Abstract
Insufficient suppression and connectivity of the default mode network (DMN) is a potential mediator of cognitive dysfunctions across various disorders, including attention deficit/hyperactivity disorder (ADHD). However, it remains unclear if alterations in sustained DMN suppression, variability and connectivity during prolonged cognitive engagement are implicated in adult ADHD pathophysiology, and to which degree methylphenidate (MPH) remediates any DMN abnormalities. This randomized, double-blinded, placebo-controlled, cross-over clinical trial of MPH (clinicaltrials.gov/ct2/show/NCT01831622) explored large-scale brain network dynamics in 20 adults with ADHD on and off MPH, compared to 27 healthy controls, while performing a reward based decision-making task. DMN task-related activation, variability, and connectivity were estimated and compared between groups and conditions using independent component analysis, dual regression, and Bayesian linear mixed models. The results show that the DMN exhibited more variable activation patterns in unmedicated patients compared to healthy controls. Group differences in functional connectivity both between and within functional networks were evident. Further, functional connectivity between and within attention and DMN networks was sensitive both to task performance and case-control status. MPH altered within-network connectivity of the DMN and visual networks, but not between-network connectivity or temporal variability. This study thus provides novel fMRI evidence of reduced sustained DMN suppression in adults with ADHD during value-based decision-making, a pattern that was not alleviated by MPH. We infer from multiple analytical approaches further support to the default mode interference hypothesis, in that higher DMN activation variability is evident in adult ADHD and associated with lower task performance.
Keywords: Adult ADHD, Dopamine, Reward, Decision-making, fMRI, Functional networks, DMN
Highlights
-
•
Increased temporal variance of the default mode is associated with poor decision-making.
-
•
Ability to sustain default mode suppression is reduced in adult ADHD.
-
•
The DMN is more highly connected to attention networks in ADHD.
-
•
Methylphenidate increased DMN precuneus connectivity, but has otherwise weak effects.
-
•
Excessive activity of the DMN is evident in ADHD and associated with poor decision-making.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterised by age-inappropriate inattention and/or impulsivity/hyperactivity (American Psychiatric Association, 2013). Individuals with ADHD display deficits in higher order decision-making processes (Sagvolden et al., 2005, Sonuga-Barke et al., 2016). In childhood and adolescence, psycho-stimulant medication effectively reduces ADHD symptoms (Fredriksen and Peleikis, 2015) and ameliorate abnormal functioning in dopamine (DA) dense fronto-striatal and mesolimbic brain networks in ADHD due to the inhibition of synaptic reuptake of DA in these networks (Arnsten, 2006, Volkow et al., 2009, Volkow et al., 2005) – effects that are reflected in improved functioning in executive functions and reward processing in ADHD (Barkley et al., 2001, Mowinckel et al., 2015). ADHD persists into adulthood for many patients (Biederman et al., 2011), but it is currently unclear whether normalization of brain function from stimulant medication is equivalent in adults and children with the condition (Bush et al., 2008, Epstein et al., 2007, Schrantee et al., 2016).
Brain-behaviour relations are built on the interaction between multiple intrinsic functional neural networks comprised of spatially distributed regions through temporally correlated activity (Beckmann and Smith, 2004, Smith et al., 2009). Disruptions within or between such networks may result in behavioural abnormalities (Sonuga-Barke and Castellanos, 2007, Sripada et al., 2014). These intrinsic functional networks are most commonly studied during rest, but show coherence during both rest and task engagement (Smith et al., 2009). One approach to elucidating the involvement of functional brain networks on cognitive operations is to study how strongly specific networks are engaged during cognitive tasks, for instance, by analysing the correlation between network time series and known task parameters. Another way of assessing the relevance of functional networks is by studying temporal BOLD signal variance. It is presumed that signal variance reflects the dynamic allocation of resources to brain regions utilized during cognitive operations, such that greater variability is indicative of greater flexibility or adaptability of those regions (Faisal et al., 2008, Grady and Garrett, 2014).
It has been proposed that DA modulates signal integrity in the brain (Garrett et al., 2015). A recent study found that younger, higher performing adults had greater blood-oxygen level dependent (BOLD) signal variability in task-relevant cortical regions than older (likely DA depressed) adults during a working-memory task (Garrett et al., 2015). Interestingly, d-amphetamine (which increases DA availability) increased signal variability in the older participants, which also improved task performance. This raises the question whether abnormal signal variability may be found in psychopathologies with known DA deficits, such as ADHD, and whether a DA agonist will increase BOLD variability in task-positive networks and task performance.
Disrupted interplay within and between “task-positive” (i.e. networks essential for task execution) and “task-negative” functional networks (i.e. networks supporting off-task processing) is implicated in ADHD where it manifests as inattention and impulsivity (Castellanos and Proal, 2012, Sonuga-Barke and Castellanos, 2007). In particular, the task-negative default mode network (DMN), which includes the posterior cingulate cortex, precuneus, and medial frontal cortex, has been hypothesized to play a pivotal role. The DMN is mostly active during rest, attenuated during externally oriented cognition, and is thought to be involved in self-referential or internally oriented processes (Andrews-Hanna et al., 2010). Specifically, the default mode interference hypothesis proposes that inattentiveness in ADHD may be caused by insufficient down-regulation of the DMN during goal-oriented processes (Castellanos and Proal, 2012, Sonuga-Barke and Castellanos, 2007). Reduced suppression of the DMN may disrupt task-positive networks and lead to lapses of attention and excessive “mind-wandering”. Weissman et al. (2006), for instance, showed that reduced deactivation of the precuneus (posterior DMN) preceded attentional lapses during a goal-oriented task. Indeed, patients with ADHD have documented weakened within-network connectivity in the DMN and several task-positive networks (Kessler et al., 2014, Uddin et al., 2009), as well as increased functional and structural connectivity between the DMN and task-positive networks during rest (Kessler et al., 2014).
The pattern of decreased within network connectivity and increased between network connectivity has furthermore lead to the proposal that functional networks are less clearly segregated in ADHD (Hoekzema et al., 2013, Kessler et al., 2014). Functional connectivity networks are more strongly differentiated in adults than children (Fair et al., 2008, Rubia, 2013), and it has been suggested that reduced differentiation of functional networks in patients with ADHD is caused by a brain maturation lag (Fair et al., 2010, Sripada et al., 2014). Reports of more diffuse functional networks also in adults with ADHD suggest that network differentiation reaches an earlier plateau than in healthy controls (Hoekzema et al., 2013), and the disruptions in functional connectivity thus become more established features of the mature ADHD brain.
Functional connectivity is suggested to be improved by increased DA availability (Nagano-Saito et al., 2008). Liddle et al. (2011) found that DMN suppression during task was improved by MPH, and others have found that MPH increases DMN suppression as a function of task difficulty (Metin et al., 2015). Hence, the therapeutic effect of MPH, a DA reuptake inhibitor frequently prescribed for treating ADHD symptoms, may partly be mediated through its effects on large-scale brain connectivity. Importantly, while MPH is often only discussed in the context of DA, it also has an agonistic effect on the noradrenaline (NA) system (Ziegler et al., 2016). There are therefore two routes by which increased striatal DA can influence cortical functioning, despite low expression of the DAT in the cortex. First, manipulation of cortical activity through striatal DA via the cortico-striatal loop neurons (e.g. Grace et al., 2007, Krugel et al., 2009). And secondly, due to the relatively stronger expression of the NA transporter (Schroeter et al., 2000), via heightened NA availability in the cortex.
More generally, Sidlauskaite et al. (2016) question whether documented abnormalities in DMN suppression in ADHD are in fact reflecting difficulties in sustaining DMN suppression, as their study shows that initial DMN suppression when cued to execute a task is not impaired in ADHD. However, this hypothesized dysfunction in sustaining DMN suppression has yet to be explored in ADHD. Moreover, a study of network connectivity found that MPH reduced the connectivity between the DMN and attention networks during rest in healthy adults (Sripada et al., 2013). Only one study, however, has explored disruptions both within and between functional networks in adults with ADHD (Kessler et al., 2014), and the possible amelioration of such disruptions by MPH remains unexplored.
Taken together research suggests that patients with ADHD struggle to engage with tasks due to weak down-regulation of the DMN during task transitions, and that MPH increases the ability to suppress the DMN and also reduces its temporal coherence with task-positive networks. In this double-blinded, placebo-controlled, crossover trial of MPH on adults with ADHD, we aimed to characterise functional brain-network abnormalities during decision-making in adult patients with ADHD and the possible ameliorating effects of MPH. To this end, based on event-related fMRI data obtained from 20 patients and 27 controls, where the patients were randomly assigned to MPH (short: medicated patients) or placebo (short: unmedicated patients) in a repeated-measures cross-over design. We used group independent component analysis (ICA) (Beckmann and Smith, 2004) to define the group level brain network nodes and dual regression (Filippini et al., 2009) to estimate the individual spatial maps and associated time series. A multiple analysis approach was adopted to illuminate the subject from different angles, where we assessed the intrinsic networks' engagement by known task variables, the temporal variance of the networks, and both the within and between network connectivity of the networks. We expected typical task-positive networks to have BOLD time series that correlate with decision phases of the task GLM, indicating that these networks are involved in task execution. Further, we anticipated reduced task related engagement of functional networks in adults with ADHD compared to controls during task transitions, and that MPH would improve task related modulations of networks. We additionally anticipated that temporal variability in task-positive networks would be reduced in the patient group compared to controls. Given the documented abnormalities in DMN activation during task in ADHD, we also predicted increased temporal DMN variability in the patient group, indicating that the DMN is overactive when it should be suppressed. Because of previous reports of DA agonists increasing BOLD variability, we also predicted that MPH would increase temporal variability in task-positive networks. In order to support previous reports of less segregated functional networks in ADHD, we lastly investigate within-network connectivity in the posterior DMN (precuneus) and between-network connectivity between the DMN and task positive networks in ADHD unmedicated patients, and also expected an amelioration of any connectivity abnormalities as a result of MPH.
2. Methods and materials
This is a secondary investigation of a randomized double-blinded placebo-controlled clinical trial of methylphenidate on adults with ADHD (clinicaltrials.gov/ct2/show/NCT01831622), and we only briefly describe the study protocol here. Participants were tested at the Intervention Centre at Oslo University Hospital, Rikshospitalet. The entire testing procedure lasted 2–3 h, including two 30-min sessions in the scanner. The study was approved by the Regional Committee of Medical Health Research Ethics (South-East Norway; identifiers: 2011/1585 and 2012/1105), and the Norwegian Medicines Agency (EudraCT: 2012-005246-38). Informed consent was obtained from all participants, and the research was carried out in compliance with the Helsinki Declaration.
