Abstract
Malnutrition results in serious consequences for growth and cognitive development in children. We studied select child and maternal biological factors, socio-economic factors, enteric pathogenic burden, and gut function biomarkers in 402 children 6–24 months of age in North-eastern Brazil. In this prospective case-control study, not being fed colostrum (odds ratio [OR] = 3.29, 95% confidence interval [CI] 1.73–6.26), maternal age ≥18 years (OR = 1.88, 95% CI 1.10–3.22), and no electrical fan (OR = 2.46, 95% CI 1.22–4.96) or bicycle (OR = 1.80, 95% CI 1.10–2.95) in the household were positively associated, and higher birth weight (OR = 0.27, 95% CI 0.19–0.38), larger head circumference (OR = 0.74, 95% CI 0.66–0.82), and shortness of breath in the last two weeks (OR = 0.49, 95% CI 0.27–0.90) were negatively associated with malnutrition. Subclinical enteric pathogen infections were common, and enteroaggregative Escherichia coli infections were more prevalent in malnourished children (p = 0.045). Biomarkers such as the lactulose:mannitol test, myeloperoxidase, neopterin, and calprotectin were highly elevated in both malnourished and nourished children. Nourished children had a better systemic immune response than the malnourished children, as detected by elevated serum amyloid A-1 (SAA-1) and soluble cluster of differentiation protein 14 (sCD14) biomarkers (P < 0.001). SAA-1 and sCD14 were also associated with better nutritional z-scores. Neonatal, maternal, and socio-economic factors were associated with malnutrition in children. There was a substantial subclinical enteric pathogen burden, particularly with EAEC, in malnourished children.
Keywords: malnutrition, growth, inflammatory biomarkers, gut function, enteric pathogens
Introduction
Malnutrition is a public health problem among young children in the developing countries, directly and indirectly increases the risk of morbidity and mortality in children younger than 5 years old, and is associated with more than one-third of all causes of deaths among these children globally.1 Malnutrition is also associated with irreversible short adult height, lower levels of attained schooling, reduced physical activity, lower income as an adult, and most importantly, impaired cognitive development, which is detrimental for a competitive adult life as well as quality of life in adulthood.2–4 Studies have also shown that malnutrition increases the risk for overweight, metabolic syndrome, and cardiovascular diseases in adulthood.3–5
The determinants of malnutrition are multifactorial and differ between geographical settings.6 They include maternal and neonatal factors, socio-economic factors, food intake and enteric pathogenic burden that can affect gut function, and local and systemic immune-related inflammatory responses that increase the risk for a vicious cycle of environmental enteropathy, morbidities, and malnutrition.2,7,8 It is important to evaluate these determinant variables to develop a cost-benefit plan for prevention of and interventions for malnutrition, particularly because interventions have not successfully broken this vicious cycle or reduced the prevalence of malnutrition in many developing countries.9
Malnutrition is also associated with an increased incidence and duration of diarrhea among children; conversely, diarrheal diseases are associated with an increased risk of malnutrition.10–13 Studies published in the last few decades have reported a negative impact of diarrheal diseases on growth and the impact of specific enteric pathogens on growth faltering.2,14–18 However, the impact of a broad spectrum of enteric pathogens on gut function, local and systemic immune-related inflammatory responses, and malnutrition have not been completely investigated in the literature.
In Brazil, the most prevalent area for undernutrition and stunting is the semiarid region from north of Minas Gerais to south of the state of Piaui; this area includes the state of Ceará.19
The MAL-ED Case Control Study, which was a prospective case-control study in North-eastern Brazil, aimed at evaluating factors associated with malnutrition in children, including the enteric pathogen burden, gut function, and immune-related inflammatory response associated with malnutrition.20 In this report, we evaluate the associations between environmental enteropathy, malnutrition, and a select set of biological, care, and socio-economic factors for both mother and child; enteric pathogenic burden; and gut function biomarkers in children from Fortaleza, Ceará, Brazil.
Materials and Methods
Study design, population, and ethical approval
This prospective case-control was conducted in Fortaleza, CE, Brazil, from 19 August 2010 to 30 September 2013. Children who attended the Institute for the Promotion of Nutrition and Human Development (IPREDE) clinic for nutritional counselling were recruited. Demographic and socio-economic information is described in detail elsewhere.20
The inclusion criteria were age of 6–24 months; weight-for-age z score (WAZ) < −2 for malnourished children and > −1 for nourished children; healthy (i.e., without any specific illness or fever); and mother/primary caregiver present with legal custody of the child. The exclusion criteria were prolonged hospitalization or serious health issues, such as human immunodeficiency virus, tuberculosis, neonatal disease, kidney disease, or other diseases diagnosed by a physician and a parent or primary caregiver with cognitive deficits or <16 years of age. We initially attempted to pair controls with cases by sex and age, but difficulties with enrollment led us to enroll controls by age for inclusion between 6–24 months. Standard nutrition education and supplementation for malnourished children were provided following the World Health Organization (WHO) guideline for the management of malnutrition.9
The study protocol and consent form were approved by the local institutional review board (IRB) at the Federal University of Ceará, the national IRB Conselho Nacional de Ética em Pesquisa, and the IRB at the University of Virginia in the United States of America.
Anthropometric measurements
Anthropometry was measured at enrolment and then every 3 months during the 24-month follow-up. The health professionals used a standard board to measure length to the nearest 0.1 cm. Digital scales were used to measure weight to the nearest 100 g. The weight-for-age (WAZ), length-for-age z score (LAZ), and weight-for-length z score (WLZ) were calculated according to the WHO Multi-Centre Growth Reference Study.21
Neonatal and maternal factors and socio-economic status
The following information was obtained using a case report form developed specifically for this study and based on previous pilot data20: anthropometrics for the child, child care, mother or caregiver characteristics, house, people who usually sleep in the house, source of water, toilet facility, average monthly income, and many other related parameters.
