Abstract
Perinatal care advances emerging over the past twenty years have helped to diminish the mortality and severe neurological morbidity of extremely and very preterm neonates (e.g., cystic Periventricular Leukomalacia [c-PVL] and Germinal Matrix Hemorrhage – Intraventricular Hemorrhage [GMH-IVH grade 3–4/4]; 22 to < 32 weeks of gestational age, GA). However, motor and/or cognitive disabilities associated with mild-to-moderate white and gray matter injury are frequently present in this population (e.g., non-cystic Periventricular Leukomalacia [non-cystic PVL], neuronal–axonal injury and GMH-IVH grade 1–2/4). Brain research studies using magnetic resonance imaging (MRI) report that 50% to 80% of extremely and very preterm neonates have diffuse white matter abnormalities (WMA) which correspond to only the minimum grade of severity. Nevertheless, mild-to-moderate diffuse WMA has also been associated with significant affectations of motor and cognitive activities. Due to increased neonatal survival and the intrinsic characteristics of diffuse WMA, there is a growing need to study the brain of the premature infant using non-invasive neuroimaging techniques sensitive to microscopic and/or diffuse lesions. This emerging need has led the scientific community to try to bridge the gap between concepts or ideas from different methodologies and approaches; for instance, neuropathology, neuroimaging and clinical findings. This is evident from the combination of intense pre-clinical and clinicopathologic research along with neonatal neurology and quantitative neuroimaging research. In the following review, we explore literature relating the most frequently observed neuropathological patterns with the recent neuroimaging findings in preterm newborns and infants with perinatal brain injury. Specifically, we focus our discussions on the use of neuroimaging to aid diagnosis, measure morphometric brain damage, and track long-term neurodevelopmental outcomes.
Keywords: Preterm neonates, magnetic resonance imaging, white matter abnormalities, neonatal neurology, infants, perinatal brain injury
Highlights
-
•
MRI is a powerful tool for diagnosis and long-term prognosis in preterm infants.
-
•
Neuropathological features of brain injury patterns are essential for MRI diagnosis.
-
•
For an accurate MRI diagnosis is essential to consider the morphological evolution.
-
•
Quantitative MRI provides information very helpful to long-term motor prognosis.
1. Introduction
Over the past two decades, advances in perinatal care have helped to diminish the mortality and severe neurological morbidity of extremely and very preterm neonates (e.g., cystic Periventricular Leukomalacia [c-PVL] and Germinal Matrix Hemorrhage–Intraventricular Hemorrhage [GMH-IVH grade 3–4/4]; 22 to < 32 weeks of gestational age; GA) (Ancel et al., 2015, Grisaru-Granovsky et al., 2014, Stoll et al., 2015). However, motor and/or cognitive disabilities associated with mild-to-moderate white and gray matter injury are frequently present in this population (e.g., non-cystic Periventricular Leukomalacia [non-cystic PVL], neuronal–axonal injury and GMH-IVH grade 1–2/4) (Marret et al., 2013, Volpe, 2009a). In fact, the diffuse component of white matter injury is the most common abnormality observed in contemporary cohorts (Back and Miller, 2014). Brain research studies using magnetic resonance imaging (MRI) report that 50% to 80% of extremely and very preterm neonates have diffuse white matter abnormalities (WMA) which correspond to the minimum grade of severity (Gano et al., 2015, Inder et al., 2005, Inder et al., 2003, Reid et al., 2014, Woodward et al., 2006). Nevertheless, mild-to-moderate diffuse WMA has also been associated with significant deficits in motor and cognitive functions (Spittle et al., 2011, Woodward et al., 2012).
Due to increased neonatal survival and the intrinsic characteristics of diffuse WMA, there is a growing need to study the brain of the premature infant using non-invasive neuroimaging techniques sensitive to microscopic and/or diffuse lesions (Ment et al., 2009a). This emerging need has led the scientific community to try to bridge the gap between concepts or ideas from different methodologies and approaches (e.g., neuropathology, neuroimaging, and clinical findings). This is evident from the combination of intense pre-clinical (Back and Miller, 2014, Kinney and Volpe, 2012, Palliser et al., 2016, Riddle et al., 2011, van Tilborg et al., 2016, Volpe et al., 2011) and clinicopathologic research (Counsell et al., 2008, de Vries et al., 2015, Harmony et al., 2016, Ment et al., 2009a, Pierson et al., 2007, Rutherford et al., 2010, Volpe, 2009a) along with neonatal neurology and quantitative neuroimaging research.
In the following article, we review literature relating the most frequent neuropathological patterns and recent neuroimaging findings in preterm newborns and infants with perinatal brain injury. Specifically, we focus on the use of neuroimaging to aid diagnosis, measure morphometric brain damage, and track long-term neurodevelopmental outcomes.
2. From neuropathology to neuroimaging… and vice versa
In 1962, Banker and Larroche described PVL for first time as a form of anoxic encephalopathy characterized by necrotic lesions in the periventricular white matter (Banker and Larroche, 1962), suggesting that this neuropathological pattern was related to ischemia and hypoperfusion at the vascular border zone in deep white matter. In subsequent decades, the histopathologic study of brain injuries in preterm infants continued to provide invaluable information to understand perinatal brain injury; however, some key concepts should be reconsidered. For example, it was believed that white matter damage was almost exclusively of the fetus or premature infant, and gray matter damage was only of the term infant (Kinney, 2009, Volpe, 2014). This notion had to be reevaluated during the late twentieth century and early twenty-first century thanks to quantitative MRI findings illustrating brain damage and loss of gray matter in preterm infants and children with and without c-PVL (Abernethy et al., 2002, Ajayi-Obe et al., 2000, Hüppi et al., 1998, Inder et al., 2005, Inder and Hüppi, 1999, Kesler et al., 2004, Lin et al., 2001, Miller et al., 2003). Based on these observations, Volpe (2005) proposed the term “encephalopathy of prematurity” to refer to encephalic gray matter abnormalities and WMA occurring in preterm infants during the perinatal period. PVL and neuronal–axonal injury are the hallmarks of the encephalopathy of prematurity (Kinney and Volpe, 2012, Volpe, 2009a). Consequently, various neuroimaging techniques (cranial ultrasound, cUS; volumetric MRI, vMRI; diffusion MRI, dMRI; functional MRI, fMRI) have been applied to study the neuropathological patterns that affect preterm infant survivors.
3. Frequent neuropathological patterns in premature infants: understanding perinatal brain injury in prematurity
The main neuropathological patterns affecting premature infants, as reported in current literature (Haynes et al., 2013, Kinney and Volpe, 2012, Marín-Padilla, 1997, Palliser et al., 2016, Pierson et al., 2007, Volpe, 2009a), are PVL, diffuse white matter gliosis (DWMG), neuronal–axonal injury of the white and gray matter, GMH-IVH, and periventricular hemorrhagic infarction. Here, we briefly summarize these findings (see annexed Table 1 and Table 2):
Table 1.
Variables to assess by clinical MRI at term-equivalent age.
| Cerebral white matter |
| Presence or absence of cysts |
| Signal abnormality⁎ |
| Myelination of the PLIC and corona radiata |
| Size and morphology of the corpus callosum |
| Size and morphology of the lateral ventricles |
| Volume of the periventricular white matter |
| Cortical gray matter |
| Signal abnormality⁎ |
| Cortical fold maturation |
| Size of the extracerebral space |
| Subcortical gray matter |
| Signal abnormality⁎ |
| Symmetry and size of the basal ganglia |
| Symmetry and size of the thalamus |
| Cerebellum |
| Signal abnormality⁎ |
| Symmetry and size of the hemispheres |
Note: For a detailed review about scores by each variable according to their severity see Inder et al., 2003, Woodward et al., 2006, Kidokoro et al., 2013.
PLIC: posterior limb of internal capsule.
Could be associated with hemorrhagic and ischemic processes.
Table 2.
Clinical neuroimaging and perinatal brain injury spectrum in premature infants.
| Neuroimaging findings | Diagnosis by |
Neuropathological findings | Major clinical outcome | |
|---|---|---|---|---|
| cUS | MRI | |||
| Cystic white matter abnormalities | ✓ | ✓ | Cystic PVL. Often bilateral cysts | Cerebral palsy with diplegia or quadriplegia |
| Porencephalic cyst secondary to periventricular hemorrhagic infarction. Often unilateral | Location-dependent. Motor cortex: hemiplegic cerebral palsy | |||
| Diffuse white matter abnormalities | X | ✓ | Diffuse component of cystic PVL (moderate-to-severe WMA) | Cognitive impairment/behavioral problems |
| Non cystic PVL (mild-to-moderate WMA) | Cognitive impairment/behavioral problems | |||
| Diffuse white matter gliosis (normal-to-mild WMA?) | Unknown | |||
| Gray matter abnormalities | X | ✓ | Neuronal loss and gliosis of the gray matter Subcortical gray matter and cerebellum are the most affected |
Cognitive impairment/behavioral problems |
| Germinal matrix hemorrhage - intraventricular hemorrhage | ✓ | ✓ | Breaking of the germinal matrix vessels | Depends on the location and severity Grade III/Periventricular hemorrhagic infarction: cerebral palsy, cognitive, behavioral and visual problems |
| Punctate white matter lesions | X/✓ | ✓ | Ischemic lesion Hemorrhagic lesion/congestion of medullary veins |
Not clear yet. Maybe cognitive impairment/behavioral problems |
| Diffuse excessive high signal intensity | X | ✓ | Unknown | Currently not found an association between diffuse excessive high signal intensity and abnormal long-term neurodevelopmental outcome |
| Encephalopathy of prematurity | X | ✓ | PVL + neuronal and axonal loss | Cerebral palsy and autistic spectrum disorders Motor, cognitive, attentional, behavioral and socialization problems |
✓: Diagnosis is possible X: Diagnosis is not possible X/✓: Punctate white matter lesions are suggested by inhomogeneous echogenicity seen on cUS, but can only be reliably detected by MRI.
PVL: Periventricular Leukomalacia. WMA: White Matter Abnormalities.
3.1. Periventricular leukomalacia
By definition, periventricular leukomalacia (PVL) has two neuropathological components: 1) a focal periventricular necrotic component and 2) a component with diffuse gliosis in the surrounding cerebral white matter. Cystic lesions secondary to necrotic foci in the white matter characterize the focal periventricular necrotic component. There are two types of necrotic foci with different histopathological/neuroimaging evolutions: macroscopic (necrosis > 1 mm that evolves over several weeks into a cyst) and microscopic (necrosis ≤ 1 mm that evolves over several weeks into glial scars) (Volpe, 2009a). The nomenclature to differentiate PVL subtypes depends on necrotic foci size but not on the diffuse component. The macroscopic focal necrotic component of PVL with diffuse gliosis is referred to as cystic PVL (c-PVL). In contrast, the term non-cystic PVL is often used to denote the microscopic focal necrotic component of PVL plus a component of diffuse gliosis in cerebral white matter (Kinney, 2009, Volpe, 2008). Therefore, it is a common error to refer to non-cystic PVL as “diffuse PVL” with the purpose of differentiating it from c-PVL, because both c-PVL and non-cystic PVL have one component with “diffuse” astrogliosis, microglial activation, and large or small cysts.
3.2. Diffuse white matter gliosis
Diffuse white matter gliosis (DWMG) is a rather common neuropathological finding in postmortem studies of preterm infants (41%) (Pierson et al., 2007) and in animal models (100% in the first week after hypoxic-ischemic event) (Riddle et al., 2011). Nevertheless, the incidence for this pattern in preterm infant survivors is unknown, as well as its impact on long-term neurodevelopmental outcome (Haynes et al., 2013). DWMG has been defined by diffuse astrocytic activation in cerebral white matter. However, unlike PVL it lacks a focal necrotic component and associated neuronal–axonal loss (Haynes et al., 2013, Pierson et al., 2007). It seems that DWMG is a prelude to pathologies of greater severity, such as PVL, although this has not been demonstrated (Kinney and Volpe, 2012).
3.3. Neuronal – axonal injury of the white and gray matter
Neuronal–axonal injuries are present in 30–40% of PVL cases confirmed by autopsy (Marín-Padilla, 1997, Pierson et al., 2007). Currently, neuronal–axonal injuries are considered over a wide neuropathological spectrum, which can occur in cerebral white matter (subplate neurons and axons) (Kinney et al., 2012), cortical gray matter, subcortical gray matter (thalamus, basal ganglia), and cerebellum (cortex, dentate nucleus) (Pierson et al., 2007, Volpe, 2009a). White matter necrosis generates abnormalities in gray matter as a result of axon terminal loss. Moreover, some neurons are unable to reach their subcortical targets due to injury. Other neurons present the appearance of “small stellate neurons,” while others suffer from dendritic resorption (centripetal) (Kinney, 2009, Marín-Padilla, 1997). In the cortex, abnormalities are mainly located in layer V with reduced neuron density in all areas and a significant reduction in pyramidal neuron density in layer V of the koniocortex (Andiman et al., 2010). Pyramidal neurons in particular may lose connectivity with layer I due to progressive distal absorption (Marín-Padilla, 1997). The subcortical structures experiencing significant neuronal loss are the thalamus, globus pallidus, and dentate nucleus of the cerebellum. The structures typically presenting significant gliosis are the thalamus, basal ganglia, and pons. Neuronal loss and gliosis in the thalamus and basal ganglia are lesions almost exclusive to PVL (for an extensive review see Andiman et al., 2010, Kinney and Volpe, 2012, Pierson et al., 2007). The presence of neuronal–axonal injury in conjunction with PVL is defined as encephalopathy of prematurity (Volpe, 2009a, Volpe, 2005).
