Abstract
Direct oral anticoagulants (DOACs) are a relatively recent addition to the oral anticoagulant armamentarium, and provide an alternative to the use of vitamin K antagonists such as warfarin. Regardless of the type of agent used, bleeding is the major complication of anticoagulant therapy. The decision to restart oral anticoagulation following a major hemorrhage in a previously anticoagulated patient is supported largely by retrospective studies rather than randomized clinical trials (mostly with vitamin K antagonists), and remains an issue of individualized clinical assessment: the patient’s risk of thromboembolism must be balanced with the risk of recurrent major bleeding. This review provides guidance for clinicians regarding if and when a patient should be re-initiated on DOAC therapy following a major hemorrhage, based on the existing evidence.
Keywords: Direct-acting oral anticoagulants, Gastrointestinal bleeding, Intracranial hemorrhage, Major hemorrhage, Restart oral anticoagulants, Warfarin
The incidence rates of atrial fibrillation (AF) in North America were estimated at 264 per 100,000 person-years for men and 196 per 100,000 person-years for women in 2010,1 and approximately 76 million prescriptions for oral anticoagulant (OAC) therapy for all indications were dispensed in the United States during 2013.2 Yet OACs are underused in many patients with AF, and an elevated risk of stroke,3 contrary to the recommendations of multiple current guidelines,4–6 with rates of OAC prescribing in appropriately risk-stratified patients ranging from 40% to 60%.7,8 The most common complication of OAC therapy is gastrointestinal (GI) bleeding, but the main cause of bleeding-related morbidity and mortality is intracranial hemorrhage (ICH).9–11 Physicians consistently underestimate the risk of stroke in patients with AF and overestimate the risk of hemorrhage with OAC therapy, leading to under-treatment, despite evidence of the benefits of OACs.8,12 This bias is exacerbated once a patient suffers a major hemorrhage while receiving OAC therapy, particularly for clinicians involved in the acute care of these episodes, as the bleeding is apparent and dramatic, while the stroke that may be prevented by OAC therapy is not. Although often counterintuitive, restarting OACs after OAC-associated major hemorrhage is usually appropriate; however, the main issue concerns the timing of the restart. Evidence-based data from prospective, randomized, controlled clinical trials to address this question are needed, particularly in direct oral anticoagulant (DOAC)-treated patients but are unavailable at present.
There are multiple definitions for assessing the severity of bleeding episodes. Major hemorrhage is defined by the International Society on Thrombosis and Haemostasis as fatal bleeding, or symptomatic bleeding in a critical area or organ, or bleeding causing a fall in hemoglobin level of 20 g/L (1.24 mmol/L or 2 g/dL) or more, or leading to transfusion of ≥ 2 units of whole blood or red cells.13 Consequently, patients enrolled into studies of OAC-associated International Society on Thrombosis and Haemostasis-defined major bleeding consist of a heterogeneous population arising from different clinical specialties, which compounds the difficulties of studying these scenarios. Estimates of the risk of major hemorrhage related to OAC range from 2% to 3% in clinical trials to approximately 1% to 7% in population cohort studies.10,11,14 The exact incidence of major hemorrhage is unknown because of uncertainty regarding the intensity of OAC therapy, and patient-related factors such as history of bleeding, concomitant disease, alcohol use, age, and risk of falls.10 Regarding types of major hemorrhage related to OAC, the largest amount of published data is for ICH and GI bleeding, and this review will focus on these 2 clinical entities. Recommendations for restarting OAC therapy in other major bleeding situations, which are relatively rare, will remain as risk–benefit decisions for the individual clinician and patient.
For many decades, OAC therapy consisted of vitamin K antagonists (VKAs), typically warfarin in the United States, although other VKAs (eg, phenprocoumon and acenocoumarol) are used in other geographical areas. VKAs act by blocking vitamin K epoxide reductase to inhibit the activation of clotting factors (F) II, VII, IX, and X, and natural anticoagulant proteins C and S. However, in recent years, small-molecule DOACs have become available, the first of which was the direct thrombin inhibitor, dabigatran, which gained U.S. Food and Drug Administration approval in 2010 for the risk reduction of stroke and systemic embolism in patients with nonvalvular AF (NVAF). This was quickly followed by the arrival of drugs that directly inhibit FXa (apixaban, rivaroxaban, and edoxaban), which is 1 step proximal to the action of direct FIIa inhibitors such as dabigatran in the clotting cascade. Data from phase 3 clinical trials in patients with NVAF demonstrated that these 4 DOACs were either noninferior or superior to warfarin in terms of efficacy (ie, reducing the rates of stroke and systemic embolism),15–18 and showed equivalence or improved safety (ie, major hemorrhage and clinically relevant nonmajor hemorrhage) vs warfarin.15–18 DOACs were associated with an approximately 30%–70% reduction in the rates of ICH vs warfarin,15–18 although they were associated with generally higher rates of GI bleeding (not further defined; annualized rate ranged from approximately 0.8% to 3.2% for DOACs [depending on the agent and dose] vs approximately 1.0% to 2.2% for warfarin).15,16,18 DOACs are also approved for the treatment and prevention of venous thromboembolism (VTE), for which they were noninferior to conventional therapy in terms of efficacy outcomes, and showed equivalence or improvement in the overall safety profile.19–22
To date, comparatively few data have been published on restarting OAC therapy after a major hemorrhage and the data that do exist are almost exclusively from patients receiving VKAs, with very few data concerning DOACs. Furthermore, some expert opinion recommends approaching the re-initiation of DOACs similarly to restart scenarios with warfarin.23 This is reflected in the discussion below. This review aims to summarize the key evidence and provide guidance for clinicians regarding if and when a patient should be restarted on DOAC therapy following a major hemorrhage.