Morbidities
Maternal-reported child illnesses and antibiotic use for the previous two weeks, including the day of collection, were collected from the enrolment surveillance form and then every 3 months. For this report, we used the first reported information at the enrolment surveillance for diarrhea, acute lower respiratory infections, and fever. The definitions for onset and episodes are detailed elsewhere.22
Microbiology
Surveillance stool samples were collected at enrolment and at each 3-month follow-up. They were tested for an expansive panel of bacterial, viral, and parasitic enteric pathogens using a standardized microbiology protocol, as previously described.23 Briefly, the bacteriology protocol was optimized for Salmonella, Shigella, Vibrio, Yersinia, Aeromonas, and Plesiomonas. Culture media was from BD (Sparks, Maryland) and it was purchased and prepared in house. Up to five suspect colony morphologies were selected and screening using the Analytical Profile Index (API) 20E (bioMérieux, Craponne, France) identification system. For Escherichia coli we selected a pool of five lactose-fermenting colonies resembling E. coli, and characterize them for virulence genes using a multiplex polymerase chain reaction (PCR) assay. The virulence genes selected for PCR probes are detailed on reference 23. For Campylobacter jejuni/coli diagnosis we used a TaqMan probe to detect the Campylobacter adhesin to fibronectin (CadF) gene, as previously reported.24,25 For Cryptosporidium (oocyst protein), E. histolytica (surface adhesin molecule) and Giardia (cyst wall protein) detection on stool samples, we used the protozoa kits from Techlab (Blacksburg, Virginia)23. The ProSpecT kits (Oxoid Ltd, Ely, United Kingdom) were used for rotavirus (VP6), adenovirus (detects all human adenovirus serotypes via a genus-specific adenovirus hexon antigen), and astrovirus kits23.
Gut function tests
The lactulose:mannitol test was used to evaluate intestinal permeability, malabsorption, and damage. At enrolment, children fasted for two hours and were encouraged to void prior to administration of a solution containing lactulose (250 mg/mL) and mannitol (50 mg/mL), in a dose of 2 mL/kg (maximum administered dose, 20 mL) at a concentration of 1002 mOsm/L. Healthcare professionals collected and measured the urine following voiding for five hours; 1–2 drops of chlorhexidine (2.35%) were added. Lactulose and mannitol were measured as previously described.26
Stool samples were collected in a sterile container, aliquoted, and stored in cryovials at −20°C until assay. Samples for alpha-1-antitrypsin (A1AT) and product of regenerating gene 1β (Reg1β) were diluted 10×, 100×, 500×, and 1000× in buffer with protease inhibitors. The A1AT enzyme linked immunosorbent assay (ELISA) kit was from Bioventor (Candler, NC), and the ELISA kit for Reg1β was from TechLab (Blacksburg, VA).
Plasma samples were collected, aliquoted, and frozen at −80°C until tested for fatty acid-binding protein (I-FABP; HYcult Biotech, Uden, Netherlands).
Standard curves provided by the ELISA kits were used for quantification.
Myeloperoxidase was measured in stool samples, using a kit from Immundiagnostik (Bensheim, Germany). Neopterin and calprotectin were measured from plasma samples using ELISA kits (Immundiagnostik, Bensheim, Germany; R & D Systems, Minneapolis, MN, respectively). Plasma samples were used to measure high sensitivity C-reactive protein, serum amyloid A-1 (SAA-1), lipopolysaccharide (LPS) binding protein, and soluble cluster of differentiation protein 14 (sCD14). The ELISA kits were provided by Hycult Biotech (Uden, Netherlands). Immunoglobulin (Ig)A and IgG against LPS (LPS IgA and LPS IgG) were measured based on the assays, as described elsewhere.27 Plasma samples were diluted, and the manufacturer recommended standard curves were used.
Outcome variables
The outcome variables were WAZ, LAZ, and WLZ. WAZ was used to categorize children as malnourished or nourished. We also stratified the nutritional z-scores (WAZ, LAZ, and WLZ) for the lower 25th percentile of the malnourished children) versus the upper 75th percentile of the nourished.
Statistical analysis
The MAL-ED Case Control Study was part of a multicenter, longitudinal, case-control study, and the Fortaleza, CE, Brazil site used a standardized protocol and data collection tools.20,28 Inbuilt training, quality assurance, and quality control protocols enabled this study to maintain a quality database for analysis. The data were entered twice using Microsoft Access software (Microsoft Corporation, Redmond, Washington) and validated by cross-matching the two databases.
Bivariate analysis of 69 variables was initially conducted. Chi-squared or Fisher exact tests were conducted to compare categorical variables between malnourished and nourished groups. Student t tests for data with a normal distribution and Mann Whitney U tests for data without a normal distribution were used to compare continuous variables between these groups.
As there are multiple determinants of the selected outcomes and some of the determinant variables are dichotomous, we performed multivariable logistic regression analysis using a backward stepwise regression strategy and including variables with a significance level ≤ 25% in the bivariate analyses: logit[θ(x)]=log [θ (x)/1−θ(x)]=α + β1x1 + β2x2 +……+ βixi.29 The Wald test, which calculates a Z statistic (z = β/SE), was used to evaluate the statistical significance of each coefficient (β) in the model. The values of significance level (p value) was assumed a higher value of p that suggests a greater number of variables to be tested as potential explanatory variables using the multivariate logistic regression model. This was the criteria adopted in the stepwise backward regression analysis. The initial set of multiple explanatory variables were selected based on p ≤ 0.25 and those in the final model remained if they met the p < 0.05 significance level. However, we noted a borderline significance (p = 0.06 for the electric fan) for one variable and observed that withdrawal of this variable from the model significantly changed the odds ratio of other variables that remained in the model. We therefore decided to leave this variable in the final model as described in Table 3. For the anthropometric variables, sex and age were matched in a further multivariable logistic regression analysis; there was no significant difference between the malnourished and nourished groups. Results are presented as the odds ratio (OR) for malnutrition.
Table 3.
Variable association with malnourished and nourished children selected from those in the group analysis at significance level ≤ 6% were used for the general multivariable logistic regression model.
| Variables | p | Odds ratio | 95% CI (OR) | |
|---|---|---|---|---|
| Birth weight | 0.000 | 0.381 | 0.252 – 0.576 | |
| Short of breath in the last two weeks? | No | - | 1.000 | - |
| Yes | 0.012 | 0.344 | 0.149 – 0.793 | |
| Does your household have an electric fan? | No | 0.057 | 2.173 | 0.978 – 4.828 |
| Yes | - | 1.000 | - |
In addition, subgroup analysis was performed for children with subclinical EAEC infection to compare the plasma concentrations of IgA and IgG against LPS between the malnourished and nourished children.
