Abstract
Changes in levels of the stress-sensitive hormone cortisol from morning to evening are referred to as diurnal cortisol slopes. Flatter diurnal cortisol slopes have been proposed as a mediator between chronic psychosocial stress and poor mental and physical health outcomes in past theory and research. Surprisingly, neither a systematic nor a meta-analytic review of associations between diurnal cortisol slopes and health has been conducted to date, despite extensive literature on the topic. The current systematic review and meta-analysis examined associations between diurnal cortisol slopes and physical and mental health outcomes. Analyses were based on 179 associations from 80 studies for the time period up to January 31, 2015. Results indicated a significant association between flatter diurnal cortisol slopes and poorer health across all studies (average effect size, r = .147). Further, flatter diurnal cortisol slopes were associated with poorer health in 10 out of 12 subtypes of emotional and physical health outcomes examined. Among these subtypes, the effect size was largest for immune/inflammation outcomes (r = .288). Potential moderators of the associations between diurnal cortisol slopes and health outcomes were examined, including type of slope measure and study quality indices. The possible roles of flatter slopes as either a marker or a mechanism for disease etiology are discussed. We argue that flatter diurnal cortisol slopes may both reflect and contribute to stress-related dysregulation of central and peripheral circadian mechanisms, with corresponding downstream effects on multiple aspects of biology, behavior, and health.
Keywords: Hypothalamic pituitary adrenal (HPA) axis, Diurnal cortisol slopes, Mental health, Physical health, Circadian rhythms
1. Introduction
1.1 Overview
The glucocorticoid hormone cortisol is a primary product of the hypothalamic-pituitary-adrenal (HPA) axis, a key biological stress response system. Cortisol is one of the most frequently employed biomarkers in psychobiological research for several reasons. First, cortisol levels are responsive to social and psychological stress (Dickerson and Kemeny, 2004; Gunnar et al., 2009a). Cortisol levels respond to both acute stress (e.g., acute loneliness or negative social evaluation) and chronic stress (e.g., the stress of poverty or ongoing family conflict) (Adam, 2012). Second, the development and adult functioning of the HPA axis is profoundly influenced by prior developmental experience (Lupien et al., 2009). Third, cortisol has pervasive effects throughout the body and brain, and is thought to play important roles in daily cognitive and behavioral functioning (Lupien et al., 2009). Fourth, cortisol has also been implicated in the etiology of a wide range of mental and physical health outcomes (Chrousos and Gold, 1992). As a result, researchers have suggested that stress-related alterations in cortisol regulation may play a role in mediating associations between stress exposure and later developmental and health outcomes (Lupien et al., 2009; Davis and Sandman, 2010), including both the onset and progression of mental and physical health disorders (Heim et al., 2008).
Past research on cortisol and health has focused on cortisol reactivity to acute stress (Granger et al., 1996; Heim et al., 2008) as well as variations in average basal cortisol levels (Chrousos and Gold, 1992). More recently, researchers have appreciated the importance of circadian variability in cortisol levels, by examining influences on, and consequences of, individual differences in the diurnal (daytime) cortisol rhythm. The current meta-analysis examined associations between one aspect of the diurnal cortisol rhythm – the diurnal cortisol slope (DCS) - and mental and physical health outcomes.
1.2 Diurnal Cortisol Rhythms: Description and Background Research
Cortisol levels typically follow a strong diurnal rhythm: levels are high on waking, surge an average of 50–60% in the 30–40 minutes after waking, drop rapidly in subsequent few hours after the awakening surge and then drop more slowly until reaching a nadir around bedtime (Pruessner et al., 1997; Adam and Kumari, 2009). Variation in cortisol levels as a function of time of day is substantial. In one study, time of day accounted for 72% of the variance in salivary cortisol levels (Adam, 2006). Early research often considered this time-of-day variation to be “nuisance” variation. Over the past 15 years, however, individual differences in the diurnal cortisol rhythm have emerged as a construct of interest (Adam et al., 2008). Researchers have examined the genetic, developmental, and psychosocial determinants of individual differences in the diurnal cortisol rhythm (Adam, 2012), as well as the potential health consequences of variation in the diurnal cortisol rhythm (Sephton et al., 2000).
The diurnal cortisol rhythm has been divided into several key components which provide complementary information. Most often examined are: the average level of cortisol across the day (daily average cortisol or DAC); the size of the post-awakening surge, called the cortisol awakening response (CAR); and the diurnal cortisol slope (DCS), the degree of change in cortisol from morning to evening over the waking day (Adam and Kumari, 2009).
Early research on cortisol rhythms generally focused on DAC (Yehuda et al., 1990; Gunnar et al., 2001; Nicolson, 2004). Since its discovery in the late 90’s (Pruessner et al., 1997), the CAR has also received extensive research attention, with reviews and meta-analyses D Steptoe, 2009; Fries et al., 2009; Clow et al., 2010).
An accumulating body of research focusing on the DCS suggests that it is sensitive to emotional and psychosocial stress (Adam and Gunnar, 2001; Adam et al., 2006; Doane and Adam, 2010) and related to health outcomes (Sephton et al., 2000; Matthews et al., 2006; Kumari et al., 2009; Doane et al., 2013), with both adverse experience and worse health being associated with a flatter DCS across the waking day. It has therefore been proposed that a flattened DCS may be one mechanism by which stress influences negative health outcomes (Sephton and Spiegel, 2003; Adam and Kumari, 2009).
Cortisol has important regulatory effects throughout the body and brain, impacting arousal, energy and metabolic processes, immune and inflammatory system functioning, and mood and sexual behavior (Sapolsky et al., 2000). Cortisol’s diurnal variation may be an important element of its regulatory actions; indeed, cortisol is one pathway by which central circadian rhythms are signaled to multiple peripheral biological systems (Bass and Lazar, 2016; Man et al., 2016). We argue here that disruption of cortisol’s circadian pattern and signaling may affect the functioning of a diverse set of central and peripheral systems, with these effects cascading over time to contribute to a wide variety of negative health outcomes.
For example, prior studies have found associations between flatter cortisol rhythms and depression (Doane et al., 2013), fatigue (Bower et al., 2005b; Kumari et al., 2009), cardiovascular disease (Matthews et al., 2006), and mortality among both breast cancer patients and in community samples (Sephton et al., 2000; Kumari et al., 2011). Findings have, however, been inconsistent, and researchers have not systematically summarized the existing research, or fully explicated the meaning of the DCS or the potential mechanisms by which it may be related to mental and physical health outcomes. Since the early 2000’s (Gunnar and Vazquez, 2001), no systematic reviews on the DCS have been conducted. Moreover, no meta-analyses have been conducted either on the effects of psychosocial experience on DCS or on its associations with health outcomes. The current manuscript addresses the latter question, with an eye to better understanding: a) what is the association between DCS and health (in particular, the average magnitude and direction of the associations as well as its consistency across studies) b) whether the DCS relates to certain types of health outcomes more strongly than to others, c) the meaning of the DCS and the mechanisms by which it may relate to health outcomes, and d) how methodological variations in study design and DCS measurement may contribute to variations in study effect sizes.
1.3 Diurnal Cortisol Slopes: Measurement, Modeling and Moderators
Researchers have referred to the DCS in a wide variety of ways, including diurnal cortisol slopes (Adam and Kumari, 2009), diurnal cortisol declines (Cohen et al., 2006), diurnal cortisol variability (Sannes et al., 2013), diurnal cortisol rhythms (Bower et al., 2005b), and the amplitude of the circadian cortisol rhythm (Goel et al., 2009). Likewise, researchers have quantified the DCS in different ways, which vary in the number and timing of the cortisol samples across the waking day, and in approaches to calculating slope measures from those samples. For the purposes of this review, any measure that provides an indication of the magnitude of the difference between morning and evening cortisol values is considered a measure of the DCS.
Common types of slopes include: 1) wake-to-bed slopes, which examine the absolute change or rate of change in cortisol from immediately upon waking to late evening or bedtime (e.g., Adam et al., 2010; Turner-Cobb et al., 2011); 2) peak-to-bed slopes, which examine the absolute change or rate of change in cortisol from the peak of the CAR to late evening or bedtime, (e.g., Hsiao et al., 2010; Vammen et al., 2014); 3) short daytime slopes, which measure slopes over a shorter portion of the waking day, typically from several hours after waking to evening or bedtime (late decline measures are one example of this; see Hajat et al., 2013); 4) fixed time point slopes (e.g., Bosch et al., 2007; Den Hartog et al., 2003), in which samples are gathered at fixed clock times across the day (e.g., 0800h and 2000h), rather than in relation to time of waking; and 5) amplitude measures, which estimate the peak-to-trough difference of the diurnal cortisol rhythm from intensive repeated measures of cortisol values across the day (e.g., Bao et al., 2004; Fidan et al., 2013a, Fidan et al., 2013b).
For the first three cortisol slope types (i.e., wake-to-bed, peak-to-bed, and short daytime slopes), samples are timed relative to each individual’s sleep-wake schedule, or more specifically, relative to person- and day-specific time of waking. These slopes typically are quantified in one of three ways: a) taking a simple difference between the morning measure and the evening measure; b) taking a simple difference divided by the total time between the two samples; or c) using regression or multilevel growth curve modeling to predict cortisol levels across the day from time of day of measurement for each individual, with the slope obtained from the size of the person-specific beta coefficient for the effect of time of day on cortisol (e.g., Adam et al., 2006; Doane et al., 2013). Fixed-time point slopes are typically calculated using either a simple difference score from morning to evening cortisol levels or with repeated-measures ANOVA examining within-person changes in cortisol from one sampling point to the next. Amplitudes are measured using the cosinor method, which fits a cosine curve to the repeated measures cortisol data and then calculates the characteristics of the curve, including its amplitude.
Although these different types of slope measures allow researchers to measure DCS across a variety of study designs, this heterogeneity has the potential to obscure associations between DCS and health outcomes. One important debate is whether DCS should be calculated from the peak of the CAR value to evening values or from waking values to evening values, excluding the CAR. Researchers have argued for excluding the CAR from DCS measures because the CAR is influenced by different biological mechanisms than the rest of the diurnal cortisol rhythm (Clow et al., 2010; Adam et al., 2015). Although current recommendations suggest that measures should be gathered relative to individual wake times (e.g., Adam and Kumari, 2009), rather than at fixed clock time points, studies have not examined the implications of this choice. The current meta-analysis, through examining type of slope measure as a moderator of the associations between DCS and health outcomes, provides important insights into these and other measurement debates.
Beyond type of slope, other study design factors that may have implications for the size of the association found between DCS and health include the number of samples across the day used to define the slope (Hoyt et al., 2016), the number of days of salivary cortisol data collection (Adam and Kumari, 2009), whether key health behavior confounds are covaried (Adam and Kumari, 2009), and the presence of objective measurement of compliance with sampling times (Kudielka et al., 2003). In addition, given developmental changes in the HPA axis (Gunnar et al., 2009b), the age or developmental stage of participants is another factor that should be considered as a moderator of associations between DCS and health.
1.4 The Current Study
1.4.1 Study Goals
The primary goal of the current study was to provide a meta-analysis of the literature (up until January 31, 2015) assessing the associations between diurnal cortisol slopes and health outcomes. Specifically, we examined the associations between DCS and 12 subtypes of mental and physical health outcomes, namely: 1) anxiety symptoms or disorders; 2) depression symptoms or disorders (excluding bipolar depression); 3) internalizing disorders (symptom scales reflecting a mixture of anxiety and depression symptoms); 4) externalizing symptoms or disorders (a spectrum of behaviors involving anger expression, aggression and delinquency); 5) fatigue symptoms or disorders; 6) immune or inflammatory disorders; 7) obesity (including measures of body mass index or BMI, obesity, and adiposity); 8) cardiovascular disease symptoms and diagnoses; 9) cancer disease status or progression; 10) other mental health outcomes (mental health symptoms or disorders not classified as one of the above disease subtypes); 11) other physical health outcomes (physical health symptoms or disorders not classified as one of the above subtypes); and 12) mortality (death from any cause). While it is challenging to capture the existing literature on DCS and these varied health outcomes in a single meta-analysis, this comprehensive approach allows comparisons of relative effect sizes across different types of health outcomes. Through shedding light on the types of health symptoms and disorders most strongly associated with flatter cortisol slopes, this analysis may provide insights into the key biological pathways linking flattened cortisol slopes to multiple indices of poor health.
A secondary goal was to test whether the size of associations between the DCD and health outcomes would be moderated by the following factors: age of participants, type of slope measure, number of cortisol samples measured per day, number of days of data collection, and a study quality index based on the number of relevant confounds measured and accounted for in design and/or analysis.
1.4.2 Study Hypotheses
We hypothesized that a flatter DCS would be associated with worse health outcomes, across a range of health outcomes. We did not have strong hypotheses regarding which specific types of health outcomes would be most strongly associated with flatter cortisol slopes, although we expected immune and inflammatory outcomes to show robust associations, given the key role played by glucocorticoids in regulating inflammation (Silverman and Sternberg, 2012). We expected that wake-bed cortisol slopes would show stronger associations with health than other types of slope measures, that studies with more samples per day and more days of measurement would show stronger associations, and that studies utilizing objective monitoring of sampling compliance would reveal stronger associations than studies not utilizing objective monitoring. We expected to see DCS-health associations across multiple age groups, with effects potentially being larger in older age groups due to longer histories of stress exposure or more advanced disease processes.
2. Methods
2.1 Data Sources and Searches
Under the direction and guidance of the first author, doctoral students and postdoctoral fellows conducted electronic searches between April 2013 and January 2015. Electronic searches were done in Medline and Web of Science (both via Endnote X4 (2010) program search tool), PubMed, Psych Info, and Social Science Abstracts (via website or EbscoHost). Search terms for each database were: “cortisol rhythm”, “cortisol rhythms”, “cortisol slope”, “cortisol slopes”, “cortisol diurnal slope”, “cortisol diurnal slopes”, and “diurnal cortisol” with any article published prior to February 1, 2015 included in the search.
2.2 Study Selection
Inclusion criteria were English language publications in a peer-reviewed journal that investigated human diurnal cortisol slopes in association with a quantifiable mental or physical health or disease outcome, including studies of mortality. To be included, studies needed to measure variation in a health outcome in relation to a DCS (either by comparing a disease group and a healthy control group or by measuring symptom variation in relation to cortisol slopes in a community group or disease group, or both). Further, studies needed to examine associations between cortisol and concurrent or later mental/physical health outcomes, including mortality. Multiple health outcomes within the same study were utilized, if available, but effects were averaged rather than treated as independent effects when results from the same study were included together in the same meta-analysis.