3.4. Germinal matrix hemorrhage – intraventricular hemorrhage
Germinal matrix vessels have an endothelial layer with tight junctions, few pericytes, and fibronectin deficiency in the immature basal lamina. Furthermore, astrocyte end-feet show decreases in glial fibrillary acidic protein expression, which supports astrocyte structure. All such features contributing to the vasculature of the germinal matrix are prone to rupture and extravasation of blood into the subependymal zone (Ballabh, 2014).
The implicated or presumed vessels in these types of injuries are mainly the recurrent artery of Heubner (Hambleton and Wigglesworth, 1976) and the lateral striate artery (Kuban and Gilles, 1985). Intraventricular hemorrhage is one of the major complications in extremely and very preterm neonates. The main cause of intraventricular hemorrhage is the breaking of germinal matrix vessels, which underlies germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) (Ballabh, 2014). Indeed, GMH-IVH is the most common intracranial bleeding in the perinatal period, and there are many risk factors associated with this pathology, including very low birth weight (< 1500 g) and extremely low birth weight (< 1000 g) (Kenet et al., 2011). GMH-IVH rarely occurs during birth; however, up to 50% of cases occur in the first 24 h of life and 80–90% of cases occur in the first 72 h of life (Kenet et al., 2011). This should be considered, especially when using conventional neuroimaging to study high-risk premature neonates. Currently, GMH-IVH is classified by neuroimaging into three grades according to their severity (Volpe, 2008).
3.5. Periventricular hemorrhagic infarction
Gould et al. (1987) have suggested that severe intraventricular hemorrhage associated with intraparenchymal hemorrhage (previously grade 4 of Papile et al., 1978) is actually a venous infarction secondary to GMH-IVH with hemorrhagic evolution in periventricular white matter. Gould et al. (1987) referred to this neuropathological pattern as “periventricular hemorrhagic infarction.” While the incidence of c-PVL has declined, periventricular hemorrhagic infarction has remained stable over decades. What is more, around 11% of infants < 32 weeks GA and 8% of infants < 1500 g develop periventricular hemorrhagic infarction (Soltirovska Salamon et al., 2014). The incidence can reach up to 45% in infants weighing between 500 and 750 g at birth (Wilson-Costello et al., 2005).
3.6. Encephalopathy of prematurity
Encephalopathy of prematurity is a result of a heterogeneous and multifactorial neuropathological spectrum (hypoxia, ischemia, infectious/inflammatory processes) that affects the white and gray matter of the premature infant (Haynes et al., 2013, Kinney, 2009). As previously mentioned, the neuropathological patterns comprising encephalopathy of prematurity are PVL and neuronal–axonal injury. However, other pathological processes such as GMH-IVH, periventricular hemorrhagic infarction, and cerebellar disorders may accompany the neuropathological spectrum in encephalopathy of prematurity (Kinney and Volpe, 2012). Because of this variety of histopathologic presentations, the study of encephalopathy of prematurity is complex and intricate, especially considering that a single neuropathological pattern (mainly GMH-IVH) may be a “pathogenetic mechanism” for the development of other brain injuries (e.g., PVL, neuronal–axonal injury, and/or periventricular hemorrhagic infarction) (Kuban et al., 1999, Larroque et al., 2003, Volpe et al., 2011) including remote lesions (e.g., cerebellar underdevelopment and atrophy) (Kinney and Volpe, 2012, Limperopoulos et al., 2005, Volpe, 2009b). As a result, mixed patterns with two or more lesions in different stages and with dissimilar evolution in each type describing a “true” instance of encephalopathy of prematurity are observed. In fact, GMH-IVH without ventricular dilation (grade 1–2) has been associated with an up to 9-fold increased risk of PVL or periventricular hemorrhagic infarction. What is more, GMH-IVH with ventricular dilation (grade 3) has been shown to increase up to 29-fold the risk of PVL or periventricular hemorrhagic infarction in premature infants (Kuban et al., 1999).
4. Neonatal and pediatric conventional neuroimaging
Cranial ultrasound (cUS) and conventional MRI (using a clinical/qualitative approach) are the main neuroimaging techniques used in hospital environments for the diagnosis and characterization of perinatal brain injury (de Vries et al., 2015, Rutherford et al., 2010, Tusor et al., 2014). cUS is a noninvasive and low-cost means of study to examine infants at high risk for neurological damage without mobilizing them outside the neonatal intensive care unit (NICU) —even though the MRI global market is moving in this direction to get high-quality diagnostic imaging inside the NICU (Tkach et al., 2014). This way it is possible to obtain brain images in the early neonatal period to aid the physician in determining the time and evolution of the injury (Sarkar et al., 2015, van Wezel-Meijler et al., 2010). cUS allows the detection of cerebellar damage and cystic lesions in the supratentorial white matter (Steggerda et al., 2009), and it enables the evolutionary tracking of GMH-IVH (Kwon et al., 2014). However, cUS is not effective in detecting subtle lesions and/or diffuse white and gray matter injuries (Parodi et al., 2013). This technique represents only a first-line option for monitoring premature neonates in the NICU. On the other hand, conventional MRI is a non-ionizing imaging technique that allows the detailed assessment of encephalic structures that are commonly affected in extremely and very preterm infants. Overall, MRI allows the evaluation of: 1) the contrast between encephalic white and gray matter (e.g., T1 weighting, “T1w”); 2) the myelination process (longitudinal T2w and T1w) (Childs et al., 2001); 3) the evolution of ischemic pathologies of arterial origin (using diffusion-weighted imaging, DWI) (van der Aa et al., 2013); 4) vascular disorders of the Circle of Willis and its efferent branches (MR angiography 3D “Time of Flight”) (Lequin et al., 2009); 5) hemorrhages (via susceptibility weighted imaging, SWI) (Kersbergen et al., 2014); and 6) macroscopic focal lesions (> 1 mm) and diffuse WMA (using FLAIR and T2w images) (Kwon et al., 2014, Rutherford et al., 2010). Thus, MRI is more sensitive than cUS for detecting subtle and/or diffuse neonatal brain disorders (Benders et al., 2014, Miller et al., 2003, Sie et al., 2000). Currently, MRI is considered the gold standard for clinical diagnosis of diffuse WMA in the absence of histological information (de Vries et al., 2015).
4.1. Conventional MRI of the neonatal brain: sequences and technical aspects
The neonatal brain is different from the adult brain because it has higher water content, especially in the preterm population. Hence, the majority of sequences for imaging the neonatal brain need to be adapted to optimize the image quality (Rutherford et al., 2006). In this sense, T1w sequences in the neonatal period are usually acquired with short repetition time (TR) and short echo time (TE), while T2w sequences are acquired with long TR and long TE. Long inversion times are recommended for the inversion recovery sequences (Dubois et al., 2014). The main neonatal MRI sequences used routinely in clinical assessments are T1w, T2w, DWI, and inversion recovery sequence (e.g., FLAIR) (Devi et al., 2015, Kwon et al., 2014). SWI and MR angiography can also be considered. Diffusion parameters, tractography, and fMRI are some emerging modalities with potential use in the clinical environment (see Section 5.1 Quantitative MRI).
4.2. Is it valid to infer a neuropathological diagnosis using conventional neuroimaging?
While it is true that cUS and conventional MRI provide valuable information for the study of the brain in premature infants, accurate diagnosis of any lesion pattern of perinatal brain injury is made by histopathological confirmation and/or postmortem “neuropathological diagnosis” (Back, 2014, Kinney, 2009, Volpe, 2009a). Obviously, since this form of diagnosis is not available in the population of preterm survivors, the clinical use of conventional MRI has become increasingly common in the last 10–15 years (Smyser et al., 2012). However, clear and comprehensive correlations between conventional neuroimaging and the neuropathological spectrum of surviving premature infants do not yet exist (Haynes et al., 2013, van Tilborg et al., 2016). Clinical MRI currently used on 1.5–3 T MRI systems does not allow the differentiation of the most subtle and frequent neuropathological patterns such as non-cystic PVL, neuronal–axonal injury, and DWMG (Back, 2014, Haynes et al., 2013, Palliser et al., 2016, Riddle et al., 2011, Volpe, 2008). This is due in part to the resolution of whole-brain MR, because conventional qualitative MRI works at a resolution of millimeters, whereas the processes associated with microcystic necrosis (e.g., focal component of non-cystic PVL) or gliosis (e.g., diffuse component of non-cystic PVL and DWMG) are present in microns. Therefore, until additional technological improvements are available, it is ill advised to infer a neuropathological diagnosis in most cases based upon neuroimaging evidence alone.
4.3. Terminology for conventional neuroimaging
Macroscopic necrotic lesions (c-PVL) and severe hemorrhagic processes (GMH-IVH grade 3 and periventricular hemorrhagic infarction) can be well characterized with the combined use of cUS and conventional MRI (Benders et al., 2014). Thus, neuropathological findings can be inferred in these types of lesions in the absence of histopathological studies. By contrast, microscopic and diffuse lesions cannot be distinguished from each other in cUS and/or qualitative MRI, so it is suggested to adopt a consistent nomenclature according to the neuroradiological findings without neuropathological inferences (van Tilborg et al., 2016).
4.3.1. Conventional MRI
Currently, several terms are used to denote brain damage detected by conventional MRI in the early days of life and/or at the term-equivalent age (TEA; 37–42 weeks postmenstrual age). The terms most commonly used are white matter abnormalities (cystic and diffuse), gray matter abnormalities (GMA), cerebellar abnormalities, punctate white matter lesions, and diffuse excessive high signal intensity (DEHSI) (Benders et al., 2014, de Vries et al., 2015, Kwon et al., 2014, Rutherford et al., 2010, van Tilborg et al., 2016). This terminology emerges from the characteristic abnormal changes in signal intensity, from its distribution and morphology (punctate white matter lesions and DEHSI), or by evaluating and grouping a set of abnormal variables (WMA, GMA, and cerebellar abnormalities).
White and gray matter abnormalities are frequently classified according to degree of severity through a standardized score system that assesses the MRI findings at TEA (Hintz et al., 2015, Horsch et al., 2010, Inder et al., 2003, Kidokoro et al., 2013, Woodward et al., 2006). This clinical assessment consists in graduating each variable independently (the most used are listed in Table 1) and then adding up the score of all variables. The final score allows situating the MRI findings in a category according to severity of the lesion, usually divided into none, mild, moderate, and severe. Thus, the extension and characterization of brain abnormalities is mainly based on their severity and not on the neuropathological pattern. Although this classification does not have a clear correlation with the neuropathology of diffuse patterns or encephalopathy of prematurity, it is very useful for establishing an imaging diagnosis and as a prognostic basis for long-term neurodevelopmental outcome (Anderson et al., 2015, Murray et al., 2014, Omizzolo et al., 2014, Reidy et al., 2013, Spittle et al., 2011). Here we summarize the context of these terms (see annexed Table 2):
4.3.1.1. Cystic white matter abnormalities
As previously mentioned the cystic lesions of cerebral white matter, mainly c-PVL and periventricular hemorrhagic infarction, can be well characterized throughout the neonatal period. The macroscopic focal lesions of c-PVL are visible on MRI at TEA (see Fig. 1-C/4-A) as one or several cystic images in periventricular white matter at the ventricular trigone level, and may extend to the centrum semiovale (Rutherford, 2001). Periventricular hemorrhagic infarction can be seen mainly in two forms on MRI at TEA: 1) as a cystic image indistinguishable from c-PVL (unless serial neonatal cUS has been obtained) or 2) as a porencephalic cyst (Benders et al., 2014). The latter is usually seen as asymmetric unilateral ventriculomegaly (porencephalic cyst with ventricular communication) or rarely, as a communication between the lateral ventricular system and the subarachnoid space (Grant et al., 1982, Volpe, 2008). Other MRI findings that may accompany cystic lesions are alterations of morphology and diminished size of the corpus callosum, dilated lateral ventricles (sometimes asymmetric and with irregular boundaries) (Martinez-Biarge et al., 2016), prominent sulci near the ventricles (Rutherford, 2001), severe myelination delay, diffuse or focal signal abnormalities, white matter loss (usually higher than that observed in diffuse patterns) (Inder et al., 2003, Kidokoro et al., 2013, Woodward et al., 2006), and some cases with reduced size/volume of the cerebellar hemispheres (Limperopoulos et al., 2005). The concomitant presence of cystic WMA and cerebellar alterations, like atrophy or underdevelopment, suggests infratentorial lesions to distance by trans‑synaptic degeneration of the corticopontocerebellar pathways (Kinney and Volpe, 2012, Limperopoulos et al., 2005). In cases with an important unilateral cystic lesion (usually periventricular hemorrhagic infarction), it is possible to see a decreased size of the cerebellar hemisphere contralateral to the cyst (Limperopoulos et al., 2005, Volpe, 2009b) (see Fig. 2-D). All cystic lesions of the white matter are considered moderate to severe due to their destructive nature.