P values ≤ 0.05 were considered statistically significant. Statistical analyses were conducted using SPSS, version 20 (IBM Corp., Armonk, NY).
Results
Participant selection and enrolment
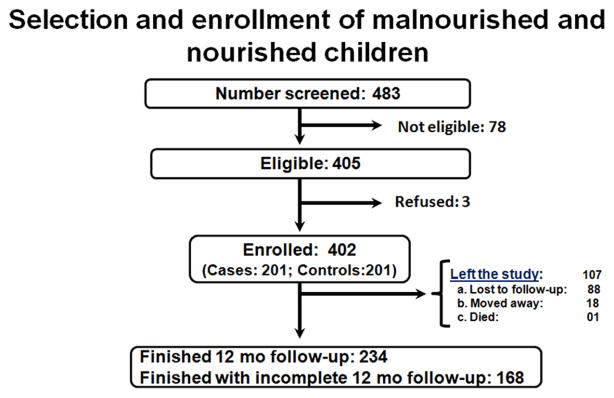
Of the 402 children (201 malnourished and 201 nourished) who were enrolled, 88 were lost to follow-up, 18 moved away, and 1 died during the study period (Figure 1). For determinant variables we analyzed initially all 402 children at the time of enrolment using the univariable analysis and the results are summarized in the Supplement Digital Content 1 (Table). A total of 358 children were the total number of valid observations in the multivariate logistic regression analysis. Children at enrolment who have not provided information on all the variables included in the first model adjustment were eliminated from the analysis (44/402; 11%).
Figure 1.
Flow diagram for the study protocol and selection of malnourished and nourished children.
Comparisons between the malnourished and nourished at enrolment
Table 1 summarizes the select baseline characteristics included in the bivariate analysis of the 69 variables (see also Supplemental Digital Content 1, Table). The nourished children were significantly younger than the malnourished children. The nourished children had greater birth weight, larger head circumference, greater weight, greater length, and higher WAZ, LAZ, and WLZ than the malnourished children. Greater proportions of the nourished children were fed colostrum and were still breastfeeding. The malnourished children were younger than the nourished children when they were first given plain water, sugar water, honey water, juice, or cow’s or goat’s milk.
Table 1.
Selected baseline characteristics of the malnourished and nourished children at enrolment included in the univariate analysis of sixty nine (see also Supplement Table 1) association factors.
| Factors | Study groups
|
|||
|---|---|---|---|---|
| Total N = 402 |
Nourished N = 201 |
Malnourished N = 201 |
p values | |
| Child anthropometrics | ||||
| Age (months; mean ± sd) | 13.6 ± 5.56 | 12.4 ± 5.45 | 14.8 ± 5.4 | <0.001 |
| Male | 193 (48%) | 98 (49%) | 95 (47%) | 0.842 |
| Birth weight (kg; mean ± sd) | 2.824 ± 0.749 | 3.125 ± 0.636 | 2.528 ± 0.734 | <0.001 |
| Current weight of the child (kg; mean ± sd) | 8.283 ± 1.714 | 9.335 ± 1.511 | 7.230 ± 1.176 | <0.001 |
| Current length of the child (cm; mean ± sd) | 71.610 ± 6.393 | 72.695 ± 6.367 | 70.530 ± 6.249 | <0.001 |
| LAZ (mean ± sd) | −1.762 ± 1.404 | −0.827 ± 1,050 | −2.693 ± 1.050 | <0.001 |
| WAZ (mean ± sd) | −1.341 ± 1.589 | 0.010 ± 0,940 | −2.692 ± 0.714 | <0.001 |
| WLZ (mean ± sd) | −0.546 ± 1.487 | 0.602± 1.036 | −1.688 ± 0.851 | <0.001 |
| Current head circumference (cm; mean ± sd) | 44.592 ± 2.265 | 45.285 ± 1.996 | 43.903 ± 2.311 | <0.001 |
| Child care | ||||
| Was the child fed the first milk (colostrum)? (n/total; %) | 340/400 (85%) | 185/200 (93%) | 155/200 (78% | <0.001 |
| At what age (in days) did you first give your child plain water, sugar water, honey water, or juice or cow’s or goat’s milk? (median; range) | 90; 1–270 | 120; 1–240 | 90; 1–270 | 0.001 |
| Are you still breastfeeding? (n/total; %) | 220/401 (55%) | 122/200 (61%) | 98/201 (49%) | 0.016 |
| Characteristics of the mother/caregiver | ||||
| (Mother) How many years of schooling have you completed? (mean ± sd) | 8.309 ± 3.021 | 7.881 ± 3.055 | 8.794 ± 2.918 | 0.014 |
| (Mother) How many pregnancies have you had in your lifetime? (mean ± sd) | 3.000 ± 1.958 | 3.315 ± 1.952 | 2.640 ± 1.911 | 0.006 |
| (Mother) How many live births have you had in your lifetime? (mean ± sd) | 2.709 ± 1.722 | 2.923 ± 1.674 | 2.464 ± 1.748 | 0.032 |
| (Mother) Weight (kg) (mean ± sd) | 58.343 ± 13.399 | 59.343 ± 12.698 | 57.321 ± 14.041 | 0.050 |
| (Mother) Height (cm) (mean ± sd) | 151.889 ± 6.087 | 152.604 ± 5.701 | 151.158 ± 6.392 | 0.024 |
| Socio-economic status | ||||
| What is the main source of drinking water for members of your household? (n=Piped into dwelling or to yard/plot or public tap/stand pipe/Total; %) | 246/271 (91%) | 134/143 (94%) | 112/128 (88%) | 0.018 |
| Does your household have an electric fan? (n/total; %) | 230/271 (85%) | 129/143 (90%) | 101/128 (79%) | 0.011 |
| Does your household have a bicycle? (n/total; %) | 160/271 (59%) | 94/143 (66%) | 66/128 (52%) | 0.019 |
| Morbidity | ||||
| Short of breath? (in the last two weeks) | 53/375 (13%) | 34/185 (18%) | 19/190 (10%) | 0.026 |
The Student t test was used for normally distributed variables and the Mann-Whitney test for variables whose distribution was not normal. The Chi-square analysis was used for contingency table analyses. LAZ, WAZ and WHZ mean length-for-age, weight-for-age and weight-for-length z-scores, respectively.
