Abstract
Non-homologous end joining (NHEJ) is the major pathway for the repair of ionizing radiation induced DNA double strand breaks (DSBs) in human cells. Critical to NHEJ is the DNA-dependent interaction of the Ku70/80 heterodimer with the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to form the DNA-PK holoenzyme. However, precisely how Ku recruits DNA-PKcs to DSBs ends to enhance its kinase activity has remained enigmatic, with contradictory findings reported in the literature. Here we address the role of the Ku80 C-terminal region (CTR) in the DNA-dependent interaction of Ku70/80 with DNA-PKcs using purified components and defined DNA structures. Our results show that the Ku80 CTR is required for interaction with DNA-PKcs on short segments of blunt ended 25 bp dsDNA or 25 bp dsDNA with a 15-base poly dA single stranded (ss) DNA extension, but this requirement is less stringent on longer dsDNA molecules (35 bp blunt ended dsDNA) or 25 bp duplex DNA with either a 15-base poly dT or poly dC ssDNA extension. Our work clarifies the role of the Ku80 CTR and dsDNA ends on the interaction of DNA-PKcs with Ku and provides key information to guide assembly and biology of NHEJ complexes.
Keywords: Ku, DNA-PK, NHEJ, protein-DNA interaction, protein-protein interaction
Introduction
DNA double strand breaks (DSBs) are considered the most lethal form of DNA damage. If unrepaired or mis-repaired, they can lead to chromosomal loss or rearrangements, reducing cellular viability and increasing the potential for cellular transformation. In eukaryotes, DSBs can be repaired by either non-homologous end joining (NHEJ), alternative non-homologous end-joining (A-NHEJ) or homologous recombination repair (HRR) [1]. NHEJ is the major pathway for repairing ionizing radiation (IR)-induced DSBs in human cells and mechanistically, can be divided into three steps: (i) DSB detection and synapsis, (ii) DNA end processing, and (iii) DNA end ligation. The Ku70/80 heterodimer plays a critical role in NHEJ, detecting DSB ends and recruiting other NHEJ pathway components to the break including the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), X-ray repair cross-complementing 4 (XRCC4), XRCC4-like factor (XLF), Aprataxin and polynucleotide kinase/phosphatase-like factor (APLF) and Paralog of XRCC4 and XLF (PAXX). Following end processing, the DNA ends are ligated by the XRCC4-DNA ligase IV complex [2, 3]. Whereas early models envisioned a pathway in which individual NHEJ components are sequentially recruited to DSBs, recent studies suggest that NHEJ proceeds via the coordinated assembly of NHEJ proteins into a dynamic multi-protein complex rather than in a stepwise fashion [4, 5]. Nevertheless, each of these models stresses the importance of the Ku70/80 heterodimer in the initial detection of the DSB as well as for interaction with and recruitment of other NHEJ proteins, including DNA-PKcs.
Human Ku is composed of 69 kDa and 83 kDa subunits, also called Ku70 (XRCC6) and Ku80 (XRCC5), respectively. The Ku70/80 heterodimer binds with high affinity to ends of double stranded (ds) DNA in a largely DNA sequence independent manner [6]. Eukaryotic Ku70 and Ku80 subunits contain three domains: an N-terminal von Willebrand A (vWA) domain, a central core domain required for DNA binding and dimerization and unique C-terminal domains (Supplementary Fig. 1A). The vWA and core domains of Ku70/Ku80 form an asymmetric ring with an expansive base and a narrow bridge that encircles dsDNA [7]. This preformed ring is capable of accommodating two turns (approximately 14 base pairs) of dsDNA. Ku loads onto the DNA such that Ku70 is proximal to the DNA end and Ku80 is distal, further away from the end [7]. The C-terminal region (CTR) of Ku70 contains a SAP (SAF-A/B, Acinus and PIAS) domain, which is a putative chromatin/DNA binding domain [8], while the Ku80 CTR (residues 545–732) is composed of an α-helical bundle (residues 594 – 704) flanked by two disordered, flexible linkers (residues 546 – 593 and 705–732) [9, 10] (Supplementary Fig. 1B), and forms a highly flexible arm that extends away from the DNA binding core [11]. The extreme C-terminus of Ku80 contains a conserved region (residues 719–732) shown to interact directly with DNA-PKcs in vitro [12, 13]. The recent crystal structure of DNA-PKcs in complex with the Ku80 CTR supports the importance of the Ku80 CTR in interaction with DNA-PKcs [14] and our small angle X-ray scattering (SAXS) structures and structure-based models of DNA-PKcs-Ku70/80-dsDNA and NHEJ complexes suggest that the extended conformation of the Ku80 CTR is critical to the flexible tethering and dynamic recruitment of DNA-PKcs to DNA ends [5]. Moreover, Ku80-deficient xrs6 cells engineered to re-express a human Ku80 C-terminal truncation mutant composed of residues 1–569 were as radiosensitive as Ku null xrs6 cells [15] consistent with the importance of the Ku80 CTR in vivo. Furthermore, work by Turchi and colleagues has revealed the importance of the Ku80 CTR in activation of DNA-PKcs in in vitro kinase assays using purified proteins, synthetic dsDNA oligonucleotides and a short peptide substrate derived from the N-terminus of p53 [16–20]. However, another study using the same p53 peptide substrate but sonicated calf thymus (CT) DNA as activator concluded that the Ku80 CTR is not required for Ku-dependent activation of DNA-PKcs’ kinase activity in vitro [21], leading to ambiguity in the field.