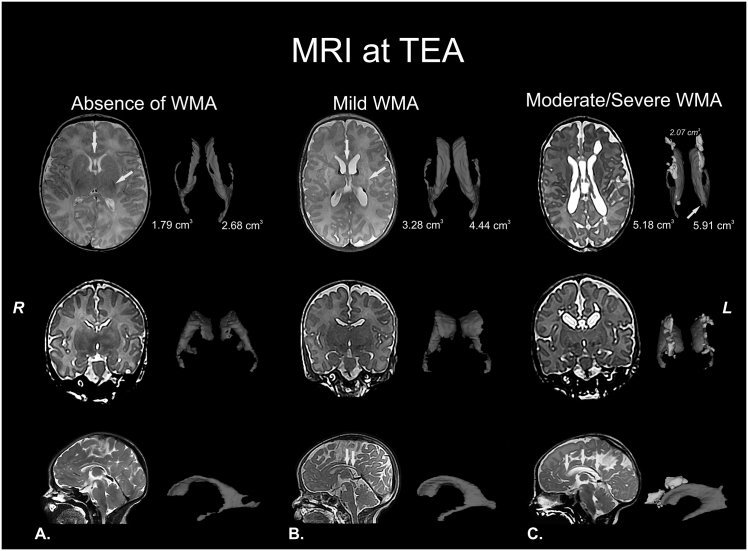
Fig. 1.
White matter abnormalities (WMA). T2 structural MRI with corresponding volumetric measurements of the lateral ventricles (in blue) at term-equivalent age (TEA) or nearly TEA. A) Normal MRI at TEA. Healthy neonate at 41 weeks of postmenstrual age with normal MRI: absence of cystic abnormalities, volume and size of corpus callosum (yellow arrow in upper panel) and lateral ventricles are normal, myelination of the corpus callosum, posterior limb of internal capsule (PLIC; yellow arrow in upper panel) and corona radiata corresponding with the age and cerebral white matter signal and volume are normal. B) Mild WMA at nearly TEA. Preterm infant (born at 28 gestational weeks) at 43 weeks of postmenstrual age with mild diffuse WMA: absence of cystic abnormalities, partial thinning of the corpus callosum (yellow arrows in upper and lower panel), mild dilated lateral ventricles, PLIC with myelination delay and focal signal abnormalities in relation to punctate white matter lesions (orange arrows in upper panel), probably associated with hemorrhagic etiology and congestion of medullary veins. C) Moderate-to-severe WMA at TEA. Preterm infant (born at 29 gestational weeks) at 41 weeks of postmenstrual age with cystic WMA: bilateral presence of multiple cysts (3D reconstruction and corresponding volumetric measurement are in yellow in upper panel), global thinning of the corpus callosum (orange arrow in upper panel and yellow arrows in lower panel), dilated lateral ventricles, severe myelination delay, diffuse and extensive signal abnormalities, white matter loss (double direction arrow in upper panel), prominent sulci near the ventricles (orange arrow in upper panel) and communication between small cyst and left lateral ventricle (yellow arrow in upper panel). The features of the last structural MRI are compatible with cystic-PVL. Images in radiological convention. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
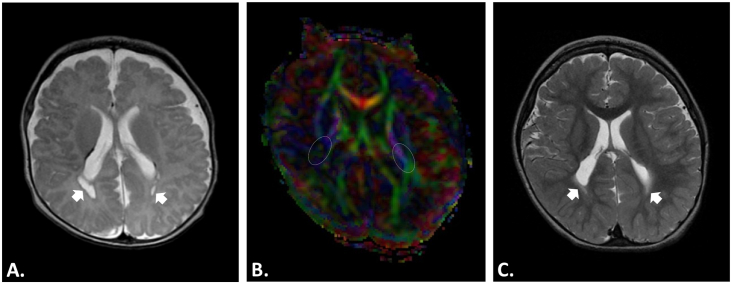
Fig. 4.
Structural MRI and Diffusion Tensor Imaging (DTI). Male preterm infant (born at 30 gestational weeks) with cystic-PVL diagnosis and neurological follow-up in the first 2 years. A-B) T2 structural MRI and DTI at nearly term-equivalent age (TEA), around 47 weeks of postmenstrual age. A) T2 structural MRI with bilateral cystic lesions (arrows) and punctate white matter lesions. B) Color-coded fractional anisotropy (FA) map with evident white matter loss in the right posterior limb of the internal capsule (PLIC; right white oval) and bilateral reduction of FA values (right PLIC FA 0.21 vs. left PLIC FA 0.36), mainly in right side. A motor prognosis for this type of cases is possible; the major clinical outcome in these cases is a location-dependent spastic cerebral palsy (hemiplegia, diplegia, quadriplegia, etc.). C) T2 structural MRI at 24 months of corrected age with loss of cysts by cyst walls adjoining or “ventriculomegaly ex-vacuo.” The evidence of cyst in cystic-PVL can be lost at this stage or before (this is one reason why MRI at TEA and follow-up for years is suggested). However, the diffuse component of cystic-PVL (arrows) is still evident at this age. The current diagnosis of this child is triparetic cerebral palsy with left side more affected. Images in radiological convention.
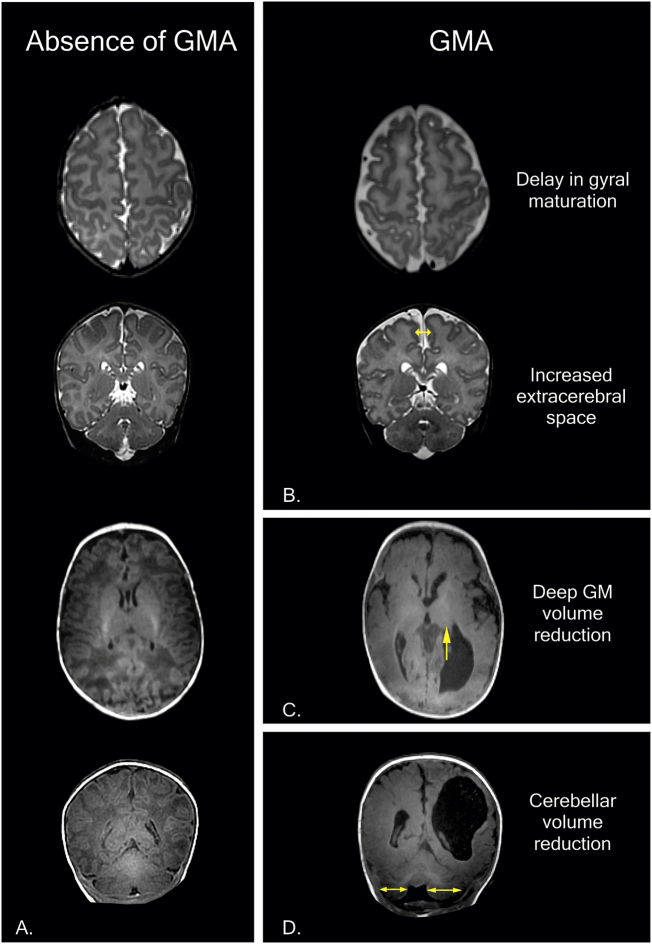
Fig. 2.
Examples of gray matter abnormalities (GMA) in MRI at term-equivalent age (TEA) in the first year of life. A) Normal T2 and T1 structural MRI at TEA. Healthy neonate at 41 weeks of postmenstrual age with normal MRI: cortical gray matter (signal, gyral maturation and subarachnoid space), deep/subcortical gray matter and cerebellum (signal and volume) are normal. B) Preterm infant (born at 31 gestational weeks) at 38 weeks of postmenstrual age with mild-to-moderate GMA: delay in gyral maturation and increase in extracerebral space (double direction arrow). C-D) Preterm infant (born at 31 gestational weeks) at 9 months of corrected age with porencephalic cyst and severe GMA. C) Deep GMA with volume reduction and asymmetry in the thalamus (arrow). D) Cerebellar volume reduction and asymmetry in cerebellar hemispheres (double direction arrow). The features of the last structural MRI (C-D) are compatible with a complex spectrum of encephalopathy of prematurity (diagnosis: periventricular hemorrhagic infarction + diffuse WMA compatible with PVL + severe GMA = encephalopathy of prematurity; mixed patterns with two or more lesions in different stages and with distinct evolution in each one). Images in radiological convention.
4.3.1.2. Diffuse white matter abnormalities
Non-cystic PVL, DWMG, and neuronal–axonal injury of the white matter are the main patterns of diffuse white matter abnormalities (diffuse WMA) currently described in literature (Haynes et al., 2013, Kinney and Volpe, 2012, Palliser et al., 2016). These patterns show at least one diffuse neuropathological component in absence of one focal macroscopic component (cysts); however, one pattern cannot be differentiated from another using conventional endogenous contrast MRI. Thus, a simple way to refer to them, collectively or individually, is “diffuse white matter abnormality” (see Fig. 1-B). As previously mentioned, diffuse WMA can be classified according to degree of severity through the evaluation of MRI findings. The main qualitative MRI findings that have been associated with diffuse WMA are: absence of cystic abnormalities > 1 mm in white matter (major criterion) (Volpe et al., 2011); diffuse signal abnormalities in white matter, posterior limb of internal capsule (PLIC), and/or corona radiata with myelination delay; thinning of the corpus callosum; dilated lateral ventricles; and/or white matter loss (Inder et al., 2003, Woodward et al., 2006). Some cases could show bilateral and symmetrical decreases in cerebellar size/volumes by bilateral crossed trans‑synaptic degeneration (Limperopoulos et al., 2005).
4.3.1.3. Gray matter abnormalities
Neuronal loss and gliosis of the gray matter are the main neuropathological abnormalities detected in just over one third of infants with PVL (see Section 3.3 Neuronal – axonal injury of the white and gray matter). These abnormalities can occur in any region of the encephalic gray matter, although subcortical regions and cerebellum tend to be the most affected structures (Pierson et al., 2007). Like WMA, qualitative GMA are usually stratified according to their degree of severity and distribution (cortical, subcortical, and/or cerebellar) (Inder et al., 2003, Kidokoro et al., 2013, Woodward et al., 2006). Although GMA in preterm infants have often been associated with PVL, these could be difficult to detect by conventional MRI and, consequently, most neuroradiologists do not report these findings in clinical practice (Slaughter et al., 2016).
4.3.1.3.1. Cortical gray matter abnormalities
Abnormalities in the cerebral cortex of preterm infants are related to cortical maturation impairment such as cortical volume loss and cortical folding delay (Dubois et al., 2008, Lodygensky et al., 2010). According to neuropathological findings, the cortical abnormalities in PVL are mainly located in layer V with a reduction in the density of neurons, especially pyramidal neurons (Andiman et al., 2010). However, this apparently does not affect cortical thickness and/or volume in this population. Dean et al. (2013) found an association between decreased cortical volume and increased neuronal density in a sheep model of ischemia, and they concluded that cortical growth impairments are related to deficits in dendritic arbor maturation and synapse formation of cortical neurons.
The delay in cortical folding has been related to antenatal (lower birth weight and smaller occipitofrontal circumference at birth) and postnatal (higher critical illness in the first 24 h of life, steroids, and prolonged endotracheal intubation) risk factors (Engelhardt et al., 2015). Nevertheless, the neuropathological substrate that explains the abnormalities in cortical folding detected by MRI in premature infants is still unclear. In summary, the main qualitative MRI findings that have been used as cortical abnormality indicators are: 1) abnormalities in the cerebral cortex signal intensity (e.g., focal or extensive; unilateral or bilateral); 2) maturity of cortical folds (e.g., delay of at least 2 weeks); and/or 3) augmented subarachnoid space (e.g., increased interhemispheric distance; see Fig. 2-B) (Inder et al., 2003, Kidokoro et al., 2013).
4.3.1.3.2. Subcortical gray matter abnormalities and cerebellar abnormalities
The deep gray matter and cerebellum tend to be the most affected structures with neural loss and gliosis. The gray matter structures with significant neuronal loss are the thalamus, globus pallidus, and cerebellar dentate nucleus. The structures with significant gliosis are the thalamus, basal ganglia, and pons. However, gliosis without obvious neuronal loss is more common than neuronal loss and gliosis combined, and it primarily affects the basis pontis, inferior olive, globus pallidus, thalamus, hippocampus, and brainstem tegmentum, respectively (Kinney and Volpe, 2012, Pierson et al., 2007). This is consistent with numerous MRI studies in preterm infants that show reductions in size and/or signal abnormalities of the thalamus, basal ganglia, or cerebellum (Hintz et al., 2015, Inder et al., 2005, Kidokoro et al., 2013, Limperopoulos et al., 2005, Martinez-biarge et al., 2016, Omizzolo et al., 2014, Tich et al., 2009). Based on the above, the aspects to consider in conventional MRI at TEA are 1) signal intensity abnormalities and 2) structural reductions in size by visual analysis (see Fig. 2-C/D) and or semi-quantitative analysis (measures by anatomic references in one or two planes) of the thalamus, basal ganglia, and cerebellum (Kidokoro et al., 2013, Tich et al., 2009).