Exclusion criteria were: 1) studies with a sample size of less than 10; 2) studies of endocrine disorders; 3) studies of genetic disorders; 4) non-empirical papers (i.e., review or methods papers); 5) studies that examined health indices as a predictor of subsequent cortisol slopes; 6) studies focusing on either morning or evening cortisol samples only (i.e., no assessment of degree of change in cortisol across the day); 7) studies focusing on daily average cortisol (DAC), area under the curve (AUC) measures of salivary cortisol, or other integrated cortisol measures such as overnight urinary cortisol or hair cortisol and; 8) slope measures that covered only the first few hours after waking, given that these typically measure CAR reactivity or recovery (e.g., early decline measures were excluded, but late decline measures examining change from mid-morning to evening cortisol were included, e.g., Hajat et al., 2013).
2.3 Data Extraction and Study Quality Assessment
A coding scheme was developed to facilitate data extraction. Studies were classified as one of the 12 subtypes of mental and physical health outcomes described above: 1) anxiety symptoms or disorders; 2) depression symptoms or disorders; 3) internalizing disorders; 4) externalizing disorders; 5) fatigue symptoms or disorders; 6) immune or inflammatory disorders; 7) obesity/adiposity; 8) cardiovascular disease symptoms and diagnoses; 9) cancer disease status or progression; 10) other mental health outcomes; 11) other physical health outcomes; and 12) mortality.
Type of slope measure was coded into the categories described in the introduction: 1) wake to late evening or bedtime slopes (wake); 2) peak to late evening or bedtime slopes (peak); 3) short slopes in which the slope covers only a portion of the waking day (including late declines from spline analyses) (short); 4) fixed time point measurements (FTP); and 5) amplitude measures from cosinor analysis (amp).
Data extraction for each study also included information on publication year, sample size, age of study sample, population description, cross sectional or prospective study design, cortisol measurement protocols (including times of measurement, number of measurements per day, number of days) statistical results and effect size (directly reported or calculated from statistics provided). If insufficient data were present to quantify an effect size, first authors were contacted to obtain more information. Data extraction was initially conducted by second through last authors and subsequently verified by the first author. When discrepancies were found, studies were presented and conferenced in consensus meetings.
The coding scheme for study quality was based primarily on the number of confounding variables assessed and accounted for in either study design or analysis. Similar approaches have been taken in prior meta-analyses of cortisol data (e.g., Chida & Steptoe, 2009). Study quality scores could range from 0 (low quality) to 9 (high quality) and were based on whether each of the following were accounted for within each study: 1) age; 2) gender; 3) smoking; 4) use of steroid-based medications; 5) wake time; 6) sampling day (weekday or weekend); 7) self-reported adherence with sampling times; 8) objective adherence based on electronic monitoring; and 9) clear sampling instructions provided to participants (e.g., to refrain from brushing their teeth, drinking, or eating 15 minutes prior to sampling).
2.4 Data Synthesis and Analysis
Effect size calculations were based on DCS differences between the healthy control group and the mental or physical health disorder group or, alternatively, the continuous association between the DCS and a mental/physical health symptom or mortality measure. Effect sizes were expressed as Pearson correlations (r values) for all studies except mortality studies, which were expressed as Hazard Ratios (HRs). When r values were available, they were used directly in the meta-analysis; otherwise, they were converted from available statistics. When regression techniques were used to determine slopes, standardized beta coefficients were transformed into r values (Peterson and Brown, 2005). The mortality studies all employed hazard ratios; for this reason, we used HRs as the effect size index in a separate meta-analysis for that outcome. If no other appropriate statistics were provided, we back-converted the effect size from a one-tailed p-value and sample size information (Hunter et al., 1982; Rosenthal and Rubin, 2003). When two-tailed p-values were reported they were converted to one-tailed p-values for these calculations. When a paper reported p < .05, p < .01, or n.s., we computed the effect size from sample sizes and p-values of .025, .005, or .50 respectively, which yield a conservative estimate of the effect size given that the upper limit of the significance value was utilized.
When multiple models predicting the same health outcome within the same population were available in an article, we selected the statistics from the model that included the most complete set of covariates. This allowed us to examine, where possible, the size of association between cortisol slopes and health outcomes net of the effect of key confounding influences on cortisol such as health behaviors. Exceptions to this rule were made when earlier models provided more accurate effect size data, such as when an earlier model provided an r value and later models provided only rough significance levels (e.g., p <.05 or n.s.). When results predicting the same health outcome were reported separately for subpopulations (e.g., men and women), both of the subpopulation results were included if the average effect size across the full population was not available.
We first conducted an overall meta-analysis across all types of mental and physical health outcomes (overall health outcome) and then conducted subgroup analyses examining associations between DCS measures and each of the twelve different subtypes of health outcomes. For the overall health outcome analysis, if more than one type of health outcome was assessed in a single study, the results were not treated as independent; rather, the average of the effects for the multiple health outcomes within each study was used. When subgroup analyses were performed for the specific health outcomes, and multiple findings for the same health outcome were reported in the same study, the average of the effects within each study was used.
We employed the Q test for homogeneity across studies. Where significant variability in effect size across studies was found, random effects models were used to determine effect sizes. In addition, tests for the moderating effects of various study characteristics were employed. Publication bias was assessed by visual inspection of a funnel plot of effect size vs. standard error, and using Egger’s unweighted regression asymmetry test (Egger et al., 1997). Duval and Tweedie’s trim and fill procedures (Duval and Tweedie, 2000b) were also used to calculate an adjusted effect size after replacing missing studies. Meta-analyses and bias analyses were performed using Comprehensive Meta-Analysis (CMA) Software 3.3 (Borenstein et al., 2014).
3. Results
3.1 Study Characteristics and Quality
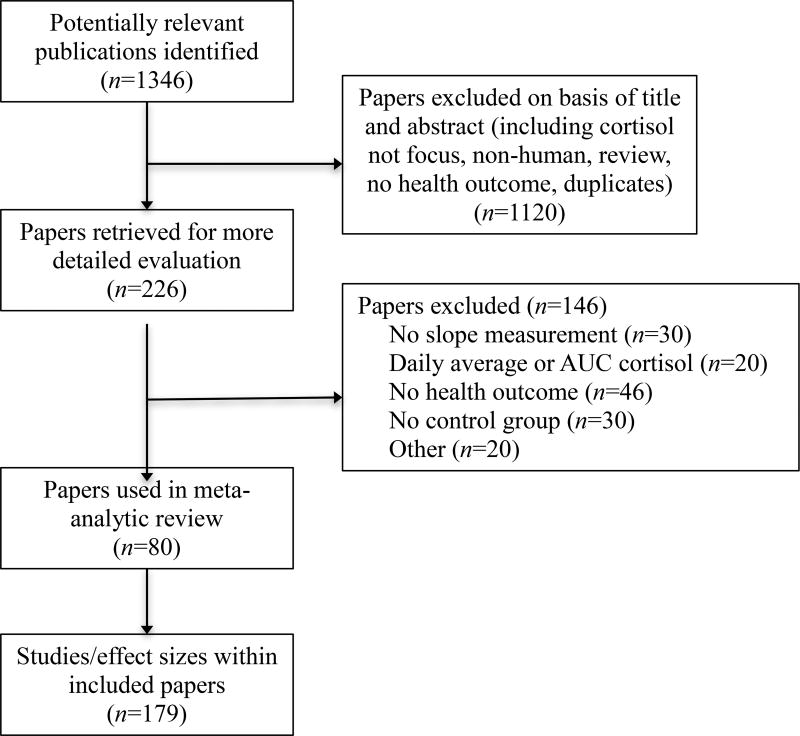
Figure 1 shows the details of the PRISMA flow diagram for this systematic review and meta-analysis (Moher et al., 2009). Table 1 details the studies and findings from each study included in this analysis. It also includes the coded characteristics, sample size, and effect size of each included finding.
Figure 1.
Flow Diagram of Systematic Review Search.
Table 1.
Summary of Papers and Findings Included in Meta-analysis of Associations between Diurnal Cortisol Slopes and Health
| No. | Authors (Year) | Outcome Categorya |
Specific Outcomeb | Study Quality (0 – 9)c | Slope Typed |
Days, Samplese |
Agef | Cross/ Prosp.g |
Nh | r or (HR)i |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | Abercrombie et al., (2004) | Cancer | Metastatic breast cancer diagnosis | 3: g, sm, st | wake | 3,4 | adult | cross | 48 | 0.308 |
| 1.2 | Abercrombie et al., (2004) | Cancer | Severity of metastatic spread | 3: g, sm, st | wake | 3,4 | adult | cross | 17 | 0.450 |
| 1.3 | Abercrombie et al., (2004) | Obesity | Waist circumference in controls | 3: g, sm, st | wake | 3,4 | adult | cross | 31 | 0.510 |
| 1.4 | Abercrombie et al., (2004) | Obesity | Waist circumference in metastatic breast cancer patients | 3: g, sm, st | wake | 3,4 | adult | cross | 17 | −0.300 |
| 2.1 | Adam (2006) | Anxiety | Anxiety symptoms | 8: ag, g, s, sm, st, i, wt, d | wake | 2,7 | adol. | cross | 52 | −0.062 |
| 2.2 | Adam (2006) | Depr. | Depressive symptoms | 8: ag, g, s, sm, st, i, wt, d | wake | 2,7 | adol. | cross | 52 | 0.003 |
| 3 | Adam et al., (2010) | Depr. | MDD | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | prosp. | 230 | −0.126 |
| 4 | Baird et al., (2012) | Extern. | Attention-deficit hyperactivity disorder | 3: ag, g, s | amp. | 1,6 | adult | cross | 32 | 0.517 |
| 5 | Bao et al., (2004) | Depr. | MDD | 5: ag, g, s, i, wt | amp. | 4,12 | adult | cross | 27 | −0.001 |
| 6.1 | Bhattacharyya et al., (2008) | CVD | Coronary artery disease | 5: ag, g, sm, st, i | FTP | 1,4 | older adult | cross | 84 | −0.018 |
| 6.2 | Bhattacharyya et al., (2008) | Depr. | Depressive symptoms in coronary artery disease patients | 5: ag, g, sm, st, i | FTP | 1,4 | older adult | cross | 52 | 0.500 |
| 6.3 | Bhattacharyya et al., (2008) | Depr. | Depressive symptoms in patients without coronary artery disease | 5: ag, g, sm, st, i | FTP | 1,4 | older adult | cross | 32 | 0.018 |
| 7.1 | Bosch et al., (2007) | Immune | Mucosal wound healing time | 2: g, s | FTP | 5,7 | adult | cross | 183 | 0.000 |
| 7.2 | Bosch et al., (2007) | Depr. | Depressive Symptoms | 2: g, s | FTP | 5,7 | adult | cross | 183 | 0.187 |
| 8 | Bower et al., (2005b) | Fatigue | Fatigue in breast cancer survivors | 3: g, sm, i | wake | 2,4 | adult | cross | 29 | 0.315 |
| 9.1 | Buss et al., (2014) | Obesity | Grhelin in obese individuals | 3: g, st, i | wake | 3,3 | adult | cross | 23 | −0.270 |
| 9.2 | Buss et al., (2014) | Obesity | Grhelin in overweight individuals | 3: g, st, i | wake | 3,3 | adult | cross | 19 | 0.480 |
| 10.1 | Carnegie et al., (2014) | Depr. | MDD | 6: g, s, sm, st, i, d | wake | 3,4 | adol. | cross | 897 | −0.010 |
| 10.2 | Carnegie et al., (2014) | Depr. | MDD | 6: ag, g, s, sm, st, i, d | wake | 3,4 | adol. | prosp. | 460 | 0.013 |
| 11 | Cicchetti et al., (2010) | Intern. | Depressive or internalizing symptoms | 4: ag, g, om, i | short | 5,2 | child | cross | 553 | 0.108 |
| 12 | Cima et al., (2008) | Other MH | Psychopathic traits | 6: ag, g, om, sm, st, i | FTP | 1,4 | adult | cross | 74 | 0.207 |
| 13.1 | Cohen et al., (2012) | Death | Death after diagnosis of metastatic renal cell carcinoma | 0 | peak | 3,5 | older adult | prosp. | 217 | (1.96) |
| 13.2 | Cohen et al., (2012) | Depr. | Depressive symptoms in metastatic renal cell carcinoma patients | 0 | peak | 3,5 | older adult | cross | 202 | 0.000 |
| 14.1 | Daniel et al., (2006) | Obesity | BMI in women with high school diploma as highest level of education | 3: ag, g, i | short | 1,2 | adult | cross | 78 | 0.450 |
| 14.2 | Daniel et al., (2006) | Obesity | BMI in women with less than a high school diploma | 3: ag, g, i | short | 1,2 | adult | cross | 16 | 0.890 |
| 14.3 | Daniel et al., (2006) | Obesity | BMI in women with education beyond a high school diploma | 3: ag, g, i | short | 1,2 | adult | cross | 35 | 0.170 |
| 15.1 | Dekker et al., (2008) | CVD | Atherosclerotic plaques | 8: ag, g, s, sm, st, i, wt, d | wake | 1,3 | older adult | cross | 1866 | −0.003 |
| 15.2 | Dekker et al., (2008) | Obesity | BMI | 8: ag, g, s, sm, st, i, wt, d | wake | 1,3 | older adult | cross | 1866 | 0.050 |
| 15.3 | Dekker et al., (2008) | Other PH | Diabetes | 8: ag, g, s, sm, st, i, wt, d | wake | 1,3 | older adult | cross | 1866 | −0.014 |
| 15.4 | Dekker et al., (2008) | CVD | Diastolic blood pressure | 8: ag, g, s, sm, st, i, wt, d | wake | 1,3 | older adult | cross | 1866 | −0.012 |