2.1. Participants
All participants had to be between 18 and 40 years old and have normal or corrected to normal vision (full list of exclusion criteria in Supplemental materials S1.1). ADHD patients were recruited through an outpatient clinic at Vestfold Hospital Trust, Norway, and were diagnosed and currently receiving care at the same clinic. The ADHD diagnosis was ascertained by a multistage and multisource procedure according to DSM-IV-TR criteria (American Psychiatric Association, 1994), described in detail in Supplemental materials S1.2. Patient participants could not currently be receiving pharmacological treatment for other psychiatric disorders.
The controls were recruited through a random selection of 3.000 men and women through the Norwegian Tax Agency (Skatteetaten), and received an invitation via mail to participate in the research project. Among the 372 persons who responded to the query, only participants matching patient participants on age and sex were contacted. The responding prospective controls had generally high levels of education, and matching for this proved difficult. Control participants were screened with the Adult ADHD self-report scale (Kessler et al., 2007) via telephone, and were excluded if they scored 15 or higher on the first 6 items (Hines et al., 2012). Thirty-three control participants were included.
Twenty-eight clinically diagnosed adults with ADHD (19 female/9 male) and 33 healthy age-matched controls (21f/12m) performed a reward-based decision-making task during functional MRI acquisition. Participants were well-matched on age before exclusions (mean [SD] age ADHD = 27.5 [1.17], controls = 27.3 [1.03]). Fourteen participants were excluded for either technical issues with their data (2 ADHD, 1 control), were lost to follow-up (1 ADHD, 1 control), responding to too few trials (3 ADHD, 1 control) or having performance at chance levels (< 60% accuracy; 2 ADHD, 1 control). A total of 20 patients (13f/7m) and 27 controls (19f/8m) were included in the final analyses. All viable datasets were included to maintain statistical power. While there was a robust difference in age after exclusion (patients being meanly 2.5 (SD 1.9) year younger than controls), the practical difference between a 27 and 29-year-old, ages where there are few expected cognitive differences related to age, should be negligible. All control participants were right-handed (missing data from one participant), and in the patient group there were 4 left, 15 right, and 1 ambidextrous participants. All male patients were diagnosed as predominantly inattentive subtype, while the female patients were both inattentive (8) and of combined type (5). Summaries of group demographic variables and task performance are summarized in Table 1.
Table 1.
Summary of posterior probabilities of group demographics and task performance. Education is measured as the years to completion of highest achieved degree. Scores from the Wechsler's Adult Intelligence Scale (WAIS-IV) are age-scaled. Difference between the groups was tested with Bayesian linear mixed models, and the posterior probabilities are summarized by the mean, standard deviation, and 95% highest density interval (HDI). The credibility of the difference distribution is assessed by the proportion of the distribution that is above or below 0 (Prop. > 0 <), which can be seen as the posterior confidence for a directed effect. Difference distributions where over 90% of posterior distribution of parameter differences are above or below zero are highlighted in bold.
| Summary of posterior probabilities | ||||||
|---|---|---|---|---|---|---|
| Measure | Group | Mean | SD | 95% HDI | Prop. ≥ 0 < | |
| Age (years) | ADHD | 29.90 | 1.41 | 27.21 | 32.68 | |
| Controls | 27.42 | 1.23 | 25.17 | 29.98 | ||
| CON-ADHD | − 2.48 | 1.86 | − 6.09 | 1.12 | 0.088 | |
| Education (years) | ADHD | 12.25 | 0.46 | 11.41 | 13.19 | |
| Controls | 14.55 | 0.39 | 13.77 | 15.29 | ||
| CON-ADHD | 2.30 | 0.60 | 1.16 | 3.51 | 1.000 | |
| Visit interval (days) | ADHD | 32.14 | 2.02 | 28.35 | 36.32 | |
| Controls | 27.08 | 1.84 | 23.50 | 30.65 | ||
| CON-ADHD | − 5.06 | 2.73 | − 10.61 | 0.41 | 0.036 | |
| WAIS Matrices | ADHD | 12.46 | 0.71 | 11.08 | 13.82 | |
| Controls | 12.44 | 0.61 | 11.25 | 13.60 | ||
| CON-ADHD | − 0.01 | 0.93 | − 1.84 | 1.76 | 0.498 | |
| WAIS Similarities | ADHD | 10.87 | 0.78 | 9.43 | 12.52 | |
| Controls | 13.03 | 0.65 | 11.81 | 14.36 | ||
| CON-ADHD | 2.16 | 1.04 | 0.11 | 4.20 | 0.982 | |
| Accuracy (log odds) | Effect of ADHD | 0.54 | 0.20 | 0.14 | 0.93 | 0.995 |
| Effect of MPH | 0.17 | 0.13 | − 0.08 | 0.42 | 0.910 | |
| Placebo | 1.87 | 0.16 | 1.55 | 2.20 | ||
| Controls | 2.33 | 0.66 | 1.04 | 3.64 | ||
| MPH | 2.04 | 0.17 | 1.69 | 2.37 | ||
| Response time (ms) | Effect of ADHD | 56.57 | 59.56 | − 61.11 | 175.90 | 0.831 |
| Effect of MPH | − 8.29 | 37.36 | − 79.50 | 65.89 | 0.410 | |
| Placebo | 1026.80 | 51.83 | 927.56 | 1132.53 | ||
| Controls | 920.52 | 197.76 | 541.24 | 1315.31 | ||
| MPH | 1018.52 | 51.25 | 917.17 | 1116.42 | ||
| RT variability (coefficient) | Effect of ADHD | 0.02 | 0.02 | − 0.02 | 0.05 | 0.862 |
| Effect of MPH | 0.00 | 0.02 | − 0.03 | 0.03 | 0.449 | |
| Placebo | 0.33 | 0.02 | 0.30 | 0.36 | ||
| Controls | 0.37 | 0.06 | 0.26 | 0.49 | ||
| MPH | 0.33 | 0.02 | 0.30 | 0.36 | ||
2.2. Experimental procedures
In a double-blinded crossover procedure, ADHD patients were administered MPH one session and placebo the other in randomized order. All patients were thus tested both on and off MPH. Randomization was un-blinded after the last participant completed the study, assuring that both study personnel and participants remained blind to the pharmacological intervention. Details on the blinding procedures are in Supplemental materials S1.3. The number of patients allocated to the different doses (close to their prescribed doses) was: 6 to instant-release tablets (IR-T) 10 mg, 3 to IR-T 20 mg, 7 to slow-release capsules (SR-C) 20 mg, and 4 to SR-C 40 mg.
Blood samples from volunteering patient participants were collected between two MRI acquisition sequences to confirm patients were unmedicated in the placebo condition and quantify the amount of ritalinic acid (metabolite of methylphenidate) in their blood serum during testing (details on the acquisition and results from the blood analyses can be found in Supplemental materials S1.4). Blood samples were batch analysed after data collection was completed.
2.3. Behavioural task
The value-based decision-making task used in this study was first introduced by Basten et al. (2010) and is comprised of two stages. Outside the scanner, participants first learn to associate 6 positive and 6 negative monetary value ranges to stimuli through trial and error. The participants train on the stimulus association until a criterion of 95% correct is achieved, with a minimum number of 180 trials and maximally for 270 trials. In the fMRI experiment, participants are presented with one composite figure consisting of both an associated positive and negative value range, and are asked to accept stimuli with summed positive values and reject the negative. Participants could win up to 250 NOK (~ 30 USD) based on their performance. The participants viewed the stimuli on a screen through mirrors, and responses were recorded using a response box with the index and middle fingers of the participants' dominant hand. Participants completed 88 trials in two runs, 176 trials per session. Stimuli were presented for maximally 2.5 s or until a response was given, and were jittered between 4 and 10 s (mean 5.7 s).
2.4. MRI
2.4.1. Image acquisition
Scanning was conducted on a 3 Tesla Philips Achieva whole-body scanner, with an 8 channel Philips SENSE head coil (Philips Medical Systems). Two sets of functional data were collected with the following sequencing parameters: 333 volumes, 3 × 3 × 3 mm, TE = 30 ms, TR = 2.3 s, resulting in 12 min of event-related functional scans. Anatomical images were acquired with a T1 weighted scan with voxel size of 1 × 1 × 1 mm; 180 sagittal slices; TR: 6.6 ms. Participants were given coil padding to reduce head movement.
2.4.2. Image processing
Removal of skull and non-brain tissue of the anatomical images was conducted with Freesurfer (Ségonne et al., 2004), and functional images were pre-processed with FMRIB's FSL (Jenkinson et al., 2012). Pre-processing included MCFLIRT motion correction, non-linear registration to MNI space, high-pass filtering (100 s), slice-timing correction, brain extraction, spatial smoothing (FWHM = 6 mm), intensity normalization, and single-session independent component analysis (ICA) using MELODIC (Beckmann and Smith, 2004). ICA-based Xnoisefier FIX (Salimi-Khorshidi et al., 2014) was used to identify and remove noise components on an individual level using a machine learning approach after initial pre-processing (with a custom training data set for Philips scanner, threshold: 20; p(component = signal) < 20%). This method has been shown to effectively reduce the impact of head motion, while retaining temporal degrees-of-freedom and increasing reproducibility of independent components (Pruim et al., 2015).
2.4.3. Analysis
A group-PCA approach (Smith et al., 2014) was conducted using MELODIC including all scans requesting 40 components. A model order of 40 should provide sufficiently low number of components for easy interpretation, while still maintaining adequate spatial segmentation. Following this, the individual time series and component spatial maps were estimated using dual regression (Filippini et al., 2009). Based on the recommendation of Kelly et al. (2010), we went through the spatial distribution of the components and identified 22 noise components. The time-series from these 22 components were regressed out of the remaining dataset. The remaining 18 clean components and associated time series constituted the nodes in the subsequent analyses with a total of 188 datasets (47 participants at two time points with two fMRI sequences from each time point). The 18 nodes were classified into functional networks according to correspondence with BrainMap 20-networks identified in Smith et al. (2009) (see Supplemental table S1.5-1 for spatial correlations and Supplemental fig. S1.5-1 for all 40 components). We additionally identified a node in the orbitofrontal cortex (OFC; IC 27), a region known to be involved in decision-making (Hare et al., 2008), and a subcortical node primarily consisting of the striata (IC 29), which are important for value-representation during decision-making (Basten et al., 2010). Both of the additional nodes include regions that have shown impaired function during decision-making in ADHD (Mowinckel et al., n.d, Ströhle et al., 2008, Wilbertz et al., 2012).