The mothers/caregivers of nourished children weighed more, were taller, had more pregnancies, had more live births, and had fewer years of schooling than those of malnourished children. Regarding socio-economic status, greater proportion of the nourished group had an electric fan or bicycle and had a better source of drinking water for the household.
Table 2 shows the results of the multivariable logistic regression analysis, and the most significant factors for malnutrition are shown in Supplemental Digital Content 2 (Figure). Children who were not fed colostrum (OR = 3.29, 95% confidence interval [CI] 1.73–6.26), did not have an electric fan (OR = 2.46, 95% CI 1.22–4.96) or bicycle (OR = 1.80, 95% CI 1.10–2.95) in the household, or had a mother ≥18 years old (OR = 1.88, 95% CI 1.10–3.22) had greater odds of being malnourished. Children with a greater birth weight (OR = 0.27, 95% CI 0.19–0.38), who experienced shortness of breath in the last two weeks (OR = 0.49, 95% CI 0.27–0.90), or with a larger head circumference (OR = 0.74, 95% CI 0.66–0.82) had lower odds of being malnourished.
Table 2.
Variable by group associated with malnourished or nourished children selected from those (See Supplement Table 1) in the univariate analysis at significance level ≤ 25% for the multivariable logistic regression analysis.
| Child anthropometrics | p | Odds ratio | 95% CI (OR) | |
|---|---|---|---|---|
| Birth weight (Kg) | 0.000 | 0.267 | 0.187 –0.382 | |
| Head circumference (cm) | 0.000 | 0.736 | 0.664 –0.816 | |
| Child care | ||||
| Took colostrum | No | 0.000 | 3.288 | 1.726 – 6.261 |
| Yes | - | 1.000 | - | |
| Characteristics of the mother/caregiver | ||||
| (Mother) How old were you when you first became pregnant? | < 18 | - | 1.000 | - |
| ≥ 18 | 0.021 | 1.883 | 1.100 – 3.222 | |
| (Mother) How many pregnancies have you had in your lifetime? | 0.022 | 0.952 | 0.914 – 0.993 | |
| Socio-economic status | ||||
| Does your household have an electric fan? | No | 0.0121 | 2.458 | 1.217 – 4.963 |
| Yes | - | 1.000 | - | |
| Does your household have a bicycle? | No | 0.0202 | 1.799 | 1.096 – 2.952 |
| Yes | - | 1.000 | - | |
| Morbidities | ||||
| Short of breath in the last two weeks? | No | - | 1.000 | - |
| Yes | 0.022 | 0.493 | 0.270 – 0.901 | |
Table 3 and Supplemental Digital Content 3 (Figure) show the results of the second multivariate logistic regression analysis with the variables that had a significance level ≤6%. Children with a greater birth weight (OR = 0.38, 95% CI 0.25–0.58) or shortness of breath in the last two weeks (OR = 0.34, 95% CI 0.15–0.79) had lower odds of being malnourished, and children in a household without an electric fan (OR = 2.17, 95% CI 0.978–4.83; P = 0.057) had borderline greater odds of being malnourished.
We ran Fisher’s test or Pearson correlation for each of our predictor variables that met the basic univariate inclusion criteria for our multivariate model. Most of our predictive variables had P > 0.05, only a few like birth weight and head circumference had P < 0.05 (in this case we leave out this last variable from the logistic model), suggesting that these variables did not have association or correlation. Collinearity is not a substantial contributing factor for our models despite the large number of variables measured in this study. The electric fan does add some increased interpretability to our final model and we prefer to include it although it does not strictly meet the 0.05 probability criterion. We ran additional analysis taking out the electric fan from the multivariable logistic regression model and it did not change much the P value and Odds ratio of the other variables, showing that electric fan it is not collinearity with these variables. We ran also the Fisher’s test or Pearson correlation between these variables included in the logistic model and did not find significant association or correlation (P > 0.05).
Prevalence of enteric pathogens in subclinical infection
The top five most prevalent enteric pathogens were atypical enteropathogenic Escherichia coli (aEPEC), enteroinvasive E. coli (EIEC), Giardia spp., enteroaggregative E. coli (EAEC), and C. jejuni/coli (Table 4 and Figure 2). A greater proportion of the malnourished group had EAEC. The plasmid-encoded dispersin translocator (aatA) gene was detected more often in the malnourished group than in the nourished group (88/278; 32% versus 66/280; 24%; p = 0.037; Chi-square test). The combination of enteric pathogens, stratified as none, 1, 2, 3 or ≥4, was not different between the malnourished and nourished groups.
Table 4.
Prevalence of enteric pathogens in subclinical infection in a prospective evaluation of malnourished and nourished children.
