Abstract
Background
Prediction of post-transplant lymphoproliferative disorder (PTLD) after pediatric lung transplant remains difficult. Use of Epstein-Barr virus (EBV) viral load (VL) in whole blood (WB) has been poorly predictive, while measurement of viral load in bronchoalveolar lavage (BAL) fluid has been suggested to have enhanced utility.
Methods
The NIH-sponsored Clinical Trials in Organ Transplantation in Children (CTOTC-03) prospectively obtained serial quantitative measurements of EBV PCR in both WB and BAL fluid after pediatric lung transplantation. Descriptive statistics, contingency analyses, and Kaplan-Meier analyses evaluated possible association between EBV and PTLD.
Results
Of 61 patients, 34 (56%) had an EBV+ PCR (at least once in WB or BAL). EBV donor (D) + patients more often had a positive PCR (D+/recipient (R) -: 13/18; D+/R+: 14/23) compared to EBV D- patients (6/17). Several D-/R- (5/12) patients developed EBV but none developed PTLD. All 4 PTLD patients were D+/R- with EBV+ PCR. Neither the time to first EBV+ PCR nor the cycle threshold for PCR positivity in BAL or WB was statistically different between those with and without PTLD.
Conclusion
Having an EBV-seropositive donor was associated with increased risk of EBV+ PCR in WB. EBV load in BAL was not predictive of PTLD.
Keywords: Lung transplantation, Pediatrics, Epstein-Barr Virus (EBV), Post-transplant Lymphoproliferative Disorder (PTLD), Bronchoalveolar lavage
Introduction
Post-transplant lymphoproliferative disorder (PTLD) is a life-threatening complication following solid organ transplantation. PTLD can range from polyclonal hyperplasia to non-Hodgkin lymphoma [1]. The majority of cases develop within the first 12 months post-transplant [2]. Additional risk factors include immunosuppression with calcineurin inhibitors, anti-T-lymphocyte globulins, young age at transplantation, and positive donor Epstein-Barr virus (EBV) serostatus [3].
Approximately 90% of PTLD cases are EBV positive based on tumor analysis [1]. EBV immortalizes B lymphocytes, a feature that is thought to promote the development of PTLD, especially in the absence of T cell regulation consequent to immunosuppressive therapies [3]. Pediatric patients are often EBV-naïve at transplantation, which places them at increased risk for PTLD, especially if they receive an EBV-seropositive donor organ [3]. The level of EBV DNA in whole blood or plasma has been identified as a possible predictor for PTLD [3]. However, consensus around the absolute viral load threshold associated with PTLD does not exist, and viral load monitoring of blood alone has not been sufficient to predict PTLD [3]. It is hypothesized that more accurate and consistent monitoring strategies may help providers stratify individual patient PTLD risk to promote early intervention to diminish risk and decrease PTLD.
Michelson et al analyzed a retrospective cohort of EBV Donor+/Recipient- (D+/R-) lung and heart-lung transplant patients at PTLD diagnosis and found that elevated EBV levels in bronchoalveolar lavage (BAL) fluid correlated with PTLD more closely than levels in peripheral blood[4]. However, the study was limited by sample size, retrospective design, and limited pre-PTLD samples. Limited samples hindered the ability to assess predictive capability; therefore, prospective, multicenter studies were suggested to verify the results [4].
The present study seeks to evaluate and compare the utility of measuring EBV in BAL fluid and whole blood (WB) to predict PTLD in a prospective cohort of pediatric lung transplant recipients.
Methods
A multi-center, prospective, observational cohort study of the 61 pediatric lung transplant patients who participated in the NIH-sponsored Clinical Trials in Organ Transplantation in Children (CTOTC-03, NCT00891865) was performed. Pediatric patients younger than 19 years old listed for first bilateral lung or heart-lung transplantation were enrolled from 2009-2013. All sites followed the International Pediatric Lung Transplant Collaborative guidelines for immunosuppression (with tacrolimus, mycophenolate mofetil and steroids) and viral prophylaxis including intent to provide prophylactic dosing of ganciclovir or valganciclovir for at least 6 months after transplant [8]. Immunosuppression induction was at the discretion of the local clinical site with IL-2 receptor inhibitors (44), anti-thymocyte globulin (14) or none (3).