The 18 nodes were classified to known functional networks both by manual inspection and through voxel-wise spatial correlation to a previously described independent component atlas (Smith et al., 2009), and are summarized in Fig. 1. Node 1 was identified as the main component of the default mode network, accompanied by node 20 (red). The cerebellum is covered by node 16 (orange), and the auditory cortices by node 26 (yellow). The visual network is comprised of nodes 4, 8, and 9 (dark green), and the sensorimotor network includes nodes 13 and 17 (light blue). The frontoparietal network is comprised of 6 nodes (purple; 2, 6, 7, 10, 11, and 12), where node 6 likely reflects the dorsal attention network (DAN) and node 7 and 12 likely reflect the right and left ventral attention networks (VAN), respectively. The executive control, or “salience”, network is identified as node 5 (pink). In addition to the nodes described by Smith et al. (2009), we identified a subcortical node 29 (light green) and an orbitofrontal cortex node 27 (dark blue).
Fig. 1.
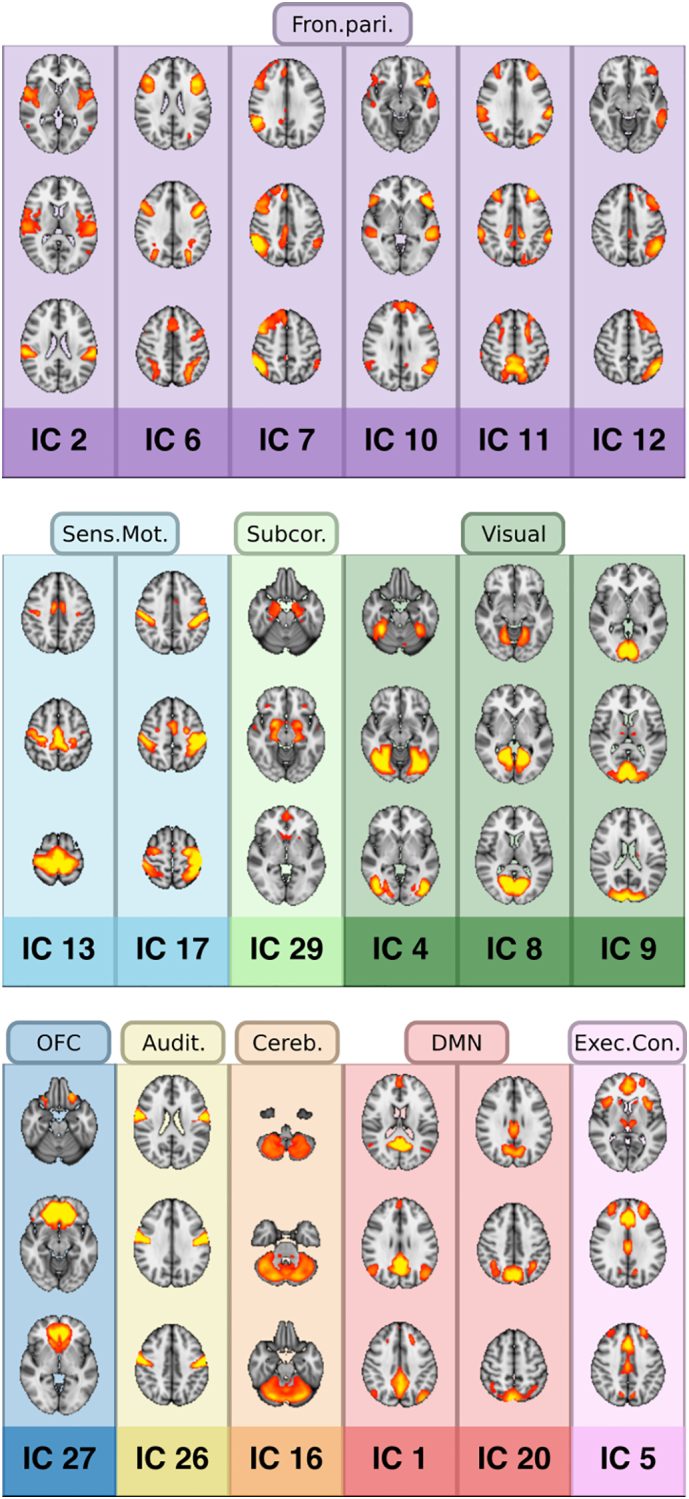
Network overview. The 18 nodes identified from the independent component analysis, classified into networks by referencing the Smith et al. (2009) published ICA atlas. Purple = frontoparietal network; Dark blue = orbitofrontal cortex (OFC); Light blue = sensorimotor; Orange = cerebellum; Red = default mode network (DMN); Dark green = visual network; Yellow = auditory network; Light green = subcortical network; Pink = executive control network (Exec.Contr.). (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
After obtaining subject-specific time series for the 18 nodes, general linear models (GLMs) with decision phase, the positive and negative value of each stimulus (gain and loss), the absolute difference of the two latter (difficulty), and trial accuracy were convolved with a double-gamma HRF and used as linear predictors for the subject specific node time series. The regressors gain, loss, and difficulty were orthogonalized with respect to decision phase, as advised in Mumford et al. (2015). The unmodulated decision phase regressor may then be interpreted as the mean activation across trials. This gave us individual-level beta-weights for the task-parameters of interest (decision phase, gain, loss, accuracy, and difficulty) for each node. Nodes relevant for task execution are expected to show task-dependent BOLD-activations, task-negative networks like the DMN are expected to show activity reductions in response to task events. Nodes showing modulation by choice and changes due to the drug-manipulation or difference between groups were targeted as nodes of interest for subsequent analyses.
Secondly, we ran analyses on the node time series variance and the pairwise node-to-node regularized partial correlations (between-network connectivity) using FSLNets (v. 06). As node variance, we calculated the subject specific time series variance of the 18 nodes. For the pairwise node correlations, we calculated partial correlations between the time series of the 18 nodes, which resulted in 153 unique combinations that we here call edges. Each edge is thus the partial correlation between two of the 18 nodes, and were estimated using L1-regularized partial correlations in FSLNets, with automatic strength regularization of lambda on the individual subject level (Kaufmann et al., 2016, Ledoit and Wolf, 2003).
For assessment of within-network connectivity, cross-subject whole-brain voxel-wise permutation tests were run on nodes correlated with task parameters using FSL's non-parametric tool randomize (Winkler et al., 2014). GLMs were run with 5000 permutations, voxel-wise correction for multiple comparisons with threshold-free cluster enhancement (TFCE) (Salimi-Khorshidi et al., 2011) and Bonferroni correction per node tested at p < 0.05. The subject specific whole-brain maps were submitted to randomize with GLMs specifying participant condition (MPH, placebo, control), with contrasts comparing each group to each other in a repeated design (control vs. placebo, control vs. MPH, placebo vs. MPH) with block-wise permutations. This provides metrics on within-node connectivity in each node that is reliably different between groups or due to the drug-manipulation.
2.5. Statistical analysis
R statistical software (v. 3.2.5) (RCore Team. R., 2013) with packages for Bayesian hierarchical mixed model analyses (Rstan [v. 2.9.0-3], Rstanarm [v. 2.9.0-4]) (Carpenter et al., 2017, Stan Development Team, 2016) was used for inference testing on the edges, node variance, and task parameter betas. Bayesian hierarchical regressions were run with behavioural measures from the task (accuracy, response time, and response time variability [standard deviation of response time divided by mean response time]), task betas, node variance, and edge correlations as dependent variables. To shrink top-level regression weights towards zero, Rstanarm's default weakly informative priors were used for analyses. By using multilevel Bayesian models, the point estimates are shifted towards each other and towards the main effect(s), resulting in posterior densities that are appropriately conservative without sacrificing power (Gelman et al., 2012), which negates the need for multiple comparison corrections (Gelman and Tuerlinckx, 2000).
Three competing models were run with a general set-up estimating an overall effect of the dependent variable and two varying terms; one allowing for varying subject specific intercepts, and another with node/edge specific intercepts, and are summarized in Inline supplementary table 1. The models for task-betas nested task parameters within each node, such that the model estimated node specific intercepts as well as parameter specific intercepts for each node. Model 1 had the basic set-up described above, and did not account for the participants being sampled from two different populations or that there was a drug-manipulation. The second model added a random group slope to the node/edge varying term, thus estimating differences between the two groups (controls and ADHD). The third model exchanged the group slope estimation with treatment slope estimation (placebo, control and MPH) in the node/edge term and additionally added treatment slope to the subject specific varying term. Similar models to model 3 were run substituting treatment slopes with mean task accuracies, to identify whether task performance could account for changes in the nodes/edges.
Model descriptions. Syntax description of the three competing models for each of the three dependent measures. The first parenthesis describes the term of interest, where one estimates intercepts for each node or edge (node pairs).
Note that for the task betas, each parameter (intercept, gain, loss, difficulty, accuracy) is hierarchically nested under each node (/), meaning a general node intercept is estimated as well for each parameter. Models 2 and 3 furthermore estimate differing slopes for groups (ADHD vs. controls) and treatments (ADHD placebo, ADHD methylphenidate, controls), allowing estimates to vary between the groups.
All models were run with four Hamiltonian Monte Carlo chains of 10,000 iterations each, whereby the first 5000 samples of each chain were discarded before further analysis (i.e. warm-up). Hence, all reported results were based on 20,000 posterior samples (5000 per chain). We assessed convergence of all models by assuring that all Rhat values were below 1.1, and that the minimum number of effective samples were above 300 (Gelman and Rubin, 1992). To control the number of divergent iterations, the target acceptance rate (adapt_delta) of Stan's Hamiltonian Monte Carlo algorithm was set to 0.99 (Stan Development Team, 2015) (see Supplemental figs. S1.6.1–3 for MCMC diagnostic plots for the three main analyses). For the Bayesian analysis, we report the mean, standard deviation, and the 95% highest density interval of the posterior distributions of the parameters of interest, as well as the posterior probability that the effect is larger than zero. If the mean of the posterior probability distribution is smaller than zero, the difference is at the opposite tail end of the distribution and we provide the posterior probability that the effect is smaller than zero, for ease of interpretation. The amount of the posterior distribution that is above or below 0 provides a measure of confidence that there is a directed effect, such that the closer the proportions are to 1 the more reliable the effect is.