| 15.5 | Dekker et al., (2008) | CVD | HDL cholesterol | 8: ag, g, s, sm, st, i, wt, d | wake | 1,3 | older adult | cross | 1866 | 0.003 |
| 15.6 | Dekker et al., (2008) | CVD | Systolic blood pressure | 8: ag, g, s, sm, st, i, wt, d | wake | 1,3 | older adult | cross | 1866 | −0.014 |
| 15.7 | Dekker et al., (2008) | CVD | Total cholesterol | 8: ag, g, s, sm, st, i, wt, d | wake | 1,3 | older adult | cross | 1866 | 0.012 |
| 16.1 | Den Hartog et al., (2003) | Immune | Allergic rhinitis | 5: ag, g, sm, st, i | FTP | 2,3 | adult | cross | 56 | 0.177 |
| 16.2 | Den Hartog et al., (2003) | Depr. | MDD | 5: ag, g, sm, st, i | FTP | 2,3 | adult | cross | 63 | 0.417 |
| 17 | Dienes et al., (2013) | Depr. | MDD in women | 8: g, s, om, sm, st, i, wt, d | peak | 5,4 | adol. | cross | 57 | 0.005 |
| 18 | Dmitrieva et al., (2013) | Other PH | Global self-rated health | 6: ag, s, om, sm, st, i | peak | 4,4 | adult | cross | 1101 | 0.339 |
| 19 | Doane & Adam (2010) | Depr. | MDD | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | cross | 108 | 0.177 |
| 20.1 | Doane et al., (2013) | Depr. | Anhedonic depression symptoms | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | cross | 300 | 0.016 |
| 20.2 | Doane et al., (2013) | Depr. | Anxious arousal symptoms | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | cross | 300 | 0.177 |
| 20.3 | Doane et al., (2013) | Intern. | Self-reported general distress | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | cross | 300 | 0.134 |
| 20.4 | Doane et al., (2013) | Anxiety | Recent Anxiety Disorder | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | cross | 300 | −0.039 |
| 20.5 | Doane et al., (2013) | Intern. | Recent comorbid MDD and Anxiety Disorder | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | cross | 300 | 0.093 |
| 20.6 | Doane et al., (2013) | Depr. | Recent MDD | 8: ag, g, s, sm, st, i, wt, d | wake | 3,6 | adol. | cross | 300 | 0.051 |
| 21 | Doane et al., (2011) | Depr. | Depressive symptoms | 7: ag, g, sm, st, i, wt, d | peak | 2,5 | adult | cross | 735 | 0.104 |
| 22 | Du et al., (2013) | Depr. | Depressive symptoms in patients with lung cancer | 3: om, st, i | FTP | 1,5 | adult | cross | 126 | 0.270 |
| 23 | Eckart et al., (2009) | Anxiety | PTSD in men | 7: ag, g, s, om, st, i, wt | FTP | 1,10 | adult | cross | 43 | −0.156 |
| 24 | Edwards et al., (2003) | Other PH | Symptoms of upper respiratory infection | 3: sm, st, i | short | 2,8 | adult | cross | 29 | −0.288 |
| 25 | Ferguson (2008) | Anxiety | Self-reported health anxiety | 4: sm, i, wt, d | short | 8,2 | adult | cross | 42 | −0.391 |
| 26 | Fidan et al., (2013a) | Immune | Allergic rhinitis | 6: ag, g, s, om, st, i | amp. | 1,6 | adult | cross | 58 | 0.661 |
| 27 | Fidan et al., (2013b) | Immune | Extensive nasal polyposis | 6: ag, g, s, om, st, i | amp. | 1,6 | adult | cross | 58 | 0.813 |
| 28 | Fiocco et al., (2006) | Depr. | Depressive symptoms | 5: ag, g, sm, st, i | wake | 2,4 | older adult | cross | 34 | 0.343 |
| 29 | Gallagher-Thompson et al., (2006) | Depr. | Depressive symptoms in dementia caregivers | 2: g, i | FTP | 3,3 | adult | cross | 83 | 0.395 |
| 30 | Goel et al., (2009) | Other MH | Night eating syndrome in women | 5: ag, g, s, om, wt | amp. | 24,20 | adult | cross | 29 | 0.130 |
| 31.1 | Gold et al., (2009) | Depr. | Depressive symptoms in patients with multiple sclerosis | 2: st, i | wake | 2,3 | adult | cross | 29 | 0.359 |
| 31.2 | Gold et al., (2009) | Other PH | Relapsing-remitting multiple sclerosis | 2: st, i | wake | 2,3 | adult | cross | 45 | 0.257 |
| 31.3 | Gold et al., (2009) | Other PH | Hippocampal volume in patients with multiple sclerosis | 2: st, i | wake | 2,3 | adult | cross | 29 | 0.460 |
| 32 | Hackett et al., (2014) | Other PH | Type 2 Diabetes | 8: ag, g, s, sm, st, i, wt, d | wake | 1,6 | older adult | cross | 3508 | 0.044 |
| 33.1 | Hagger-Johnson et al., (2010) | Other MH | Self-reported mental health | 7: ag, g, s, st, i, wt, d | wake | 2,4 | adult | cross | 68 | 0.442 |
| 33.2 | Hagger-Johnson et al., (2010) | Other PH | Self-reported physical health | 7: ag, g, s, st, i, wt, d | wake | 2,4 | adult | cross | 68 | 0.027 |
| 34.1 | Hajat et al., (2013) | CVD | Ankle-brachial index | 7: ag, s, om, st, i, wt, d | wake | 3,6 | older adult | cross | 610 | 0.220 |
| 34.2 | Hajat et al., (2013) | CVD | Coronary calcification | 8: ag, g, s, om, st, i, wt, d | wake | 3,6 | older adult | cross | 464 | 0.120 |
| 35.1 | Ho et al., (2013) | Anxiety | Anxiety symptoms in breast cancer patients | 5: ag, g, sm, i, wt | wake | 2,4 | adult | cross | 181 | −0.069 |
| 35.2 | Ho et al., (2013) | Depr. | Depressive symptoms in breast cancer patients | 5: ag, g, sm, i, wt | wake | 2,4 | adult | cross | 181 | −0.039 |
| 35.3 | Ho et al., (2013) | Other PH | Perceived health in breast cancer patients | 5: ag, g, sm, i, wt | wake | 2,4 | adult | cross | 181 | 0.190 |
| 36.1 | Hsiao et al., (2010) | Anxiety | Anxiety symptoms in MDD patients | 7: ag, g, s, sm, st, i, wt | peak | 1,5 | adult | cross | 126 | −0.339 |
| 36.2 | Hsiao et al., (2010) | Depr. | Depressive symptoms in MDD patients | 7: ag, g, s, sm, st, i, wt | peak | 1,5 | adult | cross | 126 | 0.324 |
| 36.3 | Hsiao et al., (2010) | Other PH | Self-perceived physical health in MDD patients | 7: ag, g, s, sm, st, i, wt | peak | 1,5 | adult | cross | 126 | 0.000 |
| 36.4 | Hsiao et al., (2010) | Other MH | Self-perceived suffering in MDD patients | 7: ag, g, s, sm, st, i, wt | peak | 1,5 | adult | cross | 126 | 0.181 |
| 37.1 | Jabben et al., (2011) | Other MH | Bipolar I disorder | 6: ag, g, sm, i, wt, d | wake | 1,6 | adult | cross | 437 | −0.136 |
| 37.2 | Jabben et al., (2011) | Depr. | MDD | 6: ag, g, sm, i, wt, d | wake | 1,6 | adult | cross | 1438 | −0.064 |
| 38.1 | Jarcho et al., (2013) | Other PH | Glucocorticoid (dexamethasone) suppression in women with MDD and healthy controls | 5: g, sm, st, i, wt | wake | 4,3 | adult | cross | 49 | 0.650 |
| 38.2 | Jarcho et al., (2013) | Depr. | MDD in women | 5: g, sm, st, i, wt | wake | 4,3 | adult | cross | 49 | 0.803 |
| 39.1 | Johar et al., (2014) | Other PH | Frailty status | 2: ag, g | peak | 1,3 | older adult | cross | 722 | 0.100 |
| 39.2 | Johar et al., (2014) | Other PH | Frailty status | 2: ag, g | wake | 1,3 | older adult | cross | 722 | 0.075 |
| 40.1 | Kiefte-de Jong et al., (2011) | Other PH | Abdominal pain | 6: ag, g, s, sm, i, d | wake | 1,5 | infant | cross | 483 | 0.092 |
| 40.2 | Kiefte-de Jong et al., (2011) | Other PH | Functional constipation | 6: ag, g, s, sm, i, | wake | 1,5 | infant | cross | 483 | 0.038 |
| 41.1 | Kim et al., (2012) | Cancer | Lung cancer | 5: ag, g, s, om, i | wake | 2,4 | older adult | cross | 108 | 0.172 |
| 41.2 | Kim et al., (2012) | Cancer | Lung cancer disease stage | 5: ag, g, s, om, i | wake | 2,4 | older adult | cross | 108 | 0.360 |
| 41.3 | Kim et al., (2012) | Cancer | Lung cancer performance status score | 5: ag, g, s, om, i | wake | 2,4 | older adult | cross | 108 | 0.430 |
| 42 | Knight et al., (2010) | Depr. | Depressive symptoms | 6: g, s, sm, st, i, wt | wake | 1,3 | adult | cross | 408 | 0.113 |
| 43.1 | Kumari et al., (2009) | Fatigue | Fatigue | 5: ag, sm, i, wt, d | wake | 1,6 | older adult | cross | 4299 | 0.047 |
| 43.2 | Kumari et al., (2009) | Fatigue | Persistent fatigue | 5: ag, sm, i, wt, d | wake | 1,6 | older adult | prosp. | 4299 | 0.080 |
| 44.1 | Kumari et al., (2010) | Obesity | BMI | 6: ag, g, sm, i, wt, d | wake | 1,6 | older adult | cross | 3956 | 0.044 |
| 44.2 | Kumari et al., (2010) | Obesity | Waist circumference | 6: ag, g, sm, i, wt, d | wake | 1,6 | older adult | cross | 3956 | 0.058 |
| 45.1 | Kumari et al., (2011) | Death | Cardiovascular death | 7: ag, g, s, sm, i, wt, d | wake | 1,6 | older adult | prosp. | 4047 | (1.87) |
| 45.2 | Kumari et al., (2011) | Death | Non-cardiovascular death | 7: ag, g, s, sm, i, wt, d | wake | 1,6 | older adult | prosp. | 4047 | (1.17) |
| 46.1 | Kurina et al., (2004) | Anxiety | Anxiety symptoms in men | 4: g, sm, i, d | wake | 2,6 | adult | cross | 34 | −0.222 |
| 46.2 | Kurina et al., (2004) | Anxiety | Anxiety symptoms in women | 4: g, sm, i, d | wake | 2,6 | adult | cross | 57 | 0.045 |
| 46.3 | Kurina et al., (2004) | Depr. | Depressive symptoms in men | 4: g, sm, i, d | wake | 2,6 | adult | cross | 34 | 0.072 |
| 46.4 | Kurina et al., (2004) | Depr. | Depressive symptoms in women | 4: g, sm, i, d | wake | 2,6 | adult | cross | 57 | 0.175 |
| 47.1 | Lamers et al., (2013) | Depr. | Atypical MDD | 5: ag, g, s, sm, d | wake | 1,5 | adult | cross | 665 | 0.097 |
| 47.2 | Lamers et al., (2013) | Depr. | Melancholic MDD | 5: ag, g, s, sm, d | wake | 1,5 | adult | cross | 654 | −0.120 |
| 48 | Lederbogen et al., (2011) | Other PH | Type 2 Diabetes | 6: ag, sm, st, i, wt, d | wake | 1,4 | older adult | cross | 748 | 0.065 |
| 49 | Ludescher et al., (2007) | Obesity | Visceral adipose tissue | 1: st | short | 1,2 | adult | cross | 27 | 0.451 |
| 50 | Matthews et al., (2006) | CVD | Coronary calcification | 6: ag, g, s, sm, i, d | wake | 1,6 | adult | cross | 718 | 0.253 |
| 51.1 | Nater et al., (2008) | Fatigue | Chronic fatigue symptoms in chronic fatigue syndrome patients | 5: ag, g, om, st, wt | wake | 1,6 | adult | cross | 17 | 0.559 |
| 51.2 | Nater et al., (2008) | Fatigue | Chronic fatigue syndrome | 5: ag, g, om, st, wt | wake | 1,6 | adult | cross | 67 | 0.294 |
| 52.1 | O'Conner et al., (2014) | Anxiety | Anxiety disorders | 7: ag, g, s, sm, i, wt, d | wake | 1,5 | adult | cross | 101 | 0.000 |
| 52.2 | O'Connor et al., (2014) | Depr. | MDD | 7: ag, g, s, sm, i, wt | wake | 1,5 | adult | cross | 101 | 0.192 |
| 53.1 | Patterson et al., (2013) | Immune | CD4+ T cell activation in HIV patients | 3: ag, sm, i | wake | 3,3 | adult | cross | 128 | 0.240 |
| 53.2 | Patterson et al., (2013) | Immune | CD8+ T cell activation in HIV patients | 3: ag, sm, i | wake | 3,3 | adult | cross | 128 | 0.240 |
| 54 | Possel et al., (2014) | Depr. | Depressive symptoms | 7: ag, g, sm, st, i, wt, d | wake | 3,2 | adult | cross | 257 | 0.206 |
| 55 | Ranjit et al., (2005) | Obesity | Obesity | 5: ag, g, s, sm, st | short | 1,3 | adult | cross | 188 | 0.026 |
| 56 | Rhebergen et al., (2015) | Depr. | MDD | 7: ag, g, s, sm, st, i, wt | wake | 2,5 | older adult | cross | 420 | −0.092 |
| 57.1 | Ruttle et al., (2011) | Extern. | Externalizing symptoms | 6: ag, g, sm, st, i, wt | wake | 2,8.43 | child | cross | 96 | 0.218 |
| 57.2 | Ruttle et al., (2011) | Intern. | Internalizing symptoms | 6: ag, g, sm, st, i, wt | wake | 2,8.43 | child | cross | 96 | 0.000 |
| 57.3 | Ruttle et al., (2011) | Extern. | Externalizing symptoms | 6: ag, g, sm, st, i, wt | wake | 2,8.43 | child | prosp. | 96 | 0.258 |
| 57.4 | Ruttle et al., (2011) | Intern. | Internalizing symptoms | 6: ag, g, sm, st, i, wt | wake | 2,8.43 | child | prosp. | 96 | 0.129 |
| 58.1 | Ruttle et al., (2013) | Obesity | BMI | 5: ag, g, sm, st, wt | wake | 3,3 | adol. | cross | 297 | 0.210 |
| 58.2 | Ruttle et al., (2013) | Obesity | BMI | 5: ag, g, sm, st, wt | wake | 3,3 | adol. | cross | 306 | 0.080 |
| 58.3 | Ruttle et al., (2013) | Obesity | BMI | 5: ag, g, sm, st, wt | wake | 3,3 | adol. | cross | 272 | 0.060 |
| 59 | Ruttle et al., (2014) | Immune | Atopic disorders | 6: ag, g, sm, st, i, wt | wake | 2,8.43 | child | cross | 96 | 0.197 |
| 60.1 | Sannes et al., (2013) | Anxiety | Anxiety symptoms in women with endometrial cancer | 5: g, s, | FTP | 3,4 | older adult | cross | 82 | −0.089 |
| 60.2 | Sannes et al., (2013) | Depr. | Depressive symptoms in women with endometrial cancer | 5: g, s, | FTP | 3,4 | older adult | cross | 82 | −0.143 |
| 61.1 | Saridjan et al., (2014) | Extern. | Externalizing symptoms | 6: ag, g, s, sm, i, wt | wake | 1,5 | infant | cross | 282 | −0.029 |
| 61.2 | Saridjan et al., (2014) | Extern. | Externalizing symptoms | 6: ag, g, s, sm, i, wt | wake | 1,5 | infant | prosp. | 322 | 0.460 |
| 61.3 | Saridjan et al., (2014) | Intern. | Internalizing symptoms | 6: ag, g, s, sm, i, wt | wake | 1,5 | infant | cross | 280 | −0.147 |
