Structural and functional magnetic resonance imaging (fMRI) studies have revealed many types of brain differences associated with the development of chronic pain [16,52]; however, few studies have applied these techniques to study vulvodynia. In a study using pressure (perceived as painful in patients but not in controls) to the posterior portion of the vulvar vestibule as a stimulus in women with LPV and healthy controls, women with LPV showed significantly higher activation levels in the insular and frontal cortical regions on fMRI than did controls, mirroring activation patterns observed in other chronic pain conditions [49]. In a subsequent study, patients with vulvodynia were compared to healthy controls and fibromyalgia patients (positive controls) [34]. Local (vulvar) and remote (thumb) pressure-evoked pain processing was assessed using fMRI. Both pain groups showed overlapping insular brain activations that were greater than in healthy controls during thumb stimulation. Significant differences between vulvodynia subgroups (primary/secondary, provoked/unprovoked) were observed in the posterior cingulate (thumb stimulus) and in the precuneus region (vulvar stimulus), suggesting heterogeneous neuropathologies. Using whole-brain voxel-based morphometry, increased gray matter density has been demonstrated in young women with LPV [51]. This is in contrast to studies in patients with other chronic pain conditions documenting decreased gray matter density, and it has been speculated that gray matter density might increase in young pain patients and decrease in older individuals with long-standing pain conditions.
Points and directions in vulvodynia neuroimaging:
Similar to other chronic pain syndromes, in which much work has already been done using neuroimaging techniques, this approach may help to clarify the sensory, emotional, cognitive, and interoceptive components of vulvodynia pain.
The development of “vulvodynia pain signatures” (ie, brain bio-markers) for vulvodynia sub-phenotypes would be valuable.
Neuroimaging may facilitate mechanism-based drug selection for vulvodynia patients and may serve as a predictor of placebo responders.
Recent work has provided evidence that measures of brain structure and functional activity can define a pain state and predict disease chronification in conditions such as back pain. Much more work is needed to determine the generalizability of such findings to other chronic pain conditions, including vulvodynia.
9. The future: challenges and opportunities
Vulvodynia comprises a poorly defined and understudied heterogeneous group of chronic sexual pain syndromes in women. There is an urgent need to expand both basic science as well as clinical research in this field to provide insights into the etiology, natural history, and risk factors to provide a translational foundation to facilitate future clinical intervention efforts and improve clinical management. A major confounding factor in such studies is the heterogeneity of the potential pathogenetic mechanisms of pain. In the clinical setting, this translates into the frustrating situation that the right treatment for the right patient with vulvodynia is often very difficult to predict, and may result in a long-term trial-and-error approach. This senario is not unique to vulvodynia but is typical of many chronic pain conditions, and calls for a personalized pain management approach. While research on vulvodynia is still in the “kinder-shoes” as compared to many other chronic pain syndromes, this deficit might be overcome by learning from successful approaches to other chronic pain syndromes (www.mappnetwork.org; www.oppera.org; https://www.nichd.nih.gov/publications/pubs/documents/NIH_Vulvodynia_Plan_April2012.pdf). Information resources relevant to vulvodynia are also listed in Appendix A.
Acknowledgments
This work was supported by National Institutes of Health grants UW-HD39699 (Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD], Office of Research on Women's Health), DK066641 (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]), DF-HD065740 (NICHD), and HD069313 (NICHD).
Appendix A. Resources for health care providers, patients, and researchers
American College of Obstetricians and Gynecologists (ACOG) Vulvodynia. Patient Education Pamphlet FAQ127, May 2011. Website: http://www.acog.org/For_Patients
International Association for the Study of Pain (IASP) Global Year Against Pain in Women
(Vulvodynia Fact Sheets).
IASP Secretariat, 1510 H Street NW, Suite 600, Washington, DC 20005-1020, USA. Tel: 202-524-5300.
Website: http://www.iasp-pain.org
National Vulvodynia Association (NVA), PO Box 4491, Silver Spring, MD 20914-4491, USA. Tel: 301-299-0775.
Website: http://www.nva.org
NIH Research Plan on Vulvodynia
Website: https://www.nichd.nih.gov/publications/pubs/documents/NIH_Vulvodynia_Plan_April2012.pdf
NIH Office of Research on Women's Health (ORWH).
Website (for vulvodynia): http://orwh.od.nih.gov/resources/health/researchandyourhealth/index.asp
Footnotes
Conflict of interest statement: The authors declare that they have no conflicts of interest in regard to this article.
