Highlights
-
•
The arterial supply of the future liver remnant (FLR) may be important for the FLR growth.
-
•
Huge hepatocellular carcinoma (HCC) can cause blood-stream steal from the FLR, which can result in ALPPS failure.
-
•
Transarterial embolization (TAE) of HCC can salvage failed Stage I ALPPS.
-
•
TAE can induce significant hypertrophy of FLR.
-
•
TAE-salvaged ALPPS may be suitable for huge HCC with chronic liver disease.
Keywords: Hepatocellular carcinoma, ALPPS, Transhepatic arterial embolization, Fibrosis, Case report
Abstract
Introduction
The degree of hypertrophy of the future liver remnant (FLR) induced by associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in patients with HCC and chronic liver disease is often limited as compared with patients with a healthy liver.
Presentation of case
We reported a 53-year-old male who had a huge HCC (14.8 × 12 × 9.4 cm) arising from a background of hepatitis B liver fibrosis (METAVIR score F3). The ratio of the FLR/standard liver volume (SLV) was 23.8%. After stage I ALPPS, volumetric assessment on postoperative day (POD) 7 and 13 showed insufficient FLR hypertrophy (FLR/SLV: 28.7% and 30.7%, respectively). A postoperative computed tomographic 3D reconstruction and hepatic angiography showed steal of arterial blood from the FLR to the huge tumour in the right liver. Salvage transhepatic arterial embolization (TAE) was performed to block the major arterial blood supply to the tumour on POD 13. The FLR/SLV increased to 42.5% in 7 days. Stage II ALPPS consisting of right trisectionectomy was successfully performed.
Discussion
Salvage TAE which blocked the main arterial blood supply to the huge HCC improved the arterial supply with subsequent adequate and fast hypertrophy of the FLR to allow trisectionectomy in stage II ALPPS to be carried out.
Conclusion
Salvage TAE after failed stage I ALPPS with inadequate hypertrophy of the FLR allowed trisectionectomy in stage II ALPPS to be carried out in a patient with a huge HCC with chronic liver disease.
1. Introduction
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a new operation which has been reported to provide a chance of curative liver surgery to be carried out in patients who were previously considered to have unresectable liver tumours. Its surgical indications include patients with a huge (>10 cm) hepatocellular carcinoma (HCC) with inadequate volume of the future liver remnant (FLR). Portal vein embolization is probably not suitable for these patients because of the long wait of 6–8 weeks before the FLR has hypertrophied enough to allow liver resection to be carried out for the large tumour. The huge HCC might have progress to become unresectable in the waiting process. On the other hand, as HCC often arises from a background of cirrhosis, the degree of liver hypertrophy in cirrhotic liver may not be enough to allow a second resectional surgery to be carried out after the first stage of ALPPS [1], [2], [3], [4].
We reported a patient who had a huge HCC with a background of severe liver fibrosis treated in our hospital. After stage I ALPPS, there was insufficient hypertrophy of the FLR. Investigations showed there was inadequate arterial supply to the FLR due to steal of arterial flow to the huge HCC. Transhepatic arterial embolization (TAE) was performed to block the main arterial blood supply to the HCC and improved the arterial flow to the FLR. Significant liver hypertrophy was then achieved. Stage II ALPPS in the form of right trisectionectomy was then successfully performed. We presented this patient and discussed the potential implications of using salvage TAE in patients with huge HCC who failed to have adequate liver hypertrophy after stage I ALPPS. This case report was in line with the SCARE guidelines [5].
2. Cases presentation
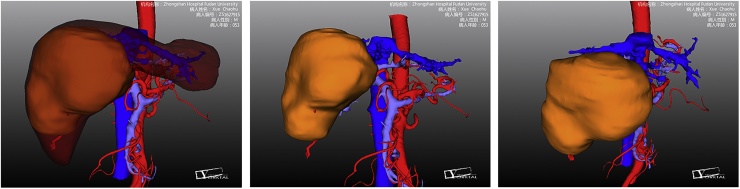
A 53-year-old male was diagnosed to have a huge HCC (14.8 × 12 × 9.4 cm) in liver segments 4, 5, 6, 7 and 8 (Fig. 1). There was no extrahepatic metastasis. The alpha-fetoprotein (AFP) level was 82.2 ng/ml. The preoperative Child-Pugh score was 5, and the MELD score was 2.58. The indocyanine green retention rate at 15 min was 5.5%. The preoperative fibroscan value of the left liver was 10.5Kpa.The estimated standard liver volume (SLV) was 1190.4 ml using the Urata formula [6]. Computed tomographic (CT) scan with volumetric assessment showed a FLR of 282.9 ml and the preoperative FLR/SLV ratio was 23.8%.The preoperative volumetric assessment showed an insufficient FLR to allow for right trisectionectomy. After a multidisciplinary team discussion, ALPPS was decided.