4.3.1.4. Punctate white matter lesions
By conventional MRI, this pattern of lesion corresponds to small periventricular lesions of ischemic or hemorrhagic nature, visible via DWI and SWI, respectively (Niwa et al., 2011). Kersbergen et al. (2014) described three different patterns of punctate white matter lesions according to their morphology: linear, cluster, and mixed. The morphology of linear punctate white matter lesion is typically the most frequently observed pattern, especially in infants < 28 weeks GA (on T1-weighted MRI at 30 weeks of postmenstrual age). This pattern tends to have a low intensity signal in SWI, with anterior and periventricular location. The linear pattern was associated with GMH-IVH, suggesting a hemorrhagic etiology or congestion of medullary veins (see Fig. 1-B). On the other hand, instances of cluster punctate white matter lesions were observed with diffusion restriction in DWI in infants > 28 weeks GA. In this pattern, there was no clear correlation with GMH-IVH, hence an inflammatory or ischemic etiology is suspected. Finally, the mixed pattern (linear and cluster combined) is located in posterior regions of the brain. According to reports, the mixed pattern is more probable in preterm infants > 28 weeks GA and it does not show an obvious correlation with GMH-IVH.
4.3.1.5. Diffuse excessive high signal intensity
The diffuse excessive high signal intensity (DEHSI) is defined in T2w images at TEA with diffuse increased signal intensity in the cerebral white matter. The term DEHSI is used only for diffuse increased signal intensity and should not be confused with mild-to-severe WMA accompanied by other structural abnormalities. No association between DEHSI and abnormal long-term neurodevelopmental outcome has been found (Iwata et al., 2012, van't Hooft et al., 2015), and DEHSI is now considered part of a normal variant of preterm infant development (Benders et al., 2014, Calloni et al., 2015, van Tilborg et al., 2016).
4.4. Motor and cognitive prognosis by conventional MRI
Conventional MRI at TEA is a helpful tool for the prediction of short- and long-term neurodevelopmental outcomes. In relation to motor outcome, WMA (cystic lesions, white matter loss, delayed myelination, thinning of the corpus callosum, ventriculomegaly) are strongly related to cerebral palsy and other motor disabilities (Anderson et al., 2015, van't Hooft et al., 2015). In the comprehensive review by, van't Hooft et al. (2015) compiled a meta-analysis of articles published between 2000 and 2013 concerning the prediction of motor sequelae in preterm infants (≤ 32 weeks GA) using qualitative MRI at TEA. One of the criteria used by these authors was the comparison of patients with normal MRI or mild MRI abnormalities vs. patients with moderate-to-severe MRI abnormalities, including WMA, DEHSI, and brain abnormality. The article reported that qualitative MRI (mainly moderate-to-severe WMA) has an acceptable prognostic value for predicting cerebral palsy (sensitivity of 77% and specificity of 79%) and motor function (sensitivity of 72% and specificity of 62%). However, DEHSI lacks prognostic value (Broström et al., 2016, van't Hooft et al., 2015).
Neonatal MRI abnormalities are also associated with cognitive outcome. WMA detected by neonatal MRI have long-term consequences. Two follow-up assessments that included measures of general intellectual ability, language development, executive functioning, and behavior at 4 and 6 years of age showed functional decline according to WMA severity across all domains. Signs of WMA, such as punctuate lesions and white matter loss, were associated with later neurocognitive outcomes (Woodward et al., 2012). It has also been reported that neonatal moderate-to-severe WMA predicts executive functioning at preschool age (Woodward et al., 2011) and other cognitive abilities including language development, attention, and processing speed learning capacity at 7 years of age (Murray et al., 2014, Reidy et al., 2013). However, language abilities are differentially associated with neonatal WMA; for instance, phonological awareness, semantics, grammar, and discourse were affected by WMA, but this abnormality was not significantly associated with pragmatics (Reidy et al., 2013). Macrostructural and microstructural corpus callosum abnormalities that result from prematurity in very preterm infants also correlated with adverse neurodevelopmental outcomes at 2 years of age measured with the Bayley Scales of Infant Development (BSID-II) (Thompson et al., 2012). On the other hand, GMA have also been associated with language, cognitive and motor delays and cerebral palsy at 18–24 months of age (Slaughter et al., 2016, Woodward et al., 2006). Omizzolo et al. (2014) reported that abnormalities of the basal ganglia and the thalamus in very preterm infants were the strongest predictor of memory and learning performance outcomes at 7 years of age. These structures are linked to memory and subsequently to learning. Nevertheless, future research is still needed to establish the relationship between qualitative GMA detected by MRI at TEA and long-term neurocognitive disorders in children and adolescents with a history of prematurity.
5. Neonatal and pediatric quantitative neuroimaging
Quantitative neuroimaging consists of a set of imaging techniques that allow one to make elemental numerical measurements (e.g., distances, perimeters, areas, thickness, volumes, indexes), construct quantitative maps (e.g., diffusion maps, fractional anisotropy maps), and obtain complex statistical images of the brain (e.g., statistical parametric maps). Hence, techniques such as US (Cruz-Martínez et al., 2011), EEG (Bosch-Bayard et al., 2012, Harmony et al., 1995), MRI (Hüppi et al., 1998, Kesler et al., 2004, Ment et al., 2009a, Tusor et al., 2014), PET/CT (Basu et al., 2011), and SPECT (Yamauchi et al., 2014) can be considered quantitative neuroimaging techniques. However, quantitative MRI (advanced neuroimaging techniques) seems to be the most promising tool for the neurological diagnosis and prognosis of surviving preterm infants (Anderson et al., 2015, Benders et al., 2014, de Vries et al., 2013, Setänen et al., 2016). For this reason, this review focuses on quantitative MRI.
5.1. Quantitative MRI
The main objectives of quantitative neonatal and pediatric MRI are the formal computational analysis and interpretation of images (Morel et al., 2016), the measurement and characterization of “subtle” pathologies (de Vries et al., 2015, Hüppi and Dubois, 2006, Inder and Hüppi, 1999, Ment et al., 2009a), and the use of sophisticated algorithms to predict motor, neurocognitive, and behavioral disabilities (Rathbone et al., 2011, Rennie and Kendall, 2015, Rose et al., 2015). Essentially, quantitative MRI of the brain seeks to measure and model brain structure, function, and connectivity, and in the case of perinatal brain injury, it seeks to provide more detailed macrostructural (vMRI), microstructural (dMRI), and functional (fMRI) information that is not considered by subjective “qualitative” approaches. Currently, quantitative MRI uses modern scientific workflow approaches that can draw together neuroimaging data processing routines from across multiple software packages, linking them together to implement end-to-end processing and analytic solutions. These solutions not only lead to detailed mathematical and statistical results but also help to improve the reproducibility of measurements and reduce the post-processing duration (Dinov et al., 2010, Van Horn and Toga, 2014). In this manner, diffusion MR data can be reconstructed and used to measure the degree of connectivity between brain regions delineated with structural MRI, which can analyze blood flow or metabolic changes seen in functional imaging. In the following sections, we describe several of these imaging methods commonly used to quantify brain alteration in pediatric patients.
5.1.1. Volumetric MRI
Volumetric MRI (vMRI) is a very helpful tool with potential use in the clinical environment. It is primarily used to detect “subtle” cerebral and cerebellar abnormalities that are visually undetectable in premature population. Numerous MRI studies at TEA have reported reductions in brain tissue and cerebellar volumes in premature infants with perinatal risk factors and WMA (Inder et al., 2005, Keunen et al., 2012, Limperopoulos et al., 2005, Lind et al., 2011, Thompson et al., 2007). For example, in a large prospective longitudinal cohort study of 202 preterm and 36 term infants, Thompson et al. (2007) found reductions in the parieto-occipital, premotor, orbitofrontal, and sensorimotor regions of preterm infants. These last two regions showed marked reductions in cortical gray matter and unmyelinated white matter volumes. Deep gray matter was more affected in parieto-occipital and subgenual regions than in term controls. Interestingly, the authors of this study (Thompson et al., 2007) conclude that perinatal risk factors alter regional brain volumes in the preterm infant. The alteration patterns vary depending on the risk factors suffered by the infant. In addition to supporting the diagnosis, vMRI allows an objective and quantitative longitudinal follow-up to assess the impact of clinical risk factors and/or the evolution of perinatal brain injury (see Fig. 3-A) (Guo et al., 2017, Kersbergen et al., 2016). Recently, in another large longitudinal cohort study of extremely preterm infants with MRI studies at around 30 weeks postmenstrual age and again at around TEA (n = 210; 131 with serial data), authors (Kersbergen et al., 2016) reported decreased brain volumes on both scans. The longitudinal follow-up study showed that brain growth in this period is crucial and that risk factors such as low birth weight z-score, sex, prolonged mechanical ventilation, and surgery affect global brain volume. On the other hand, the brain injuries (GMH-IVH grade III, periventricular hemorrhagic infarction, c-PVL, cerebellar hemorrhages, or infarctions) had local effects on some structures including lateral ventricles (enlarged) and cerebellum (volume reduction). The correlation of these measurements with clinical outcomes of the premature neonate at different ages has been very useful for researchers to establish neurodevelopmental prognosis at early ages. In fact, reductions in brain volumes at TEA are correlated with motor and neurodevelopmental outcomes in the first 2 years of life, as well as in late childhood and adolescence (Guo et al., 2017) (see Section 5.2 Motor and cognitive prognosis by quantitative MRI). Moreover, vMRI analysis also helps to detect structural abnormalities in late childhood and adolescence of populations with a history of prematurity and no previous vMRI studies when compared to healthy full-term children (de Kieviet et al., 2012, Ment et al., 2009b).
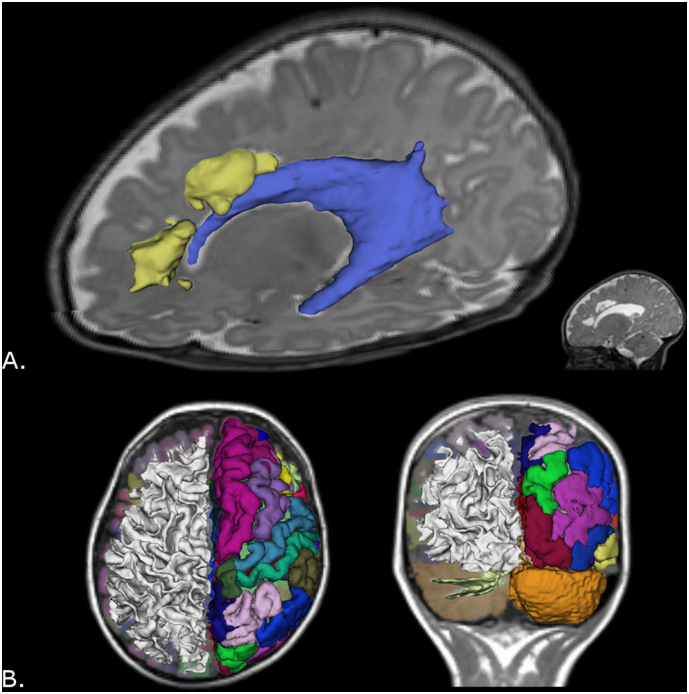
Fig. 3.
A) Volumetric MRI at term-equivalent age in preterm infant with cystic-PVL. Note the cysts or focal component of cystic-PVL (yellow) and dilated left lateral ventricle (blue). B) Automated segmentation, parcellation and 3D reconstruction of the brain and cerebellum in healthy child at 8 years old. Right cerebral and cerebellar hemispheres: white matter 3D reconstruction. Left cerebral and cerebellar hemispheres: cortical parcellation and 3D reconstruction. Image B in radiological convention. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A correct segmentation is necessary for accurate measurements and volumetric 3D reconstruction. Manual segmentation is monotonous and time consuming, as well as subjective and operator-dependent; whereas automated segmentation allows researchers to identify, parcel, and create reproducible 3D reconstructions of more brain regions and in less time (for an example, see Fig. 3-B) (Devi et al., 2015). However, automated segmentation is a challenge in neonatal and infant MRI due to poor contrast between white matter and gray matter in T1- and T2-weighted MRI. Besides, the intrinsic complexity of volumetric processing requires specialists in neonatal neuroimaging.
5.1.1.1. Overcoming the neonatal and pediatric volumetric MRI challenges
It is essential for neonatal image processing (volumetric or other quantitative techniques) to accurately classify/segment tissue and delineate the white matter/gray matter boundary. This is challenging in neonates and infants due to poor contrast between white matter and gray matter in T1- and T2-weighted MRI, but sophisticated techniques are actively being developed to address this shortcoming. Specifically, recent efforts have led to the development of longitudinal atlases for mapping the infant neocortex, and such resources are likely to play a particularly valuable role in future pediatric neuroimaging research. Zhang et al. (2016), for example, developed a spatiotemporal, longitudinal atlas of the developing infant brain by decomposing longitudinally acquired MRI volumes using wavelets, and then accommodating spatial and temporal variability into these volumes via group-sparse construction. Similarly, Kim et al. (2016) developed multi-atlas approaches for tissue classification and obtained encouraging results that demonstrate the ability of their method to map cortical development and to reveal abnormalities of cortical folding associated with preterm birth. Researchers such as Moeskops et al. (2015) have successfully distinguished gray matter from white matter in very young brains by leveraging a multimodal—rather than a unimodal—approach to tissue classification. Since multimodal imaging is becoming increasingly commonplace in neonatal imaging studies, it is likely that the impact of image analysis methods that leverage several modalities and weightings will be of substantial and growing benefit to the field.