The three competing models were fitted with a step-wise increase of model complexity, and compared with leave-one-out (LOO) cross-validation with Pareto-smoothed importance sampling (Vehtari et al., 2015). Best fitting models were determined by having the highest expected log point-wise predictive density (ELPD), and if several models were of equal fit, the model with the lowest complexity was deemed best fitting. Of the three linear mixed models we fit (modelling participants as belonging to one, two, or three groups), model 3 (which included all three conditions: control, placebo, and methylphenidate) had the highest LOO-estimates, and all reported values are from this model (see Supplemental tables S1.7.1–2 for summary of the expected predictive accuracy of all the models). We measure the effect of having ADHD by comparing healthy controls to the patients when in the placebo condition, and the effect of methylphenidate by comparing patients on and off methylphenidate.
2.5.1. Possible confounding subject motion
The functional data was pre-processed in several steps to reduce the influence of noise on the results (i.e. motion correction and FIX). To assess possible retaining effects of subject motion on the subsequent analyses, we tested volume-wise relative motion with Bayesian linear mixed models across the three conditions with a varying term allowing for subject specific intercept. As these data were less complex than the main models described above and the purpose was to do data checks, the model was run with 2000 samples of which half were warm-up. This model was also run on subject specific numbers of estimated independent components (ICs), proportion of classified noise ICs, and relative and absolute variance removed by the FIX noise reduction. Subject specific mean relative motion was also added as a confounder in the mixed models of node variance, edge connectivity and task modulation and compared to models without the confound to assess the importance of motion on the model-fit. However, while several extra steps were employed to minimize the effects of head motion on statistical inference, we cannot completely rule out that some residual head motion may still influence the results.
3. Results
3.1. Task performance
Table 1 summarizes the results from the Bayesian hierarchical linear mixed model regressions on task performance that revealed that unmedicated patients had lower accuracy than controls, and that they made fewer errors in the MPH condition compared to placebo. Unmedicated patients had slower responses than controls, and this was not changed by MPH. There were no credible differences between controls and unmediated patients or between drug conditions in response time variability (standard deviation of RT divided by mean RT).
3.2. In-scanner subject motion
Results from the motion analyses indicated that when on methylphenidate, the patients moved less in the scanner than on placebo. Also, the patients when on methylphenidate had fewer individual level IC's estimated and less absolute variance removed by FIX than when they were unmedicated. There was no difference between patients on placebo and controls (Supplemental fig. S1.8.1). In light of these results, we added mean relative motion as a linear predictor to the linear mixed models of node variance, edge correlations and task related node activations. These models were compared to the models without the motion predictor with LOO to assess which models were a better fit to the data. Of the linear mixed models for the node variance, the model that included mean relative motion had greater ELPD estimates and was thus the best fitting model to the data. No other models were improved by adding mean relative motion.
3.3. Task related node activation
To assess node modulation by the decision-making task, node time series were tested with GLMs with task parameters, such as gain and loss value of the stimuli. All nodes but one (frontoparietal node 7) showed either positive (nodes 4–6, 8–9, 16–17, 20) or negative (nodes 1–2, 10–13, 26–27, 29) activity changes by the decision phase, 15 with trial-by-trial accuracy (positive: 2, 4, 9, 11–13, 16–17, 20, 26, 29; negative: 1, 5, 7, 10), and 6 nodes with difficulty (positive: 2, 4–5, 8–10). No nodes were associated with stimulus gain or loss value (see Supplemental table S1.9.1 for summary of the posterior probabilities with credible differences between groups, and Supplemental table S2.1 for all results from the task GLM). There were seven nodes where the decision phase modulation additionally showed credible differences between controls and patients on and off medication (Fig. 2), and seven nodes where the change by trial accuracy was credibly different between controls and patients. None of the task modulations were altered by methylphenidate.
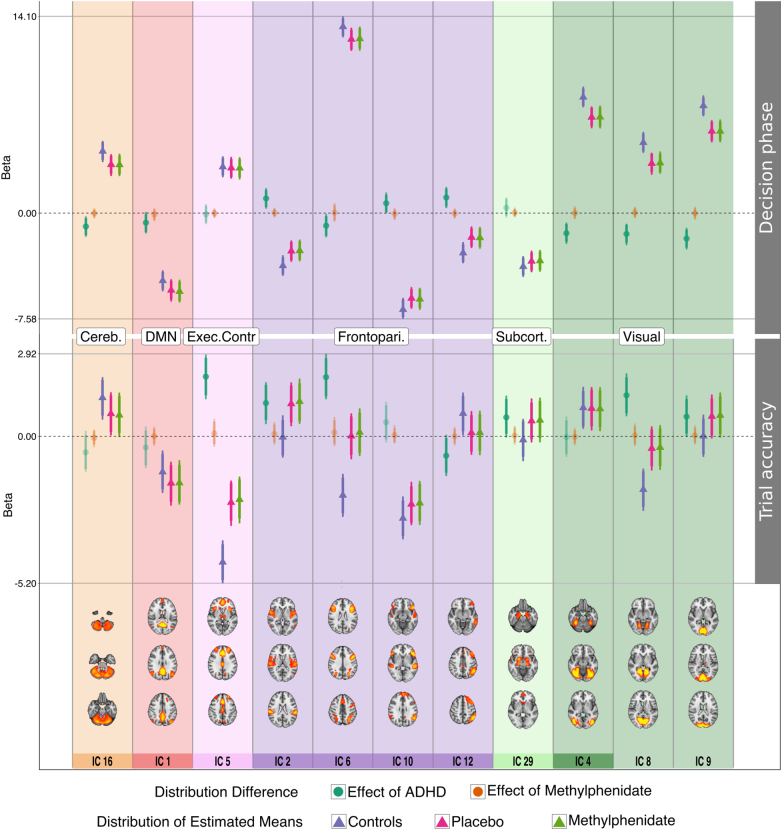
Fig. 2.
Node change by decision-making task parameters. Eleven nodes showed credible difference between groups in relation to decision phase and/or trial accuracy. Triangles are the estimated mean distributions for the groups, circles are the calculated difference distributions between groups (effect of ADHD: controls-placebo; effect of MPH: methylphenidate-placebo). The error bars denote the 95% highest density interval of the distributions. Solid horizontal lines are the value limits of the plots. Particularly DMN node 1 showed interesting negative co-variation with both decision phase and accuracy, which was stronger in the patient placebo condition compared to controls in the decision-phase. The node with the strongest positive association with choice onset was frontoparietal node 6 (DAN), where controls show increased activity compared to patients. This node encompasses the intraparietal sulcus, an area implicated in evidence accumulation in value-based decision-making (Basten et al., 2010). Activation in node 5 (executive control) showed a strong negative association with trial accuracy, which was more pronounced in controls compared to patients. Cereb. = cerebellum; DMN = default mode network; Exec.Contr. = executive control network; Frontopari. = frontoparietal network; Subcort. = subcortical network.
3.3.1. Decision phase
Comparing the controls to unmedicated patients, the cerebellum (node 16) was less activated by the decision phase in the patients off medication (difference distribution summary; mean: − 0.96; 95% highest density interval: − 1.69 to − 0.24; proportion of the difference distribution that is above/below zero: 1.00), and so was the DAN (node 6) (M: − 0.90; HDI: − 1.73 to − 0.07; prop. < 0: 0.98), and the three visual nodes (node 4 [M: − 1.44; HDI: − 2.20 to − 0.68; prop. < 0: 1.0], node 8 [M: − 1.49; HDI: − 2.3 to − 0.77; prop. < 0: 1.0], node 9 [M: − 1.82; HDI: − 2.60 to − 1.06; prop. < 0: 1.0]). The patients off medication had weaker negative modulation of frontoparietal nodes 10 (M: 0.71; HDI: − 0.01–1.47; prop. > 0: 0.97), 2 (M: 1.05; HDI: 0.32–1.79; prop. > 0: 1.00), and 12 (left VAN) (M: 1.12; HDI: 0.36–1.87; prop. > 0: 1.0) compared to controls. Unmedicated patients showed stronger negative modulation of DMN node 1 by the decision phase compared to controls (M: − 0.69; HDI: − 1.45–0.08; prop. < 0: 0.96).
3.3.2. Trial-by-trial accuracy
In trials where participants answered correctly, unmedicated patients had weaker negative activation change of the executive control network than controls (node 5, M: 2.11; HDI: 1.32–2.89; prop. > 0: 1.0), and weaker positive activation change of the left VAN (node 12, M: − 0.69; HDI: − 1.40–0.05; prop. < 0: 0.97). Patients off medication weakly activated the DAN (node 6) in correctly answered trials while controls disengaged it (M: 2.10; HDI: 1.31–2.92; prop. > 0: 1.0). Controls did not show any activity change relating to trial accuracy, whereas unmedicated patients positively activated the OFC (M: 0.67; HDI: − 0.04–1.44; prop. > 0: 0.97) and frontoparietal node 2 (M: 1.18; HDI: 0.43–1.91; prop. > 0: 1.0) on correct trials. The opposite was true for visual node 8, which showed deactivation on correct trials in controls but not in patients off medication (M: 1.45; HDI: 0.71–2.23; prop. > 0: 1.0).
3.4. Node temporal variance
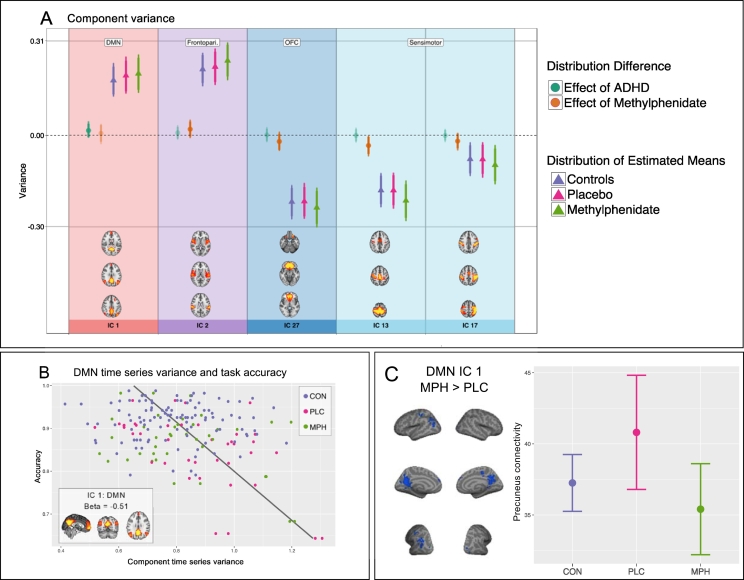
Of the linear mixed models of component variance, model 3 with mean relative motion as an additional fixed factor, was the best fitting model for the data (model comparisons including mean relative motion are in Supplemental materials S1.7.1). As can be seen in Fig. 3A, in the main DMN component (node 1) patients had higher DMN variance than controls (M: 0.02; HDI: − 0.01–0.05; prop. > 0: 0.90). MPH reduced node variance in the two sensorimotor nodes (node 13 [M: − 0.03; HDI: − 0.07–0.0; prop. < 0: 0.99], node 17 [M: − 0.02; HDI: − 0.05–0.01; prop. < 0: 0.92]), and the OFC (node 27 [M: − 0.02; HDI: − 0.05–0.01; prop. < 0: 0.99]). MPH increased the variance of frontoparietal node 2 (M: 0.02; HDI: − 0.01–0.05; prop. > 0: 0.90). Summary of estimates of node variance are in Supplemental table S2.2. The DMN (node 1) was the only node whose variance was related to overall task accuracy, where participants with low DMN variance also had high overall task accuracy (M: − 0.51; HDI: − 0.85 to − 0.77; prop. < 0: 1.0; Fig. 3B), an effect which was not reliably different between the groups. Summary of node variance models for group differences and task accuracy are shown in Supplemental fig. S1.10.1 and Supplemental table S2.3.