Fig. 1.
Preoperative 3D CT scan reconstruction shows a huge HCC measuring 14.8 × 12 × 9.4 cm in liver segments 4, 5, 6, 7 and 8.
2.1. Stage I ALPPS
Using a right subcostal abdominal incision, complete liver parenchymal transection was performed along the right border of the falciforn ligament using a CUSA. The right portal vein was doubly ligated with 3-0 Prolene sutures. A silicone drain was placed on the transection plane of the liver and the abdomen was closed. The operating time was 140 min and the estimated blood loss was 100 ml.
2.2. Volumetric assessment after stage I ALPPS
Volumetric assessment using 3-Dimensional (3D) CT reconstruction on postoperative day (POD) 7 after the Stage I operation showed the FLR increased from 282.9 ml to 342.2 ml. The kinetic growth rate (KGR) was 8.5 ml/d. The FLR/SLV ratio increased to 28.7%. The volumetric assessment was repeated on POD 13. The FLR increased from 342.2 ml to 365.3 ml and the FLR/SLV ratio was only 30.7%. The KGR was 3.8 ml/d. Since the FLR still did not meet the requirement for stage II ALPPS in our Department which required a FLR/SLV ratio of at least 35% in patients with liver fibrosis and cirrhosis, further investigations were carried out.
2.3. TAE to the supplying arteries of the tumour
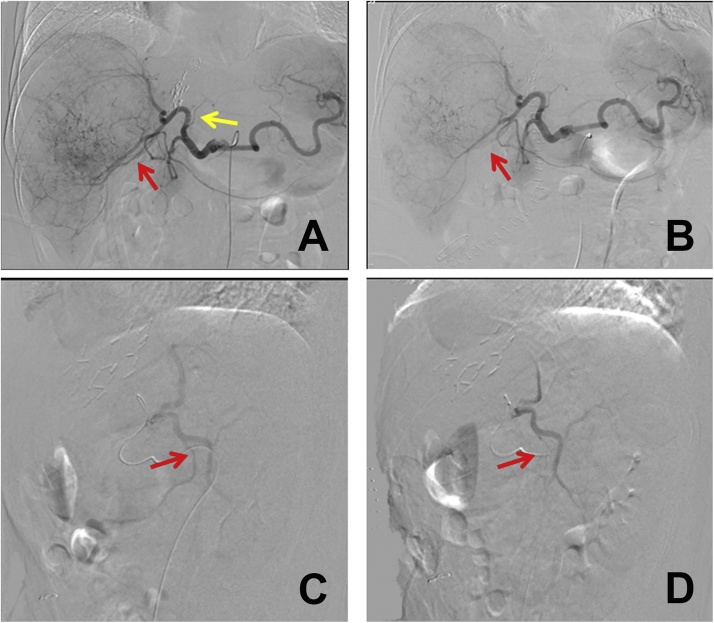
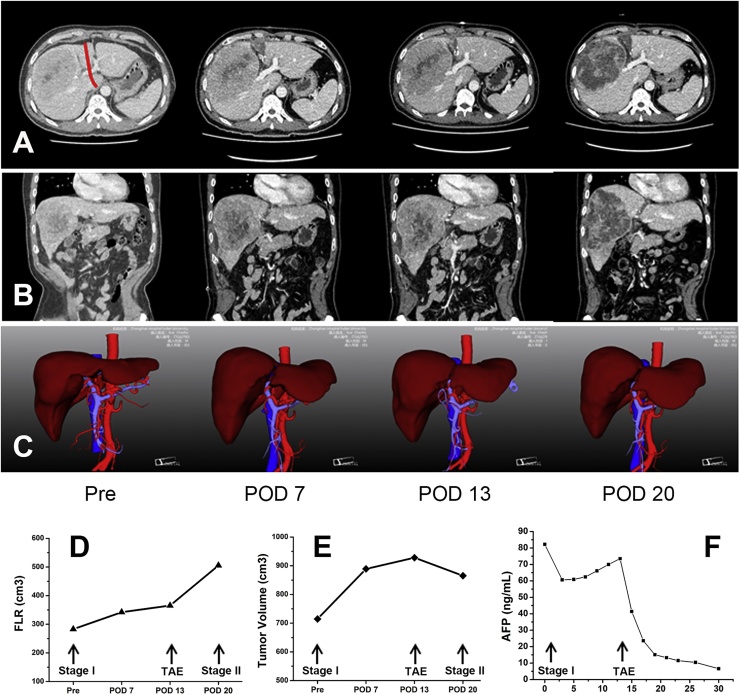
Investigations with hepatic angiography showed there was inadequate arterial blood supply to the FLR due to steal of the arterial flow to the huge tumour in the right liver which caused the limited hypertrophy of the FLR (Fig. 2). TAE was used to block the main arterial supply of the tumour which mainly originated from the anterior branch of the right hepatic artery. The flow in the left hepatic artery significantly improved after TAE. Volumetric assessment was repeated 7 days after TAE. The FLR significantly increased (Fig. 3) from 365.3 ml to 505.5 ml and the FLR/SLV ratio reached 42.5%. The KGR was as high as 20.0 ml/day. The FLR met the requirement and stage II ALPPS was performed.