| Microorganism | Study group stool samples
|
p values | ||
|---|---|---|---|---|
| Total samples N = 558 |
Nourished N = 280 |
Malnourished N = 278 |
||
| Enteropathogenic E. coli (EPEC; bfpA + eae) | 49/558 (8.8) | 24/280 (8.6) | 25/278 (9.0) | 0.882 |
| Atypical enteropathogenic E. coli (aEPEC; eae only) | 131/558 (23.5) | 68/280 (24.3) | 63/278 (22.7) | 0.690 |
| Enteroaggregative E. coli (EAEC; aatA + aaiC) | 102/558 (18.3) | 43/280 (15.4) | 59/278 (21.2) | 0.046 |
| Shiga toxin producing E. coli (STEC; stx1 or stx2) | 56/558 (10.0) | 30/280 (10.7) | 26/278 (9.4) | 0.673 |
| Entero invasive E. coli (EIEC; ipaH) | 97/558 (17.4) | 47/280 (16.8) | 50/278 (18.0) | 0.738 |
| Enterotoxigenic E. coli (ETEC; LT and/or ST) | 59/558 (10.6) | 28/280 (10.0) | 31/278 (11.2) | 0.682 |
| Campylobacter jejuni/coli (TacMan probes) | 34/288 (11.8) | 21/141 (14.9) | 13/147 (8.8) | 0.143 |
| Shigella spp | 9/558 (1.6) | 4/280 (1.4) | 5/278 (1.8) | 0.751 |
| Salmonella spp | 0/558 (0.0) | 0/280 (0.0) | 0/278 (0) | - |
| Aeromonas spp | 0/557 (0) | 0/0/280 (0) | 0/277 (0) | - |
| Adenovirus | 17/556 (3.1) | 6/279 (2.2) | 11/277 (4.0) | 0.229 |
| Astrovirus | 9/554 (1.6) | 3/277 (1.1) | 6/277 (2.2) | 0.504 |
| Rotavirus | 4/553 (0.7) | 2/278 (0.7) | 2/275 (0.7) | 1.000 |
| E. histolytica | 4/586 (0.7) | 2/279 (0.7) | 2/277 (0.7) | 1.000 |
| Giardia spp | 109/558 (19.5) | 57/280 (20.4) | 46/278 (16.5) | 0.276 |
| Cryptosporidium spp | 19/558 (3.4) | 12/280 (4.3) | 7/278 (2.5) | 0.351 |
ns means “not significant” by Fisher’s exact test (one or two-sided p values).
Figure 2.

Pathogens detected in malnourished and nourished stool samples.
EAEC=enteroaggregative Escherichia coli; EIEC=enteroinvasive E. coli; aEPEC=atypical enteropathogenic E. coli; tEPEC=typical enteropathogenic E. coli; LT/ST-ETEC=LT/ST-producing enterotoxigenic E. coli; STEC=Shiga-toxin-producing E. coli. Pathogens present in less than 1% of stool samples are not shown.
Gut function and local and systemic immune-related inflammatory responses
Nourished children had more vigorous systemic innate immune responses than the malnourished children, as indicated by the higher concentrations of SAA-1 (P < 0.001) and sCD14 (P < 0.001) (Table 5). Calprotectin concentration was borderline (P = 0.056) higher in the malnourished group than in the nourished group. The SAA-1 and sCD14 biomarker concentrations were higher in the upper 75th percentile of nourished children than in the lower 25th percentile of malnourished children, based on WAZ, LAZ, or WLZ (Table 6). I-FABP and anti-LPS IgG concentrations were higher in the lower 25th percentile of the malnourished children based on LAZ and WAZ, respectively, than in the upper 75th percentile of nourished children. Malnourished children with subclinical EAEC infection had higher concentrations of IgA and IgG against LPS than the nourished children with the same subclinical infection (Figure 3).
Table 5.
Gut function, local and systemic immune-inflammatory response biomarkers in malnourished and nourished children
| Study groups
|
||||
|---|---|---|---|---|
| Biomarkers (median; range) | Total N = 402 |
Nourished N = 201 |
Malnourished N = 201 |
p values |
| Intestinal absorption, permeability, damage and repair | ||||
| % Lactulose excretion (%L) | 0.27 | 0.27 | 0.27 | 0.574 |
| 0.00–1.65 | 0.01–1.15 | 0.00–1.65 | ||
| % Mannitol excretion (%M) | 3.38 | 3.21 | 3.62 | 0.157 |
| 0.03–23.55 | 0.03–23.55 | 0.05–12.91 | ||
| Lactulose:mannitol ratio (LM ratio) | 0.08 | 0.09 | 0.07 | 0.151 |
| 0.01–2.83 | 0.02–2.45 | 0.01–2.83 | ||
| Alpha-1-antitrypsin (AAT; ng/ml) | 10.06 | 9.87 | 10.38 | 0.851 |
| 0.06–89.43 | 0.17–88.25 | 0.06–89.43 | ||
| Fatty acid-binding protein (I-FABP; pg/mL) | 851.71 | 751.73 | 903.99 | 0.207 |
| 0.00–16999.41 | 0.00–10848.90 | 0.00–16999.41 | ||
| Lithostathine-1-beta (Reg1β; ng/mL) | 27.01 | 31.44 | 23.77 | 0.662 |
| 7.38–118.89 | 7.52–118.89 | 7.38–100.20 | ||
| Enteric immune and inflammatory responses | ||||
| Myeloperoxidase (MPO; ng/mL) | 3480.78 | 3770.00 | 3169.72 | 0.605 |
| 8.88–42070.24 | 20.37–42070.24 | 8.88–39846.90 | ||
| Neopterin (Neo; nmol/L) | 966.98 | 1040.71 | 954.39 | 0.150 |
| 6.16–1931.30 | 30.03–2271.96 | 6.16–1793.44 | ||
| Calprotectin (Cal; ng/mL) | 1006.21 | 979.59 | 1025.31 | 0.056 |
| 0.00–18737.80 | 0.00–18737.80 | 0.00–8960.12 | ||
| Systemic immune and inflammatory responses | ||||
| High sensitivity c-reactive protein (hsCRP; ng/mL) | 1.13 | 1.21 | 1.09 | 0.640 |
| 0.20–78.70 | 0.20–69.90 | 0.20–78.70 | ||
| Serum amyloid A-1 (SAA-1; ng/mL) | 219.54 | 313.24 | 0.00 | <0.001 |
| 0.00–11627.05 | 73.52–11627.05 | 0.00–9288.50 | ||
| Lipopolysaccharide binding protein (LBP; ng/mL) | 9664.09 | 9493.04 | 9723.04 | 0.544 |
| 0.00–44628.54 | 0.00–42122.81 | 2745.50–44628.54 | ||
| Soluble cluster of differentiation protein 14 (sCD14; ng/mL) | 4070.18 | 4886.42 | 3492.70 | <0.001 |
| 0.00–18737.80 | 0.00–18737.80 | 0.00–11014.38 | ||
| Immunoglobulin A against LPS (LPSIgA; AMU/mL) | 0.43 | 0.41 | 0.46 | 0.336 |
| 0.06–3.24 | 0.06–1.92 | 0.06–3.24 | ||
| Immunoglobulin G against LPS ( LPSIgG; GMU/mL) | 0.65 | 0.61 | 0.67 | 0.272 |
| 0.02–2.58 | 0.02–2.23 | 0.06–2.58 | ||
The Student t test was used for normally distributed variables and the Mann-Whitney test for variables whose distribution was not normal.
