Abstract
The retinal pigment epithelium (RPE) plays imperative roles in normal retinal function by photoreceptor protection from light and phagocytosis of rod and cone outer segments during disc shedding. Melatonin is the free radical scavenger and circadian determinant to protect the RPE and retina from oxidative stress and regulate the circadian clock. The current study tested the hypothesis whether melatonin could affect cytoskeletal structure within RPE. Our Western blot analysis demonstrated that melatonin treatment up-regulated prohibitin 3-fold compared to control. β-tubulin levels were also up-regulated by melatonin but to a lesser extent. Initial cell shape of ARPE-19 is epitheloid, however, after 30-minute treatment with melatonin, RPE cells undergo a morphological change to a fusiform shape with spindle outgrowth. Cells return to epitheloid shape after 12 hours in untreated medium. Melatonin treated cells showed increased and dissimilar distribution of prohibitin and β-tubulin compared to non-treated cells, thus altered cytoskeletal and mitochondrial structure in the RPE. Our data implies that melatonin may play a protective role under oxidative stress, which is shown by the marker prohibitin in terms of increased expression and nuclear distribution. During the protective process, cells change their morphology. Our results suggest that melatonin treatment could be beneficial to protect mitochondria under oxidative stress and treat certain ocular diseases, including age-related macular degeneration.
Index Terms: age-related diseases, circadian rhythms, cytoskeleton structure, melatonin, photoreceptors, prohibitin, β-tubulin, mitochondria, retinal pigment epithelium
1 INTRODUCTION
The photoreceptor cells are very effective in trapping photons that initiate the visual transduction, and remain vulnerable to cellular damage as a result of excessive amount of light rays entering the retina. The physiological and pathological effects of light in the retina are triggered by the rhodopsin bleaching in the retina, which is the first site for photon absorption in the rod outer segments [1]. Intense light has been reported to be a potential risk factor for many ocular diseases, including retinitis pigmentosa (RP) and age-related macular degeneration (AMD) [2]. Long exposure to excessive photons under sun and artificial light causes damage to the photoreceptors, or even their death.
Apoptosis is responsible for the photoreceptor and RPE cell death which occurs as a result of DNA fragmentation and nuclear chromatin condensation. The proapoptotic c-fos and calcium alteration may initiate the apoptotic process [3]. Exposure to intense (>10,000 lux) or constant light (24 hours) causes excessive oxidative stress in the retina and RPE. Extended exposure to intense light also leads to the loss of polyunsaturated fatty acids from the membranes, DNA fragmentation, and increased expression of a 32-kDa antioxidative prohibitin [1], [4]–[6].
Circadian clock is endogenously generated rhythm with a periodicity of approximately 24 hours and the rhythmicity that continues at constant environmental conditions that include light and temperature. Most behavioral (performance, mood, sleep, alertness) and physiological (electrolytes, body temperature, hormones) measures are regulated by circadian clock to enable organisms to predict and adapt to changes in their environment [7].
Melatonin (N-acetyl-5-methoxytryptamine) is an antioxidant and circardian clock pacemaker, produced nocturnally from tryptophan in the retina and pineal gland of mammals [2], [8]. Retinal melatonin is down-regulated by light and barely detectable during the day. Decreased melatonin in the retina under a long period of exposure to light contributes to the retinal degeneration [2].
Biochemical reactions of light-electrical energy conversion in the retina and RPE require the increased metabolism rate to satisfy the demand for high energy. Light induced degeneration of photoreceptors depends on rhodopsin bleaching and photo transductions [1], [2], [4], [7]–[10]. Damage associated with light exposure increases production of reactive oxygen species that have been implicated as an early event in light-induced retinal degeneration.
Previously, we showed that the visual cycle might be coordinated in a circadian-dependent manner to effectively protect the retina from the detrimental effects of light-induced ROS damage [2], [3], [13]. We hypothesize that these conditions may regulate protection mechanisms in the retina by anti-apoptosis, and that melatonin-targeted molecules may play crucial roles in maintaining neuroretinal architecture. Our results demonstrated that melatonin could affect cytoskeletal structure via the mechanisms involving up-regulation of prohibitin and β-tubulin. We suggest that melatonin treatment could protect mitochondria under oxidative stress and therefore be beneficial for the treatment of light-induced ocular diseases, including age-related macular degeneration.