| 61.4 | Saridjan et al., (2014) | Intern. | Internalizing symptoms | 6: ag, g, s, sm, i, wt | wake | 1,5 | infant | prosp. | 322 | 0.610 |
| 62.1 | Schrepf et al., (2014) | Other PH | Interstitial cystitis | 2: sm, wt | wake | 3,3 | adult | cross | 72 | 0.273 |
| 62.2 | Schrepf et al., (2014) | Immune | TLR-4 inflammation score | 2: sm, wt | wake | 3,3 | adult | cross | 58 | 0.340 |
| 63 | Segebladh et al., (2013) | Depr. | Premenstrual dysphoric disorder | 2: g, i | FTP | 1,4 | adult | cross | 56 | 0.179 |
| 64.1 | Sephton et al., (2009) | Depr. | Depressive symptoms | 3: ag, g, st | FTP | 3,4 | adult | cross | 72 | −0.230 |
| 64.2 | Sephton et al., (2009) | Immune | Induration size | 3: ag, g, st | FTP | 3,4 | adult | cross | 72 | −0.127 |
| 64.3 | Sephton et al., (2009) | Immune | Positive antigen response | 3: ag, g, st | FTP | 3,4 | adult | cross | 72 | 0.041 |
| 65 | Sephton and Spiegel, (2003) | Death | Death in lung cancer patients | 3: g, s, i | wake | 2,4 | older adult | prosp. | 62 | (68052.82) |
| 66.1 | Sephton et al., (2000) | Immune | Absolute circulating NK cells | 1: i | FTP | 3,4 | adult | cross | 104 | 0.250 |
| 66.2 | Sephton et al., (2000) | Death | Death in women with metastatic breast cancer | 1: i | FTP | 3,4 | adult | prosp. | 104 | (464.90) |
| 66.3 | Sephton et al., (2000) | Immune | Suppression of NK cell activity | 1: i | FTP | 3,4 | adult | cross | 104 | 0.170 |
| 67.1 | Shirtcliff and Essex, (2008) | Other MH | Mental health symptom severity | 5: g, sm, st, i, wt | wake | 3,3 | child | cross | 294 | 0.170 |
| 67.2 | Shirtcliff and Essex, (2008) | Other MH | Mental health symptom severity | 5: g, sm, st, i, wt | wake | 3,3 | child | prosp. | 294 | 0.155 |
| 68.1 | Sugaya et al., (2010) | Other PH | Bowel movement | 1: sm | wake | 8,2 | adult | cross | 11 | −0.165 |
| 68.2 | Sugaya et al., (2010) | Other PH | Degree of abdominal pain | 1: sm | wake | 8,2 | adult | cross | 11 | −0.373 |
| 68.3 | Sugaya et al., (2010) | Other PH | Duration of abdominal pain | 1: sm | wake | 8,2 | adult | cross | 11 | −0.504 |
| 68.4 | Sugaya et al., (2010) | Other PH | Stool property | 1: sm | wake | 8,2 | adult | cross | 11 | −0.210 |
| 69 | Tell et al., (2014) | Fatigue | Fatigue | 4: s, sm, st, wt | wake | 2,5 | adult | cross | 130 | 0.185 |
| 70 | Tomiyama et al., (2012) | Other PH | Telomere length | 5: ag, g, s, st, i | wake | 3,3 | older adult | cross | 23 | 0.438 |
| 71 | Tordjman et al., (2014) | Other MH | Autism | 5: sm, st, i, wt, d | FTP | 1,5 | child | cross | 87 | 0.417 |
| 72.1 | Tu at el., (2013) | Obesity | Obesity in Canadian older adults | 2: sm, i | peak | 2,5 | older adult | cross | 60 | 0.220 |
| 72.2 | Tu et al., (2013) | Immune | Arthritis in older adults from Brazil and Canada | 2: sm, i | peak | 2,5 | older adult | cross | 124 | 0.250 |
| 72.3 | Tu et al., (2013) | Depr. | Depressive symptoms in Brazilian and Canadian older adults | 2: sm, i | peak | 2,5 | older adult | cross | 124 | −0.028 |
| 72.4 | Tu et al., (2013) | Obesity | Obesity in Brazilian older adults | 2: sm, i | peak | 2,5 | older adult | cross | 64 | 0.148 |
| 73 | Turner-Cobb et al., (2011) | Other PH | Upper respiratory infection | 4: sm, i, wt, d | short | 2,2 | child | prosp. | 70 | −0.230 |
| 74.1 | Vammen et al., (2014) | Depr. | Depressive symptoms | 4: ag, g, s, sm | peak | 1,2 | adult | cross | 3536 | 0.008 |
| 74.2 | Vammen et al., (2014) | Depr. | Depressive symptoms | 4: ag, g, s, sm | peak | 1,2 | adult | cross | 3536 | −0.014 |
| 74.3 | Vammen et al., (2014) | Depr. | Depressive symptoms | 4: ag, g, s, sm | peak | 1,2 | adult | prosp. | 2789 | −0.086 |
| 74.4 | Vammen et al., (2014) | Depr. | Depressive symptoms | 4: ag, g, s, sm | peak | 1,2 | adult | prosp. | 2253 | −0.065 |
| 74.5 | Vammen et al., (2014) | Depr. | Depressive symptoms | 4: ag, g, s, sm | peak | 1,2 | adult | cross | 376 | 0.043 |
| 74.6 | Vammen et al., (2014) | Depr. | Depressive symptoms | 4: ag, g, s, sm | peak | 1,2 | adult | cross | 474 | −0.038 |
| 74.7 | Vammen et al., (2014) | Depr. | MDD | 4: ag, g, s, sm | peak | 1,2 | adult | cross | 376 | 0.061 |
| 74.8 | Vammen et al., (2014) | Depr. | MDD | 4: ag, g, s, sm | peak | 1,2 | adult | cross | 474 | −0.135 |
| 75 | Van den Bergh et al., (2009) | Depr. | Depressive symptoms | 2: g, d | wake | 1,3 | adol. | cross | 58 | 0.267 |
| 76.1 | Van den Bergh et al., (2008) | Extern. | Aggressive behaviors | 3: sm, st, d | wake | 1,3 | adol. | cross | 58 | 0.111 |
| 76.2 | Van den Bergh et al., (2008) | Anxiety | Self-reported anxiety | 3: sm, st, d | wake | 1,3 | adol. | cross | 58 | 0.277 |
| 76.3 | Van den Bergh et al., (2008) | Depr. | Self-reported depressive symptoms | 3: sm, st, d | wake | 1,3 | adol. | cross | 58 | 0.002 |
| 77.1 | Vedhara et al., (2006) | Other MH | General psychological distress in breast cancer patients | 3: g, sm, i | wake | 2,4 | adult | cross | 76 | 0.166 |
| 77.2 | Vedhara et al., (2006) | Other MH | General psychological distress in healthy control women | 3: g, sm, i | wake | 2,4 | adult | cross | 55 | −0.164 |
| 77.3 | Vedhara et al., (2006) | Anxiety | Anxiety symptoms in breast cancer patients | 3: g, sm, i | wake | 2,4 | adult | cross | 76 | 0.110 |
| 77.4 | Vedhara et al., (2006) | Anxiety | Anxiety symptoms in healthy control women | 3: g, sm, i | wake | 2,4 | adult | cross | 55 | −0.175 |
| 77.5 | Vedhara et al., (2006) | Cancer | Breast cancer patient status | 3: g, sm, i | wake | 2,4 | adult | cross | 131 | 0.012 |
| 77.6 | Vedhara et al., (2006) | Depr. | Depressive symptoms in breast cancer patients | 3: g, sm, i | wake | 2,4 | adult | cross | 76 | −0.053 |
| 77.7 | Vedhara et al., (2006) | Depr. | Depressive symptoms in healthy women | 3: g, sm, i | wake | 2,4 | adult | cross | 55 | −0.133 |
| 78.1 | Veen et al., (2011) | Depr. | Anhedonic depression symptoms in patients with MDD, anxiety, or comorbid | 5: ag, g, sm, st, i | FTP | 2,4 | adult | cross | 48 | −0.144 |
| 78.2 | Veen et al., (2011) | Anxiety | Anxiety disorder | 5: ag, g, sm, st, i | FTP | 2,4 | adult | cross | 63 | −0.146 |
| 78.3 | Veen et al., (2011) | Anxiety | Anxious arousal symptoms in patients with MDD, anxiety, or comorbid | 5: ag, g, sm, st, i | FTP | 2,4 | adult | cross | 48 | 0.094 |
| 78.4 | Veen et al., (2011) | Intern. | Comorbid MDD & anxiety diagnosis | 5: ag, g, sm, st, i | FTP | 2,4 | adult | cross | 64 | −0.041 |
| 78.5 | Veen et al., (2011) | Intern. | General distress symptoms in patients with MDD, anxiety, or comorbid | 5: ag, g, sm, st, i | FTP | 2,4 | adult | cross | 48 | −0.084 |
| 78.6 | Veen et al., (2011) | Depr. | MDD | 5: ag, g, sm, st, i | FTP | 2,4 | adult | cross | 81 | −0.269 |
| 79.1 | Weinrib et al., (2010) | Depr. | Depressive symptoms in benign patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 77 | −0.070 |
| 79.2 | Weinrib et al., (2010) | Depr. | Depressive symptoms in ovarian cancer patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 100 | 0.260 |
| 79.3 | Weinrib et al., (2010) | Fatigue | Fatigue in benign patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 77 | 0.080 |
| 79.4 | Weinrib et al., (2010) | Fatigue | Fatigue in ovarian cancer patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 100 | 0.280 |
| 79.5 | Weinrib et al., (2010) | Cancer | Ovarian cancer diagnosis (compared to healthy controls) | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 133 | 0.330 |
| 79.6 | Weinrib et al., (2010) | Cancer | Ovarian cancer diagnosis (compared to benign pelvic disease) | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 177 | 0.168 |
| 79.7 | Weinrib et al., (2010) | Other PH | Physician rating of impaired functioning in ovarian cancer patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 100 | 0.280 |
| 79.8 | Weinrib et al., (2010) | Other PH | Physician rating of impaired functioning in benign patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 77 | 0.010 |
| 79.9 | Weinrib et al., (2010) | Other PH | Physical wellbeing in benign patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 77 | 0.210 |
| 79.10 | Weinrib et al., (2010) | Other PH | Physical wellbeing in ovarian cancer patients | 5: ag, g, sm, st, i | wake | 3,3 | adult | cross | 100 | 0.290 |
| 80 | Wolf et al., (2008) | Immune | Asthma diagnosis | 6: ag, g, om, sm, st, i | peak | 2,4 | adol. | cross | 92 | −0.053 |
Outcome Category: CVD = Cardiovascular Disease; Depr. = Depression; Extern. = Externalizing; Immune = Inflammation/immune; Intern. = Internalizing; Obesity = Obesity/BMI/Adipose; Other MH = Other Mental Health; Other PM = Other Physical Health
Specific Outcome: MDD = Major Depressive Disorder;
Study Quality: number indicates overall score; ag = controlled for age; g = controlled for gender; s = controlled for smoking; om = used objective monitoring of sampling time adherence; sm = used subjective monitoring of sampling time adherence; st = controlled for steroid medications; i = provided sampling instructions; wt = controlled for wake time; d = used consistent sampling days
Slope Type: amp. = amplitude; FTP = fixed time point; peak = peak to evening; short = short slope; wake = wake to evening
Days, samples: first number indicates number of cortisol sampling days, second number indicates number of samples per day
Age: adol. = adolescent;
Cross/prosp.: cross = cross-sectional; prosp. = prospective
N: sample size for analysis of each outcome
r or (HR): effect size given as r; number in parenthesis indicates hazard ratio
A total of 36,823 participants (26,167 unique individuals, when overlap in samples across studies is considered) from 80 studies were included in this meta-analysis. From the 80 studies, 179 different associations between DCS and health outcomes were tested and reported, which reflects an average of 2.24 associations per study (range of 1 to 10 associations per study). Depression was the most common overall health outcome and most common mental health outcome assessed in relation to DCS, with 52 of the 179 findings (29.1%) focusing on depressive symptoms or diagnoses. Additional mental health outcomes included 8.4% (n = 15) of findings focusing on anxiety outcomes and 5.0% (n = 9) focused on internalizing problems more generally (a mixture of depression and anxiety symptoms typically assessed in children and adolescents). Externalizing symptoms were assessed in 3.4% (n = 6) of findings, and other mental health problems (e.g., bipolar I disorder, self-reported mental health) were assessed in 5.6% (n = 10) of findings. All told, just over half of findings focused on mental health outcomes.
The most common physical health outcome subtype was obesity/BMI/adiposity (9.5% of findings, n = 17), followed by inflammatory or immune outcomes (7.8%, n = 14), and cardiovascular disease (5.0%, n = 9). Fatigue (n = 8), cancer (n = 8), and mortality (n = 5) outcomes each accounted for less than 5% (4.5%, 4.5% and 2.8%, respectively) of the associations. The remaining findings included other physical health outcomes (14.5%, n = 26) not falling into any of the above subtypes (e.g., diabetes, abdominal pain, self-reported physical health).
In terms of type of slope assessed, most findings (64.2%, n = 115) were based on wake-to-bedtime slopes (excluding any CAR data points), with only 12.8% (n = 23) of studies including CAR data points in their slope measure. Notably, 15.1% (n = 27) of findings used fixed time points, rather than basing cortisol sampling around participants’ individual sleep wake schedules. Five percent (n = 9) of findings estimated slopes over a shorter portion of the day than the full wake-to-bedtime span (e.g., 2 hours post-awakening to bedtime), and 2.8% (n = 5) used a formal circadian cosinor analysis, extracting an amplitude measure reflecting the distance from the imputed peak and trough of the fitted cosine curve.
An overwhelming majority of the findings reported (91.1%, n = 163) were cross sectional in nature, with only 8.9% (n = 16) of the findings based on prospective studies, with the cortisol measurement preceding the health outcome (fewer still controlled for baseline symptoms or diagnoses in these prospective analyses).