Table 6.
Gut function, systemic immune and inflammatory responses association with nutritional z-scores for lower 25th percentile malnourished versus higher 75th percentile nourished children in the Mal-Ed case-control study.
| Biomarkers | N | Median | Range | |
|---|---|---|---|---|
| Fatty acid-binding protein (I-FABP; pg/mL) | Percentile LAZ
|
|||
| ≥ −0.1200 | 120 | 722.31 | 0.00 – 10848.90 | |
| ≤ −3.2300 | 37 | 1239.92 * | 268.61 – 16999.41 | |
|
| ||||
| Soluble cluster of differentiation protein 14 (sCD14; ng/mL) | ≥ −0.1200 | 114 | 4974.28 * | 0.00 – 18737.80 |
| ≤ −3.2300 | 37 | 3567.35 | 1138.18 – 7786.70 | |
|
| ||||
| Serum amyloid A-1 (SAA-1; ng/mL) | ≥ −0.1200 | 120 | 313.24 * | 80.57 – 11357.95 |
| ≤ −3.2300 | 37 | 0.00 | 0.00 – 8173.74 | |
|
| ||||
| Soluble cluster of differentiation protein 14 (sCD14; ng/mL) | Percentile WAZ
|
|||
| ≥ 0.6250 | 64 | 4974.28 * | 0.00 – 10418.30 | |
| ≤ −2.9400 | 37 | 3883.17 | 0.00 – 11014.38 | |
|
| ||||
| Serum amyloid A-1 (SAA-1; ng/mL) | ≥ 0.6250 | 64 | 319.03 * | 80.57 – 9295.29 |
| ≤ −2.9400 | 37 | 0.00 | 0.00 – 9288.50 | |
|
| ||||
| Immunoglobulin G against LPS ( LPSIgG; GMU/mL) | ≥ 0.6250 | 58 | 0.57 | 0.09 – 2.13 |
| ≤ −2.9400 | 39 | 0.83 * | 0.06 – 2.17 | |
|
| ||||
| Soluble cluster of differentiation protein 14 (sCD14; ng/mL) | Percentile WLZ
|
|||
| ≥ 1.1525 | 38 | 4505.19 * | 795.13 – 10418.30 | |
| ≤ −2.1650 | 40 | 3437.08 | 0.00 – 11014.38 | |
|
| ||||
| Serum amyloid A-1 (SAA-1; ng/mL) | ≥ 1.1525 | 39 | 289.36 * | 84.09 – 4381.41 |
| ≤ −2.1650 | 40 | 7.31 | 0.00 – 9288.50 | |
The Student t test was used for normally distributed variables and the Mann-Whitney test for variables whose distribution was not normal. LAZ, WAZ and WHZ mean length-for-age, weight-for-age and weight-for-length z-scores, respectively.
P ≤ 0.05 was considered statistic significant.
Figure 3.

Immunoglobulin A and G antibodies against LPS on malnourished and nourished children with enteroaggregative Escherichia coli subclinical infection.
Discussion
We first study the homogeneity between the malnourished and nourished children. These results showed clearly that there are a number of variables that are relevant for a broad approach to the intervention and prevention of childhood malnutrition, such as mother/caregiver characteristics, anthropometry of the child, childcare, socio-economic status, and malnutrition-associated morbidities, not only in the bivariate analysis but also in the logistic regression analysis, which was conducted to determine the most important variables. The higher odds of finding that children of mothers ≥ 18 years old are malnourished is explained by the need for such mothers to work outside of their houses in order to provide better wages for the family, is a very common practice in urban communities in Brazil.
A study of the causes for the decreased prevalence of undernutrition in children in Brazil in 1996–2007 showed that there were four associated factors: higher levels of maternal schooling, increased purchasing power of families, expansion of healthcare, and improvements in sanitation.30 A community-based cross-sectional study conducted with children aged 0–5 years in tribal areas of India also showed that maternal literacy status, household wealth index, and morbidities were associated with underweight and stunting.31 In a cross-sectional study to evaluate the risk factors for undernutrition among children <36 months of age in the Kilimanjaro region of Tanzania, a diverse set of variables, including maternal education, child’s age, and distance to a water source, were associated with underweight.32 Recently, a survey of children aged 0–6 years in 30 cities in the Fars province, Iran showed a diverse set of variables associated with underweight, including sex, settlement area, family size, ethnicity, family income, maternal education, health services, and safe water supply.33 Collectively, these results demonstrate the diversity of the variables associated with malnutrition and the importance of identifying the important variables by specific geographic setting in a multicenter study design.
EPEC, EIEC, Giardia spp., EAEC, and C. jejuni/coli were the most prevalent pathogens in stool samples from both nourished and malnourished children in the present study. Moreover, the number of children with EAEC infection was significantly different based on nutritional status. The most prevalent enteric pathogens associated with moderate to severe diarrhea in the Global Enteric Multicenter Study, which studied the etiology and burden of diarrheal diseases in children 0–59 months old in Africa and Asia, were rotavirus, Cryptosporidium, enterotoxigenic E. coli producing heat-stable toxin (with or without heat-labile enterotoxin co-expression), and Shigella.34 The children in which these pathogens were detected had growth deficit, as measured using the height-for-age z score 50–90 days later, suggesting that these pathogens influence nutritional status. Recent data from the MAL-ED Cohort Study Network also showed that enteric pathogen burden and, in particular, high exposure to Campylobacter or EAEC was associated with a growth deficit.35 EAEC has consistently been associated with malnutrition in the present and previous studies, with effects on growth even without overt diarrhoea.16
Two systemic immune and inflammatory biomarkers, sCD1436,37 and SAA-138, were higher in the nourished than in the malnourished children, supporting that these children had chronic, endemic enteropathy with enteric pathogen contamination that resulted in LPS/bacterial translocation; however, the nourished children had a better immune response.
