Abstract
A 66 year old man was diagnosed with CNS diffuse large B-cell lymphoma, and underwent treatment with Temozolomide, Dexamethasone, Rituximab, and radiation therapy, and prolonged steroid taper with Dexamethasone. Approximately one month after this, he presented with severe acute hypoxemic respiratory failure, and was admitted to the Medical Intensive Care Unit. Imaging showed diffuse ground glass opacities. Patient underwent diagnostic bronchoalveolar lavage which was positive for Pneumocystis jiroveci. He did not respond well to appropriate therapy and was transitioned to comfort care per his family's wishes, and expired. Pneumocystis jiroveci should always be included in the differential diagnosis of pneumonia in patients treated with Temozolomide, especially when this agent is used in combination with long term, high dose corticosteroids and radiation therapy.
Keywords: Pneumocystis jiroveci, Temozolomide, CNS lymphoma
1. Case report
A 66-year-old man presented to emergency department for worsening headaches and bilateral lower extremity weakness for two weeks, leading to a fall on the day of presentation. A week prior, he was found to have a mass in the corpus callosum extending into bilateral parietal lobes. He reported insidious onset of diffuse headaches, progressively getting worse, recurrent falls, dizziness and lower extremity weakness. He was started on levetiracetam and oral dexamethasone 4 mg four times a day. Patient's past medical history was remarkable for Factor V Leiden mutation and had been on Coumadin previously. Upon initial admission, patient was afebrile, and hemodynamically stable. Neurologic exam was remarkable for markedly decreased strength in lower extremities, and patellar hyper-reflexia. Laboratory work-up revealed hemoglobin 15.1 g/dL, leukocyte count of 12.9 × 109/L with 13% lymphocytes (reference 13%–52%) and 78% neutrophils (important to note; patient was on oral dexamethasone). CT head showed 5.8 × 1.8 cm hyper-dense mass involving splenium of the corpus callosum with extension into the corona radiata of bilateral parietal lobes, and surrounding vasogenic edema causing mass effect on the adjacent lateral ventricles. Patient underwent right parieto-occipital stereotactic brain biopsy, and the results were consistent with Diffuse Large B-Cell Lymphoma (DLBCL). CALGB 50202 protocol-based chemotherapy, was initiated and patient received Methotrexate 8 mg/m2 IV on day 1 and 15, Leucovorin, Temozolomide 150 mg/m2/day PO on days 7–11 and Rituximab 375 mg/m2 IV days (3,10,17,24). Patient tolerated the chemotherapy well and was discharged home on a tapering dose of dexamethasone after a month long stay in the hospital.
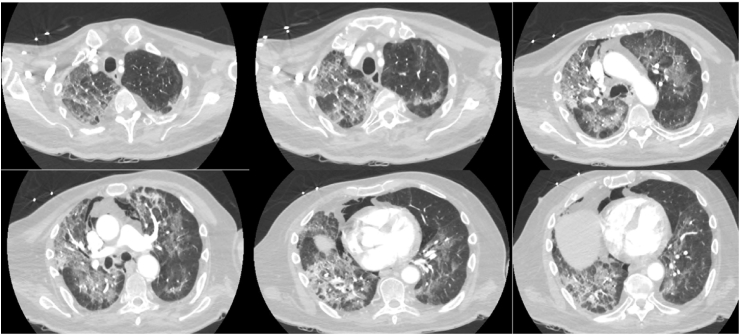
The patient continued to follow up with physical therapy and made a remarkable recovery, was able to ambulate without any support. However a month later he had syncope at home, witnessed by family. He was brought to our emergency department and was diagnosed with sepsis secondary to complicated urinary tract infection and pneumonia. Broad spectrum antibiotic therapy with piperacillin/tazobactam, vancomycin and azithromycin was started. On arrival to the emergency department, he was found to be in respiratory distress and was profoundly hypoxic (Pulse oximetry 83%), hence he was started on face-mask 50% oxygen and later switched to high flow oxygen. CT head with contrast did not show any interval change. CT thorax with contrast ruled out pulmonary emboli, however significant new ground glass opacities were noted throughout the lung fields along with pneumo-mediastinum (Fig. 1). Thoracic surgeon did not recommend any intervention at that time; it was believed to be due to possible pleural bleb rupture. Patient continued to have worsening of his oxygenation and was transitioned to non-invasive positive pressure ventilation and eventually required intubation and mechanical ventilation overnight.