The structural properties of the developing brain are substantially more dynamic than in adulthood or typical aging, which indicates that the task of pediatric MRI atlas construction must not simply be concerned with quantifying and understanding how brain structure differs between childhood and adulthood. To generate a robust anatomic atlas of the developing brain, one must also be able to map structural changes as they occur as a function of time (Shi et al., 2010). This fundamental necessity of pediatric MRI processing has been acknowledged by Li et al. (2015), who developed a robust longitudinal brain atlas of the young brain after repeatedly scanning hundreds of children of various ages over several years in order to generate a four-dimensional (space + time) atlas of brain development. Such atlases, which accommodate brain changes as a highly dynamic—rather than static—and continuous—rather than discrete—process, are likely to become the norm for pediatric MRI processing, particularly when studying brain changes that occur very rapidly over several months or even weeks, as is often the case in neonates.
5.1.2. Diffusion MRI
5.1.2.1. Diffusion tensor imaging
Diffusion tensor imaging (DTI) is an advanced quantitative MRI technique that allows a non-invasive assessment of the microstructural characteristics of brain fasciculi. The diffusion tensor model is used to obtain parameters of water diffusion in the brain tissue, such as fractional anisotropy (FA), apparent diffusion coefficient (ADC), axial diffusivity (AD), radial diffusivity (RD), and the average of ADC or mean diffusivity (MD; sum of the radial and axial diffusivity divided by three) (Basser and Pierpaoli, 1996, Le Bihan, 1995). The quantitative information provided by DTI can be used in neonatal and pediatric clinical practice to detect diffuse white matter injury, since changes in water diffusion are early indicators of cell damage (Hüppi and Dubois, 2006). As demonstrated by Beaulieu (2002), FA values are closely correlated with the microstructure of brain fasciculi (axonal structure, density, and size of the fasciculus). RD is related to molecular diffusion in the perpendicular axis of the axon, providing information regarding oligodendrocytes (Concha et al., 2006) and the neurodevelopmental process (Hüppi and Dubois, 2006).
5.1.2.1.1. Diffusion parameters in preterm neonates and infants with white matter abnormalities
Diffusion parameters are age-related; FA increases while MD and RD decrease with age (Dubois et al., 2014). It has been shown that delayed neurodevelopment in premature infants with a perinatal diagnosis of WMA, GMH-IVH, and/or periventricular hemorrhagic infarction is accompanied by consistent changes in diffusion parameters (high RD/MD and/or low FA) (Arzoumanian et al., 2003, Cheong et al., 2009, Drobyshevsky et al., 2007, Pandit et al., 2013, Rose et al., 2015). Furthermore, the diffusion parameters could be used to support diagnosis in subtle pathology (Hinojosa-Rodríguez et al., 2013). Liu et al. (2012) found significantly increased RD and MD, mainly in anterior and superior thalamic radiation, and significantly decreased FA in superior thalamic radiation in preterm infants with mild WMA. Similar results were observed in the corpus callosum (van Pul et al., 2012).
DTI parameter findings in preterm infants at TEA (or near-term) reveal microstructural alterations of major bundles of white matter in relation to risk factors and/or neurological comorbidity (Dubois et al., 2014, Kwon et al., 2014, Malavolti et al., 2016, Ment et al., 2009a, Pandit et al., 2013, Rose et al., 2014, Tusor et al., 2014). Moreover, some authors consider DTI of major white matter bundles at TEA or near-term age a more sensitive biomarker of later neurodevelopment than conventional MRI (Rose et al., 2014).
5.1.2.2. Diffusion tensor tractography
Through analysis of water anisotropic diffusion, it is possible to obtain in vivo 3D reconstructions of the white matter fasciculi based on the principle of directionality of water movement as modeled by the diffusion tensor (Ciccarelli et al., 2008). Tractography enables one to observe the morphology, location, distribution, and connectivity of white matter and its subsequent correlation with neurodevelopment (Anderson et al., 2015, Harmony et al., 2016, Hinojosa-Rodríguez et al., 2013, Kaur et al., 2014). Indeed, due to the anatomic and histological characteristics of the neuropathological spectrum that affects premature infants, the study and assessment of white matter fasciculi (primarily corticospinal tract and corpus callosum) by tractography and diffusion parameters (Braga et al., 2015) is likely an essential element of modern diagnosis.
There are other promising models for the study of white matter, including the study of crossing fibers (e.g., high-angular resolution diffusion imaging and diffusion spectrum imaging). However, the acquisition time for this analysis is an issue to consider in clinical practice (Dubois et al., 2014).
5.1.3. Functional MRI
Functional magnetic resonance imaging or fMRI is a neuroimaging technique for studying brain function using a type of MRI contrast called BOLD (Blood Oxygen Level Dependent). The nature of BOLD contrast originates mainly from changes in signal magnitude of T2*w sequences, caused by the increased amount of oxygen (diamagnetic) in hemoglobin. The increase in oxyhemoglobin (with decrease in deoxyhemoglobin) is due to increased cerebral vascular flow that aims to cover the metabolic needs of the activated neurons. Thus, the positive BOLD signal apparently corresponds to the post-synaptic electrical activity of the local group of neurons (Lauritzen, 2005). The optimum analysis of the BOLD signal depends on the model adjustment of hemodynamic response function, which varies according to subject age (Harris et al., 2011, Seghier et al., 2006). There are even reports of subtle variations in the hemodynamic response function between preterm infants and term infants (Arichi et al., 2012). Current efforts in the field of pediatric neuroimaging are focused on accurately characterizing the hemodynamic response function in fetal and neonatal population. fMRI is not yet considered “diagnostic” in evaluating or predicting the outcome of this population. However, fMRI has two modalities that are promising for the study of the motor cortex in perinatal brain injury. These modalities are fMRI with motor task and resting-state fMRI (Kirton, 2013).
5.1.3.1. fMRI with motor task
A simple motor task, such as squeezing a ball with the grasp reflex or the active or passive mobilization of any limb, can produce a detectable change in the BOLD signal (Arichi et al., 2014, Graham et al., 2015, Van de Winckel et al., 2013), which provides reliable anatomical and functional information about the motor cortex of infants with perinatal brain injury (Kirton, 2013, Staudt, 2010). In this sense, Scheef et al. (2017) found by passive stimulation with extension/flexion during an fMRI study that the lateralization of the sensorimotor cortex is present in extremely preterm infants (born at 26 weeks of GA). Most notably, the authors highlight that the neonatal fMRI (using optimized coil settings) allows a robust and reproducible sensorimotor cortex analysis at the single subject level, which increases its feasibility in clinical practice. Furthermore, the use of this technique allows the study of the evolution and/or motor recovery of patients treated with rehabilitation therapies (Bleyenheuft et al., 2015).
5.1.3.2. Resting-state fMRI
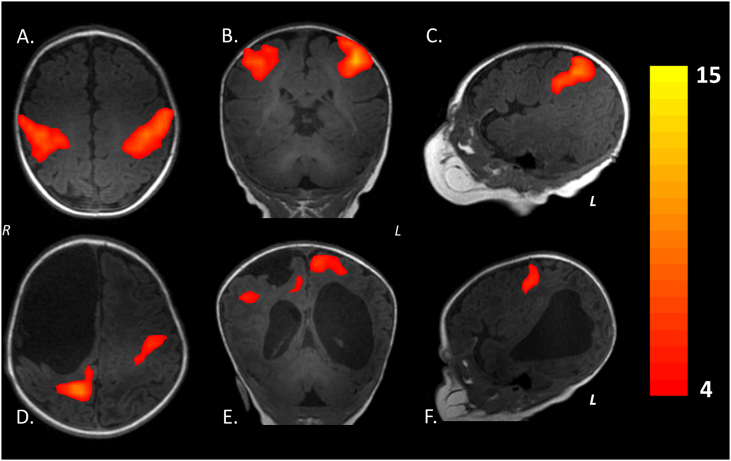
In this modality, the temporal correlations of low frequency (< 0.1 Hz) in BOLD signal fluctuations are detected. These fluctuations represent the neuronal base activity in absence of an activity or predetermined motor task. Therefore, the resting-state fMRI reflects the synchronic and spontaneous electrical activity of neural networks in resting state (Fox and Raichle, 2007). The resting-state fMRI allows the developmental assessment of the various functional brain networks (e.g., sensorimotor, auditory/language, default-mode, salience, medial occipital, occipital pole, lateral visual/parietal, and two lateralized frontoparietal) in the first months of life (see Fig. 5) (Gao et al., 2015). It also helps demonstrate neuroplastic changes (Adhikari et al., 2015, Manning et al., 2015, Smyser and Neil, 2015) associated with early therapeutic intervention. However, this procedure neither diagnoses nor predicts the outcome in premature infants.
Fig. 5.
Sensorimotor network by resting-state fMRI at term-equivalent age during physiological sleep (without anesthesia or sedation). A-C) Healthy female term infant. D-F) Male preterm infant (born at 35 gestational weeks) with clinical history of right periventricular hemorrhagic infarction. Currently with diagnosis by structural MRI of porencephalic cyst with ipsilateral communication between lateral ventricular system and the subarachnoid space. Note the change in resting-state pattern of sensorimotor network in relation to the healthy term infant. Images A, B, D and E are in radiological convention.
5.2. Motor and cognitive prognosis by quantitative MRI
Quantitative MRI is a powerful tool with a potential use in clinical practice for the prediction of short- and long-term motor and cognitive sequelae. Several authors found an association between vMRI in premature infants at TEA and motor outcome, especially in the first 18–24 months of life (Cheong et al., 2016, Guo et al., 2017, Lind et al., 2011, Shah et al., 2006). Lind et al. (2011) described that specifically the cerebellum, total brain tissue, basal ganglia, cerebrum, frontal lobes, and thalamus volumes were significantly smaller, and that ventricle volumes were significantly larger in preterm children with neurodevelopmental impairment at 24 months of corrected age. Cheong et al. (2016) also reported a correlation between cerebellar volumes with motor scores at 2 years old.
Diffusion parameters also provide valuable information for motor prognosis (Arzoumanian et al., 2003, Malavolti et al., 2016, Rose et al., 2007, Roze et al., 2012). In fact, the most studied structures are the PLIC, corticospinal tract, and corpus callosum. Malavolti et al. (2016) found in another large prospective cohort of 193 neonates (24 to 32 weeks of GA) that the corpus callosum of the preterm infant with FA significantly lower has abnormal motor outcomes at 18 months corrected age. In general, FA values from the corpus callosum at TEA correlate with psychomotor development, and lower FA in PLIC is associated with adverse neurodevelopmental outcomes at 12–24 months of life (Kwon et al., 2014).
On the other hand, the sensorimotor activation reported by Scheef et al. (2017) reflects the possibility of using fMRI to distinguish between normal and abnormal motor activation (e.g., unilateral or asymmetric) of the sensorimotor cortex in the neonatal period, which is useful for motor prognosis. Abnormal fMRI findings at TEA correlate with motor outcome at 1 year of life in patients with unilateral lesions (Arichi et al., 2014). Therefore, it is possible that in a near future fMRI could be used as a biomarker in clinical practice with prognostic utility.
Although there are numerous references about the prognostic value of quantitative MRI in relation to motor outcome, there are few regarding quantitative MRI and cognitive outcome. Volumetric measures have shown that the volume of the corpus callosum measured during the first 4 months of age correlates with the mental index of the Bayley Scales of Infant Development in a group of infants evaluated at age 1 (Fernández-Bouzas et al., 2007). The same result was obtained by Thompson et al. (2012) at age 2. Significantly smaller brain volumes at TEA in preterm children with significantly delayed cognitive performance have been observed (Lind et al., 2011). Volumes of the lateral ventricles measured at birth also correlated with the Bayley Scales measured at 18 months adjusted age (Miller et al., 2005). Peterson et al. (2003) acquired measures of cognitive and motor development between 18 and 20 months of corrected age. Positive correlations between regional brain volumes in sensorimotor and parieto-occipital regions and developmental outcome were observed in preterm infants. According to the authors, regional brain volumes near term are a promising marker for predicting disturbances of cognitive outcome in preterm infants. Guo et al. (2017) focused on quantitatively assessing white matter injury in preterm neonates. They found that high white matter injury volumes predicted poor motor outcomes and frontal white matter injury volumes predicted adverse cognitive and language outcomes at 18 months corrected age.
In relation to diffusion measures as predictors, high white matter diffusivity measures of the inferior occipital and cerebellar region at TEA have been associated with increased risk of impairments in motor and executive function at 7 years in VPT children (Thompson et al., 2014).