Fig. 3.
Component time series signal variance and the DMN. Nodes showing credible difference between groups/conditions with regards to node variance. Triangles are the estimated mean distributions for the groups, circles are the calculated difference distributions between groups (effect of ADHD: controls- placebo; effect of MPH: methylphenidate-placebo). (B) The DMN temporal variance showed negative correlation to task accuracy, participants who performed well on the task also had little variance in the DMN, with no difference between the groups. (C) The precuneus was more strongly connected to the rest of the DMN when patients were on placebo than on methylphenidate (C left: inflated brain). Mean connectivity scores (error bars are 2 * standard deviation in both directions) from this ROI (p < 0.004) also indicate that the participants on placebo had higher connectivity than the controls, and that patients on methylphenidate had closer to normal precuneus connectivity (C right: error bar plot). DMN = default mode network.
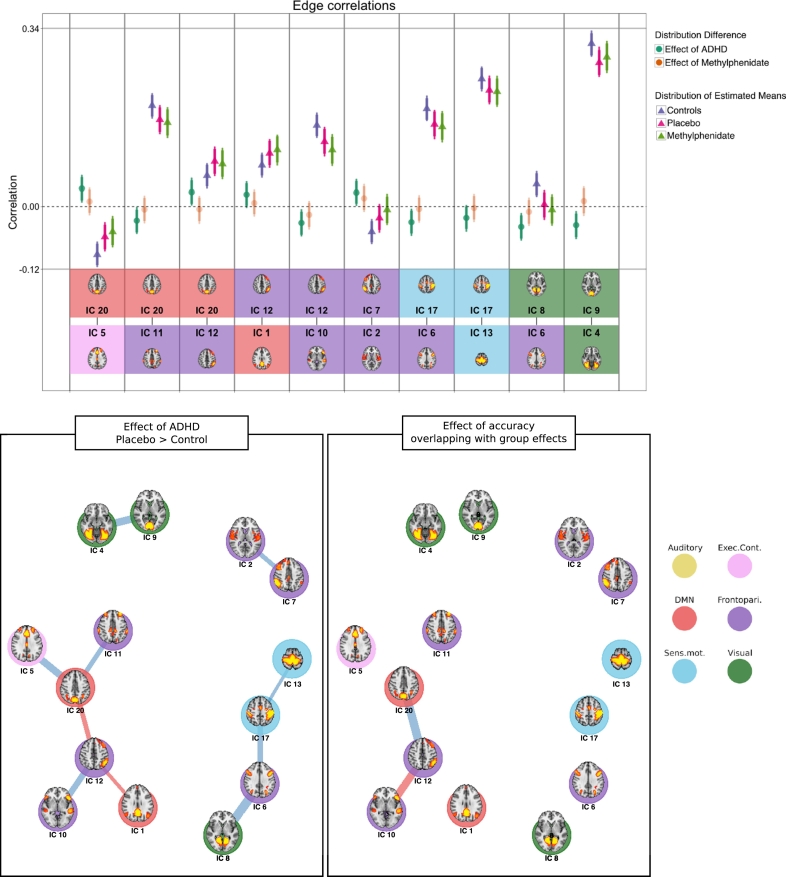
3.5. Between node connectivity (edges)
As can be seen in Fig. 4, the positive coupling between the left VAN (node 12) and DMN node 1 (M: 0.02; HDI: − 0.00–0.05; prop. > 0: 0.97) and 20 (M: 0.03; HDI: − 0.06 to − 0.01; prop. > 0: 0.99) was stronger for the patients than the controls. Otherwise, there was a general decrease of connectivity when comparing unmedicated patients with controls between several nodes, including between two visual nodes (4 and 9), two pairs of frontoparietal nodes (2 and 7, 10 and 12), and between executive, frontoparietal and sensorimotor nodes (see Supplemental table S1.11.1 for summary of the credible edges). Among these, there was weakened negative coupling when patients were unmedicated compared to controls between DMN node 20 and executive control node 5 (M: 0.03; HDI: 0.01–0.06; prop. > 0: 1.00). There was also weaker positive coupling between the DAN (node 6) and sensorimotor node 17 (M: − 0.03; HDI: − 0.5 to − 0.00; prop. < 0: 0.99), and an absence of credible coupling between the DAN and visual node 8 (M: − 0.04; HDI: − 0.07 to − 0.01; prop. < 0: 1.00) when comparing controls to unmedicated patients. The negative correlation between the auditory network (node 26) and the executive control network was weakened by MPH (M: 0.02; HDI: − 0.00–0.04; prop. < 0: 0.95). Please see Supplemental table S2.4 for summary of all posterior probabilities for between group comparisons.
Fig. 4.
Edge correlations. Top panel: Triangles are the estimated mean distributions for the groups, circles are the calculated difference distributions between groups. Error bars denote the 95% highest density interval of the distribution. Bottom two rows depict the two nodes connected by the edge. Bottom panels: Graph representation of edges between nodes for contrasts between patients when on placebo and controls (left), and edges correlated with overall task accuracy that are also different between patients and controls (right). Blue lines indicate a negative difference; red lines indicate a positive difference. The line thickness represents the magnitude of the difference. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
Several edges were correlated to overall task accuracy (see Supplemental fig. S1.11.1 for credible effects and Supplemental table S2.5 for summary of all regressions). Of particular interest were two edges connected to the left VAN, as these two edges show a similar pattern of connectivity when comparing controls to patients when on placebo. A strong positive connection between DMN node 20 and left VAN is associated with reduced task performance (M: − 0.47; HDI: − 0.68 to − 0.27; prop. < 0: 1.0), and this edge is more strongly connected in unmedicated patients than controls (M: 0.03; HDI: 0.00–0.05; prop. < 0: 0.98). Additionally, increased accuracy was associated with stronger positive coupling between frontoparietal nodes 10 and 12 (left VAN) (M: 0.41; HDI: 0.21–0.62; prop. > 0: 1.0), and this edge was weaker for the unmedicated patients than for controls (M: − 0.03; HDI: − 0.06 to − 0.01; prop. < 0: 0.99).
3.6. Voxel-wise within-node connectivity
Of the eleven nodes that showed activity change in response to the decision phase or other task parameters and showed group differences, six had regions of significant decrease or increase of within-node connectivity when comparing groups (Supplemental fig. S1.12.1 and table S2.6). In the DMN node 1, and frontoparietal nodes 2 and 6 there were patterns of both increased and decreased connectivity in controls compared to placebo in all major divisions of the brain. Patients off medication showed decreased connectivity along large parts of the anterior cingulate cortex in the executive control node compared to controls, and in subcortical node 29 and visual node 8 there were wide-spread connectivity reductions in unmedicated patients were unmedicated compared to the controls. The controls additionally had increased connectivity in superior frontal regions of frontoparietal node 10 and 12 (left VAN) compared to unmedicated patients.
MPH changed within-node connectivity in three nodes. A large area encompassing the precuneus showed strong attenuation in the MPH condition compared to placebo in DMN node 1 (Fig. 3C), and there was reduced connectivity in core visual regions of visual nodes 8 and 9.
4. Discussion
We tested the extent of large-scale brain network disruptions in adult ADHD during a decision-making task, and their remediation by MPH, using data-driven fMRI analyses in conjunction with Bayesian hierarchical linear mixed models. By studying the activation patterns and signal variance of 18 identified signal components (which we call nodes), and the connectivity between these nodes (the edges), we found aberrant activity and connectivity of the DMN in the ADHD sample.
Importantly, the nodes identified corresponded to common functional resting-state networks, and all nodes but one displayed task-dependent activity changes, suggesting the dynamics of intrinsic functional networks were altered by cognitive engagement. Several analyses indicated abnormal activation of the DMN in the patient sample. With regards to the task-dependent activity changes in the functional networks, adults with ADHD showed increased levels of trial-by-trial suppression of the DMN compared to controls. Given the frequency of choice trials and lack of rest-periods during scanning, increased DMN suppression during each trial indicates that unmedicated patients were less able to sustain DMN suppression while anticipating upcoming decisions. This result was corroborated by increased DMN variance when patients were off medication compared to controls, suggesting that adults with ADHD displayed excessive DMN activity throughout the task. Increased DMN variance was also correlated with reduced performance on the task, which further supports the negative effect of excessive DMN activity during tasks. The DMN was additionally more positively connected to the left ventral attention network (VAN), while there was a general pattern of reduced connectivity between other nodes in ADHD. Contrary to our expectations, there were few robust effects of MPH on network connectivity.
Multiple analytical approaches thus suggest that the DMN is overactive in adults with ADHD during decision-making, as indicated by increased trial-triggered DMN suppression and increased DMN signal variance. A pattern of increased DMN signal variance, reduced coupling between two attention nodes, and increased coupling between the DMN and the left ventral attention network (VAN) was evident when patients were unmedicated compared to controls, and also associated with reduced task performance across groups, indicating they are general network changes correlated with reduced performance. MPH robustly reduced precuneus connectivity in the DMN and reduced connectivity within core visual nodes.
4.1. ADHD patients show reduced DMN down-regulation that is also associated with reduced performance
The DMN was implicated in several analyses, both in regards to quantifying differences between patients and controls, but also in relation to task performance and effects of MPH.