Fig. 1.
CT thorax.
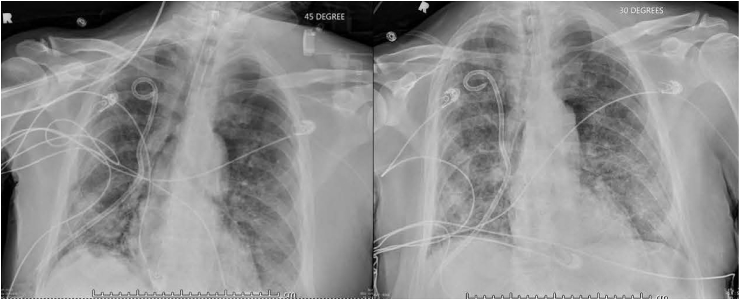
Complete blood work up revealed absolute lymphocyte count of 0.32 × 102/μL (2%, normal reference range 13–52%), which further dropped to 1% over the course of hospital stay. He was diagnosed with severe acute respiratory distress syndrome (ARDS), and did not show any improvement in the ground glass opacities after 24 hours of furosemide challenge (Fig. 2), in the setting of mildly elevated pro-BNP from baseline and concentric left ventricular hypertrophy with a normal ejection fraction of 60%. Patient continued to deteriorate clinically, requiring vasopressor support. He underwent diagnostic broncho-alveolar lavage (BAL) on day-3 and the stains were positive for Pneumocystis jiroveci. Intravenous bactrim was added to the antibiotic regimen, and at this time the patient was on 4 mg decadron twice daily. Unfortunately he did not respond to the treatment and had poor prognosis from the primary CNS lymphoma, hence patient's health-care proxy decided on comfort care measures, and patient expired.
Fig. 2.
CXR, Before and after furosemide trial.
This case report describes a 66-year-old man undergoing chemotherapy with temozolomide for primary CNS lymphoma (DLBCL), who developed isolated lymphopenia and pneumocystis jiroveci pneumonia leading to acute hypoxemic respiratory failure.
2. Discussion
Temozolomide is an alkylating agent, which was initially approved in 1999 for treatment of refractory anaplastic astrocytoma, and in March 2005 for glioblastoma multiforme, in combination with radiation therapy. Temozolomide has shown significant improvement in survival curve of these patients; however many adverse effects are reported, primarily myelosuppression, hematologic toxicity and infections [1]. However due to growing concern of neurocognitive toxicity from whole-brain radiotherapy (WBRT), researchers have been motivated to study alternate chemotherapeutic agents in patients with primary CNS lymphoma (PCNSL). Temozolomide is currently under investigation for CNS lymphomas, low-grade gliomas, melanomas and brain metastases. In one clinical trial, out of forty-four patients who received Methotrexate, Temodar and Rituximab (MT-R) for induction chemotherapy, 66% had complete remission [2]. The patient in this case also received MT-R for induction based on CALGB 50202 protocol. Dose-dependent administration schedules have showed marked antitumor activity, however such dose-dependent schedules have been associated with marked isolated lymphopenia leading to immunosuppressive states, giving rise to increasing numbers of fatal opportunistic infections [3], [4], [5], [6].
Use of corticosteroids in brain therapy causes further lymphopenia and immune-suppressed state [7], [8]. Hence it is important to rule out HIV and other causes of lymphopenia such as human T-cell lymphotropic virus type I (HTLV-1) [9]. Pneumocystis jiroveci infections have been reported in patients with brain cancer undergoing treatment with temozolomide, other less common opportunistic infections observed with temozolomide include Aspergillus pneumonia, Herpes simplex, herpes zoster and candidiasis [9], [10].