Donor and recipient (D/R) EBV serostatus was recorded. Serial quantitative measurements of EBV viral load in both whole blood (WB) and bronchoalveolar lavage fluid (BAL) after pediatric lung transplantation were obtained. Samples were collected at scheduled intervals following the transplant (0, 2, 4-6 weeks and 3, 6, 9, 12 and 18 months) and during symptomatic events for two years post-transplant, batched and sent to a central laboratory. All study sites performed standardized BAL fluid collection according to the study laboratory manual. Clinicians caring for the patients did not have access to the results of these assays, and no center performed quantitative EBV PCR on BAL fluid as part of routine clinical care. Participating sites reported local monitoring of EBV viral load in blood independently of CTOTC-03 protocols. Two of the six participating centers reported intent to adjust immunosuppression based on local EBV blood viral loads at the individual clinician's discretion; however, this was not applied consistently and one center reported no such adjustments to immunosuppression in any EBV PCR + patients other than as treatment for established PTLD.
A quantitative PCR assay was used to measure EBV viral loads (EBV VL) in samples of WB (n=158) and BAL (n=90). Briefly, total nucleic acids were extracted from whole blood specimens using the BioMerieux NucliSens easyMAG automated extractor (BioMerieux Durham, NC) and from bronchoalveolar lavage specimens using the Roche MagNA Pure Compact (Roche Life Sciences Indianapolis, IN). Extracts were amplified using a laboratory-developed quantitative real-time PCR assay. This assay was based on an assay originally described by Wandinger et al [5] that amplifies a segment of the EBV EBNA-1 gene. Primer sequences were modified using the Primer Express program, and the probe used in the original assay was replaced by a TaqMan minor groove binder (MGB) probe. The sequences of the forward (F) and reverse (R) primers and MGB probe were as follows:
| EBV-F | 5′-GGT-AGT-AAG-ACC-TCC-CTT-TAC-AAC-CTA-A-3′ |
| EBV-R | 5′-TGT-AAG-ACG-ACA-TTG-TGG-AAT-AGC-A-3′ |
| EBV-MGB | 5′-6FAM-CGA-GGA-ACT-GCC-C-MGB-3′ |
A set of quantification standards, consisting of a series of six 5-fold dilutions of a commercial quantitated EBV DNA standard (Advanced Biotechnologies Inc. Eldersberg, MD, Cat. # 08-925-000), was included in each run. The PCR assay was run on an Applied Biosystems 7300 or 7500 Real Time PCR Instrument using Applied Biosystems TaqMan Universal PCR Master Mix (Applied Biosystems/ThermoFisher Scientific Foster City, CA) and employing the reaction concentrations and cycling conditions recommended for TaqMan Universal PCR Master Mix. Results were reported as 1) cycle threshold (CT) defined as the PCR cycle number when the fluorescent signal exceeds the threshold for a positive result and 2) copies of EBV DNA per ml of starting sample. CT is inversely related to VL with lower CT (earlier detection) consistent with increased VL. Because the assay standard curve did not allow quantitation of VL <2000 copies/mL, a category into which many samples from the present study fell, CT was used for statistical comparisons between all groups with a CT of approximately >35 consistent with VL<2000 copies/mL.
Statistical Methods
Data collected from the clinical sites and core laboratories was supplied by the Statistical and Clinical Coordinating Center for CTOTC-03 (Rho, Inc., Chapel Hill, NC) and included PTLD status, D/R serostatus, EBV-related graft rejection information, and viral load measurements in WB and BAL for all patient visits.
WB and BAL sample data were catalogued to evaluate for missing samples. Descriptive statistics, chi-square tests, t-tests and Kaplan-Meier plots with log-rank tests were used to test the relationship between EBV PCR CT and the development of PTLD. Comparisons between EBV BAL and WB CT within PTLD and non-PTLD groups were estimated using a random intercepts (subject) model to meet the assumption of normality. Box-plots are used to give a visual description of CT over time (since transplant) for PTLD and non-PTLD groups. All statistical analyses were performed using Microsoft Excel 2013, JMP 12.0, and SAS 9.4 (Cary, NC).