4.1.1. Variable DMN suppression during decision-making
The task GLMs indicate that DMN activity was reduced during the decision phase in all participants and that reduced DMN activity was also related to increased accuracy. This pattern was more pronounced in patients than controls (Fig. 2). Given the difficulties with attention in ADHD, and previous studies indicating that decreased DMN suppression precedes attentional lapses (Weissman et al., 2006), it is possible that patients struggle to keep DMN suppressed between trials, and need to re-suppress it to execute the task. Such a process would result in the greater trial-by-trial variation in DMN suppression seen in the patient group.
The analyses of node signal variance corroborate this explanation, as patients showed higher DMN variance than controls. Neural variance is thought to partly reflect the flexibility of the brain to explore and adopt different network configurations (Deco et al., 2011, Ghosh et al., 2008), and, in this context, the DMN is expected to display decreased variance during externally oriented tasks. Indeed, we found that increased DMN signal variance was associated with poor task performance (Fig. 3B). While a recent study found that increased DMN variance in young adults was associated with increased performance on a spatial working memory task (Guitart-Masip et al., 2015), the current results indicate that DMN variability as measured by temporal variance of a DMN independent component is associated with lower task performance. Together these results provide novel evidence that adults with ADHD show reduced sustained DMN suppression during decision-making between trials and re-suppress the DMN at each choice, and that such excessive DMN variance is associated with reduced task performance.
The interpretation of the DMN as overactive in adult ADHD is inferred by multiple analyses pointing in that direction. However, it was not possible to test this possibility directly, as there were no longer periods of rest during the experiment, nor did we have a resting condition. We thus could not test the activation level of the DMN in non-task periods. It is therefore also possible that increased task related suppression and DMN variance result from greater dynamic range of the DMN. However, given the current literature on the DMN in ADHD, and that increased DMN variance was associated with reduced task performance, this explanation seems unlikely.
4.1.2. Abnormal DMN connectivity with the precuneus and the left VAN
The two edges between the DMN nodes and frontoparietal node 12 were the only edges with higher positive correlations in unmedicated patients than the controls Fig. 4. Frontoparietal node 12 includes the temporoparietal junction, and inferior and middle frontal gyrus, and likely reflects the left ventral attention network (VAN). The VAN is commonly described as right lateralized, but these areas in the left hemisphere have also been implicated as important to attention processes (Vossel et al., 2014). Increased structural and functional connectivity between attention networks and the DMN has been reported previously in adult ADHD (Kessler et al., 2014), and reduced segregation between task-positive networks and the DMN has been suggested to arise from reduced regulation of the DMN by task-positive networks (Fassbender et al., 2009). These results are therefore consistent with previous studies, and together suggest that the DMN is overly connected to attention networks during cognitive engagement in ADHD, possibly contributing to manifested symptoms of inattention.
Additionally, MPH decreased precuneus connectivity to the DMN in the patient group (Fig. 3C). Precuneus activation has been implicated in ADHD, where both higher activation during task engagement (Peterson et al., 2009, Tomasi et al., 2009), and lower activation during rest (Castellanos et al., 2008, Uddin et al., 2008) compared to controls were reported. MPH has been found to increase DMN suppression in precuneus during a Stroop task in adolescents with ADHD (Peterson et al., 2009). The precuneus has also been implicated in non-spatial attentional shifts, and Tomasi et al. (2009) found that precuneus deactivation was amplified with increased cognitive load in a sustained attention task. These authors furthermore showed that MPH decreased levels of DA transporter binding in the striatum (indicating increased DA in the synapse), which in turn was related to increased precuneus deactivation. MPH related precuneus connectivity may thus be mediated by striatal influences. Alternatively, as DA transporter is, compared to NA transporter, minimally expressed in the cortex (Schroeter et al., 2000), precuneus connectivity may be altered by MPH through noradrenergic influences.
More generally, while animal models of ADHD (Meneses et al., 2011, Sagvolden et al., 1992) and most neurobiologically detailed theories of ADHD propose a central role of DA (Ziegler et al., 2016), the current evidence is consistent with the view that both DA and NA levels are modulated by MPH, and their availability is associated with ADHD symptoms. For instance, catecholamine transporter blockers of DA and NA transporters affect levels of both DA and NA (Han and Gu, 2006, Bymaster et al., 2002, Seeman and Madras, 1998). Furthermore, a recent functional connectivity study found MPH affected connectivity of cortical brain regions with the ventral tegmental area but also the locus coeruleus (Kline et al., 2016).
Together with our results, this suggests that increased catecholamine signalling through MPH medication facilitates attention by modulating deactivation in precuneus, a key region of the DMN. These observations are also in line with recent studies demonstrating decreased task-related DMN suppression in patients with schizophrenia compared to controls (Haatveit et al., 2016) and in elderly compared to young healthy adults (Garrett et al., 2015), in particular considering that both schizophrenia and cognitive aging have been associated with catecholamine alterations (Bäckman et al., 2010, Dørum et al., 2016, Howes and Kapur, 2009).
4.1.3. Less segregated functional networks in ADHD
In addition to irregular connectivity in the DMN, there were patterns of reduced within-network connectivity in visual, subcortical, and frontoparietal networks in patients off medication. Unmedicated patients furthermore exhibited decreased coupling between nodes within the same network, like decreased coupling between two visual nodes and two pairs of frontoparietal nodes Fig. 4, as well as increased coupling between the DMN nodes and the left VAN. The combination of widespread reduced connectivity within multiple nodes, decreased coupling between nodes of the same network, and increased connectivity between the DMN and VAN supports the hypothesis that patients with ADHD have more diffuse functional brain networks than controls (Hoekzema et al., 2013). Indeed, using joint ICA combining resting-state fMRI with MRI indices of white matter integrity and grey matter morphology, Kessler et al. (2014) documented disruptions in brain network differentiation in adult ADHD, with reduced within and increased between network connectivity. It is possible that the more diffuse network patterns in adult ADHD are the result of persistent disruptions in brain network differentiation, and we here additionally provide evidence that these disruptions might not be alleviated by acute MPH medication.
4.2. Connectivity between nodes and task performance
While the strength of several edges displayed credible correlations with overall task accuracy, of particular interest are two edges that in addition were different between patients and controls. Participants with high task performance had a weak connection between the DMN and the left VAN, and a strong connection between two task-positive networks (node 10 and VAN). Importantly, these effects of connectivity and task performance did not differ between the groups. Unmedicated patients had a stronger connection between the DMN and left VAN and weaker connection between the two task-positive nodes than the controls, and this is the same pattern associated with decreased task performance across all participants. Interestingly, in task-positive node 10 and the VAN controls additionally have stronger frontoparietal within–node connectivity than patients. It is thus not only that the connection between the two task-positive networks is malfunctioning in ADHD, but also that the nodes themselves are disrupted. These results again support the hypothesis that brain networks are less differentiated in ADHD and that network differentiation is connected to behaviour.
Another node of particular interest is frontoparietal node 6, which likely reflects the dorsal attention network (DAN) and includes the intraparietal sulcus and insula; areas that have been linked to evidence accumulation in perceptual and value-based decision making (Basten et al., 2010, Furl and Averbeck, 2011). The DAN shows the largest activation during the decision phase of all the nodes, and controls more actively engaged the DAN than patients. Furthermore, the DAN was less coupled to visual and sensorimotor nodes in patients off medication compared to controls (Fig. 4). Studies have shown that the frontoparietal network can dynamically change its degree of functional coupling with perceptual and sensory areas based on task demands (Alnæs et al., 2015, Chadick and Gazzaley, 2011), and that such changes in coupling may act to bias perceptual decision processes (Wang et al., 2013). While the edges connecting sensorimotor and visual nodes to the DAN were not directly related to task performance, the diminished coupling between such regions in patients off medication may be a contributing factor to reduced performance in the patient group.
4.3. Weak effects of methylphenidate on functional connectivity
Other than the reduction in precuneus connectivity in the DMN (Fig. 3C), the reduction of connectivity in two visual nodes, and decreased variance in the sensorimotor nodes, the drug manipulation yielded fewer effects than expected. Given the increase of DA availability brought about by MPH and DA's importance in reward signalling (Volkow et al., 2009, Volkow et al., 2001), we expected some effects of medication on brain networks. The primary, pre-registered analyses of this study were concerned with the representation of task-specific information and the integration of such information in the brain (Mowinckel et al., n.d.). While the results supported several of our hypotheses, the effects of MPH were also weaker than anticipated. There we found that MPH improved task accuracy, ameliorated the decreased representation of gain magnitude in the left striatum, and that functional connectivity between regions important for decision-making was improved by MPH as a function of symptom severity. Possible explanations for the weak effects of MPH, such as sample size and characteristics, are discussed in the limitations section.
With regard to network variance, MPH reduced network variance in the sensorimotor network, but had no effect on the DMN, which was the only node where patients had aberrant variance. Given the previously reported increase in BOLD variance in older participants as a result of d-amphetamine (AMPH) (Garrett et al., 2015), which also improved task performance, we expected similar effects of MPH. Here we assessed the temporal variance on dual-regression time-series, while Garrett et al. (2015) calculated the standard deviation of all voxels across task blocks, a difference which might explain result discrepancies. Also, while AMPH and MPH both inhibit DA reuptake from the synapse, AMPH additionally stimulates DA release (Fischer and Cho, 1979). AMPH thus has two mechanisms by which DA availability increases, likely resulting in higher availability of DA compared to MPH. Furthermore, Garrett et al.'s (2015) older participants had decreased BOLD variability in regions important to task execution, which was improved by AMPH. The only network with aberrant variance in patients in the current study was the task-negative DMN, and MPH did not alleviate this abnormality. In contrast, the effects of MPH on network variability in the current sample were reductions of variance in the sensorimotor network and the OFC. It is possible to interpret this reduction as an attenuation of overactive motor processes in ADHD (Mostofsky et al., 2006), but as none of these nodes exhibited abnormal variance, this seems unlikely.
While we observed within-network changes in response to MPH in the precuneus of the DMN and in core visual areas of visual networks, these changes are network specific. MPH might more successfully increase within-network connectivity, while not being strong enough to alleviate developmental disruptions in larger between-network dynamics. Although speculative, increasing connectivity within networks that are already established, but function at a reduced capacity, might be easier than changing disruptions between different networks that themselves function sub-optimally.