Despite multiple cases of opportunistic infections reported with the use of temozolomide, no specific guidelines exist for prophylaxis due to paucity of conclusive evidence, as no trials have been performed to assess the need for such prophylaxis. One retrospective review of 240 patients who received temozolomide for brain tumor reported only one patient to have been diagnosed with Pneumocystis jiroveci (PJP), without PJP prophylaxis (0.4%; 95% CI: 0.01–2.00) with a medium dose of 737 mg/m2 of temozolomide [3]. Due to adverse effects associated with use of antibiotics, there is a need for risk stratification of these patients undergoing chemotherapy with temozolomide, and guidance for PJP prophylaxis. Unfortunately only glioblastomas have been studied the most so far, hence we will use this model with an extrapolation for primary CNS lymphoma [8].
Pneumocystis jiroveci, previously known as Pneumocystis carinii, causes fatal pneumonia, especially in immune-compromised hosts, it is transmitted via air-borne route, acquired by person-to-person transmission [11]. PJP primarily exits as asymptomatic infection in general population [8], [10], [12], however it can manifest as dry cough, low-grade fever, and mild dyspnea to severe hypoxic failure in HIV-negative patients. Examination findings are often consistent with tachypnea, hypoxemia, tachycardia, and normal to near-normal lung auscultation. The incidence and mortality rates are much higher in HIV-positive with low CD-4 counts, solid-organ transplant recipients, connective tissue disorders, hematologic and solid malignancies, and inflammatory bowel disease [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. HIV positive patients, in contrast to HIV negative patients, tend to manifest symptoms at a younger age (42 ± 15 vs. 68 ± 13 years), whereas HIV negative patients are twice as likely to have an underlying pulmonary disease [23]. Mortality rate from PJP secondary to hypoxic failure in patients with HIV is approximately 10–20% compared with 35–50% in those without HIV [24], [25], [26]. PJP manifests as symmetric, atypical ground glass opacities in peri-hilar or lower zones, it can vary from interstitial infiltrates to cavity lesions [27]. Diagnostic tools include staining P jiroveci cysts in sputum or bronchoalveolar lavage (BAL) fluid, direct antigen immunofluorescence and PCR. In comparison to microscopy, PCR has a much higher sensitivity in diagnosis and quantitative PCR can be used to distinguish between PJP infection and colonization with higher accuracy. Tissue biopsy is rarely required for diagnosis, whereas new modalities of diagnosis, such as PET scan and (1,3)-beta-d-glucan are still under investigation [28], [29].
Having discussed the role of CD-4 cells, it is vital to mention that B cells play an essential role in expansion and generation of colonies of protective effector and memory CD4− T cells in response to Pneumocystis lung infection. One animal study comparing pneumocystis jiroveci/carinii in mice expressing MHC class II (MHC II) on all antigen presenting cells (APCs) and mice expressing MHC II on all APCs except B cells, showed that CD-4 T cells from B-cell deficient mice did not infiltrate the lungs efficiently and hence resulting in less clearance of Pneumocystis jiroveci from the lungs [30].
Basic laboratory research has also shown that Pneumocystis carinii in mice is dependent on B cells, one such study showed reduced number of activated CD-4 T cells in lungs and lymph nodes in chimeric mice with reduced CD 40 expression had delayed clearance of P. carinii infection [31].
Of note, our patient also received Rituximab, which impairs B-cell function. A retrospective study at Mayo clinic Rochester reviewed patients from 1998 to 2011, who were diagnosed with pneumocystis pneumonia and had received rituximab treatment. A total of 30 patients met this criteria and 29 and out of these 30 patients were not receiving any prophylaxis. Ninety percent of these patients had hematological disease and seventy-three percent were undergoing different chemotherapies. Three patients received rituximab alone, and still developed Pneumocystis pneumonia, thus highlighting the role of this agent. This study supports the importance of the role of B-cells in defense against PJP, as rituximab is a monoclonal antibody that binds to the CD20 antigen on B-lymphocytes. This study also suggested primary prophylaxis for patients receiving rituximab [32].