The presence of single stranded (ss) DNA ends also has significant effects on DNA-PK kinase activity, with dsDNA with ssDNA extensions activating DNA-PKcs better than the corresponding blunt ended dsDNA both alone [22, 23] and in the presence of Ku [17]. Furthermore, the nucleotide sequence at DNA ends also influences DNA-PK activity, with enhanced activity seen with dsDNA with a 3′-pyrimidine rich or 5′-purine rich ssDNA extension [20]. In contrast, although Turchi and colleagues showed in enzyme-linked immunosorbent (ELISA) assays that DNA-PKcs-Ku complexes form on 30, 60 and 400bp dsDNA [17], very little is known about the nature of the DNA ends on the interaction of DNA-PKcs with the Ku80 CTR or its ability to form a stable protein-DNA complex suitable for biophysical analysis and structural studies.
Given the central role of Ku and DNA-PKcs in NHEJ and the ambiguity concerning the importance of the Ku80 CTR in activating DNA-PK kinase activity [16–21], we felt compelled to examine the role of the Ku80 CTR in interaction with DNA-PKcs. Our results show that the Ku80 CTR is required for assembly of the Ku-DNA-PKcs complex on short, 25 bp blunt ended dsDNA and on 25 bp dsDNA with a 15-base poly dA ssDNA extension. However, this requirement became less stringent on longer (35bp) blunt ended dsDNA and on 25 bp dsDNA with poly dT or poly dC ssDNA extensions. Moreover, 25 bp dsDNA with a 3′ or 5′ poly dT or poly dC ssDNA extension was a better scaffold for assembly of the DNA-PKcs-Ku complex than blunt ended dsDNA or 25 bp DNA with a poly dA ssDNA extension. Together, our results clarify ambiguity in the field, confirm the importance of the Ku80 CTR and ssDNA ends in recruitment of DNA-PKcs to short segments of dsDNA in vitro and provide important information for efficient assembly of the NHEJ complex for biophysical and structural studies.
Materials and Methods
Methods for cloning and protein purification can be found in Supplementary Materials
Reagents and antibodies
Bovine serum albumin (BSA), phenylmethylsulfonyl fluoride (PMSF), Tris base, EGTA, leupeptin, and pepstatin were purchased from Sigma-Aldrich. The Ku80 antibody was from Abcam (ab33242) and the antibody to Ku70 was from Fisher Scientific (MS329P0).
Biotin DNA pull-down assays
Biotinylated oligonucleotides (listed in Supplementary Table 2) were used to determine the interaction between wild type and mutant Ku heterodimers and DNA-PKcs. Reactions were carried out in biotin pull-down buffer [50 mM HEPES–NaOH, pH 7.5, 75 mM KCl, 10 mM MgCl2, 0.05 mg/ml BSA, 1 mM DTT, 0.2 mM EGTA, 0.1 mM EDTA] containing 5% (v/v) glycerol and 0.05% (v/v) NP-40. Oligonucleotides were bound to Streptavidin MagneSphere® Paramagnetic Particles (Promega, WI, USA) at RT with end-over-end rotation for 10 min at room temperature. Beads were then pelleted using a MagneSphere Technology Magnetic Separation Stand (Promega), and washed once with biotin pull-down buffer. Ku (150 ng) and DNA-PKcs (450 ng) were incubated with the beads for an additional 20 min. at RT with end-over-end rotation. Beads were then washed 3 X with biotin pull-down buffer supplemented with 0.1% (v/v) NP 40. Two times SDS sample buffer was added to the beads and samples were heated at 95°C in a heating block for 5 min. Samples were loaded onto 8% acrylamide/low bis acrylamide SDS PAGE gels and immunoblotting was performed as described previously [24]. Imaging was carried out on a Fuji Luminescent Image Analyzer model # LAS-400 and images were quantitated using Image J software [25]. Statistical analysis was carried out using one way ANOVA with Tukey’s multiple comparison test using GraphPad Prism 6 software. Statistical significance is indicated on graphs by asterisks, where one to four asterisks indicate p ≤ 0.05, ≤ 0.01, ≤ 0.001 or ≤ 0.0001, respectively. p < 0.05 was used as a significance threshold for all tests.
Results
The role of the C-terminal region of Ku80 in recruitment and activation of DNA-PKcs is a question of critical importance to NHEJ. The effects of the Ku80 CTR and DNA ends on DNA-PK kinase activity has been addressed in several studies [16–20], although with contradictory results [21]. In contrast, very little is known about the role of the Ku80 CTR in supporting DNA-PKcs-Ku complex formation on defined dsDNA structures. To better understand both the role of the Ku80 CTR and the nature of DNA ends in recruitment of DNA-PKcs to DNA, we expressed the Ku70/80 heterodimer in baculovirus-infected insect cells with deletion of either the C-terminal DNA-PKcs interaction region (DIR, residues 719–732) or virtually the entire C-terminal region (residues 570–732) (Supplementary Figures 1 and 2) and examined its ability to support recruitment of DNA-PKcs to dsDNA with different ends. Previous studies have shown that the core DNA binding domain of the Ku70/80 heterodimer binds to 14bp dsDNA [7], while, the minimum length of DNA required to support activation of DNA-PKcs in the presence of full-length Ku70/80 heterodimer is 25–30 bp [19]. DNA crosslinking shows that the DNA-PKcs-Ku complex covers approximately 33 bp of duplex DNA [26]. Therefore, we first determined the ability of Ku and DNA-PKcs to interact with blunt ended dsDNA molecules of 25 bp and 35 bp. Oligonucleotides were synthesized with a 3′-biotin group to allow binding to streptavidin-coated magnetic beads and DNA pull-down experiments were performed as described in Materials and Methods.