2 METHODS
ARPE-19 Cells under Oxidative Stress
For in vitro experiments, retinal pigment epithelial cells (ARPE-19) were purchased from ATCC (Manassas, VA). ARPE-19 cells were cultured in a 5% CO2 incubator at 37°C in 100 mm dishes (Nalge Nunc International, Naperville, IL) using Dulbecco’s modified Eagle’s medium (DMEM) with fetal bovine serum (10%) and penicillin/streptomycin (1%). Confluent cells were trypsinized (5–7 minutes at 37 °C) using a trypsin-EDTA buffer (0.1%, Sigma-Aldrich, St. Louis, MO), followed by centrifugation (300 × g, 7 min). Cells (eight to nine passages) were grown to confluence for 2–4 days and then were treated with H2O2 (200 μM), intense light (7,000–10,000 lux, 1–24 hours) or constant light (700 lux, 48 hrs). ARPE-19 cells were rinsed (Modified Dulbecco’s PBS) and lysed using IP lysis buffer containing Tris (25 mM), NaCl (150 mM), EDTA (1 mM), NP-40 (1%), glycerol (5%), and protease inhibitor cocktail at pH 7.4 with periodic sonication (3 × 5 min), followed by centrifugation (13,000 × g, 10 minutes). Proteins were separated using SDS–PAGE and stained using Coomassie blue (Pierce, IL) or silver staining kit (Bio-Rad, Hercules, CA).
2.1 Melatonin Treatment
ARPE-19 cells were grown to 70% confluence in 10% FBS, 1% antibiotic supplemented DMEM. Cells were treated with 100 μM melatonin in DMSO and 100 μM H2O2, or 1 μM melatonin and subjected to light stress (dark, 500 lux, and 7000 lux) for 12 hours. Cells were processed for immunocytochemistry (ICC) or proteins were extracted for Western blotting analysis.
2.2 Immunocytochemistry
ARPE-19 cells grown on cover slips were probed with prohibitin or β-tubulin primary antibody using AlexaFluor488 secondary antibody. Mitochondria and nuclei were labeled fluorescently with MitoTracker Orange and DAPI, respectively. More specifically, cells were grown on sterile glass cover slips in DMEM/F12 medium supplemented with 10 % FBS and 1% penicillin/streptomycin (Hyclone) in 5 % CO2 incubator at 37 °C. Cells were washed with PBS and incubated with Mito-Tracker Orange CMTMRos (100 nM, Molecular Probes, Carlsbad, CA) in serum-free culture medium (30 min, 37 °C), followed by washing (PBS) and fixing (10 % formaldehyde, 30 min, room temperature). Membrane permeabilization was performed with 0.2 % Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS for 30 min. Blocking was done by using complete medium (10 % FBS, 0.05 % Tween-20, 1 hour). Cells were incubated using anti-prohibitin antibody (1:1000, Genemed Synthesis Inc., San Antonio, TX, overnight, 4 °C) and washed with PBS, followed by incubation with Alexa Fluor 488-conjugated anti-rabbit IgG secondary antibody (1:700; Molecular Probes, Carlsbad, CA, 1 h, room temperature). Cells were mounted with VECTASHIELD medium with DAPI (4,6-diamidino-2-phenylindole, the nucleus) and visualized using a Zeiss AxioVert fluorescent microscope (200 M Apo Tome, 639 magnification). Data were analyzed using ImageJ software (NIH) [11]–[14].