INTRACRANIAL HEMORRHAGE AND RE-INITIATION OF OACS
Intracranial hemorrhage has a heterogeneous etiology, including spontaneous ICH (eg, lobar and deep hemispheric hemorrhages, aneurismal subarachnoid hemorrhages, and bleeding arteriovenous malformations) and traumatic ICH (eg, extra-axial subdural, epidural hematomas, traumatic subarachnoid hemorrhages, and intra-axial hemorrhagic contusions). The risk of ICH recurrence can be related to etiologic factors. For example, superficial (lobar) hemorrhages are often caused by cerebral amyloid angiopathy, a condition that affects cerebral arteries and arterioles and increases the risk of hemorrhage, and is associated with recurrence rates of up to 22%.24 The incidence of nontraumatic ICH is approximately 25 per 100,000 person-years.25 It has been estimated that there are approximately 67,000 cases of spontaneous ICH per year in the United States,26 and anticoagulant-associated ICH accounts for nearly 20% of those.26 The 30-day case fatality rate is as high as 50%, and most survivors are left with some degree of disability, which is often severe.26
In cases of OAC-related ICH, the therapeutic dilemma is that stopping anticoagulation increases the risk of cerebral ischemia, while continuing or restarting treatment after stopping it increases the risk of recurrent bleeding.24 This has been referred to as “steering between Scylla and Charybdis,” meaning to have to choose between 2 evils.24 The published reports described below are all retrospective analyses of OAC-related ICH, with varying patient populations (eg, some studies focus on patients with NVAF or patients with mechanical heart valves, while other studies include patients treated for VTE). It should be noted that DOACs are not approved for use in patients with mechanical heart valves.
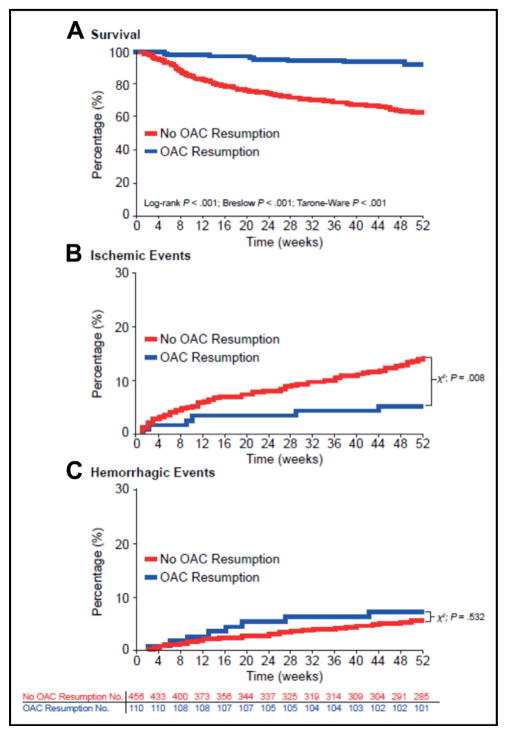
A recent report from a German multicenter, retrospective study (2006–2012) assessed the effects of OAC resumption in patients with anticoagulation-related (VKAs) spontaneous ICH.27 Of the 1176 patients with data available, 719 patients were part of the OAC resumption analysis (the remainder were analyzed for hematoma enlargement [n = 853] or long-term outcomes [n = 1083]). OAC was restarted in 172 of 719 (23.9%) patients (including 34/50 [68.0%] with mechanical heart valves, and 110/566 [19.4%] with AF).27 Median time to OAC resumption was 31 days (interquartile range [IQR] 18–65). Restarting OAC therapy was associated with fewer ischemic events (OAC 9/172 [5.2%]; no OAC 82/547 [15.0%]; P < .001), and no significant increase in hemorrhagic complications (OAC 14/172 [8.1%]; no OAC 36/547 [6.6%]; P = .48) (Figure).27 Furthermore, there was a decrease in long-term mortality in the subgroup of patients with AF who restarted OAC (propensity-matched survival analysis, hazard ratio [HR] 0.258; 95% confidence interval [CI], 0.125–0.534; P < .001).27
Figure.
Unmatched survival and event rates in atrial fibrillation patients: analyzing oral anticoagulant resumption status. (Reproduced with permission from reference27) Unmatched Kaplan-Meier survival curves, ischemic, and hemorrhagic event rates in atrial fibrillation (AF) patients with and without oral anticoagulant (OAC) resumption. (A) Kaplan-Meier survival rates of patients with AF with and without OAC resumption from index-intracranial hemorrhage (ICH) until 1-year follow-up, analyzed by log-rank, Breslow, and Tarone–Ware testing, with corresponding P values. (B) Incidence rates of new ischemic events over the 1-year follow-up period in patients with and without OAC resumption. (C) Incidence rates of hemorrhagic events over the 1-year follow-up period in patients with and without OAC resumption. Numbers for patients at risk apply to parts A–C. One year after OAC-related ICH 8.2% (n = 9/110) of resumed patients vs 37.5% (n = 171/456) of patients without OAC resumption had died (P < .001). The crude incidence of bleeding events was not significantly different among AF patients with and without OAC resumption (OAC resumed: 7.3% [n = 8/110] vs 5.7% [n = 26/456] nonresumed patients; P = .532), the incidence of new ischemic events was significantly increased in patients without OAC resumption (5.4% [n = 6/110] vs 14.9% [n = 68/456]; P = .008).