5. Limitations
In order to study deficits in adults with ADHD, without the uncertainty that follows from a patient sample with multiple comorbidities that require treatment with other psychopharmacological substances, our patient sample might not be generalizable to the whole ADHD population. While we deliberately matched patients and controls on key characteristics such as gender and age, we were more lenient when it came to educational attainment. The choice of leniency arose from the proposal that “overmatching” on certain characteristics that have arisen as a result of the disorder itself would lead to an underestimation of the abnormalities associated with the disorder (Seidman et al., 1997). Such multi-collinearity is often dealt with by statistically controlling for other regressors, but leads to uninterpretable results as naturally co-occurring phenomena are artificially separated (Miller and Chapman, 2001).
Another limitation arises from our wish to test the effects of MPH on a sample of adults who were receiving treatment with MPH; all patients had therefore been receiving MPH treatment for a minimum of one month prior to testing. Previous studies of MPH effects in healthy adults have been on MPH-naïve participants, and the PET literature suggests strong effects of acute MPH on DA transporter binding in the striatum in previously unmedicated adults with ADHD (Tomasi et al., 2009, Volkow et al., 2007). Studies on the effects of MPH in patients used patients having received long-term medication (Liddle et al., 2011). Using ADHD patients on MPH treatment has two related implications for the interpretation of our results. First, it is possible that the difference between the on and off MPH conditions is due to withdrawal effects, as opposed positive effects of MPH in the MPH condition. Second, because it is known that MPH can also have chronic effects on neurotransmitter functioning, it is possible that our results underestimate the full effects of MPH, because acute cession of MPH will not remove the chronic effects. A generalization of our results to a population of patients who have never received MPH should therefore be made cautiously. We outline elsewhere the experiments required to differentiate acute and chronic effects of MPH (Ziegler et al., 2016). The very low to unidentifiable levels of ritalinic acid in the blood of the patients in the placebo condition at least make it unlikely that patients were under the influence of MPH in the placebo condition.
Finally, due to an MRI scanner upgrade data-collection ended prematurely, resulting in a smaller ADHD sample than aimed for. While the sample size is comparable to that of other fMRI studies in ADHD (Cortese et al., 2012), it might still not be enough to robustly detect changes in network dynamics as studied here. However, the use of rigorous statistical methods through permutation testing and Bayesian inference should ensure the robustness of the results reported here, despite some of its limitations.
6. Conclusions
This study provided novel evidence for difficulties in sustaining DMN suppression during decision-making in adults with ADHD, which necessitates re-suppression of the DMN at each choice. The excessive activation of the DMN was also associated with poor task performance and corroborated by two different analytical approaches. While we found no evidence of a positive effect of MPH on the stability of DMN suppression, MPH decreased precuneus connectivity in the DMN, which is commonly found in studies of DA agonists. Moreover, a pattern of higher connectivity between and lower connectivity within the left ventral attention network and the DMN was not only associated with reduced task performance, but also found in the patient sample compared to controls. The results of this study thus support the default mode interference hypothesis, in that excessive activity of the DMN is both evident in adults with ADHD and associated with lower task performance. We also provided further support towards reduced brain network differentiation in ADHD, which here was not remediated by MPH medication.
Financial disclosures
Funding was provided by the Norwegian Research Council (#249519), the South-Eastern Norway Regional Health Authority (#2012051), and the Department of Psychology, University of Oslo (#143660). ES-B has over the last three years received speaker fees, consultancy, research funding and conference support from Shire Pharma and speaker fees from Janssen Cilag. He has received consultancy fees from Neurotech solutions, Aarhus University, Copenhagen University and Berhanderling, Skolerne, Copenhagen, KU Leuven. Book royalties from OUP and Jessica Kingsley. He is the editor-in-chief of the Journal of Child Psychology and Psychiatry for which his University receives financial support and he receives an honorarium. The remaining authors declare no competing interests.
Acknowledgements
Dag Alnæs and Athanasia Monika Mowinckel had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We thank Inge R. Groothe and Atle Bjørnerud for adjustment of MRI sequences, and Mikjel Skurdal, Svein Are Vatnehol, and Grethe Løvvold for radiological assistance during data collection.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.03.008.
Appendix A. Supplementary data
Results from all Bayesian Linear Mixed Models.
Supplementary tables
References
- Alnæs D., Kaufmann T., Richard G., Duff E.P., Sneve M.H., Endestad T., Nordvik J.E., Andreassen O.A., Smith S.M., Westlye L.T. Attentional load modulates large-scale functional brain connectivity beyond the core attention networks. NeuroImage. 2015;109:260–272. doi: 10.1016/j.neuroimage.2015.01.026. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Pub; 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychiatric Association, [APA] 1994. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the Brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Bäckman L., Lindenberger U., Li S.-C.C., Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci. Biobehav. Rev. 2010;34:670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Barkley R.A., Edwards G., Laneri M., Fletcher K., Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J. Abnorm. Child Psychol. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Basten U., Biele G., Heekeren H.R., Fiebach C.J. How the brain integrates costs and benefits during decision making. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21767–21772. doi: 10.1073/pnas.0908104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Biederman J., Petty C.R., Clarke A., Lomedico A., Faraone S.V. Predictors of persistent ADHD: an 11-year follow-up study. J. Psychiatr. Res. 2011;45:150–155. doi: 10.1016/j.jpsychires.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Spencer T.J., Holmes J., Shin L.M., Valera E.M., Seidman L.J., Makris N., Surman C.B.H., Aleardi M., Mick E., Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch. Gen. Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Bymaster F.P., Katner J.S., Nelson D.L., Hemrick-luecke S.K., Threlkeld P.G., Heiligenstein J.H., Morin S.M., Gehlert D.R., Perry K.W. 2002. Atomoxetine Increases Extracellular Levels of Norepinephrine and Dopamine in Prefrontal Cortex of Rat: A Potential Mechanism for Efficacy in Attention Deficit/Hyperactivity Disorder. [DOI] [PubMed] [Google Scholar]
- Carpenter B., Gelman A., Hoffman M., Lee D., Goodrich B., Betancourt M., Brubaker M., Guo J., Li P., Riddell A. Stan: A Probabilistic Programming Language. J. Stat. Softw. 2017;76(1):1–32. doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos X.F., Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn. Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos X.F., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., Shaw D., Shehzad Z., Di Martino A., Biswal B.B., Sonuga-Barke E.J.S., Rotrosen J., Adler L.A., Milham M.P. Cingulate-Precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadick J.Z., Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat. Neurosci. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos X.F. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Dørum E.S., Alnæs D., Kaufmann T., Richard G., Lund M.J., Tønnesen S., Sneve M.H., Mathiesen N.C., Rustan Ø.G., Gjertsen Ø., Vatn S., Fure B., Andreassen O.A., Nordvik J.E., Westlye L.T. Age-related differences in brain network activation and co-activation during multiple object tracking. Brain Behav. 2016;6(11):e00533. doi: 10.1002/brb3.533. Published online 2016 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J.N., Casey B.J., Tonev S.T., Davidson M.C., Reiss A.L., Garrett A., Hinshaw S.P., Greenhill L.L., Glover G., Shafritz K.M., Vitolo A., Kotler L.A., Jarrett M.A., Spicer J. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J. Child Psychol. Psychiatry Allied Discip. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.a., Posner J., Nagel B.J., Bathula D.R., Costa-Dias T.G., Mills K.L., Blythe M.S., Giwa A., Schmitt C.F., Joel T. Atypical default network connectivity in youth with ADHD. Biol. Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal A.A., Selen L.P.J., Wolpert D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C., Zhang H., Buzy W.M., Cortes C.R., Mizuiri D., Beckett L., Schweitzer J.B. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. PNAS. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F., Cho K. Release of dopamine release. J. Pharmacol. Exp. Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- Fredriksen M., Peleikis D.E. Long-term pharmacotherapy of adults with attention-deficit/hyperactivity disorder (ADHD): a literature review and clinical study. Basic Clin. Pharmacol. Toxicol. 2015 doi: 10.1111/bcpt.12477. [DOI] [PubMed] [Google Scholar]
- Furl N., Averbeck B.B. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J. Neurosci. 2011;31:17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D.D., Nagel I.E., Preuschhof C., Burzynska A.Z., Marchner J., Wiegert S., Jungehülsing G.J., Nyberg L., Villringer A., Li S.-C., Heekeren H.R., Bäckman L., Lindenberger U. Amphetamine modulates brain signal variability and working memory in younger and older adults. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7593–7598. doi: 10.1073/pnas.1504090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A., Rubin D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992;7:457–511. [Google Scholar]
- Gelman A., Tuerlinckx F. Type S error rates for classical and Bayesian single and multiple comparison procedures. Comput. Stat. 2000;15:373–390. [Google Scholar]
- Gelman A., Hill J., Yajima M. Why we (usually) don't have to worry about multiple comparisons. J. Res. Educ. Eff. 2012;5:189–211. [Google Scholar]
- Ghosh A., Rho Y., McIntosh A.R., Kötter R., Jirsa V.K. Noise during rest enables the exploration of the brain's dynamic repertoire. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A.A., Floresco S.B., Goto Y., Lodge D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grady C.L., Garrett D.D. Understanding variability in the BOLD signal and why it matters for aging. Brain Imaging Behav. 2014;8:274–283. doi: 10.1007/s11682-013-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Masip M., Salami A., Garrett D., Rieckmann A., Lindenberger U., Bäckman L. BOLD variability is related to dopaminergic neurotransmission and cognitive aging. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv029. [DOI] [PubMed] [Google Scholar]
- Haatveit B., Jensen J., Alnæs D., Kaufmann T., Brandt C.L., Thoresen C., Andreassen O.A., Melle I., Ueland T., Westlye L.T. Reduced load-dependent default mode network deactivation across executive tasks in schizophrenia spectrum disorders. NeuroImage Clin. 2016 doi: 10.1016/j.nicl.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.D., Gu H.H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., O'Doherty J., Camerer C.F., Schultz W., Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J.L., King T.S., Curry W.J. The adult ADHD self-report scale for screening for adult attention deficit-hyperactivity disorder (ADHD) J. Am. Board Fam. Med. 2012;25:847–853. doi: 10.3122/jabfm.2012.06.120065. [DOI] [PubMed] [Google Scholar]