Results
This prospective cohort consisted of 61 pediatric lung (n=56) or heart-lung transplant (n=5) recipients. Thirty-six (59%) were female. The mean age at transplantation was 12.2 years (range 0.7-19.1). Serostatus classifications are as follows: 18 D+/R-, 23 D+/R+, 5 D-/R+, and 12 D-/R-. Three patients had incomplete serostatus information and so were excluded. Additional demographic characteristics, including pre-transplantation diagnoses, are listed in Tables 1 and 2.
Table 1.
Clinical characteristics of CTOTC-03 patients (n=61).a
| EBVPCR + (n=34)/% | EBV PCR -(n=26)/% | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| Sex | 0.17 | ||||
| Female | 23 (68%) | 13 (50%) | |||
|
| |||||
| Ethnicity | 0.77 | ||||
| Hispanic or Latino | 2 (6%) | 3 (12%) | |||
| Not Hispanic or Latino | 25(73%) | 17 (65%) | |||
| Unknown or Not Reported | 7 (21%) | 6 (23%) | |||
|
| |||||
| Race | 0.06 | ||||
| White | 30 (88%) | 19 (73%) | |||
| Black/African-American | 2 (6%) | 3 (12%) | |||
| Asian | 2 (6%) | 0 | |||
| Multiple races | 0 | 1 (3%) | |||
| Unknown/not reported | 0 | 3 (12%) | |||
|
| |||||
| Mean age at transplant (yrs) | 13.7 (3.6-19.1) | 10.2 (0.7-18.8) | 0.02 | ||
|
| |||||
| Transplant type | 0.64 | ||||
| Lung (single or double) | 32 (94%) | 23 (88%) | |||
| Heart-lung | 2 (6%) | 3 (12%) | |||
|
| |||||
| EBV+ serostatus | Either+ | WB+ | BAL+ | BAL and WB - | 0.13 |
| D+/R- | 13 | 13 | 13 | 5 | |
| D-/R+ | 1 | 1 | 1 | 4 | |
| D+/R+ | 14 | 13 | 10 | 9 | |
| D-/R- | 5 | 5 | 4 | 7 | |
|
| |||||
| Death | 0.12 | ||||
| Yes | 8 (24%) | 11 (42%) | |||
| No | 26 (76%) | 15 (58%) | |||
One patient excluded because of incomplete EBV information.
Table 2. CTOTC-03 Pre-transplant Primary Diagnoses.
| Frequency | Percent | |
|---|---|---|
| Acute Respiratory Distress Syndrome | 1 | 2 |
| Bronchiolitis Obliterans | 6 | 10 |
| Cystic Fibrosis | 29 | 48 |
| Eisenmenger's Syn: VSD/Pul HTN/Unrepaired Vent Shunt | 1 | 2 |
| Obliterative bronchiolitis (Non-RE-TXP) | 1 | 2 |
| Other | 16 | 26 |
| Primary Pulmonary Hypertension/Idiopathic Pulmonary Hypertension | 5 | 8 |
| Pulmonary Fibrosis/Chronic Interstitial Pneumonitis | 1 | 2 |
| Pulmonary Fibrosis/Secondary Pulmonary Fibrosis | 1 | 2 |
Of 61 patients, 34 (56%) had an EBV+ PCR in one or more WB or BAL samples. EBV D+ patients more often had an EBV+ PCR compared to EBV D- patients (p<0.05). Of 18 D+/R- patients, 13 (72%) became EBV+ by PCR. Sixty-one percent of D+/R+ patients developed an EBV+ PCR (14/23). Compared to EBV D- organ recipients, donor EBV seropositivity (D+ EBV) was significantly associated with post-transplant EBV+ PCR in WB specimens (p=0.039) but not in BAL specimens (p=0.08) (Table 1). Only 1/5 D-/R+ patients became positive (20%). Additionally, 5/12 (42%) D-/R- patients developed EBV during the study period. Mean time to first EBV+ PCR was 50 days for D-/R- patients, compared to 82 and 130 day for D+/R- and D+/R+, respectively.
