Abstract
Social Disorganisation (SD) is a shared autistic and schizotypal phenotype that is present in the subclinical population. Auditory processing deficits, particularly in mismatch negativity/field (MMN/F) have been reported across both spectrum disorders. This study investigates differences in MMN/F cortical spatio-temporal source activity between higher and lower quintiles of the SD spectrum.
Sixteen low (9 female) and 19 high (9 female) SD subclinical adults (18–40years) underwent magnetoencephalography (MEG) during an MMF paradigm where standard tones (50ms) were interrupted by infrequent duration deviants (100ms).
Spatio-temporal source cluster analysis with permutation testing revealed no difference between the groups in source activation to the standard tone. To the deviant tone however, there was significantly reduced right hemisphere fronto-temporal and insular cortex activation for the high SD group (p= 0.038). The MMF, as a product of the cortical response to the deviant minus that to the standard, did not differ significantly between the high and low Social Disorganisation groups.
These data demonstrate a deficit in right fronto-temporal processing of an auditory change for those with more of the shared SD phenotype, indicating that right fronto-temporal auditory processing may be associated with psychosocial functioning.
Keywords: Mismatch negativity, Mismatch field, Magnetoencephalography, MNE, Autism, Schizotypy, Social Disorganisation
Highlights
-
•
Autism and schizotypal spectra share a trait phenotype, Social Disorganisation (SD).
-
•
Auditory mismatch paradigm demonstrates processing differences between high and low SD.
-
•
High SD scorers have reduced fronto-temporal response to auditory change.
-
•
Reduced fronto-temporal source activation in high SD is right lateralised.
-
•
Psychosocial function is related to auditory deviant processing.
1. Introduction
Social Disorganisation has previously been reported across the typically developing population (of normal mental ability) as a trait symptom phenotype that is shared by the autism and schizophrenia spectrum (Ford and Crewther, 2014). The trait phenotype comprises social and interpersonal difficulties that exist within the two spectra. The unconscious auditory change processing potential, the mismatch negativity (MMN), is elicited in response to an infrequent deviant among repeated standard stimuli. Deficits in the MMN, and its magnetoencephalography (MEG) measured counterpart the mismatch field (MMF), have been identified across the two spectrum disorders, particularly in response to auditory tone duration deviants (Shelley et al., 1991, Michie et al., 2000, Fulham et al., 2014, Andersson et al., 2013, Kujala et al., 2010). Reduced MMN/F amplitude has also been identified in those at-risk of developing schizophrenia (Brockhaus-Dumke et al., 2005, Atkinson et al., 2012, Hsieh et al., 2012, Murphy et al., 2013, Nagai et al., 2013), and those with high schizotypal spectrum traits (Hong et al., 2012).
There is increasing evidence that the MMN/F is a robust marker of global functioning (Fulham et al., 2014, Lee et al., 2014, Wynn et al., 2010), and cognitive deficits and decline (Naatanen et al., 2012). In schizophrenia studies, reduced MMN/F amplitude has also been associated with increased severity of psychosocial deficits, such as in negative symptoms (Lee et al., 2014, Fulham et al., 2014), social functioning (Fulham et al., 2014, Lee et al., 2014), social cognition (Hermens et al., 2010, Kawakubo et al., 2007, Wynn et al., 2010) and theory of mind (Lee et al., 2014). Psychosocial functioning is a core deficit of both autism spectrum disorders and Social Disorganisation, thus the relationship with the MMN/F may translate. In fact, recent evidence from our lab suggests a delay in MMF peak latency for those with more of the Social Disorganisation phenotype (Ford et al., 2017). This study utilises the cortical source analysis capacity of MEG to investigate source processing differences across the Social Disorganisation spectrum phenotype in the non-clinical population.
The MMN has been extensively studied as a fronto-central potential, and has long been suggested as a marker of the involuntary attention switch to auditory stimulus change (Naatanen, 1992, Giard et al., 1990, Alho, 1995). Due to the role of the frontal lobe in higher cognitive processes, the MMN has been suggested as a neural marker for executive function, visual-motor processing and motor speed, response inhibition and selective attention (Toyomaki et al., 2008).
Cortical generators of the MMN/F are located in the superior temporal gyrus (STG), specifically the primary auditory cortex, and are predominately right lateralised (Alho, 1995, Giard et al., 1990). Regions of the STG are responsible for the reception and processing of auditory stimuli, as well as perceptual and working memory functions (Schonwiesner et al., 2007, Opitz et al., 2005, Alho et al., 1998, Shelley et al., 1991, Giard et al., 1990).
MMN/F generators have also been identified in the prefrontal cortex, particularly the mid-ventrolateral prefrontal cortex (VLPFC) that is partially located in the inferior frontal gyrus (IFG), which indexes the involuntary attention switch to auditory change, allocation of attentional resources, response inhibition and updating of the prediction model (Schonwiesner et al., 2007, Alho, 1995, Naatanen, 1992, Giard et al., 1990, Garrido et al., 2008, Rinne et al., 2005). The right hemisphere IFG has also been suggested to have forward and backward connections with the STG, these connections are thought to be involved in establishing and modulating the prediction model (Garrido et al., 2008).