A recent meta-analysis of 11 cohort studies compared the incidence of Pneumocystis with and without rituximab. The analysis concluded convincingly that rituximab was associated with increased risk of PJP and was further increased in lymphoma patients undergoing rituximab-containing chemotherapies. Prophylaxis once again proved to be effective and resulted in significantly reduction of PJP incidence. It is pertinent to mention HIV patients were excluded from these studies [33].
Similarly steroids are associated with increased risk of PJP; a retrospective analysis of 116 patients without AIDS at Mayo Clinic with first episode of Pneumocystis carinii, between 1985 and 1991 showed that patients receiving prolonged, high-dose corticosteroid treatment (16–25 mg of prednisone per day or ≥4 mg dexamethasone daily for ≥4 weeks) are also at high risk of PJP, regardless of underlying type or stage of malignancy, or use of other chemotherapy agents [34].
The Fifth-European Conference on Infections in Lekemia (ECIL-5) recently published recommendations for prophylaxis against Pneumocystis jirovecii pneumonia (PJP) in non-HIV-infected patients in 2016. Trimethoprim/sulfamethoxazole was termed as the drug of choice of primary prophylaxis of PJP, followed by pentamidine, atovaquone and dapsone as alternatives when trimethoprim/sulfamethoxazole is poorly tolerated or contraindicated. Patients requiring prophylaxis were identified as those receiving 20 mg or higher doses of prednisone per day for 4 weeks, patients with ALL, allogeneic HSCT, and those receiving treatment with alemtuzumab, fludarabine/cyclophosphamide/rituximab combinations. Other patients groups identified as high risk are those undergoing radiation therapy for brain tumors or metastasis, along with high doses of steroids [35].
3. Conclusion
Currently the following recommendations regarding PJP prophylaxis in high-grade glioma patient being treated with a temozolomide have been suggested in a published research article [30].
-
-
Corticosteroid use with a daily dose equal to or exceeding 3 mg dexamethasone-equivalent for more than 3 weeks: prophylaxis should be continued for a month after steroid discontinuation, provided CD4+ has returned to normal
Physician discretion has been recommended in considering PJP prophylaxis for
-
-
Elderly population (>65 years); prophylaxis can be discontinued at the end of chemoradiation-provided CD4 count returns to normal
-
-
Patients with immunosuppressive treatment for solid tumors, bone marrow transplants, rheumatologic conditions, connective tissue disorders or inflammatory bowel disease
-
-
HIV- positive patients with CD4+ counts less than 200 cells/uL at the start of the treatment
-
-
History of PJP or opportunistic infections
It has also been suggested that PJP primary prophylaxis should be started if lymphocyte count falls below 500 cells/uL or CD4+ count falls below 200 cells/uL and prophylaxis should be continued until the counts return to normal. The current choice of PJP prophylaxis is TMP-SMX (sulfamethoxazole 800 mg; trimethoprim 160 mg) once daily dose, in case of intolerance aerosolized pentamidine has been suggested as an alternative [36].
Therefore we can infer from the existing published literature that in the absence of any contraindications in patients with primary CNS lymphoma treated with temozolomide especially with the concurrent use of steroids, radiation or other immunosuppressant drugs, PJP prophylaxis can be considered either at the initiation of the treatment or when lymphocyte count and CD4+ count falls below 500 cells/uL and 200 cells/uL respectively. It can be continued either until the end of the treatment or the return of the cell counts to normal.
All available and emerging evidence demonstrates that there are multiple factors that play a role in increasing the risk for acquiring this opportunistic infection, and therefore careful consideration of potential risk factors in each patient on an ongoing basis is paramount. Our patient we presented was on temozolomide, received radiation therapy, and steroid therapy, and therefore was profoundly immunocompromised, leading to severe PJP infection. We have learnt from this case that physicians should be extremely vigilant regarding these patients who are at risk for PJP pneumonia and consider starting prophylaxis on a case-by-case basis.