Information on methodological quality measures and scores, number of days assessed, and number of samples per day also is recorded in Table 1. Study quality scores varied from 0 to 8 (out of possible 9), with a mean of 4.73 (SD = 1.99). Most findings (over 60%) controlled for age and gender, and used self-reported compliance with sampling times to identify mistimed samples. The least common aspect of study quality was the use of objective/electronic monitoring devices for compliance with sample timing, with only 16.3% (n = 13) of studies employing objective adherence monitors. Studies most commonly employed one (38.8%, n = 31), two (26.3%, n = 21) or three (23.8%, n = 19) days of cortisol sampling. Requesting four samples per day was the most common sampling protocol (25%, n = 20), although a substantial number of studies requested three (21.3%, n = 17), five (15.0%, n = 12), or six samples per day (18.8%, n = 15). Fewer studies utilized protocols with two samples per day (10%, n = 8) or seven or more samples per day (10% each, n = 8). Most studies focused on adult populations (57.5%, n = 46), followed by older adult (21.3%, n = 17), adolescent (11.3%, n = 9), child (7.5%, n = 6) and infant (2.5%, n = 2) populations.
3.2 Meta-analysis of DCS and Overall Associations with Health
The overall association between DCS and health (across all health outcomes) showed significant variability in effect sizes by study (Q = 494.77, p < .001, I2 = 83.23), justifying the use of random effects analysis in our models. Across all of the findings reported in the meta-analysis, a significant association was found between a flatter DCS and negative health outcomes (r = .147; 95% confidence interval (.112 – .183), p < .001). This overall effect size was not strongly affected by inclusion of effect sizes that were calculated from p-values and sample sizes, which were less precise than other effect size estimates given that exact p-values were not always available. Excluding the findings (n = 60) that required use of p-values and sample sizes to calculate effect sizes resulted in slightly stronger overall effects (r = .180 (.128 – .230), p < .001). By contrast, the 60 findings calculated from p-values and sample sizes provided weaker (but still significant) overall effect sizes (r = .110 (.056 – .164), p < .001), such that their inclusion leads to more conservative results.
3.3 Meta-analysis of DCS and Subtypes of Health Outcomes
Differing effect sizes for the different subtypes of health outcomes were examined using a mixed effect analysis (Borenstein et al., 2009). Type of health outcome was treated as a moderator and differences in average effect sizes for health outcomes were compared. For 8 out of 12 of the individual health outcomes (anxiety, CVD, depression, inflammation, overweight/obesity, other mental health, other physical health, and mortality outcomes), we found significant variability in effect sizes across studies within health outcome (all Q’s significant at p < .05; all I2 statistics > 50%). The remaining four outcomes (internalizing disorders, externalizing disorders, fatigue, and cancer) also showed substantial (all I2 > 35%), but not significant variability in effect sizes across studies within health outcome. For ease of comparison and significance testing across outcomes, we used random effects models for all the individual health outcomes; effect sizes for internalizing disorders, externalizing disorders, fatigue, and cancer were similar (and still significant) when fixed effects models were used.
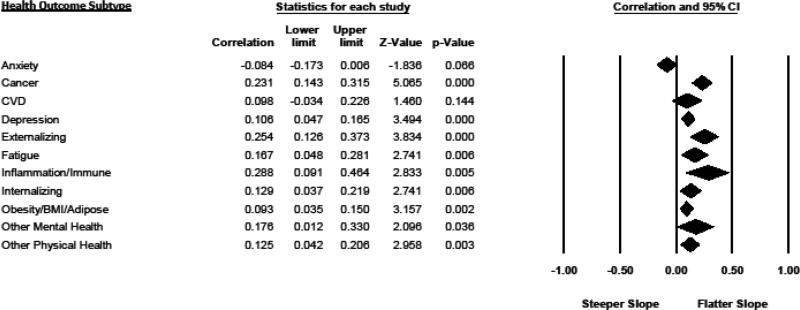
The overall test of whether effect sizes significantly varied by health outcome subgroup was significant (between-health outcomes Q = 35.135, p < .000), suggesting that certain negative health outcomes showed larger effect sizes in their meta-analytic associations with flatter DCS than other outcomes (see Figure 2). The largest effect size was found for immune and inflammatory outcomes (r = .288 (.091 – .464), p = .005), followed by the presence of externalizing symptoms or diagnoses (r = .254 (.126 – .373), p < .001), and cancer diagnoses and disease progression (r =.231 (.143 – .315), p < .001). Fatigue-related outcomes also showed significant associations with flatter DCS (r =.167 (.048 – .281), p = .006), as did internalizing symptoms and disorders (r = .129 (.037 – .219) p = .006), and the presence of depressive symptoms or diagnoses (r = .106 (.047 – .165); p < .001). Obesity/BMI/adiposity also was significantly related to flatter slopes (r = .093 (.035 – .150), p = .002), as were the catch-all categories of other mental health (r =.176 (.012 – .330), p = .036) and other physical health disorders (r = .125 (.042 – .206), p = .003).
Figure 2.
Forest Plot of Associations Between Individual Health Outcomes and Diurnal Cortisol Slopes (Pearson r)
Cardiovascular symptoms and diagnoses were not significantly associated with flatter diurnal cortisol slopes in a random effects model (r = .098 (−.034 – .226), p = .144), although the coefficient was in the expected direction, and the corresponding fixed effects model for CVD was significant (r = .043 (.004 – .081), p < .05). Finally, higher anxiety symptoms and diagnoses were not significantly associated with flatter diurnal cortisol slopes, although they neared significance (r = −.084 (−.173 – .006), p = .066). Anxiety was the only health outcome for which the coefficient was negative in direction.
Studies examining mortality as an outcome used hazard ratios (HRs) as their primary indicator of effect size. HRs cannot be converted to correlations and thus these findings were examined in a separate meta-analysis. Flatter diurnal cortisol slopes predicted a significantly increased risk for mortality over the study follow-up periods (which ranged from several years up to 10 years follow-up). Using a random effects model, the average hazard ratio of flatter cortisol slopes predicting later mortality was 2.40 (1.00 – 5.74), p = .049. Two of the mortality studies had small samples sizes and unreasonably large hazard ratios (HRs > 400). The combined HR for flatter DCS predicting increased mortality over follow-up was still significant with these two outlying studies excluded (HR = 1.630 (1.254 – 2.12), p = .000).
3.4 Moderation of Effect Size by Study Characteristics
Given significant variability in effect sizes across studies when examining the overall health effect (Q = 494.77, p < .001, I2 = 83.23), we tested whether the effect size for the association between DCS and overall health (calculated across all health outcomes) was moderated by key study characteristics.
Examining moderation by age of participant involved in the study, we found that diurnal cortisol slopes were associated with worse health for all age groups except the infant/toddler age group. There was, however, significant variability in effect sizes across the different age groups (Q = 11.030, p = .026). The largest effect sizes were found for studies of adults (r = .189 (.121 –.255), p < .001), followed by studies of school-age children (r = .145 (.020 – .265), p = .023). Average effect sizes were smaller but still significant for older adults (r = .076 (.039 – .114), p < .001), and adolescents (r = .059 (.001 – .116), p = .045). The average effect size for the infant/toddler age group was of a similar magnitude to the effect size for children, but was not statistically significant (r = .169 (−.017 – .343), p = .075). This latter finding should be interpreted with caution as there were only two studies that included participants in that age category.
Comparing the effect sizes for various types of slopes, effect sizes were largest for the five studies using amplitude measures (r = .500, (.147 – .740), p = .007). Studies using wake to bedtime or late evening slopes (the most common diurnal cortisol slope measure) and studies examining peak to late evening or bedtime slopes showed slightly smaller but significant associations with negative health outcomes (r = .118 (.082 – .153), p < .001 and r = .105 (.005. – .203), p = .039, respectively). Studies using fixed time point measures also showed significant associations (r =.156 (.062 – .247), p = .001). Studies using short daytime slopes did not show significant associations with health (r = .029 (−.168 – .224), p = .773). Although the overall variance in effect size across all slope types was not significant (Q = 5.85, p = .211), studies using amplitude measures showed significantly stronger associations with health outcomes when compared to all of the other types of slope measures combined (Q = 4.31, p = .038).
Examining moderation by number of samples per day, studies in which participants collected 3–4 samples per day or 5–6 samples per day showed significant associations between DCS and health (r = .168 (.112 – .223), p < .001 and r = .165(.107 – .221), p < .001, respectively). Conversely, studies based on 2 samples or >7 samples per day did not reveal significant associations between DCS and health outcomes (r = .065 (−.100 – .226), p = .440 and r =.060 (−.038 – .157), p = .233, respectively), although only 8 studies fell in each of these two categories. The variability in effect sizes across the samples-per-day subgroups was not significant (Q = 4.986, p = .173).
Surprisingly, studies employing only one day of data collection did not show weaker associations between health outcomes and diurnal cortisol slopes than those employing 2 or more days of cortisol data (r = .168(.115 – .220), p < .001 versus r = .130 (.081 – .179), p < .001, respectively; Q = 1.054, p = .305). In addition, effect sizes did not vary by study quality, with studies with 0 to 4 quality indicators showing similar effect sizes to those employing 5 or more quality indices (r = .146 (.087 –.204), p < .001 versus r = .148 (.103 – .193), p < .001, respectively; Q = .003, p = .954).
One aspect of study quality that did make a significant difference in effect size was the use of objective versus subjective compliance monitoring (Q = 5.825, p = .016). Studies not using an objective measure of adherence with sample timing showed an average effect size of r = .114 (.082 – .145), p < .000, while those employing objective or electronic monitoring of compliance with sample timing showed an average effect size of r = .285 (.150 – .410), p < .001.
3.5 Cross-sectional versus Prospective
Finally, we compared cross-sectional results to prospective results. Cross-sectional studies are more ambiguous with respect to causal direction than prospective studies, in which the cortisol measures are assessed prior to measurement of health outcomes and are used to prospectively predict a later health outcome. Results showed a similar average effect size for prospective studies (r = .138, .005 – .267, p = .042) as compared to cross-sectional studies (r = .141 (.106 – .176), p < .001), with no significant difference between average effect sizes for cross-sectional vs. prospective studies (Q = .001, p = .970).
3.6 Publication Bias Analysis
3.6.1 Overall Health
For the overall association between DCS and health outcomes, Egger’s regression intercept was significant, indicating the presence of bias (b = 1.22; t(82)=3.05, p(2-tailed) = .002. However, using Duval and Tweedie’s trim and fill procedure (Duval and Tweedie, 2000b, a), looking for missing studies to the left of the mean in a random effects model, 0 missing studies were identified, and the random effects coefficient for the overall association between cortisol and health remained the same (r = .147 (.111 – .183); Q = 494.77, p < .001).
3.6.2 Mortality Studies
For the mortality studies, Egger’s regression intercept was also significant, indicating the presence of bias (b = 2.82; t(2)=13.88, p (2-tailed) = .005). Duval and Tweedie’s trim and fill statistic suggested that 2 studies were missing from the analysis, and the hazard ratio was adjusted downward from 2.4 (1 to 5.7) to a still significant 1.69 (.55 – 5.2). For overall health and for mortality, it appears there is a small amount of publication bias that causes a small upward bias in our overall estimate of effect size (Sterne et al., 2001).
4. Discussion
4.1. Overview of Primary Results
Results of this systematic review and meta-analysis provide evidence to support prior assertions that flatter diurnal cortisol rhythms across the day are associated with poorer mental and physical health outcomes (Adam and Kumari, 2009). Notably, effects were both significant and in the predicted direction (flatter slopes associated with worse health outcomes) for 10 out of the 12 physical and mental health outcomes assessed. The significant outcomes included depression, internalizing disorders, externalizing disorders, fatigue, immune/inflammatory outcomes, BMI/obesity, cancer, other mental health outcomes, other physical health outcomes, and mortality.
The average effect size across all health outcomes was .147, and ranged from .09 to .29 for the significant individual health outcomes, with the largest effect size found for immune/inflammatory outcomes. These effect sizes were larger than the average effect sizes previously reported in a meta-analysis of psychosocial variables predicting the cortisol awakening response (CAR) (Chida and Steptoe, 2009), but still relatively small on average. Effects may have been small in size for a number of reasons. There was significant variability in effect directions and sizes, overall and within most of the separate health outcomes. Indeed, one purpose of the meta-analysis was to summarize these varying effect directions and sizes systematically with the goal of revealing the combined effect direction and size across studies. Potential contributions to varying and small effect sizes include: wide variation in type of study population (such as age of participant, or community vs. patient populations); variation and imprecision in DCS measurement and modeling strategies; and imprecision in measurement of health outcomes. Most studies related diurnal cortisol slopes assessed during one time period (generally with the cortisol assessment involving multiple samples over multiple days, summarized into a single DCS measure) to health outcomes measured at one point in time (usually concurrently). Both DCS measures and health measures at any point in time provide “snapshots” of complex and dynamic biological and disease processes. Both cortisol and disease symptoms or diagnoses are subject to state variation that may contribute to measurement error, particularly if not properly accounted for in study design or statistical methods. Given these methodological issues, it is possible that the effect sizes reported here underestimated the true size of DCS-health associations. On the other hand, the tendency for null results to be under-reported in the published literature may have contributed to an upward bias to our average effect size. We note, however, that our publication bias analysis suggested that publication bias had only a small effect on our results.
4.2. Implications of Primary Results
What are the implications of our finding that flatter diurnal cortisol slopes were related to such a wide variety of negative health outcomes? The broad array of health outcomes with which flatter DCS were associated argues against very specific disease processes, and towards some form of more general, shared mechanism common to multiple disease states. The generality of the DCS-health associations, along with the fact that the majority of the studies in the meta-analysis were cross-sectional in nature, led us to consider four possible explanations for the observed associations between flatter DCS and negative health outcomes: i) direct causal explanation, ii)reverse-causal explanation, iii) shared-causation explanation, and iv) cascading effects explanation. Below we describe each of these possibilities and, based on our results, suggest the likelihood that each of these mechanisms may be at work. We also propose a new concept, stress-related circadian dysregulation or SCiD. We recognize these interpretations and extrapolations are somewhat speculative; our goal in presenting them is to stimulate further research designed to more fully understand the mechanisms underlying the development of DCS-health associations.
4.2.1. Direct Causal Explanation (HPA axis dysregulation as primary)
In this frequently proposed scenario, flattened diurnal cortisol rhythms precede and contribute to dysregulations in multiple downstream biological and behavioral systems, including inflammation, metabolism, energy, appetite, etc. These dysregulations in turn contribute to the development of specific disease outcomes. Supporting this explanation, considerable evidence indicates glucocorticoids regulate multiple other aspects of biology relevant to mental and physical health.