6. Conclusions
Since neuropathological data about preterm survivors with antecedents of risk factors for perinatal brain damage are unavailable in the clinical practice, it is fundamental to bridge the gap between neuropathology and neonatal and pediatric neuroimaging. Considering the quantitative MRI findings on neonatal and pediatric neuroimaging over the past decade and the increasing acknowledgement of the perinatal brain injury spectrum in premature population, new tools at hand should be used. Quantitative techniques capable of being performed in the clinical environment, such as delineating structural, connectomic, and functional brain abnormalities, exist in noninvasive neuroimaging. These techniques for cases presenting more subtle and diffuse patterns of perinatal brain injury offer a unique opportunity for pediatric neuroimaging diagnosis. Neuroradiological organizations should begin a formal process to critically evaluate current clinical classifications and develop guidelines and “best practices” to use in pediatric neurology.
Search strategy and scope of literature
References for this review were researched in Medline/PubMed and Scopus databases. The inclusion criteria were: 1) preterm infants with perinatal risk factors and/or perinatal brain injury, 2) neuropathological patterns in extremely and very preterm infants, 3) neuroimaging of the preterm infant at term-equivalent age, 4) neuroimaging at term-equivalent age and short- and long-term neurodevelopmental outcome, and 5) motor and cognitive outcome. The search strategy was based on the following terms: “preterm infant”, “preterm neonate”, “extremely preterm”, “very preterm”, “preterm brain injury”, “leukomalacia periventricular”, “non-cystic leukomalacia periventricular”, “diffuse leukomalacia periventricular”, “encephalopathy of prematurity”, “diffuse white matter gliosis”, “white matter abnormalities”, “gray matter abnormalities”, “MRI at term-equivalent age”, “germinal matrix hemorrhage - intraventricular hemorrhage”, “periventricular hemorrhagic infarction”, “conventional MRI”, “T1- and T2-weighted MRI”, “DWI”, “structural MRI”, “quantitative MRI”, “volumetric MRI”, “diffusion tensor image”, “DTI tractography”, “diffusion parameters”, “functional MRI”, “resting-state MRI”, “neurodevelopmental outcome by MRI at term-equivalent age”, “motor outcome”, and “cognitive outcome”.
Conflicts of interest
None.
Acknowledgments
Acknowledgements
Manuel Hinojosa-Rodríguez is a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received fellowship 304834 from CONACYT. This work was partially supported by grants of CONACYT No. 166772 and PAEP-Doctorado en Ciencias Biomédicas 2016 funding project. The authors acknowledge Ms. Teresa Álvarez-Vázquez, P.T. Oliver de Leo-Jiménez, B·Sc. in Electronics Carlos Sair Flores-Bautista, M. in Sc. Juan José Ortiz-Retana, Ph.D. Jesús Edgar Barrera-Reséndiz, Eng. Héctor Belmont-Tamayo, P.T. Alejandro Aguilar-Arriaga, Eng. Paulina Álvarez-García, M. in Sc. Leonor Casanova-Rico, Bch. Ma. de Lourdes Lara Ayala, M.D. Ma. Elena Juárez-Colín and Ph.D. Erick Humberto Pasaye-Alcaraz for their technical support. Authors also acknowledge B.A Jessica González-Norris for the revision of the English version of the manuscript. I acknowledge support by PAPIIT (DGAPA UNAM) IN200917
Contributor Information
Manuel Hinojosa-Rodríguez, Email: manuelhinojosa@comunidad.unam.mx.
Thalía Harmony, Email: thaliah@unam.mx.
John Darrell Van Horn, Email: jvanhorn@usc.edu.
References
- Abernethy L.J., Palaniappan M., Cooke R.W. Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight. Arch. Dis. Child. 2002;87(4):279–283. doi: 10.1136/adc.87.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari M.H., Raja Beharelle A., Griffa A., Hagmann P., Solodkin A., McIntosh A.R., Deco G. Computational modeling of resting-state activity demonstrates markers of normalcy in children with prenatal or perinatal stroke. J. Neurosci. 2015;35(23):8914–8924. doi: 10.1523/JNEUROSCI.4560-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi-Obe M., Saeed N., Cowan F., Rutherford M., Edwards A. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356(9236):1162–1163. doi: 10.1016/s0140-6736(00)02761-6. [DOI] [PubMed] [Google Scholar]
- Ancel P.-Y., Goffinet F., Kuhn P., Langer B., Matis J., Hernandorena X.…Kaminski M. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169(3):230–238. doi: 10.1001/jamapediatrics.2014.3351. [DOI] [PubMed] [Google Scholar]
- Anderson P.J., Cheong J.L.Y., Thompson D.K. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol. 2015;39(2):147–158. doi: 10.1053/j.semperi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Andiman S.E., Haynes R.L., Trachtenberg F.L., Billiards S.S., Folkerth R.D., Volpe J.J., Kinney H.C. Analysis of pyramidal neurons. Brain Pathol. 2010;20(4):803–814. doi: 10.1111/j.1750-3639.2010.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arichi T., Fagiolo G., Varela M., Melendez-Calderon A., Allievi A., Merchant N.…Edwards A.D. Development of BOLD signal hemodynamic responses in the human brain. NeuroImage. 2012;63(2):663–673. doi: 10.1016/j.neuroimage.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arichi T., Counsell S.J., Allievi A.G., Chew A.T., Martinez-Biarge M., Mondi V.…Edwards A.D. The effects of hemorrhagic parenchymal infarction on the establishment of sensori-motor structural and functional connectivity in early infancy. Neuroradiology. 2014:985–994. doi: 10.1007/s00234-014-1412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzoumanian Y., Mirmiran M., Barnes P.D., Woolley K., Ariagno R.L., Moseley M.E.…Atlas S.W. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. Am. J. Neuroradiol. 2003;24(8):1646–1653. ( https://doi.org/13679287) [PMC free article] [PubMed] [Google Scholar]
- Back S.A. Cerebral white and gray matter injury in newborns: new insights into pathophysiology and management. Clin. Perinatol. 2014;41(1):1–24. doi: 10.1016/j.clp.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A., Miller S.P. Brain injury in premature neonates: a primary cerebral dysmaturation disorder? Ann. Neurol. 2014;75(4):469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 2014;41(1):47–67. doi: 10.1016/j.clp.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker B.Q., Larroche J.C. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch. Neurol. 1962;7(5):386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basu S., Zaidi H., Holm S., Alavi A. Quantitative techniques in PET-CT imaging. Curr. Med. Imaging Rev. 2011;7(3):216–233. [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benders M.J.N.L., Kersbergen K.J., de Vries L.S. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin. Perinatol. 2014;41(1):69–82. doi: 10.1016/j.clp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y., Dricot L., Gilis N., Kuo H.-C., Grandin C., Bleyenheuft C.…Friel K.M. Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: a combined DTI, TMS and fMRI pilot study. Res. Dev. Disabil. 2015;43–44:136–149. doi: 10.1016/j.ridd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Bayard J., Valdés-Sosa P.A., Fernández T., Otero G., Pliego Rivero B., Ricardo-Garcell J.…Harmony T. 3D statistical parametric mapping of quiet sleep EEG in the first year of life. NeuroImage. 2012;59(4):3297–3308. doi: 10.1016/j.neuroimage.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Braga R.M., Roze E., Ball G., Merchant N., Tusor N., Arichi T.…Counsell S.J. Development of the corticospinal and callosal tracts from extremely premature birth up to 2 years of age. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broström L., Bolk J., Padilla N., Skiöld B., Eklöf E., Mårtensson G.…Ådén U. Clinical implications of diffuse excessive high signal intensity (DEHSI) on neonatal MRI in school age children born extremely preterm. PLoS One. 2016;11(2):1–12. doi: 10.1371/journal.pone.0149578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloni S.F., Cinnante C.M., Bassi L., Avignone S., Fumagalli M., Bonello L.…Triulzi F. Neurodevelopmental outcome at 36 months in very low birth weight premature infants with MR diffuse excessive high signal intensity (DEHSI) of cerebral white matter. Radiol. Med. 2015:1056–1063. doi: 10.1007/s11547-015-0540-2. [DOI] [PubMed] [Google Scholar]
- Cheong J.L.Y., Thompson D.K., Wang H.X., Hunt R.W., Anderson P.J., Inder T.E., Doyle L.W. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. Am. J. Neuroradiol. 2009;30(3):623–628. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J.L.Y., Thompson D.K., Spittle A.J., Potter C.R., Walsh J.M., Burnett A.C.…Doyle L.W. Brain volumes at term-equivalent age are associated with 2-year neurodevelopment in moderate and late preterm children. J. Pediatr. 2016;174:91–97.e1. doi: 10.1016/j.jpeds.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Childs A.M., Ramenghi L.A., Cornette L., Tanner S.F., Arthur R.J., Martinez D., Levene M.I. Cerebral maturation in premature infants: quantitative assessment using MR imaging. AJNR Am. J. Neuroradiol. 2001;22(8):1577–1582. [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O., Catani M., Johansen-Berg H., Clark C., Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7(8):715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- Concha L., Gross D.W., Wheatley B.M., Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. NeuroImage. 2006;32(3) doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- Counsell S.J., Edwards A.D., Chew A.T.M., Anjari M., Dyet L.E., Srinivasan L.…Cowan F.M. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131(12):3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Cruz-Martínez R., Figueras F., Hernández-Andrade E., Oros D., Gratacos E. Fetal brain Doppler to predict cesarean delivery for nonreassuring fetal status in term small-for-gestational-age fetuses. Obstet. Gynecol. 2011;117(3):618–626. doi: 10.1097/AOG.0b013e31820b0884. [DOI] [PubMed] [Google Scholar]
- de Kieviet J.F., Zoetebier L., van Elburg R.M., Vermeulen R.J., Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev. Med. Child Neurol. 2012;54(4):313–323. doi: 10.1111/j.1469-8749.2011.04216.x. [DOI] [PubMed] [Google Scholar]
- de Vries L.S., Benders M.J.N.L., Groenendaal F. Imaging the premature brain: ultrasound or MRI? Neuroradiology. 2013;55(Suppl. 2):13–22. doi: 10.1007/s00234-013-1233-y. [DOI] [PubMed] [Google Scholar]
- de Vries L.S., Benders M.J.N.L., Groenendaal F. Progress in neonatal neurology with a focus on neuroimaging in the preterm infant. Neuropediatrics. 2015;46(4):234–241. doi: 10.1055/s-0035-1554102. [DOI] [PubMed] [Google Scholar]
- Dean J.M., McClendon E., Hansen K., Azimi-Zonooz A., Chen K., Riddle A.…Back S.A. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci. Transl. Med. 2013;5(168):168ra7. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi C.N., Chandrasekharan A., Sundararaman V.K., Alex Z.C. vol. 64. 2015. Neonatal Brain MRI Segmentation: A Review; pp. 163–178. [DOI] [PubMed] [Google Scholar]
- Dinov I., Lozev K., Petrosyan P., Liu Z., Eggert P., Pierce J.…Toga A. Neuroimaging study designs, computational analyses and data provenance using the LONI pipeline. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A., Bregman J., Storey P., Meyer J., Prasad P.V., Derrick M.…Tan S. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev. Neurosci. 2007;29(4–5):289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- Dubois J., Benders M., Borradori-Tolsa C., Cachia A., Lazeyras F., Ha-Vinh Leuchter R.…Hüppi P.S. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131(8):2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Kulikova S., Poupon C., Hu P.S. vol. 276. 2014. Review the Early Development of Brain White Matter: A Review of Imaging Studies in Fetuses, Newborns and Infants; pp. 48–71. [DOI] [PubMed] [Google Scholar]
- Engelhardt E., Inder T.E., Alexopoulos D., Dierker D.L., Hill J., Van Essen D., Neil J.J. Regional impairments of cortical folding in premature infants. Ann. Neurol. 2015;77(1):154–162. doi: 10.1002/ana.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Bouzas A., Harmony T., Porras-Kattz E., Santiago-Rodríguez E., Ricardo Garcell J., Ortega-Ávila R., Fernández T., Barrera J., Ávila-Acosta D. 50 Congreso Nacional de Ciencias Fisiológicas. México; Puebla: 2007. Evolución de las medidas volumétricas del cuerpo calloso durante el primer año de vida en lactantes con daño cerebral perinatal. [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gano D., Andersen S.K., Partridge J.C., Bonifacio S.L., Xu D., Glidden D.V.…Glass H.C. Diminished white matter injury over time in a cohort of premature newborns. J. Pediatr. 2015;166(1):39–43. doi: 10.1016/j.jpeds.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Elton A., Hernández-Castillo C.R., Smith J.K., Ramirez J., Lin W. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb. Cortex. 2015;25(9):2919–2928. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S.J., Howard S., Hope P.L., Reynolds E.O.R. Periventricular intraparenchymal cerebral haemorrhage in preterm infants: the role of venous infarction. J. Pathol. 1987;151:197–202. doi: 10.1002/path.1711510307. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.A., Lin W., Gao W., Fair D.A. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev. Cogn. Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant E.G., Kerner M., Schellinger D. Evolution of porencephalic cysts from intraparenchymal hemorrhage in neonates: sonographic evidence. Am. J. Neuroradiol. 1982;3(1):47–50. doi: 10.2214/ajr.138.3.467. [DOI] [PubMed] [Google Scholar]