DNA-PKcs was retained in DNA pull-down assays in the presence of Ku70/80 containing full-length Ku80 and 25bp blunt-ended dsDNA, whereas no interaction with DNA-PKcs was observed in reactions with either Ku70/80(1–718) or Ku70/80(1–569), revealing that the Ku80 DIR is required for interaction with DNA-PKcs under these conditions (Figure 1). In contrast, using 35bp blunt ended duplex dsDNA as scaffold, deletion of the DIR (Ku70/Ku80 (1–718)) reduced the ability of DNA-PKcs to interact with Ku by only 50%, while truncation of Ku80 at residue 569 reduced the interaction by a further 30% (Figure 2). Thus, a longer fragment of dsDNA partially alleviated the need for the DIR for interaction with DNA-PKcs compared to 25bp dsDNA. Moreover, Ku70/80(1–569) supported approximately 20% DNA-PKcs binding compared to full length Ku70/80, suggesting that Ku80 regions beyond the DIR can contribute to the interaction with DNA-PKcs on DNA. The reduced ability of Ku70/80 mutants to interact with DNA-PKcs was not due to their inability to interact with DNA, as both mutants bound 25bp dsDNA with comparable binding to wild type (Supplementary Figures 3 and 4).
Figure 1. Ku80 CTR is required for interaction with DNA-PKcs on 25 bp blunt ended double stranded (ds) DNA.
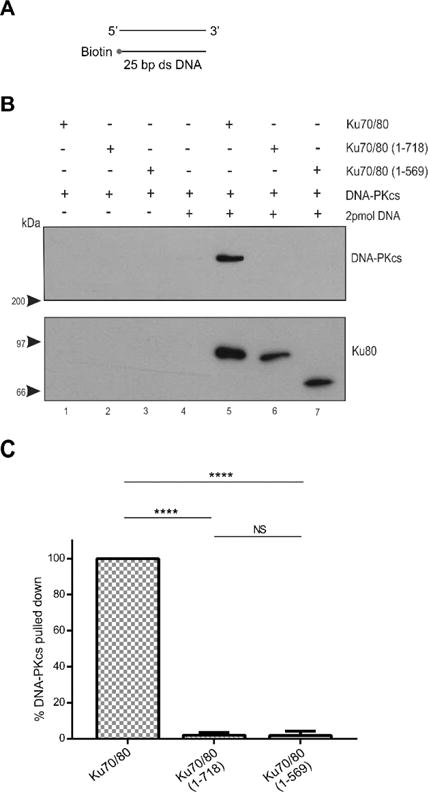
(A) Schematic of the 25bp blunt ended dsDNA with a 3′-biotin group. Oligonucleotide sequences are shown in the Supplementary Material.
(B) Biotin pull-down assays were carried out using 3′ biotin labeled 25 bp blunt ended dsDNA to determine the interaction between DNA-PKcs and Ku wild type and mutant heterodimers. DNA bound-streptavidin coated magnetic beads were incubated with purified Ku70/80 heterodimer (full-length or mutants) as indicated. Beads were pulled down, washed with binding buffer and analyzed by SDS PAGE followed by immunoblot using antibodies to DNA-PKcs or Ku80 as indicated on the right-hand side. Lanes 1 and 5 contained full-length Ku70/80, lanes 2 and 6 contained Ku70/80 (1–718), and lanes 3 and 7 contained Ku70/80 (1–569). DNA was present in lanes 4–7 as indicated. Lanes 1–3 contained DNA-PKcs incubated with Ku plus beads in the absence of DNA. Blots were probed with antibodies to Ku80 and DNA-PKcs as shown.
(C) Quantitation of three independent experiments as described in panel B. In each case, the amount of DNA-PKcs bound was normalized to the amount of Ku in the pull down and expressed as a percentage of the amount of DNA-PKcs bound to full length Ku. Error bars indicate mean ± S.D. **** = p≤0.0001. NS = non-significant.
Figure 2. The Ku80 CTR is only partially required for interaction of DNA-PKcs with Ku70/80 on 35 bp blunt ended dsDNA.
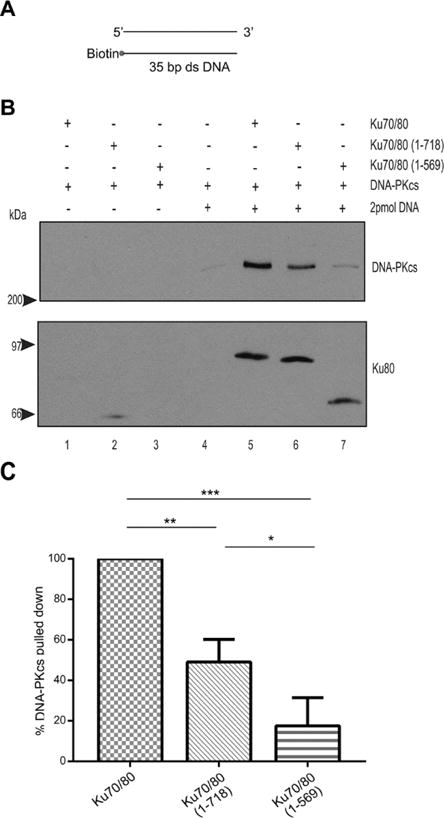
(A) Schematic of biotinylated 35bp dsDNA.
(B) Biotin pull down assay performed as described in Figure 1 but with 35 bp blunt ended dsDNA. (C) Quantitation of three independent experiments as described in panel B. *, ** and *** refer to p values of ≤0.05, ≤ 0.01 and 0.001, respectively.