Four patients (6.5%) in this cohort developed PTLD. Three received double lung transplants and one received a heart-lung transplant. One was female and all were white. The average age at transplantation was 14.9 years (range 11.9-17.6) (Table 3). One patient died secondary to PTLD while another died secondary to multi-organ failure unrelated to PTLD. All four patients had immunosuppression reduction and were treated with rituximab as part of their PTLD therapy.
Table 3. PTLD Patient Characteristics.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Gender/Age (yr) | Male/14.8 | Female/11.9 | Male/15.2 | Male/17.6 |
| Reason for Txp | PPHTN | PHTN/CHD | BO | CF |
| Txp type | Double lung | Heart-lung | Double lung | Double lung |
| Serostatus | D+/R- | D+/R- | D+/R- | D+/R- |
| Time to PTLD (days) | 328 | 124 | 130 | 244 |
| PTLD Site | Multifocal including tonsil, adenoid, gastrointestinal, lungs and cervical lymph node | Small bowel, kidney | Right hemithorax | Ethmoid Sinus |
| PTLD type | Diffuse large B-cell lymphoma | Diffuse large B-cell lymphoma | Monomorphic | Monomorphic |
| Immunosuppression management | Decreased tacrolimus, discontinued MMF | Decreased cyclosporine, discontinued azathioprine | Tacrolimus and MMF discontinued, steroids decreased | Tacrolimus and MMF decreased |
| Additional therapy | Rituximab Chemotherapy | Rituximab Chemotherapy | Rituximab Chemotherapy | Rituximab Chemotherapy |
| Mortality 2° to PTLD | Yes |
PPHTN: primary pulmonary hypertension; PHTN/CHD: pulmonary hypertension/congenital heart disease; BO: bronchiolitis obliterans; CF: cystic fibrosis.
EBV PCR CT among D+/R- patients with and without PTLD are displayed in Figures 1a-b. The four patients who developed PTLD were a subset of the 13 D+/R- EBV patients with an EBV+ PCR (in either WB or BAL). Within the D+/R- group, the mean EBV PCR CT from BAL in those with PTLD was 37.8 cycles (range 34.8-41.9) compared to 36.5 cycles (range 32-40) in those without PTLD (p=0.54). In the same group, the mean EBV WB PCR CT in those with PLTD was 36.4 cycles (range 32.6-40.1), compared to 37.3 cycles (range 32.3-40) in those without PTLD (p=0.87) (Table 4). Furthermore, EBV PCR CT in the BAL were not appreciably different from WB when the visits closest to the dates of PTLD diagnosis were compared (Table 5).
Figure 1a.
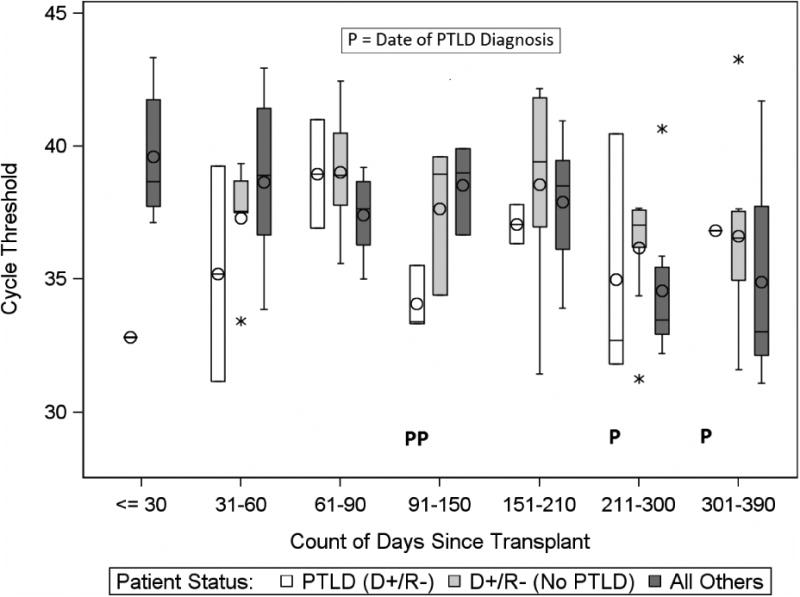
Comparison of EBV PCR Cycle Threshold (CT) in WB among study groups, by time since transplant.