In understanding associations between DCS and mental health outcomes, it is important to note that receptors for glucocorticoids are present in almost every cell and organ in the body. Glucocorticoids also cross the blood-brain barrier to reach glucocorticoid and mineralocorticoid receptors (GRs and MRs, respectively) in the brain, including in limbic regions centrally involved in emotional and behavioral functioning (de Kloet et al., 2005; McEwen, 2007). Glucocorticoids impact neural systems associated with arousal, reward, fear/threat, and loss (McEwen, 2007), all key components of the Research Domain Criteria (RDoC) dimensional approach to studying psychopathology (Cuthbert and Insel, 2013). These findings provide a basis for the association between altered diurnal cortisol functioning and symptoms of multiple mental health disorders. Higher basal cortisol, particularly in the evening hours, has been implicated in depression (Dahl et al., 1991) whereas lower basal cortisol has been connected to externalizing disorders (Adam et al., 2007). Flatter diurnal cortisol rhythms contain elements of both hypo- and hyper-cortisolism (including low morning and/or high evening cortisol levels), which may explain why they were associated with both internalizing and externalizing disorders in the current analysis. The fact that the association between DCS and anxiety disorders showed a trend in the opposite direction from the other health outcomes (steeper DCS with greater anxiety) requires further investigation. Generally, anxiety has been differentiated from many of the other health disorders discussed here in that it has been associated with hyper-arousal, particularly in the form of elevated physiological arousal (Clark and Watson, 1991), rather than hypo-arousal. Elevated morning cortisol, resulting in a steeper DCS, could contribute to the heightened arousal of anxiety. Further research should investigate this possibility, and should also distinguish between subtypes of anxiety disorders, which are heterogeneous in symptomatology, and may therefore relate in differing ways to cortisol.
In understanding associations between DCS and physical health disorders, it is notable that cortisol plays a role in regulating appetite, metabolism, fat deposition, and visceral adiposity in particular (Epel et al., 2001; Rosmond, 2005), providing a possible mechanism for associations between flatter cortisol slope and the BMI, obesity, and adiposity group of outcomes examined here. Perhaps somewhat surprising in our results was a lack of significant overall association between diurnal cortisol slopes and cardiovascular outcomes. However, only four studies were available in the cardiovascular outcome category. Interestingly, diurnal cortisol slopes significantly prospectively predicted increased CVD-related mortality in one of the mortality studies, with a notable hazard ratio of 1.87. More studies of the relationship between DCS and CVD are needed.
The strongest effect size was found for associations between cortisol and immune and inflammatory outcomes. This finding was perhaps not surprising, given that glucocorticoids are key regulators and modifiers of immune and inflammatory system biology (McEwen, 1998; Webster et al., 2002; Silverman and Sternberg, 2012). Among its immunoregulatory effects, glucocorticoids impact natural killer cells which play a key role in tumor suppression and cancer disease progression and mortality (Sephton and Spiegel, 2003). This relationship provides one plausible mechanism for the meta-analytic association between DCS and cancer progression.
Findings of associations between flatter DCS and fatigue also are not surprising, given cortisol’s important role in energy regulation and prior studies showing waking with lower cortisol levels are associated with increased fatigue (Adam et al., 2006). The presence of lower morning cortisol levels in many instances of flatter DCS help explain why flatter DCS are associated with disorders previously characterized as due to hypocortisolemia (fatigue and immune and inflammatory disorders) (Chrousos and Gold, 1992).
Another potential mechanism by which flatter DCS may have negative effects on peripheral biological systems and related health outcomes, is through the impact of glucocorticoids on peripheral clock gene expression. Diurnal rhythmicity in cortisol is thought to play an important mediating role in synchronizing peripheral biological clocks with the central circadian clock mechanism of the suprachiasmic nucleus (SCN) (Nader et al., 2010; Cermakian et al., 2014; Bass and Lazar, 2016; Man et al., 2016). A loss of HPA-axis signaling is associated with disruptions of peripheral circadian biology (Scheiermann et al., 2013).
Overall, there are plausible biological pathways supporting the possibility that dysregulation of the DCS may play an etiological role in the development of various forms of disease by way of effects on other aspects of central and peripheral biology. Our meta-analytic finding that prospective studies (those using baseline DCS measures to predict future health outcomes) were significant, with similar effect sizes to cross-sectional studies, also lends some support for the direct causal explanation.
4.2.2. Reverse-causality: HPA dysregulation as secondary
In the reverse-causality explanation, the biological changes, symptoms, and psychological stress associated with the onset or persistence of a specific disorder may lead to an alteration (flattening) of the DCS. Given that the bulk of the research examined was cross-sectional in nature, it is possible that a flattened DCS is a symptom, or a consequence, of a prior disease state. Many diseases result in physical pain and psychological stress. Consequently, a flattened DCS could be caused by pathophysiological or stress-related changes resulting from the experience of the disease itself. In one example, the past experience of depression was shown to have an ongoing or “scar effect” (Doane et al., 2013) on DCS. In this study, individuals having experienced past depression showed a flatter DCS. In the same sample, having a flatter DCS did not predict the later onset of depressive symptoms (Adam et al., 2010). Together, these findings suggest a reverse-causal explanation deserves more attention in future research.
4.2.3. Shared Causality: Primary Role for Inflammation, Clock Gene Biology, or Sleep?
In a shared causality explanation, a third factor, such as alterations in immune/inflammatory biology or dysregulations of clock gene expression and/or sleep, may cause both a flattening of the DCS and the pathophysiological changes that lead to the development of multiple disorders.
4.2.3.1. Primary Role of Inflammation?
Given that we found the largest effect sizes for the associations between DCS and immune and inflammatory outcomes in the current study, we give particular attention to a possible primary role of inflammation. Pro-inflammatory cytokines are stress-sensitive (Steptoe et al., 2007) and they impact the activity of many other central and peripheral biological systems, including the HPA axis and clock gene activity (Cavadini et al., 2007; Cermakian et al., 2013; Cermakian et al., 2014; Verburg-van Kemenade et al., 2017). Pro-inflammatory cytokines such as IL-1β and TNF-α alter the activity of clock genes (Ohdo et al., 2001; Cavadini et al., 2007) and clock genes, both central and peripheral, contribute to regulation of the DCS. Inflammation also plays a key role in the development of cardiovascular disease (Juonala et al., 2006). Inflammation has been strongly implicated in fatigue and in behavioral withdrawal; elevated inflammation is therefore hypothesized to be one pathway in the development of major depressive disorder (Dantzer et al., 2008; Miller and Raison, 2016). Importantly, it is not just elevated inflammation, but loss of appropriate circadian variability in immune/inflammatory factors that may play a role in negative health outcomes (Arjona et al., 2012; Scheiermann et al., 2013; McEwen and Karatsoreos, 2015; Man et al., 2016).
4.2.3.2. Primary Role of Clock Gene Expression?
Another potential shared causal explanation is that a primary dysregulation of central circadian biology (originating from alterations in clock gene expression in the SCN) causes downstream effects on multiple systems including the HPA axis and inflammation, with consequences for a wide range of disease processes. Estimates suggest that up to 10% of the human genome is under circadian control (Scheiermann et al., 2013). The fact that the measurement approach emerging from circadian biology studies (amplitude measures obtained from cosinor analyses) showed the strongest effect sizes suggest that greater attention to the literature and methods of circadian biology or chronobiology (Otsuka et al., 2016) is warranted in understanding DCS-health associations.
4.2.3.3 Primary Role of Sleep?
Shortened sleep, and/or social jetlag has been found to affect both the DCS as well as metabolic, inflammatory and behavioral outcomes (Spiegel et al., 1999; Sadeh et al., 2003; Doane et al., 2010; Niu et al., 2011; Zeiders et al., 2011; Rutters et al., 2014; Turek, 2016). Notably, a number of the studies reviewed in the current meta-analysis controlled for sleep hours or sleep-wake timing, and still found DCS-health associations (e.g., Doane et al., 2011; Jarcho et al., 2013; Sephton et al., 2013). However, additional sleep characteristics (e.g., night awakenings, sleep latency, sleep efficiency, and sleep architecture) deserve attention as potential contributors to dysregulations in diurnal cortisol rhythms and other health-relevant biological systems.
4.2.4 Cascading Effects Explanation
In what we are calling the cascading effects explanation, transactional and cascading changes across multiple stress-sensitive biological systems mutually reinforce each other. In this explanation, there could be multiple initial sources of dysregulation. Regardless of the initial source or system of dysregulation, interacting and cascading changes ultimately lead to multi-systemic biological dysregulation, of which a flatter DCS is both an indicator and a precipitating and reinforcing factor. Given the transactional nature of the regulation of interrelated biological systems, and our findings of associations between flatter DCS and multiple health outcomes, it seems plausible that reciprocal and cascading interactions among clock gene mechanisms, sleep, cortisol, inflammation, fatigue, appetite, behavior, and social and psychological experiences jointly contribute to the observed associations between flatter DCS and multiple types of negative health outcomes.
4.2.5 Stress-related Circadian Dysregulation (SCiD)
Importantly, given evidence that dysregulations in circadian processes may be initiated and maintained by psychosocial stress (Van Reeth et al., 2000; Hall et al., 2004; Sadeh et al., 2004), we propose that flatter DCS and alterations in other aspects of circadian biology are signs of what we are terming “stress-related circadian dysregulation (SCiD).” We suggest that SCiD, examined across multiple biological systems, should be a key focus of future research on stress and health. Future research is need both tracing the psychosocial origins of circadian dysregulations, and the pathways by which subtle initial stress-induced circadian dysregulations over time cascade into major mental and physical health disorders. Multiple co-regulatory systems are likely involved in the development of SCiD suggesting that interventions at any one of a number of levels (e.g. psychological, behavioral, or biological) could help to correct SCiD. For example, altered health behaviors, reduced stress, improved mood, better control of inflammation, and better sleep could all play a role in improved circadian regulation. Interventions simultaneously directed at multiple of these levels may be most effective. Importantly, the SCiD theory implies that in any intervention, it is the righting of rhythms that is important, more so than the righting of levels. Any therapies, including pharmacologic ones, should have the restoration of expected circadian rhythms as a key goal.
4.3 Moderator Effects
In addition to examining the effect sizes of DCS on various health outcomes and considering the role of DCS in the development of health disorders, another key goal of this study was to examine potential methodological moderators of DCS-health effect sizes. Although results were relatively robust to moderation by study characteristics, the moderation analyses provide important considerations for the design and analysis of future DCS research.
4.3.1 Age
Despite significant variability in effect sizes by age category, all of the age categories except one were significant. Moreover, all age category effect sizes were in the expected direction of a flatter DCS being associated with worse health outcomes. The one age category that was not significant was infants/toddlers, which included only two studies. Yet, even the infants/toddlers age category showed a trend in the expected direction. Effect sizes were larger for adults than for children of all ages, but were not larger for older adults, such that our hypothesis of increasing effect sizes among older age groups was not consistently supported.
4.3.2 Slope Type
In examining type of cortisol slope measure, the five studies using formal circadian measures to capture degree of circadian variation (amplitude measures derived from cosinor analysis) showed the strongest average effect size. The circadian nature of the phenomenon being studied suggests that greater use of this statistical approach is warranted for those salivary studies that have relatively intensive repeated measurement of cortisol levels across the day or multiple days. Wake-to-bedtime cortisol slopes and fixed time point slopes also showed reasonable effect sizes. Peak-to-bedtime slopes showed slightly smaller (but still significant effect sizes). Short daytime slopes were the only type of slope measure that did not reveal significant associations with health outcomes. The weaker effect sizes for peak-to-bedtime slopes and short daytime slopes could be due to the fact that these slope measurements are influenced by levels of the CAR, which is a separate index of cortisol regulation (Clow et al., 2010).
4.3.3 Numbers of Samples Per Day
Interestingly, studies using only two samples or greater than seven samples per day did not, on average, reveal significant associations with health outcomes. Studies using between three and six samples revealed significant and comparable effect sizes to each other. These results suggest 3 to 6 samples may be the optimal number of samples to gather per day. This conclusion is reinforced by a recent study showing that using 5 or 6 samples per day to estimate the DCS closely approximates slopes derived with a more intensive protocol (Hoyt et al., 2016).
4.3.4 Number of Days
It is particularly noteworthy that more days of data did not appear to increase the size of association between diurnal cortisol slopes and health outcomes (Adam et al., 2006). This is interesting because the DCS is subject to considerable state-related variation. Indeed, it has been noted that DCS measures are not highly stable across days, months or years, making it more surprising that single (or even multiple) day DCS measures were associated with negative health outcomes (Ross et al., 2014). The fact that associations were found with health outcomes, despite the established high level of state-related DCS variation, suggests that the “signal” associated with dysregulated slopes must be strong. Repeated measurement of DCSs over time could be combined with modeling approaches attempting to isolate and examine the predictive power of state vs. trait components of DCS variance.
4.3.5 Study Quality and Objective Compliance
Our index of study quality, which included factors such as inclusion of health behavior covariates and use of objective compliance indicators, did not significantly predict the size of association between DCS and health outcomes. However, when separated out as its own indicator, studies using objective compliance monitors revealed significantly and notably stronger average effect sizes for associations between DCS and health outcomes than those not employing such monitors. This meta-analytic finding was particularly noteworthy because very few studies (only 16.3% of studies and 9.5% of the findings analyzed) used objective/electronic compliance monitors, despite such methods being strongly recommended by experts in the field (Kudielka et al., 2003; Stalder et al., 2016). Our meta-analytic findings further support the importance of these recommendations.
4.3.6 Cross-sectional vs. Prospective
Of relevance to the interpretation of our findings, prospective studies showed comparable effect sizes to cross-sectional studies, and strong effects were found for the few prospective studies of mortality using hazard ratios as the effect size index. The presence of significant results for prospective studies lends support to the possibility that disruptions of the DCS may play an etiological role in the development or progression of at least some of the health outcomes assessed here. This finding is most in line with either the direct effects or cascading effects explanations outlined above.