Previous studies have shown that dsDNA with a 3′ or 5′ ssDNA extension supports higher levels of DNA-PK kinase activity than blunt ended dsDNA [17, 22, 23]. Also, IR-induced DSBs are produced from two single strand DNA breaks on opposite strands of DNA a short distance apart [27], and would therefore be unlikely to be blunt ended when generated in vivo. Therefore, we next repeated our experiments with 25bp dsDNA with either a 3′ or 5′ 15-base ssDNA extension. Neither a 3′ or 5′ poly dA ssDNA extension supported interaction with DNA-PKcs using either Ku70/80 (1–718) or (1–569) mutants (Figures 3 and 4). In contrast, when a 15-base poly dT or poly dC ssDNA extension was used, the ability of DNA-PKcs to interact with Ku70/80 (1–718) and Ku70/80 (1–569) was partially restored (Figures 5 – 8). Thus, the presence of a poly dT or poly dC ssDNA extension can partially compensate for loss of the Ku80 CTR whereas a poly dA ssDNA extension cannot (summarized in Table 1).
Figure 3. The Ku80 CTR is required for recruitment of DNA-PKcs to 25 bp dsDNA with a 15-base poly-dA 3′-extension.
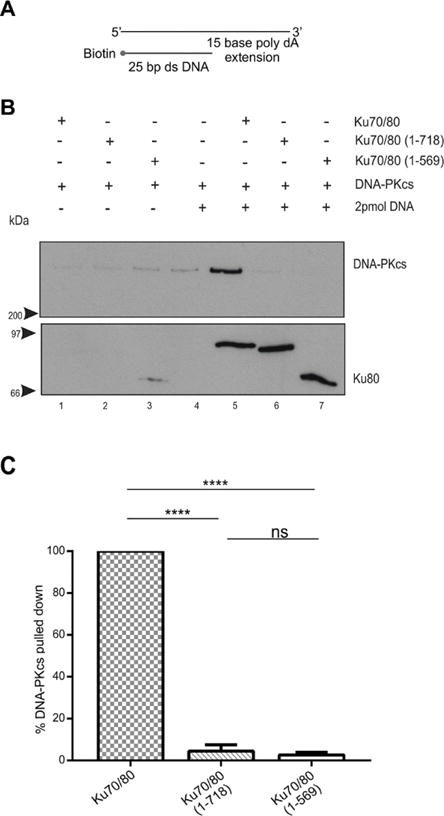
(A) Schematic of biotin labeled 25bp dsDNA with a 15-base poly-dA 3′ extension.
(B) Biotin pull down assays were performed as described in Figure 1 except that 25 bp dsDNA with a 15-base poly-dA 3′ extension was used.
(C) Quantitation of three independent experiments as described in panel B.
Figure 4. The Ku80 CTR is required for recruitment of DNA-PKcs to 25bp dsDNA with a 15-base poly-dA 5′-extension.

(A) Schematic of biotin labelled 25bp dsDNA with a 15 base 5′-extension.
(B) Biotin pull down assays were performed as described in Figure 1 but with 25 bp dsDNA with a 15-base poly dA 5′ extension.
(C) Quantitation of three independent experiments as described in panel B.
Figure 5. The presence of a 15-base poly dT 3′ extension partially restores the ability of DNA-PKcs to interact with Ku70/80 lacking the Ku80 CTR.
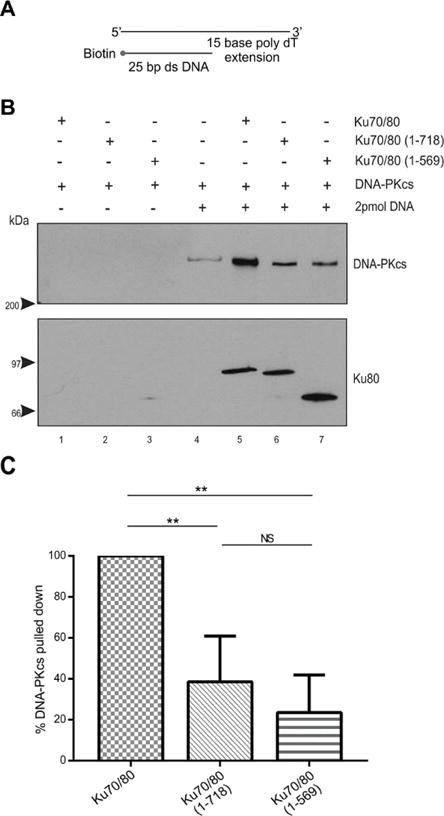
(A) Schematic of biotin labelled 25bp dsDNA with a 15 base 3′-extension.
(B) Biotin pull down assays were performed as described in Figure 1 but with 25 bp dsDNA with a 15-base poly dT 3′ extension.
(C) Quantitation of three independent experiments as described in panel B.
Figure 8. The presence of a 15-base poly dC 5′ extension partially restores the ability of DNA-PKcs to interact with Ku70/80 lacking the Ku80 CTR.
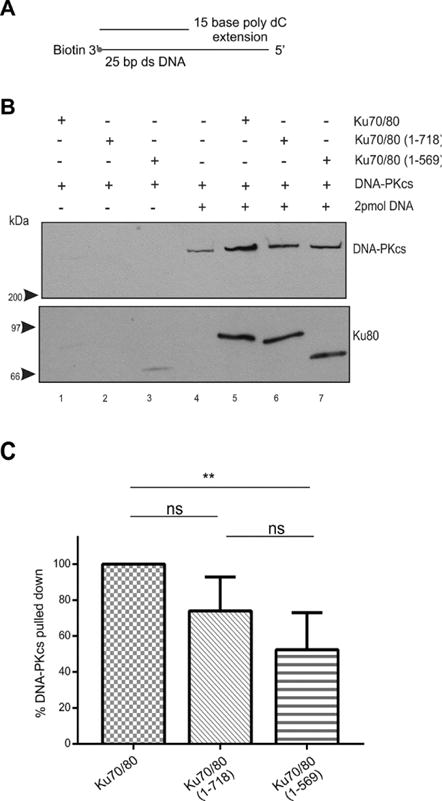
(A) Schematic of biotin labelled 25 bp dsDNA with a 15-base poly dC 5′ extension.