Biomarkers for intestinal and systemic inflammation, including myeloperoxidase, neopterin, and calprotectin, were present in high concentrations in both groups, with or without breastfeeding (data not shown), suggesting chronic, endemic environmental enteropathy. Plasma calprotectin, which has been used as a biomarker for systemic neutrophil elevation,39–41 was higher in the malnourished than the nourished children. To examine the associations between these biomarkers and the clinical growth parameters (LAZ, WAZ, and WLZ), we compared the results between the lower 25th percentile of malnourished children and the upper 75th percentile of nourished children. sCD14 and SAA-1 were consistently higher, across all z scores, in the nourished than in the malnourished children, while IgG against LPS was higher in children with higher WAZ, suggesting a better immune response against LPS/gram-negative bacteria translocation in the nourished children.
Furthermore, I-FABP, a biomarker associated with intestinal damage,42 was higher in children with lower LAZ scores, suggesting intestinal damage. In the sub-analysis of children infected with EAEC, the malnourished children had higher IgA and IgG against LPS concentrations than the nourished children, which is consistent with intestinal damage, as measured by I-FABP, and potential translocation of LPS/EAEC specific enteric pathogens in malnourished children. We have recently also published extensive biomarker findings also analyzed with growth data were available in these children and find that higher fecal MPO, alpha-1-antitrypsin, L/M, plasma LPS, I-FABP and SAA associated with impaired subsequent growth, while better growth was predicted by plasma citrulline (in girls) and tryptophan (in boys)43.
This study had some limitations. Because the MAL-ED protocol was not designed to assess the association between illness and malnutrition in children, daily morbidity data were not collected. Data regarding the quantity and quality of complementary food intake that could influence malnutrition were also not collected. When we ran the multivariate model we lost some of these children in the analysis and this is also a limitation of this study. Our protocol design was to match subjects on age and gender. We set our inclusion criteria for age to 6–24 months, which age range we considered close enough to declare that an index subject’s age is ‘matched’ with a reference subject’s age if within that range. Our goal was met with all children successfully enrolled within that age range. The result was that mean months of age of control children (12.4 mos (sd=5.45) was 2.4 months younger compared to case children (14.8 mos (sd=5.4). In response to this observation, all results presented were statistically adjusted for child age. Note that inclusion of child age added little predictive variance. Despite these limitations, the study also had several strengths. First, the MAL-ED study comprehensively collected information for neonatal factors, anthropometry, childcare, mother/caregiver characteristics, socio-economic status, and morbidities that enabled identification of key variables associated with malnutrition. Second, quality control was ensured by the standardized protocol and data collection by a trained team. Third, the study used an expansive panel of bacterial, viral, and parasitic enteric pathogens and a standardized microbiology protocol.20
In summary, neonatal, maternal, and socio-economic status factors were significantly associated with malnutrition in children. Enteric inflammatory biomarkers were elevated, suggesting environmental enteropathy. Plasma concentrations of the systemic immune-related inflammatory biomarkers SAA-1 and sCD14 protein were elevated in both malnourished and nourished children and were significantly higher in the nourished children, suggesting a better systemic immune response. EAEC was more prevalent in malnourished children, who also had higher plasma I-FABP concentrations, suggesting intestinal epithelial damage. Malnourished children with EAEC colonization had higher plasma IgA and IgG against LPS concentrations, suggesting damage to intestinal barrier function with LPS/EAEC bacterial translocation. These data emphasize the need to improve maternal and neonatal care as well as piped water supply and monthly income factors.
Supplementary Material
Characteristics of child anthropometrics, child care, mother or caregiver, socio-economic status and child morbidities factors in malnourished and nourished children on the univariate analysis.
Odds of being in the malnourished category at enrolment for the top risk and protective association factors.
General model of multivariable logistic regression analysis for association factors of being in the malnourished group of children.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation case-control component of the “Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development” Project (MAL-ED), and in part as a carried out as a collaborative Biomarker Grants No. OPP1066140 entitled, “Novel metabonomic biomarkers of gut function and health: Modeling enteropathy (EE) and field validation”, project also supported by the Bill & Melinda Gates Foundation, the Foundation for the NIH, and the National Institutes of Health, Fogarty International Center. The authors thank the staff and participants of the MAL-ED Network for their important contributions. No authors declare a conflict of interest.
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
References
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Child Health Epidemiology Reference Group of WHO and UNICEF. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Guerrant RL, Oriá RB, Moore SR, et al. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoddinott J, Behrman JR, Maluccio JA, et al. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013;98:1170–1178. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBoer MD, Lima AA, Oría RB, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70:642–653. doi: 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Checkley W, Buckley G, Gilman RH, et al. Childhood Malnutrition and Infection Network. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–30. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keusch GT, Denno DM, Black RE, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59(Suppl 4):S207–S212. doi: 10.1093/cid/ciu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaible UE, Kaufmann SHE. Malnutrition and Infection: Complex Mechanisms and Global Impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Guideline: Updates on the management of severe acute malnutrition in infants and children. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 10.Mata LJ, Kronmal RA, Garcia B, et al. Breast-feeding, weaning and the diarrhoeal syndrome in a Guatemalan Indian village. Ciba Found Symp. 1976;42:311–338. doi: 10.1002/9780470720240.ch17. [DOI] [PubMed] [Google Scholar]
- 11.Guerrant RL, Kirchhoff LV, Shields DS, et al. Prospective study of diarrhoeal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis. 1983;148:986–997. doi: 10.1093/infdis/148.6.986. [DOI] [PubMed] [Google Scholar]