4.4 Suggestions for Future Research
Based on our review of the literature as well as results of moderator analyses, we have several suggestions for the design and reporting of future DCS research. First, because this is the first meta-analysis of the associations between diurnal cortisol slopes and health outcomes, we took a “forest” approach of examining associations across multiple health outcomes rather than choosing only one health outcome to examine in detail. Building from the current analysis, future meta-analyses can focus on individual health outcomes/domains, updating findings for particular health outcomes as the latest studies in that domain become available, and exploring specific mechanisms for particular health domains in more detail. Second, additional meta-analyses are also needed to identify what types of stress-related or psychosocial variables are most predictive of a flatter DCS, and over what time frames. Third, researchers need to be cognizant of which type of DCS they are measuring, from the design phase through the analysis and reporting phases of their research. This includes researchers being aware of, and justifying their choices regarding whether CAR variance is included or excluded from the DCS measure. Fourth, it may be helpful to disaggregate contributions of low morning cortisol and high evening cortisol levels to a flatter DCS for understanding mechanisms associated with different disease outcomes. Are associations with particular health outcomes due to lower morning levels, higher evening levels, or a combination of both? Fifth, studies should use more than two cortisol samples per day to estimate slopes. This meta-analysis research suggests 3 to 6 samples per day are sufficient for demonstrating links between the DCS and health outcomes. Other recent work suggests including 5–6 samples per day is a good balance between accuracy of DCS measurement and participant demand (Hoyt et al., 2016). When chronometric approaches are employed, more intensive sampling will be required. Sixth, this study highlights the need for improved reporting of details of study results. Examples of improved reporting includes provision of raw correlations between constructs of interest, standardized coefficients in the case of regression and HLM models, and means accompanied by SD or SE measures in the case of group comparisons. Seventh, there is a dearth of studies using prospective approaches (less than 10% of the findings reviewed), limiting interpretation of the causal direction of effects. Future research should employ longitudinal designs, with repeated measures of both DCS and health outcomes to assess causal and potentially cascading effects. Eighth, future studies should measure potential downstream biological pathways by which a flatter DCS relates to health outcomes – including not just fixed but diurnal/circadian measures of factors such as arousal, activity, cognition, health behaviors, sleep, metabolism and inflammation. Finally, it will be important to determine whether naturalistic or experimental reductions in stress, or chronobiologically-informed behavioral (e.g., sleep, light therapy) and pharmacological interventions, can cause reversal of DCS flattening, with resulting improvement in health outcomes.
4.5 Limitations
There are a number of limitations to the current study. First, we searched for combined terms, such as ‘diurnal cortisol’ rather than employing a more flexible approach of using separate word combinations such as ‘cortisol’ and ‘diurnal’, which may have resulted in some missed studies. In addition, other potential search terms such as ‘decline’, ‘variability’ and ‘drop’ became apparent later in our analysis; again, omission of these search terms may have resulted in some missed studies.
Second, we had to rely on back-calculating some effect sizes from p-values and sample size information, in some cases using non-exact p-values (e.g., p < .05). However, we decided utilizing these estimated effect sizes was superior to excluding a large number of studies. Upon exclusion of studies for which we used p-values and sample sizes to derive effect sizes, a similar (to slightly larger) overall effect size was found. We caution that average effect sizes should not be regarded as exact. Rather, they should be seen as indicators of general patterns of results and of the relative strengths of associations across various health outcomes.
Third, we utilized covariate-adjusted models in some cases (where relevant covariate-adjusted effect sizes were available), but not in others. We believe covariate adjusted models provided the best test of the unique association between cortisol and the health outcome, net of confounders. Utilizing covariates or not did not make a difference in effect sizes, so we are less concerned about lack of comparability across studies on this variable. However, it should be noted that we did not examine inclusion of demographic factors such as socioeconomic status or race/ethnicity as potential effect size moderators; whether or not these variables matter for associations between DCS and health should be addressed in future research.
4.6 Conclusions
This systematic review and meta-analysis provides evidence that reduced diurnal variation in cortisol (i.e., loss of circadian amplitude or a flattening of the DCS) is related to a wide range of negative health outcomes. Flatter diurnal cortisol slopes were associated with worse health for overall health, and for 10 of 12 subtypes of mental and physical health outcomes examined. Cardiovascular disease and anxiety outcomes were not significantly associated with a flatter DCS in meta-analytic analyses, whereas immune and inflammatory system dysregulation, fatigue, cancer, obesity/BMI/adiposity, externalizing symptoms, internalizing symptoms, depression symptoms and disorders, other mental health, other physical health, and mortality outcomes were all associated with a flatter DCS. Whether changes in the DCS are a mechanism by which disease states emerge, an effect of emerging disease symptoms, or a marker of other forms of circadian dysregulation remain to be determined in future research, and may vary for differing disease states. Regardless, our meta-analysis makes it clear that diurnal cortisol slopes are meaningfully related to health, and that a flatter DCS is a strong candidate mechanism for explaining associations between psychosocial stress and a wide range of mental and physical health outcomes.
Highlights.
Meta-analysis shows flatter diurnal cortisol slopes are associated with worse health
Results are significant for 10 out of 12 health outcomes and across multiple age groups
Strongest effects are found for Inflammation and immune system outcomes
Effects are found across most types of diurnal cortisol slope measurement
Use of objective compliance monitors is associated with larger effect sizes
Acknowledgments
The authors’ time on this project was supported, in part, by Faculty and Graduate Fellowships from the Institute for Policy Research at Northwestern University. The funder played no role in the conduct of this research, the analyses, interpretations or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to report.
References
*Studies included in the meta-analysis are indicated with a star symbol.
- *.Abercrombie HC, Giese-Davis J, Sephton S, Epel E, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- *.Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK. Emotion-cortisol transactions occur over multiple time scales in development: Implications for research on emotion and the development of emotional disorders. In: Dennis TA, Buss KA, Hastings PD, editors. Physiological Measures of Emotion from a Developmental Perspective: State of the Science. Monographs of the Society for Research in Child Development. 2. Vol. 77. 2012. pp. 17–27. [Google Scholar]
- *.Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, Levy DJ, Kemeny M, Brodish AB, Malanchuk O. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology. 2015;62:279–291. doi: 10.1016/j.psyneuen.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Klimes-Dougan B, Gunnar MR. Social regulation of the adrenocortical response to stress in infants, children and adolescents: Implications for psychopathology and education. In: Coch D, Dawson G, Fischer K, editors. Human Behavior, Learning, and the Developing Brain: Atypical Development. Guilford Press; 2007. pp. 264–304. [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic–pituitary–adrenal axis measures into preventive interventions for adolescent depression: Are we there yet? Development and Psychopathology. 2008;20:975–1001. doi: 10.1017/S0954579408000461. [DOI] [PubMed] [Google Scholar]
- Arjona A, Silver AC, Walker WE, Fikrig E. Immunity's fourth dimension: approaching the circadian–immune connection. Trends in immunology. 2012;33:607–612. doi: 10.1016/j.it.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatr. 2012;17:988–995. doi: 10.1038/mp.2011.149. [DOI] [PubMed] [Google Scholar]
- *.Bao A-M, Ji Y-F, Van Someren EJW, Hofman MA, Liu R-Y, Zhou J-N. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Hormones and behavior. 2004;45:93–102. doi: 10.1016/j.yhbeh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354:994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- *.Bhattacharyya MR, Molloy GJ, Steptoe A. Depression is associated with flatter cortisol rhythms in patients with coronary artery disease. Journal of psychosomatic research. 2008;65:107–113. doi: 10.1016/j.jpsychores.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive metaanalysis version 3. Englewood, NJ: Biostat; 2014. [Google Scholar]
- Borenstein M, Hedges LV, Higgins J, Rothstein HR. Subgroup analyses. Introduction to Meta-analysis. 2009:149–186. [Google Scholar]
- *.Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Depressive symptoms predict mucosal wound healing. Psychosom. Med. 2007;69:597–605. doi: 10.1097/PSY.0b013e318148c682. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N. Altered Cortisol Response to Psychologic Stress in Breast Cancer Survivors With Persistent Fatigue. Psychosom Med. 2005a;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- *.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005b;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- *.Buss J, Havel PJ, Epel E, Line J, Blackburn E, Daubenmier J. Associations of ghrelin with eating behaviors, stress, metabolic factors, and telomere length among overweight and obese women: Preliminary evidence of attenuated ghrelin effects in obesity? Appetite. 2014;76:84–94. doi: 10.1016/j.appet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Carnegie R, Araya R, Ben-Shlomo Y, Glover V, O'Connor TG, O'Donnell KJ, Pearson R, Lewis G. Cortisol awakening response and subsequent depression: prospective longitudinal study. Brit J Psychiat. 2014;204:137–143. doi: 10.1192/bjp.bp.113.126250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proceedings of the National Academy of Sciences. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, Mazzoccoli G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int. 2013;30:870–888. doi: 10.3109/07420528.2013.782315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N, Westfall S, Kiessling S. Circadian clocks and inflammation: reciprocal regulation and shared mediators. Archivum immunologiae et therapiae experimentalis. 2014;62:303–318. doi: 10.1007/s00005-014-0286-x. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Journal of American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- *.Cicchetti D, Rogosch FA, Howe ML, Toth SL. The effects of maltreatment and neuroendocrine regulation on memory performance. Child Development. 2010;81:1504–1519. doi: 10.1111/j.1467-8624.2010.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cima M, Smeets T, Jelicic M. Self-reported trauma, cortisol levels, and aggression in psychopathic and non-psychopathic prison inmates. Biological psychology. 2008;78:75–86. doi: 10.1016/j.biopsycho.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–36. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress: The International Journal on the Biology of Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- *.Cohen L, Cole SW, Sood AK, Prinsloo S, Kirschbaum C, Arevalo JMG, Jennings NB, Scott S, Vence L, Wei Q, Kentor D, Radvanyi L, Tannir N, Jonasch E, Tamboli P, Pisters L. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11:1–8. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Ryan N, Puig-Antich J, Nguyen N, Al-Shabbout M, Meyer V, Perel J. 24-Hour cortisol measures in adolescents with major depression: A controlled study. Biological Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- *.Daniel M, Moore DS, Decker S, Belton L, DeVellis B, Doolen A, Campbell MK. Associations among education, cortisol rhythm, and BMI in blue-collar women. Obesity (Silver Spring) 2006;14:327–335. doi: 10.1038/oby.2006.42. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Neuroscience Reviews. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- *.Dekker MJHJ, Koper JW, van Aken MO, Pols HAP, Hofman A, de Jong FH, Kirschbaum C, Witteman JCM, Lamberts SWJ, Tiemeier H. Salivary cortisol is related to atherosclerosis of carotid arteries. The Journal of Clinical Endocrinology and Metabolism. 2008;93:3741–3747. doi: 10.1210/jc.2008-0496. [DOI] [PubMed] [Google Scholar]
- *.den Hartog HM, Nicolson NA, Derix MMA, van Bemmel AL, Kremer B, Jolles J. Salivary cortisol patterns and cognitive speed in major depression: a comparison with allergic rhinitis and healthy control subjects. Biological Psychology. 2003;63:1–14. doi: 10.1016/s0301-0511(03)00050-4. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- *.Dienes KAH, N A, Hammen CL. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology. 2013;38:927–940. doi: 10.1016/j.psyneuen.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dmitrieva NOA, D M, Dmitrieva J, Loken E, Pieper CF. A day-centered approach to modeling cortisol: Diurnal cortisol profiles and their associations among U.S. adults. Psychoneuroendocrinology. 2013;38:2354–2365. doi: 10.1016/j.psyneuen.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35:430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Doane LD, Franz CE, Prom-Wormley E, Eaves LJ, Mendoza SP, Hellhammer DH, Lupien S, Xian H, Lyons MJ, Kremen W, Jacobson KC. Negative emotionality, depressive symptoms and cortisol diurnal rhythms: Analysis of a community sample of middle-aged males. Hormones and behavior. 2011;60:202–209. doi: 10.1016/j.yhbeh.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Kremen WS, Eaves LJ, Eisen SA, Hauger R, Hellhammer D, Levine S, Lupien S, Lyons M, Mendoza S. Associations between jet lag and cortisol diurnal rhythms after domestic travel. Health Psychology. 2010;29:117–123. doi: 10.1037/a0017865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and psychopathology. 2013;25:629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- *.Du YJ, Zhang HY, Li B, Wu X, Lv YB, Jin HL, Cao YX, Sun J, Luo QL, Gong WY, Liu BJ, Wu JF, Shi SX, Dong JC. Sputum interleukin-6, tumor necrosis factor-alpha and salivary cortisol as new biomarkers of depression in lung cancer patients. Prog Neuro-Psychoph. 2013;47:69–76. doi: 10.1016/j.pnpbp.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association. 2000a;95:89–98. [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000b;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- *.Eckart C, Engler H, Riether C, Kolassa S, Elbert T, Kolassa I-T. No PTSD-related differences in diurnal cortisol profiles of genocide survivors. Psychoneuroendocrinology. 2009;34:523–531. doi: 10.1016/j.psyneuen.2008.10.012. [DOI] [PubMed] [Google Scholar]
- *.Edwards S, Hucklebridge F, Clow A, Evans P. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosomatic medicine. 2003;65:320–327. doi: 10.1097/01.psy.0000033123.70631.8e. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- *.Ferguson E. Health anxiety moderates the daytime cortisol slope. Journal of Psychosomatic Research. 2008;64:487–494. doi: 10.1016/j.jpsychores.2008.01.011. [DOI] [PubMed] [Google Scholar]
- *.Fidan V, Alp HH, Gozeler M, Karaaslan O, Binay O, Cingi C. Variance of melatonin and cortisol rhythm in patients with allergic rhinitis. Am J Otolaryng. 2013a;34:416–419. doi: 10.1016/j.amjoto.2013.03.004. [DOI] [PubMed] [Google Scholar]
- *.Fidan V, Alp HH, Kalkandelen S, Cingi C. Melatonin and cortisol rhythm in patients with extensive nasal polyposis. Am J Otolaryng. 2013b;34:61–64. doi: 10.1016/j.amjoto.2012.09.001. [DOI] [PubMed] [Google Scholar]
- *.Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: relation to cognitive functioning. Stress. 2006;9:143–152. doi: 10.1080/10253890600965674. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- *.Gallagher-Thompson D, Shurgot GR, Rider K, Gray HL, McKibbin CL, Kraemer HC, Sephton SE, Thompson LW. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: A preliminary study of family dementia caregivers and noncaregivers. Am J Geriatr Psychiatry. 2006;14:334–342. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- *.Goel N, Stunkard AJ, Rogers NL, Van Dongen HPA, Allison KC, O'Reardon JP, Ahima RS, Cummings DE, Heo M, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gold SM, Kern KC, Montag M, O'Connor MF, Giesser BS, Sicotte N. Flatter diurnal cortisol slope is associated with regional hippocampal atrophy and depressive symptoms in multiple sclerosis. Mult Scler. 2009;15:1390–1390. [Google Scholar]