References
- 1.Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107:617–24. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babula O, Danielsson I, Sjoberg I, Ledger WJ, Witkin SS. Altered distribution of mannose-binding lectin alleles at exon I codon 54 in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2004;191:762–6. doi: 10.1016/j.ajog.2004.03.073. [DOI] [PubMed] [Google Scholar]
- 3.Basson R. The recurrent pain and sexual sequelae of provoked vestibulodynia: a perpetuating cycle. J Sex Med. 2012;9:2077–92. doi: 10.1111/j.1743-6109.2012.02803.x. [DOI] [PubMed] [Google Scholar]
- 4.Berkley KJ, Wood E, Scofield SL, Little M. Behavioral responses to uterine or vaginal distension in the rat. PAIN®. 1995;61:121–31. doi: 10.1016/0304-3959(94)00150-D. [DOI] [PubMed] [Google Scholar]
- 5.Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gyn Scand. 2001;80:638–44. [PubMed] [Google Scholar]
- 6.Bohm-Starke N, Hilliges M, Blomgren B, Falconer C, Rylander E. Increased blood flow and erythema in the posterior vestibular mucosa in vulvar vestibulitis. Obstet Gynecol. 2001;98:1067–74. doi: 10.1016/s0029-7844(01)01578-2. [DOI] [PubMed] [Google Scholar]
- 7.Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. PAIN®. 2001;94:177–83. doi: 10.1016/S0304-3959(01)00352-9. [DOI] [PubMed] [Google Scholar]
- 8.Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1998;46:256–60. doi: 10.1159/000010045. [DOI] [PubMed] [Google Scholar]
- 9.Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1999;48:270–5. doi: 10.1159/000010198. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein J, Goldschmid N, Sabo E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol Obstet Invest. 2004;58:171–8. doi: 10.1159/000079663. [DOI] [PubMed] [Google Scholar]
- 11.Bullones Rodriguez MA, Afari N, Buchwald DS National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2013;189(Suppl 1):S66–74. doi: 10.1016/j.juro.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadha S, Gianotten WL, Drogendijk AC, Weijmar Schultz WC, Blindeman LA, van der Meijden WI. Histopathologic features of vulvar vestibulitis. Int J Gynecol Pathol. 1998;17:7–11. doi: 10.1097/00004347-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Chaim W, Meriwether C, Gonik B, Qureshi F, Sobel JD. Vulvar vestibulitis subjects undergoing surgical intervention: a descriptive analysis and histopathological correlates. Eur J Obstet Gynecol Reprod Biol. 1996;68:165–8. doi: 10.1016/0301-2115(96)02502-x. [DOI] [PubMed] [Google Scholar]
- 14.Eva LJ, Rolfe KJ, MacLean AB, Reid WM, Fong AC, Crow J, Perrett CW. Is localized, provoked vulvodynia an inflammatory condition? J Reprod Med. 2007;52:379–84. [PubMed] [Google Scholar]
- 15.Farage MA, Singh M, Ledger WJ. Investigation of the sensitivity of a cross-polarized light visualization system to detect subclinical erythema and dryness in women with vulvovaginitis. Am J Obstet Gynecol. 2009;201:20–6. doi: 10.1016/j.ajog.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett. 2012;520:197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, Crissman HP, Bennett GJ, Ribeiro-da-Silva A, Binik YM, Mogil JS. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Trans Med. 2011;3:101ra91. doi: 10.1126/scitranslmed.3002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster DC, Sazenski TM, Stodgell C. Impact of genetic variants of the interleukin receptor antagonist and melanocortin-1 receptor genes on vulvar vestibulitis syndrome (vestibulodynia) J Reprod Med. 2004;49:503–9. [PubMed] [Google Scholar]
- 19.Foster DC, Dworkin RH, Wood RW. Effects of intradermal foot and forearm capsaicin injections in normal and vulvodynia-afflicted women. PAIN®. 2005;117:128–36. doi: 10.1016/j.pain.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Foster DC, Hasday JD. Elevated tissue levels of interleukin-1 beta and tumor necrosis factor-alpha in vulvar vestibulitis. Obstet Gynecol. 1997;89:291–6. doi: 10.1016/S0029-7844(96)00447-4. [DOI] [PubMed] [Google Scholar]
- 21.Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196:e1–8. doi: 10.1016/j.ajog.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Foster DC, Robinson JC, Davis KM. Urethral pressure variation in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 1993;169:107–12. doi: 10.1016/0002-9378(93)90141-5. [DOI] [PubMed] [Google Scholar]