The MMN has been shown to be larger in the right hemisphere, which has been thought to indicate right lateralisation of sensory working memory in relation to the physical and temporal features of sound (Alho, 1995, Giard et al., 1995). Furthermore, the right hemisphere is heavily involved in the processing of prosody and the paralinguistic aspects of language (Lindell, 2006), and the MMN itself has been related to the perception of prosody (Kujala et al., 2007, Korpilahti et al., 2007). In sum, bilateral auditory change detection in the primary auditory cortex triggers a preattentive reorienting response in the right frontal lobes, which then feeds back to the auditory sensory regions (Garrido et al., 2008, Rinne et al., 2005, Giard et al., 1990).
The MMF recorded using MEG allows for improved signal to noise (Thonnessen et al., 2008), as well as a more source specific analysis of auditory cortex response (Alho, 1995, Hillebrand and Barnes, 2002). Typically, MEG studies investigating MMF differences in autism and schizophrenia spectrum samples report specific source current dipoles. However, due to the temporal (time) and spatial progression of cortical change detection from temporal to frontal regions it is important to investigate such spatio-temporal differences in the processing of auditory change.
There are a number of factors that may contribute to the inconsistency in MMN/F characteristics within autism studies. First, there is significant heterogeneity in autism, which leads to within and between study sample variability (Ford and Crewther, 2016). Second, psychoactive medication effects neurotransmitter systems involved in the MMN/F (Gomot et al., 2000, Javitt et al., 1996). Third, typically developing children tend to have increased temporal component of the MMN/F compared to adults (Gomot et al., 2000). Eliminating the effects of disorder heterogeneity, age and medication is necessary to identify specific neural correlates of symptom phenotypes. Identification of such markers is important for the development of targeted symptom treatment.
The Social Disorganisation trait phenotype was isolated through factor analysis of schizotypal and autistic traits within a non-clinical, unmedicated, adult population (Ford and Crewther, 2014). Due to the spectrum nature of autism and schizophrenia, utilising groups with varying degrees of the Social Disorganisation phenotype might prove valuable to elucidate neural correlates of the interpersonal and social impairments that are pervasive across the autism and schizophrenia spectra, while removing the complication of psychiatric medications.
As MMN/F deficits have been identified across the autism and schizophrenia spectra, and have been associated with increased severity in psychosocial functioning (Fulham et al., 2014, Lee et al., 2014, Hermens et al., 2010, Kawakubo et al., 2007, Wynn et al., 2010), this study investigated differences in spatio-temporal source activation to an auditory deviant in duration for those with a high degree of the Social Disorganisation phenotype compared to those with low Social Disorganisation. It was hypothesised that cortical response to the deviant tone would be larger across temporal and frontal regions compared to the standard tone response for both Social Disorganisation groups. With respect to group differences, it was predicted that those with high Social Disorganisation would have a reduced superior temporal and inferior frontal response to the deviant, particularly over the right hemisphere, compared to the low Social Disorganisation. Finally, in combination with no difference in response to the standard tone, it was predicted that those with high Social Disorganisation would have a reduced MMF in the superior temporal and inferior frontal regions, particularly within the right hemisphere.
2. Methods
The Swinburne University Human Research Ethics Committee approved this study in accordance with the 1964 Declaration of Helsinki. All participants provided written informed consent to participate in the study. MMF data were acquired within a larger experimental battery; only the MMF-related methodology is reported below. Participant recruitment and data collection methods have been detailed elsewhere (Ford et al., 2017).
2.1. Participants
In brief, items from the Autism Spectrum Quotient (AQ), Schizotypal Personality Questionnaire (SPQ), Coolidge Axis II Inventory (CATI+) Schizotypy and Schizoid scales, and Short Eysenck Personality Questionnaire–Revised Lie scale (EPQ–RL) were combined and pseudo-randomised to create an Autism Schizotypy Questionnaire (ASQ). The ASQ was presented on a 4-point Likert scale from 1 (strongly disagree) to 4 (strongly agree) and was completed by 428 males and 1250 females, aged 18 to 40 years. Only AQ and SPQ data underwent further analysis.
Principal axis factor analysis of the AQ and SPQ subscales with a promax (oblimin) rotation revealed the Social Disorganisation factor as reported in Ford and Crewther (2014). The Social Disorganisation factor comprised the AQ subscales Social Skills, Communication and Attention Switching, and SPQ subscales No Close Friends, Constricted Affect, Social Anxiety and Odd Behaviour (see Ford et al., 2017, for full factor structure), as well as weaker contributions from Suspiciousness, Ideas of Reference and Imagination. For each participant, the standardised regression score (z-score) for Social Disorganisation was calculated, with those in the top (high group) and bottom (low group) quintile of z-scores recruited to participate in the MEG study.
A total of 18 low Social Disorganisation and 19 high Social Disorganisation scorers that participated underwent MEG (demographics in Table 1). All participants were free of illicit drug and cigarette effects at the time of scan and none reported hearing difficulties. None of the participants was taking psychiatric medication at the time of the study, although five participants in the high Social Disorganisation group reported a past history of psychiatric illness (3 depression, 1 bipolar, 1 anorexia).
Table 1.