The box (bar) represents the interquartile range (IQR) with the bar in the middle of each box being the median. The circle in each box represents the mean. The whiskers frame values that are <= 1.5 × IQR below Q1 or above Q3. An asterisk above or below the whiskers represents an outlier. “P” indicates a subject diagnosed with PTLD. Note the inverse relationship between CT and the level of EBV DNA in the specimen, with higher CTs indicating lower levels of EBV DNA.
Figure 1b.
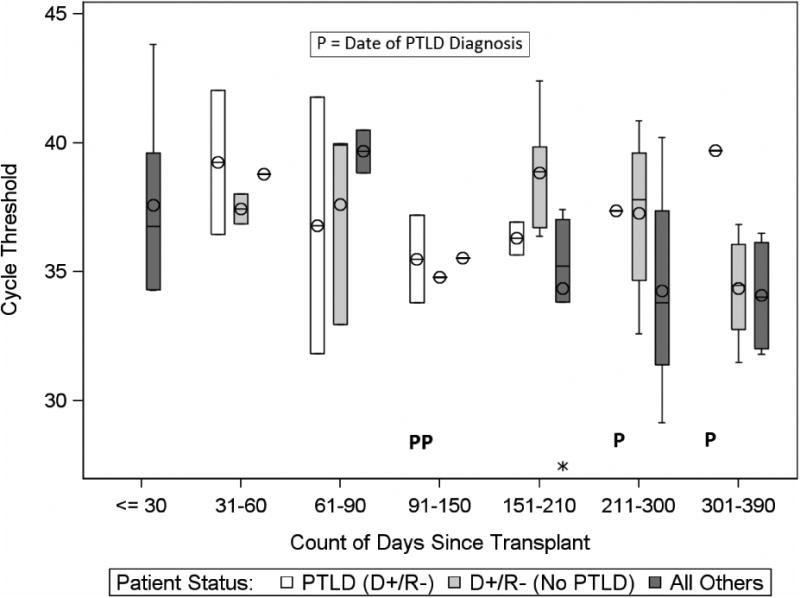
Comparison of EBV PCR Cycle Threshold (CT) in BAL among study groups, by time since transplant.
The box (bar) represents the interquartile range (IQR) with the bar in the middle of each box being the median. The circle in each box represents the mean. The whiskers frame values that are <= 1.5 × IQR below Q1 or above Q3. An Asterisk above or below the whiskers represents an outlier.“P” indicates a subject diagnosed with PTLD. Note the inverse relationship between CT and the level of EBV DNA in the specimen, with higher CTs indicating lower levels of EBV DNA
Table 4.
EBV PCR CT for D+/R- grouped by BAL / WB (values in cycle number). Mean and range for each specimen type are based on subject-means (due to uneven numbers of observations among subjects). Differences between PTLD and non-PTLD groups were estimated based on linear random (subject) effects model (using actual, observation-level data).
| Specimen Type | Mean Crossing Threshold (CT) (Range) | p-value | |
|---|---|---|---|
| PTLD | No PTLD | ||
| BAL | 37.8 (34.8-41.9) |
36.5 (32.0-40.0) |
0.34 |
| WB | 36.4 (32.6-40.1) |
37.3 (32.3-40.0) |
0.53 |
Table 5. WB and BAL CT in specimens obtained closest in time to PTLD diagnosis (in cycle number).