Another study linked 3 large Danish registries (1997–2013), and assessed the risk of recurrent stroke and mortality when restarting OAC in patients with AF and OAC-associated ICH (n = 1752).28 The majority of patients received VKA (65%) or VKA plus antiplatelet therapy (33%), and a small proportion received DOACs (2%) or DOACs plus antiplatelet therapy (<1%). The overall event rates (using 1 year of follow-up) of the combined end point of ischemic stroke/systemic embolism and all-cause mortality (per 100 person-years) for patients treated with OAC was 13.6 vs 27.3 for nontreated patients (HR 0.55; 95% CI, 0.39–0.78; no P-value stated).28 Of patients who resumed OAC treatment after ICH (n = 621), the overall median time from ICH to the first claimed prescription was 34 days.28
A Canadian registry study of 284 spontaneous warfarin-related ICH (intracerebral or subarachnoid hemorrhage) cases, in which warfarin was restarted in 91 (32%) patients, reported that there was no increase in 30-day mortality (adjusted odds ratio 0.49; 95% CI, 0.26–0.93; P = .03) in patients who restarted warfarin.29 This trend continued at 1 year but was no longer significant (adjusted odds ratio 0.79; 95% CI, 0.43–1.43; P = .43).29 This study included VTE indications and valve prosthesis for OAC therapy, in addition to AF.
A retrospective, 3-center analysis of 234 patients with warfarin-associated ICH found a 5-fold increased risk of recurrent ICH with the resumption of OAC in the immediate period (median time: 5.6 weeks; IQR 2.6–17) after the index event (HR 5.6; 95% CI, 1.8–17.2; P = .0029), and the HR for ischemic stroke was 0.11 (95% CI, 0.014–0.87; P = .036).30 The combined risk of recurrent ICH and ischemic stroke reached its lowest point if OAC therapy was restarted between 10 and 30 weeks after the index event.30
A further report, in which 7 clinical experts assessed scenarios concerning acute reversal and resumption of OAC in the setting of warfarin-associated ICH, revealed that expert opinion favored OAC resumption within 3–10 days of ICH if the patient was stable and anticoagulation was mandatory.31 A shorter time to restarting OAC therapy, as early as 72 hours post-bleed, was also recommended in a review of 63 publications that described 492 patients with warfarin-associated central nervous system hemorrhage (including spinal hemorrhage).32
Lastly, a retrospective review (1976–1999) of 141 patients with ICH at high thromboembolic risk (OAC indications: mechanical heart valve, AF, and prior stroke) found that discontinuation of warfarin for 1–2 weeks (median time not receiving warfarin 10 days; range 0–30 days) had a comparatively low probability of embolic events, and there was no recurrence of ICH at 30 days for the 35 patients who were restarted on OAC.33
GASTROINTESTINAL HEMORRHAGE AND RE-INITIATION OF OACS
Gastrointestinal bleeding also has a diverse etiology (eg, hemorrhagic gastritis, peptic ulcer disease, arteriovenous malformations, and diverticulosis), but has generally been studied as a single cohort. Again, etiology plays a role in recurrence risk, but it is difficult to parse out. Acute GI bleeds related to both VKA34 and DOAC18 therapy are more common in the upper GI tract. Data from an open cohort study (upper GI bleed n = 21,641) gave an age-standardized incidence rate (per 1000 person-years) for upper GI tract bleeding of 5.8 in those prescribed warfarin and 2.7 in those prescribed DOACs.34 A meta-analysis of data from 11 phase 3 randomized controlled trials reported no significant difference in the overall incidence of major GI bleeding between DOACs and VKAs (relative risk 0.94; P = .62).35 There is evidence, some of it prospective, that restarting OAC therapy after GI hemorrhage is beneficial.