Participant demographic descriptives.
| Low |
High |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| n | 7 | 9 | 10 | 9 |
| Age | 24.7(7.02) | 22.6(4.64) | 22.2(4.61) | 22.0(4.95) |
| Social Dis. (z-score) | −1.30(0.29) | −1.48(0.33) | 1.50(0.54) | 1.42(0.53) |
| AQ (/200) | 92.6(7.41) | 92.2(3.83) | 130.2(10.16) | 132.6(11.15) |
| SPQ (/296) | 112.3(10.55) | 107.2(8.79) | 187.1(16.53) | 188.0(19.22) |
| RAPM-S (/12) | 5.6(2.76) | 8.0(1.58) | 7.1(1.85) | 6.8(2.54) |
Notes. Social Dis. = Social Disorganisation, AQ = Autism Spectrum Quotient, SPQ = Schizotypal Personality Questionnaire, RAPM-S = Raven's Advanced Progressive Matrices–Short form.
The 12-item Raven's Advanced Progressive Matrices–Short form (RAPM-S) was used as a general intelligence measure (Cronbach's α= 0.69, test-retest reliability= 0.75, average score= 8.1 (SD= 2.5)) (Arthur and Day, 1994, Raven et al., 1998). RAPM-S scores were obtained by summing the number of correct responses given within 10 min.
2.2. MMF stimuli
The MMF paradigm consisted of three blocks of repeated standard tones (50 ms, 1000 Hz, 85% of stimuli) randomly interrupted by 60 duration deviants (100 ms, 1000 Hz, 15% of stimuli). The inter-stimulus interval was 500 ms and each block lasted 4–5 min. MMF paradigm blocks were presented between stimulus blocks of an attention-directed auditory paradigm not discussed herein. Stimuli were created using VPixx version 3.0 with DataPixx (VPixx Technologies Inc., 2016) and calibrated with a Jay Cam sound pressure level meter to 85 dB SPL. Tones were presented binaurally through Etymotic headphones. During the MMF blocks, participants watched a silent landscapes film. The MMF paradigm adhered to the recommendations of Duncan et al. (2009).
2.3. MEG recording
MEG recording methods have been described elsewhere (Ford et al., 2017). In brief, continuous MEG recordings were performed within the Neuroimaging Facility at Swinburne University of Technology with a whole-head 306 channel Elekta Neuromag® TRIUX magnetometer system (Helsinki, Finland: Elekta, 2016). Recordings were taken at a sampling rate of 1000 Hz with online high-pass filtering at 0.1 Hz, and were stored for later offline analysis. Recordings took place in magnetically shielded room (MSR) with active external shielding on. Head position with respect to the magnetometers and gradiometers was tracked with five continuous head position indicator (cHPI) coils; three coils attached to the forehead and one to each mastoid. Three fiducial positions (nasion and pre-auricular points), the cHPIs, and participant head shape (including the forehead, nose and eye sockets) were digitised using the Polhemus FASTRAK head digitising system (Polhemus Inc., Colchester, VT, United States of America). Electrocardiography (ECG) was recorded from the left and right wrist for heart beat artefact removal, and electrooculography (EOG) was recorded from the supraorbital ridge and the infraorbital ridge of the right eye for blink artefact rejection. A ground electrode was positioned on the right elbow.
2.4. Structural T1 acquisition
Participant structural T1 images were acquired from a 3T Siemens TIM Trio magnetic resonance imaging (MRI) system (Siemens, 2016, Erlangen, Germany, 32-channel head coil acquisition system) in order to co-register individual MEG data to cortical surfaces. T1-weighted images were acquired on a sagittal plane with a magnetisation preprepared rapid gradient echo (MP-RAGE) pulse sequence with an inversion recovery (176 slices, slice thickness = 1.0 mm, voxel resolution = 1.0 mm3, TR = 1900 ms, TE = 2.52 ms, TI = 900 ms, bandwidth = 170 Hz /Px, flip angle= 9°, field of view 350 mm x 263 mm x 350 mm, orientation sagittal, acquisition time = 5 min).
2.5. MEG data analysis
Temporal signal space separation filtering with initial head position, bad channels removed and default parameters was conducted on raw data with MaxFilter Version 2.1 (Helsinki, Finland Elekta, 2016). Following MaxFilter, remaining bad channels were identified using in-built software that plots the variance of each channel and event epoch; on average 12% of channels were excluded per participant. The data of one male and one female participant from the low scoring group was corrupted. Noisy epochs (−200 ms pre-stimulus to 500 ms post-stimuli onset) were identified by eye using the software above; on average 18% of epochs were excluded per participant. Head movement from the beginning to end of block was minimal and did not differ between groups (in mm, low group = 3.63(5.33), high group = 3.41(4.53)).
Preprocessing, coregistration and source space analysis of MEG data was conducted using the MNE-Python software (Gramfort et al., 2013). MaxFiltered raw data were filtered offline with a 40 Hz low-pass and epochs were taken from −200 ms pre-stimulus to 500 ms post-stimuli onset. Epochs were baseline corrected from −200 ms to 0 ms and all noisy epochs identified previously were removed. The data were then grouped according to stimulus type and averaged for each participant. Only the deviant stimuli (171 per participant, on average) and the standard stimuli immediately preceding a deviant (176 per participant, on average) were used for subsequent analyses.
T1-weighted images were processed with Freesurfer, where cortical reconstruction and volumetric segmentation were performed (http://surfer.nmr.mgh.harvard.edu/). Segmentation of grey and white matter was conducted by defining the border between grey and white matter as the surface boundary. The source space was obtained by tessellation and inflation of the cortical surface using the MNE-Python software (Gramfort et al., 2014).