References
- 1.Vincent Ganière G.C., Bally Frank, Guillou Louis, Pica Alessia, Ribaupierre Sandrine de, Stupp Roger. Listeria brain abscess, Pneumocystis pneumonia and Kaposi's sarcoma after temozolomide. Nat. Clin. Pract. Oncol. 2006;3:339–343. doi: 10.1038/ncponc0514. [DOI] [PubMed] [Google Scholar]
- 2.BCe Al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58:4363–4367. [PubMed] [Google Scholar]
- 3.Tolcher A.W., Gerson S.L., Denis L. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br. J. Cancer. 2003;88(7):1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahindra A.K., Grossman S.A. Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors. J. Neurooncol. 2003;63(3):263–270. doi: 10.1023/a:1024217527650. [DOI] [PubMed] [Google Scholar]
- 5.Hughes M.A., Parisi M., Grossman S., Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int. J. Radiat. Oncol. Biol. Phys. 2005;62(5):1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 6.Su Y.B., Sohn S., Krown S.E. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J. Clin. Oncol. 2004;22(4):610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R., Dietrich P.Y., Ostermann Kraljevic S. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J. Clin. Oncol. 2002;20(5):1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 8.De Vos FY1 G.J., Bleeker-Rovers C.P., van Herpen C.M. Pneumocystis jirovecii pneumonia prophylaxis during temozolomide treatment for high-grade gliomas. Crit. Rev. Oncol. Hematol. 2013 Mar;85(3):373–382. doi: 10.1016/j.critrevonc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 9.MAaG S.A. Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors. J. Neurooncol. 2003;63:263–270. doi: 10.1023/a:1024217527650. [DOI] [PubMed] [Google Scholar]
- 10.Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 11.Choukri F., Menotti J., Sarfati C. Quantification and spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin. Infect. Dis. 2010;51(3):259–265. doi: 10.1086/653933. [DOI] [PubMed] [Google Scholar]
- 12.Simpson J.R., Horton J., Scott C. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 1993;26(2):239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 13.Gianella S., Haeberli L., Joos B. Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl. Infect. Dis. 2010;12:1–10. doi: 10.1111/j.1399-3062.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 14.Godeau B., Coutant-Perronne V., Le Thi Huong D. Pneumocystis carinii pneumonia in the course of connective tissue disease: report of 34 cases. J. Rheumatol. 1994;21(2):246–251. [PubMed] [Google Scholar]
- 15.Mori S., Cho I., Sugimoto M. A cluster of Pneumocystis jirovecii infection among outpatients with rheumatoid arthritis. J. Rheumatol. 2010;37:1547–1548. doi: 10.3899/jrheum.091294. [DOI] [PubMed] [Google Scholar]
- 16.Lertnawapan R., Totemchokchyakarn K., Nantiruj K., Janwityanujit S. Risk factors of Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus. Rheumatol. Int. 2009;29:491–496. doi: 10.1007/s00296-008-0721-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.C., Bell D.C., Guinness R.M., Ahmad T. Pneumocystis jirovecii pneumonia and pneumomediastinum in an anti-TNFalpha naive patient with ulcerative colitis. World J. Gastroenterol. 2009;15:1897–1900. doi: 10.3748/wjg.15.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velayos F.S., Sandborn W.J. Pneumocystis carinii pneumonia during maintenance anti-tumor necrosis factor-alpha therapy with infliximab for Chrohn's disease. Inflamm. Bowel Dis. 2004;10:657–660. doi: 10.1097/00054725-200409000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Worth L.J., Dooley M.J., Seymour J.F., Mileshkin L., Slavin M.A., Thursky K.A. An analysis of the utilization of chemoprophylaxis against Pneumocystis jirovecii in patients with malignancy receiving corticosteroid therapy at a cancer hospital. Br. J. Cancer. 2005;92:867–872. doi: 10.1038/sj.bjc.6602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres H.A., Chemaly R.F., Storey R. Influence of type of cancer and hematopoietic stem cell transplantation on clinical presentation of Pneumocystis jirovecii pneumonia in cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 2006;25:382–388. doi: 10.1007/s10096-006-0149-4. [DOI] [PubMed] [Google Scholar]