2.3 Western Blot and Analysis
ARPE-19 whole cell lysate was used to extract proteins. 1D and 2D SDS-PAGE gels were run and proteins were transferred to Western blot membranes. Prohibitin and β-tubulin were tagged with primary and HRP-conjugated secondary antibodies. Protein amount was quantitated using a BSA standard curve with a BCA protein assay kit (Pierce). Samples were solubilized in Laemmli buffer (5X, 240 mM Tris, pH 6.8, 8 % SDS, 40 % glycerol, 0.4 % bromophenol blue, 10 % β-mercaptoethanol) and denatured at 90 °C for 10 min. Proteins were separated using a gradient polyacrylamide precast gel (8–16 %, Bio-Rad, Hercules, CA), followed by semidry electrotransfer (15 V, 30 min) onto the methanol-activated PVDF membrane (Bio-Rad). Nonspecific proteins were blocked (5 % nonfat dry milk, 2 h, 4 °C) in Tween-20 (0.1 %). Membranes were incubated using a primary antibody (rabbit polyclonal, 1:1000, Genemed Synthesis, San Antonio, TX, 4 °C, overnight) and an anti-rabbit HRP conjugated secondary antibody (1:10,000,
Agrisera, Vännäs, Sweden, 2 h, room temperature). Protein bands were visualized using West Pico Chemiluminescent reagent (Pierce, 1–2 min) and a LAS 4000 mini luminescent image analyzer (GE, Piscataway, NJ). Membranes were incubated again using an anti-β-actin antibody (mouse monoclonal, 1:5000, Sigma-Aldrich, St. Louis, MO) followed by a secondary antibody (1:7000, antimouse, horse radish peroxidase conjugated, Santa Cruz Biotechnology, Santa Cruz, CA) as a loading/positive control. Protein relative amount was analyzed quantitatively based on pixels (area and intensity) using Quantity One software (Bio-Rad) and GraphPad Prism software [11]–[14].
3 RESULTS AND DISCUSSION
Previously, our studies identified proteins that showed a reversal of the constant light-induced expression changes after melatonin administration. Proteomic data showed that constant light-regulated proteins include molecular chaperones (crystalline family), antioxidative proteins (peroxiredoxins), G-protein coupled receptors (G proteins and Rab), and neuroretinal filament proteins (vimentin, tubulin) [2]. Identifications of melatonin-targeted proteins lay the foundation for the current studies of melatonin’s potential for preventing or treating light induced retinal degeneration.
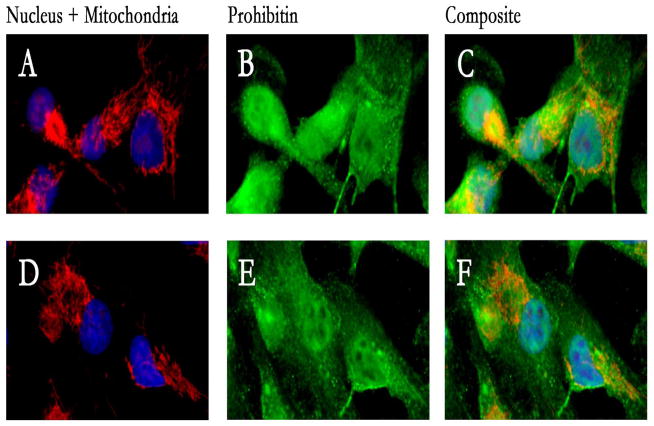
In the current study, we treated ARPE-19 cells with melatonin to test the hypothesis that melatonin induces expression of prohibitin and alters cytoskeletal structure. Immunocytochemical analysis showed that melatonin increased levels of prohibitin and promoted prohibitin nuclear localization (Figure 1).
Figure 1.
Melatonin up-reguated prohibitin concentration and promotednuclear localization of prohibitin in ARPE-19 cells. ARPE-19 cells were fluorescently labeled with DAPI, MitoTracker Orange, and AlexaFluor488 to show the nucleus (blue), mitochondria (red) and prohibitin (green), in melatonin treatment (100 μM, A, B, C) versus control (D, E, F). Prohibitin localization is up-regulated in the nucleus and prohibitin concentration is increased. Mitochondria was altered into ring-orientated structure.
The nucleus, mitochondria, and prohibitin in ARPE-19 cells were fluorescently labeled with DAPI, MitoTracker Orange, and AlexaFluor488 in melatonin treatment (100 μM, A, B, C) versus control (D, E, F). Nuclear localization of prohibitin was increased in a melatonin-dependent manner and mitochondrial structure was changed into ring-orientated structure.