Coregistration of the MEG data to the T1-weighted structural image was conducted by aligning the digitised cortical landmark points (nasion, and right and left pre-auricular points) to the structural image. The digitised head shape was then coregistered to individual participant scalp surfaces using an iterative closest point algorithm (Gramfort et al., 2014).
The forward solution was obtained automatically, with 5 mm separation between dipole sources to obtain the gain matrix (Gramfort et al., 2014). The gain matrix was then used to obtain the inverse solution. The density of the cortical current source was calculated using the minimum-norm estimate (MNE), which accounts for the superficial source bias by applying a depth-weighting scheme that is based on the source covariance matrix (Gramfort et al., 2014). Additionally, dynamic statistical parametric mapping (dSPM) was used to calculate the noise-normalised linear inverse estimates, which further reduces source location bias (Dale et al., 2000, Gramfort et al., 2014).
2.6. MEG statistical analysis
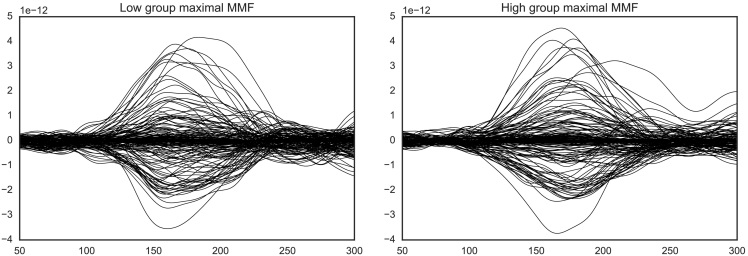
To examine group spatio-temporal cluster differences in response to the standard and deviant stimuli, as well as differences in the MMF, a non-parametric spatio-temporal clustering method, controlling for multiple comparisons (Maris and Oostenveld, 2007), was implemented with MNE-Python (Gramfort et al., 2014). The MMF for each group was calculated by subtracting the deviant stimuli source estimate from the standard stimuli source estimate. Briefly, source clusters were defined based on their temporal and spatial adjacency; sensors were considered neighbours when they were within 4 cm from each other. The time window of interest was defined based on the time series of the MMF; Fig. 1 illustrates the MMF between 100 ms and 250 ms.
Fig. 1.
The MMF from bilateral frontal and temporal magnetometers (top) and gradiometers (bottom) demonstrating maximal activation between 100ms and 250ms for low (left) and high (right) Social Disorganisation groups.
The source-cluster permutation test was conducted by constructing a distribution of F-statistics for each stimulus condition at each cortical source vertex, this was done by random partitioning of the source data over 1000 times (Monte Carlo estimation). The F-statistic of the actual group difference was significant when it was larger than the F-threshold (6.73), which was calculated based on the number of participants and the primary threshold (0.0005). The p-value indicated the proportion of permuted F-statistics that were greater than the actual F-statistic, p-values less than the critical alpha (0.05) indicated a significant group difference between spatio-temporal clusters.
3. Results
3.1. Demographic data
There was no significant difference in age (t(28.9)=−0.78, p= 0.44) or RAPM-S performance (t(30.3)= 0.01, p= 0.99) between the high and low Social Disorganisation groups. Independent samples t-tests confirmed no differences in RAPM-S performance, or AQ, SPQ and Social Disorganisation scores between males and females within each group (all p > 0.05, Table 1).
3.2. Standard and deviant stimulus group differences
Non-parametric spatio-temporal cluster-based analyses with Monte Carlo permutations to correct for multiple comparisons were employed to examine the cortical source activation differences between the high and low Social Disorganisation groups in response to standard and deviant stimuli. Cortical source cluster differences between the group were defined based on their spatial (within 4 cm of an active neighbour) and temporal (within 100 ms–250 ms time window) adjacency.
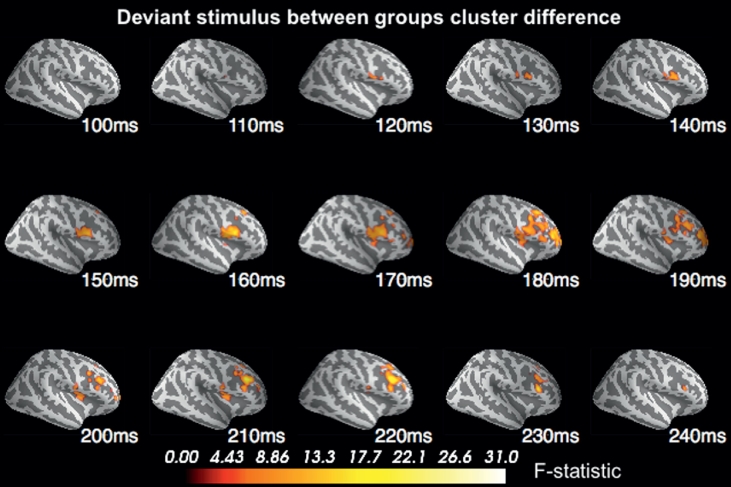
In response to the standard stimulus, the F-test revealed no significant source cluster differences between the high and low Social Disorganisation groups (p > 0.05). In response to the deviant stimulus however, the F-test revealed one cluster of sources in which the response was significantly lower for the high compared to the low Social Disorganisation group (p= 0.038). The difference in source activity between the Social Disorganisation groups was lateralised to the right hemisphere. Fig. 2 illustrates the temporal progression of the difference in source activation, beginning at 120 ms at the right medial IFG, with the difference between the groups increasing and becoming more widespread superiorly over time to encapsulate more of the VLPFC and extend to the dorsolateral prefrontal cortex (DLPFC) by 180 ms, as well as extending to the insular cortex. Reduced source activity for the high Social Disorganisation group was sustained in the mid-IFG region from 130 ms to 170 ms and the mid-DLPFC region from 180 ms to 220 ms.