- 21.Barbounis V., Aperis G., Gambletsas E. Pneumocystis carinii pneumonia in patients with solid tumors and lymphomas: predisposing factors and outcome. Anticancer Res. 2005;25(1B):651–655. [PubMed] [Google Scholar]
- 22.Dall'oglio S., D'Amico A., Pioli F. Dose-intensity temozolomide after concurrent chemoradiotherapy in operated high-grade gliomas. J. Neurooncol. 2008;90(3):315–319. doi: 10.1007/s11060-008-9663-9. [DOI] [PubMed] [Google Scholar]
- 23.Enomoto T., Azuma A., Kohno A. Differences in the clinical characteristics of Pneumocystis jirovecii pneumonia in immunocompromized patients with and without HIV infection. Respiratology. 2010;15:126–131. doi: 10.1111/j.1440-1843.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- 24.Sepkowitz K.A. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin. Infect. Dis. 2002;34:1098–1107. doi: 10.1086/339548. [DOI] [PubMed] [Google Scholar]
- 25.Mansharamani N.G., Garland R., Delaney D., Koziel H. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest. 2000;118(3):704–711. doi: 10.1378/chest.118.3.704. [DOI] [PubMed] [Google Scholar]
- 26.Festic E., Gaijc O., Limper A.H., Aksamit T.R. Acute respiratory failure due to Pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest. 2005;128:573–579. doi: 10.1378/chest.128.2.573. [DOI] [PubMed] [Google Scholar]
- 27.Vogel M.N., Vatlach M., Weissgerber P. HRCT-features of Pneumocystis jirovecii pneumonia and their evolution before and after treatment of non-HIV immunocompromised patients. Eur. J. Radiol. 2012 Jun;81(6):1315–1320. doi: 10.1016/j.ejrad.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 28.Nakazato T., Mihara A., Sanada Y. Pneumocystis jirovecii pneumonia detected by FDG-PET. Ann. Hematol. 2010;89:839–840. doi: 10.1007/s00277-009-0888-2. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu Y., Sunaga N., Dobashi K. Serum markers in interstitial pneumonia with or without Pneumocystis jirovecii colonization: a prospective study. BMC Infect. Dis. 2009;9:47–52. doi: 10.1186/1471-2334-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund F.E., Hollifield M., Schuer K., Lines J.L., Randall T.D., Garvy B.A. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J. Immunol. 2006;176(10):6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 31.Lund F.E., Schuer K., Hollifield M., Randall T.D., Garvy B.A. Clearance of Pneumocystis carinii in mice is dependent on B cells but not on P carinii-specific antibody. J. Immunol. 2003;171(3):1423–1430. doi: 10.4049/jimmunol.171.3.1423. [DOI] [PubMed] [Google Scholar]
- 32.Martin-Garrido I., Carmona E.M., Specks U., Limper A.H. Pneumocystis pneumonia in patients treated with rituximab. Chest. 2013;144(1):258–265. doi: 10.1378/chest.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X., Mei X., Feng D., Wang X. Prophylaxis and treatment of pneumocystis jiroveci pneumonia in lymphoma patients subjected to rituximab-contained therapy: a systemic review and meta-analysis. PLoS One. 2015;10(4):e0122171. doi: 10.1371/journal.pone.0122171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yale S.H., Limper A.H. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin. Proc. 1996;71(1):5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Maertens J., Cesaro S., Maschmeyer G. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J. Antimicrob. Chemother. 2016;71(9):2397–2404. doi: 10.1093/jac/dkw157. [DOI] [PubMed] [Google Scholar]
- 36.De Vos F.Y., Gijtenbeek J.M., Bleeker-Rovers C.P., van Herpen C.M. Pneumocystis jirovecii pneumonia prophylaxis during temozolomide treatment for high-grade gliomas. Crit. Rev. Oncol. Hematol. 2013;85(3):373–382. doi: 10.1016/j.critrevonc.2012.08.002. [DOI] [PubMed] [Google Scholar]