A prospective observational study in the United States identified 197 patients who developed GI bleeding while receiving systemic anticoagulation (145/197 [74%] received warfarin), of whom 76 (39%) discontinued anticoagulation upon hospital discharge (ie, interruption of anticoagulation for ≥72 hours after discharge).36 Restarting OAC therapy at hospital discharge was associated with a lower risk of major thrombotic episodes within 90 days (HR 0.121; 95% CI, 0.006–0.812; P = .03), and no significant difference in mortality was observed (at 90 days, HR 0.632; 95% CI, 0.216–1.89; P = .40).36 Furthermore, restarting OAC was not significantly associated with an increased risk of recurrent GI bleeding at 90 days (HR 2.17; 95% CI, 0.861–6.67; P = .10).36
A retrospective United States cohort study enrolled patients with AF who developed GI bleeding while receiving anticoagulation (n = 1329).37 Warfarin was restarted in 653 (49%) patients, after a median duration of 50 days (IQR 21–78). Restarting warfarin was associated with reduced mortality (adjusted HR 0.67; 95% CI, 0.56–0.81; P < .0001) and decreased risk of thromboembolism (adjusted HR 0.71; 95% CI, 0.54–0.93; P = .01), but not recurrent GI bleeding (adjusted HR 1.18; 95% CI, 0.94–1.10; P = .47).37 When the outcomes were stratified by duration of warfarin interruption, restarting warfarin after 7 days was not associated with increased risk of GI bleeding, but was associated with decreased risk of mortality and thromboembolism compared with resuming after 30 days of interruption.37 These data are in agreement with other studies.36,38 These findings were extended in a recent meta-analysis that included this study from the United States, and concluded that the resumption of warfarin following interruption because of GI bleeding is associated with a reduction in thromboembolic events and mortality without a statistically significant increase in recurrent GI bleeding.39
RISK STRATIFICATION AND CLINICAL DECISION-MAKING
The overall annual risk of any major hemorrhage for patients receiving OACs is 2% to 3%, with the annual risk of OAC-related ICH at 0.3% to 0.5%.11 This must, however, be considered against the annual risk of arterial thromboembolism in the absence of OAC therapy, which is 12% to 22% for patients with mechanical heart valves, and 6% to 18% in patients with AF plus a CHA2DS2-VASc score of ≥3, and there is a 5% to 7% risk of VTE recurrence in the first 3 months for patients receiving OAC for previous VTE.40–43 The clinical consequences of a thrombotic or bleeding event must also be taken into consideration when deciding to restart OACs. For example, mechanical heart valve thrombosis is fatal in approximately 12% of patients, embolic stroke results in death in up to 27% of cases, while VTE has a case-fatality rate of approximately 4% to 14%, and major bleeding has a case-fatality rate of approximately 9% to 13%.44–49 Most of the major guidelines, including those from the American Heart Association/American Stroke Association, the American College of Chest Physicians, and the European Stroke Organisation, provide advice on whether to restart OAC therapy after major hemorrhage in appropriately risk stratified patients, although they differ over the timing (Table 1).30,31,50–52 For example, the American Heart Association/American Stroke Association guidelines for the management of spontaneous ICH had previously advised restarting OAC at ≥1 week after ICH,53 but recently revised their guidance and now recommend avoidance of OACs for at least 4 weeks in patients without mechanical heart valves.51
Table 1.
Major Guideline Recommendations on Re-Initiation of OAC Following a Major Bleed
| Guideline and Citation | Recommendation |
|---|---|
| European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage, 201450 |
Recommendation 18: Unable to make firm recommendations about whether and when to resume antithrombotic drugs after ICH in the absence of RCTs to address treatment dilemmas Additional information: Suggested timings for restarting these drugs range from not earlier than 14 days up to 30 weeks (data from observational studies)30,31 |
| Guidelines for the management of spontaneous intracerebral hemorrhage (American Heart Association/American Stroke Association), 201551 | Prevention of Recurrent ICH, Recommendation 6: Optimal timing to resume OAC after OAC-related ICH is uncertain. Avoidance of OAC for at least 4 weeks, in patients without mechanical heart valves, might decrease the risk of ICH recurrence. If indicated, aspirin monotherapy can probably be restarted in the days after ICH, although the optimal timing is uncertain |
| Antithrombotic and thrombolytic therapy for ischemic stroke, (Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians), 201252 |
Recommendation 4.3: In patients with a history of a symptomatic primary ICH, we suggest against the long-term use of antithrombotic therapy for the prevention of ischemic stroke Remarks: Patients with a history of ICH who might benefit from antithrombotic therapy are those at relatively low risk of recurrent ICH (eg, with deep hemorrhages) and relatively high risk (>7% per year) of cardiac thromboembolic events (eg, with mechanical heart valves or CHADS2* score of ≥4 points) |
ICH = intracranial hemorrhage; OAC = oral anticoagulant; RCT = randomized controlled trial.
CHADS2 (score for atrial fibrillation stroke risk) congestive heart failure history, hypertension history, age ≥75 years, diabetes mellitus history, stroke or transient ischemic attack previously (see also Table 2).
Evaluation of an individual’s risk factors for stroke, bleeding, and VTE recurrence is essential to understanding the risks and benefits of OAC therapy for that individual, and several risk-stratification tools are available (Table 2,41,54–58 Table 3,57,59–61 and Table 462–65). The CHA2DS2-VASc score54 to assess stroke risk and the HAS-BLED score59 to assess bleeding risk are the most commonly used tools, as recommended by the current guidelines.5,6 The HAS-BLED score was used to identify modifiable risk factors for bleeding.5 It should be noted that these schemes were developed in populations with AF and did not include patients with VTE. The same is true of the HEMORR2HAGES score for bleeding risk,60 whereas the Outpatient Bleeding Risk Index (OBRI) validation did include patients with VTE.66 Validated scores for predicting VTE recurrence include HERDOO2,62 the Vienna model,63 and DASH.64 Unfortunately, bleeding scores (OBRI, HEMORR2HAGES, HAS-BLED, and ATRIA61) have shown poor discriminatory ability to predict major bleeding and clinically relevant nonmajor bleeding in subsequent external validation studies.67
Table 2.