Fig. 2.
The cluster of significantly different source activation from 100 ms to 250 ms to the duration deviant, with reduced activation for the high Social Disorganisation group compared to the low group (p= 0.038).
3.3. Mismatch field group differences
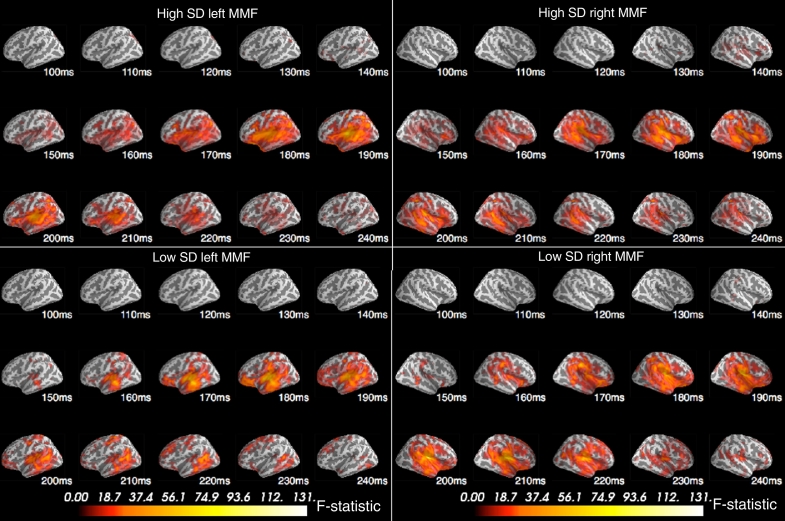
For both high and low Social Disorganisation groups, source activity was significantly higher in response to the deviant compared to the standard tone in a right and left hemisphere cluster, which is indicative of a significant MMF (p < 0.001, see Fig. 3). The difference in response initially reached significance at 150 ms for both the low and high Social Disorganisation groups, and was generally located in the superior temporal, and inferior frontal and parietal regions, which indicates deviant-specific activation in these areas. Despite visual differences between the high and low group MMF clusters, there were no significant differences in MMF (deviant minus standard) spatio-temporal source clusters between the groups (p > 0.05).
Fig. 3.
High (top) and low (bottom) Social Disorganisation significant spatio-temporal cluster differences between standard and deviant stimuli (mismatch field; MMF) for the left (left) and right (right) hemisphere from 100 ms to 250 ms (p < 0.001).
4. Discussion
This study is the first to investigate differences in cortical sources involved in auditory change processing between those high and low on the shared trait phenotype, Social Disorganisation. The key finding of this study was of significantly reduced cortical source response to the duration deviant in the frontal and insular cortex regions of the right hemisphere for those high on Social Disorganisation. These findings extend from those reporting an association between reduced MMN/F amplitude and increased impairment in psychosocial functioning (Lee et al., 2014, Fulham et al., 2014, Hermens et al., 2010, Kawakubo et al., 2007, Wynn et al., 2010). Additional findings were of larger and stronger activation across temporal, frontal and parietal sources in response to the deviant stimulus compared to the standard for both groups, reflecting a significant MMF as expected. Finally, contrary to expectations, there was no significant difference in MMF source cluster activity between the high and low Social Disorganisation groups.
Reduced right hemisphere activation difference in response to the duration deviant tone for the high Social Disorganisation group was first apparent in the mid-IFG, which is triggered by sensory detection in temporal regions via afferent projections and is involved in change processing (Schonwiesner et al., 2007). The left hemisphere mid-IFG is home to Broca's area, the speech production centre of the cortex. The role of the equivalent right hemisphere region is less well understood, but is thought to play a role in inhibitory control (Hampshire et al., 2010) and repetition suppression and enhancement (Recasens et al., 2015). These processes are required for effective feed-forward and feed-backward connections, which operate between the right STG and right IFG to establish a prediction model according to the predictive coding framework (Garrido et al., 2008, Schonwiesner et al., 2007). According to this framework, the right IFG activation deficit found in this study indicates disruption to these feed-forward and/or feed-back connections. Disruption to feed-forward connections compromise the establishment of the prediction model in the IFG, thereby reducing the prediction error response in the event of a deviant. Conversely, feed-back deficits disrupt formation of the prediction model in the STG, leading to a deficit in prediction error at the sensory level.
Reduced spatio-temporal activation for the high Social Disorganisation group was also detected in the right insular cortex from 160 ms. The insular cortex is widely connected in the cortex, relaying information to and from somatosensory, auditory and visual areas, as well as the amygdala, which is largely implicated in emotion and fear processing (Craig, 2009). In fact, the insular cortex itself plays a central role in social cognition and emotion processing (Radeloff et al., 2014, Craig, 2009), which are key deficits of Social Disorganisation. Structural changes in the insular cortex have also been identified in autism and schizophrenia patients (Radeloff et al., 2014). Finally, the insular cortex makes up part of the secondary auditory cortices, and has connections to the STG and dorsal superior temporal sulcus (Hackett, 2015). Connections to or from these regions may therefore be disrupted for those high on Social Disorganisation, which may subsequently disrupt feed-back pathways from the IFG to the STG. Together, these data demonstrate a reduced response to the deviant that is initially located in the mid-IFG for those with more of the Social Disorganisation phenotype. This may suggest a deficit in the feed-forward connection from the auditory processing regions to the IFG, as well as a feed-back deficit as indicated by later insular cortex activity reduction.