| PTLD Case | Days from Transplant to PTLD Diagnosis | Time of Sample in Relation to PTLD Diagnosis | WB EBV Viral Load | BAL EBV Viral Load |
|---|---|---|---|---|
| 1 | 328 | 28 days post | 36.8 | 37.9 |
| 2 | 124 | 17 days pre | 33.4 | 37.2 |
| 3 | 130 | 43 days pre | 41.0 | 41.8 |
| 4 | 244 | 7 days post | 32.7 | 0 |
Kaplan-Meier estimates assessed time to EBV+ PCR and its association to PTLD. Initially, the WB and BAL compartments were compared separately to assess time to EBV+ PCR. There was no difference between the WB and BAL compartments when all patients who had at least one measurable EBV PCR in either sample type were included (p=0.18). Additionally, a comparison of the time to EBV PCR positivity separated by EBV D/R serostatus groups showed no difference between combinations (p=0.25). The ‘high-risk’ D+/R- patients did not become EBV PCR positive more quickly than other lower risk serostatus groups (D+/R+, D-/R+, D-/R-). Furthermore, the four PTLD patients did not become EBV PCR positive in either compartment (BAL, WB) earlier compared to non-PTLD patients (p=0.99). Among the D+/R- patients, there was no difference in time to EBV PCR positivity in either WB or BAL comparing PTLD to non-PTLD patients (p=0.80, 0.43).
Discussion
PTLD is a life-threatening complication of pediatric lung and heart-lung transplantation for which a biomarker for disease onset and progression is lacking; to date monitoring strategies inadequately predict the development of PTLD in this patient population. This study sought to evaluate serial monitoring of EBV DNA levels in BAL fluid of pediatric lung or heart-lung transplant patients as a predictor of PTLD and to investigate whether measuring EBV load in BAL fluid provides additional value to monitoring in blood [4].
For another important transplant-related virus, cytomegalovirus (CMV), similar monitoring strategies of BAL fluid have shown promise, with BAL sampling being superior to plasma to predict CMV pneumonitis in an adult lung transplant cohort [6]. This particular study followed 43 patients for 12 months post-transplant with serial BAL and blood CMV assays. CMV DNA was detected in BAL and not in plasma for several patients but never in plasma alone. CMV BAL positivity results correlated well with histopathologic diagnosis of CMV infection highlighting the potential of BAL viral monitoring as a diagnostic tool for lung transplant recipients.
While the relationship between CMV pneumonitis and a CMV infection is well-established, the relationship between EBV and PTLD is less straightforward. However, EBV VL monitoring at the site of transplantation appeared promising, especially in light of the findings of Michelson et al. In that study, two of the three PTLD patients had low EBV peripheral blood levels, while all three had greatly increased EBV in the BAL. However, that cohort of pediatric lung transplant patients was small and did not have prospective BAL samples that were used for predictive analyses.
The present study, which prospectively monitored both BAL and WB EBV PCR in the largest pediatric lung transplant cohort to date, found no association of EBV PCR in either BAL or WB with PTLD. A significant relationship between donor EBV seropositivity was detected for EBV PCR positivity in WB with a trend toward significance for BAL. Interestingly, EBV BAL PCRs were not quantitatively different from WB EBV PCRs; however, the primary site of PTLD may have influenced this result with two PTLD events presenting as disseminated disease. The prior study by Michelson et al. did not report the site of PTLD. Neither the BAL nor WB compartment was predictive of PTLD. These findings suggest that monitoring of EBV in either whole blood or BAL may not be effective in predicting the occurrence of PTLD in this population, especially when PTLD is not isolated to the lung.
In the D-/R- serostatus group, 5/12 (42%) became EBV PCR positive during the study. Possible explanations include either nosocomial or community acquisition or inaccurate serostatus assignment. Serostatus assignment is complex, especially in young children who may have detectable maternal antibody for more than a year[7]; however, this would more likely lead to false assignment as EBV seropositive. None of the D-/R- patients in this study were under 12 months of age at the time of transplantation. Administration of blood products that were not screened for EBV may also have confounded the interpretation of EBV+ serostatus [7]. Blood products may also have been a source for EBV acquisition in these patients. Additionally, patients may have been exposed to EBV at home, day care or in the healthcare setting [7]. The ubiquity of EBV infection in the general population increases this possibility. Interestingly, none of the D-/R- patients went on to develop PTLD during the period of follow-up, regardless of whether or not EBV DNA was detected in WB or BAL. This observation combined with an absence of PTLD in patients with negative EBV DNA testing underscores the previously reports of poor positive predictive value and highly reliable negative predictive value of EBV DNA testing [3].