- 12.Lima AA, Moore SR, Barboza MS, et al. Persistent diarrhoea signals a critical period of increased diarrhoea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis. 2000;181:1643–1651. doi: 10.1086/315423. [DOI] [PubMed] [Google Scholar]
- 13.Moore SR, Lima AA, Conaway MR, et al. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 14.Black RE, Brown KH, Becker S. Effects of diarrhoea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 15.Checkley W, Gilman RH, Epstein LD, et al. Asymptomatic and symptomatic cryptosporidiosis: Their acute effect on weight gain in Peruvian children. American Journal of Epidemiology. 1997;145:156–163. doi: 10.1093/oxfordjournals.aje.a009086. [DOI] [PubMed] [Google Scholar]
- 16.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 17.da Silva Quetz J, Lima IF, Havt A, et al. Campylobacter jejuni and Campylobacter coli in children from communities in Northeastern Brazil: molecular detection and relation to nutritional status. Diagn Microbiol Infect Dis. 2010;67:220–227. doi: 10.1016/j.diagmicrobio.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee G, Paredes Olortegui M, Peñataro Yori P, et al. Effects of Shigella-, Campylobacter- and ETEC-associated diarrhoea on childhood growth. Pediatr Infect Dis J. 2014;33:1004–1009. doi: 10.1097/INF.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 19.Correia LL, Silva AC, Campos JS, et al. Prevalence and determinants of child undernutrition and stunting in semiarid region of Brazil. Rev Saude Publica. 2014;48(1):19–28. doi: 10.1590/S0034-8910.2014048004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima AA, Oriá RB, Soares AM, et al. Geography, population, demography, socioeconomic, anthropometry, and environmental status in the MAL-ED cohort and case-control study Sites in Fortaleza, Ceará, Brazil. Clin Infect Dis. 2014;59(Suppl 4):S287–S294. doi: 10.1093/cid/ciu438. [DOI] [PubMed] [Google Scholar]
- 21.WHO Multicentre Growth Reference Study Group. Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr. 2006;450:38–46. doi: 10.1111/j.1651-2227.2006.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 22.Richard SA, Barrett LJ, Guerrant RL, Checkley W, et al. Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin Infect Dis. 2014;59(Suppl 4):S220–S224. doi: 10.1093/cid/ciu435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houpt E, Gratz J, Kosek M, et al. Microbiologic Methods Utilized in the MAL-ED Cohort Study. Clinical Infectious Diseases. 2014;59:S225–S232. doi: 10.1093/cid/ciu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham SA, Sloan LM, Nyre LM, et al. Three-hour molecular detection of Campylobacter, Salmonella, Yersinia, and Shigella species in feces with accuracy as high as that of culture. J Clin Microbiol. 2010;48:2929–2933. doi: 10.1128/JCM.00339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Gratz J, Amour C, et al. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol. 2013;51(2):472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barboza MS, Guerrant RL, Lima AA. Measurement of intestinal permeability using mannitol and lactulose in children with diarrhoeal diseases. Braz J Med Biol Res. 1999;32:1499–1504. doi: 10.1590/s0100-879x1999001200008. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler TR, Luo M, Estívariz CF, et al. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294:R402–R410. doi: 10.1152/ajpregu.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The MAL-ED Network Investigators. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014;59(Suppl 4):S193–S206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- 29.Pagano’s M, Gauvreau’s K. Principles of Biostatistics. 2. Duxbury Press; Pacific Grove, CA: 2000. [Google Scholar]
- 30.Monteiro CA, Benicio MH, Konno SC, et al. Causes for the decline in child under-nutrition in Brazil, 1996–2007. Rev Saude Publica. 2009;43(1):35–43. doi: 10.1590/s0034-89102009000100005. [DOI] [PubMed] [Google Scholar]
- 31.Meshram II, Arlappa N, Balakrishna N, et al. Trends in the prevalence of undernutrition, nutrient and food intake and predictors of undernutrition among under five year tribal children in India. Asia Pac J Clin Nutr. 2012;21(4):568–576. [PubMed] [Google Scholar]
- 32.Abubakar A, Uriyo J, Msuya SE, et al. Prevalence and risk factors for poor nutritional status among children in the Kilimanjaro region of Tanzania. Int J Environ Res Public Health. 2012;9(10):3506–3518. doi: 10.3390/ijerph9103506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavosi E, Hassanzadeh Rostami Z, Kavosi Z, et al. Prevalence and determinants of under-nutrition among children under six: a cross-sectional survey in Fars province, Iran. Int J Health Policy Manag. 2014;3(2):71–6. doi: 10.15171/ijhpm.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 35.The MAL-ED contributors. Factors affecting growth velocity and risk factors for stunting in the first 24 months of life: Results from the MAL-ED study. Am J Trop Med Hyg. 2015;93(Supplement 4):242. [Google Scholar]
- 36.Chevalier MF, Petitjean G, Dunyach-Remy C, et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS pathogens. 2013;9:e1003453. doi: 10.1371/journal.ppat.1003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voss S, Welte S, Fotin-Mleczek M, et al. A CD14 domain with lipopolysaccharide-binding and -neutralizing activity. Chembiochem. 2006;7(2):275–286. doi: 10.1002/cbic.200500257. [DOI] [PubMed] [Google Scholar]
- 38.Reisinger KW, Kramer BW, Van der Zee DC, et al. Non-invasive serum amyloid A (SAA) measurement and plasma platelets for accurate prediction of surgical intervention in severe necrotizing enterocolitis (NEC) PLoS One. 2014;9(6):e90834. doi: 10.1371/journal.pone.0090834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cayatte C, Joyce-Shaikh B, Vega F, et al. Biomarkers of Therapeutic Response in the IL-23 Pathway in Inflammatory Bowel Disease. Clin Transl Gastroenterol. 2012;3:e10. doi: 10.1038/ctg.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramma W, Buhimschi IA, Zhao G, et al. The elevation in circulating anti-angiogenic factors is independent of markers of neutrophil activation in preeclampsia. Angiogenesis. 2012;15(3):333–340. doi: 10.1007/s10456-012-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung SY, Park YB, Ha YJ, et al. Serum calprotectin as a marker for disease activity and severity in adult-onset Still’s disease. J Rheumatol. 2010;37(5):1029–1034. doi: 10.3899/jrheum.091120. [DOI] [PubMed] [Google Scholar]
- 42.Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility Clin Biochem. 2003;36(7):529–535. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 43.Guerrant RL, Leite AM, Pinkerton R, et al. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in Northeast Brazil. PLOS ONE. 2016 Sep 30; doi: 10.1371/journal.pone.0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of child anthropometrics, child care, mother or caregiver, socio-economic status and child morbidities factors in malnourished and nourished children on the univariate analysis.
Odds of being in the malnourished category at enrolment for the top risk and protective association factors.
General model of multivariable logistic regression analysis for association factors of being in the malnourished group of children.