Compromised feed-forward and feed-back connections between sensory detection and processing regions may have a downstream effect on higher order processing, as indicated by reduced DLPFC source activation for the high group. The DLPFC is more specifically involved in higher order processes, such as memory and decision making. Furthermore, the right frontal lobe has been associated with the processing of prosody and the paralinguistic aspects of language (Lindell, 2006). Altogether, these data suggest that disruption in the feed-forward and feed-back connections from the right auditory cortices to right frontal regions might have downstream effects on higher order change processing and attention switching.
Group differences in auditory change processing are in line with studies that demonstrate MMN amplitude reduction with increased severity of global functioning and social and interpersonal deficits (Lee et al., 2014, Fulham et al., 2014, Hermens et al., 2010, Kawakubo et al., 2007, Wynn et al., 2010). Therefore, the downstream effect of disrupted duration deviant processing may manifest itself as psychosocial functioning and behavioural characteristics of the Social Disorganisation phenotype.
For both groups, MMF source cluster activation progressed bilaterally from superior and middle temporal regions to frontal, parietal and inferior temporal regions. This progression of activity is the function of the difference in cortical response to the deviant minus that of the standard, indicating the auditory processing path unique to deviance detection and processing. These regions of activation are consistent with deviant-related activity in Heschl's gyrus, the STG, superior temporal sulcus and planum temporale, and the mid-VLPFC in the IFG (Schonwiesner et al., 2007, Recasens et al., 2014); areas that are involved in auditory change detection, processing, and novelty assessment for attention, respectively (Schonwiesner et al., 2007).
Despite clear differences in source cluster response to the deviant stimulus alone, the MMF source cluster (deviant minus standard response) differed slightly visually, but not statistically, between the high and low Social Disorganisation groups. This lack in significant difference may be due to a sub-threshold difference in response to the standard stimulus, or due to the reduction in the signal as a function of subtraction leading to a reduced signal to noise for the MMF data. Visual inspection indicates less regional specificity of the MMF for those with more of the Social Disorganisation phenotype, with the high group recruiting additional occipital and parietal resources. In addition, progression of cortical activation occurred slightly earlier for those with more of the Social Disorganisation phenotype. Together, this may indicate a lack of regional specificity for change processing, leading to aberrant processing of consistent and/or novel stimuli. Although there were no differences in cluster response to the standard stimuli between the two groups, the lack of significance difference in MMF source clusters between the groups may have been due to sub-threshold differences in source cluster activation to the standard stimulus.
Potential limitations to this study are as follows: first, the objective of the current research was to identify source activation differences between those at the high and low ends of the Social Disorganisation spectrum. Although it would be interesting to investigate stepwise progression of spatio-temporal change processing by including a mid-scoring group, it was not the aim of this paper; comparisons between high and low groups provided optimal conditions for identifying group source cluster differences to the standard and deviant stimuli. Second, the recruitment of participants was dependent upon accurate self-report responses to the AQ and SPQ. Such accuracy requires introspection, a quality that may be lacking to some degree for those with more Social Disorganisation characteristics. However, selecting groups of those at the high and low ends of the spectrum is more than likely to minimise the small degree of inaccuracy.
This study is the first to investigate cortical source cluster differences in response to an auditory change between non-clinical, unmedicated individuals with high and low levels of the shared autistic and schizotypal trait, Social Disorganisation. Results demonstrate that those with high Social Disorganisation traits have deviant processing deficits across the right fronto-temporal processing network, particularly in the right IFG and insular cortex. These deficits may have downstream effects on psychosocial functioning and behaviour. These data highlight the need to measure or control for spectrum traits in clinical and non-clinical studies, and to further investigate trait-level differences in cortical processes related to shared and differential symptom phenotypes as potential targets for clinical interventions.
Abbreviations
AQ = Autism Spectrum Quotient, ASQ = Autism Schizotypy Questionnaire, cHPI = continuous head position indicator, DLPFC = dorsolateral prefrontal cortex, IFG = inferior frontal gyrus, MMN/F = mismatch negativity/field, RAPM–S = Raven's Advanced Progressive Matrices–Short form, SPQ = Schizotypal Personality Questionnaire, STG = superior temporal gyrus, VLPFC = ventrolateral prefrontal cortex.
Acknowledgements
This work was supported by the National Health and Medical Research Council of Australia, APP1004740.
Contributor Information
Talitha C. Ford, Email: tcford@swin.edu.au.
Will Woods, Email: wwoods@swin.du.au.
David P. Crewther, Email: dcrewther@swin.edu.au.