- Granger DA, Weisz JR, McCracken JT. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Dev. 1996;67:3259–3262. [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Long-term effects of institutional rearing on cortisol levels in adopted Romanian children. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009a;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. [Special Issue] Stress & Development: Behavioral and Biological Consequences. Development and Psychopathology. 2001;13:516–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in HPA activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009b;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II Study. Journal of Clinical Endocrinology & Metabolism. 2014;99:4625–4631. doi: 10.1210/jc.2014-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hagger-Johnson GE, Whiteman MC, Wawrzyniak AJ, Holroyd WG. The SF-36 component summary scales and the daytime diurnal cortisol profile. Qual Life Res. 2010;19:643–651. doi: 10.1007/s11136-010-9626-4. [DOI] [PubMed] [Google Scholar]
- *.Hajat A, Diez-Roux AV, Sanchez BN, Holvoet P, Lima JA, Merkin SS, Polak JF, Seeman TE, Wu MH. Examining the association between salivary cortisol levels and subclinical measures of atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2013;38:1036–1046. doi: 10.1016/j.psyneuen.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, Kupfer D, Thayer JF. Acute stress affects heart rate variability during sleep. Psychosomatic medicine. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- *.Ho RTH, Fong TCT, Chan CKP, Chan CLW. The associations between diurnal cortisol patterns, self-perceived social support, and sleep behavior in Chinese breast cancer patients. Psychoneuroendocrinology. 2013;38:2337–2342. doi: 10.1016/j.psyneuen.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Hoyt LT, B EK, Cham H, Adam EK. Balancing scientific accuracy and participant burden: Testing the impact of sampling intensity on diurnal cortisol indices. Stress. 2016;19:476–485. doi: 10.1080/10253890.2016.1206884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hsiao F-H, Yang T-T, Ho RTH, Jow G-M, Ng S-M, Chan CLW, Lai Y-M, Chen Y-T, Wang K-C. The self-perceived symptom distress and health-related conditions associated with morning to evening diurnal cortisol patterns in outpatients with major depressive disorder. Psychoneuroendocrinology. 2010;35:503–515. doi: 10.1016/j.psyneuen.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Schmidt FL, Jackson GB. Meta-analysis : Cumulating research findings across studies. Sage; Beverly Hills [Calif.]: 1982. [Google Scholar]
- *.Jabben N, Nolen WA, Smit JH, Vreeburg SA, Beekman ATF, Penninx BWJH. Co-occurring manic symptomatology influences HPA axis alterations in depression. J Psychiatr Res. 2011;45:1208–1213. doi: 10.1016/j.jpsychires.2011.03.010. [DOI] [PubMed] [Google Scholar]
- *.Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biological psychology. 2013;93:150–158. doi: 10.1016/j.biopsycho.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johar HE, R T, Bidlingmaier M, Reincke M, Thorand B, Peters A, Heier M, Ladwig KH. Blunted Diurnal Cortisol Pattern Is Associated With Frailty: A Cross-Sectional Study of 745 Participants Aged 65 to 90 Years. Journal of Clinical Endocrinology & Metabolism. 2014;99:E464–E468. doi: 10.1210/jc.2013-3079. [DOI] [PubMed] [Google Scholar]
- Juonala M, Viikari JSA, Ronnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-Reactive Protein in Predicting CRP and Carotid Intima-Media Thickness in Adulthood: The Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006;26:1883–1888. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- *.Kiefte-de Jong JC, Saridjan NS, Escher JC, Jaddoe VWV, Hofman A, Tiemeier H, Moll HA. Cortisol diurnal rhythm and stress reactivity in constipation and abdominal pain: the Generation R Study. J Pediatr Gastroenterol Nutr. 2011;53:394–400. doi: 10.1097/MPG.0b013e31821e73cf. [DOI] [PubMed] [Google Scholar]
- *.Kim KS, Kim YC, Oh IJ, Kim SS, Choi JY, Ahn RS. Association of worse prognosis with an aberrant diurnal cortisol rhythm in patients with advanced lung cancer. Chronobiol Int. 2012;29:1109–1120. doi: 10.3109/07420528.2012.706767. [DOI] [PubMed] [Google Scholar]
- *.Knight JM, Avery EF, Janssen I, Powell LH. Cortisol and depressive symptoms in a population-based cohort of midlife women. Psychosomatic medicine. 2010;72:855–861. doi: 10.1097/PSY.0b013e3181f4ab87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- *.Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, Kirschbaum C, Kivimaki M. Cortisol secretion and fatigue: associations in a community based cohort. Psychoneuroendocrinology. 2009;34:1476–1485. doi: 10.1016/j.psyneuen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- *.Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. The Journal of clinical endocrinology and metabolism. 2010;95:4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. Journal of Clinical Endocrinology & Metabolism. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kurina LM, Schneider B, Waite LJ. Stress, symptoms of depression and anxiety, and cortisol patterns in working parents. Stress and Health. 2004;20:53–63. [Google Scholar]
- *.Lamers FV, N, Merikangas KR, de Jonge P, Beekman ATF, Penninx BWJH. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatr. 2013;18:692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- *.Lederbogen F, Hummel J, Fademrecht C, Krumm B, Kuhner C, Deuschle M, Ladwig KH, Meisinger C, Wichmann HE, Lutz H, Breivogel B. Flattened circadian cortisol rhythm in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119:573–575. doi: 10.1055/s-0031-1275288. [DOI] [PubMed] [Google Scholar]
- *.Ludescher B, Najib A, Baar S, Machann J, Thamer C, Schick F, Buchkremer G, Claussen CD, Eschweiler GW. Gender specific correlations of adrenal gland size and body fat distribution: a whole body MRI study. Horm Metab Res. 2007;39:515–518. doi: 10.1055/s-2007-982518. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Man K, Loudon A, Chawla A. Immunity around the clock. Science. 2016;354:999–1003. doi: 10.1126/science.aah4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosomatic Medicine. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Karatsoreos IN. Sleep deprivation and circadian disruption: stress, allostasis, and allostatic load. Sleep medicine clinics. 2015;10:1–10. doi: 10.1016/j.jsmc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Interactions of the Circadian CLOCK System and the HPA Axis. Trends in endocrinology and metabolism: TEM. 2010;21:277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Nater UM, Youngblood LS, Jones JF, Unger ER, Miller AH, Reeves WC, Heim C. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosomatic Medicine. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- Nicolson N. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Niu S-F, Chung M-H, Chen C-H, Hegney D, O'Brien A, Chou K-R. The effect of shift rotation on employee cortisol profile, sleep quality, fatigue, and attention level: a systematic review. Journal of Nursing Research. 2011;19:68–81. doi: 10.1097/JNR.0b013e31820c1879. [DOI] [PubMed] [Google Scholar]
- *.O'Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: Short-term longitudinal study. Biological Psychology. 2014;96:35–41. doi: 10.1016/j.biopsycho.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nature Medicine. 2001;7:356–360. doi: 10.1038/85507. [DOI] [PubMed] [Google Scholar]
- Otsuka K, Cornelissen G, Halberg F. From Chronobiology to Chronomedicine: Early Days, Chronomics and Continuous Ambulatory Blood Pressure Monitoring: Vascular Chronomics: From 7-Day/24-Hour to Lifelong Monitoring. Springer; Japan, Tokyo: 2016. pp. 1–51. [Google Scholar]
- *.Patterson SM, P, Epel E, Sinclair E, Kemeny ME, Deeks SG, Bacchetti P, Acree M, Epling L, Kirschbaum C, Hecht FM. Cortisol patterns are associated with T cell activation in HIV. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. Journal of Applied Psychology. 2005;90:175–181. doi: 10.1037/0021-9010.90.1.175. [DOI] [PubMed] [Google Scholar]
- *.Pössel P, Mitchell AM, Sjögren E, Kristenson M. Do depressive symptoms mediate the relationship between hopelessness and diurnal cortisol rhythm? International Journal of Behavioral Medicine. 2015;22:251–257. doi: 10.1007/s12529-014-9422-6. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- *.Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30:615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- *.Rhebergen DK, N CM, Penninx BWJH, Stek ML, van der Mast RC, Oude Voshaar R, Comijs HC. Hypothalamic–pituitary–adrenal axis activity in older persons with and without a depressive disorder. Psychoneuroendocrinology. 2015;51:341–350. doi: 10.1016/j.psyneuen.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rubin DB. r equivalent: A simple effect size indicator. Psychological Methods. 2003;8:492–496. doi: 10.1037/1082-989X.8.4.492. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Adam TC, Bremmer MA, Elders PJ, Nijpels G, Dekker JM. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29:377–383. doi: 10.1177/0748730414550199. [DOI] [PubMed] [Google Scholar]
- *.Ruttle PL, Serbin LA, Martin-Storey A, Stack DM, Schwartzman AE. Longitudinal associations between infections and atopic disorders across childhood and dysregulated adrenocortical functioning in early adolescence. Developmental psychobiology. 2014;56:897–907. doi: 10.1002/dev.21163. [DOI] [PubMed] [Google Scholar]
- *.Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DB-D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Hormones and behavior. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ruttle PLJ, K N, Klein MH, Armstrong JM, Burk LR, Essex MJ. Concurrent and Longitudinal Associations Between Diurnal Cortisol and Body Mass Index Across Adolescence. Journal of Adolescent Health. 2013;52:731–737. doi: 10.1016/j.jadohealth.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The Effects of Sleep Restriction and Extension on School-Age Children: What a Difference an Hour Makes. Child Development. 2003;74:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Keinan G, Daon K. Effects of stress on sleep: the moderating role of coping style. Health Psychology. 2004;23:542. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- *.Sannes TS, Jensen SE, Dodd SM, Kneipp SM, Garey Smith S, Patidar SM, Marsiske MM, Lutgendorf SM, Morgan LS, Pereira DB. Depressive symptoms and cortisol variability prior to surgery for suspected endometrial cancer. Psychoneuroendocrinology. 2013;38:241–249. doi: 10.1016/j.psyneuen.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- *.Saridjan NS, Velders FP, Jaddoe VWV, Hofman A, Verhulst FC, Tiemeier H. The longitudinal association of the diurnal cortisol rhythm with internalizing and externalizing problems in pre-schoolers. The Generation R Study. Psychoneuroendocrinology. 2014;50:118–129. doi: 10.1016/j.psyneuen.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nature Reviews Immunology. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schrepf A, O'Donnell M, Luo Y, Bradley CS, Kreder K, Lutgendorf S, Multi disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. Pain. 2014;155:1755–1761. doi: 10.1016/j.pain.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Segebladh B, Bannbers E, Moby L, Nyberg S, Bixo M, Backstrom T, Sundstrom Poromaa I. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16:131–137. doi: 10.1007/s00737-013-0327-1. [DOI] [PubMed] [Google Scholar]
- *.Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, Spiegel D. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain, behavior, and immunity. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- *.Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behavior and Immunity. 2013;30:S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- *.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain, behavior, and immunity. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- *.Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. The Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Dockray S, Smyth N, Evans P, Hellhammer DH. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sugaya N, Izawa S, Ogawa N, Shirotsuki K, Kobayashi H, Yamada KC, Tsumura H, Nomura S, Shimada H. Effect of day-to-day variations in adrenal cortex hormone levels on abdominal symptoms. Biopsychosoc Med. 2010;4:2. doi: 10.1186/1751-0759-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tell D, Mathews HL, Janusek LW. Day-to-Day Dynamics of Associations Between Sleep, Napping, Fatigue, and the Cortisol Diurnal Rhythm in Women Diagnosed as Having Breast Cancer. Psychosomatic Medicine. 2014;76:519–528. doi: 10.1097/PSY.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tomiyama AJ, O'Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Dhabhar FS, Wolkowitz O, Kirschbaum C, Blackburn E, Epel E. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiology & Behavior. 2012;106:40–45. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tordjman S, Anderson GM, Kermarrec S, Bonnot O, Geoffray MM, Brailly-Tabard S, Chaouch A, Colliot I, Trabado S, Bronsard G, Coulon N, Botbol M, Charbuy H, Camus F, Touitou Y. Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology. 2014;50:227–245. doi: 10.1016/j.psyneuen.2014.08.010. [DOI] [PubMed] [Google Scholar]
- *.Tu MT, Zunzunegui MV, Guerra R, Alvarado B, Guralnik JM. Cortisol profile and depressive symptoms in older adults residing in Brazil and in Canada. Aging Clin Exp Res. 2013;25:527–537. doi: 10.1007/s40520-013-0111-0. [DOI] [PubMed] [Google Scholar]
- Turek FW. Circadian clocks: Not your grandfather’s clock. Science. 2016;354:992–993. doi: 10.1126/science.aal2613. [DOI] [PubMed] [Google Scholar]
- *.Turner-Cobb JM, Rixon L, Jessop DS. Hypothalamic–pituitary–adrenal axis activity and upper respiratory tract infection in young children transitioning to primary school. Psychopharmacology. 2011;214:309–317. doi: 10.1007/s00213-010-1965-x. [DOI] [PubMed] [Google Scholar]
- *.Vammen MA, Mikkelsen S, Hansen AM, Grynderup MB, Andersen JH, Bonde JP, Buttenschon HN, Kolstad HA, Kaergaard A, Kaerlev L, Mors O, Rugulies R, Thomsen JF. Salivary cortisol and depression in public sector employees: Cross-sectional and short term follow-up findings. Psychoneuroendocrinology. 2014;41:63–74. doi: 10.1016/j.psyneuen.2013.12.006. [DOI] [PubMed] [Google Scholar]
- *.Van den Bergh BRH, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children's Depression Inventory. Psychoneuroendocrinology. 2009;34:791–794. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- *.Van den Bergh BRH, Van Calster B, Pinna Puissant S, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Hormones and Behavior. 2008;54:253–257. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Weibel L, Spiegel K, Leproult R, Dugovic C, Maccari S. Physiology of sleep (review)–interactions between stress and sleep: from basic research to clinical situations. Sleep Medicine Reviews. 2000;4:201–219. [Google Scholar]
- *.Vedhara K, Tuinstra J, Stra JT, Miles JNV, Sanderman R, Ranchor AV. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology. 2006;31:299–311. doi: 10.1016/j.psyneuen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- *.Veen G, van Vliet IM, DeRijk RH, Giltay EJ, van Pelt J, Zitman FG. Basal cortisol levels in relation to dimensions and DSM-IV categories of depression and anxiety. Psychiatry Research. 2011;185:121–128. doi: 10.1016/j.psychres.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Verburg-van Kemenade BML, Cohen N, Chadzinska M. Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Developmental & Comparative Immunology. 2017;66:2–23. doi: 10.1016/j.dci.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Webster JK, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annual Review of Immunology. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- *.Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, Zimmerman B, Kirschbaum C, Sood AK, Lubaroff DM, Lutgendorf SK. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010;116:4410–4419. doi: 10.1002/cncr.25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and [alpha]-amylase in children with asthma and healthy children. Biological Psychology. 2008;78:20–28. doi: 10.1016/j.biopsycho.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Jr, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. The Journal of nervous and mental disease. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Adam EK. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescents. Journal of Adolescent Health. 2011;48:566–571. doi: 10.1016/j.jadohealth.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]