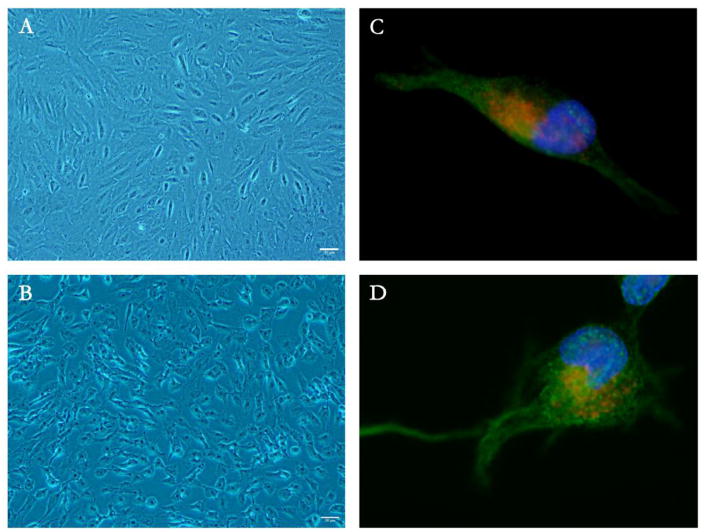
Next, we tested the hypothesis whether melatonin could affect cytoskeletal structure via regulation of β-tubulin. Immunocytochemistry showed that cytoskeletal structure was modified in melatonin-supplemented ARPE-19 cells (Figure 2). Melatonin treated cells demonstrated distinct morphological changes (Figure 2A and 2B). β-tubulin structure was altered and neurite-like outgrowth was induced in melatonin-treated cells (Figure 2C and 2D).
Figure 2.
Cytoskeletal structure is modified in melatonin-supplemented ARPE-19 cells. A,C. Control; B,D. Melatonin 100 μM; AlexaFluor488 labeled β-tubulin (green) of control (C) and melatonin treatment (D). Melatonin treatment shows distinct morphological changes in ARPE-19 (A, B). Melatonin induced the formation of neurite-like outgrowth by altering β-tubulin levels (C, D).
Initial cell shape of ARPE-19 was epitheloid; however, with 100 μM melatonin the cells underwent morphological changes to fusiform shape with neurite-like outgrowths. Immunocytochemistry of melatonin treated cells illustrates an increase in and higher nuclear localization of prohibitin and a rearrangement in β-tubulin compared to non-treated cells.
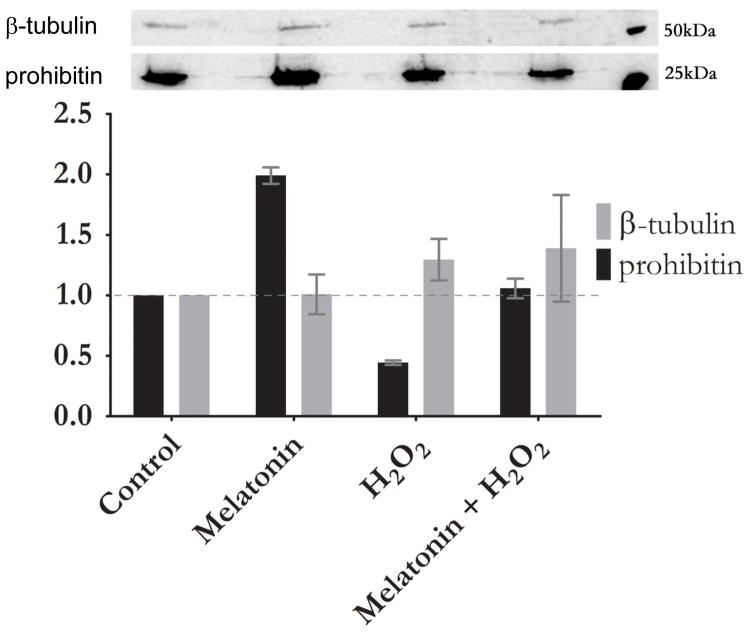
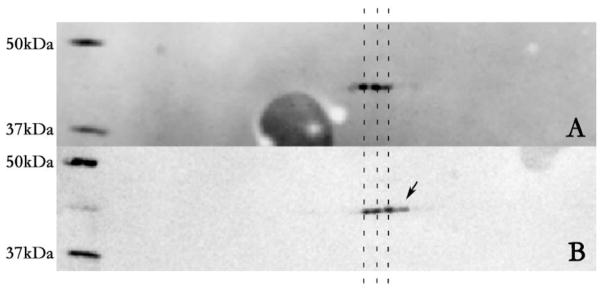
Western blot analysis shows a 3-fold up-regulation of prohibitin with melatonin treatment versus control. A comparable level of prohibitin was detected when ARPE-19 cells were subjected to treatment of both melatonin and H2O2. β-tubulin levels were up-regulated under melatonin to a lesser extent (Figure 3).