References
- Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Alho K., Winkler I., Escera C., Huotilainen M., Virtanen J., Jaaskelainen I.P., Pekkonen E., Ilmoniemi R.J. Processing of novel sounds and frequency changes in the human auditory cortex: magnetoencephalographic recordings. Psychophysiology. 1998;35:211–224. [PubMed] [Google Scholar]
- Andersson S., Posserud M.-B., Lundervold A.J. Early and late auditory event-related potentials in cognitively high functioning male adolescents with autism spectrum disorder. Res. Autism Spectr. Disord. 2013;7:815–823. [Google Scholar]
- Arthur W., Day D.V. Development of a short form for the Raven Advanced Progressive Matrices test. Educ. Psychol. Meas. 1994;54:394–403. [Google Scholar]
- Atkinson R.J., Michie P.T., Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol. Psychiatry. 2012;71:98–104. doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A., Tendolkar I., Pukrop R., Schultze-Lutter F., Klosterkotter J., Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr. Res. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel-now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Liu A.K., Fischl B.R., Buckner R.L., Belliveau J.W., Lewine J.D., Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Duncan C.C., Barry R.J., Connolly J.F., Fischer C., Michie P.T., Naatanen R., Polich J., Reinvang I., Van Petten C. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Elekta . 2016. Elekta instrument AB Stockholm. [Google Scholar]
- Ford T.C., Crewther D.P. Factor analysis demonstrates a common schizoidal phenotype within autistic and schizotypal tendency: implications for neuroscientific studies. Front. Psych. 2014;5 doi: 10.3389/fpsyt.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford T.C., Crewther D.P. A comprehensive review of the 1H-MRS metabolite spectrum in autism spectrum disorder. Front. Mol. Neurosci. 2016;9 doi: 10.3389/fnmol.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford T.C., Woods W., Crewther D.P. Mismatch field latency, but not power, may mark a shared autistic and schizotypal trait phenotype. Int. J. Psychophysiol. 2017;116:60–67. doi: 10.1016/j.ijpsycho.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Fulham W.R., Michie P.T., Ward P.B., Rasser P.E., Todd J., Johnston P.J., Thompson P.M., Schall U. Mismatch negativity in recent-onset and chronic schizophrenia: a current source density analysis. PLoS One. 2014;9:e100221. doi: 10.1371/journal.pone.0100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido M.I., Friston K.J., Kiebel S.J., Stephan K.E., Baldeweg T., Kilner J.M. The functional anatomy of the MMN: A DCM study of the roving paradigm. Neuroimage. 2008;42:936–944. doi: 10.1016/j.neuroimage.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard M.H., Lavikahen J., Reinikainen K., Perrin F., Bertrand O., Pernier J., Näätänen R. Separate representation of stimulus frequency, intensity, and duration in auditory sensory memory: an event-related potential and dipole-model analysis. J. Cogn. Neurosci. 1995;7:133–143. doi: 10.1162/jocn.1995.7.2.133. [DOI] [PubMed] [Google Scholar]
- Giard M.-H., Perrin F., Pernier J., Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Gomot M., Giard M.-H., Roux S., Barthelemy C., Bruneau N. Maturation of frontal and temporal components of mismatch negativity (MMN) in children. Neurophysiol. Basic Clin. 2000;11:3109–3112. doi: 10.1097/00001756-200009280-00014. [DOI] [PubMed] [Google Scholar]
- Gramfort A., Luessi M., Larson E., Engemann D.A., Strohmeier D., Brodbeck C., Goj R., Jas M., Brooks T., Parkkonen L., Hämäläinen M. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013;7 doi: 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A., Luessi M., Larson E., Engemann D.A., Strohmeier D., Brodbeck C., Parkkonen L., Hämäläinen M.S. MNE software for processing MEG and EEG data. NeuroImage. 2014;86:446–460. doi: 10.1016/j.neuroimage.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett T.A. Anatomic organization of the auditory cortex. Handb. Clin. Neurol. 2015;129:27–53. doi: 10.1016/B978-0-444-62630-1.00002-0. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens D.F., Ward P.B., Hodge M.A., Kaur M., Naismith S.L., Hickie I.B. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G. A quantitative assessment of the sensitivity of whole-head MEG to activity in the adult human cortex. NeuroImage. 2002;16:638–650. doi: 10.1006/nimg.2002.1102. [DOI] [PubMed] [Google Scholar]
- Hong L.E., Moran L.V., Du X., O’Donnell P., Summerfelt A. Mismatch negativity and low frequency oscillations in schizophrenia families. Clin. Neurophysiol. 2012;123:1980–1988. doi: 10.1016/j.clinph.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M.H., Shan J.C., Huang W.L., Cheng W.C., Chiu M.J., Jaw F.S., Hwu H.G., Liu C.C. Auditory event-related potential of subjects with suspected pre-psychotic state and first-episode psychosis. Schizophr. Res. 2012;140:243–249. doi: 10.1016/j.schres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Javitt D.C., Steinschneider M., Schroeder C.E., Arezzo J.C. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc. Natl. Acad. Sci. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y., Kamio S., Nose T., Iwanami A., Nakagome K., Fukuda M., Kato N., Rogers M.A., Kasai K. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007;152:261–265. doi: 10.1016/j.psychres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Korpilahti P., Jansson-Verkasalo E., Mattila M.L., Kuusikko S., Suominen K., Rytky S., Pauls D.L., Moilanen I. Processing of affective speech prosody is impaired in Asperger syndrome. J. Autism Dev. Disord. 2007;37:1539–1549. doi: 10.1007/s10803-006-0271-2. [DOI] [PubMed] [Google Scholar]