- 23.Frasson E, Graziottin A, Priori A, Dall'ora E, Didone G, Garbin EL, Vicentini S, Bertolasi L. Central nervous system abnormalities in vaginismus. Clin Neurophysiol. 2009;120:117–22. doi: 10.1016/j.clinph.2008.10.156. [DOI] [PubMed] [Google Scholar]
- 24.Gentilcore-Saulnier E, McLean L, Goldfinger C, Pukall CF, Chamberlain S. Pelvic floor muscle assessment outcomes in women with and without provoked vestibulodynia and the impact of a physical therapy program. J Sex Med. 2010;7:t-22. doi: 10.1111/j.1743-6109.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerber S, Bongiovanni AM, Ledger WJ, Witkin SS. Defective regulation of the proinflammatory immune response in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2002;186:696–700. doi: 10.1067/mob.2002.121869. [DOI] [PubMed] [Google Scholar]
- 26.Gerber S, Bongiovanni AM, Ledger WJ, Witkin SS. Interleukin-1beta gene polymorphism in women with vulvar vestibulitis syndrome. Eur J Obstet Gynecol Reprod Biol. 2003;107:74–7. doi: 10.1016/s0301-2115(02)00276-2. [DOI] [PubMed] [Google Scholar]
- 27.Giamberardino MA, Wesselmann U, Costantini R, Czakanski P. Animal Models of Urogenital Pain. In: Handwerker HO, Arendt-Nielsen L, editors. Pain Models: Translational Relevance and Applications. Washington, DC: IASP Press; 2013. pp. 183–200. [Google Scholar]
- 28.Giesecke J, Reed BD, Haefner HK, Giesecke T, Clauw DJ, Gracely RH. Quantitative sensory testing in vulvodynia patients and increased peripheral pressure pain sensitivity. Obstet Gynecol. 2004;104:126–33. doi: 10.1097/01.AOG.0000129238.49397.4e. [DOI] [PubMed] [Google Scholar]
- 29.Glazer HI, Jantos M, Hartmann EH, Swencionis C. Electromyographic comparisons of the pelvic floor in women with dysesthetic vulvodynia and asymptomatic women. J Reprod Med. 1998;43:959–62. [PubMed] [Google Scholar]
- 30.Glazer HI, Rodke G, Swencionis C, Hertz R, Young AW. Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature. J Reprod Med. 1995;40:283–90. [PubMed] [Google Scholar]
- 31.Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164:1609–14. doi: 10.1016/0002-9378(91)91444-2. [DOI] [PubMed] [Google Scholar]
- 32.Granot M, Friedman M, Yarnitsky D, Tamir A, Zimmer EZ. Primary and secondary vulvar vestibulitis syndrome: systemic pain perception and psychophysical characteristics. Am J Obstet Gynecol. 2004;191:138–42. doi: 10.1016/j.ajog.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 33.Halperin R, Zehavi S, Vaknin Z, Ben-Ami I, Pansky M, Schneider D. The major histopathologic characteristics in the vulvar vestibulitis syndrome. Gynecol Obstet Inves. 2005;59:75–9. doi: 10.1159/000082112. [DOI] [PubMed] [Google Scholar]
- 34.Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented central pain processing in vulvodynia. J Pain. 2013;14:579–89. doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heddini U, Bohm-Starke N, Gronbladh A, Nyberg F, Nilsson KW, Johannesson U. GCH1-polymorphism and pain sensitivity among women with provoked vestibulodynia. Mol Pain. 2012;8:68. doi: 10.1186/1744-8069-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latini C, Frontini A, Morroni M, Marzioni D, Castellucci M, Smith PG. Remodeling of uterine innervation. Cell Tissue Res. 2008;334:1–6. doi: 10.1007/s00441-008-0657-x. [DOI] [PubMed] [Google Scholar]
- 37.Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstet Gynecol. 2011;117:1307–13. doi: 10.1097/AOG.0b013e31821c33dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2009;200:303–6. doi: 10.1016/j.ajog.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 39.Lundqvist EN, Hofer PA, Olofsson JI, Sjoberg I. Is vulvar vestibulitis an inflammatory condition? A comparison of histological findings in affected and healthy women. Acta Derm Venereol. 1997;77:319–22. doi: 10.2340/0001555577319322. [DOI] [PubMed] [Google Scholar]
- 40.Marson L, Giamberardino MA, Costantini R, Czakanski P, Wesselmann U. Animal Models for the Study of Female Sexual Dysfunction. Sex Med Rev. 2013;1(1):108–22. doi: 10.1002/smrj.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meana M, Binik YM, Khalife S, Cohen D. Psychosocial correlates of pain attributions in women with dyspareunia. Psychosomatics. 1999;40:497–502. doi: 10.1016/S0033-3182(99)71188-6. [DOI] [PubMed] [Google Scholar]
- 42.Meana M, Binik YM, Khalife S, Cohen DR. Biopsychosocial profile of women with dyspareunia. Obstet Gynecol. 1997;90:583–9. doi: 10.1016/s0029-7844(98)80136-1. [DOI] [PubMed] [Google Scholar]