Fig. 2.
Hepatic angiography confirmed the “arterial steal” and TAE was performed to block the main arterial supply to the tumour. The left haptic artery (yellow arrow) was almost not shown due to the “arterial steal” to the huge tumour, whereas the blood supply to the tumour was rich (A). The main artery supplying the tumour (red arrow) was embolized by super-selective TAE (B). The lateral view showed the main artery of the tumour (red arrow) after embolization using super-selective TAE (C, D).
Fig. 3.
CT scan and 3D reconstruction show volumes of the future liver remnant (FLR) before and on POD7, POD 13 and POD20 after stage I ALPPS (A, B, C). The FLR slowly increased after stage I ALPPS and rapidly enlarged after TAE (D). Meanwhile, the tumour volume increased after stage I ALPPS and decreased after TAE (E). The AFP levels decreased shortly after stage IALPPS but increased subsequently (F). Salvage TAE in ALPPS significantly decreased the AFP level to normal. (Pre, preoperative; POD, postoperative day).
2.4. Stage II ALPPS
Re-laparotomy was performed through the previous incision. The right hepatic artery, the right portal vein and the right bile duct were ligated and divided, followed by right trisectionectomy. The operating time was 160 min and the estimated blood loss was 300 ml. No blood transfusion was required. The liver function recovered well (Fig. 3). Histopathology confirmed a 14.8 × 12.0 × 9.4 cm HCC in the right liver. The liver parenchyma showed severe fibrosis (METAVIR score F3). The patient was discharged home on POD 10 after stage II ALPPS.
3. Discussion
In this patient, the HCC was accompanied with severe fibrosis (METAVIR score F3) and there was slow and limited hypertrophy of the FLR after stage I ALPPS. 3D CT reconstruction and hepatic angiography showed an enlarged HCC with steal of arterial blood flow from the FLR to the tumour. TAE, by blocking the main arterial supply of the tumour, successfully salvaged the failed stage I ALPPS. TAE significantly induced the hypertrophy of the FLR. The International ALPPS Registry reported that the absolute KGR in patients with fibrosis grade 1, 2, 3 were 12.3 ± 4.4, 4.7 ± 0.6, and 3.0 ± 0.7 ml/d, respectively [4]. The absolute KGR after TAE in our patient reached 20.0 ml/d, which was significantly higher than that reported in classical ALPPS on patients with HCC and fibrosis.
Portal vein embolization (PVE) has been shown to be useful in some patients with HCC and chronic liver disease. It is the standard preoperative procedure used in some centers to induce hypertrophy of the FLR. However, there are two major concerns on the use of PVE for such a purpose. These include the high failure rate and tumour progression during the long waiting time for resectional surgery. Furthermore, increased compensatory arterial flow after PVE has been shown to encourage tumour progression [7], [8], which may be one of the reasons for the failure of PVE. Our patient also showed tumour progression after stage I ALPPS. The volume of the tumour increased from preoperative 714.7 ml to 928.0 ml on POD 13 (Fig. 3E). The increase reached to 29.9% before stage II ALPPS. The AFP level decreased shortly after stage I ALPPS but increased from POD 7 onwards (Fig. 3F). After TAE, the tumour volume significantly decreased to 865.4 ml while the AFP level also rapidly decreased (Fig. 3F). TAE, by blocking the main arterial supply of the tumour, induced more rapid and significant hypertrophy which allowed subsequent successful resection of the tumour before it further progressed to unresectability. Recent systematic reviews showed that the median interval from PVE to surgery was 28 days (range 21–45 days) [9]. The mean hypertrophy rate of the FLR after PVE was 37.9 ± 0.1% with a median of 25.9 ± 10.1 days [10], [11]. In our patient, the hypertrophy rate of the FLR after salvage TAE reached 38.4% in 7 days. Salvage TAE clearly induced a greater degree of liver hypertrophy in the FLR within a shorter period of time than PVE.