Risk Stratification Tools for Stroke Risk in Atrial Fibrillation57
| Tool and Citation | Risk Factor* | Score | Tool Score (if Stated) | Annual Event Rate, % (if Stated) |
|---|---|---|---|---|
| CHADS241 | CHADS2 score | Adjusted stroke rate | ||
| CHF (recent) | 1 | 0 | 1.9 | |
| Hypertension (history of) | 1 | 1 | 2.8 | |
| Age ≥75 y | 1 | 2 | 4.0 | |
| DM | 1 | 3 | 5.9 | |
| Stroke/TIA | 2 | 4 | 8.5 | |
| 5 | 12.5 | |||
| (6 = max. score) | 6 | 18.2 | ||
| CHA2DS2-VASc54 | CHA2DS2-VASc score | TEE rate | ||
| CHF/LV dysfunction | 1 | 0 | 0 | |
| Hypertension | 1 | 1 | 0.6 | |
| Age ≥75 y | 2 | 2 | 1.6 | |
| DM | 1 | 3 | 3.9 | |
| Stroke/TIA/TE | 2 | 4 | 1.9 | |
| Vascular disease (prior MI, PAD, or aortic plaque) | 1 | 5 | 3.2 | |
| Age 65–74 y | 1 | 6 | 3.6 | |
| Sex category (female) | 1 | 7 | 8.0 | |
| 8 | 11.1 | |||
| (9 = max. score) | 9 | 100 | ||
| R2CHADS255 | ||||
| Renal dysfunction (CrCl <60 mL/min) | 2 | |||
| CHF (recent) | 1 | |||
| Hypertension | 1 | |||
| Age ≥75 y | 1 | |||
| DM | 1 | |||
| Stroke/TIA | 2 | |||
| (8 = max. score) | ||||
| QStroke (QResearch database Stroke)56,57 | Age (at entry) y | Range, 25–84 | ||
| Sex | Separate models for male and female | |||
| Treated hypertension (diagnosis of hypertension and ≥1 current prescription for ≥1 antihypertensive agent) | Yes/No | |||
| T1DM | Yes/No | |||
| T2DM | Yes/No | |||
| AF | Yes/No | |||
| CHF | Yes/No | |||
| CHD | Yes/No | |||
| Self-assigned ethnicity (White/not recorded, Indian, Pakistani, Bangladeshi, other Asian, Black Caribbean, Black African, Chinese, other/mixed) | 9 categories | |||
| Townsend Deprivation Score | Continuous | |||
| Smoking status (nonsmoker, ex-smoker, light smoker [<10 cigarettes/day], moderate smoker [10–19 cigarettes/day], heavy smoker [≥20 cigarettes/day]) | 5 categories | |||
| SBP | Continuous | |||
| TC:HDL-C ratio | Continuous | |||
| BMI | Continuous | |||
| Family history of coronary disease (in first-degree relative age <60 y) | Yes/No | |||
| RA | Yes/No | |||
| CKD | Yes/No | |||
| Valvular heart disease | Yes/No | |||
| (99% = max. score) | ||||
| ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Stroke58 | Prior stroke | |||
| Without | With | |||
| Age, y | ||||
| ≥85 | 6 | 9 | ||
| 75–84 | 5 | 7 | ||
| 65–74 | 3 | 7 | ||
| <65 | 0 | 8 | ||
| Female sex | 1 | 1 | ||
| DM | 1 | 1 | ||
| CHF | 1 | 1 | ||
| Hypertension | 1 | 1 | ||
| Proteinuria | 1 | 1 | ||
| eGFR <45 mL/min/1.73 m2 or ESRD | 1 | 1 | ||
| (12 = max. score) | (15 = max. score) | |||
AF = atrial fibrillation; BMI = body mass index; CHD = coronary heart disease; CHF = congestive heart failure; CKD = chronic kidney disease; CrCl = creatinine clearance; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; HDL-C = high-density lipoprotein cholesterol; LV = left ventricular; MI = myocardial infarction; PAD = peripheral artery disease; RA = rheumatoid arthritis; SBP = systolic blood pressure; T1 = type 1 (DM); T2 = type 2 (DM); TC = total cholesterol; TE = thromboembolism; TEE = thromboembolic event; TIA = transient ischemic attack.
First letter of each row spells out the acronym, unless otherwise stated.
Table 3.
Risk Stratification Tools for Bleeding Risk in Atrial Fibrillation57
| Tool and Citation | Risk Factor* | Score |
|---|---|---|
| HAS-BLED59 | Hypertension (SBP >160 mm Hg) | 1 |
| Abnormal renal or liver function | 1 or 2 | |
| Stroke | 1 | |
| Bleeding history or predisposition | 1 | |
| Labile INRs (if on warfarin) | 1 | |
| Elderly (eg, age >65 y, frail condition) | 1 | |
| Drugs (eg, concomitant antiplatelet or NSAIDs) or alcohol excess/abuse | 1 or 2 | |
| (9 = max. score) | ||
| HEMORRHAGES60 | Hepatic or renal disease | 1 |
| Ethanol abuse | 1 | |
| Malignancy | 1 | |
| Older age (>75 y) | 1 | |
| Reduced platelet count or function | 1 | |
| Re-bleeding risk | 2 | |
| Hypertension (uncontrolled) | 1 | |
| Anemia | 1 | |
| Genetic factors (CYP2C9 SNP) | 1 | |
| Excessive fall risk | 1 | |
| Stroke | 1 | |
| (12 = max. score) | ||
| ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) bleeding61 | Anemia | 3 |
| Severe renal disease (eGFR <30 mL/min/1.73 m2 or dialysis dependent) | 3 | |
| Age ≥75 y | 2 | |
| Prior hemorrhage | 1 | |
| Diagnosed hypertension | 1 | |
| (10 = max. score) |
CYP2C9 = cytochrome P450 2C9; eGFR = estimated glomerular filtration rate; INR = international normalized ratio; NSAIDs = nonsteroidal antiinflammatory drugs; SBP = systolic blood pressure; SNP = single nucleotide polymorphism.