Figure 3.
Melatonin reestablishes prohibitin concentration under oxidative stress. Prohibitin (32 kDa) and β-tubulin (55 kDa) levels were examined in relation to 100 μM Melatonin and 100 μM H2O2 treated cells. Quantitative analysis of Western blot shows up-regulation of prohibitin in melatonin treatment, down-regulation in oxidative stress (H2O2), and recover when treated simultaneously. β-tubulin shows no statistically different changes from control under H2O2 treatment. Western blot analysis (upper panel) shows control (Lane 1), melatonin treatment (Lane 2), oxidative stress (Lane 3), melatonin treatment under oxidative stress (Lane 4), and molecular markers (50 and 25 kDa), respectively.
Previously, our proteomic data demonstrated that mitochondrial-nuclear shuttle scaffold prohibitin was down-regulated under oxidative stress [11]. The current study showed that melatonin reestablishes prohibitin levels under oxidative stress.
Western blotting analysis shows up-regulation of prohibitin in melatonin treatment, whereas down-regulation in oxidative stress (H2O2). However, when melatonin is treated under oxidative stress simultaneously, melatonin reestablishes prohibitin levels as control concentration. β-tubulin shows no statistically different changes from control under oxidative stress.
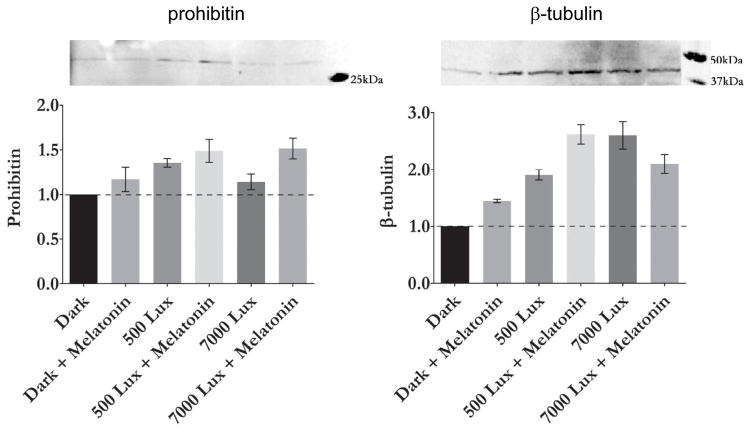
ARPE-19 cells that underwent light stress and 100 μM melatonin supplement demonstrated that up-regulation of prohibitin compared to control in the dark (Figure 4). For both normal room light or intense light conditions (500 vs. 7,000 lux), prohibitin was up-regulated as previously observed in mouse retina in vivo. β-tubulin was also up-regulated by melatonin and light stress. A 2.5-fold increase of β-tubulin from dark control was observed in melatonin treated cells with light (500 lux).
Figure 4.
Light exposure and melatonin up-regulate prohibitin and β-tubulin levels in ARPE-19 cells. Prohibitin and β-tubulin levels were examined in dark control, light (500 lux), light with melatonin (500 lux + 1 μM melatonin), intense light (7000 lux) and intense light with melatonin (7,000 lux + 1 μM melatonin). Prohibitin and β-tubulin levels were up-regulated under light and melatonin conditions.
2D SDS-PAGE demonstrated that β-tubulin undergoes dephosphorylation or basic modification in melatonin treatment conditions, showing pI value of approximately 5.9 which is not seen in the control.