(B) Biotin pull down assay performed as described in Figure 1 but with 25 bp blunt ended dsDNA with a 15-base poly dC 5′ extension.
(C) Quantitation of three independent experiments as described in panel B.
Table 1. Summary of the interaction of DNA-PKcs with baculovirus expressed Ku70/80 full length and Ku80 CTR mutants Ku70/80 (1–718) and Ku70/80 (1–569) on defined dsDNA scaffolds.
For each dsDNA species used (25 bp blunt etc), DNA-PKcs bound was normalized to Ku bound (by western blot signal) as described in Figures 1–8. Binding to Ku70/80 full length was set as 100 and DNA-PKcs binding to Ku70/80 (1–718) and (1–569) mutants is shown as percentage of binding in the presence of full length Ku70/80. Values in parenthesis indicate standard deviation over three separate experiments
| Protein | 25 bp blunt | 35 bp blunt | 3′-poly dT | 3′-poly dC | 3′-poly dA | 5′-poly dT | 5′-poly dC | 5′-poly dA |
|---|---|---|---|---|---|---|---|---|
| Ku70/80 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
|
Ku70/80
(1–718) |
1.93 (± 1.55) |
49.03 (± 11.15) |
38.57 (± 22.32) |
31.67 (± 42.66) |
4.47 (± 2.99) |
24.73 (± 21.54) |
73.89 (± 18.93) |
7.35 (± 6.38) |
|
Ku70/80 (1–569) |
1.78 (± 2.35) |
17.53 (± 13.89) |
23.52 (± 18.33) |
14.72 (± 9.62) |
2.61 (± 1.58) |
10.34 (± 8.34) |
52.34 (± 20.61) |
6.68 (± 5.32) |
Previous studies have shown that the nature of the ssDNA extension affects the ability of DNA-PK to phosphorylate a synthetic peptide substrate, with a 2-fold enhancement in activity observed in the activity in the presence of dsDNA with a 15-base poly dT 3′-extension, compared to a 15 base poly dA extension, suggesting that a pyrimidine rich ssDNA extension preferentially activates DNA-PK kinase activity [20]. Therefore, we next compared the relative abilities of the panel of oligonucleotides used in this study to pull down DNA-PKcs (in the presence of full length Ku). The most efficient dsDNA constructs for pulling down DNA-PKcs were 25 bp duplex DNA with a 3′ or 5′ poly dT or poly dC ssDNA extension (Figure 9 and Table 2). These constructs pulled down 2–4 × more DNA-PKcs than either 25bp dsDNA with a poly dA extension, blunt ended 25bp or 35 bp dsDNA (Figure 9 and Table 2). Thus, the presence of a poly-pyrimidine ssDNA 3′ or 5′ extension enhances the ability of DNA-PKcs to interact with Ku on DNA. Moreover, the requirement for the Ku80 CTR was inversely correlated with the ability of Ku70/80 to interact with DNA-PKcs (Table 1), suggesting that the KU80 CTR contributes to stabilization of DNA-PKcs with Ku on short segments of DNA with blunt ends or poly dA ssDNA extensions but this requirement is, at least in part, circumvented when DNA-PKcs interacts with Ku bound to a DNA end with a 3′ or 5′ poly-pyrimidine ssDNA end.
Figure 9. DNA-PKcs preferentially interacts with Ku70/80 heterodimer on dsDNA with poly-pyrimidine single stranded DNA extensions.

(A) Biotin pull down assay performed as described in Figure 1 but with different DNA structures as described below. Lane 1 contained 25 bp blunt ended dsDNA. Lane 2 contained 35 bp blunt ended dsDNA. Lane 3 contained 25 bp dsDNA with a 15-base poly dA 5′ extension. Lane 4 contained 25 bp dsDNA with a 15-base poly dC 5′ extension. Lane 5 contained 25 bp dsDNA with a 15-base poly dT 5′ extension. Lane 6 contained 25 bp dsDNA with a 15-base poly dA 3′ extension. Lane 7 contained 25 bp dsDNA with a 15-base poly dC 3′ extension. Lane 8 contained 25 bp dsDNA with a 15-base poly dT 3′ extension.
(B) Quantitation of three independent experiments as described in Fig. 1B. Error bars indicate mean ± S.D. Statistically significant differences were detected between 25 bp blunt DNA and 5′ poly dC, 3′ -poly dC, 5′-poly dT and 3′-poly dC (red asterisks); between 35bp blunt DNA and 5′ poly dC and 3′-poly dT (green asterisks); between 5′-poly dA and 3′-poly dT (purple asterisks) and between 3′-poly dA and 5′ poly dC and 3′ poly dT (yellow asterisks). One asterisks indicates significance to 0.05, two to 0.01 and three to 0.001. See also Table 2.
Table 2. Ability of various dsDNA oligonucleotides to support formation of DNA-PKcs-Ku complex, relative to 25 bp DNA and full length Ku70/80.