- Grisaru-Granovsky S., Reichman B., Lerner-Geva L., Boyko V., Hammerman C., Samueloff A., Schimmel M.S. Population-based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum. Dev. 2014;90(12):821–827. doi: 10.1016/j.earlhumdev.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Guo T., Duerden E.G., Adams E., Chau V., Branson H.M., Chakravarty M.M.…Miller S.P. Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurology. 2017;88(7):614–622. doi: 10.1212/WNL.0000000000003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton G., Wigglesworth J.S. Origin of intraventricular haemorrhage in the preterm infant. Arch. Dis. Child. 1976;51(9):651–659. doi: 10.1136/adc.51.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmony T., Marosi E., Becker J., Rodríguez M., Reyes A., Fernández T., Silva J., Bernal J. Longitudinal quantitative EEG study of children with different performances on a reading-writing test. Electroencephalogr. Clin. Neurophysiol. 1995;95(6):426–433. doi: 10.1016/0013-4694(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Harmony T., Barrera-Reséndiz J., Juárez-Colín M.E., Carrillo-Prado C., del Consuelo Pedraza-Aguilar M., Asprón Ramírez A.…Ricardo-Garcell J. Longitudinal study of children with perinatal brain damage in whom early neurohabilitation was applied: preliminary report. Neurosci. Lett. 2016;611:59–67. doi: 10.1016/j.neulet.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Harris J.J., Reynell C., Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev. Cogn. Neurosci. 2011;1(3):199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R.L., Sleeper L.A., Volpe J.J., Kinney H.C. Neuropathologic studies of the encephalopathy of prematurity in the late preterm infant. Clin. Perinatol. 2013;40(4):708–722. doi: 10.1016/j.clp.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Rodríguez M., González-Frankenberger B., Harmony-Baillet T., Fernández-Bouzas A., Barragán-Campos H. Organization for Human Brain Mapping; Seattle, WA, USA: 2013. Tractography in Preterm Infants with Perinatal Risk Factors for Hypoxic-ischemic Encephalopathy. (Poster Listings #3454) [Google Scholar]
- Hintz S.R., Barnes P.D., Bulas D., Slovis T.L., Finer N.N., Wrage L.A.…Higgins R.D. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch S., Skiöld B., Hallberg B., Nordell B., Nordell A., Mosskin M.…Blennow M. Cranial ultrasound and MRI at term age in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2010;95(5):F310–F314. doi: 10.1136/adc.2009.161547. [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Dubois J. Diffusion tensor imaging of brain development. Semin. Fetal Neonatal Med. 2006;11(6):489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Warfield S., Kikinis R., Barnes P.D., Zientara G.P., Jolesz F.A.…Volpe J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998;43(2):224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Inder T.E., Hüppi P.S. Periventricular white matter injury in the premature infants id followed by reduced cerebral cortical gray matter volume at term. Ann. Neurol. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Inder T.E., Wells S.J., Mogridge N.B., Spencer C., Volpe J.J. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J. Pediatr. 2003;143(2):171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- Inder T.E., Warfield S.K., Wang H., Hu P.S. vol. 115. 2005. Abnormal Cerebral Structure is Present at Term in Premature Infants; p. 2. [DOI] [PubMed] [Google Scholar]
- Iwata S., Nakamura T., Hizume E., Kihara H., Takashima S., Matsuishi T., Iwata O. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics. 2012;129(5):e1138–e1147. doi: 10.1542/peds.2011-1735. [DOI] [PubMed] [Google Scholar]
- Kaur S., Powell S., He L., Pierson C.R., Parikh N.A. Reliability and repeatability of quantitative tractography methods for mapping structural white matter connectivity in preterm and term infants at term-equivalent age. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet G., Kuperman A.A., Strauss T., Brenner B. Neonatal IVH - mechanisms and management. Thromb. Res. 2011;127(Suppl. 3):S120–S122. doi: 10.1016/S0049-3848(11)70032-9. [DOI] [PubMed] [Google Scholar]
- Kersbergen K.J., Benders M.J.N.L., Groenendaal F., Koopman-Esseboom C., Nievelstein R.A.J., Van Haastert I.C., De Vries L.S. Different patterns of punctate white matter lesions in serially scanned preterm infants. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0108904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersbergen K.J., Leroy F., Igum I., Groenendaal F., de Vries L.S., Claessens N.H.P.…Benders M.J.N.L. Relation between clinical risk factors, early cortical changes, and neurodevelopmental outcome in preterm infants. NeuroImage. 2016;142:301–310. doi: 10.1016/j.neuroimage.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Kesler S.R., Ment L.R., Vohr B., Pajot S.K., Schneider K.C., Katz K.H.…Reiss A.L. Volumetric analysis of regional cerebral development in preterm children. Pediatr. Neurol. 2004;31(5):318–325. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen K., Kersbergen K.J., Groenendaal F., Isgum I., de Vries L.S., Benders M.J.N.L. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J. Matern. Fetal Neonatal Med. 2012;25(S1):89–100. doi: 10.3109/14767058.2012.664343. [DOI] [PubMed] [Google Scholar]
- Kidokoro H., Neil J.J., Inder T.E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am. J. Neuroradiol. 2013;34(11):2208–2214. doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Lepage C., Maheshwary R., Jeon S., Evans A.C., Hess C.P.…Xu D. NEOCIVET: towards accurate morphometry of neonatal gyrification and clinical applications in preterm newborns. NeuroImage. 2016;138:28–42. doi: 10.1016/j.neuroimage.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H.C. The encephalopathy of prematurity: one pediatric neuropathologist's perspective. Semin. Pediatr. Neurol. 2009;16(4):179–190. doi: 10.1016/j.spen.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H.C., Volpe J.J. Modeling the encephalopathy of prematurity in animals: the important role of translational research. Neurol. Res. Int. 2012;2012 doi: 10.1155/2012/295389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H.C., Haynes R.L., Xu G., Andiman S.E., Folkerth R.D., Sleeper L.A., Volpe J.J. Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann. Neurol. 2012;71(3):397–406. doi: 10.1002/ana.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirton A. Modeling developmental plasticity after perinatal stroke: defining central therapeutic targets in cerebral palsy. Pediatr. Neurol. 2013;48(2):81–94. doi: 10.1016/j.pediatrneurol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kuban K., Gilles F.H. Human telencephalic angiogenesis. Ann. Neurol. 1985;17(6):539–548. doi: 10.1002/ana.410170603. [DOI] [PubMed] [Google Scholar]
- Kuban K., Sanocka U., Leviton A., Allred E.N., Pagano M., Dammann O.…Schonfeld S. White matter disorders of prematurity: association with intraventricular hemorrhage and ventriculomegaly. The developmental epidemiology network. J. Pediatr. 1999;134(5):539–546. doi: 10.1016/s0022-3476(99)70237-4. (http://doi.org/S0022347699002073) [DOI] [PubMed] [Google Scholar]
- Kwon S.H., Vasung L., Ment L.R., Hüppi P.S. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin. Perinatol. 2014;41(1):257–283. doi: 10.1016/j.clp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Larroque B., Marret S., Ancel P.Y., Arnaud C., Marpeau L., Supernant K.…Bréart G. White matter damage and intraventricular hemorrhage in very preterm infants: the epipage study. J. Pediatr. 2003;143(4):477–483. doi: 10.1067/S0022-3476(03)00417-7. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat. Rev. Neurosci. 2005;6(1):77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995;8(7–8):375–386. doi: 10.1002/nbm.1940080711. [DOI] [PubMed] [Google Scholar]
- Lequin M.H., Dudink J., Tong K.A., Obenaus A. Magnetic resonance imaging in neonatal stroke. Semin. Fetal Neonatal Med. 2009;14(5):299–310. doi: 10.1016/j.siny.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Li G., Wang L., Shi F., Gilmore J.H., Lin W., Shen D. Construction of 4D high-definition cortical surface atlases of infants: methods and applications. Med. Image Anal. 2015;25(1):22–36. doi: 10.1016/j.media.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C., Soul J.S., Haidar H., Huppi P.S., Bassan H., Warfield S.K.…Volpe J.J. vol. 116. 2005. Among Preterm Infants; p. 4. [DOI] [PubMed] [Google Scholar]
- Lin Y., Okumura A., Hayakawa F., Kato K., Kuno T., Watanabe K. Quantitative evaluation of thalami and basal ganglia in infants with periventricular leukomalacia. Dev. Med. Child Neurol. 2001;43(7):481–485. doi: 10.1017/s0012162201000883. http://www.ncbi.nlm.nih.gov/pubmed/11463180 (Retrieved from) [DOI] [PubMed] [Google Scholar]
- Lind A., Parkkola R., Lehtonen L., Munck P., Maunu J., Lapinleimu H., Haataja L. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr. Radiol. 2011;41(8):953–961. doi: 10.1007/s00247-011-2071-x. [DOI] [PubMed] [Google Scholar]
- Liu Y., Aeby A., Balériaux D., David P., Absil J., De Maertelaer V.…Metens T. White matter abnormalities are related to microstructural changes in preterm neonates at term-equivalent age: a diffusion tensor imaging and probabilistic tractography study. Am. J. Neuroradiol. 2012;33(5):839–845. doi: 10.3174/ajnr.A2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodygensky G.A., Vasung L., Sizonenko S.V., Hüppi P.S. Neuroimaging of cortical development and brain connectivity in human newborns and animal models. J. Anat. 2010;217(4):418–428. doi: 10.1111/j.1469-7580.2010.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavolti A.M., Chau V., Poskitt M.B.K.J. 2016. Association between Corpus Callosum Development on Magnetic Resonance Imaging and Diffusion Tensor Imaging, and Neurodevelopmental Outcome in Neonates Born Very Preterm; pp. 433–440. [DOI] [PubMed] [Google Scholar]
- Manning K.Y., Menon R.S., Gorter J.W., Mesterman R., Campbell C., Switzer L., Fehlings D. Neuroplastic sensorimotor resting state network reorganization in children with hemiplegic cerebral palsy treated with constraint-induced movement therapy. J. Child Neurol. 2015;31(2):1–7. doi: 10.1177/0883073815588995. [DOI] [PubMed] [Google Scholar]
- Marín-Padilla M. Developmental neuropathology and impact of perinatal brain damage. II white matter lesions of the neocortex. J. Neuropathol. Exp. Neurol. 1997;56(3):219–235. doi: 10.1097/00005072-199703000-00001. [DOI] [PubMed] [Google Scholar]
- Marret S., Marchand-Martin L., Picaud J.C., Hascoët J.M., Arnaud C., Rozé J.C.…Ancel P.Y. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One. 2013;8(5):1–9. doi: 10.1371/journal.pone.0062683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-biarge M., Groenendaal F., Kersbergen K.J., Manon J.N. 2016. MRI Based Preterm White Matter Injury Classification: The Importance of Sequential Imaging in Determining Severity of Injury; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment L.R., Hirtz D., Hüppi P.S. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8(11):1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Ment L.R., Kesler S., Vohr B., Katz K.H., Baumgartner H., Schneider K.C.…Reiss A.L. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123(2):503–LP-511. doi: 10.1542/peds.2008-0025. http://pediatrics.aappublications.org/content/123/2/503.abstract (Retrieved from) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.P., Cozzio C.C., Goldstein R.B., Ferriero D.M., Partridge J.C., Vigneron D.B., Barkovich A.J. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. Am. J. Neuroradiol. 2003;24(8):1661–1669. [PMC free article] [PubMed] [Google Scholar]
- Miller S.P., Ferriero D.M., Leonard C., Piecuch R., Glidden D.V., Partridge J.C.…Barkovich A.J. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J. Pediatr. 2005;147(5):609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Moeskops P., Benders M.J.N.L., Chi S.M., Kersbergen K.J., Groenendaal F., de Vries L.S.…Igum I. Automatic segmentation of MR brain images of preterm infants using supervised classification. NeuroImage. 2015;118:628–641. doi: 10.1016/j.neuroimage.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Morel B., Antoni G., Teglas J.P., Bloch I., Adamsbaum C. Neonatal brain MRI: how reliable is the radiologist's eye? Neuroradiology. 2016;58(2):189–193. doi: 10.1007/s00234-015-1609-2. [DOI] [PubMed] [Google Scholar]
- Murray A.L., Scratch S.E., Thompson D.K., Inder T.E., Doyle L.W., Anderson J.F.I., Anderson P.J. Neonatal brain pathology predicts adverse attention and processing speed outcomes in very preterm and/or very low birth weight children. Neuropsychology. 2014;28(4):552–562. doi: 10.1037/neu0000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa T., De Vries L.S., Benders M.J.N.L., Takahara T., Nikkels P.G.J., Groenendaal F. Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging. Neuroradiology. 2011;53(9):669–679. doi: 10.1007/s00234-011-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omizzolo C., Scratch S.E., Stargatt R., Kidokoro H., Thompson D.K., Lee K.J.…Anderson P.J. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory (Hove, England) 2014;22(6):605–615. doi: 10.1080/09658211.2013.809765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliser H.K., Bennett G.A., Kelleher M.A., Cumberland A.L., Walker D.W., Hirst J.J. Prenatal and postnatal determinants of development. NeuroMethods. 2016;109 [Google Scholar]