4 CONCLUSION
Melatonin up-regulates the anti-apoptotic, mitochondrial-nuclear shuttle protein prohibitin in human retinal pigment epithelium. Application of melatonin to RPE during oxidative stress reinstitutes normal prohibitin levels in the cells, indicating that melatonin has a regulatory effect on this anti-apoptic mechanism. A high concentration of melatonin restructures RPE cytoskeleton by β-tubulin modification and rearrangement. The current study suggests that re-organization of the β-tubulin framework may play a role in cellular stabilization during oxidative stress. Melatonin treatment alters cytoskeletal and mitochondrial structure in the RPE, implying potential therapeutic function of melatonin toward light-induced retinal diseases, including age-related macular degeneration.
Figure 5.
β-tubulin is dephosphorylated by melatonin and light stress. Dark control shows β-tubulin with pI values ranging from 5.6 to 5.8 (A), whereas 1 μM melatonin treatment shows a basic pI value of 5.9 (B).
Acknowledgments
We would like to thank Parrisha Louis, Joey Smith, Jackie Pribyl, Dr. Ruonan Zhang for their technical assistance, as well as Dr. Mike Gibson and Dr. Jeremy Goldman for use of their equipment. This study was supported by the Century II Equipment Fund and the Research Excellence Fund from Michigan Technological University, Research Assistantship and Teaching Assistantship from American University of Nigeria. The authors thank Dr. Brian K. Reed for his critical reading and suggestions.
References
- 1.Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian-Dependent Retinal Light Damage in Rats. Invest Ophthalmol Vis Sci. 2000 Nov;41(12):3694–3701. [PubMed] [Google Scholar]
- 2.Zhang R, Hrushesky WJM, Wood PA, Lee SH, Hunt RC, Jahng WJ. Melatonin reprogrammes proteomic profile in light-exposed retina in vivo. Int J Biol Macromol. 2010 Aug;47(2):255–260. doi: 10.1016/j.ijbiomac.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung H, Lee H, Lamoke F, Hrushesky WJM, Wood PA, Jahng WJ. Neuroprotective Role of Erythropoietin by Antiapoptosis in the Retina. J Neurosci Res. 2009 Aug;87(10):2365–2374. doi: 10.1002/jnr.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noell WK, Walker VS, Kang BS, Berman S. Retinal Damage by Light in Rats. Invest Ophthalmol Vis Sci. 1966 Oct;5(5):450–473. [PubMed] [Google Scholar]
- 5.Organisciak DT, et al. Light history and age-related changes in retinal light damage. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1107–1116. [PubMed] [Google Scholar]
- 6.Shahinfar S, Edward DP, Tso MO. A pathologic study of photoreceptor cell death in retinal photic injury. Curr Eye Res. 1991 Jan;10(1):47–59. doi: 10.3109/02713689109007610. [DOI] [PubMed] [Google Scholar]
- 7.Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006 Sep;43(5):344–353. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 8.Wiechmann AF, Summers JA. Circadian rhythms in the eye: The physiological significance of melatonin receptors in ocular tissues. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Organisciak DT, et al. Light history and age-related changes in retinal light damage. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1107–1116. [PubMed] [Google Scholar]
- 10.Tosini G, Menaker M. Circadian Rhythms in Cultured Mammalian Retina. Science. 1996;272(5260):419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, et al. Prohibitin as an oxidative stress biomarker in the eye. Int J Biol Macromol. 2010 Dec;47(5):685–690. doi: 10.1016/j.ijbiomac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sripathi SR, et al. Mitochondrial-nuclear communication by prohibitin shuttling under oxidative stress. Biochemistry (Mosc) 2011 Oct;50(39):8342–8351. doi: 10.1021/bi2008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sripathi SR, et al. Altered Cytoskeleton as a Mitochondrial Decay Signature in the Retinal Pigment Epithelium. Protein J. 2016;35(3):179–192. doi: 10.1007/s10930-016-9659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sripathi SR, et al. Prohibitin as the Molecular Binding Switch in the Retinal Pigment Epithelium. Protein J. 2016 Feb;35(1):1–16. doi: 10.1007/s10930-015-9641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]