Values for DNA-PKcs binding to Ku70/80 (top row, in bold) represent quantitation of results shown in Figure 9. Results for Ku70/80(1–718) and (1–569) mutants with 25bp blunt ended dsDNA are from Figure 1 (bold). The relative interaction of DNA-PKcs with Ku70/80 (1–718) and (1–569) mutants with other DNA molecules was inferred from binding relative to 25bp blunt ended DNA and Ku70/80 full length from Figures 1–8 (shown in italics).
| Protein | 25 bp blunt | 35 bp blunt | 3′-poly dT | 3′-poly dC | 3′-poly dA | 5′-poly dT | 5′-poly dC | 5′-poly dA |
|---|---|---|---|---|---|---|---|---|
| Ku70/80 | 100 |
137.74 (± 61.22) |
420.38 (± 99) |
300.59 (± 109.19) |
124.52 (± 30.42) |
288.81 (± 32.42) |
323.52 (± 16.29) |
202.41 (± 55.17) |
|
Ku70/80 (1–718) |
1.93 (± 1.55) |
67.53 | 162.14 | 95.20 | 5.57 | 71.42 | 239.05 | 14.88 |
|
Ku70/80 (1–569) |
1.78 (± 2.35) |
24.15 | 98.87 | 44.25 | 3.25 | 29.86 | 169.33 | 13.52 |
Discussion
Previous reports have shown that the Ku80 CTR plays an important role in NHEJ and is important for DNA-PKcs’ kinase activity [12, 13, 16–18]. In keeping with this, Ku null xrs6 cells expressing Ku80 C-terminal truncations (deletion of residues 555–732) are as radiosensitive as Ku80 null cells [16, 21]. Our recent structural studies show that the Ku80 CTR forms an extended, flexible arm that connects DNA-PKcs to the DNA bound Ku70/80 core through the conserved interaction region (DIR) at the extreme C-terminus of Ku80 (residues 719–732) [5, 11]. Indeed, the recent crystal structure of DNA-PKcs reveals binding sites for the Ku80 CTR (residues 539 to 732) within the base of DNA-PKcs [14], further supporting this model. The isolated Ku80 DIR peptide itself does not support DNA-PKcs activity [18], speaking to the importance of the KU80 CTR as a tether to recruit DNA-PKcs to the DNA-bound heterodimer rather than directly activating DNA-PKcs [5, 11]. However, despite several studies reporting that the Ku80 CTR is required for DNA and Ku-dependent activation of DNA-PKcs [16–20], one group has reported that the Ku80 CTR is not required for activation of DNA-PKcs [21] but the reason for this discrepancy was not known.
Here, using purified components, we show that the Ku80 CTR is required for interaction of DNA-PKcs with Ku in the presence of either 25 bp blunt ended DNA or 25 bp dsDNA with a poly dA ssDNA extension. In contrast, the requirement for the Ku80 CTR was partially alleviated when 25 bp duplex DNA with either a poly dC or poly dT 15 base ssDNA extension or 35 bp blunt ended DNA duplex was used. Moreover, the DNA-PKcs-Ku complex formed preferentially on dsDNA with a 15-base poly-pyrimidine ssDNA extension and the nature of the DNA ends affects DNA-PKcs-Ku-DNA complex formation, with 25bp blunt ended dsDNA being the least optimal and 25bp dsDNA with a 3′ or 5′ poly dT or poly dC extension being the preferred scaffold for DNA-PKcs-Ku complex assembly. These results support previous studies in which the Ku80 CTR was shown to be required for activation of DNA-PKcs and where dsDNA with ss DNA extensions, in particular poly pyrimidine extensions were shown to be optimal for stimulating DNA-PK kinase activity [17–20, 28].
The X-ray structure of DNA-PKcs reveals the presence of multiple channels that could interact with ssDNA [14] as suggested from earlier cryo-EM structures [29]. We speculate that the preference for interaction with ssDNA with pyrimdine rich sequences over purine rich sequences may reflect binding of ssDNA within a narrow channel that accommodates the smaller pyrimidine bases over the more bulky purine bases. Since the oligonucleotides that are best able to support DNA-PKcs-Ku complex formation also display a less stringent requirement for the Ku80 CTR, our data are also consistent with the suggestion that pyrimidine rich ssDNA extensions can compensate for loss of the KU80 CTR in stabilizing the DNA-PKcs-Ku complex on DNA as proposed by Pawelczak and Turchi from DNA-PK kinase assays [19, 20]. It is also possible that acidic amino acids in the DIR may act as a DNA mimic [30], partially overcoming the need for the Ku80 CTR on certain DNA scaffolds.
Together, our studies show that the requirement for the Ku80 CTR for the DNA-dependent interaction of Ku with DNA-PKcs depends on both the length of the ds DNA and the composition of the ssDNA extension as found by Turchi and colleagues for DNA-PK activity [19, 20]. However, in preliminary studies, we found that, as reported by Weterings et al [21], in the presence of sonicated calf thymus (CT)-DNA, the Ku80 DIR was not required for DNA-PK kinase activity and that loss of the entire Ku80 CTR only reduced DNA-PK kinase activity by about 50% (Supplementary Fig. 5). Sonicated CT-DNA is a crude mixture of DNA molecules of diverse lengths and sequence composition and we speculate that the complexity of the DNA species present in CT-DNA may have masked the clear requirement for the Ku80 CTR observed with defined DNA molecules. Together, our data clarify ambiguities in the field and informs on DNA preference for future structural and biophysical studies of the NHEJ complex.
Supplementary Material
Figure 6. The presence of a 15-base poly dT 5′ extension partially restores the ability of DNA-PKcs to interact with Ku70/80 lacking the Ku80 CTR.