- Pandit A.S., Ball G., Edwards A.D., Counsell S.J. Diffusion magnetic resonance imaging in preterm brain injury. Neuroradiology. 2013;55(Suppl. 2) doi: 10.1007/s00234-013-1242-x. [DOI] [PubMed] [Google Scholar]
- Papile L.A., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- Parodi A., Morana G., Severino M.S., Malova M., Natalizia A.R., Sannia A.…Ramenghi L.A. Low-grade intraventricular hemorrhage: is ultrasound good enough? J. Matern. Fetal Neonatal Med. 2013;7058(January) doi: 10.3109/14767058.2013.796162. [DOI] [PubMed] [Google Scholar]
- Peterson B.S., Anderson A.W., Ehrenkranz R., Staib L.H., Tageldin M., Colson E.…Ment L.R. Regional brain volumes and their neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111(5):939–948. doi: 10.1542/peds.111.5.939. http://pediatrics.aappublications.org/content/111/5/939.abstract (Retrieved from) [DOI] [PubMed] [Google Scholar]
- Pierson C.R., Folkerth R.D., Billiards S.S., Trachtenberg F.L., Drinkwater M.E., Volpe J.J., Kinney H.C. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114(6):619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone R., Counsell S.J., Kapellou O., Dyet L., Kennea N., Hajnal J.…Edwards A.D. Perinatal cortical growth and childhood neurocognitive abilities. Neurology. 2011;77(16):1510–1515. doi: 10.1212/WNL.0b013e318233b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S.M., Dagia C.D., Ditchfield M.R., Carlin J.B., Reddihough D.S. Population-based studies of brain imaging patterns in cerebral palsy. Dev. Med. Child Neurol. 2014;56(3):222–232. doi: 10.1111/dmcn.12228. [DOI] [PubMed] [Google Scholar]
- Reidy N., Morgan A., Thompson D.K., Inder T.E., Doyle L.W., Anderson P.J. Impaired language abilities and white matter abnormalities in children born very preterm and/or very low birth weight. J. Pediatr. 2013;162(4):719–724. doi: 10.1016/j.jpeds.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie J.M., Kendall G.S. Advanced magnetic resonance techniques provide more accurate prognostic information than conventional imaging. Dev. Med. Child Neurol. 2015;57(5):403–404. doi: 10.1111/dmcn.12680. [DOI] [PubMed] [Google Scholar]
- Riddle A., Dean J., Buser J.R., Gong X., Maire J., Chen K.…Back S.A. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann. Neurol. 2011;70(3):493–507. doi: 10.1002/ana.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J., Mirmiran M., Butler E.E., Lin C.Y., Barnes P.D., Kermoian R., Stevenson D.K. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev. Med. Child Neurol. 2007;49(10):745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- Rose J., Vassar R., Cahill-Rowley K., Stecher Guzman X., Hintz S.R., Stevenson D.K., Barnea-Goraly N. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. NeuroImage Clin. 2014;5:169–177. doi: 10.1016/j.nicl.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J., Cahill-Rowley K., Vassar R., Yeom K.W., Stecher X., Stevenson D.K.…Barnea-Goraly N. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18–22 months of age: an MRI and DTI study. Pediatr. Res. 2015;78:1–9. doi: 10.1038/pr.2015.157. (1530-0447 (Electronic)) [DOI] [PubMed] [Google Scholar]
- Roze E., Harris P.A., Ball G., Elorza L.Z., Braga R.M., Allsop J.M.…Counsell S.J. Tractography of the corticospinal tracts in infants with focal perinatal injury: comparison with normal controls and to motor development. Neuroradiology. 2012;54(5):507–516. doi: 10.1007/s00234-011-0969-5. [DOI] [PubMed] [Google Scholar]
- Rutherford M.A. 4th ed. Saunders Ltd.; 2001. MRI of the Neonatal Brain.http://www.mrineonatalbrain.com/ [Google Scholar]
- Rutherford M., Srinivasan L., Dyet L., Ward P., Allsop J., Counsell S., Cowan F. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr. Radiol. 2006;36(7):582–592. doi: 10.1007/s00247-006-0164-8. [DOI] [PubMed] [Google Scholar]
- Rutherford M.A., Supramaniam V., Ederies A., Chew A., Bassi L., Groppo M.…Ramenghi L.A. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology. 2010;52(6):505–521. doi: 10.1007/s00234-010-0700-y. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Shankaran S., Laptook A.R., Sood B.G., Do B., Stoll B.J.…Barks J. Screening cranial imaging at multiple time points improves cystic periventricular leukomalacia detection. Am. J. Perinatol. 2015;32(10):973–979. doi: 10.1055/s-0035-1545666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L., Nordmeyer-Massner J.A., Smith-Collins A.P., Müller N., Stegmann-Woessner G., Jankowski J.…Boecker H. Functional laterality of task-evoked activation in sensorimotor cortex of preterm infants: an optimized 3 T fMRI study employing a customized neonatal head coil. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Lazeyras F., Hüppi P.S. Functional MRI of the newborn. Semin. Fetal Neonatal Med. 2006;11(6):479–488. doi: 10.1016/j.siny.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Setänen S., Lehtonen L., Parkkola R., Aho K., Haataja L., Ahtola A.…Ylijoki M. Prediction of neuromotor outcome in infants born preterm at 11 years of age using volumetric neonatal magnetic resonance imaging and neurological examinations. Dev. Med. Child Neurol. 2016;58(7):721–727. doi: 10.1111/dmcn.13030. [DOI] [PubMed] [Google Scholar]
- Shah D.K., Anderson P.J., Carlin J.B., Pavlovic M., Howard K., Thompson D.K., Inder T.E. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr. Res. 2006;60(1):97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- Shi F., Fan Y., Tang S., Gilmore J.H., Lin W., Shen D. Neonatal brain image segmentation in longitudinal MRI studies. NeuroImage. 2010;49(1):391–400. doi: 10.1016/j.neuroimage.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie L.T.L., Van Der Knaap M.S., Van Wezel-Meijler G., Taets Van Amerongen A.H.M., Lafeber H.N., Valk J. Early MR features of hypoxic-ischemic brain injury in neonates with periventricular densities on sonograms. Am. J. Neuroradiol. 2000;21(5):852–861. [PMC free article] [PubMed] [Google Scholar]
- Slaughter L.A., Bonfante-Mejia E., Hintz S.R., Dvorchik I., Parikh N.A. Early conventional MRI for prediction of neurodevelopmental impairment in extremely-low-birth-weight infants. Neonatology. 2016;43215:47–54. doi: 10.1159/000444179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Neil J.J. Use of resting-state functional MRI to study brain development and injury in neonates. Semin. Perinatol. 2015;39(2):130–140. doi: 10.1053/j.semperi.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Kidokoro H., Inder T.E. Magnetic resonance imaging of the brain at term equivalent age in extremely premature neonates: to scan or not to scan? J. Paediatr. Child Health. 2012;48(9):794–800. doi: 10.1111/j.1440-1754.2012.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltirovska Salamon A., Groenendaal F., van Haastert I.C., Rademaker K.J., Benders M.J.N.L., Koopman C., de Vries L.S. Neuroimaging and neurodevelopmental outcome of preterm infants with a periventricular haemorrhagic infarction located in the temporal or frontal lobe. Dev. Med. Child Neurol. 2014;56(6):547–555. doi: 10.1111/dmcn.12393. [DOI] [PubMed] [Google Scholar]
- Spittle A.J., Cheong J., Doyle L.E.X.W., Roberts G., Lee K.J., Lim J.…Anderson P.J. 2011. Neonatal White Matter Abnormality Predicts Childhood Motor Impairment in Very Preterm Children; pp. 4–6. (Ci) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M. Reorganization after pre- and perinatal brain lesions. J. Anat. 2010;217(4):469–474. doi: 10.1111/j.1469-7580.2010.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggerda S.J., Leijser L.M., Walther F.J., van Wezel-Meijler G. Neonatal cranial ultrasonography: how to optimize its performance. Early Hum. Dev. 2009;85(2):93–99. doi: 10.1016/j.earlhumdev.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Stoll B.J., Hansen N.I., Bell E.F., Walsh M.C., Carlo W.A., Shankaran S.…Higgins R.D. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Warfield S.K., Carlin J.B., Pavlovic M., Wang H.X., Bear M.…Inder T.E. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(3):667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- Thompson D.K., Inder T.E., Faggian N., Warfield S.K., Anderson P.J., Doyle L.W., Egan G.F. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. NeuroImage. 2012;59(4):3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Lee K.J., Egan G.F., Warfield S.K., Doyle L.W., Anderson P.J., Inder T.E. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex. 2014;52:60–74. doi: 10.1016/j.cortex.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tich S.N.T., Anderson P.J., Shimony J.S., Hunt R.W., Doyle L.W., Inder T.E. A novel quantitative simple brain metric using MR imaging for preterm infants. Am. J. Neuroradiol. 2009;30(1):125–131. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach J.A., Merhar S.L., Kline-Fath B.M., Pratt R.G., Loew W.M., Daniels B.R.…Dumoulin C.L. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. Am. J. Roentgenol. 2014;202(1) doi: 10.2214/AJR.13.10613. [DOI] [PubMed] [Google Scholar]
- Tusor N., Arichi T., Counsell S.J., Edwards A.D. Brain development in preterm infants assessed using advanced MRI techniques. Clin. Perinatol. 2014;41(1):25–45. doi: 10.1016/j.clp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Van de Winckel A., Klingels K., Bruyninckx F., Wenderoth N., Peeters R., Sunaert S.…Feys H. How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Res. Dev. Disabil. 2013;34(1):183–197. doi: 10.1016/j.ridd.2012.07.030. [DOI] [PubMed] [Google Scholar]
- van der Aa N.E., Benders M.J.N.L., Vincken K.L., Groenendaal F., de Vries L.S. The course of apparent diffusion coefficient values following perinatal arterial ischemic stroke. PLoS One. 2013;8(2):1–6. doi: 10.1371/journal.pone.0056784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn J.D., Toga A. Human neuroimaging as a “big data” science. Brain Imaging Behav. 2014;8(2) doi: 10.1007/s11682-013-9255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pul C., van Kooij B.J.M., De Vries L.S., Benders M.J.N.L., Vilanova A., Groenendaal F. Quantitative fiber tracking in the corpus callosum and internal capsule reveals microstructural abnormalities in preterm infants at term-equivalent age. Am. J. Neuroradiol. 2012;33(4):678–684. doi: 10.3174/ajnr.A2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilborg E., Heijnen C.J., Benders M.J., van Bel F., Fleiss B., Gressens P., Nijboer C.H. Impaired oligodendrocyte maturation in preterm infants: potential therapeutic targets. Prog. Neurobiol. 2016;136:28–49. doi: 10.1016/j.pneurobio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- van Wezel-Meijler G., Steggerda S.J., Leijser L.M. Cranial ultrasonography in neonates: role and limitations. Semin. Perinatol. 2010;34(1):28–38. doi: 10.1053/j.semperi.2009.10.002. [DOI] [PubMed] [Google Scholar]
- van't Hooft J., van der Lee J.H., Opmeer B.C., Aarnoudse-Moens C.S.H., Leenders A.G.E., Mol B.W.J., de Haan T.R. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst. Rev. 2015;4(1):71. doi: 10.1186/s13643-015-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116(1):221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. 5th ed. Saunders/Elsevier; Philadelphia: 2008. Neurology of the Newborn. [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol. 2009;24(9):1085–1104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Neonatal neurology—my personal journey and some lessons learned. Pediatr. Neurol. 2014;51(6):753–757. doi: 10.1016/j.pediatrneurol.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Volpe J.J., Kinney H.C., Jensen F.E., Rosenberg P.A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 2011;29(6):565–582. doi: 10.1016/j.ijdevneu.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D., Friedman H., Minich N., Fanaroff, A. a, & Hack, M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115(4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- Woodward L.J., Anderson P.J., Austin N.C., Howard K., Inder T.E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Woodward L.J., Clark C.A., Pritchard V.E., Anderson P.J., Inder T.E. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev. Neuropsychol. 2011;36(1):22–41. doi: 10.1080/87565641.2011.540530. [DOI] [PubMed] [Google Scholar]
- Woodward L.J., Clark C.A.C., Bora S., Inder T.E. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M., Imabayashi E., Matsuda H., Nakagawara J., Takahashi M., Shimosegawa E.…Iida H. Quantitative assessment of rest and acetazolamide CBF using quantitative SPECT reconstruction and sequential administration of 123I-iodoamphetamine: comparison among data acquired at three institutions. Ann. Nucl. Med. 2014;28(9):836–850. doi: 10.1007/s12149-014-0879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shi F., Wu G., Wang L., Yap P.-T., Shen D. Consistent spatial-temporal longitudinal atlas construction for developing infant brains. IEEE Trans. Med. Imaging. 2016;35(12):2568–2577. doi: 10.1109/TMI.2016.2587628. [DOI] [PMC free article] [PubMed] [Google Scholar]