First letter of each row spells out the acronym, unless otherwise stated.
Table 4.
Risk Stratification Tools for Predicting Venous Thromboembolism Risk Recurrence65
| Tool and Citation | Risk Factor | Score |
|---|---|---|
| HERDOO2 (Hyperpigmentation, Edema, Redness, D-dimer, Obesity, Older age, 2 scores)62 | Clinical decision rule to identify patients at low risk of recurrent VTE after 5–7 months of OAC therapy | |
| Men | Always long-term AC | |
| Women | Long-term AC if score ≥2 | |
| Predictive factors for women | Score | |
| Post-thrombotic signs (hyperpigmentation, edema, or redness in either leg) | 1 | |
| D-dimer level ≥250 μg/L (during anticoagulation) | 1 | |
| BMI ≥30 kg/m2 | 1 | |
| Age ≥65 years | 1 | |
| Vienna (Medical University of Vienna)63 | Sex | |
| Male | 60 | |
| Female | 0 | |
| Site of VTE | ||
| Distal DVT | 0 | |
| Proximal DVT | 70 | |
| Pulmonary embolism | 90 | |
| D-dimer levels | ||
| Continuous (Low risk of recurrence with score ≤180) | 0–100 | |
| DASH (D-dimer level, young Age, male Sex, and Hormonal therapy associated with the index VTE event)64 | Elevated D-dimer levels 1 month after stopping VKAs | 2 |
| Age <50 y | 1 | |
| Male sex | 1 | |
| Women taking oral contraceptives (Low risk of recurrence with score ≤180) | −2 |
AC = anticoagulation; BMI = body mass index; DVT = deep vein thrombosis; OAC = oral anticoagulant; VKA = vitamin K antagonist; VTE = venous thromboembolism.
A broad clinical assessment must be performed following an OAC-related major hemorrhage, including identification of the underlying reason for which the patient originally received anticoagulation therapy and their risk of stroke or VTE and bleeding. Factors that would favor restarting OAC therapy include the presence of deep ICH, a mechanical heart valve, secondary prevention, or high risk of stroke or VTE.68 A corrected cause of bleeding (eg, a clipped aneurysm or a repaired aortoenteric fistula) would also facilitate OAC restart. Factors that would confer an unfavorable benefit–risk profile for restarting OAC therapy include lobar ICH, multiple microbleeds on gradient-recalled-echo-magnetic resonance imaging (correlating with cerebral amyloid angiopathy and an increased risk of ICH recurrence), and a low risk of stroke or VTE.68
If a patient is deemed appropriate to restart OAC therapy, the clinician must decide when re-initiation should occur, and how rapidly therapeutic anticoagulation is needed. The decision pathway for re-initiation of OAC therapy must recognize when the increasing risk of thromboembolism outweighs the decreasing risk of recurrent hemorrhage. Thus, restarting OAC might be considered earlier in patients with mechanical heart valves or stabilized GI bleeds, later with inpatients with ICH or a low risk of stroke/VTE, and probably disregarded in cases of lobar ICH or in the presence of intracranial microbleeds. As evidenced from the studies described, the recommendations vary widely (eg, from 3 days31 to 30 weeks30 for ICH), and are based on retrospective data rather than prospective data from randomized, controlled clinical trials. A recent nonsystematic review of antithrombotic treatment and ICH by Hofmeijer et al24 concluded that OAC therapy should be resumed after 1–2 weeks in patients with deep ICH and high risk of cerebral ischemia (ie, patients with NVAF and a CHA2DS2-VASc score of ≥4 or a mechanical heart valve), but restart should be later (ie, after 4 weeks) in other patients. A further consideration when re-initiating OAC therapy is the time to onset of action, which is much faster for DOACs than for warfarin (0.5–4 hours vs 36–72 hours, respectively).69
Lastly, it should also be noted that the risk of thromboembolism is still high in the immediate period after a major hemorrhage, and that rapid reversal of OAC, regardless of the method, can be attended by thromboembolic events in some patients. A post hoc analysis of thromboembolic complications after warfarin reversal, examining data from 388 patients presenting with acute major hemorrhage or in need of urgent surgical intervention, reported that the incidence of thromboembolic events in the first 45 days was similar following VKA reversal with either 4-factor prothrombin complex concentrate or plasma (approximately 7% for each agent).70
CONCLUSIONS
In summary, the decision to restart OAC therapy in a patient receiving chronic OAC who has suffered from a major bleed is a highly individualized assessment. The risk of thromboembolism must be balanced with the risk of recurrent major bleeding in that individual, while taking into account the morbidity and case-fatality of a thrombotic/bleed outcome, in addition to the optimal time frame of when to restart OAC. It is also dependent upon the original indication for OAC, and the type of major bleed from which the patient suffered. Many patients with OAC-associated ICHs can restart OAC at some point between 1 and 30 weeks, but careful risk stratification must be performed. Most patients with OAC-associated clinically stable GI hemorrhages can restart OAC at 1 week post index bleed. Currently, there are few data on how DOACs might change the risk–benefit analysis of when to restart therapy after a major bleed event, particularly in ICH. However, the available data are reassuring in that practitioners would expect approximately 50% fewer of these events vs VKA-treated patients, which has major implications from a public health perspective. A larger number of well-designed studies are needed in this area for both VKA and, especially, for DOAC-treated patient groups.