This case showed that a huge HCC can steal arterial blood from the FLR and cause slow and limited hypertrophy of the FLR which potentially can lead to failure of stage I ALPPS. Salvage TAE, by blocking the main arterial supply to the tumour, should be carried out in an attempt to improve the arterial flow and hypertrophy of the FLR.
4. Conclusions
Salvage TAE should be considered in patients with a huge HCC and chronic liver disease in case of insufficient hypertrophy of the FLR after stage I ALPPS. We name this procedure as TAE-salvaged ALPPS. More studies are needed to confirm our findings.
Conflict of interest
The authors declare no conflict of interest.
Funding
National Science and Technology Major Project (2017ZX10203204).
National Natural Science Foundation of China (No. 81572296).
Ethical approval
The study was approved by Ethics committee of Zhongshan hospital of Fudan university.
Consent
Consent for publication has been obtained.
Author contributions
Jian Zhou, Zheng Wang, Yuanfei Peng, Qiman Sun, and Xudong Qu: patient management, surgery, TAE treatment.
Jia Fan, Jian Zhou, Zheng Wang, Yuanfei Peng, Min Tang, and Yajie Dai: analysis and interpretation of data.
Jian Zhou, Zheng Wang, and Yuanfei Peng: interpreted the results, and wrote the paper.
Zhaoyou Tang and Wan Yee Lau: critical comments and revised the manuscript.
Registration of research studies
Name of the registry: Salvage Transhepatic Arterial Embolization after Failed Stage I ALPPS in Patient with a Huge HCC with Chronic Liver Disease.
The unique identifying number (UIN): researchregistry2595.
Guarantor
Jian Zhou.
References
- 1.Chan A.C., Poon R.T., Chan C., Lo C.M. Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann. Surg. 2016;263:e14–e16. doi: 10.1097/SLA.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 2.Vennarecci G., Grazi G.L., Sperduti I., Busi Rizzi E., Felli E., Antonini M. ALPPS for primary and secondary liver tumors. Int. J. Surg. 2016;30:38–44. doi: 10.1016/j.ijsu.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Hong de F., Zhang Y.B., Peng S.Y., Huang D.S. Percutaneous microwave ablation liver partition and portal vein embolization for rapid liver regeneration: a minimally invasive first step of ALPPS for hepatocellular carcinoma. Ann. Surg. 2016;264:e1–2. doi: 10.1097/SLA.0000000000001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Haese J.G., Neumann J., Weniger M., Pratschke S., Björnsson B., Ardiles V. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann. Surg. Oncol. 2016;23:1335–1343. doi: 10.1245/s10434-015-5007-0. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P., Afifi R. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Urata K., Hashikura Y., Ikegami T., Terada M., Kawasaki S. Standard liver volume in adults. Transplant. Proc. 2000;32:2093–2094. doi: 10.1016/s0041-1345(00)01583-9. [DOI] [PubMed] [Google Scholar]
- 7.Ribero D., Curley S.A., Imamura H., Madoff D.C., Nagorney D.M., Ng K.K. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann. Surg. Oncol. 2008;15:986–992. doi: 10.1245/s10434-007-9731-y. [DOI] [PubMed] [Google Scholar]
- 8.Shindoh J.D., Tzeng C.W., Vauthey J.N. Portal vein embolization for hepatocellular carcinoma. Liver Cancer. 2012;1:159–167. doi: 10.1159/000343829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glantzounis G.K., Tokidis E., Basourakos S.P., Ntzani E.E., Lianos G.D., Pentheroudakis G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur. J. Surg. Oncol. 2016;43:32–41. doi: 10.1016/j.ejso.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Schadde E., Ardiles V., Robles-Campos R., Malago M., Machado M., Hernandez-Alejandro R. Early survival and safety of ALPPS: first report of the international ALPPS registry. Ann. Surg. 2014;260:829–838. doi: 10.1097/SLA.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 11.van Lienden K.P., van den Esschert J.W., de Graaf W., Bipat S., Lameris J.S., van Gulik T.M. Portal vein embolization before liver resection: a systematic review. Cardiovasc. Intervent. Radiol. 2013;36:25–34. doi: 10.1007/s00270-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]