- Hoekzema E., Carmona S., Ramos-Quiroga J.A., Richarte Fernández V., Bosch R., Soliva J.C., Rovira M., Bulbena A., Tobeña A., Casas M., Vilarroya O. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum. Brain Mapp. 2013;35:1261–1272. doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III - the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaufmann T., Elvsåshagen T., Alnæs D., Zak N., Pedersen P., Norbom L.B., Quraishi S.H., Tagliazucchi E., Laufs H., Bjørnerud A., Malt U.F., Andreassen O.A., Roussos E., Duff E.P., Smith S.M., Groote I.R., Westlye L.T. The brain functional connectome is robustly altered by lack of sleep. NeuroImage. 2016;127:324–332. doi: 10.1016/j.neuroimage.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R.E., Wang Z., Alexopoulos G.S., Gunning F.M., Murphy C.F., Morimoto S.S., Kanellopoulos D., Jia Z., Lim K.O., Hoptman M.J. Hybrid ICA-seed-based methods for fMRI functional connectivity assessment: A feasibility study. Int. J. Biomed. Imaging. 2010:2010. doi: 10.1155/2010/868976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Adler L.A., Gruber M.J., Sarawate C.a., Spencer T.J., Van Brunt D.L., Brunt D.L. Van. Validity of the World Health Organization adult ADHD self-report scale (ASRS) screener in a representative sample of health plan members. Int. J. Methods Psychiatr. Res. 2007;16:52–65. doi: 10.1002/mpr.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D., Angstadt M., Welsh R.C., Sripada C.S., Kessler X.D., Angstadt X.M., Welsh R.C., Sripada X.C., Kessler D., Angstadt M., Welsh R.C., Sripada C.S., Kessler X.D., Angstadt X.M., Welsh R.C., Sripada X.C., Kessler D., Angstadt M., Welsh R.C., Sripada C.S., Kessler X.D., Angstadt X.M., Welsh R.C., Sripada X.C., Kessler D., Angstadt M., Welsh R.C., Sripada C.S., Kessler X.D. Modality-spanning deficits in attention-deficit/hyperactivity disorder in functional networks, gray matter, and white matter. J. Neurosci. 2014;34:16555–16566. doi: 10.1523/JNEUROSCI.3156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R.L., Zhang S., Farr O.M., Hu S., Zaborzsky L., Samanez-Larkin G.R., Li C.R. The effects of methylphenidate on resting-state functional connectivity of the basal nucleus of Meynert, locus coeruleus, and ventral tegmental area in healthy adults. Front. Hum. Neurosci. 2016;10:1–16. doi: 10.3389/fnhum.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel L.K., Biele G., Mohr P.N.C., Li S.-C., Heekeren H.R. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17951–17956. doi: 10.1073/pnas.0905191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoit O., Wolf M. Improved estimation of the covariance matrix of stock returns with an application to portfolio selection. J. Empir. Financ. 2003;10:603–621. [Google Scholar]
- Liddle E.B., Hollis C., Batty M.J., Groom M.J., Totman J.J., Liotti M., Scerif G., Liddle P.F. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J. Child Psychol. Psychiatry Allied Discip. 2011;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A., Perez-Garcia G., Ponce-Lopez T., Tellez R., Gallegos-Cari A., Castillo C. Spontaneously hypertensive rat (SHR) as an animal model for ADHD: a short overview. Rev. Neurosci. 2011;22:365–371. doi: 10.1515/RNS.2011.024. [DOI] [PubMed] [Google Scholar]
- Metin B., Krebs R.M., Wiersema J.R., Verguts T., Gasthuys R., Van Der Meere J.J., Achten E., Roeyers H., Sonuga-Barke E.J.S. Dysfunctional modulation of default mode network activity in attention-deficit/hyperactivity disorder. J. Abnorm. Psychol. 2015;124:208–214. doi: 10.1037/abn0000013. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Rimrodt S.L., Schafer J.G., Boyce A., Goldberg M.C., Pekar J.J., Denckla M.B. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol. Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Mowinckel A.M., Pedersen M.L., Eilertsen E., Biele G. A meta-analysis of decision-making and attention in adults with ADHD. J. Atten. Disord. 2015;19:355–367. doi: 10.1177/1087054714558872. [DOI] [PubMed] [Google Scholar]
- Mowinckel, A.M., Pedersen, M.L., Ziegler, S., Fredriksen, M., Bjørnerud, A., Endestad, T., Biele, G., n.d. Methylphenidate Alleviates Aberrant Processing of Gain Information During Value-based Decision-making in Adult ADHD: A Randomized Placebo-controlled Clinical Trial. (submitted).
- Mumford J.A., Poline J.B., Poldrack R.A. Orthogonalization of regressors in fMRI models. PLoS One. 2015;10:1–11. doi: 10.1371/journal.pone.0126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A., Leyton M., Monchi O., Goldberg Y.K., He Y., Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J. Neurosci. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.S., Potenza M.N., Wang Z., Zhu H., Martin A., Marsh R., Plessen K.J., Yu S. An fMRI study of the effects of psychostimulants on default- mode processing during Stroop task performance in youths with ADHD. Am. J. Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., Buitelaar J.K., Beckmann C.F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage. 2015;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- RCore Team R . R Found. Stat. Comput; Vienna, Austria: 2013. A Language and Environment for Statistical Computing. [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry. 2013;22:719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T., Metzger M.A., Schiorbeck H.K., Rugland A.L., Spinnangr I., Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav. Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sagvolden T., Aase H., Johansen E.B., Russell V.A. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 2005;28:397–486. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Smith S.M., Nichols T.E. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. NeuroImage. 2011;54:2006–2019. doi: 10.1016/j.neuroimage.2010.09.088. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrantee A., Tamminga H.G.H., Bouziane C., Bottelier M.A., Bron E.E., Mutsaerts H.-J.M.M., Zwinderman A.H., Groote I.R., Rombouts S.A.R.B., Lindauer R.J.L., Klein S., Niessen W.J., Opmeer B.C., Boer F., Lucassen P.J., Andersen S.L., Geurts H.M., Reneman L. Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter S., Apparsundaram S., Wiley R.G., Miner L.H., Sesack S.R., Blakely R.D. Immunolocalization of the cocaine- and antidepressant-sensitive 1-norepinephrine transporter. J. Comp. Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Seeman P., Madras B.K. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol. Psychiatry. 1998;3:386–396. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seidman L.J., Biederman J., Faraone S.V., Weber W., Ouellette C. Toward defining a neuropsychology of attention deficit-hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J. Consult. Clin. Psychol. 1997;65:150–160. doi: 10.1037/0022-006X.65.1.150. [DOI] [PubMed] [Google Scholar]
- Sidlauskaite J., Sonuga-Barke E.J..S., Roeyers H., Wiersema J.R. Default mode network abnormalities during state switching in attention deficit hyperactivity disorder. Psychol. Med. 2016;46:519–528. doi: 10.1017/S0033291715002019. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Hyvärinen A., Varoquaux G., Miller K.L., Beckmann C.F. Group-PCA for very large fMRI datasets. NeuroImage. 2014;101:738–749. doi: 10.1016/j.neuroimage.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S.S., Castellanos X.F. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J..S., Cortese S., Fairchild G., Stringaris A. Annual research review: transdiagnostic neuroscience of child and adolescent mental disorders - differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. J. Child Psychol. Psychiatry Allied Discip. 2016;57:321–349. doi: 10.1111/jcpp.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C.S., Kessler D., Welsh R., Angstadt M., Liberzon I., Phan K.L., Scott C. Distributed effects of methylphenidate on the network structure of the resting brain: a connectomic pattern classification analysis. NeuroImage. 2013;81:213–221. doi: 10.1016/j.neuroimage.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C.S., Kessler D., Angstadt M. Lag in maturation of the brain's intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc. Natl. Acad. Sci. U. S. A. 2014;111:14259–14264. doi: 10.1073/pnas.1407787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan Development Team . 2015. Pkg{Stan} Modeling Language: User's Guide and Reference Manual. Interact. Flow Model. Lang. [Google Scholar]
- Stan Development Team . 2016. Rstan: The R Interface to Stan, Version 2.9.0. [Google Scholar]
- Ströhle A., Stoy M., Wrase J., Schwarzer S., Schlagenhauf F., Huss M., Hein J., Nedderhut A., Neumann B., Gregor A., Juckel G., Knutson B., Lehmkuhl U., Bauer M., Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. NeuroImage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D., Wang R., Telang F., Wang G.J., Chang L., Ernst T., Fowler J.S. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M.C., Biswal B.B., Margulies D.S., Shehzad Z., Shaw D., Ghaffari M., Rotrosen J., Adler L.A., Castellanos X.F., Milham M.P. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M.C., Biswal B.B., Castellanos X.F., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehtari A., Gelman A., Gabry J. 2015. Efficient Implementation of Leave-One-Out Cross-validation and WAIC for Evaluating Fitted Bayesian Models. [Google Scholar]
- Volkow N.D., Wang G., Fowler J.S., Logan J., Gerasimov M., Maynard L., Ding Y.S., Gatley S.J., Gifford A., Franceschi D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. (doi:20014896 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Fowler J.S., Ding Y.S. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Newcorn J., Fowler J.S., Telang F., Solanto M.V., Logan J., Wong C., Ma Y., Swanson J.M., Schulz K.P., Pradhan K. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. NeuroImage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Kollins S.H., Wigal T.L., Newcorn J.H., Telang F.W., Fowler J.S., Zhu W., Logan J., Ma Y., Pradhan K., Wong C., Swanson J.M. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems distinct neural circuits but collaborative roles. Neurosci. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Arteaga D., He B.J. Brain mechanisms for simple perception and bistable perception. Proc. Natl. Acad. Sci. 2013;110:E3350–E3359. doi: 10.1073/pnas.1221945110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wilbertz G., Tebartz van Elst L., Delgado M.R., Maier S., Feige B., Philipsen A., Blechert J. Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. NeuroImage. 2012;60:353–361. doi: 10.1016/j.neuroimage.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S., Pedersen M.L., Mowinckel A.M., Biele G. Modelling ADHD: a review of ADHD theories' predictions for computational models of decision-making and reinforcement learning. Neurosci. Biobehav. Rev. 2016;71 doi: 10.1016/j.neubiorev.2016.09.002. 10.1016/j.neubiorev.2016.09.002 (under revision) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model descriptions. Syntax description of the three competing models for each of the three dependent measures. The first parenthesis describes the term of interest, where one estimates intercepts for each node or edge (node pairs).
Note that for the task betas, each parameter (intercept, gain, loss, difficulty, accuracy) is hierarchically nested under each node (/), meaning a general node intercept is estimated as well for each parameter. Models 2 and 3 furthermore estimate differing slopes for groups (ADHD vs. controls) and treatments (ADHD placebo, ADHD methylphenidate, controls), allowing estimates to vary between the groups.
Results from all Bayesian Linear Mixed Models.
Supplementary tables