Acknowledgments
Funding: This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). Editorial support was provided by Debra Brocksmith, MB, ChB, PhD, of Envision Scientific Solutions, which was contracted and funded by BIPI. The authors received no direct compensation related to the development of the manuscript.
Footnotes
Authorship: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors were responsible for all content and editorial decisions, were involved at all stages of manuscript development, and approved the final version. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Conflict of Interest: TJM receives personal fees from Boehringer Ingelheim, Janssen, CSL Behring, and Portola outside of the submitted work. ACS receives personal fees from Boehringer Ingelheim, Janssen, Bristol-Myers Squibb, Pfizer, Portola, and Daiichi Sankyo, and nonfinancial support from Janssen outside of the submitted work.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IMS Health. [Accessed July 22, 2016];Medicine use and shifting costs of healthcare: a review of the use of medicines in the United States in 2013. Available at: http://www.imshealth.com/en/thought-leadership/ims-institute/reports/use-of-medicines-in-the-us-2013.
- 3.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638e4–645e4. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 4.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 7.Fitch K, Broulette J, Pyenson B, Iwasaki K, Kwong WJ. Utilization of anticoagulation therapy in medicare patients with nonvalvular atrial fibrillation. Am Health Drug Benefits. 2012;5:157–168. [PMC free article] [PubMed] [Google Scholar]
- 8.Sen S, Dahlberg KW. Physician’s fear of anticoagulant therapy in nonvalvular atrial fibrillation. Am J Med Sci. 2014;348:513–521. doi: 10.1097/MAJ.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervera A, Amaro S, Chamorro A. Oral anticoagulant-associated intracerebral hemorrhage. J Neurol. 2012;259:212–224. doi: 10.1007/s00415-011-6153-3. [DOI] [PubMed] [Google Scholar]
- 10.Wiedermann CJ, Stockner I. Warfarin-induced bleeding complications – clinical presentation and therapeutic options. Thromb Res. 2008;122(suppl 2):S13–S18. doi: 10.1016/S0049-3848(08)70004-5. [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Beyth RJ, Kearon C, Levine MN American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 suppl):257S–298S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 12.Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40:675–683. doi: 10.1093/ageing/afr097. [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 14.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 17.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 19.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 20.Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 21.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 22.Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 23.Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 24.Hofmeijer J, Kappelle LJ, Klijn CJ. Antithrombotic treatment and intracerebral haemorrhage: between Scylla and Charybdis. Pract Neurol. 2015;15:250–256. doi: 10.1136/practneurol-2015-001104. [DOI] [PubMed] [Google Scholar]
- 25.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 26.Flaherty ML. Anticoagulant-associated intracerebral hemorrhage. Semin Neurol. 2010;30:565–572. doi: 10.1055/s-0030-1268866. [DOI] [PubMed] [Google Scholar]
- 27.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–836. doi: 10.1001/jama.2015.0846. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen PB, Larsen TB, Skj⊘th F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation. 2015;132:517–525. doi: 10.1161/CIRCULATIONAHA.115.015735. [DOI] [PubMed] [Google Scholar]
- 29.Yung D, Kapral MK, Asllani E, Fang J, Lee DS Investigators of the Registry of the Canadian Stroke Network. Reinitiation of anticoagulation after warfarin-associated intracranial hemorrhage and mortality risk: the Best Practice for Reinitiating Anticoagulation Therapy After Intracranial Bleeding (BRAIN) study. Can J Cardiol. 2012;28:33–39. doi: 10.1016/j.cjca.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke. 2010;41:2860–2866. doi: 10.1161/STROKEAHA.110.593087. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:82–92. doi: 10.4065/82.1.82. [DOI] [PubMed] [Google Scholar]
- 32.Hawryluk GW, Austin JW, Furlan JC, Lee JB, O’Kelly C, Fehlings MG. Management of anticoagulation following central nervous system hemorrhage in patients with high thromboembolic risk. J Thromb Haemost. 2010;8:1500–1508. doi: 10.1111/j.1538-7836.2010.03882.x. [DOI] [PubMed] [Google Scholar]
- 33.Phan TG, Koh M, Wijdicks EF. Safety of discontinuation of anticoagulation in patients with intracranial hemorrhage at high thromboembolic risk. Arch Neurol. 2000;57:1710–1713. doi: 10.1001/archneur.57.12.1710. [DOI] [PubMed] [Google Scholar]