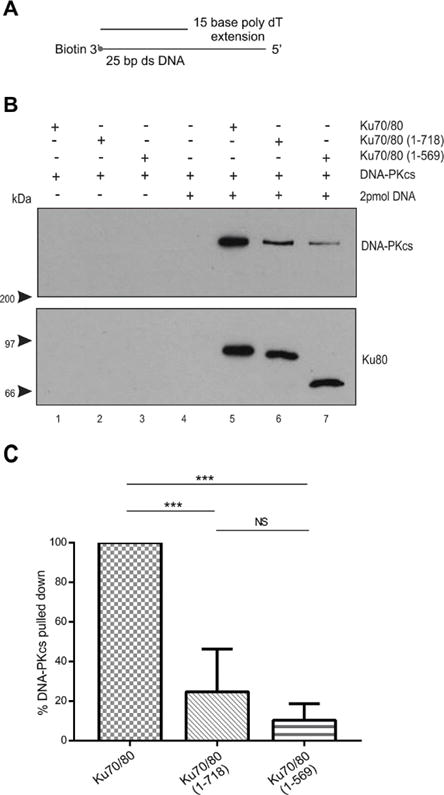
(A) Schematic of biotin labelled 25bp dsDNA with a 15 base 5′-extension.
(B) Biotin pull down assays were performed as described in Figure 1 but with 25 bp dsDNA with a 15-base poly dT 5′ extension.
(C) Quantitation of three independent experiments as described in panel B.
Figure 7. The presence of a 15-base poly dC 3′ extension partially restores the ability of DNA-PKcs to interact with Ku70/80 lacking the Ku80 CTR.
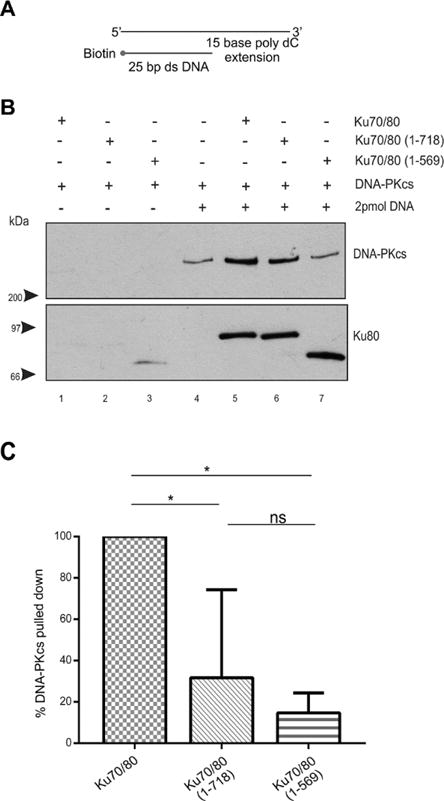
(A) Schematic of biotin labelled 25 bp dsDNA with a 15-base poly dC 3′ extension.
(B) Biotin pull down assay performed as described in Figure 1 but with 25 bp dsDNA with a 15-base poly dC 3′ extension.
(C) Quantitation of three independent experiments as described in panel B.
Highlights.
The Ku70/80 heterodimer and DNA dependent protein kinase catalytic subunit (DNA-PKcs) play critical roles in mammalian DNA double strand repair by the non-homologous end joining pathway (NHEJ). Previous studies have examined the effects of the Ku80 C terminal region and DNA end groups on the ability to activate DNA-PK kinase activity with conflicting results. Here we have examined the requirement for KU80CTR and DNA end groups for formation of a stable complex with DNA-PKcs and show that, as in kinase assays, dsDNA with overhanging poly pyrimidine ends is the best scaffold for interaction of DNA-PKcs with Ku on DNA, providing important information for structural studies of the NHEJ complex.
Acknowledgments
We thank Shujuan Fang for technical assistance, Dr. Yaping Yu for purification of DNA-PKcs, Drs. Aaron Goodarzi and Karolin Klement for helping with the baculovirus expression system, the University of Calgary DNA synthesis core for oligonucleotides, Dr. George Korza, Department of Molecular Biology and Biophysics, University of Connecticut Health Centre, Farmington, CT for SPR analysis and Dr. J. A. Tainer for helpful discussions.
Grant Funding
This study was supported by the Canadian Institutes of Health Research grant # MOP13639, The Engineered Air Chair in Cancer Research and NIH PO1 CA092584.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest.
The authors declare they have no conflicts of interest.