- Kujala T., Aho E., Lepistö T., Jansson Verkasalo E., Nieminen von Wendt T., von Wendt L., Näätänen R. Atypical pattern of discriminating sound features in adults with Asperger syndrome as reflected by the mismatch negativity. Biol. Psychol. 2007;75:109–114. doi: 10.1016/j.biopsycho.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Kujala T., Kuuluvainen S., Saalasti S., Jansson-Verkasalo E., von Wendt L., Lepisto T. Speech-feature discrimination in children with Asperger syndrome as determined with the multi-feature mismatch negativity paradigm. Clin. Neurophysiol. 2010;121:1410–1419. doi: 10.1016/j.clinph.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Sung K., Lee K.S., Moon E., Kim C.G. Mismatch negativity is a stronger indicator of functional outcomes than neurocognition or theory of mind in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:213–219. doi: 10.1016/j.pnpbp.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Lindell A.K. In your right mind: right hemisphere contributions to language processing and production. Neuropsychol. Rev. 2006;16:131–148. doi: 10.1007/s11065-006-9011-9. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Michie P.T., Budd T., Todd J., Rock D., Wichmann H., Box J., Jablensky A.V. Duration and frequency mismatch negativity in schizophrenia. Clin. Neurophysiol. 2000;111:1054–1065. doi: 10.1016/s1388-2457(00)00275-3. [DOI] [PubMed] [Google Scholar]
- Murphy J., Rawdon C., Kelleher I., Twomey D., Markey P., Cannon M., Roche R. Reduced duration mismatch negativity in adolescents with psychotic symptoms: further evidence for mismatch negativity as a possible biomarker for vulnerability to psychosis. BMC Psych. 2013;13:45. doi: 10.1186/1471-244X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R. L. Erlbaum; Hillsdale, N.J: 1992. Attention and brain function. [Google Scholar]
- Naatanen R., Kujala T., Escera C., Baldeweg T., Kreegipuu K., Carlson S., Ponton C. The mismatch negativity (MMN)-A unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin. Neurophysiol. 2012;123:424–458. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Nagai T., Tada M., Kirihara K., Yahata N., Hashimoto R., Araki T., Kasai K. Auditory mismatch negativity and P3a in response to duration and frequency changes in the early stages of psychosis. Schizophr. Res. 2013;150:547–554. doi: 10.1016/j.schres.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Opitz B., Schroger E., von Cramon D.Y. Sensory and cognitive mechanisms for preattentive change detection in auditory cortex. Eur. J. Neurosci. 2005;21:531–535. doi: 10.1111/j.1460-9568.2005.03839.x. [DOI] [PubMed] [Google Scholar]
- Radeloff D., Ciaramidaro A., Siniatchkin M., Hainz D., Schlitt S., Weber B., Poustka F., Bolte S., Walter H., Freitag C.M. Structural alterations of the social brain: a comparison between schizophrenia and autism. PLoS One. 2014;9(9):e106539. doi: 10.1371/journal.pone.0106539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J.C., Raven J., Court J.H. Harcourt Assessment; San Antonio, TX: 1998. Manual for Raven's Progressive Matrices and Vocabulary Scales. Section 4: The Advanced Progressive Matrices. [Google Scholar]
- Recasens M., Grimm S., Capilla A., Nowak R., Escera C. Two sequential processes of change detection in hierarchically ordered areas of the human auditory cortex. Cereb. Cortex. 2014;24(1):143–153. doi: 10.1093/cercor/bhs295. [DOI] [PubMed] [Google Scholar]
- Recasens M., Leung S., Grimm S., Nowak R., Escera C. Repetition suppression and repetition enhancement underlie auditory memory-trace formation in the human brain: an MEG study. Neuroimage. 2015;108:75–86. doi: 10.1016/j.neuroimage.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Rinne T., Degerman A., Alho K. Superior temporal and inferior frontal cortices are activated by infrequent sound duration decrements: an fMRI study. Neuroimage. 2005;26:66–72. doi: 10.1016/j.neuroimage.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Schonwiesner M., Novitski N., Pakarinen S., Carlson S., Tervaniemi M., Naatanen R. Heschl's gyrus, posterior superior temporal gyrus, and mid-ventrolateral prefrontal cortex have different roles in the detection of acoustic changes. J. Neurophysiol. 2007;97:2075–2082. doi: 10.1152/jn.01083.2006. [DOI] [PubMed] [Google Scholar]
- Shelley A.-M., Ward P.B., Catts S.V., Michie P.T., Andrews S., McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol. Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- Siemens . 2016. Siemens Medical Solutions. [Google Scholar]
- Thonnessen H., Zvyagintsev M., Harke K.C., Boers F., Dammers J., Norra C., Mathiak K. Optimized mismatch negativity paradigm reflects deficits in schizophrenia patients. a combined eeg and meg study. Biol. Psychol. 2008;77:205–2016. doi: 10.1016/j.biopsycho.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Toyomaki A., Kusumi I., Matsuyama T., Kako Y., Ito K., Koyama T. Tone duration mismatch negativity deficits predict impairment of executive function in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:95–99. doi: 10.1016/j.pnpbp.2007.07.020. [DOI] [PubMed] [Google Scholar]
- VPixx Technologies Inc . 2016. VPixx Technologies. [Google Scholar]
- Wynn J.K., Sugar C., Horan W.P., Kern R., Green M.F. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol. Psychiatry. 2010;67:940–947. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]