The number of PTLD patients (n=4) and the diversity of PTLD sites including two with disseminated disease in the cohort limit the generalizability of the present study. Additionally, the patients with PTLD ranged from 11 to 17 year of age, which is older than previously reported cohorts of pediatric solid organ transplant recipients. Further, several patients were discharged home three to six months after transplantation; “home” may be quite remote from the study center due to the regional nature of pediatric lung transplantation. Thus, samples were not obtained during all symptomatic events if the patients were not evaluated at the study site during a symptomatic event, especially if the event was more than 3-6 months post-transplant. All PTLD events occurred at least 4 months post-transplant suggesting that early identification through WB or BAL screening could have been missed, especially in PTLD events further removed from the early post-transplant period. Nonetheless, this is the largest study in a pediatric lung transplant population to date and is the only prospective study performed that contains a cohort large enough to investigate the predictive power of EBV DNA levels for early recognition of PTLD. Since the pediatric lung transplant centers that participated in this study followed a shared protocol for care, the opportunity for evaluation during symptomatic events was greatest within the first three months post-transplantation. Furthermore, study centers followed standard immunosuppressive protocols and a difference in rejection episodes between the PTLD and non-PTLD patient populations was not detected.
In conclusion, further investigation is needed to identify screening methods for PTLD. Monitoring of the BAL fluid for EBV DNA was not useful in this study, and so other methods should be pursued. Possible avenues for investigation include refining immunosuppression protocols, increasing surveillance for signs and symptoms of PTLD, or identification of other biomarkers predictive of PTLD.
Acknowledgments
This research was performed as a project of the Clinical Trials in Organ Transplantation in Children a collaborative clinical research project headquartered at the National Institute of Allergy and Infectious Diseases (U01 Grant AI077810 awarded to S. Sweet).
The CTOT-03 consortium members thank the following personnel for the support of the work: Boston Children's Hospital, Boston MA: Dawei Jiang; Children's Hospital of Philadelphia: Rosa Kim, Sara Nguyen; Lucille Packard Children's Hospital at Stanford, Palo Alto, CA: Elisabeth Merkel; Nationwide Children's Hospital, Columbus, OH: Todd Astor, Stephen Kirkby, Ashley Nance, Kerri Nicholson, Susan Meyer; St. Louis Children's Hospital, St. Louis, MO: Colleen Eisenbarger; Texas Children's Hospital, Houston, TX: George Mallory, Mea Ebenbichler.
Abbreviations
- BAL
bronchoalveolar lavage
- CT
cycle threshold
- D/R
donor/recipient (in reference to respective EBV serostatus)
- EBV
Epstein-Barr virus
- PCR
polymerase chain reaction
- PTLD
post-transplant lymphoproliferative disorder
- VL
viral load
- WB
whole blood
Footnotes
Disclosures: None of the authors have any disclosures.
Previously presented at the Research & Service Symposium, University of Cincinnati College of Medicine, Cincinnati, OH and at the International Society of Heart and Lung Transplantation 36th Annual Meeting, Washington D.C.
References
- 1.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 2.Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 2010;23(2):350–66. doi: 10.1128/CMR.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen UD, Preiksaitis JK. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):107–20. doi: 10.1111/ajt.12104. [DOI] [PubMed] [Google Scholar]
- 4.Michelson P, et al. Screening for PTLD in lung and heart-lung transplant recipients by measuring EBV DNA load in bronchoalveolar lavage fluid using real time PCR. Pediatr Transplant. 2008;12(4):464–8. doi: 10.1111/j.1399-3046.2007.00835.x. [DOI] [PubMed] [Google Scholar]
- 5.Wandinger K, et al. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55(2):178–84. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- 6.Westall GP, et al. Human cytomegalovirus load in plasma and bronchoalveolar lavage fluid: a longitudinal study of lung transplant recipients. J Infect Dis. 2004;190(6):1076–83. doi: 10.1086/422327. [DOI] [PubMed] [Google Scholar]
- 7.Danziger-Isakov LA, et al. The risk, prevention, and outcome of cytomegalovirus after pediatric lung transplantation. Transplantation. 2009;87(10):1541–8. doi: 10.1097/TP.0b013e3181a492e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldfarb S, et al. ISHLT Monograph Series: Pediatric Lung Transplantation. 2013;7:98. [Google Scholar]