References
- 1.Ceccaldi R, Rondinelli B, D’Andrea AD. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends in cell biology. 2016;26:52–64. doi: 10.1016/j.tcb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Lees-Miller SP. Detection and repair of ionizing radiation-induced DNA double strand breaks: new developments in nonhomologous end joining. International journal of radiation oncology, biology, physics. 2013;86:440–449. doi: 10.1016/j.ijrobp.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radhakrishnan SK, Jette N, Lees-Miller SP. Non-homologous end joining: emerging themes and unanswered questions. DNA repair. 2014;17:2–8. doi: 10.1016/j.dnarep.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yano K, Chen DJ. Live cell imaging of XLF and XRCC4 reveals a novel view of protein assembly in the non-homologous end-joining pathway. Cell cycle (Georgetown, Tex) 2008;7:1321–1325. doi: 10.4161/cc.7.10.5898. [DOI] [PubMed] [Google Scholar]
- 5.Hammel M, Yu Y, Radhakrishnan SK, Chokshi C, Tsai MS, Matsumoto Y, Kuzdovich M, Remesh SG, Fang S, Tomkinson AE, Lees-Miller SP, Tainer JA. An Intrinsically Disordered APLF Links Ku, DNA-PKcs and XRCC4-DNA Ligase IV in an Extended Flexible Non-Homologous End Joining Complex. Journal of Biological Chemistry. 2016 doi: 10.1074/jbc.M116.751867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blier PR, Griffith AJ, Craft J, Hardin JA. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. The Journal of biological chemistry. 1993;268:7594–7601. [PubMed] [Google Scholar]
- 7.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 8.Aravind L, Koonin EV. SAP - a putative DNA-binding motif involved in chromosomal organization. Trends in biochemical sciences. 2000;25:112–114. doi: 10.1016/s0968-0004(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Hu W, Cano L, Lee TD, Chen DJ, Chen Y. Solution structure of the C-terminal domain of Ku80 suggests important sites for protein-protein interactions. Structure (Camb) 2004;12:495–502. doi: 10.1016/j.str.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Harris R, Esposito D, Sankar A, Maman JD, Hinks JA, Pearl LH, Driscoll PC. The 3D solution structure of the C-terminal region of Ku86 (Ku86CTR) Journal of molecular biology. 2004;335:573–582. doi: 10.1016/j.jmb.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 11.Hammel M, Yu Y, Mahaney BL, Cai B, Ye R, Phipps BM, Rambo RP, Hura GL, Pelikan M, So S, Abolfath RM, Chen DJ, Lees-Miller SP, Tainer JA. Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial non-homologous end joining complex. The Journal of biological chemistry. 2010;285:1414–1423. doi: 10.1074/jbc.M109.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gell D, Jackson SP. Mapping of protein-protein interactions within the DNA-dependent protein kinase complex. Nucleic acids research. 1999;27:3494–3502. doi: 10.1093/nar/27.17.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 14.Sibanda BL, Chirgadze DY, Ascher DB, Blundell TL. DNA-PKcs structure suggests an allosteric mechanism modulating DNA double-strand break repair. Science (New York, NY) 2017;355:520–524. doi: 10.1126/science.aak9654. [DOI] [PubMed] [Google Scholar]
- 15.Singleton BK, Priestley A, Steingrimsdottir H, Gell D, Blunt T, Jackson SP, Lehmann AR, Jeggo PA. Molecular and biochemical characterization of xrs mutants defective in Ku80. Molecular and cellular biology. 1997;17:1264–1273. doi: 10.1128/mcb.17.3.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singleton BK, Torres-Arzayus MI, Rottinghaus ST, Taccioli GE, Jeggo PA. The C terminus of Ku80 activates the DNA-dependent protein kinase catalytic subunit. Molecular and cellular biology. 1999;19:3267–3277. doi: 10.1128/mcb.19.5.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods DS, Sears CR, Turchi JJ. Recognition of DNA Termini by the C-Terminal Region of the Ku80 and the DNA-Dependent Protein Kinase Catalytic Subunit. PloS one. 2015;10:e0127321. doi: 10.1371/journal.pone.0127321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett SM, Woods DS, Pawelczak KS, Turchi JJ. Multiple protein-protein interactions within the DNA-PK complex are mediated by the C-terminus of Ku 80. Int J Biochem Mol Biol. 2012;3:36–45. [PMC free article] [PubMed] [Google Scholar]
- 19.Pawelczak KS, Turchi JJ. A mechanism for DNA-PK activation requiring unique contributions from each strand of a DNA terminus and implications for microhomology-mediated nonhomologous DNA end joining. Nucleic acids research. 2008;36:4022–4031. doi: 10.1093/nar/gkn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawelczak KS, Andrews BJ, Turchi JJ. Differential activation of DNA-PK based on DNA strand orientation and sequence bias. Nucleic acids research. 2005;33:152–161. doi: 10.1093/nar/gki157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weterings E, Verkaik NS, Keijzers G, Florea BI, Wang SY, Ortega LG, Uematsu N, Chen DJ, van Gent DC. The Ku80 carboxy terminus stimulates joining and artemis-mediated processing of DNA ends. Molecular and cellular biology. 2009;29:1134–1142. doi: 10.1128/MCB.00971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martensson S, Hammarsten O. DNA-dependent protein kinase catalytic subunit. Structural requirements for kinase activation by DNA ends. The Journal of biological chemistry. 2002;277:3020–3029. doi: 10.1074/jbc.M106711200. [DOI] [PubMed] [Google Scholar]
- 23.Hammarsten O, DeFazio LG, Chu G. Activation of DNA-dependent protein kinase by single-stranded DNA ends. The Journal of biological chemistry. 2000;275:1541–1550. doi: 10.1074/jbc.275.3.1541. [DOI] [PubMed] [Google Scholar]
- 24.Douglas P, Zhong J, Ye R, Moorhead GB, Xu X, Lees-Miller SP. Protein phosphatase 6 interacts with the DNA-dependent protein kinase catalytic subunit and dephosphorylates gamma-H2AX. Molecular and cellular biology. 2010;30:1368–1381. doi: 10.1128/MCB.00741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol Reprod Dev. 2015;82:518–529. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo S, Kimzey A, Dynan WS. Photocross-linking of an oriented DNA repair complex. Ku bound at a single DNA end. The Journal of biological chemistry. 1999;274:20034–20039. doi: 10.1074/jbc.274.28.20034. [DOI] [PubMed] [Google Scholar]
- 27.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 28.Hammarsten O, Martensson S. DNA-dependent protein kinase catalytic subunit: Structural requirements for kinase activation by DNA ends. The Journal of biological chemistry. 2001 doi: 10.1074/jbc.M106711200. [DOI] [PubMed] [Google Scholar]
- 29.Williams DR, Lee KJ, Shi J, Chen DJ, Stewart PL. Cryo-EM structure of the DNA-dependent protein kinase catalytic subunit at subnanometer resolution reveals alpha helices and insight into DNA binding. Structure (London, England : 1993) 2008;16:468–477. doi: 10.1016/j.str.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putnam CD, Tainer JA. Protein mimicry of DNA and pathway regulation. DNA repair. 2005;4:1410–1420. doi: 10.1016/j.dnarep.2005.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