- 34.Hippisley-Cox J, Coupland C. Predicting risk of upper gastrointestinal bleed and intracranial bleed with anticoagulants: cohort study to derive and validate the QBleed scores. BMJ. 2014;349:g4606. doi: 10.1136/bmj.g4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014;124:2450–2458. doi: 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 36.Sengupta N, Feuerstein JD, Patwardhan VR, et al. The risks of thromboembolism vs. recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. Am J Gastroenterol. 2015;110:328–335. doi: 10.1038/ajg.2014.398. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113:662–668. doi: 10.1016/j.amjcard.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 38.Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172:1484–1491. doi: 10.1001/archinternmed.2012.4261. [DOI] [PubMed] [Google Scholar]
- 39.Chai-Adisaksopha C, Hillis C, Monreal M, Witt DM, Crowther M. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819–825. doi: 10.1160/TH15-01-0063. [DOI] [PubMed] [Google Scholar]
- 40.Salem DN, O’Gara PT, Madias C, Pauker SG American College of Chest Physicians. Valvular and structural heart disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:593S–629S. doi: 10.1378/chest.08-0724. [DOI] [PubMed] [Google Scholar]
- 41.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 42.Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110:515–519. doi: 10.1016/s0002-9343(01)00661-1. [DOI] [PubMed] [Google Scholar]
- 43.Lobo JL, Jimenez D, Teresa Orue M, et al. Recurrent venous thromboembolism during coumarin therapy. Data from the computerised registry of patients with venous thromboembolism. Br J Haematol. 2007;138:400–403. doi: 10.1111/j.1365-2141.2007.06679.x. [DOI] [PubMed] [Google Scholar]
- 44.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578–589. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 45.Douketis JD, Gu CS, Schulman S, Ghirarduzzi A, Pengo V, Prandoni P. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med. 2007;147:766–774. doi: 10.7326/0003-4819-147-11-200712040-00007. [DOI] [PubMed] [Google Scholar]
- 46.Longstreth WT, Jr, Bernick C, Fitzpatrick A, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 47.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 48.Deviri E, Sareli P, Wisenbaugh T, Cronje SL. Obstruction of mechanical heart valve prostheses: clinical aspects and surgical management. J Am Coll Cardiol. 1991;17:646–650. doi: 10.1016/s0735-1097(10)80178-0. [DOI] [PubMed] [Google Scholar]
- 49.Arboix A, Alio J. Acute cardioembolic cerebral infarction: answers to clinical questions. Curr Cardiol Rev. 2012;8:54–67. doi: 10.2174/157340312801215791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage - part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis. 2006;22:294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 51.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 52.Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e601S–e636S. doi: 10.1378/chest.11-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 54.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 55.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 56.Hippisley-Cox J, Coupland C, Brindle P. Derivation and validation of QStroke score for predicting risk of ischaemic stroke in primary care and comparison with other risk scores: a prospective open cohort study. BMJ. 2013;346:f2573. doi: 10.1136/bmj.f2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dzeshka MS, Lane DA, Lip GY. Stroke and bleeding risk in atrial fibrillation: navigating the alphabet soup of risk-score acronyms (CHADS2, CHA2DS2-VASc, R2CHADS2, HAS-BLED, ATRIA, and more) Clin Cardiol. 2014;37:634–644. doi: 10.1002/clc.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 60.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179:417–426. doi: 10.1503/cmaj.080493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121:1630–1636. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 64.Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH) J Thromb Haemost. 2012;10:1019–1025. doi: 10.1111/j.1538-7836.2012.04735.x. [DOI] [PubMed] [Google Scholar]
- 65.Poli D, Palareti G. Assessing recurrence risk following acute venous thromboembolism: use of algorithms. Curr Opin Pulm Med. 2013;19:407–412. doi: 10.1097/MCP.0b013e328363ed7c. [DOI] [PubMed] [Google Scholar]
- 66.Wells PS, Forgie MA, Simms M, et al. The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch Intern Med. 2003;163:917–920. doi: 10.1001/archinte.163.8.917. [DOI] [PubMed] [Google Scholar]
- 67.Burgess S, Crown N, Louzada ML, Dresser G, Kim RB, Lazo-Langner A. Clinical performance of bleeding risk scores for predicting major and clinically relevant non-major bleeding events in patients receiving warfarin. J Thromb Haemost. 2013;11:1647–1654. doi: 10.1111/jth.12352. [DOI] [PubMed] [Google Scholar]
- 68.Molina CA, Selim MH. The dilemma of resuming anticoagulation after intracranial hemorrhage: little evidence facing big fears. Stroke. 2011;42:3665–3666. doi: 10.1161/STROKEAHA.111.631689. [DOI] [PubMed] [Google Scholar]
- 69.Ment J. Direct oral anticoagulants: key considerations for use to prevent stroke in patients with nonvalvular atrial fibrillation. Vasc Health Risk Manag. 2015;11:317–332. doi: 10.2147/VHRM.S79065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milling TJ, Jr, Refaai MA, Goldstein JN, et al. Thromboembolic events after vitamin K antagonist reversal with 4-factor prothrombin complex concentrate: exploratory analyses of two randomized, plasma-controlled studies. Ann Emerg Med. 2016;67(1):96–105.e5. doi: 10.1016/j.annemergmed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]