- 43.Morin M, Bergeron S, Khalifé S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J Sex Med. 2014;11:776–85. doi: 10.1111/jsm.12367. [DOI] [PubMed] [Google Scholar]
- 44.Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49:772–7. [PubMed] [Google Scholar]
- 45.Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response Part 2 of 3 – inflammatory profile of pain syndromes. Med Hypotheses. 2007;69:1169–78. doi: 10.1016/j.mehy.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 47.Pukall CF, Baron M, Amsel R, Khalife S, Binik YM. Tender point examination in women with vulvar vestibulitis syndrome. Clin J Pain. 2006;22:601–9. doi: 10.1097/01.ajp.0000210903.67849.af. [DOI] [PubMed] [Google Scholar]
- 48.Pukall CF, Binik YM, Khalife S, Amsel R, Abbott FV. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. PAIN®. 2002;96:163–75. doi: 10.1016/s0304-3959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 49.Pukall CF, Strigo IA, Binik YM, Amsel R, Khalife S, Bushnell MC. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. PAIN®. 2005;115:118–27. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 50.Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, Haefner HK. Relationship between vulvodynia and chronic comorbid pain conditions. Obstet Gynecol. 2012;120:145–51. doi: 10.1097/AOG.0b013e31825957cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. PAIN®. 2008;140:411–9. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009;66:375–90. doi: 10.1007/s00018-008-8428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonni L, Cattaneo A, De MA, De MA, Carli P, Marabini S. Idiopathic vulvodynia Clinical evaluation of the pain threshold with acetic acid solutions. J Reprod Med. 1995;40:337–41. [PubMed] [Google Scholar]
- 54.Sutton KS, Pukall CF, Chamberlain S. Pain ratings, sensory thresholds, and psychosocial functioning in women with provoked vestibulodynia. J Sex Marital Ther. 2009;35:262–81. doi: 10.1080/00926230902851256. [DOI] [PubMed] [Google Scholar]
- 55.Sutton KS, Pukall CF, Chamberlain S. Diffuse noxious inhibitory control function in women with provoked vestibulodynia. Clin J Pain. 2012;28:667–74. doi: 10.1097/AJP.0b013e318243ede4. [DOI] [PubMed] [Google Scholar]
- 56.Ting AY, Blacklock AD, Smith PG. Estrogen regulates vaginal sensory and autonomic nerve density in the rat. Biol Reprod. 2004;71:1397–404. doi: 10.1095/biolreprod.104.030023. [DOI] [PubMed] [Google Scholar]
- 57.Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Norman HR. Vaginal pressure-pain thresholds: Initial validation and reliability assessment in healthy women. Clin J Pain. 2008;24:45–50. doi: 10.1097/AJP.0b013e318156db13. [DOI] [PubMed] [Google Scholar]
- 58.Tympanidis P, Casula MA, Yiangou Y, Terenghi G, Dowd P, Anand P. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur J Pain. 2004;8:129–33. doi: 10.1016/S1090-3801(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 59.Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148:1021–7. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- 60.van Lankveld JJ, Granot M, Weijmar Schultz WC, Binik YM, Wesselmann U, Pukall CF, Bohm-Starke N, Achtrari C. Women's sexual pain disorders. J Sex Med. 2010;7(1 Pt 2):615–31. doi: 10.1111/j.1743-6109.2009.01631.x. [DOI] [PubMed] [Google Scholar]
- 61.Wesselmann U, Sanders C, Czakanski PP. Progress in Pain Research and Management. Vol. 16. Seattle: IASP Press; 2000. Altered CNS processing of nociceptive messages from the vagina in rats, that have recovered from uterine inflammation; pp. 581–88. [Google Scholar]
- 62.Wesselmann U, Burnett AL, Heinberg LJ. The urogenital and rectal pain syndromes. PAIN®. 1997;73:269–94. doi: 10.1016/S0304-3959(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 63.Wesselmann U, Lai J. Mechanisms of referred visceral pain: uterine inflammation in the adult virgin rat results in neurogenic plasma extravasation in the skin. PAIN®. 1997;73:309–17. doi: 10.1016/S0304-3959(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 64.Westrom LV, Willen R. Vestibular nerve fiber proliferation in vulvar vestibulitis syndrome. Obstet Gynecol. 1998;91:572–6. [PubMed] [Google Scholar]
- 65.Zhang Z, Zolnoun DA, Francisco EM, Holden JK, Dennis RG, Tommerdahl M. Altered central sensitization in subgroups of women with vulvodynia. Clin J Pain. 2011;27:755–63. doi: 10.1097/AJP.0b013e31821c98ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain. 2012;13:910–20. doi: 10.1016/j.jpain.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


